Abstract
This research was undertaken to assess the results of repeated exposure to the insecticide; imidacloprid (IMI)-contaminated feed on testicular tissue, spermatogenic cell population, Leydig cell number, and sperm morphology in adult male rabbits (n = 24). The treatment groups received IMI (Bildor® 100 mg/L water spray on green grass)-contaminated green grass without wash (n = 8, not-washed-feed rabbit group) and after wash (n = 8, washed-feed rabbit group) once daily for two weeks on an alternate day basis. The rest of the rabbits, as control, received a normal pesticide-free standard feed. During the exposure time, there was no evident toxic symptom found on regular monitoring of IMI-treated rabbits. Histopathologically, the thickness of tunica albuginea of testes reduced significantly with loosely arranged connective tissues in IMI-treated rabbits. Within the testes, the bizarre-shaped seminiferous tubules were seen with increased lumen diameter in IMI-treated rabbits. The spermatogenic cells were disorganized and detached from the basement membrane in seminiferous tubules of IMI-exposed testes of rabbits. The spermatogenic cell population decreased significantly (P < 0.05) in IMI-treated rabbits compared to control rabbits. Leydig cell number decreased significantly (P < 0.05) in IMI-treated rabbits. A high percentage of morphologically abnormal spermatozoa was seen in IMI-treated rabbits. The degree of the histopathological changes was more prominent in the testes of IMI-exposed not-washed-feed rabbits. The results showed that insecticide-IMI has toxicological effects on testicular tissues, mainly spermatogenic and Leydig cell population of adult rabbits which may cause infertility.
A short running title: Effect of imidacloprid on testicular tissue of rabbits.
Keywords: Imidacloprid, Histopathology, Spermatogenic cells, Leydig cells, Testes, Rabbit
1. Introduction
Pesticide is one of the top ten substances that pose a serious risk to the public's health (WHO, 2020). Imidacloprid (IMI) is a neonicotinoid synthetic insecticide and was most frequently used by farmers in agro-based areas of Bangladesh (Kobir et al., 2020a). Bayer (a multinational pharmaceutical company in Germany) first introduced imidacloprid in 1991 in the USA to protect crops as an alternative to organophosphorus insecticides. IMI ((E)-1- (6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine) has a strong agonistic effect on insect nicotinic acetylcholine receptors (nAChRs), particularly the nicotinic receptor's -subunits and affects the central nervous system of the insect (Matsuda et al., 2005, Bal, 2012). It is registered in more than 120 countries and used to control more than 140 crops, including rice, maize, cotton, potatoes, tomatoes, sugar beets, and fruit plants, from insect attacks (Lewis et al., 2016, Iturburu, et al., 2017). There is widespread evidence that most crop, vegetable, and fruit farmers misuse pesticides throughout the world and the scenario is worst in developing and least developing countries like Bangladesh (Miah et al., 2014, Sharma et al., 2019, Kobir et al., 2020a). Consequently, pesticide has entered our food chain, and it is difficult to avoid its exposure , especially in occupational life (Damalas et al., 2011).
IMI causes oxidative stress, which was detected by oxidative stress marker enzymes such as lipid peroxidation levels, catalase, superoxide dismutase, and glutathione peroxidase in adult male mice (El-Gendy et al., 2010). IMI affects vital organs of multiple systems of mammals and causes inflammation (Komal, 2018). It affects the blood cells and cardiovascular system in rats (Bhardwaj et al., 2010), mice (Bagri et al., 2013), and rabbits (Kobir et al., 2020b). It has also hepatotoxic, nephrotoxic, neurotoxic, and gonadotoxic effects in rats (Mazdak et al., 2009, Duzguner and Erdogan, 2010, Lonare et al., 2014, Mohamed et al., 2018).
Testicular toxicity of insecticides and other toxicants results in male infertility in mammals (Mantovani and Maranghi, 2005). IMI causes vacuolation, degeneration of seminiferous tubules, detachment of germinal cells from the basement membrane, increased interstitial spaces, disrupted basement membrane, decreased Leydig cells number, severe congestion in interstitial spaces, and affects the tunica albuginea in rats (Soujanya et al., 2013). The Japanese quails treated with 1/50 LD50 of IMI revealed degenerative changes in testes, including bizarre cells, the disappearance of spermatogenic cells, devoid of sperm in the tubules, and thickened tunica albuginea of testis (Eissa, 2004).
Reported literature on the effects of IMI using a wide range of doses, routes, and formulations has opened the scope to study from Bangladesh’s perspective. Menon et al. (2014) used IMI 45 mg/bdwt (1/10th of LD50) orally, mixed with 10 ml distilled water for 10–20 days consecutively in rabbits and observed empty seminiferous tubules, and interstitial space became widened. Pyknosis of the spermatocytes and vacuolation in the seminiferous tubules were also observed. Najafi et al. (2010) used IMI @ 225 and 112 mg/kg body weight for two months which caused atrophied seminiferous tubules, arrested spermatogenesis, negative tubular differentiation and repopulation indexes, decreased Leydig cells/mm2 of interstitial tissue, hypertrophy and cytoplasmic granulation of the Leydig cells in mature male rats. Bal et al. (2012) used IMI @ 0.5 and 8 mg/kg body weight for 3 months of oral gavage in adult male rats and showed a significantly increased apoptotic spermatogenic cells in the seminiferous tubules with an elevated level of oxidative stress marker enzymes. These literature reflects that IMI may affect spermatogenic cells and the Leydig cell population in the testes. No study and data have been found to evaluate the residual effects of IMI in the spermatogenic cells population and Leydig cell number, and the morphology of spermatozoa through contaminated feed exposure. Therefore, the present study has been designed to investigate the effect of repeated IMI-exposed feed on the testicular tissues in adult rabbits (Oryctolagus cuniculus). Rabbits were chosen for this research because they are frequently used as models in various laboratory studies, and the outcomes can be extrapolated to other mammalian species.
2. Materials and methods
2.1. Ethic al approval
The present study and all experimental protocols were approved and performed according to the guidelines for the care and use of animals as established by the Animal Welfare and Experimentation Ethics Committee, Bangladesh Agricultural University, Mymensingh, Bangladesh (AWEEC/BAU/2019–41).
2.2. Experimental animal
A total of twenty-four (24) adult male rabbits (Oryctolagus cuniculus) aged greater than 6 months were collected from the Rabbit Palli, Muktagacha, Mymensingh, Bangladesh. The rabbits were subsequently acclimated for seven days in the animal shade of the Bangladesh Agricultural University's Department of Anatomy and Histology, which had enough light and ventilation. The rabbits were given unlimited amounts of fresh, green grass, wheat bran, and water. The rabbits were divided into control (n = 8) and pesticides exposed (n = 16) rabbits. Rabbits exposed to pesticides were split into two groups: not-washed-feed rabbits (n = 8; pesticide-sprayed green grass was fed right away without washing) and washed-feed rabbits (n = 8; pesticide-sprayed green grass was fed after washing). Regular pesticide-free fresh green grass and wheat bran were given to the control rabbits. Dietary habits concerning water and food consumption were recorded during the study. All rabbits were observed regularly for health conditions and different toxic symptoms (any signs which seemed as abnormal due to IMI- exposure including feeding, defecation, movement, gathering, chirping, alertness, response to any kind of external stimuli, etc.) were recorded as well.
2.3. Chemical preparation and exposure of animals
Imidacloprid (marketed under the trade name Bildor® by Corbel International ltd.) was acquired from the licensed pesticide vendor at City Corporation Market in Mymensingh, Bangladesh, and properly handled using the necessary safety measures. According to the datasheet for agricultural pest management, the imidacloprid (IMI) 0.5 ml (100 mg)/liter was adequately diluted with fresh tap water. In the evening, the properly mixed pesticide water was sprayed on green grass in a fenced and restricted field using Suja Global Hand Sprayer Machine (SEGARTEX, Dhaka-1230, Bangladesh). The IMI-sprayed green grass was harvested the following morning and given to the rabbits (classified as not-washed-feed rabbits) and after washing (classified as washed-feed rabbits), with unlimited access to water for up to 15 days every other day. On pesticide-free days supplied regular pesticide free green grass and wheat bran with drinking water ad libitum. All rabbits were observed regularly, and their health condition was recorded. At the end of the pesticide exposure, imidacloprid-exposed and control rabbits were sacrificed.
2.4. Collection of testes
After the exposure period, on day 16, the rabbits were weighed and euthanized under chloroform anesthesia. Then, testes from all rabbits were surgically removed (Bal et al., 2012) and examined carefully to record the gross anatomy (color, texture, and any abnormality visible grossly). After gross examination, the weight of the testes was measured, and a small incision was carried out on the body and epididymal parts of the left or right testes for impression smear using clear glass slides (Gatimel et al., 2017). Then, a suitable small slice from the testes was collected and preserved in 10 % neutral buffered formalin (NBF).
2.5. Sperm staining
Impression smears were fixed into methanol, followed by air drying. The next day, the smear was stained with hematoxylin and eosin (HE).
2.6. Tissue processing and staining
After washing the NBF-fixed tissues in running tap water, dehydration was done using ascending grades of alcohol, followed by clearance using xylene, then embedding in paraffin, and sectioning (6 μm) was done using a sliding microtome (EuromaxR Japan). Hematoxylin and eosin were used to stain the sections that had been deparaffinized in xylene and Goldner's trichrome stain for histopathological examination (Luna, 1968). Goldner's trichrome stain was used to visualize the connective tissues of the testicular capsule. The connective tissue collagen fibers appeared greenish-blue under a light microscope. IMI-induced histological changes and morphology of spermatozoa were examined using a light microscope (LABOMED, Labo America Inc., CA94538).
2.7. Spermatogenic (SG) cell count
Quantification of SG cells was done considering at least 10 (ten) seminiferous tubules (clear cross sections which were sharply rounded) of each rabbit using a light microscope (LABOMED, Labo America Inc., CA94538). The oblique or longitudinal sections were not under consideration during the spermatogenic cell count.
2.8. Leydig (LG) cell count
For the Quantification of LG cells, all the stromal cells in the interstitial spaces (at least ten interstitial spaces for each rabbit) were counted using a light microscope (LABOMED, Labo America Inc., CA94538) and represented as Leydig cells.
2.9. Statistical analysis
To analyze the body weight, weight of the testes, the thickness of tunica albuginea, total number of spermatogenic and Leydig cells count, and the percentage of morphologically abnormal spermatozoa,utilized the Microsoft Excel program (version 2013). Mean ± Standard Error (S.E.) was used to express the values. Student t-test was considered to quantify the significant difference. * p < 0.05, a significant difference between control and IMI-exposed rabbits, and †p < 0.05, a significant difference between IMI-exposed not-washed and washed-feed rabbits.
3. Results
3.1. Effect of IMI on body weight and testicular weight of rabbits
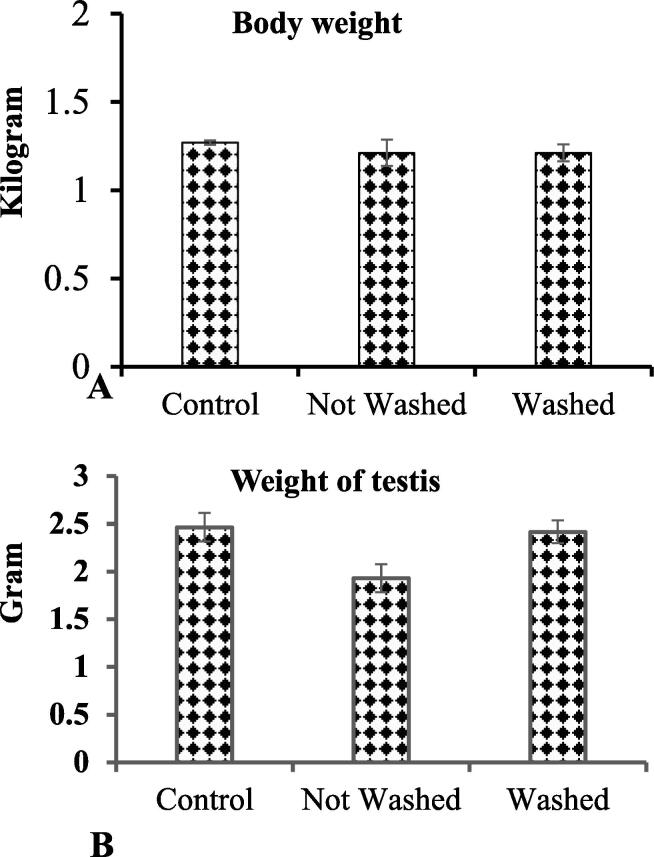
During the exposure time, there was no toxic symptom found on regular monitoring of IMI-exposed rabbits. Comparing the IMI-exposed rabbits to the control s, neither the mean body weight nor the weight of the testes substantially altered. (Fig. 1A-B).
Fig. 1.
A-B: The body weight and testicular weight did not differ significantly comparing imidacloprid exposed and control rabbits.
3.2. Effect of IMI on histoarchitecture of testes of rabbits
3.2.1. Effect of IMI on tunica albuginea
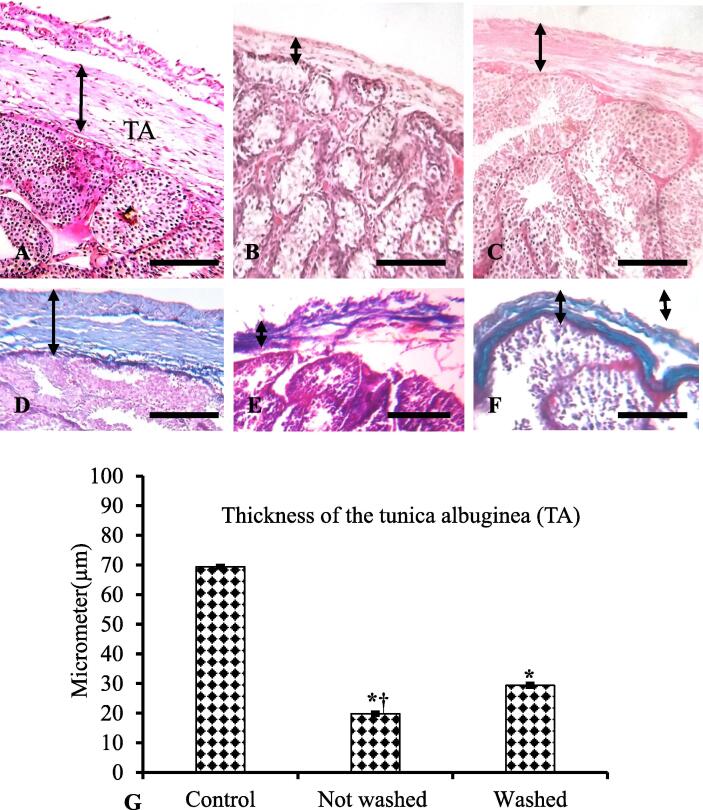
Hematoxylin Eosin and Goldner's trichome stained sections revealed that the capsule (tunica albuginea and tunica vaginalis) covers the testes, and composed of dense fibrous connective tissue with homogenously distributed collagen fibers (green color collagen with blue to the dark nucleus) in control rabbits (Fig. 2A & 2D). In IMI-exposed rabbits, the thickness of tunica albuginea reduced severely with the disruption of regular arrangements of connective tissue fibers in both not-washed and washed-feed rabbits (Fig. 2B-C). But the reduction of the thicknes of the TA was more prominent in not-washed rabbits (Fig. 2B). Goldner's trichome stain exposed loosely arranged collagen fibers (green color) with an irregular pattern of distribution of the tunica albuginea of IMI-exposed rabbits (Fig. 2E-F).
Fig. 2.
A-F: Histopathology of the capsule of testes using H and E (A-C) and Goldner's trichome staining (D-F). The thickness of the testicular capsule was reduced in both imidacloprid (IMI) exposed not-washed and washed-feed rabbits (B-C). Goldner's trichome stain visualizes loosely arranged collagen fibers (green color) with an irregular distribution pattern of the testicular capsule (D-F). G: The thickness of the capsule reduced significantly in IMI-exposed rabbits in comparison with control rabbits. Student t-test, *p ≤ 0.05, significantly different between control and IMI exposed rabbits; †p ≤ 0.05, significantly different between IMI exposed not-washed and washed-feed rabbits. Scale bar: A-F = 200 µm.
Statistical analysis showed that the thickness was significantly (P < 0.05) reduced in IMI-exposed rabbits in comparison with control rabbits (Fig. 2G). In between IMI-treated groups, the thickness of the tunica albuginea was significantly (P < 0.05) reduced in not-washed-feed rabbits.
3.2.2. Effect of IMI on histo-architecture of seminiferous tubules
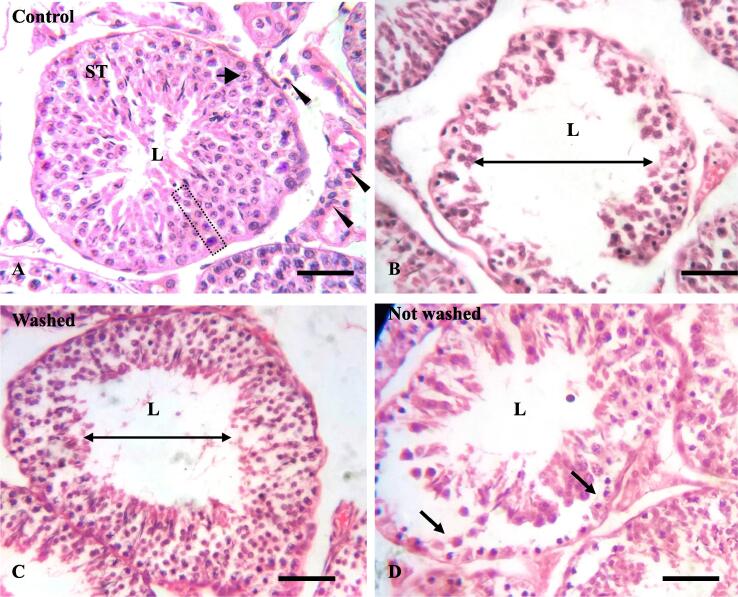
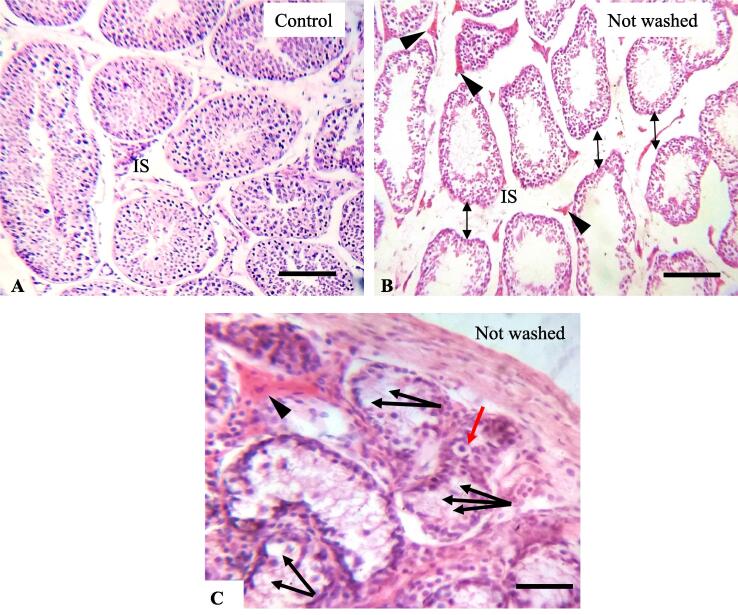
In the testes of control rabbits, regular histological structure with regular distribution of spermatogenic cells was seen (Fig. 3A). In transverse sections, testes contain numerous seminiferous tubules of different sizes and shapes such as round, oblique, and longitudinal sections (Fig. 3A). Each seminiferous tubule is surrounded by thin basement membrane where the Sertoli cells and the stratified layer of spermatogenic cells (spermatogonia, spermatocytes, spermatids, and spermatozoa) lined the seminiferous tubules (Fig. 3A). The Sertoli cells were tall cells with oval nuclei at the basal region, while the spermatogonia were rounded cells with rounded nuclei (Fig. 3A). In the interstitial space, interstitial cells (Leydig cells) and loose connective tissues with blood capillaries were found as normal distribution pattern (Fig. 5A).
Fig. 3.
Histopathology of testis of control (A) and imidacloprid (IMI) exposed (B-D) rabbits. A. Normal appearance of seminiferous tubules (S.T.) and regular distribution of spermatogenic cells (S.C., rectangular dotted area), Leydig cells (L.C., arrowheads), and Sertoli cells (black arrows) in the testis of the control rabbits. B-C: The lumen diameter of the S.T. became enlarged (double arrow line) in both IMI-exposed rabbits. D: IMI-exposed, not-washed-feed rabbit shows SC cells were separated from their basement membrane. Hematoxylin and Eosin stain. Scale Bar = 100 µm.
Fig. 5.
A: Testicular histopathology of control rabbits demonstrate seminiferous tubules with normal size and shape and uniform interstitial spaces (IS). B: The interstitial space has been increased in imidacloprid (IMI) exposed rabbit (double-headed arrows) with numerous congestion (arrowheads) in the interstitial space and Leydig cells area (B-C). C: Vacuolization (black arrows) and pyknotic cells (red arrow) are evident in IMI-exposed rabbits. Hematoxylin and Eosin stain. Scale Bars A-B = 200 µm, C = 100 µm.
Testes of both IMI-treated rabbits revealed distorted seminiferous tubules, depletion of spermatogenic cells, and degenerative changes in seminiferous tubules with increased lumen diameter (Fig. 3B-C). The severity of the damage histo-pathologically was more pronounced in the testes of not-washed-feed rabbits compared to washed-feed rabbits (Fig. 3B-D and Fig. 5B-C). The spermatogenic cells were detached from the basement membrane with irregular arrangements in both IMI-exposed rabbits (Fig. 3D). Vacuolization and pyknotic cells were found in the seminiferous tubules of IMI-exposed rabbits (Fig. 5D). Widening of the interstitial spaces and low integrity of interstitial tissues were found in both IMI exposed rabbits (Fig. 5B). Profuse congestion and hemorrhage were seen in the interstitial space, close to the Leydig cells in IMI-exposed rabbits (Fig. 5B-C).
3.2.3. Effect of IMI on the spermatogenic (SG) cell population
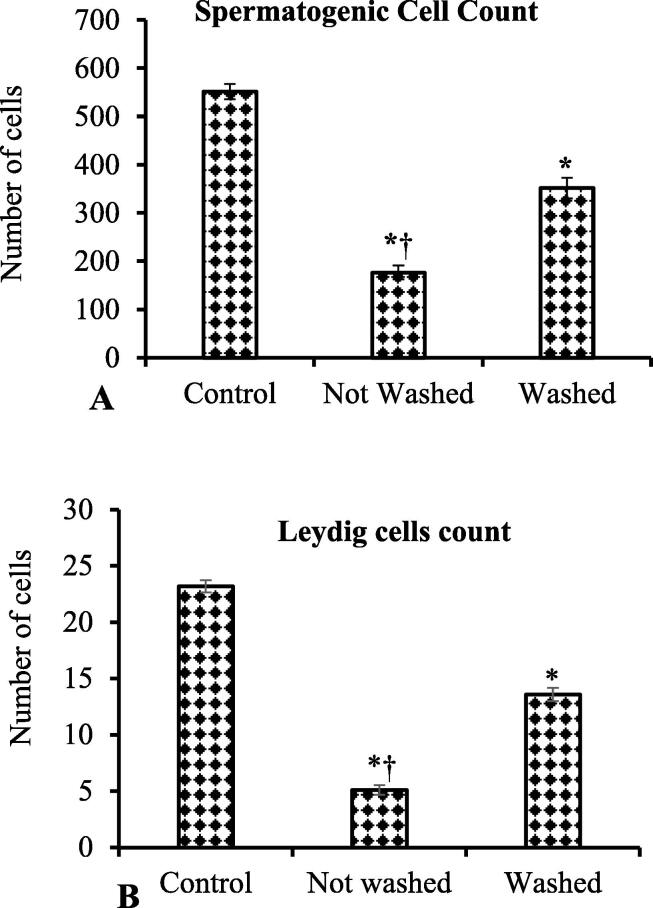
According to the quantification of spermatogenic cells, seminiferous tubules of rabbits exposed to IMI had substantially less (P < 0.05) SG cells than control rabbits (Fig. 4A). In between the IMI exposed rabbits, the number of SG cells was significantly reduced (P < 0.05) in testes of not-washed-feed rabbits compared to washed-feed rabbits (Fig. 4A).
Fig. 4.
Population of the spermatogenic cells (A) and frequency of Leydig cell (B) distribution significantly reduced in imidacloprid (IMI) exposed rabbits compared to control rabbits. In between IMI-treated groups, spermatogenic cells (A) and Leydig cell (B) numbers were significantly reduced in not-washed-feed rabbits. Student t-test, *p ≤ 0.05, significantly different between control and IMI exposed rabbits; †p ≤ 0.05, significantly different between IMI exposed not-washed and washed-feed rabbits.
3.2.4. Effect of IMI on the Leydig (LG) cell population
Leydig cells (LG) were found as a cord or cluster in the interstitial spaces of control rabbit's testes (Fig. 3A). The quantification of LG cells showed that the numbers were significantly (P < 0.05) decreased in IMI exposed rabbit's testes in comparison with control rabbits. Comparing the testes of IMI-exposed rabbits, the LG cell count was significantly lower (P < 0.05) in not-washed-feed rabbits (Fig. 4B).
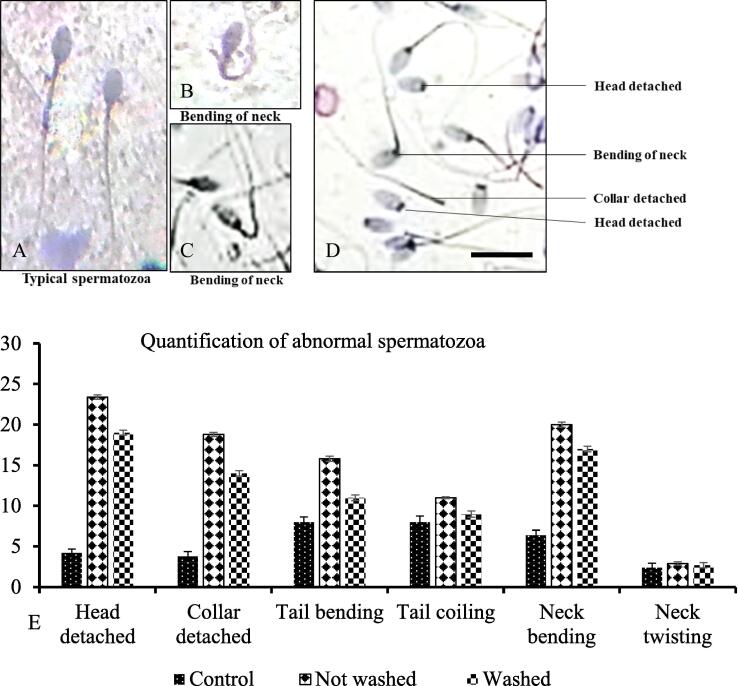
3.2.5. The morphological abnormalities of spermatozoa
The spermatozoa encountered the toxic effects of pesticides, which resulted in the formation of morphologically abnormal spermatozoa (Fig. 6A-D). The quantification of morphologically abnormal spermatozoa revealed that the number of abnormal spermatozoa, particularly head detached, collar detached, tail bending, and neck bending was the highest percentage in impression smear of testes and epididymis of IMI-contaminated rabbit's testes (not-washed and washed-feed rabbits) compared to control rabbits (Fig. 6E). It is noted that not-washed-feed rabbit had more pronounced abnormalities than the washed-feed rabbit.
Fig. 6.
Normal (A) and abnormal (B-D) morphology of spermatozoa of rabbit. E: The abnormal morphology of the spermatozoa is high in imidacloprid-exposed rabbits. The percentage of abnormal spermatozoa is highest in not-washed-feed rabbits compared to washed-feed rabbits. Hematoxylin and Eosin stain. Scale Bar A-D = 100 µm.
4. Discussion
4.1. Effect of IMI on body weight and testicular weight
The body weight and the weight of testes of IMI-contaminated feed-exposed rabbits were not changed significantly, although showing a decreasing tendency. In contrast, Bagri et al. (2013) determined that IMI reduces body weight significantly at the dose rate of 110 mg/kg body weight or more (oral gavage) in Swiss albino rats for 14 days. The body weight and testicular weight were significantly reduced in male rats treated with IMI orally (20 mg/kg body weight) for 90 days (Bal et al., 2012). Najafi et al. (2017) reported that the body and testicular weight reduction depends on the dose, route, duration, and mode of insecticide administration in rats. The variation in the present result may be due to dose, formulation, or species differences.
4.2. Effect of IMI on histo-architectural alterations
4.2.1. Tunica albuginea
The reduced thickness of tunica albuginea and loosely arranged connective tissue with the disruption of collagen fibers arrangement were seen in IMI-exposed testes of adult rabbits. Manas et al. (2013) stated that a low dose (53 mg/kg body weight) of pyridaben causes reduced thickness of tunica albuginea in mice for 45 days. This finding supports the result of the present study.
4.2.2. Seminiferous tubules
In the present study, the testes of IMI-exposed rabbits revealed distorted seminiferous tubules with a low number of spermatogenic cells detached from the basement membrane. In addition, the spermatogenic cells were disorganized with an increased lumen diameter of seminiferous tubules. Vacuolization and pyknotic cells were evident in seminiferous tubules. These histo-architectural alterations might be attributed to IMI-triggered oxidative stress generated from reactive oxygen species (ROS) consistent with those reported by Bal, 2012, Soujanya et al., 2013, Najafi et al., 2017 in testes of adult male rats. Eissa (2004) stated that IMI causes different degenerative changes, including bizarre-shaped cells and spermatogenic cell disappearance in the testes of Japanese quail. The author also showed that the seminiferous tubules were devoid of sperms with vacuolation and pyknotic changes , which coincides with the findings of the present study. Insecticide cypermethrin oral exposure caused distorted seminiferous tubules with deformed and disorganized germ cells in mice at different doses (Fang et al., 2013).
When compared to the testes of IMI-exposed rabbits, the histopathological alterations in the not-washed-feed rabbits were more pronounced. This result indicates that IMI-contaminated, not-washed-feed contains a high level of IMI, and IMI-contaminated washed-feed losses the IMI, and a low level of IMI enters into the body system of rabbits.
The extended interstitial spaces with numerous hemorrhages and congestion were seen in the testes of IMI-exposed rabbits. Similarly, Soujanya et al., 2013, Menon et al., 2014 reported widening of the interstitial space with profuse hemorrhage on the area of the Leydig cell in IMI-treated testes of rats and rabbits, respectively.
4.2.3. Spermatogenic (SG) cell population
Evaluation of SG cell populations of the current study was reduced significantly in IMI-exposed rabbits compared to control rabbits. Similarly, Menon et al. (2014) reported that IMI disrupts the testicular tissues and spermatogenic cell populations in rabbits. Pesticide chlorpyrifos causes cessation of spermatogenesis, and it harms tubular differentiation and repopulation of the spermatogenic cells in adult rats (Najafi et al., 2017).
Interestingly, the SG cell population was affected severely in not-washed-feed rabbits compared to washed-feed rabbits in the present study. This finding coincides with the results of Menon et al. (2014) that IMI exerts mild to moderate effects on testicular tissues depending on a low to high dose of exposure in rabbits. Grewal et al. (2010) also revealed that cypermethrin at a higher dose could disrupt the cycle of various stages of spermatogenesis in albino rats.
In the present study, the degeneration and depletion in the SG cell population and its extent vary. This is might be due to the two different ways to expose the rabbits to IMI (i.e., not-washed and washed-feed).
4.2.4. Leydig (LG) cell population
The number of Leydig cells was significantly decreased in IMI-exposed testes compared to control rabbits. Between the IMI-exposed rabbits' Leydig cell was significantly decreased in not-washed-feed rabbits compared to washed-feed rabbits. Similarly, it was reported that the number of LG cells reduced in IMI (Soujanya et al., 2013) and chlorpyrifos (Najafi et al., 2017) treated adult male rats. Manas et al. (2013) stated that Leydig cell distribution reduced significantly in mm2 of testicular interstitial tissue in high doses (212 mg/kg) of pyridaben-exposed mice for up to 45 days.
Insecticides can pass through the blood-testes barrier. Leydig cells present biologically relevant to the effects of IMI (Zhao et al., 2021). IMI has direct degenerative effects on Leydig cells and/or indirectly affects nicotine, like suppressing luteinizing hormone (LH) secretion from the pituitary gland (Mohamed et al., 2016, Ngoula et al., 2007). Moreover, cytochrome P- 450 3A4 (CYP3A4) is an essential metabolic enzyme responsible for metabolizing exogenous substances (Zhao et al., 2021). The impairment of CYP-450 enzymatic activities disrupts steroid hormone (testosterone) synthesis (Bernhardt and Waterman, 2007). Depending on the dose and repeated exposure, partial to complete dissolution of Leydig cells occur. Degeneration and depletion of Leydig cells result in a decreased testosterone level in the blood. A low level of blood testosterone fails to maintain the functions of Sertoli cells leading to germinal cell degeneration and detachment from the basement membrane.
Degeneration and detachment of germinal cells cause the dissolution of seminiferous tubules resulting in arrest in spermatogenesis (Soujanya et al., 2013, Najafi et al., 2017). IMI-induced oxidative stress that causes degeneration of the Leydig cells from interstitial space (Soujanya et al., 2013, Najafi et al., 2017) may cause depopulation of LG cells in male rabbits in the present study.
4.3. Morphological abnormalities
In the present study, head detached, tail detached, bending of the neck, twisting of the neck, tail bending, and tail coiling morphological abnormalities of spermatozoa were high percentages in IMI-exposed rabbits compared to control rabbits. These findings coincided with the results of Kalra (2018) in male rats intoxicated with cypermethrin, where sperm abnormalities were categorized into tail detached, defective head, bending and coiling of the middle piece, and bending plus coiling of the tail. Sperm abnormalities were significantly increased by oxidative damage due to reactive oxygen species generated from cypermethrin (Kumar and Kiran, 2010, Joshi et al., 2011). Li et al. (2015) identified that the number of abnormal sperms, particularly head shape abnormality, was significantly increased in cypermethrin-exposed rats. A significantly increased percentage of morphologically abnormal spermatozoa was also reported in chlorpyrifos-treated adult rats (Dutta et al., 2013). Therefore, IMI-induced reactive oxygen species leading to oxidative stress (Kumar and Kiran, 2010, Joshi et al., 2011), may affect the morphology of spermatozoa in rabbits.
5. Conclusion
IMI induced testicular damage, including distorted seminiferous tubules with depleted spermatogenic cells, separation of the basement membrane, and less frequency of distribution in Leydig cells, and gravitated in the production of morphologically abnormal spermatozoa in adult male rabbits. The finding of the present study suggests that repeated oral exposure to IMI-contaminated feed produces testicular toxicity in adult male rabbits, which potentially can cause infertility. Therefore, suitable measures must be implemented to reduce the negative effects of IMI on humans and animals.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We acknowledge the professional support from Dr Imam Hasan, Dr Md. Asabur Rahman, and Dr Ziaul Haque, Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202.
Funding
The study was supported by Bangladesh Agricultural University; Grant for Advance Research in Education (GARE), Bureau of Educational Information & Statistics (BANBEIS), Ministry of Education (MoE), Government of the People's Republic of Bangladesh (Grant no. LS2018773, SD20211598 to MRK).
Author Contribution
MRK, MAK, and MP designed the experiment. MAK undertook the experiment. Recording, analysis of data, and interpretation of results done by MAK, MRK, and LA MAK, MRK, MAA, NS, and MP made significant revisions to the text. The final manuscript was read and approved by all writers.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Md. Alamgir Kobir, Email: alamgir41172@bau.edu.bd.
Latifa Akter, Email: latifa.20210101@bau.edu.bd.
Nazneen Sultana, Email: nazneen41151@bau.edu.bd.
Munmun Pervin, Email: munmunpervin@bau.edu.bd.
Md. Abdul Awal, Email: awal.vah@bau.edu.bd.
Mohammad Rabiul Karim, Email: mrabiulkarim@bau.edu.bd.
References
- Bagri, P., Kumar, V., Sikka, A.K., and Punia, J.S., 2013. Preliminary acute toxicity study on imidacloprid in Swiss albino mice, Veterinary World 6(12): 955-959. https://doi.org/10.14202/vetworld.2013.955-959.
- Bal R., et al. Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell Biochem. Funct. 2012;6:492–499. doi: 10.1002/cbf.2826. [DOI] [PubMed] [Google Scholar]
- Bernhardt R., Waterman M.R. Cytochrome P450 and Steroid Hormone Biosynthesis. Met. Ions. Life Sci. 2007;3:361–396. [Google Scholar]
- Bhardwaj S., et al. A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem. Toxicol. 2010;48(5):1185–1190. doi: 10.1016/j.fct.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Damalas C.A., Eleftherohorinos I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health. 2011;8(5):1402–1419. doi: 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A.L., Sahu Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. Spring. Plus. 2013;2(1):541. doi: 10.1186/2193-1801-2-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzguner V., Erdogan S. Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pest. Biochem. Physiol. 2010;97:13–18. doi: 10.1016/j.pestbp.2009.11.008. [DOI] [Google Scholar]
- Eissa O.S. Protective effect of vitamin C and glutathione against the histopathological changes induced by imidacloprid in the liver and testes of Japanese quail. Egypt. J. Hospital Med. 2004;16:39–54. doi: 10.21608/EJHM.2004.18174. [DOI] [Google Scholar]
- Fang et al. 2013. Effects of cypermethrin on male reproductive system in adult Rats. Biomed. and Environ. Sci. 26(3):201-8. https://doi.org//10.3967/0895-3988.2013.03.007 [DOI] [PubMed]
- El-Gendy K.S., Aly N.M., Mahmoud F.H., Kenawy A., El-Sebae The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food and Chem. Toxicol. 2010;48(1):215–221. doi: 10.1016/j.fct.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gatimel N., Moreau J., Parinaud J., Leandri R.D. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Androl. 2017;5:845–862. doi: 10.1111/andr.12389. [DOI] [PubMed] [Google Scholar]
- Grewal et al. 2010. Toxic impacts of cypermethrin on behavior and histology of certain tissues of albino rats. Toxicol Int. 17(2): 94–98. https://doi.org/10.4103/0971-6580.72679 [DOI] [PMC free article] [PubMed]
- Iturburu, F. G., et al. 2017. Uptake, distribution in different tissues, and genotoxicity of imidacloprid in the freshwater fish Australoheros facetus. Environ. Toxicol. Chem. 36, 699-708. https://doi.org/ 10.1002/etc.3574. [DOI] [PubMed]
- Joshi S.C., et al. Evaluation of reproductive and developmental toxicity of cypermethrin in male albino rats. Toxicol. Environ. Chem. 2011;93(3):593–602. doi: 10.1080/02772248.2010.537441. [DOI] [Google Scholar]
- Kalra S., 2018. An MS thesis on biological monitoring in cypermethrin-induced albino rats. http://krishikosh.egranth.ac.in/handle/1/5810045054.
- Kobir et al. 2020b. Effects of imidacloprid-contaminated feed exposure on hematological parameters in adult rabbits (Oryctolagus Cuniculus). Res. in Agril. Lives. and Fis.7(3):439-444. https://doi.org/10.3329/ralf. v7i3.51363
- Kobir et al. 2020a. Ubiquitous use of agricultural pesticides in six agro-based districts of Bangladesh and its impact on public health and environment. J. of Food Agril. Environ. 1(3):47-52. https://doi.org/ 10.47440/JAFE.2020.1307.
- Komal 2018. A Review: Pathological studies on imidacloprid toxicity and its amelioration with vitamin C. 7(4): 999-1002 hhtp//www.thepharmajournal.com.
- Kumar, B.K., and Kiran, D.G., 2010. Chronic exposure to pesticides- neurological, neurobehavioral and molecular targets of neurotoxicity. https://www.intechopen.com/books/pesticides-in-the-modern-world-effects-of-pesticides-exposure.
- Lewis K.A., et al. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016;22:1050–1064. doi: 10.1080/10807039.2015.1133242. [DOI] [Google Scholar]
- Li J., et al. A review on phospholipids and their main applications in drug delivery systems. Asian. J. Pharma. Sci. 2015;10(2):81–98. doi: 10.1016/j.ajps.2014.09.004. [DOI] [Google Scholar]
- Lonare et al. 2014. Evaluation of imidacloprid-induced neurotoxicity in male rats: A protective effect of curcumin. J. Neurochem. Internl. https://doi.org/10.1016/j.neuint.2014.09.004 [DOI] [PubMed]
- Luna L.G. Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3rd Edition. McGraw-Hill; New York: 1968. [Google Scholar]
- Manas et al. 2013. The effects of pyridaben pesticide on the histo-morphometric, hormonal alternations and reproductive functions of BALB/c Mice. Iranian J. of Basic Medi. Sci. 16(10):1055-1064 [PMC free article] [PubMed]
- Mantovani A., Marangh F. Risk assessment of chemicals potentially affecting male fertility. Contracept. 2005;72(4):308–313. doi: 10.1016/j.contraception.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Matsuda K., et al. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci. Biotechnol. Biochem. 2005;69:1442–1452. doi: 10.1271/bbb.69.1442. [DOI] [PubMed] [Google Scholar]
- Mazdak et al. 2009. The effect of chronic exposure with imidacloprid insecticide on fertility in mature male rats. Internl. J. of Ferti. Steri. 9(1)
- Menon S.A., et al. Histopathological changes in the gonads of male rabbits (Oryctolagus cuniculus) on exposure to imidacloprid insecticide. J. Entomo. Zoolo. Stud. 2014;2(4):159–163. [Google Scholar]
- Miah S.J., Hoque A., Paul A., Rahman A. Unsafe use of pesticide and its impact on health offarmers: a case study in Burichong Upazila, Bangladesh. Cancer. 2014;21(3):22–30. [Google Scholar]
- Mohamed H.B., et al. Sub-acute oral toxicity of imidacloprid and fipronil pesticide mixture in male albino rats; biochemical and reproductive toxicity evaluation. J. Mater. Environ. Sci. 2018;9(8):2431–2437. [Google Scholar]
- Mohamed A.R., Mohamed W.A.M., Khater S.I. Imidacloprid induces various toxicological effects related to the expression of 3β-HSD, NR5A1, and OGG1 genes in mature and immature rats. Environ. Pollut. 2016;221:15–25. doi: 10.1016/j.envpol.2016.08.082. [DOI] [PubMed] [Google Scholar]
- Najafi G., et al. Effect of chlorpyrifos on sperm characteristics and testicular tissue changes in adult male rats. Veteri. Res. For. 2017;8(4):319–326. [PMC free article] [PubMed] [Google Scholar]
- Najafi G., Razi M., Hoshyar A., Shahmohamadloo S., Feyzi S. The effect of chronic exposure with imidacloprid insecticide on fertility in mature male rats. Internat. J. of Ferti. & Steri. 2010;4(1):9–16. [Google Scholar]
- Ngoula F., Watcho P., Dongmo M.C., Kenfack A., Kamtchouing P.T., Choumboue J. Effects of pirimiphos emethyl (an organophosphate insecticide) on the fertility of adult male rats. Afr. Health Sci. 2007;7:3–9. doi: 10.5555/afhs.2007.7.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Kumar V., Shahzad B., et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019;1:1446. doi: 10.1007/s42452-019-1485-1. [DOI] [Google Scholar]
- Soujanya S., et al. Protective role of vitamin-c against the histopathological and ultrastructural changes induced by imidacloprid in testes of male rats. Int. J. Life Sci. Biotechnol. Pharma. Res. 2013;2(1):92–97. [Google Scholar]
- WHO 2020. 10 chemicals of public health concern. https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern.
- Zhao G.P., Li J.W., Yang F.W., Yin X.F., Ren F.Z., Fang B., Pang G.F. Spermiogenesis toxicity of imidacloprid in rats, possible role of CYP3A4. Chemosphere. 2021;282:131120. doi: 10.1016/j.chemosphere.2021.131120. [DOI] [PubMed] [Google Scholar]