Abstract
Background
Epithelial ion transport regulates hydration of airway mucosal surfaces, and thus promotes effective mucociliary clearance (MCC). Decreased transepithelial Cl− transport may contribute to epithelial dysfunction by abrogating MCC and increasing mucus viscosity in chronic rhinosinusitis (CRS). The objective of the current study is to evaluate Cl− channel transport properties from cultures of human sinonasal epithelia.
Methods
Human sinonasal epithelia (HSNE) from patients undergoing sinus surgery were cultured at an air-liquid interface to confluence and full differentiation. The epithelial monolayers were mounted in Ussing Chambers to investigate pharmacological manipulation of ion transport. Epithelial Na+ channel (via Amiloride), CFTR (via forskolin), and Ca2+-activated Cl− channel (CaCC, via UTP) transport were investigated among three different patient groups: Control, CRS and CRS with polyposis. CFTR mRNA levels were evaluated with quantitative RT-PCR.
Results
HSNE cultures from 18 patients (Control = 9, CRS = 6, CRS with polyposis = 3) were evaluated in 142 experiments. Summary data from the 18 patients demonstrated that stimulated CFTR-mediated anion transport (Δ ISC) was significantly lower with CRS (7.58+/−2.24 µA/cm2) compared to control (25.86+/−3.44 µA/cm2) and CRS with polyposis (20.16+/−4.0 µA/cm2) (p = 0.004). No statistically significant difference was found for CaCC anion transport between groups (p = 0.39). Significantly decreased mRNA (relative expression) was noted in CRS cultures (CRS = 40.83+/−1.76 vs. control = 116.2+/−24.27, p = 0.03).
Conclusions
A substantial decrease in the Cl− secretory capacity of HSNE monolayers was demonstrated in CRS subjects. Data suggest that CFTR may contribute more to abnormal ion transport in CRS than CaCC.
Keywords: chronic rhinosinusitis, CFTR, cystic fibrosis, potential difference, transepithelial ion transport, sinusitis, chloride secretion, chloride channels, nasal mucosa, ussing chamber analysis
Introduction
The sinonasal epithelium is comprised of airway surface liquid (ASL) containing a low viscosity periciliary fluid layer (sol) around the respiratory cilia and a superficial mucus (gel) layer, which function to trap and sweep inhaled particles into the digestive tract through coordinated mucociliary clearance (MCC).1 The cystic fibrosis transmembrane conductance regulator (CFTR) functions as a chloride (Cl−) and bicarbonate (HCO3) channel and is critical for the normal height of the ASL. Decreased CFTR-mediated Cl− secretion across the mucosal surface contributes to the development of airway disease by depletion of ASL, which hinders effective MCC by increasing the viscosity and adhesion of mucus resulting in persistent infection and/or inflammation.2–6 These effects are pronounced in patients with cystic fibrosis (CF) who exhibit CFTR dysfunction and chronic lower and upper airway infection. An enriched understanding of the role of CFTR in the maintenance of normal epithelial function has revealed that wild-type (WT) CFTR processing, endocytic recycling, and function can also be significantly inhibited by environmental insults, including cigarette smoke exposure, high altitude/hypoxia, inflammation, and infectious agents, and may be a contributing factor in CRS and other disease of MCC such as chronic obstructive pulmonary disease (COPD).7–12 Our previous study measured functional CFTR-mediated Cl− transport activity and detected a decrease in the stimulatory effect of CFTR mediated Cl- secretion across the sinonasal epithelia from CRS patients compared to normal controls.2 However, additional apical channels (e.g. Calcium activated Chloride Channel, CaCC also known as TMEM16A) and transporters contribute to transepithelial ion transport in sinonasal epithelium, and could be potential therapeutic targets for sinusitis treatment.2,13
Air-liquid interface (ALI) cultures of airway epithelial cells reproduce key aspects of in vivo airway epithelial structure and function and have proved to be invaluable for understanding airway diseases.3,14 Well-differentiated ALI cultures of normal and chronic sinusitis epithelia from surgical specimens are well established models for studying airway ion transport properties.11,15,16 The objective of the current study is to evaluate apical ion transport characteristics from cultures of human sinonasal epithelia from controls and CRS with and without polyposis by assessing Na+ absorptive and Cl− secretory properties. Based on previous data from ex vivo specimens, the hypothesis of the current study is that recapitulated HSNE cultures from CRS patients would exhibit decreased CFTR-mediated anion transport.
Methods
Study Population
This study was approved by the institutional review board at University of Alabama at Birmingham. All subjects enrolled in the study provided informed consent. Normal sinonasal mucosa was obtained intraoperatively from patients undergoing endoscopic surgery for pituitary tumors, facial trauma, benign sinonasal tumors, or lacrimal obstruction. Sinonasal tissue was also procured from patients with medically refractory chronic rhinosinusitis (CRS) with and without polyposis undergoing endoscopic sinus surgery (ESS). All CRS patients fulfilled diagnostic criteria endorsed by the American Academy of Otolaryngology.17
Primary Cell Culture
After tissue harvest, human sinonasal epithelial (HSNE) cells were grown on Costar 6.5-mm diameter permeable filter supports (Corning Life Sciences, Lowell, MA), and submerged in culture medium as previously described.18–21 Media was removed from the monolayers on day 4 after the epithelium reached confluence, and cells fed via the basal chamber. Differentiation and ciliogenesis occurred in all cultures within 10 to 14 days. Cultures were used for experiments when fully differentiated with widespread ciliogenesis and transepithelial resistances (Rt) >300 Ω·cm2.
Ussing Chamber Analysis
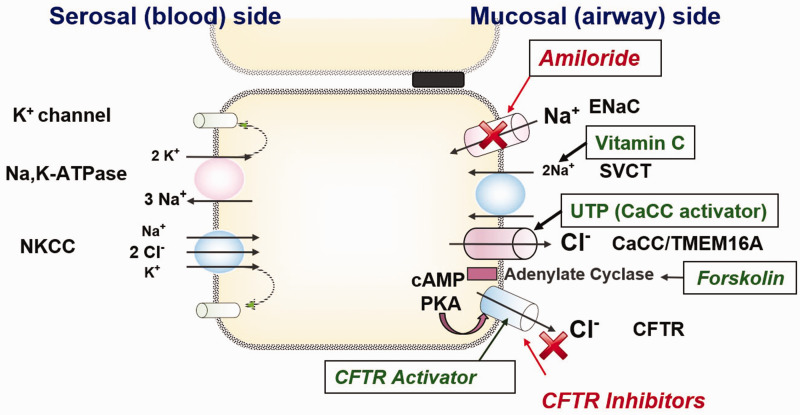
Transwell inserts (Costar) were mounted in Ussing chambers to investigate pharmacologic manipulation of vectorial ion transport as previously described.7,10,11,15,16,22–26 Monolayers were evaluated under short circuit current conditions following fluid resistance compensation using automatic voltage clamping (VCC 600; Instruments, San Diego, CA). Serosal bath solutions contained (in mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose. Baths for transwell filters were warmed to 37°C and gassed continuously with a 95%O2-5%CO2 mixture that provides a pH of 7.4 under conditions studied here. Chemicals were obtained from Sigma (St. Louis, MO). To increase the sensitivity of the assay for detecting chloride currents across the apical membrane, a serosal to mucosal Cl− secretory gradient was applied. The mucosal bathing solution was changed to a low Cl− solution containing (in mM) 1.2 NaCl and 115 Na gluconate. Stock concentrations of the following pharmacologic agents were used: amiloride (100 mM) - blocks epithelial Na+ channels, as a means to isolate changes in short-circuit current (ΔISC) secondary to effects on Cl− channel activity;27 forskolin (20 mM) - activates CFTR by elevating intracellular cAMP, which results in protein kinase A (PKA)-dependent phosphorylation of the CFTR regulatory domain; INH-172 (10 mM)- a CFTR inhibitor that permits determination of CFTR-dependent contributions to ISC;22 uridine triphosphate (UTP) (150 mM)- stimulates the CaCC, TMEM16A, by triggering P2Y receptor-mediated increases in intracellular Ca2+. Each solution was produced as 1000x stock and studied at 1x in the Ussing chamber. The ISC was continuously assessed at one current measurement per second. Amiloride was added first to track ENaC response. The total CFTR response was measured as the difference between baseline after the effect of amiloride had stabilized to the peak of the forskolin response. INH-172 was then administered to block CFTR, and finally UTP was added to measure CaCC function. The ISC was continuously assessed at one current measurement per second. By convention, a positive deflection in ISC under conditions tested here was defined as the net movement of anions from the serosal to mucosal direction. A schematic drawing of ion transport in airway epithelium is demonstrated in Figure 1.
Figure 1.
Ion transport and pharmacologic manipulation in airway epithelium. Transepithelial Na+ reabsorption mediated by concerted activity of apical epithelial Na+ channels (ENaC: Blocker = Amiloride) and the basolateral Na+/K+ ATPase. Apical located sodium dependent vitamin C transporter might also contribute to Na+ reabsorption. In addition to Na+ reabsorption airway, epithelia display a prominent apical Cl− secretion that is mainly mediated by the cystic fibrosis transmembrane conductance regulator (CFTR) in humans and to a lesser extent by Ca2+-dependent Cl− channels (CaCC) such as the TMEM channels. This secretion is kept up by the basolateral Na+/K+/2Cl− cotransporter (NKCC). In the basolateral membrane, several voltage-dependent K+ channels have been identified.
Gene Expression
RNA Isolation
Total RNA was isolated with RNeasy mini kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions from HSNE cultures. To prevent possible DNA contamination, samples were pretreated with RNase-free DNase (Qiagen, Germantown, MD) and column purified.
Primers and Probes
Sequences used for human CFTR and 18S rRNA were purchased from Assays on Demand (ABI); with assay ID for human CFTR, Hs00357011_m1.
Quantitative RT-PCR
A one-step Applied Biosystems PCR protocol was used to quantify CFTR mRNA transcripts on ABI Prism 7500 sequence detection system on six serial dilutions of RNA isolates according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). TaqMan OneStep PCR Master Mix Reagents Kit (ABI) was used for reverse transcription and PCR. The thermocycler conditions were as follows: Stage 1: 48 ° C for 30 min; Stage 2: 95 ° C for 10 min; Stage 3: 95 ° C for 15 sec; Stage 4: 60 ° C for 1 min; 40 cycles. All CFTR values were normalized to 18S rRNA (from the same sample) according to the Applied Biosystems relative quantification method as described in ABI manual to establish specific changes in CFTR mRNA levels as opposed to more general effects on widespread suppression of cellular transcription. All experiments were performed in triplicate.
Statistical Analysis
Statistical analyses were conducted using Microsoft Excel 2016 and GraphPad Prism Version 7.01 and significant statistical difference was set at p < 0.05. Statistical analyses were performed using paired and unpaired Student’s t-tests and Tukey-Kramer as appropriate.
Results
Patient Characteristics
Sinonasal tissue from 21 patients were included in this study. Mean ages were 51.7 ± 15.4 years in the control (n = 11, 5 males, 6 females), 58.8 ± 7.1 years in the CRS group (n = 8, 3 males, 5 females), and 39.0 ± 7.1 years in the CRS group with polyposis (n = 3, 1 male, 2 females) (p > 0.05).
Ussing Chamber Analysis
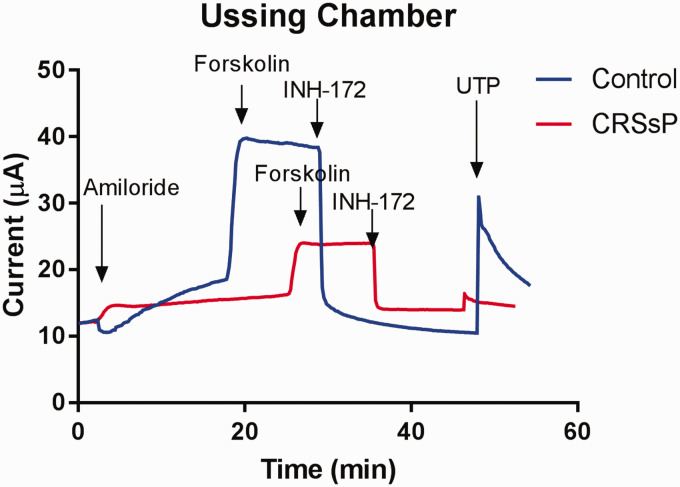
HSNE cultures from 18 patients (Control = 9, CRS = 6, CRS with polyposis = 3) were evaluated in 142 experiments. Transepithelial Cl− currents (ICl) across the monolayer of HSNE were measured in the presence of the sodium channel blocker amiloride and a serosal-to-mucosal directed Cl− gradient (−Clmuc) developed. Gradient driven Cl− currents (ICl) stabilized after ∼30 minutes. Exposure of the apical surface to the cAMP agonist forskolin (20 μM) gradually stimulated ICl in a sustained fashion over the course of 15–30 minutes. Subsequent additions of the CFTR transport inhibitor INH-172 significantly reduced forskolin-stimulated ICl to baseline levels indicating that the cAMP agonist stimulated Cl− transport across the epithelium. Exposure of the apical surface to UTP activated Cl− transport across the epithelium and represented CaCC function (Figure 2).
Figure 2.
Representative Ussing chamber tracings—transepithelial Cl− currents (ICl) across the monolayer of HSNE were measured in the presence of the sodium channel blocker amiloride and a serosal-to-mucosal directed Cl− gradient (−Clmuc). Exposure of the apical surface to the cAMP agonist forskolin (20 μM) gradually stimulated ICl in a sustained fashion over the course of 15–30 minutes. Subsequent additions of the CFTR transport inhibitor INH-172 significantly reduced forskolin-stimulated ICl to baseline levels indicating that the cAMP agonist stimulated Cl− transport across the epithelium. Exposure of the apical surface to UTP activated Cl− transport across the epithelium.
Enac
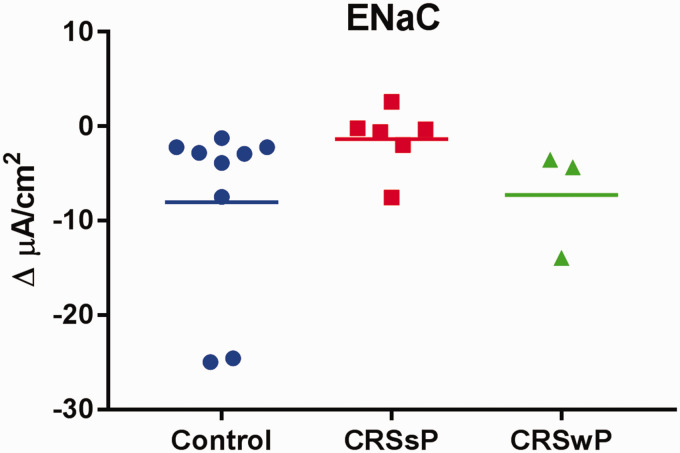
The epithelial sodium channel, ENaC, plays a key role in apical sodium absorption and is important in regulating ASL hydration and mucociliary transport.13 ENaC activity is upregulated in CF, which leads to Na+ hyper-absorption and contributes to ASL dehydration.28 The goal of this study was to test whether ENaC activity is impaired in CRS. Changes in basal Isc after inhibition of Na+ transport with 100 μM amiloride in 3 groups are demonstrated in Figure 3. Interestingly, a notable decrease in amiloride-sensitive ISC was noted in CRS HSNE compared to control and CRS with NP patients, although statistical significance was lacking (ΔISC = Controls, −8.04 ± 3.22 µA/cm2 vs. CRS with polyposis, −7.27 ± 3.34 µA/cm2 vs. CRS without polyposis, −1.36 ± 1.38 µA/cm2) (p = 0.26). ENaC activity was inhibited rather than up-regulated in CRS without polyposis compared to controls.
Figure 3.
No significant difference was observed in ENaC channel function in control, CRS without polyps (CRSsP), and CRS with polyps (CRSwP) (p = 0.26). Average amiloride-sensitive ISC in controls was due to the contributions of 2 outliers.
Cftr
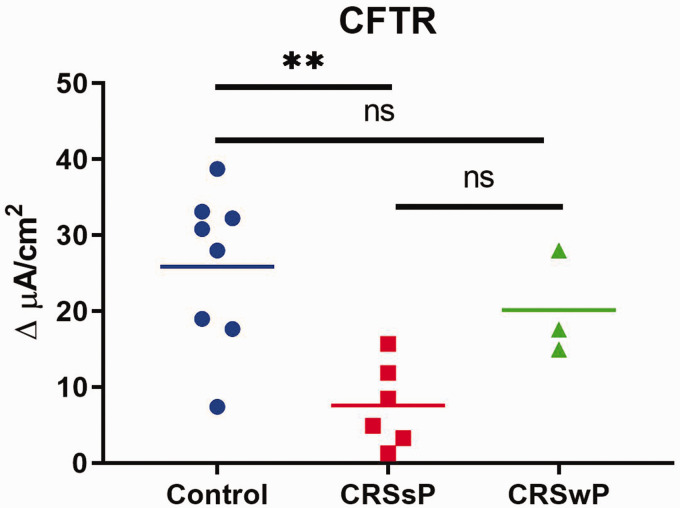
Decreased CFTR activity leads to defective apical chloride and bicarbonate transport into the airway lumen. The goal of this experiment was to identify the CFTR-mediated anion transport capacity in CRS with and without polyposis. Stimulated CFTR-mediated anion transport (Δ ISC) was significantly lower with CRS (7.58 ± 2.24 µA/cm2) compared to controls (25.86 ± 3.44 µA/cm2) and CRS with polyposis (20.16 ± 4.0 µA/cm2) (one way ANOVA, p = 0.004; post hoc Tukey-Kramer analysis, p = 0.003 (control vs CRS)) demonstrated in Figure 4.
Figure 4.
Stimulated CFTR-mediated anion transport was significantly lower in CRS cultures than those generated from nasal polyps or control patients (**p = 0.004).
Cacc
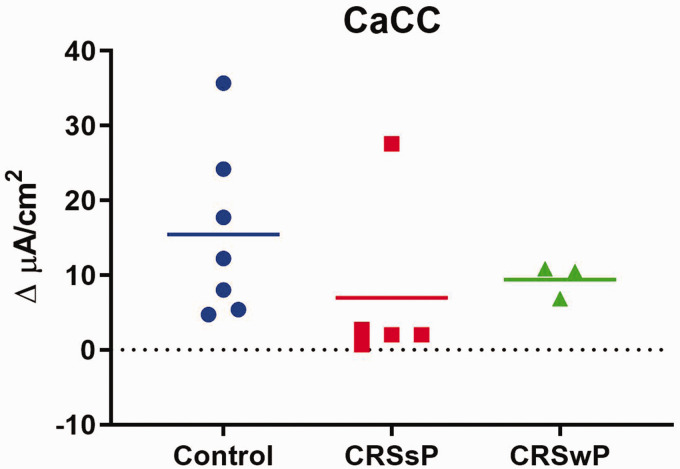
TMEM16A (ANO1) is a calcium-activated chloride channel (CaCC) expressed in airway epithelium and has shown higher activity in CF airways.13 Ano1 knockout mice develop a CF-like phenotype, highlighting its importance in Cl− transport and potential role for therapy. Even though cells from CRS with and without polyposis exhibited relatively smaller UTP-mediated CaCC anion transport compared to normal controls, statistical significance was lacking: control = 15.43 ± 3.78 µA/cm2; CRS without polyposis = 7.00 ± 4.70 µA/cm2; CRS with polyposis = 9.39 ± 1.27 µA/cm2 (p = 0.39) (Figure 5).
Figure 5.
No significant difference was observed in stimulated CaCC-mediated anion transport between disease states (p = 0.39).
Relative CFTR mRNA expression
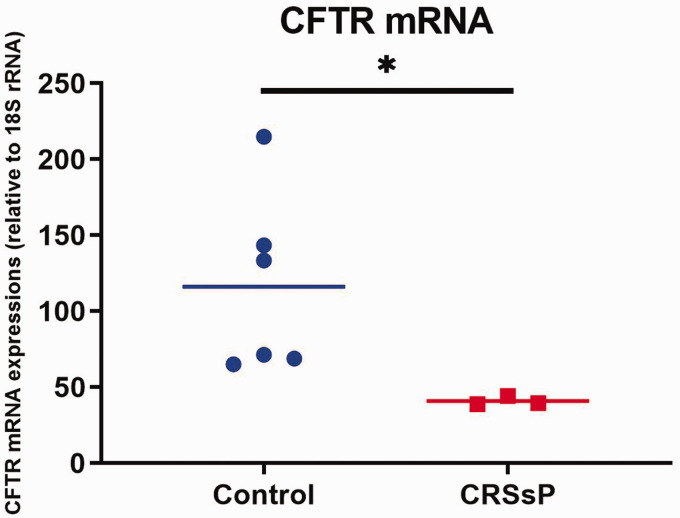
As CFTR-mediated Cl transport was down-regulated in HSNE from CRS without polyposis from above experiments, mRNA expression of CFTR was assessed in normal control (n = 6) and CRS without polyposis (n = 3). RT-PCR after RNA isolation from primary HSNE cultures showed significantly decreased CFTR mRNA expression in CRS without polyposis (40.83 ± 1.76) compared to normal controls (116.2 +/− 24.3) (t test with Welch’s correction p = 0.03) (Figure 6).
Figure 6.
CFTR mRNA expressions relative to 18S rRNA in HSNE derived from CRS without polyps were significantly decreased compared to controls (*p = 0.03).
Discussion
Airway epithelial ion transport regulates the ASL homeostasis, essential for airway mucosal immunity and sinonasal host defense. This study was designed to investigate 3 apical ion channels (ENaC, CFTR, and CaCC) in controls and CRS patients with and without polyposis in recapitulated HSNE cultures to clarify potential contributions to the pathogenesis of CRS. The markedly diminished Cl− secretion across sinonasal epithelial cells from human subjects with CRS suggests acquired CFTR dysfunction may be present in CRS without polyposis. This finding is also consistent with PCR analysis demonstrating decreased mRNA in CRS without polyposis. The decreased CFTR function observed in our analysis could be secondary to decreased transcription. A recent study demonstrated that exoproducts from P. aeruginosa impacted CFTR function in human airway epithelial cells isolated from non-CF patients with a decrease in CFTR protein expression, likely in part from enhanced protein degradation and protein synthesis.29 However, it is still unclear whether the relationship that is suggested between mRNA levels and CFTR function in CRS patients is a result of CRS or a predisposing factor to developing CRS. In addition, there was no accounting for the presence of CFTR heterozygotes since genetic testing was not performed in these subjects.
The precise underlying mechanisms for ENaC upregulation in CF are not fully understood and CFTR dysfunction is thought to be linked to an increase in ENaC activity.13 This has not been demonstrated consistently in all experimental models and their connection remains in question.30 Dejima et al. demonstrated that cells from CRS exhibited relatively large Isc responses to amiloride, suggesting increased Na+ transport rates.3 However, in the current study, there was no statistical difference between controls and CRS. Wang et al.’s in vivo nasal potential difference (PD) measurement also showed a mean ΔPD (changes in PD) that was similar to that of controls after exposure to amiloride.31 ENaC activation occurs through a number of other mechanisms, including the cleavage of its subunits by endogenous serine proteases.32 Its expression and function is influenced by diverse factors, such as oxygen tension, glucocorticoids, and cytoskeletal proteins.33 In addition, ENaC dysfunction has been shown to be induced by reactive species (such as, in acute lung injury) and neutrophil elastase, which are abundant in inflamed sinusitis.33,34 Therefore, it may contribute to the various ENaC activities demonstrated in cells from CRS patients in our study. It is important to note that interpreting the effect of amiloride on ENaC-specific ISC is difficult in the presence of a Cl− gradient. Changes in the apical membrane and paracellular driving force, as well as depolarization of the electrochemical potential of Cl− close to the potential of Na+, will impact amiloride-sensitive ISC.35
Salomon et al. observed substantial differences in the Cl− secretory capacity of human nasal epithelial cell monolayers from patients.36 UTP-induced responses reflecting Ca2+−-activated Cl- secretion were substantially lower in CRS compared to normal controls, which was consistent with data in the present study. Pretreatment of cells with IL-13 increased TMEM16A transcript levels in cultured human nasal epithelial cells. Thus, upregulation of CaCC could be associated with allergic inflammation or individuals with CRS with polyposis and T helper type 2 (Th2)-skewed cytokine profiles37 These cytokines induce eosinophilic inflammation, which is not typically exhibited in CRS without polyposis. CFTR function appears to be significantly more suppressed in CRS patients indicating non-eosinophilic disease may have greater susceptibility to environmental perturbations that induce acquired CFTR deficiency.
In this study, the CFTR-mediated chloride secretion that was stimulated by the cAMP agonist forskolin was reduced on average by 70% in cultured cells from CRS subjects when compared to controls. The The diminished CFTR function demonstrated in CRS subjects could serve as a therapeutic target for small molecules identified in high-throughput screening that act as Cl− secretagogues.38 This strategy has been pursued for therapy of other diseases of mucus clearance, including CF and chronic obstructive pulmonary disease. One such agent, ivacaftor (Vertex Pharmaceuticals, Boston MA), represents a novel CF therapeutic approved for treatment of CF patients with at least one copy of the G551D mutation.39 In this CF population, ivacaftor improves CFTR channel opening and thereby augments endpoints predictive of benefit, including MCC, sweat Cl−, and lung function.40 Importantly, the same strategy being applied to activate Cl− secretion in the lower airways of CF patients is also applicable to acquired CFTR dysfunction in CRS patients with impaired sinus and nasal mucus clearance.38,40
There are several shortcomings of this study. The present data included a small number of tissue samples from patients with CRS with polyposis. A larger patient cohort is required to examine CaCC anion transport effects in this population. The lack of CF genetic testing is another limitation that could skew cultures of CRS patients towards decreased CFTR function if heterozygotes were present in the study population.
Conclusion
A substantial decrease in the Cl− secretory capacity of HSNE monolayers was demonstrated in CRS subjects. Data suggest that CFTR may contribute more to abnormal ion transport in CRS than CaCC and indicates Cl− secretagogues could be useful as potential therapy for these individuals.
Authors’ Note
Manuscript presented at the American Rhinologic Society Annual Meeting at AAOHNS, San Diego, CA, September 17, 2016.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bradford A. Woodworth, MD, is a consultant for Olympus and Cook Medical. He also receives grant support from Cook Medical.
Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-04) to B.A.W.; National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-02) to S.M.R.; the Cystic Fibrosis Foundation research grant (ILLEK16G0) to B.I.; and NIH/National Institutes of Allergy and Infectious disease (K08AI146220), John W. Kirklin Research and Education Foundation Fellowship Award, UAB Faculty Development Research Award, Cystic Fibrosis Foundation K08 Boost Award (CHO20A0-KB), and Cystic Fibrosis Foundation Research Development Pilot grant (ROWE15R0) to D.Y.C.
ORCID iDs
Justin McCormick https://orcid.org/0000-0001-6027-4976
Harrison Thompson https://orcid.org/0000-0001-7030-6388
Bradford A. Woodworth https://orcid.org/0000-0003-3292-9167
References
- 1.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy 2013; 27: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho DY, Hwang PH, Illek B. Effect of L-ascorbate on chloride transport in freshly excised sinonasal epithelia. Am J Rhinol Allergy 2009; 23: 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejima K, Randell SH, Stutts MJ, Senior BA, Boucher RC. Potential role of abnormal ion transport in the pathogenesis of chronic sinusitis. Arch Otolaryngol Head Neck Surg 2006; 132: 1352–1362. [DOI] [PubMed] [Google Scholar]
- 4.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med 2014; 20: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy 2013; 27: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birket SE, Chu KK, Liu L., et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 2014; 190: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander NS, Blount A, Zhang S., et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope 2012; 122: 1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bebok Z, Varga K, Hicks JK, et al. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia. J Biol Chem 2002; 277: 43041–43049. [DOI] [PubMed] [Google Scholar]
- 9.Bomberger JM, Ye S, Maceachran DP, et al. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog 2011; 7: e1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope 2009; 119: 2269–2274. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One 2014; 9: e104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy 2012; 26: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax 2016; 71: 284–287. [DOI] [PubMed] [Google Scholar]
- 14.Matsui H, Grubb BR, Tarran R, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998; 95: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 15.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope 2011; 121: 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope 2010; 120: 1051–1056. [DOI] [PubMed] [Google Scholar]
- 17.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 2003; 129: S1–32. [DOI] [PubMed] [Google Scholar]
- 18.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol 2008; 22: 7–12. [DOI] [PubMed] [Google Scholar]
- 19.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol 2007; 21: 533–537. [DOI] [PubMed] [Google Scholar]
- 20.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol 2008; 22: 235–238. [DOI] [PubMed] [Google Scholar]
- 21.Antunes MB, Woodworth BA, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques 2007; 43: 195–196, 198, 200 passim. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy 2009; 23: 149–152. [DOI] [PubMed] [Google Scholar]
- 23.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg 2010; 143: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope 2010; 120: 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope 2011; 121: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy 2011; 25: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazrak A, Jurkuvenaite A, Ness EC, et al. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. American journal of respiratory cell and molecular biology 2014; 50: 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest 2007; 132: 1631–1636. [DOI] [PubMed] [Google Scholar]
- 29.Trinh NT, Bilodeau C, Maille E, et al. Deleterious impact of Pseudomonas aeruginosa on cystic fibrosis transmembrane conductance regulator function and rescue in airway epithelial cells. The European respiratory journal 2015; 45: 1590–1602. [DOI] [PubMed] [Google Scholar]
- 30.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 2012; 302: L1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Moylan B, Leopold DA, et al. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA 2000; 284: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Caballero A, Rasmussen JE, Gaillard E, et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci U S A 2009; 106: 11412–11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis IC, Matalon S. Epithelial sodium channels in the adult lung–important modulators of pulmonary health and disease. Advances in experimental medicine and biology 2007; 618: 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaillard EA, Kota P, Gentzsch M, Dokholyan NV, Stutts MJ, Tarran R. Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflugers Arch 2010; 460: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 2009; 284: 29320–29325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomon JJ, Albrecht T, Scheuermann H, Baumann I, Mall MA. Evidence For Reduced Epithelial Cl- Secretion In Primary Human Nasal Epithelial Cells Of Patients With Chronic Rhinosinusitis. Am J Respir Crit Care Med 2016; 193: 287. [Google Scholar]
- 37.Drake-Lee AB, McLaughlan P. Clinical symptoms, free histamine and IgE in patients with nasal polyposis. Int Arch Allergy Appl Immunol 1982; 69: 268–271. [DOI] [PubMed] [Google Scholar]
- 38.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. Laryngoscope 2015; 125 Suppl 7:S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger J, Macek M, Jr., Stuhrmann M, Reis A, Krawczak M, Schmidtke J. Genetic influences in the formation of nasal polyps. Lancet 1991; 337: 974. [DOI] [PubMed] [Google Scholar]
- 40.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009; 106: 18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]