Abstract
Chemokines are recognized as the major contributor to various tumorigenesis, tumor heterogeneity, and failures of current cancer therapies. The tumor microenvironment (TME) is enriched with chemokines and cytokines and plays a pivotal role in cancer progression. Chronic inflammation is also considered an instructive process of cancer progression, where chemokines are spatiotemporally secreted by malignant cells and leukocyte subtypes that initiate cell trafficking into the TME. In various cancers, prostate cancer (PCa) is reported as one of the leading cancers in the worldwide male population. The chemokines-mediated signaling pathways are intensively involved in PCa progression and metastasis. Emerging evidence suggests that chemokines and cytokines are responsible for the pleiotropic actions in cancer, including the growth, angiogenesis, endothelial mesenchymal transition, leukocyte infiltration, and hormone escape for advanced PCa and therapy resistance. Chemokine’s system and immune cells represent a promising target to suppress tumorigenic environments and serve as potential therapy/immunotherapy for the PCa. In this review, an attempt has been made to shed light on the alteration of chemokine and cytokine profiles during PCa progression and metastasis. We also discussed the recent findings of the diverse molecular signaling of these circulating chemokines and their corresponding receptors that could become future targets for therapeutic management of PCa.
Keywords: Chemokines, Cytokines, Prostate cancer, Tumor microenvironment
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed human malignancy and the second leading cause of cancer-related death in men worldwide. The annual mortality rate of PCa has increased up to more than three hundred thousand [1]. The impact of this lethal disease depends on several factors such as diverse geographic regions, genetic distinction, epigenetic alteration, nutrition, lifestyle, and ecological factors [2]. However, aging, family genetics, and racial disparities are the most recognized risk factors implicated in the pathobiology of PCa [3]. Growing evidence suggested that exacerbated chronic inflammation caused by hormones, chemicals, radiation, stress, infectious agent, or other environmental factors contributes to the progression and development of PCa. As the prostate is more susceptible to infection, it displayed an association between chronic prostate inflammation and PCa development. It is also evident by histological characteristics of inflammation in prostate specimens [4]. Several epidemiological studies have revealed the direct interaction of inflammatory genes with the risk of PCa and the reverse relation of the anti-inflammatory drugs with PCa [5,6]. Therefore, inflammation is also one of the instructive processes of cancer progression. Chemokines are spatiotemporally secreted by malignant cells and leukocyte subtypes initiate cell trafficking into the TME [7]. The recruitment of cells is complicated and implies distinct leukocyte subsets with cancer stimulating/inhibiting functions by coordinating the movement of immune cells to the inflammation site.
Chemokines projected as a member of cytokines superfamily that have chemoattractant properties, are primarily secretory in nature (8–12 kDa). Chemokines are associated with inflammatory response and mediate particular action by binding to their corresponding receptors [8]. Chemokines and their respective receptors are classified into four subclasses depending on the motif of their first two conserved cysteine residues within their structure as CXC, CC, C, or CX3C [9–11], where C and X are represented by cysteine and non-cysteine residue, respectively. Chemokine-mediated signal transduction and other regulatory functions are governed by their motifs specific amino acid sequences [7,12]. As chemokines are generated by tumor cells, the stromal microenvironment in the primary tumor and distant metastatic sites also produce chemokines. These chemokines are also responsible for the pleiotropic actions, including the growth, angiogenesis, epithelial-mesenchymal transition (EMT), leukocyte infiltration, and hormone escape for advanced cancer development and therapy resistance [7,12–14]. Concerning the role of chemokine-mediated PCa progression and development, a large body of work has focused on the chemokines CXCL8, CXCL12, and CCL2. These studies displayed the differential expression of chemokines and their receptor in PCa progression [13]. Remarkably, PCa also comprises the lymphocytes infiltration and an elevated level of pro-inflammatory cytokines [15]. TME contains tumor-related fibroblasts, smooth muscle cells, bone marrow mesenchymal stem cells (MSCs), and numerous inflammatory cells such as lymphocytes, macrophages, and endothelial cells. The TME directly affects cancer progression and invasion by synthesizing different cytokines/chemokines and growth factors. Various pro-inflammatory cytokines, such as interferon-gamma (IFN-γ), transforming growth factor-beta (TGF-β), tumor necrosis factor-alpha (TNF-α), and interleukins (ILs) showed their contributions in the initiation, progression, and establishment of metastatic PCa [15]. However, the mechanism by which these circulating molecules are responsible for progression and metastasis is elusive.
This review compiles all possible pre-clinical or clinical aspects of chemokines and cytokines that regulate the PCa progression. By compiling all these studies, we have also attempted to show the diverse molecular signaling(s) of these circulating molecules involved in the PCa development and progression.
2. Chemokines and prostate cancer pathogenesis
Chemokines primarily involve in the chemotaxis of immune cells by binding to their receptors and fine-tuned regulating immune responses. Approximately 50 diverse chemokines accomplish their task through 25 different seven transmembrane G-protein coupled receptors. Some receptors required stimulation, while some constitutively expressed sustaining homeostasis amongst cells and tissues. The chemokines, such as CXC (α), CC (β), C (γ), or CX3C (δ) regulate several molecular signaling pathways and exhibit a significant role in numerous biological processes. In addition, chemokines modulate the host–tumor connections and enable cancer cells to evade the immune system via employing T regulatory cells (Tregs) and tumor-associated macrophages (TAMs), producing an immune oppressive TME [9,16]. These are the main reasons for the up-regulation of the different chemokines in various cancers.
CC and CXC chemokines and their receptors were reported in the progression of PCa. Recently, several chemokine networks have been reviewed in different carcinogenesis, including PCa, and they delineate the functional role of chemokines in bone metastasis [17]. In vitro co-culture study of PCa cells with macrophages suggested the enhanced CCL2 and CCR2 in the PCa cells. Further, the elevated CCL22 and CCR4 in the PCa cells was evident after exogenous exposure to CCL2 [18]. Mechanistically, it was revealed that the CCL2 and CCL17 stimulated the phosphorylation of AKT that eventually led to the enhanced invasion and metastasis of PCa cells [18]. High CCL2 expression was reported in bone pathophysiological conditions, such as osteoporosis and PCa-bone metastasis, suggesting the essential role of CCL2 in bone metabolism [11,19,20]. Furthermore, the CXCL1 and CXCL2 chemokines encourage inflammation and help to sustain the tumorigenic microenvironment after binding to their CXCR2 receptor [21]. Overall, the increased expression of various chemokines like CCL2 and CXCL8 augmented the migration of PCa cells. However, the expression of these chemokines was noticed to be reduced via androgen receptor (AR) signaling in cancer-associated fibroblasts (CAFs) like cells [22]. Importantly, CXCL8 and CXCL13 bind to CXCR1/CXCR2 heterodimer and CXCR5 receptor, respectively [12]. Moreover, CXLC5 enhances the level of inflammation, invasion, and metastasis in PCa cells, mutually in vitro and in vivo.
Hardaway et al. demonstrated that increasing the level of adiposity corresponds to the enhanced level of pro-inflammatory factors such as CCL2 and cyclo-oxygenase (COX2). It was suggested that these pro-inflammatory factors are associated with osteodastogenesis and tumorigenesis of the bone [23]. In addition, increased marrow adipocytes investigational conditions enhance CXCL1 and CXCL2 in prostate bone tumors. It provided the link between CXCL1 and CXCL2 chemokines with bone marrow adiposity via CXCR2 signaling [23]. Li et al. investigated the CXCR2 as a cell surface marker for neuroendocrine (NE) cells and drove NE phenotype, which assets aggressiveness and therapy failure for PCa [24]. Thus, CXCR2 was used to target the purification of NE cells from fresh primary human PCa tissue and was considered a therapeutic target for advanced and therapy resistance of PCa. Mechanistically, the CXCR2 in NE cells excites excretion of pro-angiogenic components and enhances angiogenesis in PCa [24]. The high mobility group boxl (HMGB1) protein interacts with CXCL12 and activates CXCR4-dependent or an independent mechanism to stimulate neutrophil release in the tumor immune microenvironment that is required for tumor relapse [7,10,25]. Cabozantinib is a receptor tyrosine kinase (RTK) inhibitor implicated in eradicating phosphatase and tensin homolog (PTEN)/p53-deficient invasive murine adenocarcinoma. This tumor clearance effect was associated with augmented neutrophil infiltration and chemokine milieu into the tumor bed via a CXCL12/HMGB1/CXCR4 axis. Interestingly, blockage of chemotaxis response using HMGB1-neutralizing monoclonal antibody or the CXCR4 inhibitor attenuated cabozantinib induced anticancer immune response coupled with tumor regression [7,10,25].
Emerging evidence suggested that CXCR2 could be targeted and used as a prognostic marker for human PCa. The up-regulation of CXCR4 was associated with the invasion and metastasis of various cancers, including leukemia, breast cancer, and PCa. Jang et al. showed that dihydrotestosterone (DHT) targets the AR and modulates CXCR4 pathways for PCa progression and development. In this regard, DHT stimulated-PCa development was abrogated using a CXCR4 inhibitor such as AMD3100. It was confirmed by the decreased expression of CXCR4, AR, p-PI3K, p-AKT, other downstream target genes involved in the cell cycle, and EMT, along with the increased expression of apoptosis-related genes. Hence, the study suggests that resveratrol, an anti-inflammatory/anti-cancerous phyto-compound in conjunction with antagonists of AR and CXCR4, suppresses the DHT-induced progression and metastatic behavior of PCa [26].
Following this, Baci et al. showed the protective role of acetyl-l-carnitine (ALCAR), the acetylated derivative of carnitine . They proposed ALCAR as a “repurposed agent’ for cancer therapy. ALCAR inhibits cellular proliferation, promotes cell death, adhesion, migration, and invasion of human PCa cell lines models such as DU-145, LNCaP, PC-3, 22Rv1, and BPH. ALCAR-mediated inhibitory effect was associated with obstructing the synthesis of pro-inflammatory chemokines (CCL2, CXCL12), and cytokines (TNF-α and IFN-γ). Remarkably, the expression of CXCR1, CXCR2, CXCR4, and CCR2 was found to be repressed in the endothelial cells-treated with conditioned media from PCa cells pre-incubated with ALCAR [27]. In this way, ALCAR also decreases the synthesis/release of pro-angiogenic factors and exhibits angio-preventive action on PCa cells via vascular endothelial growth factor (VEGF) and CXCR4/CXCL12 cascade [27].
Recently, Yu et al. elucidated that the TME of PCa cells consists of the chemokine CXCL12 and its receptors CXCR4 and CXCR7 promote metastasis of PCa to lymph nodes, lungs, and bone via CXCL12/CXCR4/CXCR7- chemokine axis. Putative androgen-responsive features as androgen-responsive element (ARE) consensus binding sites were examined in CXCR4, 7 genes. The elevated CXCR4/7 mRNA ratio was observed in the malignant PCa tissue, while a higher level of CXCR7 mRNA was identified in the androgen-sensitive LNCaP cells. Exposure of DHT and flagellin enhanced CXCR4 mRNA and reduced CXCR7 mRNA; however, CXCR7 protein, but not CXCR4, was decreased in LNCaP cells. In addition, the knockdown of CXCR7 increased the migration potency of androgen-sensitive LNCaP cells toward CXCL12. Hence, CXCR7 is recognized as a decoy receptor in PCa cell migration regulated by androgen, and inflammatory stimuli counteract CXCL12/CXCR4 metastatic promoting effects [28]. Bai et al. demonstrated that the RUNX2-CXCR7-AKT axis might be a potential target for effective PCa treatment. Uncovering the cooperative function of overexpressed RUNX2 with prostate-specific PTEN heterozygous deletion established intense prostatic intraepithelial neoplasia (HGPIN) and cancerous abrasions. Concurrently, it was associated with the increased AKT phosphorylation and the CXCR7 in metastatic glands at the age of less than one year in mice. Increased expression of RUNX2 also promoted the growth of PCa cells, and CXCR7 primarily mediated this effect. CXCR7 expression also positively correlated with AKT phosphorylation in PCa patient specimens. Similarly, in PTEN-deficient human PCa cell lines, RUNX2 activation initiates transcription of CXCR7, membrane relocation, and CXCR7-mediated hyperactivation of AKT. These findings corroborated that RUNX2 is pivotal for prostate tumorigenesis via upregulation of CXCR7 expression [29]. Furthermore, studies identified the role of CXCR7-mediated MAPK activation as a resistance mechanism for second-generation anti-androgen therapy and assessed the therapeutic potential of MAPK/ERK inhibitors in castration-resistant prostate cancer (CRPC) [30]. Miyake et al. explored the correlation amongst CXCL1, IL-6, and tissue inhibitor of metalloproteinase 4 (TIMP4) in solid tumors biology. The high expression of CXCL1 was found in the PC3 cells; likewise, increased expression of its receptor CXCR2 was also observed in DU145 and PC3 cells. The mechanistic insight of the study demonstrated that CXCL1 is crucial in nourishing prostate tumors’ progression through the up and down-regulation of IL6 and TIMP4, respectively. Therefore, systemic administration of CXCL1 monoclonal antibodies, HL2401 against these crucial associations impedes prostate tumorigenesis via inhibiting the cellular proliferation, angiogenesis, and apoptosis stimulation. It provided a novel therapeutic approach by targeting tumor-associated CXCL1 to manage PCa [31].
Chronic inflammation causes 20 % of all cancer progression worldwide, including PCa encouraged by several histopathological, molecular, epidemiological, and genetic studies [32]. However, the major cause of intraprostatic inflammation is unknown. Still, the possible sources are infection such as sexually transmitted agents, cell injury due to chemical and physical trauma from urine reflux and prostatic calculi generation, hormonal exposures, and dietary imbalance factors [33]. The Trichomonas vaginalis (Tv), the most common sexually transmitted parasite, facilitates chronic prostatitis and creates an accommodating microenvironment to accelerate the growth, invasion, and migration of PCa cells. The study by Han et al. displayed an increased level of inflammatory mediators such as IL-1β, CCL2, CXCL8, IL-6, prostaglandin-E2 (PGE2), and COX2 in a normal human prostatic epithelial cell line (RWPE-1) after stimulation with the Tv [34]. Resultant, it promoted EMT of PCa cells through these inflammatory mediators coupled receptor via the instigation of signal pathways such as Janus kinase (JAK), Nuclear factor kappa B (NF-κB), and Zinc finger protein Snail [34]. Fascinatingly, it was elucidated that blockage of receptors including CXCR1, CXCR2, CCR2, glycoprotein 130, EP2, and EP4 inhibited the PCa progression via decreased production of CCL2, IL-6, CXCL8, and PGE2 [34].
In various studies, the enhanced level of G Protein Subunit Alpha 13 (GNA13) is found in many solid tumors comprising PCa [35]. The GNA13 is an alpha subunit of a heterotrimeric G protein that can promote tumor initiation, drug resistance, and metastasis via signaling through G protein-coupled receptors (GPCRs) [35,36]. Lim et al. performed the whole transcriptome analysis of GNA13 silenced PC3 and parental PC3 cell line model, i.e., highly metastatic PCa cell line, and revealed that GNA13 contributes to tumor progression and metastasis via affecting multiple CXC-family chemokines [37]. Furthermore, to explore the underlying mechanism, the pro-tumorigenic CXCL5 emerged as a direct target of GNA13 signaling where the CXCL5 expression was found to be consistently induced by the elevated levels of GNA13 in three different PCa cell lines (PC3, DU145, and LNCaP). Furthermore, the investigation of the CXCL5 promoter indicated that the −505/+62 region was highly active and influenced by GNA13 as well as a single NF-κB site in this region. The stimulation of the expression of GNA13 enhances the tenure of the p65 component of NF-κB at the CXCL5 promoter. Whereas the knockdown of GNA13 attenuated inhibited the NF-κB p65 phosphorylation and the action of a particular NF-κB reporter. In addition, the silencing of NF-κB p65 diminished the GNA13- boosted expression of CXCL5 [37]. Intriguingly, the effect of GNA13 on NF-κB transcriptional activity and CXCL5 expression was abolished via obstruction of Rho GTPase activity. Collectively, this study indicated that GNA13 drives CXCL5 expression via transactivating NF-κB in a manner that depends on Rho GTPase in PCa cells and suggested that targeting GNA13 might lead to new therapeutic options for PCa [37].
Di Mitri et al. recognized TAMs, a major component of the TME, as a therapeutic approach based on the obstruction of macrophage receptor CXCR2 and CXCL2 signaling that triggers re-education of TAMs polarization led to tumor eradication. Macrophage polarization in the direction of an anti-inflammatory phenotype was achieved by stimulating the CXCR2 receptor through CXCL2 expressed on TAMs, which are infiltrated in prostate tumors. Interestingly, the Ptenpc−/−; Trp53pc−/− mice infused with CXCR2 knockout monocytes leads to senescence and tumor suppression via differentiation into pro-inflammatory macrophages that release TNF-α [38]. Moreover, PTEN null tumor showed high sensitization to TNF-α-mediated senescence and growth inhibition via upregulation of TNFR1.
Tregs play an essential role in the anti-tumor immune responses. The effector Tregs serves as a site for the high expression of CCR4, where CCR4 promotes the migration of effector Tregs into cancer tissues through chemotaxis. The studies deciphered the increased infiltration of the CCR4+Tregs in the prostate tissue of poorly prognosed patients, establishing CRPC. In addition, this poor prognosis of PCa is associated with the amount of infiltration of CCR4+Tregs [39]. Therefore, effector Tregs-targeting immunotherapy is anticipated as the new therapeutic approach for PCa patients. Xiang et al. investigated that infiltrated CD4+ T cells activate the CCL5/STAT3 signaling pathway that promotes PCa chemotherapy resistance. In this study, qPCR analysis and cytokine arrays demonstrated that the co-culture of CD4+ T cells with PCa cells could release a high volume of CCL5 that activates p-STAT3 signaling to promote PCa chemo resistance against docetaxel; contrary to this, an anti-CCL5 antibody or p-STAT3 inhibitor attenuates this action [40].
The study of chemokine signaling pathways responsible for cancer progression and metastasis is an alternate investigation approach that utilizes the activation of downstream signaling proteins. The role of downstream signaling proteins such as PKC and PKD (Protein Kinase C and Protein Kinase D) isoforms in the CXCL12 dependent metastasis was evaluated in the PC3 cells. Out of these, the inhibition of PKCζ isoform exhibited the highest suppression in metastasis. The suppression of the PKC and PKD results in the morphological changes of the PCa cells, particularly the inhibition of the PKC corresponds to the round shape PCa cells; in contrast, PKD inhibition corresponds to the elongated PCa cells [41] (Fig. 1; Table 1).
Fig. 1.
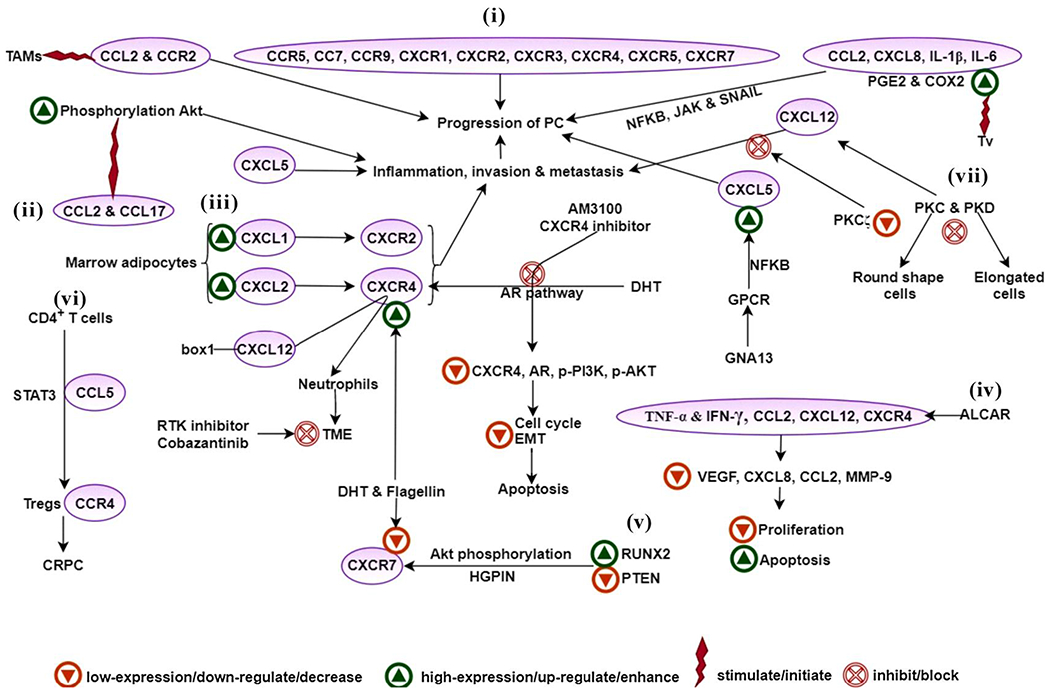
Role of chemokines, interleukins, TNF-α and IFN-γ in PCa. (i) Chemokines CCL2 (CCR5, CCR7, and CCR9) and CXCL8 (CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR7) play a crucial role in PCa progression, (ii) CCL2 and CCL17 stimulated the phosphorylation of AKT, resulting in the enhanced invasion and metastasis of PCa cells, (iii) CXCL1 and CXCL2 uphold inflammation and tumor development after binding to the receptor CXCR2, (iv) ALCAR obstructs the synthesis of pro-inflammatory chemokines (CCL2, CXCL12 and receptor CXCR4) and cytokines (TNF-α and IFN-γ), this reduces invasion, proliferation, and migration of DU-145, LNCaP, PC-3,22Rv1, and BPH cells, (v) RUNX2 initiates transcription of CXCR7 and membrane locality and AKT phosphorylation in PTEN-deficient human PCa cell lines, (vi) High infiltration of the CCR4+Tregs was found in the CRPC and the CD4+T cells activated the CCL5/STAT3 signaling pathway during this resistance, (vii) In the CXCL12 dependent metastasis PKCζ isoform of PKC showed the highest suppression in metastasis.
Abbreviations- PCa- prostate cancer, TAMs- tumor-associated macrophages, PGE2- prostaglandin-E2, COX2- cyclo-oxygenase-2, NFKB- nuclear factor kappa-light-chain-enhancer of activated B cells, JAK- janus kinase pathway, AKT- protein kinase B pathway, PKC- protein kinase C, PKD-protein kinase D, DHT- dihydrotestosterone, AR- androgen receptor, GPCR- G protein-coupled receptor, GNA13- G protein subunit alpha 13, TME- tumor microenvironment, STAT3- signal transducer and activator of transcription 3, Tregs- regulatory T cells, CRPC- castration-resistant prostate cancer, RTK- receptor tyrosine kinase, PI3K- phosphatidylinositol 3-kinase, EMT- epithelial-mesenchymal transition, ALCAR- acetyl-l-carnitine, VEGF- vascular endothelial growth factor, MMP-9- angiogenin, metal-loprotease 9, RUNX2- runt-related transcription factor 2, PTEN- phosphatase and tensin homolog, HGPIN- high-grade prostatic intraepithelial neoplasia.
Table 1.
List of various chemokines with their corresponding receptors, and interleukins, involved in the development, invasion, and metastasis of PCa.
| Chemokines & Interleukins | Functions in PCa | Study details | References |
|---|---|---|---|
| CCL2, 8, 16/CCR2 | Metastasis | CCL2 acts as a paracrine and autocrine factor for PCa growth and invasion and is mediated by CCR2. | [160] |
| CCL2& CXCL8/CXCR1, CXCR2 & CCR2 | Tumor progression and metastasis via NF-κB, JAK, and Snail signaling pathways | PCa cells infected with Trichomonas vaginalis (Tv) showed increased levels of CCL2 and CXCL8. | [34] |
| CCL5, 3, 4/CCR5 | Metastasis | High expressions of CCL5 and CCR5 are detected in human PCa tissues. CCR5 expression was demonstrated on the cell surface of PCa cells, and incubation with CCL5 induced PCa cell proliferation, and the CCR5 antagonist TAK-779 inhibits CCL5-induced proliferation. CCL5 was found to stimulate PCa cell invasion, and TAK-779 blocks the effects of CCL5. | [161] |
| Infiltration of CD4+T cells leads to CRPC | qPCR analysis and cytokine arrays demonstrated that the co-culture of CD4+T cells with PCa cells might be able to release a high volume of CCL5. Variable expression of chemokine receptors may be associated with organ-specific patterns of metastasis. Expression of CCR7 may have accounted for lymph nodes metastasis of PCa cells. |
[40] | |
| CCL19, 21/CCR7 | Metastasis | [162] | |
| CCL25/CCR9 | Migration and invasion | The migration of tumor cells shares many similarities with leukocyte trafficking, which is regulated by chemokine receptor-ligand interactions. This study evaluated the molecular mechanisms of CCL25 and CCR9 in PCa cell migration and invasion. | [163] |
| CXCL1 & CXCL2/CXCR2 | Inflammation, tumor development, aggressiveness, resistance and angiogenesis | Activation of the CXCLS/CXCR2 axis activates multiple signalling pathways, including the PI3K, p38/ERK, and JAK pathways, and regulates cell survival and migration. | [164] |
| CXCL5/CXCR2 | Recurrence and metastasis | CXCL5/CXCR2 axis can stimulate tumor growth and angiogenesis, and promote the infiltration and activation of host cells. | [165] |
| CXCL6/CXCR1/2 | Proliferation and metastasis | The interaction of CXCL6 has been evaluated with CXCR1/2 and correlates with the growth and metastasis of osteosarcoma. | [166] |
| CXCL8/CXCR1/2 | Metastasis | The CXCL8-CXCR1/2 axis may play an important role in tumor progression and metastasis by regulating cancer stem cell (CSC) proliferation and self-renewal. | [167] |
| CXCL8, 12/CXCR4 | Adhesion, proliferation, and metastasis | AR expression regulates the migratory responses of human PCa cells via the CXCL8, 12/CXCR4 axis. | [168] |
| CXCL9, 10, 11/CXCR3 | Growth | Binding of CXCL9, 10, 11 with CXCR3 activates pro-tumoral signaling events and increases tumor progression from a low to a highly aggressive phenotype. Activation of CXCL11, 12/CXCR7 axis promotes the growth and proliferation of PCa cells. |
[169] |
| CXCL11, 12/CXCR7 | Growth and proliferation | Overexpression of CXCR7 in normal prostate cells increased their proliferation with increased levels of phospho-EGFR and phospho-ERK1/2. | [170, 171, 172, 173] |
| CXCL12/CXCR4 | Imply neutrophils to the tumor microenvironment | The high mobility group box1 protein linked CXCL12 interacts with CXCR4 ligand to intervene its effects such as employment of neutrophils to the tumor microenvironment and acquiring an anti-cancer reaction prompted by treatment with cabozantinib, a RTK inhibitor. | [96, 174] |
| CXCL13/CXCR5 | Tumor progression and immune modulation in tumor microenvironment | Activation of the CXCL13/CXCR5 axis is associated with G protein-coupled receptor (GPCR) responsiveness, invasion/migration, immune checkpoint, and innate immunity. | [175] |
| Invasion and MMP modulation | Activation of CXCL13/CXCR5 axis increases the expression of MMP-1, MMP-10, and MMP-11 and increases the invasiveness and metastasis of PCa. | [176] | |
| CXCL16/CXCR6 | Adhesion and chronic inflammation | CXCR6/AKT/mTOR pathway plays a central role in the development of PCa, and CXCL16/CXCR6 might be another essential axis involved in PCa bone metastasis. | [177] |
| CCL22/CCR4 | Phosphorylation of Akt and enhanced the metastasis and invasion | CCL2 and CCR2 were reported in the PCa cells in the in vitro co-culture study of PCa cells with macrophages.The increased level of CCL22 and CCR4 in the PCa cells was achieved via the addition of CCL2. | [18] |
| IL-Ιβ | Chronic inflammation, | The IL-Ιβ expression showed the transformed glandular architecture that phenocopies human PIA. | [178] |
| IL-2 | Improve the therapeutic potential of an anti-cancer vaccine | Specifically targeted the regulatory T cells in the tumor microenvironment and eliminated these cells via a chimeric protein, which consists of the cytokine IL-2 merged to the binding mutant of the highly toxic bacterial toxin proaerolysin (IL2-R336A). | [119] |
| IL-6 | Chronic inflammation, EMT, resistance to radiotherapy leads to CRPC | IL-6 increased proliferation and decreased apoptosis in the PCa cells via targeting several signaling pathways such as JAK-STAT, ERK1/2-MAPK, and PI3-K pathway. | [179] |
| IL-8 | Inhibition of GSK-3β leads to cancer cell defense | IL-8 acts as a connecting molecule between the inflammation and cancer cell oxidative stress-induced death; IL-8 and mTOR defend PCa cells and mitigate the oxidative stress via inhibition of GSΚ-3β. | [180] |
| IL-15 | Activation of NK cells | Lymphocytes co-cultured with PCa/noncancer cells with IL- 15, IL- 2, IL-12, IL-15, interferon (IFN)-c, or IL-21 for seven days showed the high growth level of NK cells/NKT cells and CD8T cells in the presence of PCa cell lines stimulated by IL-15 cytokine. | [116] |
| IL-17 | Increased expression of MMP7 | IL-17 induces cancer via induced expression of MMP7. | [118] |
| IL-23 | Resistance to ADT | The increased concentration of IL-23 and enhanced permeation of MDSC was found in the blood and tumor samples of PCa patients. In mice, the inactivation of the IL-23 via specific antibody results in the sensitivity to the ADT. | [120] |
| IL-24 | Anti-tumor activity | The DU-145 cells transfected with the IL-24 gene using a retroviral vector showed inhibition of cell growth and viability together with the induction of apoptosis and regulation of anchorage dependence via intracellular signaling. | [121] |
3. Chemokines and tumor heterogeneity
In past decades, several studies have emphasized the chemotactic response of chemokines, including their role as natural antagonists or synergistic effects on selective receptors by making heterocomplexes [42,43]. Initially, its function was elucidated as regulating immune cells migration during inflammation, but now the relevance of heterocomplexes is established in the TME. In this way, chemokines are released in the microenvironment in a heterocomplex of two chemokines or chemokine and chemokine receptors that interact with inflammatory mediators of malignant cells or stromal cells and amplify cellular responses [42]. The clinical presentation of PCa differs widely, ranging from the localized slow-growing tumor, which is clinically harmless, to the aggressive metastatic form of cancer, which progresses rapidly and leads to lethal disease [44,45]. From the clinical, morphological, and molecular studies, PCa has been known to be a heterogeneous disease [44]. In this context, the neoplastic cell population interacts with several other types of complex resident cells, including fibroblasts, vascular endothelium, infiltrating immune cell types, growth factors, and other nutrient constituents. These cells contribute to tumor heterogeneity and cancer cell survival [46]. Tumor heterogeneity has been established as a characteristic event for cancers, defined as monitoring different tumor cells showing distinct morphological and phenotypical properties [47]. Investigations at genomic, histopathological, and molecular levels have shown a high level of genetic and phenotypic diversity within the tumor [47]. This divergence of cells at the genetic level is the primary driver of tumor heterogeneity [48]. This complex heterogeneity of molecular alterations causes obstacles in the diagnosis of PCa and provokes a resistant environment for therapy. Fortunately the advent of genome sequencing (next-generation sequencing) technologies, it has become more common to unravel the genetic heterogeneity from primary to metastatic PCa [47]. The relationship between the primary and the metastatic tumor is the critical aspect of tumor heterogeneity that impacts the disease progression and affects the efficacy of current and future treatment strategies [47].
The metastatic PCa originates from a single clone but tends to exhibit sub-clonal heterogeneity at the genetic, epigenetic, and phenotypic levels [44]. With this, the heterogeneity has been characterized in two ways; intra-tumor heterogeneity (present within tumors) and inter-tumor heterogeneity (present between tumors) [47]. The intra-tumor heterogeneity results from various types of cell populations in an individual tumor that differs in genetic and phenotypic behavior [49]. It is considered complex with spatial and temporal heterogeneity [50]. In comparison, inter-tumor heterogeneity defines the entirely different genotype of the tumors in one patient [45]. Intra-tumor heterogeneity affects diverse treatment responses, including drug resistance [51] due to variations at the genomic level [47]. However, the non-genetic factors such as epigenetic and TME are also implicated in the heterogeneity [48], where the heterogeneity of clones can vary depending upon the TME surrounding the PCa cells [51].
The TME plays a pivotal role in the progression of PCa and is recognized as the major contributor of tumor heterogeneity as well as implicated in the failures of current cancer therapies [52,53]. The occurrence and progression of PCa are caused by the intrinsic alterations in tumor cells and the complicated crosstalk between cancer cells and the altered microenvironment components that leads to the metastasis of PCa [54]. TME components involving the CAFs, TAMs, cytokines, and chemokines, cancer stem cells (CSCs), immune cells (B and T lymphocytes), extracellular matrix (ECM), myeloid-derived suppressor cells (MDSCs), mesenchymal stromal cells (MSCs), proteins, and growth factors. These microenvironments work together to promote tumor survival, leading to malignancy [55].
3.1. Role of chemokines/cytokines in cancer-associated fibroblast
CAFs are most abundant in the stromal cell population in TME is, and their function is of great concern. The CAFs are the spindle-shaped cells distinguished by the elevated expression of the markers involving the fibroblast-specific protein 1 (FSP1), α-smooth muscle actin (α-SMA), platelet-derived growth factor receptor (PDGFR) α and β, vimentin, and fibroblast activation protein (FAP) [56]. In addition, the derivation of CAFs can be from the normal fibroblasts (NFs), endothelial-mesenchymal transition (EndMT), and epithelial cells [57]. The NFs, which are usually inactive, get activated via their altered intrinsic signaling pathways through the activation of TGF-β and IL-1β signaling that results in CAFs, suggesting the modulation of the TME by secreting various growth factors [57,58]. As discussed earlier, CAFs are the most abundant components in the TME that contribute to the progression of prostate tumor growth, where several chemokines (CCL2, CCL5, CXCL8, and CXCL12) are involved in the regulation of CAFs. The chemokine-mediated supervision of CAFs is dependent on the activation of different signaling pathways (mitogen-activated protein kinases; MAPKs and PI3K/AKT), which help in the succession of CAFs malignancy in the TME [59].
The CCL2 present in the prostate TME, which has been derived from CAF, was found to induce the recruitment of macrophages to the primary stage of tumor development and assist in the recruitment of myeloid cells to the TME [60,61]. Another chemokine, CXCL12, was found to activate numerous responses in the TME through the activation of several pathways such as migration, cell survival, and cell proliferation. CXCL12 also plays a role in the maintenance of the phenotypes that are directly involved in tumor progression and metastasis [62]. In a recent study, Parol-Kulczyk et al. showed the interesting involvement of the SDF-1- α (stromal-derived factor 1-alpha) together with the CXCR4 and CXCR7 in the EMT of the PCa cells [63]. Moreover, CXCL12 binds with two distinct receptors that are CXCR4 and CXCR7, in which CXCR4 is highly conserved. The interaction of CXCR4 with the CXCL12 induces various downstream signaling cascade in TME that ultimately leads to cancer cell survival, proliferation, and metastasis. In addition to CXCR4, another chemokine receptor, CXCR7 binds with the CXCL12 more strongly than CXCR4. Their interaction triggers the accumulation of non-G protein-mediated β-arrestin that leads to activation of the ERK pathway. The binding of CXCL12 to CXCR7 induces non-G protein-mediated β-arrestin accumulation and subsequent ERK activation [64]. The chemokine CXCL5, secreted by endothelial cells of various organs, plays a significant role in the recruitment of immune cells, enhancing tumor progression [64]. The CXCL8 chemokine has been known to play an essential role in the invasion and migration of various cancer, including PCa. The CXCL8 was released by the PTEN deficient PCa cells, which results in the overexpression of CCL2 and CXCL12 in the stromal cells that leads to the migration of PC3 cells. The overexpression of the CXCL8 in the PCa cells regulates the expression of cydin D1, which promotes tumor growth and cell cycle progression [22].
A prominent study by Shen et al. has reported that the Yes-associated protein (YAP-1) is responsible for cell invasion, proliferation, apoptosis inhibition, and EMT induction. Moreover, YAP-1 plays a crucial role in converting NFs into CAFs in the TME that encourages the aggressiveness of PCa. Hence, targeting YAP-1 protein is suggested as potential therapeutic management for PCa [57]. In addition, another study by Cheteh et al. has analyzed that the IL-6 produced from the CAFs play a vital role in regulating tumor cell proliferation, migration, and angiogenesis. These processes are dependent on attenuating the p53 via the JAK/STAT signaling pathway and cell death upon treating the PCa cells with doxorubicin. CAFs derived IL-6 protects cancer cells from chemotherapy; hence, IL-6 also serves as a therapeutic target for PCa [65]. In contrast, in a recent study, Zhang et al. showed that low expression of the IL-6 in the system resulted in the inhibition of the PCa cell invasion. Zhang et al. also identified the high expression of the G protein-coupled receptor 30 (GPR30) in the prostate CAFs and showed that it plays an essential role in the stromal cell activation of the prostate. Moreover, the downregulation of the GPR30 contributes to the inhibition of PCa cell invasion via suppressing infiltration and M2 polarization of TAMs [66].
3.2. Tumor-associated macrophages in heterogeneity
Another critical component of the TME contributing to the heterogeneity of PCa is TAMs, which are highly heterogeneous cells originating from dwelling tissue-specific macrophages and newly recruited monocytes [67]. These are the essential constituents that participate in cell-to-cell communication inside the TME and remarkably show their pro-tumoral role in the metastasis of PCa and response to androgen deprivation therapy (ADT) [68]. Therefore, the role of TAMs is multi-factorial in the progression of PCa, causing tumor invasion, promoting angiogenesis, remodeling of ECM, releasing growth factors, tumor proliferation, metastasis, and ultimately instigating the immunosuppressive microenvironment. These all establish favorable conditions for tumor cells to metastasize in the secondary organs [67,69].
Two major phenotypes of TAMs are tumor-inhibiting M1 (classically activated) and tumor-promoting M2 (alternatively activated). The main markers of M1 are HLA-DR, CD80/86, and the key representative markers of M2 are CD206, CD163, CD204, stabilin-1. The M1 macrophages manifest antitumoral activity that activates the adaptive immune response and inflammation by releasing IFN-γ and IL-12. In contrast, M2-like macrophages act as immune suppressors in TME by inducing angiogenesis leading to metastasis [67]. The presence of M2 macrophages was found to be clinically worse in PCa because it has a unique pattern of infiltration [45]. During the progression of PCa, the infiltration of TAMs increases because of the presence of the M2 phenotype in TAMs, which suppress the antitumor-immune response by promoting tumor growth., Therefore, high infiltration of TAMs leads to poor survival of PCa patients [68,70]. In addition, studies have reported that high numbers of CD163-positive M2 macrophages in prostate tumors are associated with poor outcomes [69]. The immunosuppressive cytokines and chemokines can also be produced from the M2 macrophages involved in the recruitment of lymphocytes and stimulating them to develop into Tregs. The Tregs are involved in tumor development by producing elevated IL-10, IL-32, and TGF-β that further inhibit the anti-tumor inflammatory response and stimulate M2 macrophages [69,71]. TAMs are the essential cells responsible for the immunosuppressive microenvironment by favoring the generation of pro-inflammatory cytokines and the chemokines that drive the progression and the metastasis of PCa [54,72]. The M1 macrophages produce inflammatory cytokines such as IL-1β, IL-6, IL-12, IL-23, and TNF-α [73]; in contrast, the M2 macrophages have anti-inflammatory activity and produce immunosuppressive chemokines IL-13, IL-10, IL-8, IL-6, IL-4, and TGF-β. The immunosuppressive chemokines produced by TAMs increases the motility, invasion, and proliferation of tumor [73]. The M2 phenotype was found to significantly increase in the prostatic TME via initiating the complex paracrine signaling through activation of different chemokines networking such as CXCL-10, CCL-22 [66], CCL2, CCL3, CCL4, CCL5, CXCL1, CXCL2, CXCL8, CXCL9, CXCL10, CCL25, and CCL27. Under the influence of these chemokines, the TAMs are recruited to the cancer site [7]. The overexpression of most of these chemokines and their receptors are involved in cancer cell survival, proliferation, epithelial to mesenchymal transition (CXCL8/CXCR1), metastasis (CCL2, CCL5), angiogenesis (CXCL1–3, CXCL5–6, CXCL8, CXCL12, CCL2, CCL11, and CCL16), and in the immune evasion (CXCL5 and CXCL8). In contrast, some chemokines such as CCR1/CCR5 are known to support the inhibitory signals [64], and CXCL9 produced by the CAFs has a role in inhibiting anti-tumor T cell response [73].
The differentiation of macrophages into TAMs is recruited by promoting CAF-derived factors such as CCL2, IL-6, and IL-8, in which the CCL2 has strong chemotactic activity for the macrophage’s recruitment. The TAMs infiltration in TME is highly dependent on the presence of the above-described chemokines, which play a crucial role in the overall heterogeneity of the tumor [73]. Many studies have also reported the supportive role of TAMs and CAFs in promoting tumor metastasis in which the M2 phenotype assists tumor growth and converts healthy fibroblast into CAF. Further, the CAF gets activated and starts secreting factors that accelerate TAM, EMT, and tumor aggressiveness. Reports also suggested that CCL5 derived from TAMs promotes PCa stemness and metastasis with the involvement of β-catenin/STAT3 signaling [74].
Moreover, the chemokines and their receptors, including CCL2, are involved in the TAMs recruitment, which later facilitates tumor cell survival and metastasis [75]. Therefore, TAMs influence diverse processes during tumor progression in PCa, including TAMs infiltration is projected with poorer response ADT response and tumor angiogenesis [68,76]. Additionally, the amount of TAM is considered with prognosis where increased TAMs infiltration was correlated with poor cancer-specific survival and recurrence-free survival. In contrast, reports also suggested that the enhanced TAM infiltration in prostate tumors is prognostic of improved disease-free survival [68]. However, for better understanding and precise interpretation of TAMs in the PCa microenvironment, further studies with a more significant number of patients and extended follow-up are required. Still, current evidence provides the clinical significance and therapeutic management of TAMs in PCa.
3.3. Chemokines and their receptors in cancer cell stemness
The chemokines and their associated receptors are not only involved in cellular motility, tumor angiogenesis, and proliferation but are implicated in the maintenance of CSCs [77]. It leads to tumor malignancy by promoting the release of matrix metalloproteinases (MMPs), EMT in cancer cells, and enabling therapy-resistant response [78]. The CSC’s proliferative capability facilitates tumor initiation and produces various differentiated cell populations. These cell populations constitute the phenotypically heterogeneous tumor [79] and are highly resistant to chemotherapy [78,80]. As discussed earlier, tumor heterogeneity plays a crucial role in enhancing cancer aggressiveness and drug resistance [79]. Recent reports elucidated that genetic and phenotypic heterogeneity is the major challenge during the treatment [77].
CSCs and epithelial-mesenchymal plasticity (EMP) are nongenetic heterogeneity and the two principal interconnected axes. These are involved in different sarcomas, including PCa [79,81]. The molecular interaction between the EMT and CSC has reflected that the process of EMT constitutes the cancer stemness properties [82]. Bocci F et al. have demonstrated the correlation between the EMT, Notch signaling, and CSCs in the progression and maintenance of cancer aggressiveness. It was suggested that the stemness traits co-exist with an epithelial/mesenchymal phenotype and associate with the Notch signaling [82]. Furthermore, the role of inflammatory cytokines was found in the activation of Notch signaling, such as IL-6 is involved in the acquisition of various subpopulations of cancer cells that exhibit the hybrid of EMT/EMP phenotype. This hybrid EMT/EMP possesses the properties of cancer stemness [81]. In addition, many other studies have also shown that cytokines increase the level of CSCs in the tumor [83].
An imperative cytokine IL-30 plays a significant role in regulating PCa stem-like cell (PCSLC) behavior by synchronizing the cytokine-induced autocrine and paracrine signaling [84]. The cytokine-mediated signaling activation alters the TME and promotes the metastasis of PCa. The property of PCSLC is regulated by various factors such as attachment factors like annexins II, growth factors, hypoxia, angiogenic factors like VEGFs, chemokines such as CXCL12. The up regulation of IL-30 promotes the viability of PCSCL, self-renewal tumorigenicity, inflammatory immune response via activating the STAT1/3 signaling. The overproduction of IL-30 increases the expression of CXCR5 and CXCL13, which helps PCSCL to metastasis into the lymph node and bone marrow, while the increased expression of CXCR4/CXCL12 metastasizes to the lungs [84]. The CXCR4/CXCL12 are well-studied chemokine receptors that regulate the cancer stemness in various cancer, including PCa, where the higher expression of CXCR4 was observed in the tumor-initiating markers CD44+/CD133+ PCSLCs. That promotes the extracellular fibronectin proteins adhesion and its proliferation in CXCL12 dependent manner by activating the PI3k/AKT signaling cascade [77,85].
The continuous production of inflammatory and angiogenic responses promotes the hypoxic condition in the TME, which leads to the generation of reactive oxygen species, further supports the tissue damage events by raising the DNA damage of neighboring cells [86,87]. Hypoxia is a condition of low oxygen level in the cell that alters the microenvironment and stimulates various molecular responses that support the tumor growth and survival controlled by hypoxia-induced factor-1 α (HIF-1α), a transcription factor [55,87]. Hypoxia-induced factors interact with the hypoxia-response element and regulate cellular events such as cell proliferation, angiogenesis, invasion, metastasis, and EMT [88]. The hypoxic condition in the TME causes the increased expression of chemokines ligands and their receptors such as CXCL12, CCL28, and CXCR1, CXCR2, CXCR4, CCR2, in various cancer such as breast cancer, ovarian cancer, and PCa [88]. In PCa, the hypoxic condition enhances the CXCR1 and CX3CR1 expression that regulates the invasion and migration of PCa cells [88,89]. Thus, targeting these chemokine receptors may result in improved treatment outcomes.
4. Chemokines and their receptors in drug resistance
The evolution of chemoresistance is one of the hallmarks in the course of cancer treatment. However, the mechanism that underlies chemoresistance is still a matter of research. Several lines of evidence suggested that TME and its components such as CAFs, TAMs, cytokines/chemokines, and immune cells play a major role in the inauguration of chemoresistance by protecting the tumor niche against chemotherapeutic drugs [90,91]. Interestingly, cancer progression to the secondary organ is instructed by the secretion of immune cells at the tumor site by interacting with the chemokines and their receptors [92]. Chemokines and their receptors were found to show a strong relation and result in poor clinical outcomes in various cancer such as oral squamous cell carcinoma (OSCC), breast cancer, and PCa. For instance, the increased level of CCR7 and its ligand CCL21 were found in OSCC [93], while the overexpression of CXCR4, along with its ligand CXCL12, serves as the potent driver of breast cancer progression [94]. Notably, the chemokine CCL5 was found to play an important role in drug resistance in patients with PCa [95]. Chemokines that belong to the CC subfamily have been implicated in chemoresistance; as CXCL12 gets elevated during cancer chemotherapy [96], CCL5 activates the STAT3 through an autocrine loop by inhibiting the caspase9/PARP and modulates the Bcl-2. The autocrine regulation avoids the response of drugs and other signaling pathways activated by tumor-derived cytokines, which are involved in cancer cell survival and proliferation, which further prevents chemotherapy [97].
Studies have revealed that the CCL2-CCR2 plays a crucial role in the drug resistance of PCa. Increased expression of CCL2 was found in cabazitaxel-resistant cell line DU145-TxR/CxR and Paclitaxel-resistant cell line DU145-TxR compared with the vehicle control, where the CCL2-CCR2 reduced the apoptosis by inhibiting the caspase-3 [98]. Another prominent study reported the role of chemokines in PCa to bone metastasis and drug resistance by activating the CXCR4 receptor. Mechanistically, through the bone-borne TGF-β induced acetylated transcription factor Krüppel-like factor 5 (Ac-KLF5) that stimulates the secretion of IL-11, which further triggers the SHH/IL-6 paracrine signaling. SHH/IL-6 cascades are also crucial for tumorigenicity and mesenchymal phenotype maintenance. The Ac-KLF5 was also found in docetaxel resistance in PCa to bone metastasis, where the high expression of KLF5 in the bone metastasis of PCa in vitro and in vivo models. Hence, this chemoresistance of PCa can be conquered by targeting KLF5 and the chemokine receptor CXCR4 [99]. A recent study also demonstrated that CCL20/CCR6 axis disruption in mice with syngeneic PCa bone metastases relieves T cell exhaustion and extends animal survival [100].
Increasing evidence has suggested the role of obesity in PCa progression and chemoresistance by activating the chemokines. The periprostatic white adipose tissue (PPAT) surrounding the prostate gland consists of adipocytes and other cells such as macrophages, endothelial, and epithelial cells. The combination of PPAT with PCa cells increases the extra-prostatic extension. In the presence of PPAT, obesity was found to increase the extra-prostatic extension as PPAT is elevated in obesity, promoting PCa progression [101]. However, Laurent et al. have reported that the chemokine CCL7, secreted by these adipocytes, gets dispersed from the PPAT to the periphery of the prostate gland. This CCL7 binds to its receptor CCR3 which is expressed on the PCa cell surface, leading to extra-prostatic extension [102]. In PCa, the adipose-derived stem cells (ASCs) derived adipocytes help in the migration of tumors and induce the EMT, resulting in the aggressiveness of PCa [103]. Further, the effect of these ASCs on PCa cells sensitivity has been evaluated to chemotherapeutic drugs such as cabazitaxel, cisplatin, and docetaxel which showed resistance against these chemotherapeutic drugs in the presence of ASCs [101].
As chemokines and chemokine receptors are represented by malignant cells, leukocyte infiltrate is considered an ideal target for immunotherapy. In the context of PCa, chemokine receptor inhibitors in combination with chemotherapy or with antibodies against immune checkpoints were evident for killing tumor cells [104]. Altogether, targeting these chemokines and their receptors, especially CCL5, CXC12, CXCR4, and CXCR7, would be a promising target as chemo or immunotherapy to reduce metastasis and drug resistance of PCa.
5. Cytokines in prostate cancer
Cytokines are small polypeptides/glycoproteins (low molecular weight) act as molecular messengers of innate/adaptive immunity. Also, it functions to transfer signals of development, differentiation, and pro-inflammatory/anti-inflammatory to various types of cells [105,106]. Cytokines are secreted for a distinct period (short half-life) in response to stimuli and predominantly work in an autocrine or paracrine manner. The association of cytokines with the immune system significantly increases the interest to target these cytokines to treat cancer [106]. The alteration of the cytokine signaling in the cancer cells affects their connections with the immune system. The presence of inflammatory cytokines in the TME recommends their crucial role in cancer progression and therapeutic efficiency [106,107].
5.1. Interleukins in the development and progression of prostate cancer
ILs are a class of cytokines that play a role in signaling and are subdivided into four major types and around 50 subtypes. The major ILs involved in the proliferation of cancer are IL-1 and IL-2 superfamilies, IL-6, IL-10, IL-12, IL-17. The IL-1 is known to act on various levels in the initiation and progression of tumor development, such as driving tumor angiogenesis, chronic inflammation, MDSC induction, and invasion and metastasis [108].
IL-6 has been reported in high concentrations and is generally overexpressed in almost all types of cancer cells. Moreover, IL-6 protects the cancer cells from chemo/radiotherapy-induced DNA damage and apoptosis by activating the anti-apoptotic pathway [109]. In PCa, the IL-6 signaling is involved in the resistance to radiotherapy. The IL-6 upregulates the DNA repair-related signaling molecules ataxia–telangiectasia and Rad3 related (ATR), ataxia–telangiectasia mutated (ATM), and breast cancer gene (BRCA1/2), responsible for developing the therapy resistance. Chen et al. showed that IL-6 expressing cell lines survive better after irradiation than vehicle control cells. It was further confirmed by the xenograft mouse model that showed radiation sensitivity of C4-2-IL-6 cell-derived tumors vs. C4-2-vec cell-derived tumors, suggesting the role of IL-6 in developing radiation-resistant PCa cells [110]. In pre-clinical studies, IL-6 increased proliferation and decreased apoptosis in the PCa cells via targeting several signaling pathways such as JAK-STAT ERK1/2-MAPK and PI3-K pathway. Moreover, IL-6 regulates the EMT involved in the aggressive PCa phenotype [111].
Another inflammatory cytokine, IL-8, plays an imperative role in developing many human malignancies [112]. A meta-analysis study by Chen et al. also suggested that IL-8 rs4073polymorphism was significantly associated with a high risk of PCa [113]. The IL-8 endorses proliferation and decreases apoptosis in PCa cells. The IL-8 works as the connecting molecule between the inflammation and tumor cell oxidative stress-mediated cell death. It also showed a connection with the mTOR and glycogen synthase kinase-3 beta (GSK-3β). Sim et al. showed that GSK-3β enhanced the oxidative stress in PCa cells via stimulating ROS production; in contrast, IL-8 and mTOR defend these cells and mitigate the oxidative stress via inhibition of GSK-3β [114]. Chronic inflammation is associated with PCa development, and IL-1β plays an essential role in inflammation. The expression of IL-1β leads to human prostatic proliferative inflammatory atrophy (PIA) lesions. The IL-1β expression was studied in the transgenic mouse, which resulted in a transformed glandular architecture that phenocopies human PIA. The human IL-1β in the mouse prostate caused acute and chronic inflammation, which was categorized via the permeation of CD4+ T cells that feature an adaptive immune response [115].
The IL-15 is the only one in the group of cytokines that trigger natural killer (NK) cells in the presence of PC3 and LNCaP PCa cells [116]. Sakellariou et al. isolated the lymphocytes and co-cultured cancer/-non-cancer PCa cells with cytokines like IL-2, IL-12, IL-15, IFN-c, or IL-21 for one week. They compared the growth level of NK cells/NKT cells and CD8T cells in the normal and cancer cells and found that the growth level was high in the presence of PCa cell lines stimulated by IL-15 cytokine [116]. Yuanyuan et al. studied the vulnerability of the polymorphism of IL-18-607 C/A towards PCa. In this study, the meta-analysis of IL-18-607 C/A polymorphism showed that IL-18-607 C/A polymorphism resulted in the reduced threat of PCa in the Asian population augmented threat in the Caucasian population [117].
The pro-inflammatory cytokine IL-17 is involved in the initiation and progression of various cancers, including PCa. In the mouse prostate, the IL-17 stimulates the cancer progression with the simultaneously increased expression of MMP7; later, one facilitates the role of IL-17 in the advancement of PCa via initiation of EMT. Zhang et al. proved the role of IL-17 in the development of PCa via MMP7-induced EMT. They have used in vitro and in vivo knockdown strategies to validate this. Therefore, the IL-17-MMP7-EMT axis can be served as a potential therapeutic target for the prevention and control of PCa [118]. In another study, Rogers et al. try to improve the therapeutic potential of an anticancer vaccine (GMCSF-expressing CT-26 GVAX) via specifically targeting the immunosuppressive cells, i.e., Tregs in the TME. They eliminate these cells via a chimeric protein consisting of the cytokine IL-2 merged to the binding mutant of the highly toxic bacterial toxin proaerolysin (IL2-R336A) [119].
In addition, IL-23 promotes the progression and persistence of the PCa cells in androgen-deprived conditions via activating the AR pathway. In this way, IL-23, which is derived by the MDSCs, serves as a cause for ADT resistance. The increased concentration of IL-23 and enhanced permeation of MDSC was reported in the blood and tumor samples of PCa patients. In mice, the inactivation of the IL-23 via specific antibody results in the sensitivity to the ADT. These studies confirmed the role of IL-23 in the proliferation and enhancement of the PCa [120].
In contrast, some ILs such as IL-24 and IL-6 showed anti-tumor activity in the PCa cells. The DU-145 cells transfected with the IL-24 gene using a retroviral vector showed inhibition of cell growth and viability together with the induction of apoptosis and regulation of anchorage dependence via intracellular signaling. Furthermore, a reduced level of stress fiber formation and reduced fibronectin expression affect the motility of the PCa cells [121]. Zhang et al. showed the anticancer activity of Polyphyllin I in PC3 and DU145 PCa cells. In this study, the polyphyllin I increased the expression of p21 and caused cell cycle arrest at the G0/G1 phase via increased expression of IL-6, suggesting the role of IL-6 in the anti-tumor activity [122] (Fig 2; Table 1).
Fig. 2.
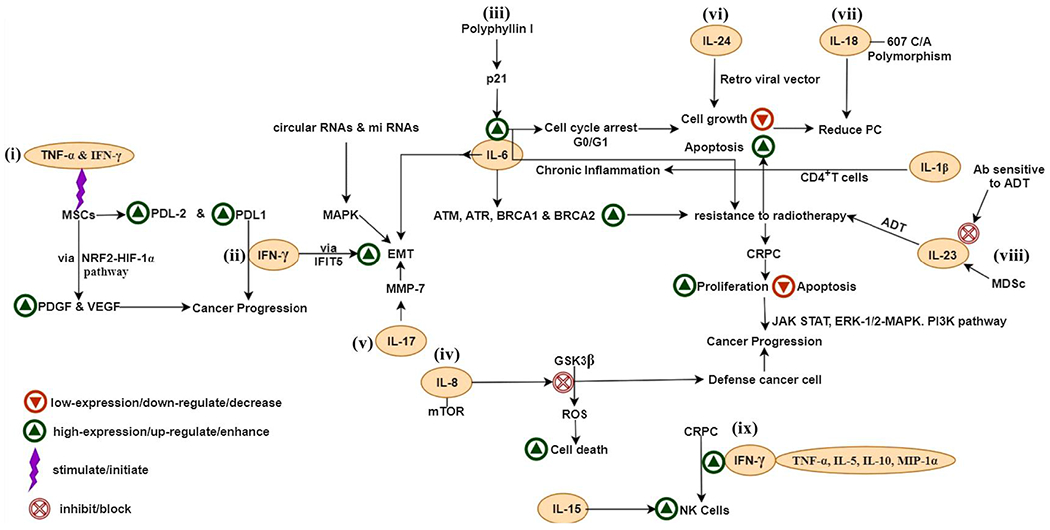
Signaling of interleukins,IFN-γ and TNF-α in PCa. (i) TNF-α and IFN-γ increase PDGF and VEGF levels via the NRF2-HIF-1α pathway and stimulate PCa growth, (ii) Occurrence of IFN-γ and TNF-α, causes mesenchymal cells to have increased levels of PDL1 and PDL2, (iii) Polyphyllin I increase the expression of p21 and cause cell cycle arrest at the G0/G1 phase via increased expression of IL-6, (iv) IL-8 and mTOR mitigate the oxidative stress via inhibition of GSK-3β, (v) IL-17 stimulates expression of matrix metalloproteinase 7 (MMP7); which causes the advancement of PCa via initiation of EMT, (vi) IL-24 also inhibit the cell growth, (vii) IL-18-607 C/A polymorphism resulted in the reduced threat of PCa, (viii) MDSCs derived IL-23 serves as a cause for the resistance to ADT, (ix) IFN-γ secreted by NK cells and high expression of the IFN-γ together with the TNF-α, IL-5, IL-10, and MIP-1α was found in CRPC and IL-15 is the only one in the group of cytokines that can trigger NK cells in the presence of PC3 and LNCaP PCa cells.
Abbreviations- IL-interleukin, TNF-α- tumor necrosis factor α, IFN-γ-interferon γ, PCa- prostate cancer, CRPC- castration-resistant prostate cancer, MSCs- mesenchymal stem cells, PDL1 & PDL2- programmed death-ligand 1 & ligand 2, ADT- androgen deprivation therapy, MDSc- myeloid-derived suppressor cells, IFIT5-interferon-inducible transmembrane protein 5, Ab- antibody, EMT- epithelial-mesenchymal transition, MMP-7- matrix metalloproteinase 7, GSK-3β- glycogen synthase kinase-3 beta, PDGF- platelet-derived growth factor, VEGF- vascular endothelial growth factor, ROS- reactive oxygen species, MAPK- mitogen-activated protein kinase.
5.2. Transforming growth factor β and prostate Cancer
The TGF-β showed divergent character in cancer development. It serves as a tumor suppressor at the early stage of tumor development, whereas at later stages, it promotes tumor development via initiating progression and metastasis [123]. TGF-β is of three types viz. β1, β2, and β3 that function via TGF-β receptor (TGF-βR). The most abundantly expressed TGF-βR is the TGF-βRIII. However, its expression is frequently lost or reduced in PCa cells suggesting its function as a tumor suppressor. It may hint towards a possible mechanism via which cancer cells evade TGF-β-mediated tumor suppression. Previously, It was known that growth differentiation factor-15 (GDF15) facilitates tissue repair after acute injuries via TGF-β signaling. But recently, a cognate receptor glial-derived neurotrophic factor receptor alpha-like (GFRAL) has been discovered for the cytokine GDF15, which plays an intense role in obesity, cancer, and cachexia [124–126]. GDF15 facilitates PCa bone metastasis through osteoblastic CCL2 and RANKL activation [127].
Similarly, the loss of the TGF-βRII expression in the epithelium, whether through gene methylation silencing or transcriptional blockage, has been associated with TGF-β insensitivity, promoting metastatic spread [128]. Liu et al. demonstrated the role of the TGF-β-Smads signaling pathway in the PCa cells progression via elevated expression of interferon-inducible transmembrane protein 3 (IFITM3). The knockdown and overexpression of IFITM3 showed an effect on the activation of the MAPK pathway. This alteration was more prominent after the exogenous stimulation of TGF-β, showing the role of IFITM3 in PCa development and progression of bone metastasis via the TGF-β-Smads-MAPK pathway [129]. The TGF-β1 signaling emerged as a critical regulator in the enhanced proliferation and migration of the PCa cells via CAFs in pre-clinical studies. It has been reported that CAFs significantly enhanced PCa cell proliferation. The inhibition of TGF-β signaling with TGF-β receptor inhibitor (LY2109761) reduced the CAF-mediated PCa cell proliferation and migration [130]. Moreover, TGF-β1 is highly expressed in PCa cells, and the progression of these cells was also suppressed by using LY2109761 [131].
The long non-coding RNAs (LncRNAs) have been associated with the TGF-β signaling and TGF-β mediated cell migration and invasion in various cancers [132–135]. The plasma level of TGF-β1, together with the MIR4435-2HG, was high in PCa patients. The LncRNA MIR4435-2HG also reported stimulating lung cancer. The overexpression of the MIR4435-2HG increased the level of TGF-β1 and led to enhanced cancer cell progression and metastasis, but the inhibitor of the TGF-β diminished this enhancing effect. In this regard, the MIR4435-2HG might enhance the progression of PCa via upregulating TGF-β1 [136]. The TGF-β1 activates the TGF-β signaling after binding with the TGF-βR2, which leads to the stimulation of EMT in PCa. A prominent study by Qi et al. explored the regulatory function of a loop consisting of miR-20b-5p, TGF-βR2, and E2F1 in the modulation of EMT induced by TGF-β1. This study uncovers a unique mechanism that demonstrates the connection of miR-20b-5p/TGF-βR2/E2F1 alliance in TGF-β1 prompted EMT of PCa cells [137]. The Afdal et al. found the difference in the expression pattern of both IL-6 and TGF-β1 between prostate hyperplasia and PCa. The TGF-β1 was spotted in the stromal as well as in the epithelial part. The two most common pathological conditions of the prostate are hyperplasia and cancer, which show the mutual pathogenesis comprising inflammation of prostatic tissues [138]. The high expression of stromal TGF-β1 was reported in prostate hyperplasia compared to PCa (Fig. 3). TGF-β plays a crucial role in bone metastasis, an advanced stage of PCa, with very few therapies. The TGF-β prompts EMT and also contributes to bone metastasis.
Fig. 3.
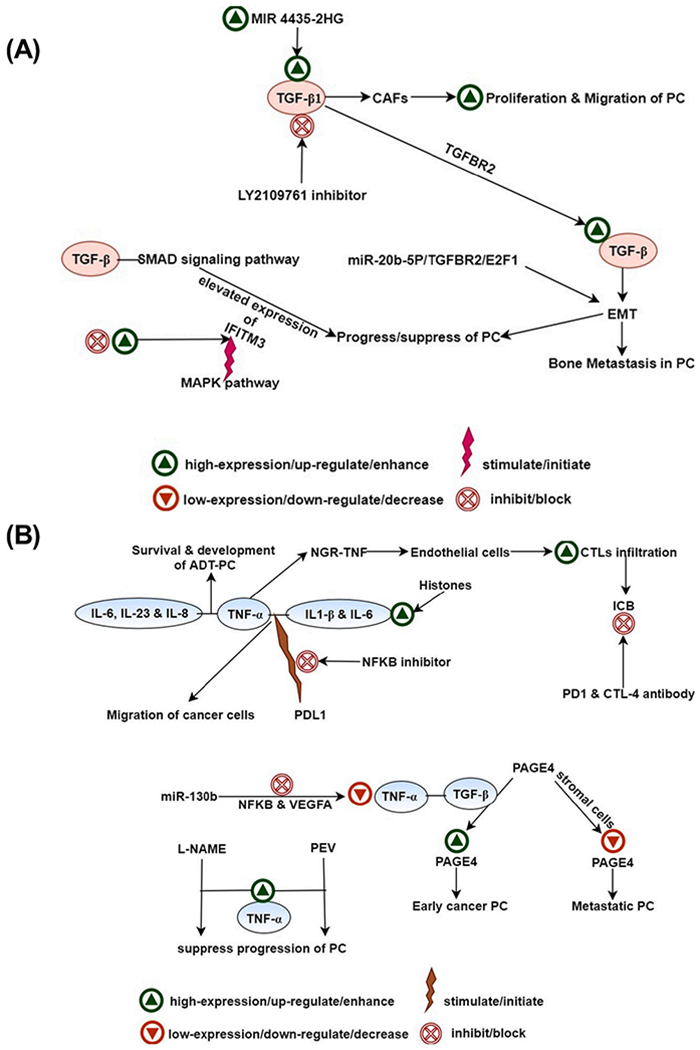
(A) Signaling of TGF-β in PCa. The TGF-β1 activates the TGF-β signaling after binding with the TGF-beta receptor 2 (TGF-βR2), which leads to the stimulation of EMT in PCa. miR-20b-5p, TGF-βR2, and E2F1 are known to promote the TGF-β1 prompted EMT of PCa cells, (B) Signaling of TNF-α with TGF-β and interleukins in PCa. (i) High levels of TNF-α, together with the IL-β, and IL-6 in the presence of extracellular histones, causes enhanced migration rate of PCa cells, (ii) MiR-130b, inhibit the expression of TNF-α via diminishing NF-κB axis and its downstream target gene vascular endothelial growth factor-A (VEGFA), (iii) Nω-nitro-l-arginine methyl ester (L-NAME), increases the expression of TNF-α, which is leveraged by plant enzyme validux (PEV) to suppress the progression of PCa.
Abbreviations- PCa- prostate cancer, ADT-PCa-ADT androgen deprivation therapy receiving prostate cancer, IL- interleukin, TNF-α- tumor necrosis factor α, TGF-β- transforming growth factor β, NGR-TNF- tumor necrosis factor-α (TNF-α) to the tumor endothelium with Cys–Asn–Gly–Arg–Cys–Gly–TNF, NFKB- nuclear factor kappa-light-chain-enhancer of activated B cells, PDL-1-programmed death-ligand 1, CTLs- cytotoxic T lymphocytes, ICB- immune checkpoint blockade, PD-1- Programmed cell death protein 1, CTT-4- cytotoxic T-lymphocyte-associated protein 4, VEGFA- vascular endothelial growth factor-A, PAGE4- prostate-associated gene 4, l-NAME-Nω-nitro-l-arginine methyl ester, PEV- plant enzyme validux, CAFs-cancer-associated fibroblast, TGF-βR2- transforming growth factor-beta receptor 2, EMT-epithelial-mesenchymal transition, IFITM3-interferon-inducible transmembrane protein 3, MAPK- mitogen-activated protein kinase, E2F1-E2F Transcription Factor 1.
5.3. Interferons and prostate cancer
Interferons can be broadly categorized into three classes Type I (IFN-α, β, ε, κ, ω), Type II (IFN-γ), and Type III (IFN-λ) based on differences in their properties, such as a corresponding receptor, sequence, and cell secreting them [139]. IFN-γ secreted by NK cells was found to be dropped to an abnormal level in PCa patients [140]. In contrast, IFN-γ also upholds cancer cell survival and prompts adaptive immune resistance in PCa cells. Increased level of programmed death-ligand 1 (PD-L1) showed the involvement of IFN-γ in cancer progression. In another interesting study, Lo et al. showed that IFN-γ enhances EMT via regulation of miRNA turnover in PCa. An IFN-induced IFIT5, a tetra-tricopeptide repeat (IFIT) family member, develops a complex through the exoribonuclease-XRN1 to process miRNA maturation. Together, these findings revealed a novel IFN-γ–STAT1–IFIT5–miRNA–EMT pathway in PCa development and metastasis [141].
Xia et al. demonstrate the high secretion of IFN-γ in the GM-CSF-modified cell vaccine group of splenocytes (from the experimental animals C57BL/6 mice) against RM-1 cells. This secretion was further decreased after vaccination in males compared to females [142,143]. The IFN-γ induced EMT in PC-3MIE8 cells suggested that the EMT is crucial for the progression and invasion of PCa. The high-throughput sequencing of differentially expressed circular RNAs (circRNAs) and miRNAs in the cells with or without IFN-γ treatment revealed that differentially expressed circRNAs and differentially expressed miRNAs were augmented in the MAPK signaling pathway associated with EMT [143]. IFN-γ may impart crucial roles in cytotoxic T cell functions and anticancer activity. The increased level of IFN-γ was found in the treatment-sensitive metastatic CRPC patients. The level of IFN-γ, together with the TNF-α, IL-5, IL-10, and MIP-1α, was further increased after week eight compared to the baseline [144]. The initiation of MSCs via pro-inflammatory cytokines like TNF-α and IFN-γ increases PDGF and VEGF levels via the NRF2-HIF-1α pathway that stimulates PCa growth in the C57BL/6 syngeneic mouse model [15]. Moreover, in the occurrence of IFN-γ and TNF-α, these PCa-infiltrating MSCs enhance the level of PD-L1 and PD-L2 on their cell surface [145] (Fig. 3).
5.4. Tumor necrosis factor in prostate Cancer
TNF-α is most expansively reported in various cancers; however, this superfamily also consists of other cytokines. The cytokines TNF-α, IL-6, IL-23, and IL-8 (originated from myeloid), and different immunosuppressive cellular compartments showed decreased survival and development of the androgen-independent PCa. In a study by Hawley et al., TNF-α emerged as a prospective negative predictive biomarker for irregular ADT receiving patients. This study proposed the requirement of straight cure growth or a constant course of ADT for patients having a high TNF-α level [146]. The TNF-α levels, together with the IL-1β and IL-6, were found to be increased in the PC3 and C4-2B cells after stimulation with the extracellular histones. It is also a possible cause of the enhanced migration rate of PCa cells [147]. The TH17 cells co-expressed TNF-α and IL-17 in many tumors, but both cytokines act independently in PCa cells. TNF-α induced PD-L1 mRNA and protein expression in LNCaP cells but could not prompt PD-L2 mRNA or protein expression in the same. In addition, the PD-L1 protein expression induced by TNF-α could be diminished by the NF-κB inhibitor. The TNF-α activates NF-κB signaling in LNCaP cells [148]. Fu et al. suggested the protective role of prostate-associated gene 4 (PAGE4) in terms of decreased signaling via TNF-α and TGF-β pathways in the stromal cells. The up-regulation of PAGE 4 was found in early-stage PCa and its precursor lesions. On the contrary, its downregulation was reported in highly metastatic PCa. This study suggests the role of TNF-α signaling in the migration and invasion of the PCa epithelial cells [149]. The targeted delivery of a small fusion protein comprising TNF-α to the tumor endothelium with Cys–Asn–Gly–Arg–Cys–Gly–TNF (NGR-TNF) can activate endothelial cells and enhance the tumor infiltration by CTLs [150–152]. In addition to this, NGR-TNF could sensitize the tumors to immune checkpoint blockade (ICB) together with the PD-1 and CTLA-4 receptors specific antibodies. In this way, tumor vasculature targeting with the low-dose TNF associated with adoptive T-cell therapy can serve as an innovative approach to disable resistance to immune checkpoint blockers and improve the permeation of T-cells in tumors [152].
The microRNAs (miRNAs) play a crucial role in numerous biological and physiological processes in different cancers. Angiogenesis is one of the biological processes necessary for the development and metastasis of solid cancer. The downregulation of the miR-130b was found in PCa cell lines, and the enforced expression of miR-130b obstructed the in vitro and in vivo angiogenesis of PCa. Mu et al. found that TNF-α was regulated by miR-130b, and the TNF-α expression was inhibited by MiR-130b via diminishing NF-κB axis and its downstream target gene VEGFA. This study demonstrated that miR-130b might serve as a potential therapeutic target for PCa treatment, and miR-130b/TNF-α/NF-γB/VEGFA feedback loop is considerably associated with PCa angiogenesis [153].
The level of TNF-α was also increased in various anticancer studies against the PCa. The high expression level of TNF-α was found in the DU145 PCa cells after treatment with the Nω-nitro-l-arginine methyl ester (L-NAME), which is a nitric oxide-based treatment [154]. Gu et al. explored the anti-PCa effect of plant enzyme validux (PEV), which suppressed the progression of PCa via increasing the level of TNF-α [155] (Fig. 3).
6. Chemokines and cytokines targeted for PCa in clinical trials
In cancer therapy, the role of cytokines and chemokines has been long studied by researchers over the years. Cytokines have been known to stimulate the TME and recruit immune cells such as CD4+T cells, which then can target the tumor cells; thus, the cytokines may not influence the target tumors directly but via the enhancement of various cytotoxic therapies [156]. Recent research on cytokines has shown positive results in some clinical trials, mainly in combination with other therapies. Moreover, cytokines such as GM-CSF enhanced the activation of antigen-presenting cells such as dendritic cells (DCs), macrophages, and NKT cells, thereby improving tumor antigen presentation [157]. A clinical study under the identifier- NCT02250014, which started in 2014 and is still ongoing, has shown increased effects of sargramostim (the synthetic form of GM-CSF) after cryotherapy (NCT02250014). Another ongoing study for treating solid tumors (including PCa) under the identifier- NCT03970382 is under investigation in phase-I, where IL-2 is being used in combination with nivolumab NeoTCR-P1 adoptive cell therapy (NCT03970382). Concerning chemokine, studies related to primarily the blockers of chemokine receptors have been employed in trials that were not found to be of much relevance as not many chemokine-related studies were undertaken. Studies were undertaken regarding the chemokine CCL2, which was targeted using anti-chemokine drugs such as carlumab targeting CCL2. A clinical trial was also set up, which was later discontinued due to a lack of visible results in the trials (NCT00992186). Recently, a study on the chemokine IFN-α-2b is also being monitored and is currently under phase-II for its effect on PCa before surgery in combination with other drugs, namely Aspirin and rintatolimod, in patients with localized PCa undergoing radical prostatectomy (NCT03899987). Moreover, Morales and Emerson reported an intralesional recombinant IFN-α-based study for localized PCa, where they directly administered the IFN-α into the prostate. The results of this phase I trial indicated a beneficial effect of recombinant IFN-α [158]. A similar study was undertaken using lower doses of IFN-α-2b combined with docetaxel in patients with CRPC [159]. Evidence shows there have not been many studies in the recent past that directly deal with the use of cytokines or chemokines either alone or even in combination with other drugs or therapies. Although the study of cytokines and chemokines has shown results in vitro, in vivo, and pre-clinical studies, the overall effort has not led to much translation at the clinical level.
7. Concluding remarks and future prospective
PCa is a solid tumor triggered by chronic inflammation, inappropriate hormones stimulation, altered expression of distinct chemokines/cytokines, or imbalance in the homeostatic mechanisms coupled with chemokines and other regulatory signaling axis. Remarkably, both chemokines and cytokines play critical roles in modulating the immune response, promoting the trafficking of immune cells in TME, and significantly contributing to tumor heterogeneity. These immunomodulators have a pleiotropic effect and perform various functions depending on environmental cues.
The functionality of these chemokines relies on the binding of their receptors that influence cellular trafficking and regulate several vital aspects of the cellular process. In the primary PCa and metastatic locations, the chemokines/receptors axis regulates multistep processes requiring activation of tumor-promoting signals. These signals provide survival, proliferation, angiogenesis, EMT, invasion characteristics, and acquisition of resistance to chemo-hormonal therapy of PCa cells. In addition, chemokines modulate the host–tumor connections and enable cancer cells to evade the immune system for promoting PCa development and progression. Hence, it is critical to understand the responsive factors that trigger the dysregulated function of chemokine receptors in PCa. Notably, cell types and physiological states determine the redundancy between chemokines/receptors complex functionality as pro-inflammatory/anti-inflammatory effects in certain circumstances. Therefore, therapeutic strategies targeted at one chemokine are primarily ineffective in clinical trials.
In the future, research can be directed toward a better understanding of the regulation of different cascades that govern the altered expression of these chemokines/cytokines in PCa cells. The development of chemokines/cytokines-based drugs is still a challenge. The mechanism by which these chemokines/cytokines manage the invasion and metastasis of specific cancer can uncover the whole layout of their role in the disease progression. In addition, it will also be helpful to design a possible therapeutic approach that can have the potential to fight in a well-organized and targeted manner against the different types of cancer. Moreover, potential inhibitors/monoclonal antibodies can be synthesized and optimized against these targets to overcome the treatment resistance to better recover PCa patients. In conclusion, modulation of the chemokine and cytokine secretion and related signaling may potentially approach to PCa therapies.
Acknowledgments
We thank our colleagues for their valuable suggestions and critical reading of this review. Also, we thank Ms. Faizia Bano for her help with the illustrations.
Funding source
This work and the authors are, in part, supported by grants from the U.S. Department of Defense (DOD) through the Prostate Cancer Research Program under Award No. W81XWH-21-1-0640 (JAS), DOD W81XWH-21-1-0340 (JBK), and National Institutes of Health (NIH) U01 CA185148, DOD W81XWH-18-1-0308 (SKB).
Interpretations, opinions, conclusions, and recommendations presented in this manuscript are those of the author and are not necessarily endorsed by the Department of Defense and other funding agencies.
Abbreviations:
- PCa
prostate cancer
- TME
tumor microenvironment
- EMT
epithelial-mesenchymal transition
- AR
androgen receptor
- MSCs
mesenchymal stromal cells
- NE
neuroendocrine
- CAFs
cancer-associated fibroblasts
- TAMs
tumor-associated macrophages
- ECM
extracellular matrix
- CSCs
cancer stem cells
- LncRNAs
long non-coding RNAs
- TGF-β
transforming growth factor-beta
- ILs
interleukins
- TNF-α
tumor necrosis factor-alpha
Footnotes
Declaration of Competing Interest
SKB is co-founder of Sanguine Diagnostics and Therapeutics, Inc. Other authors declare no competing interests.
Data availability
Not applicable, all information in this review can be found in the reference list.
References
- [1].Rawla P, Epidemiology of prostate cancer, World J. Oncol 10 (2) (2019) 63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Taitt HE, Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location, Am. J. Men’s Health 12 (6) (2018) 1807–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A, Recent global patterns in prostate cancer incidence and mortality rates, Eur. Urol 77 (1) (2020) 38–52. [DOI] [PubMed] [Google Scholar]
- [4].Ugge H, Downer MK, Carlsson J, Bowden M, Davidsson S, Mucci LA, Fall K, Andersson SO, Andren O, Circulating inflammation markers and prostate cancer, Prostate 79 (11) (2019) 1338–1346. [DOI] [PubMed] [Google Scholar]
- [5].Balistreri CR, Candore G, Lio D, Carruba G, Prostate cancer: from the pathophysiologic implications of some genetic risk factors to translation in personalized cancer treatments, Cancer Gene Ther. 21 (1) (2014) 2–11. [DOI] [PubMed] [Google Scholar]
- [6].Scaglia N, Frontini-Lopez YR, Zadra G, Prostate cancer progression: as a matter of fats, Front. Oncol 11 (2021), 719865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rani A, Dasgupta P, Murphy JJ, Prostate cancer: the role of inflammation and chemokines, Am. J. Pathol 189 (11) (2019) 2119–2137. [DOI] [PubMed] [Google Scholar]
- [8].Thobe MN, Gurusamy D, Pathrose P, Waltz SE, The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells, Oncogene 29 (2) (2010) 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].King J, Mir H, Singh S, Association of cytokines and chemokines in pathogenesis of breast cancer, Prog. Mol. Biol. Transl. Sci 151 (2017) 113–136. [DOI] [PubMed] [Google Scholar]
- [10].Hughes CE, Nibbs RJB, A guide to chemokines and their receptors, FEBS J. 285 (16) (2018) 2944–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Siddiqui JA, Partridge NC, CCL2/monocyte chemoattractant protein 1 and parathyroid hormone action on bone, Front. Endocrinol. (Lausanne) 8 (2017) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Salazar N, Castellan M, Shirodkar SS, Lokeshwar BL, Chemokines and chemokine receptors as promoters of prostate cancer growth and progression, Crit. Rev. Eukaryot. Gene Expr 23 (1) (2013) 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vindrieux D, Escobar P, Lazennec G, Emerging roles of chemokines in prostate cancer, Endocr. Relat. Cancer 16 (3) (2009) 663–673. [DOI] [PubMed] [Google Scholar]
- [14].Adekoya TO, Richardson RM, Cytokines and chemokines as mediators of prostate cancer metastasis, Int. J. Mol. Sci 21 (12) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang KQ, Liu Y, Huang QH, Mo N, Zhang QY, Meng QG, Cheng JW, Bone marrow-derived mesenchymal stem cells induced by inflammatory cytokines produce angiogenetic factors and promote prostate cancer growth, BMC Cancer 17 (1) (2017) 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh R, Lillard JW Jr., Singh S Chemokines: key players in cancer progression and metastasis, in: Frontiers in Bioscience (Scholar Edition), vol. 3, 2011, pp. 1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sharma G, Pothuraju R, Kanchan RK, Batra SK, Siddiqui JA, Chemokines network in bone metastasis: vital regulators of seeding and soiling. Seminars in Cancer Biology, Elsevier, 2022, 10.1016/j.semcancer.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maolake A, Izumi K, Shigehara K, Natsagdorj A, Iwamoto H, Kadomoto S, Takezawa Y, Machioka K, Narimoto K, Namiki M, Lin WJ, Wufuer G, Mizokami A, Tumor-associated macrophages promote prostate cancer migration through activation of the CCL22-CCR4 axis, Oncotarget 8 (6) (2017) 9739–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Siddiqui JA, Johnson J, Le Henaff C, Bitel CL, Tamasi JA, Partridge NC, Catabolic effects of human PTH (1-34) on bone: requirement of monocyte chemoattractant protein-1 in murine model of hyperparathyroidism, Sci. Rep 7 (1) (2017) 15300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Siddiqui JA, Le Henaff C, Johnson J, He Z, Rifkin DB, Partridge NC, Osteoblastic monocyte chemoattractant protein-1 (MCP-1) mediation of parathyroid hormone’s anabolic actions in bone implicates TGF-beta signaling, Bone 143 (2021), 115762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sokol CL, Luster AD, The chemokine system in innate immunity, Cold Spring Harb. Perspect. Biol 7 (5) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cioni B, Nevedomskaya E, Melis MHM, van Burgsteden J, Stelloo S, Hodel E, Spinozzi D, de Jong J, van der Poel H, de Boer JP, Wessels LFA, Zwart W, Bergman AM, Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration, Mol. Oncol 12 (8) (2018) 1308–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I, Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer, Clin. Exp. Metastasis 32 (4) (2015) 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, He Y, Butler W, Xu L, Chang Y, Lei K, Zhang H, Zhou Y, Gao AC, Zhang Q, Taylor DG, Cheng D, Farber-Katz S, Karam R, Landrith T, Li B, Wu S, Hsuan V, Yang Q, Hu H, Chen X, Flowers M, McCall SJ, Lee JK, Smith BA, Park JW, Goldstein AS, Witte ON, Wang Q, Rettig MB, Armstrong AJ, Cheng Q, Huang J, Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer, Sci. Transl. Med 11 (521) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patnaik A, Swanson KD, Csizmadia E, Solanki A, Landon-Brace N, Gehring MP, Helenius K, Olson BM, Pyzer AR, Wang LC, Elemento O, Novak J, Thornley TB, Asara JM, Montaser L, Timmons JJ, Morgan TM, Wang Y, Levantini E, Clohessy JG, Kelly K, Pandolfi PP, Rosenblatt JM, Avigan DE, Ye H, Karp JM, Signoretti S, Balk SP, Cantley LC, Cabozantinib eradicates advanced murine prostate cancer by activating antitumor innate immunity, Cancer Discov. 7 (7) (2017) 750–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jang YG, Go RE, Hwang KA, Choi KC, Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway, J. Steroid Biochem. Mol. Biol 192 (2019), 105406. [DOI] [PubMed] [Google Scholar]
- [27].Baci D, Bruno A, Cascini C, Gallazzi M, Mortara L, Sessa F, Pelosi G, Albini A, Noonan DM, Acetyl-L-Carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells: rationale for prevention and interception strategies, J. Exp. Clin. Cancer Res 38 (1) (2019) 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yu L, Pham Q, Yu LL, Wang TTY, Modulation of CXC-motif chemokine receptor 7, but not 4, expression is related to migration of the human prostate cancer cell LNCaP: regulation by androgen and inflammatory stimuli, Inflamm. Res 69 (2) (2020) 167–178. [DOI] [PubMed] [Google Scholar]
- [29].Bai Y, Yang Y, Yan Y, Zhong J, Blee AM, Pan Y, Ma T, Karnes RJ, Jimenez R, Xu W, Huang H, RUNX2 overexpression and PTEN haploinsufficiency cooperate to promote CXCR7 expression and cellular trafficking, AKT hyperactivation and prostate tumorigenesis, Theranostics 9 (12) (2019) 3459–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li S, Fong KW, Gritsina G, Zhang A, Zhao JC, Kim J, Sharp A, Yuan W, Aversa C, Yang XJ, Nelson PS, Feng FY, Chinnaiyan AM, de Bono JS, Morrissey C, Rettig MB, Yu J, Activation of MAPK signaling by CXCR7 leads to enzalutamide resistance in prostate cancer, Cancer Res. 79 (10) (2019) 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miyake M, Furuya H, Onishi S, Hokutan K, Anai S, Chan O, Shi S, Fujimoto K, Goodison S, Cai W, Rosser CJ, Monoclonal antibody against CXCL1 (HL2401) as a novel agent in suppressing IL6 expression and tumoral growth, Theranostics 9 (3) (2019) 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Multhoff G, Molls M, Radons J, Chronic inflammation in cancer development, Front. Immunol 2 (2011) 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG, Inflammation in prostate carcinogenesis, Nat. Rev. Cancer 7 (4) (2007) 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Han IH, Kim JH, Jang KS, Ryu JS, Inflammatory mediators of prostate epithelial cells stimulated with Trichomonas vaginalis promote proliferative and invasive properties of prostate cancer cells, Prostate 79 (10) (2019) 1133–1146. [DOI] [PubMed] [Google Scholar]
- [35].Rasheed SAK, Leong HS, Lakshmanan M, Raju A, Dadlani D, Chong FT, Shannon NB, Rajarethinam R, Skanthakumar T, Tan EY, Hwang JSG, Lim KH, Tan DS, Ceppi P, Wang M, Tergaonkar V, Casey PJ, Iyer NG, GNA13 expression promotes drug resistance and tumor-initiating phenotypes in squamous cell cancers, Oncogene 37 (10) (2018) 1340–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rasheed SAK, Subramanyan LV, Lim WK, Udayappan UK, Wang M, Casey PJ, The emerging roles of Gα12/13 proteins on the hallmarks of cancer in solid tumors, Oncogene 41 (2) (2022) 147–158, 10.1038/s41388-021-02069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lim WK, Chai X, Ghosh S, Ray D, Wang M, Rasheed SAK, Casey PJ, Gα-13 induces CXC motif chemokine ligand 5 expression in prostate cancer cells by transactivating NF-κB, J. Biol. Chem 294 (48) (2019) 18192–18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Di Mitri D, Mirenda M, Vasilevska J, Calcinotto A, Delaleu N, Revandkar A, Gil V, Boysen G, Losa M, Mosole S, Pasquini E, D’Antuono R, Masetti M, Zagato E, Chiorino G, Ostano P, Rinaldi A, Gnetti L, Graupera M, Martins Figueiredo Fonseca AR, Pereira Mestre R, Waugh D, Barry S, De Bono J, Alimonti A, Re-education of tumor-associated macrophages by CXCR2 blockade drives senescence and tumor inhibition in advanced prostate cancer, Cell Rep. 28 (8) (2019), 2156–2168 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Watanabe M, Kanao K, Suzuki S, Muramatsu H, Morinaga S, Kajikawa K, Kobayashi I, Nishikawa G, Kato Y, Zennami K, Nakamura K, Tsuzuki T, Yoshikawa K, Ueda R, Sumitomo M, Increased infiltration of CCR4-positive regulatory T cells in prostate cancer tissue is associated with a poor prognosis, Prostate 79 (14) (2019) 1658–1665. [DOI] [PubMed] [Google Scholar]
- [40].Xiang P, Jin S, Yang Y, Sheng J, He Q, Song Y, Yu W, Hu S, Jin J, Infiltrating CD4+ T cells attenuate chemotherapy sensitivity in prostate cancer via CCL5 signaling, Prostate 79 (9) (2019) 1018–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hamshaw I, Ajdarirad M, Mueller A, The role of PKC and PKD in CXCL12 directed prostate cancer migration, Biochem. Biophys. Res. Commun 519 (1) (2019) 86–92. [DOI] [PubMed] [Google Scholar]
- [42].D’Agostino G, Cecchinato V, Uguccioni M, Chemokine heterocomplexes and cancer: a novel chapter to be written in tumor immunity, Front. Immunol 9 (2018) 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cecchinato V, D’Agostino G, Raeli L, Uguccioni M, Chemokine interaction with synergy-indudng molecules: fine tuning modulation of cell trafficking, J. Leukoc. Biol 99 (6) (2016) 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haffner MC, Zwart W, Roudier MP, True LD, Nelson WG, Epstein JI, De Marzo AM, Nelson PS, Yegnasubramanian S, Genomic and phenotypic heterogeneity in prostate cancer, Nat. Rev. Urol 18 (2) (2021) 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tolkach Y, Kristiansen G, The heterogeneity of prostate cancer: a practical approach, Pathobiology 85 (1–2) (2018) 108–116. [DOI] [PubMed] [Google Scholar]
- [46].Brady L, Kriner M, Coleman I, Morrissey C, Roudier M, True LD, Gulati R, Plymate SR, Zhou Z, Birditt B, Meredith R, Geiss G, Hoang M, Beechem J, Nelson PS, Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling, Nat. Commun 12 (1) (2021) 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yadav SS, Stockert JA, Hackert V, Yadav KK, Tewari AK, Intratumor heterogeneity in prostate cancer, Urol. Oncol 36 (8) (2018) 349–360. [DOI] [PubMed] [Google Scholar]
- [48].Yang L, Lin PC, Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression, Semin. Cancer Biol 47 (2017) 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Turashvili G, Brogi E, Tumor heterogeneity in breast cancer, Front. Med. (Lausanne) 4 (2017) 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stanta G, Bonin S, Overview on clinical relevance of intra-tumor heterogeneity, Front. Med. (Lausanne) 5 (2018) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yun JW, Lee S, Ryu D, Park S, Park WY, Joung JG, Jeong J, Biomarkers associated with tumor heterogeneity in prostate cancer, Transl. Oncol 12 (1) (2019) 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hass R, von der Ohe J, Ungefroren H, Impact of the tumor microenvironment on tumor heterogeneity and consequences for cancer cell plasticity and stemness, Cancers (Basel) 12 (12) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Maurya SK, Khan P, Rehman AU, Kanchan RK, Perumal N, Mahapatra S, Chand HS, Santamaria-Barria JA, Batra SK, Nasser MW, Rethinking the chemokine cascade in brain metastasis: preventive and therapeutic implications, Semin. Cancer Biol (2021), 10.1016/j.semcancer.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lin Y, Xu J, Lan H, Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications, J. Hematol. Oncol 12 (1) (2019) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mughees M, Sengupta A, Khowal S, Wajid S, Mechanism of tumour microenvironment in the progression and development of oral cancer, Mol. Biol. Rep 48 (2) (2021) 1773–1786. [DOI] [PubMed] [Google Scholar]
- [56].Ortiz-Otero N, Clinch AB, Hope J, Wang W, Reinhart-King CA, King MR, Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression, Oncotarget 11 (12) (2020) 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shen T, Li Y, Zhu S, Yu J, Zhang B, Chen X, Zhang Z, Ma Y, Niu Y, Shang Z, YAP1 plays a key role of the conversion of normal fibroblasts into cancer-associated fibroblasts that contribute to prostate cancer progression, J. Exp. din. Cancer Res 39 (1) (2020) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eiro N, Fernandez-Gomez J, Sacristán R, Fernandez-Garcia B, Lobo B, Gonzalez-Suarez J, Quintas A, Escaf S, Vizoso FJ, Stromal factors involved in human prostate cancer development, progression and castration resistance, J. Cancer Res. Clin. Oncol 143 (2) (2017) 351–359. [DOI] [PubMed] [Google Scholar]
- [59].Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S, Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives, Mol. Cancer 20 (1) (2021) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vickman RE, Franco OE, Hayward SW, Contributions of carcinoma-associated fibroblasts to the prostate cancer microenvironment, Curr. Opin. Endocr. Metab. Res 10 (2020) 1–6. [Google Scholar]
- [61].Hao Q, Vadgama JV, Wang P, CCL2/CCR2 signaling in cancer pathogenesis, Cell Commun. Signal 18 (1) (2020) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lang J, Zhao X, Qi Y, Zhang Y, Han X, Ding Y, Guan J, Ji T, Zhao Y, Nie G, Reshaping prostate tumor microenvironment to suppress metastasis via cancer-associated fibroblast inactivation with peptide-assembly-based nanosystem, ACS Nano 13 (11) (2019) 12357–12371. [DOI] [PubMed] [Google Scholar]
- [63].Parol-Kulczyk M, Gzil A, Ligmanowska J, Grzanka D, Prognostic significance of SDF-1 chemokine and its receptors CXCR4 and CXCR7 involved in EMT of prostate cancer, Cytokine 150 (2022), 155778. [DOI] [PubMed] [Google Scholar]
- [64].Caligiuri A, Pastore M, Lori G, Raggi C, Di Maira G, Marra F, Gentilini A, Role of chemokines in the biology of cholangiocarcinoma, Cancers (Basel) 12 (8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cheteh EH, Sarne V, Ceder S, Bianchi J, Augsten M, Rundqvist H, Egevad L, Östman A, Wiman KG, Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells, Cell Death Discov. 6 (2020) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang R, Zong J, Peng Y, Shi J, Du X, Liu H, Shen Y, Cao J, Jia B, Liu F, Zhang J, GPR30 knockdown weakens the capacity of CAF in promoting prostate cancer cell invasion via reducing macrophage infiltration and M2 polarization, J. Cell. Biochem (2021), 10.1002/jcb.29938. [DOI] [PubMed] [Google Scholar]
- [67].Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, Choinzonov E, Kzhyshkowska J, Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers, Front. Oncol 10 (2020), 566511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yuri P, Shigemura K, Kitagawa K, Hadibrata E, Risan M, Zulfiqqar A, Soerohaijo I, Hendri AZ, Danarto R, Ishii A, Yamasaki S, Yan Y, Heriyanto DS, Fujisawa M, Increased tumor-associated macrophages in the prostate cancer microenvironment predicted patients’ survival and responses to androgen deprivation therapies in Indonesian patients cohort, Prostate Int. 8 (2) (2020) 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Erlandsson A, Carlsson J, Lundholm M, Falt A, Andersson SO, Andren O, Davidsson S, M2 macrophages and regulatory T cells in lethal prostate cancer, Prostate 79 (4) (2019) 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Werneck-Gomes H, Campolina-Silva GH, Maria BT, Barata MC, Mahecha GAB, Hess RA, Oliveira CA, Tumor-Associated Macrophages (TAM) are recruited to the aging prostate epithelial lesions and become intermingled with basal cells, Andrology 8 (5) (2020) 1375–1386. [DOI] [PubMed] [Google Scholar]
- [71].Li C, Jiang P, Wei S, Xu X, Wang J, Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects, Mol. Cancer 19 (1) (2020) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Powell IJ, Chinni SR, Reddy SS, Zaslavsky A, Gavande N, Pro-inflammatory cytokines and chemokines initiate multiple prostate cancer biologic pathways of cellular proliferation, heterogeneity and metastasis in a racially diverse population and underlie the genetic/biologic mechanism of racial disparity: update, Urol. Oncol 39 (1) (2021) 34–40. [DOI] [PubMed] [Google Scholar]
- [73].Raskov H, Orhan A, Gaggar S, Gögenur I, Cancer-associated fibroblasts and tumor-associated macrophages in cancer and cancer immunotherapy, Front. Oncol 11 (2021), 668731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, Wang X, Zhang J, Guo L, Wang S, Chen Z, Wang Z, Xiang S, CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating beta-catenin/STAT3 signaling, Cell Death Dis. 11 (4) (2020) 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].O’Connor T, Heikenwalder M, CCL2 in the tumor microenvironment, Adv. Exp. Med. Biol 1302 (2021) 1–14. [DOI] [PubMed] [Google Scholar]
- [76].Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A, Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival, Int. J. Oncol 17 (3) (2000) 445–451. [DOI] [PubMed] [Google Scholar]
- [77].Saxena S, Singh RK, Chemokines orchestrate tumor cells and the microenvironment to achieve metastatic heterogeneity, Cancer Metastasis Rev. 40 (2) (2021) 447–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Morein D, Erlichman N, Ben-Baruch A, Beyond cell motility: the expanding roles of chemokines and their receptors in malignancy, Front. Immunol 11 (2020) 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jolly MK, Celia-Terrassa T, Dynamics of phenotypic heterogeneity associated with EMT and sternness during cancer progression, J. Clin. Med 8 (10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zheng X, Dai F, Feng L, Zou H, Feng L, Xu M, Communication between epithelial-mesenchymal plasticity and cancer stem cells: new insights into cancer progression, Front. Oncol 11 (2021), 617597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bocci F, Gearhart-Serna L, Boareto M, Ribeiro M, Ben-Jacob E, Devi GR, Levine H, Onuchic JN, Jolly MK, Toward understanding cancer stem cell heterogeneity in the tumor microenvironment, Proc. Natl. Acad. Sci. U. S. A 116 (1) (2019) 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bocci F, Jolly MK, George JT, Levine H, Onuchic JN, A mechanism-based computational model to capture the interconnections among epithelial-mesenchymal transition, cancer stem cells and Notch-Jagged signaling, Oncotarget 9 (52) (2018) 29906–29920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pietilä M, Ivaska J, Mani SA, Whom to blame for metastasis, the epithelial-mesenchymal transition or the tumor microenvironment? Cancer Lett. 380 (1) (2016) 359–368. [DOI] [PubMed] [Google Scholar]
- [84].Sorrentino C, Ciummo SL, Cipollone G, Caputo S, Bellone M, Di Carlo E, Interleukin-30/IL27p28 shapes prostate cancer stem-like cell behavior and is critical for tumor onset and metastasization, Cancer Res. 78 (10) (2018) 2654–2668. [DOI] [PubMed] [Google Scholar]
- [85].Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, Yan F, Wang Y, Bouchez LC, Kularatne SA, Watson J, Trussell C, Reddy VA, Cho CY, Schultz PG, CXCR4 expression in prostate cancer progenitor cells, PLoS One 7 (2) (2012), e31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Muz B, de la Puente P, Azab F, Azab AK, The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy, Hypoxia (Auckl) 3 (2015) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y, Role of hypoxia in cancer therapy by regulating the tumor microenvironment, Mol. Cancer 18 (1) (2019) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Singh SK, Mishra MK, Singh R, Hypoxia-indudble fador-1α induces CX3CR1 expression and promotes the epithelial to mesenchymal transition (EMT) in ovarian cancer cells, J. Ovarian Res 12 (1) (2019) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Xiao LJ, Chen YY, Lin P, Zou HF, Lin F, Zhao LN, Li D, Guo L, Tang JB, Zheng XL, Yu XG, Hypoxia increases CX3CR1 expression via HIF-1 and NF-κB in androgen-independent prostate cancer cells, Int. J. Oncol 41 (5) (2012) 1827–1836. [DOI] [PubMed] [Google Scholar]
- [90].Jena BC, Das CK, Bharadwaj D, Mandal M, Cancer as so dated fibroblast mediated chemoresistance: a paradigm shift in understanding the mechanism of tumor progression, Biochim. Biophys. Acta Rev. Cancer 1874 (2) (2020), 188416. [DOI] [PubMed] [Google Scholar]
- [91].Ireland LV, Mielgo A, Macrophages and fibroblasts, key players in cancer chemoresistance, Front. Cell Dev. Biol 6 (2018) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Neophytou CM, Panagi M, Stylianopoulos T, Papageorgis P, The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities, Cancers (Basel) 13 (9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen Y, Shao Z, Jiang E, Zhou X, Wang L, Wang H, Luo X, Chen Q, Liu K, Shang Z, CCL21/CCR7 interaction promotes EMT and enhances the sternness of OSCC via a JAK2/STAT3 signaling pathway, J. Cell. Physiol 235 (9) (2020) 5995–6009. [DOI] [PubMed] [Google Scholar]
- [94].Mizejewski GJ, Breast cancer, chemokines, and metastases: a search for decoy ligands of the CXCR4 receptor, J. Neoplasms 1 (2018) 1–9. [Google Scholar]
- [95].Huang R, Guo L, Gao M, Li J, Xiang S, Research trends and regulation of CCL5 in prostate cancer, Onco. Ther 14 (2021) 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shi Y, Riese DJ 2nd, Shen J, The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer, Front. Pharmacol 11 (2020), 574667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Reyes ME, de La Fuente M, Hermoso M, Ili CG, Brebi P, Role of CC chemokines subfamily in the platinum drugs resistance promotion in cancer, Front. Immunol 11 (2020) 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Izumi K, Mizokami A, Suppressive role of androgen/androgen receptor signaling via chemokines on prostate cancer cells, J. Clin. Med 8 (3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu C, Li X, Wu D, Xia S, Chen J, Qian WP, Yang L, Osunkoya AO, Boise L, Vertino PM, Zhao Y, Li M, Chen HR, Kowalski J, Kucuk O, Zhou W, Dong JT, Acetylation of KLF5 maintains EMT and turnorigenicity to cause chemoresistant bone metastasis in prostate cancer, Nat. Commun 12 (1) (2021) 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kfoury Y, Baryawno N, Severe N, Mei S, Gustafsson K, Hirz T, Brouse T, Scadden EW, Igolkina AA, Kokkaliaris K, Choi BD, Barkas N, Randolph MA, Shin JH, Saylor PJ, Scadden DT, Sykes DB, Kharchenko PV, C. part of the Boston Bone Metastases, Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment, Cancer Cell 39 (11) (2021), 1464–1478 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bhagirath D, Saini S, Coping with chemoresistance in prostate cancer-co-targeting of adipose stromal cells? Transl. Androl. Urol 8 (Suppl. 3) (2019) S250–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Laurent V, Guérard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, Zaidi F, Majed B, Garandeau D, Socrier Y, Golzio M, Cadoudal T, Chaoui K, Dray C, Monsarrat B, Schiltz O, Wang YY, Couderc B, Valet P, Malavaud B, Muller C, Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity, Nat. Commun 7 (2016) 10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Su F, Ahn S, Saha A, DiGiovanni J, Kolonin MG, Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance, Oncogene 38 (11) (2019) 1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mollica Poeta V, Massara M, Capucetti A, Bonecchi R, Chemokines and chemokine receptors: new targets for cancer immunotherapy, Front. Immunol 10 (2019) 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, Rodriguez-Ruiz ME, Ponz-Sarvise M, Castanon E, Melero I, Cytokines in clinical cancer immunotherapy, Br. J. Cancer 120 (1) (2019) 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Conlon KC, Miljkovic MD, Waldmann TA, Cytokines in the treatment of cancer, J. Interferon Cytokine Res 39 (1) (2019) 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zheng J, Gao P, Toward normalization of the tumor microenvironment for cancer therapy, Integr. Cancer Ther 18 (2019), 1534735419862352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mantovani A, Barajon I, Garlanda C, IL-1 and IL-1 regulatory pathways in cancer progression and therapy, Immunol. Rev 281 (1) (2018) 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kumari N, Dwarakanath BS, Das A, Bhatt AN, Role of interleukin-6 in cancer progression and therapeutic resistance, Tumour Biol. 37 (9) (2016) 11553–11572. [DOI] [PubMed] [Google Scholar]
- [110].Chen X, Chen F, Ren Y, Weng G, Xu L, Xue X, Keng PC, Lee SO, Chen Y, IL-6 signaling contributes to radioresistance of prostate cancer through key DNA repair-associated molecules ATM, ATR, and BRCA 1/2, J. Cancer Res. Clin. Oncol. 145 (6) (2019) 1471–1484. [DOI] [PubMed] [Google Scholar]
- [111].Nguyen DP, Li J, Tewari AK, Inflammation and prostate cancer: the role of interleukin 6 (IL-6), BJU Int. 113 (6) (2014) 986–992. [DOI] [PubMed] [Google Scholar]
- [112].Fousek K, Horn LA, Palena C, Interleukin-8: a chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression, Pharmacol. Ther. 219 (2021), 107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chen CH, Ho CH, Hu SW, Tzou KY, Wang YH, Wu CC, Association between interleukin-8 rs4073 polymorphism and prostate cancer: a meta-analysis, J. Formos. Med. Assoc. 119 (7) (2020) 1201–1210. [DOI] [PubMed] [Google Scholar]
- [114].Sun Y, Ai JZ, Jin X, Liu LR, Lin TH, Xu H, Wei Q, Yang L, IL-8 protects prostate cancer cells from GSK-3beta-induced oxidative stress by activating the mTOR signaling pathway, Prostate 79 (10) (2019) 1180–1190. [DOI] [PubMed] [Google Scholar]
- [115].Ashok A, Keener R, Rubenstein M, Stookey S, Bajpai S, Hicks J, Alme AK, Drake CG, Zheng Q, Trabzonlu L, Yegnasubramanian S, De Marzo AM, Bieberich CJ, Consequences of interleukin 1beta-triggered chronic inflammation in the mouse prostate gland: altered architecture associated with prolonged CD4(+) infiltration mimics human proliferative inflammatory atrophy, Prostate 79 (7) (2019) 732–745. [DOI] [PubMed] [Google Scholar]
- [116].Sakellariou C, Elhage O, Papaevangelou E, Giustarini G, Esteves AM, Smolarek D, Smith RA, Dasgupta P, Galustian C, Prostate cancer cells enhance interleukin-15-mediated expansion of NK cells, BJU Int. 125 (1) (2020) 89–102. [DOI] [PubMed] [Google Scholar]
- [117].Yuanyuan G, Xue Y, Yachao L, Xiao F, Xu C, Association between IL-18-607 C/A polymorphism and the risk of prostate cancer: a meta-analysis of case-control studies, Asian Pac. J. Cancer Prev 20 (6) (2019) 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang Q, Liu S, Parajuli KR, Zhang W, Zhang K, Mo Z, Liu J, Chen Z, Yang S, Wang AR, Myers L, You Z, Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition, Oncogene 36 (5) (2017) 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rogers O, Yen H, Solomon A, Drake C, Denmeade S, An IL-2 proaerolysin fusion toxin that selectively eliminates regulatory t cells to enhance antitumor immune response, Prostate 79 (10) (2019) 1071–1078. [DOI] [PubMed] [Google Scholar]
- [120].Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, Rinaldi A, Sumanasuriya S, Lambros MB, Neeb A, Lucianò R, Bravi CA, Nava-Rodrigues D, Dolling D, Prayer-Galetti T, Ferreira A, Briganti A, Esposito A, Barry S, Yuan W, Sharp A, de Bono J, Alimonti A, IL-23 secreted by myeloid cells drives castration-resistant prostate cancer, Nature 559 (7714) (2018) 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Maehana S, Matsumoto Y, Kojima F, Kitasato H, Interleukin-24 transduction modulates human prostate cancer malignancy mediated by regulation of anchorage dependence, Anticancer Res. 39 (7) (2019) 3719–3725. [DOI] [PubMed] [Google Scholar]
- [122].Zhang D, Liu S, Liu Z, Ma C, Jiang Y, Sun C, Li K, Cao G, Lin Z, Wang P, Zhang J, Xu D, Kong F, Zhao S, Polyphyllin I induces cell cycle arrest in prostate cancer cells via the upregulation of IL6 and P21 expression, Medicine 98 (44) (2019), e17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Oshimori N, Oristian D, Fuchs E, TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma, Cell 160 (5) (2015) 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM, GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates, Nat. Med. 23 (10) (2017) 1150–1157. [DOI] [PubMed] [Google Scholar]
- [125].Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Norgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jorgensen SB, GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand, Nat. Med. 23 (10) (2017) 1158–1166. [DOI] [PubMed] [Google Scholar]
- [126].Siddiqui JA, Pothuraju R, Khan P, Sharma G, Muniyan S, Seshacharyulu P, Jain M, Nasser MW, Batra SK, Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia, Cytokine Growth Factor Rev. (2021), 10.1016/j.cytogfr.2021.ll.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Siddiqui JA, Seshacharyulu P, Muniyan S, Pothuraju R, Khan P, Vengoji R, Chaudhary S, Maurya SK, Lele SM, Jain M, Datta K, Nasser MW, Batra SK, GDF15 promotes prostate cancer bone metastasis and colonization through osteoblastic CCL2 and RANKL activation, Bone Res. 10 (1) (2022) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Syed V, TGF-β signaling in cancer, J. Cell. Biochem 117 (6) (2016) 1279–1287. [DOI] [PubMed] [Google Scholar]
- [129].Liu X, Chen L, Fan Y, Hong Y, Yang X, Li Y, Lu J, Lv J, Pan X, Qu F, Cui X, Gao Y, Xu D, IFITM3 promotes bone metastasis of prostate cancer cells by mediating activation of the TGF-β signaling pathway, Cell Death Dis. 10 (7) (2019) 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sun DY, Wu JQ, He ZH, He MF, Sun HB, Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway, Life Sci. 235 (2019), 116791. [DOI] [PubMed] [Google Scholar]
- [131].Sun DY, Wu JQ, He ZH, He MF, Sun HB, Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway, Life Sci. 235 (2019), 116791. [DOI] [PubMed] [Google Scholar]
- [132].Li C, Zheng H, Hou W, Bao H, Xiong J, Che W, Gu Y, Sun H, Liang P, Long non-coding RNA linc00645 promotes TGF-βinduced epithelial-mesenchymal transition by regulating miR-205-3p-ZEB1 axis in glioma, Cell Death Dis. 10 (10) (2019) 717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [133].Richards EJ, Zhang G, Li ZP, Permuth-Wey J, Challa S, Li Y, Kong W, Dan S, Bui MM, Coppola D, Mao WM, Sellers TA, Cheng JQ, Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) β: LncRNA-hit-mediated TGFβ-induced epithelial to mesenchymal transition in mammary epithelia, J. Biol. Chem. 290 (11) (2015) 6857–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Li Z, Dong M, Fan D, Hou P, Li H, Liu L, Lin C, Liu J, Su L, Wu L, Li X, Huang B, Lu J, Zhang Y, LncRNA ANCR down-regulation promotes TGF-β-induced EMT and metastasis in breast cancer, Oncotarget 8 (40) (2017) 67329–67343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, Wang SB, Wang YZ, Yang Y, Yang N, Zhou WP, Yang GS, Sun SH, A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma, Cancer Cell 25 (5) (2014) 666–681. [DOI] [PubMed] [Google Scholar]
- [136].Zhang H, Meng H, Huang X, Tong W, Liang X, Li J, Zhang C, Chen M, lncRNA MIR4435-2HG promotes cancer cell migration and invasion in prostate carcinoma by upregulating TGF-β1, Oncol. Lett. 18 (4) (2019) 4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Qi JC, Yang Z, Zhang YP, Lu BS, Yin YW, Liu KL, Xue WY, Qu CB, Li W, miR-20b-5p, TGFBR2, and E2F1 form a regulatory loop to participate in epithelial to mesenchymal transition in prostate cancer, Front. Oncol 9 (2019) 1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Afdal A, Darwin E, Yanwirasti Y, Hamid R, The expression of transforming growth factor beta-1 and interleukin-6 on human prostate: prostate hyperplasia and prostate cancer, Open Access Maced. J. Med. Sd 7 (12) (2019) 1905–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Pestka S, Krause CD, Walter MR, Interferons, interferon-like cytokines, and their receptors, Immunol. Rev. 202 (2004) 8–32. [DOI] [PubMed] [Google Scholar]
- [140].Hansen TF, Nederby L, Zedan AH, Mejlholm I, Henriksen JR, Steffensen KD, Thomsen CB, Raunkilde L, Jensen LH, Jakobsen A, Correlation between natural killer cell activity and treatment effect in patients with disseminated cancer, Transl. Oncol 12 (7) (2019) 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lo UG, Pong RC, Yang D, Gandee L, Hernandez E, Dang A, Lin CJ, Santoyo J, Ma S, Sonavane R, Huang J, Tseng SF, Moro L, Arbini AA, Kapur P, Raj GV, He D, Lai CH, Lin H, Hsieh JT, IFNgamma-induced IFIT5 promotes epithelial-to-mesenchymal transition in prostate cancer via miRNA processing, Cancer Res. 79 (6) (2019) 1098–1112. [DOI] [PubMed] [Google Scholar]
- [142].Xia H, Luo X, Yin W, Inhibition of prostate cancer growth by immunization with a GM-CSF-modified mouse prostate cancer RM-1 cell vaccine in a novel murine model, Oncol. Lett. 15 (1) (2018) 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yan Z, Xiao Y, Chen Y, Luo G, Screening and identification of epithelial-to-mesenchymal transition-related circRNA and miRNA in prostate cancer, Pathol. Res. Pract 216 (2) (2020), 152784. [DOI] [PubMed] [Google Scholar]
- [144].Pal SK, Moreira D, Won H, White SW, Duttagupta P, Luda M, Jones J, Hsu J, Kortylewski M, Reduced T-cell numbers and elevated levels of immunomodulatory cytokines in metastatic prostate cancer patients de novo resistant to abiraterone and/or enzalutamide therapy, Int. J. Mol. Sci 20 (8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Krueger TE, Thorek DLJ, Meeker AK, Isaacs JT, Brennen WN, Tumor-infiltrating mesenchymal stem cells: drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 79 (3) (2019) 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hawley JE, Pan S, Figg WD, Lopez-Bujanda ZA, Strope JD, Aggen DH, Dallos MC, Lim EA, Stein MN, Hu J, Drake CG, Association between immunosuppressive cytokines and PSA progression in biochemically recurrent prostate cancer treated with intermittent hormonal therapy, Prostate 80 (4) (2020) 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chen L, Yang F, Li T, Xiao P, Han ZJ, Shu LF, Yuan ZZ, Liu WJ, Long YQ, Extracellular histone promotes prostate cancer migration and epithelial-mesenchymal transition through NF-κB-mediated inflammatory responses, Chemotherapy 64 (4) (2019) 177–186. [DOI] [PubMed] [Google Scholar]
- [148].Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, You Z, Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells, Immunol. Lett. 184 (2017) 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Fu S, Liu T, Lv C, Fu C, Zeng R, Kakehi Y, Kulkami P, Getzenberg RH, Zeng Y, Stromal-epithelial interactions in prostate cancer: overexpression of PAGE4 in stromal cells inhibits the invasive ability of epithelial cells, J. Cell. Biochem 121 (11) (2020) 4406–4418. [DOI] [PubMed] [Google Scholar]
- [150].Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A, Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13), Nat. Biotechnol. 18 (11) (2000) 1185–1190. [DOI] [PubMed] [Google Scholar]
- [151].Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, Corti A, Bellone M, Targeting TNF-α to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy, J. Immunol. 188 (6) (2012) 2687–2694. [DOI] [PubMed] [Google Scholar]
- [152].Elia AR, Grioni M, Basso V, Curnis F, Freschi M, Corti A, Mondino A, Bellone M, Targeting tumor vasculature with TNF leads effector t cells to the tumor and enhances therapeutic efficacy of immune checkpoint blockers in combination with adoptive cell therapy, Clin. Cancer Res. 24 (9) (2018) 2171–2181. [DOI] [PubMed] [Google Scholar]
- [153].Mu HQ, He YH, Wang SB, Yang S, Wang YJ, Nan CJ, Bao YF, Xie QP, Chen YH, MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis, Clin. Transl. Oncol 22 (1) (2020) 111–121. [DOI] [PubMed] [Google Scholar]
- [154].Kacar S, Kar F, Hacioglu C, Kanbak G, Sahinturk V, The effects of L-NAME on DU145 human prostate cancer cell line: a cytotoxidty-based study, Hum. Exp. Toxicol 39 (2) (2020) 182–193. [DOI] [PubMed] [Google Scholar]
- [155].Gu YH, Yamashita T, Yamamoto H, Matsuo T, Washino N, Song JH, Kang KM, Plant enzymes decrease prostate cancer cell numbers and increase TNF-α in vivo: a possible role in immunostimulatory activity, Int. J. Food Sci 2019 (2019), 8103480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I, Cytokines in clinical cancer immunotherapy, Br. J. Cancer 120 (1) (2019) 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM, Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy, Front. Immunol. 9 (2018) 3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Emerson L, Morales A, Intralesional recombinant alpha-interferon for localized prostate cancer: a pilot study with follow-up of &10 years, BJU Int. 104 (8) (2009) 1068–1070. [DOI] [PubMed] [Google Scholar]
- [159].Li YF, Wang QZ, Zhang TT, Li L, Wang JP, Ding GF, He DL, Low dose of interferon-α improves the clinical outcomes of docetaxel in patients with castration-resistant prostate cancer: a pilot study, Oncol. Lett. 7 (1) (2014) 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, Zhang J, CCR2 expression correlates with prostate cancer progression, J. Cell. Biochem. 101 (3) (2007) 676–685. [DOI] [PubMed] [Google Scholar]
- [161].Vaday GG, Peehl DM, Kadam PA, Lawrence DM, Expression of CCL5 (RANTES) and CCR5 in prostate cancer, Prostate 66 (2) (2006) 124–134. [DOI] [PubMed] [Google Scholar]
- [162].Heresi GA, Wang J, Taichman R, Chirinos JA, Regalado JJ, Lichtstein DM, Rosenblatt JD, Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: report of a case, review of the literature, and analysis of chemokine receptor expression, Urol Oncol 23 (4) (2005) 261–267. [DOI] [PubMed] [Google Scholar]
- [163].Singh S, Singh UP, Stiles JK, Grizzle WE, Lillard JW Jr., Expression and functional role of CCR9 in prostate cancer cell migration and invasion, Clin. Cancer Res. 10 (24) (2004) 8743–8750. [DOI] [PubMed] [Google Scholar]
- [164].Cheng Y, Ma XL, Wei YQ, Wei XW, Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases, Biochim. Biophys. Acta Rev. Cancer 1871 (2) (2019) 289–312. [DOI] [PubMed] [Google Scholar]
- [165].Zhang W, Wang H, Sun M, Deng X, Wu X, Ma Y, Li M, Shuoa SM, You Q, Miao L, CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target, Cancer Commun (Lond) 40 (2–3) (2020) 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Liu G, An L, Zhang H, Du P, Sheng Y, Activation of CXCL6/CXCR1/2 axis promotes the growth and metastasis of osteosarcoma cells in vitro and in vivo, Front. Pharmacol 10 (2019) 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ha H, Debnath B, Neamati N, Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases, Theranostics 7 (6) (2017) 1543–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Tong Z, Guha S, CXCL8/IL-8 and CXCL12/SDF-1 alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer, Int. J. Cancer 124 (4) (2009) 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H, Lenz HJ, CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy, Cancer Treat. Rev 63 (2018) 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS, The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer, J. Biol Chem. 283 (7) (2008) 4283–4294. [DOI] [PubMed] [Google Scholar]
- [171].Singh RK, Lokeshwar BL, The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth, Cancer Res. 71 (9) (2011) 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Begley LA, MacDonald JW, Day ML, Macoska JA, CXCL12 activates a robust transcriptional response in human prostate epithelial cells, J. Biol. Chem. 282 (37) (2007) 26767–26774. [DOI] [PubMed] [Google Scholar]
- [173].Van Rechem C, Rood BR, Touka M, Pinte S, Jenal M, Guerardel C, Ramsey K, Monte D, Begue A, Tschan MP, Stephan DA, Leprince D, Scavenger chemokine (CXC motif) receptor 7 (CXCR7) is a direct target gene of HIC1 (hypermethylated in cancer 1), J. Biol Chem. 284 (31) (2009) 20927–20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Peng C, Rabold K, Mulder WJM, Jaeger M, Netea-Maier RT, Kinase inhibitors’ effects on innate immunity in solid cancers, Cancers (Basel) 13 (22) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Singh S, Singh R, Singh UP, Rai SN, Novakovic KR, Chung LW, Didier PJ, Grizzle WE, Lillard JW Jr., Clinical and biological significance of CXCR5 expressed by prostate cancer specimens and cell lines, Int. J. Cancer 125 (10) (2009) 2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Ohandjo AQ, Liu Z, Dammer EB, Dill CD, Griffen TL, Carey KM, Hinton DE, Meller R, Lillard JW Jr., Transcriptome network analysis identifies CXCL13-CXCR5 signaling modules in the prostate tumor immune microenvironment, Sci. Rep. 9 (1) (2019) 14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wang J, Lu Y, Wang J, Koch AE, Zhang J, Taichman RS, CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamydn signaling pathway, Cancer Res. 68 (24) (2008) 10367–10376. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [178].Ashok A, Keener R, Rubenstein M, Stookey S, Bajpai S, Hicks J, Alme AK, Drake CG, Zheng Q, Trabzonlu L, Yegnasubramanian S, De Marzo AM, Bieberich CJ, Consequences of interleukin 1β-triggered chronic inflammation in the mouse prostate gland: altered architecture associated with prolonged CD4(+) infiltration mimics human proliferative inflammatory atrophy, Prostate 79 (7) (2019) 732–745. [DOI] [PubMed] [Google Scholar]
- [179].Culig Z, Puhr M, Interleukin-6: a multifunctional targetable cytokine in human prostate cancer, Mol. Cell. Endocrinol 360 (1–2) (2012) 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Sun Y, Ai JZ, Jin X, Liu LR, Lin TH, Xu H, Wei Q, Yang L, IL-8 protects prostate cancer cells from GSK-3β-induced oxidative stress by activating the mTOR signaling pathway, Prostate 79 (10) (2019) 1180–1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable, all information in this review can be found in the reference list.


