Abstract
Objective:
Although infantile hypertrophic pyloric stenosis (IHPS) is a well-known disease, there is no systematic review regarding the optimal diagnostic strategy. We conducted a systematic review and meta-analysis to obtain diagnostic accuracy of all methods to diagnose IHPS.
Methods:
According to the Preferred Reported Items for Systematic Reviews and Meta-Analysis guidelines, we searched MEDLINE and Embase to identify studies reporting sensitivity and specificity of all methods used to diagnose IHPS. Inclusion criteria were infants with suspicion of/or diagnosed with IHPS who underwent pyloromyotomy or had clinical follow-up. A random-effects model was used to obtain pooled estimates of sensitivity, specificity and area under the receiver operating characteristic curve.
Results:
After screening 5364 studies, we included 43 studies with in total 6085 infants (n = 4241 IHPS; n = 1844 controls). The diagnostic sensitivity of palpation ranged from 10.0 to 93.4% and decreased over time. Different parameters for ultrasonography were found. Most used parameters were pyloric muscle thickness (PMT) ≥ 3 mm (pooled sensitivity 97.6% and specificity 98.8%), PMT ≥ 4 mm (pooled sensitivity 94.0% and specificity 98.0%) or a combination of PMT ≥ 4 mm and/or pyloric canal length ≥16 mm (pooled sensitivity 94.0% and specificity 91.7%). The AUC showed high diagnostic accuracy (0.997, 0.966 and 0.981 respectively), but large heterogeneity exists. Due to the large differences in cut-off values no meta-analysis could be conducted for pyloric canal length and pyloric diameter.
Conclusion:
Palpation has limited sensitivity in diagnosing IHPS. We showed that ultrasonography has highest diagnostic accuracy to diagnose IHPS and we advise to use PMT ≥ 3 mm as cut-off.
Advances in knowledge:
This is the first systematic review and meta-analysis on diagnosing IHPS, which summarizes the available literature and may be used as a guideline.
Introduction
Infantile hypertrophic pyloric stenosis (IHPS) is a condition which develops in the first weeks of life and requires surgical treatment. 1 Infants with IHPS typically present with projectile vomiting and may suffer from dehydration due to a gastric outlet obstruction. The exact pathophysiology which leads to muscular wall thickening and the inability of the pyloric canal to relax is still unknown, but thought to be multifactorial. 1,2 Well-known risk factors are male sex, prematurity, bottle-feeding and delivery by cesarean section. 3–5
In children presenting with vomiting, physical examination and imaging are key in the diagnostic process. Before ultrasound made its introduction, medical history and physical examination played a major role in diagnosing infants with IHPS. Important signs suspect for IHPS are non-bilious projectile vomiting, dissatisfaction after feeding and inability to gain weight. Physical examination may consist of careful palpation of the abdomen to search for pyloric thickening (‘olive sign’) and visualization of peristaltic waves. Although the guideline of the American College of Radiologists still states that in case of new-onset non-bilious vomiting where a classic ‘olive’ is palpated, the diagnosis of IHPS can be made clinically, experience has shown that nowadays this sign is often absent. 6,7 Furthermore, test feedings and palpation are invasive and stress enhancing for both infants and parents.
Since Teele and Smith first described the use of ultrasonography to diagnose IHPS in 1977, the incidence of ultrasound diagnosis of IHPS increased. 8 Over the subsequent years, ultrasound has become the diagnostic modality of choice due to its non-invasiveness and the ability to directly observe the pyloric canal and all its assets. Although ultrasound is a common practice in most institutions today, many different parameters are used and the evidence is limited by single studies. Another, historic diagnostic modality for diagnosing obstructive causes of vomiting such as IHPS is an upper gastrointestinal (UGI) studies. 6 However, the use of ionizing radiation makes this test less favorable for young infants.
The aim of this systematic review is to evaluate the evidence base for the diagnostic strategy with respect for physical examination and imaging for IHPS. We have studied the diagnostic accuracy of physical examination and imaging of IHPS and aimed to develop the first evidence-based guideline for diagnosing IHPS in order to ensure highest possible quality of care.
Methods
Protocol
A systematic review was conducted according to the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines on all literature regarding diagnosing IHPS. The protocol was registered at PROSPERO (2021, CRD42021227343).
Literature search
We performed a search in PubMed and EMBASE (Ovid). Keywords were hypertrophic pyloric stenosis, infant, diagnostic imaging, diagnostic techniques, physical examination, radiography, fluoroscopy, ultrasonography, Computed Tomography and Magnetic Resonance Imaging. The detailed search strategy is available in the Supplementary Material 1. The reference lists of the included articles were examined for additional publications. The search was conducted in November 2017 and updated in December 2020 and July 2022.
Eligibility criteria
All original studies reporting on sensitivity and specificity of any method used to diagnose IHPS were considered eligible, with language restriction to English publications. Case reports, reviews, letters, and congress abstracts were excluded. Inclusion criteria were infants (<3 months) with suspicion of or diagnosed with IHPS who underwent open or laparoscopic pyloromyotomy after diagnosis or had clinical follow-up. Studies with no clinical follow-up or surgical treatment to confirm or exclude diagnosis of IHPS and/or studies describing infants with other (congenital) gastrointestinal conditions were excluded from data analysis.
Study selection and methodological quality assessment
Studies were included according to the criteria listed above. Two reviewers (JD, RvR) independently screened title and abstract of the studies retrieved using the search strategy. After first selection, full text was screened for final selection. The methodological quality of the included studies was assessed by using the Quality Assessment of Diagnostic Accuracy Studies v. 2 (QUADAS-2) tool (FvdB, JD and RvR). Inconsistencies were solved by second joint review.
Data extraction and analysis
We systematically extracted the data regarding study design characteristics, patient characteristics and test characteristics from all the included studies and recorded them in a data extraction form. If incomplete, missing data were calculated or, if possible, retrieved by contacting the authors. Surgery, i.e. open or laparoscopic pyloromyotomy, was used as the reference standard following positive index test results. Negative index test results were followed by surgery or clinical follow-up.
Statistical analysis
Statistical analysis was performed using Review Manager v. 5.4 and Meta-DiSc v. 1.4. The available data were inserted in 2 × 2 tables to compute sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV) for each study. A random-effects model was used to obtain pooled estimates of sensitivity and specificity with 95% confidence intervals (95% CIs). Heterogeneity between studies was assessed using the χ2 and I two statistic. The summary receiver operating characteristic (SROC) curve was used to estimate the area under the curve (AUC) that represented overall diagnostic performance of the index test.
Results
Literature search
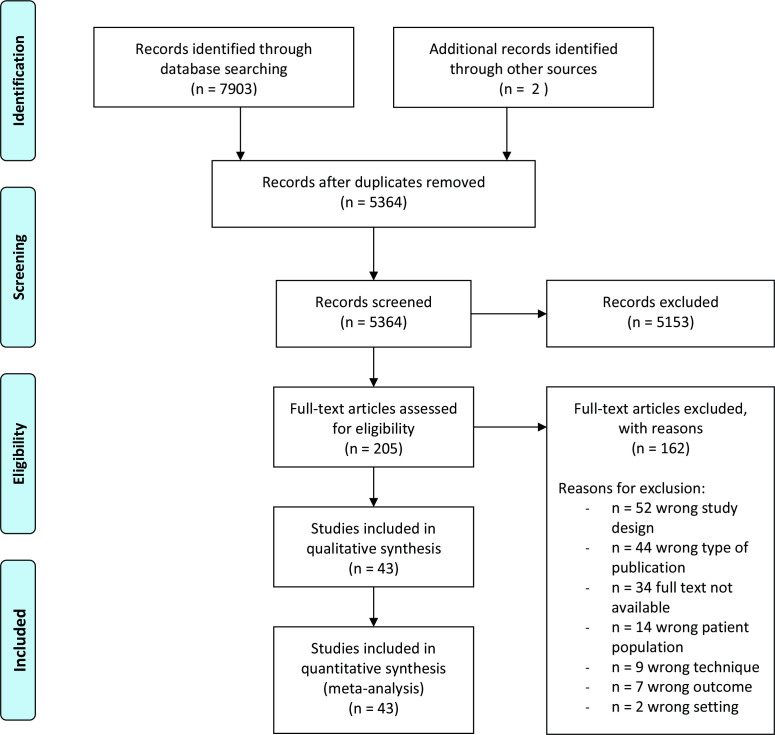
The literature search initially provided 7903 potentially suitable studies. Two studies were added after examination of reference lists and hand searching. After exclusion of duplicates, 5364 studies remained, of which 5153 studies were excluded subsequent to first screening. After full text screening, 162 more studies were excluded and 43 were included. See Figure 1 for the detailed PRISMA chart.
Figure 1.
PRISMA flowchart. PRISMA, Preferred Reported Items for Systematic Reviews and Meta-Analysis.
Study characteristics
The 43 included studies were published between 1968 and 2021. In total, 6085 infants were included, of which 4241 infants had IHPS and 1844 were controls. The boys–girls ratio of positive cases was described in 25 articles and was 5:1 (boy n = 2040 vs. girl n = 436). The mean age of infants with IHPS was described in only 14 publications and ranged between 34 and 46 days. 23 studies (n = 3164 infants) investigated the sensitivity of palpation of a pyloric mass 9–31 and 31 studies the sensitivity and specificity of ultrasonography (n = 3685 infants; n = 1914 with IHPS vs n = 1771 controls). 12–16,20,23,26,28,30–51 The presence of a pyloric mass was assessed during clinical examination by any doctor. However, information in regard to the performer of physical examination was not available for all studies. Optionally, an additional test feed was conducted to observe for the presence of peristaltic waves. The ultrasound was (if mentioned) performed by a (pediatric) radiologist, ultrasonographer or other doctor. Measurements performed during ultrasonography included pyloric muscle thickness (PMT), pyloric canal length (PCL), pyloric muscle diameter (PD) and in a few cases pyloric ratio (PR) or pyloric muscle index (PMI). The upper limits of normal of PMT, PCL and PD differed between studies and varied between ≥ 3–4 mm, ≥ 11–17 mm and ≥ 13–20 mm respectively. Some studies used combined measurements to diagnose IHPS.
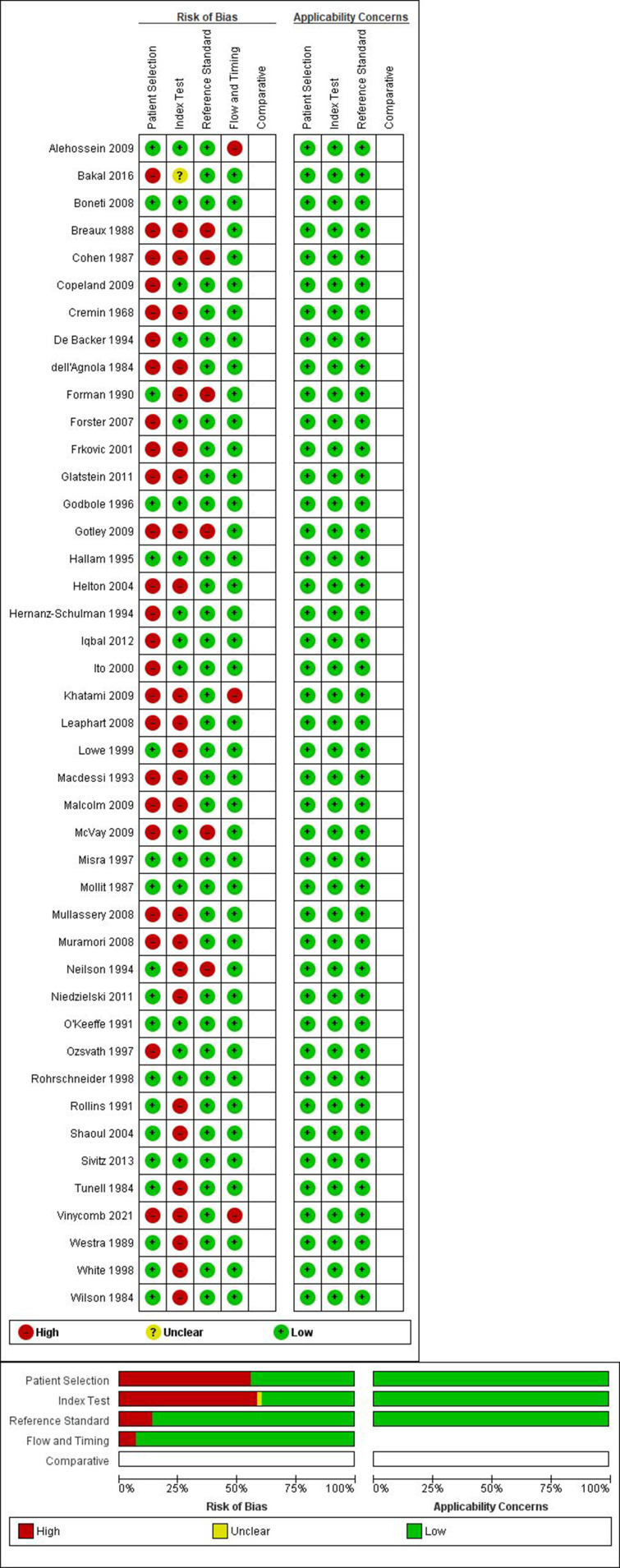
Methodological quality
Quality assessment by using the QUADAS-2 tool showed a high risk regarding both patient selection and index test in more than half of the studies (Figure 2). Many studies only included patients with IHPS and no control group, 9–12,16–19,22–30,37,44 some excluded infants with a palpable pyloric mass 33,39,42 and others did not describe in- and exclusion criteria. 49,50 In regard to the index test, it was often unknown who performed palpation of the pyloric mass or ultrasound and/ or whether this person had experience with this technique, 9–12,14,15,17,18,21,24–30,40,41 sometimes no pre-determined cut-off values were available in regard to ultrasonography 14,42,51 and some authors documented a learning curve during performing ultrasounds. 23,46 The risk of bias concerning reference test and flow and timing were considered low to moderate. Assessment of bias regarding the overall applicability was considered low.
Figure 2.
Quality assessment.
Palpation
23 papers reporting on the sensitivity of palpation of the ‘olive’ were found (Table 1). The diagnostic sensitivity ranged widely from 10.0 to 93.4%. It is of interest to note that over the years, the sensitivity of palpation seems to drop. Only six studies included infants without IHPS, of which four studies reported one or more false-positive cases, leading to a specificity ranging from 0.0 to 100.0%. 12–14,20,21,26 No meta-analysis could be conducted due to lack of control patients in most studies.
Table 1.
Percentage of ‘olives’ palpated
| Author | Yeara | Cases b | Proportion IHPS | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| Cremin 9 | 1956–1967 | 165 | 1.0 | 0.84 [0.77–0.89] | n/e |
| Macdessi 10 | 1974–1977 and 1988–1991 |
402 | 1.0 | 0.90 [0.86–0.92] | n/e |
| Breaux 11 | 1980–1984 | 363 | 1.0 | 0.85 [0.80–0.88] | n/e |
| dell'Agnola 12 | 1981–1982 | 17 | 0.82 | 0.15 [0.02–0.45] | 1.00 [0.29–1.00] |
| Mollitt 13 | 1983–1986 | 101 | 0.57 | 0.47 [0.33–0.60] | 0.98 [0.88–1.00] |
| Forman 14 | 1985–1988 | 101 | 0.77 | 0.77 [0.66–0.86] | 1.00 [0.29–1.00] |
| Rollins 15 | 1986–1988 | 100 | 0.44 | 0.86 [0.73–0.95] | n/e |
| De Backer 16 | 1986–1991 | 63 | 1.0 | 0.60 [0.47–0.72] | n/e |
| Muramori 17 | 1986–1996 | 103 | 1.0 | 0.79 [0.69–0.86] | n/e |
| Shaoul 18 | 1990–2000 | 70 | 1.0 | 0.50 [0.38–0.62] | n/e |
| Ozsvath 19 | 1991–1995 | 60 | 1.0 | 0.33 [0.22/0.47 | n/e |
| Godbole 20 | 1993–1995 | 116 | 0.65 | 0.80 [0.69–0.88] | 0.98 [0.87–1.00] |
| White 21 | 1994–1996 | 234 | 0.64 | 0.74 [0.66–0.81] | 0.99 [0.94–1.00] |
| Gotley 22 | 1994–2004 | 329 | 1.0 | 0.44 [0.39–0.50] | n/e |
| Frkovic 23 | 1995–1999 | 14 | 1.0 | 0.15 [0.02–0.45] | n/e |
| Bakal 24 | 1996–2015 | 56 | 1.0 | 0.54 [0.40–0.67] | n/e |
| Helton 25 | 2000–2002 | 175 | 1.0 | 0.32 [0.25–0.39] | n/e |
| Mullassery 26 | 2000–2004 | 343 | 1.0 | 0.94 [0.89–0.96] | 0.00 [0.00–0.97] |
| Leaphart 27 | 2001–2006 | 314 | 1.0 | 0.63 [0.57–0.68] | n/e |
| Khatami 28 | 2001–2008 | 84 | 1.0 | 0.15 [0.09–0.25] | n/e |
| Glatstein 29 | 2006–2008 | 118 | 1.0 | 0.13 [0.07/0.20] | n/e |
| Malcolm 30 | n/a (published in 2009) | 8 | 1.0 | 0.25 [0.03–0.65] | n/e |
| Sivitz 31 | 2009–2012 | 67 | 0.15 | 0.10 [0.00–0.45] | n/e |
IHPS, Infantile hypertrophic pyloric stenosis.
Range of data collection
Total number of cases included in the study. n/e means not estimable, 95% CI means 95% confidence interval
Ultrasonography
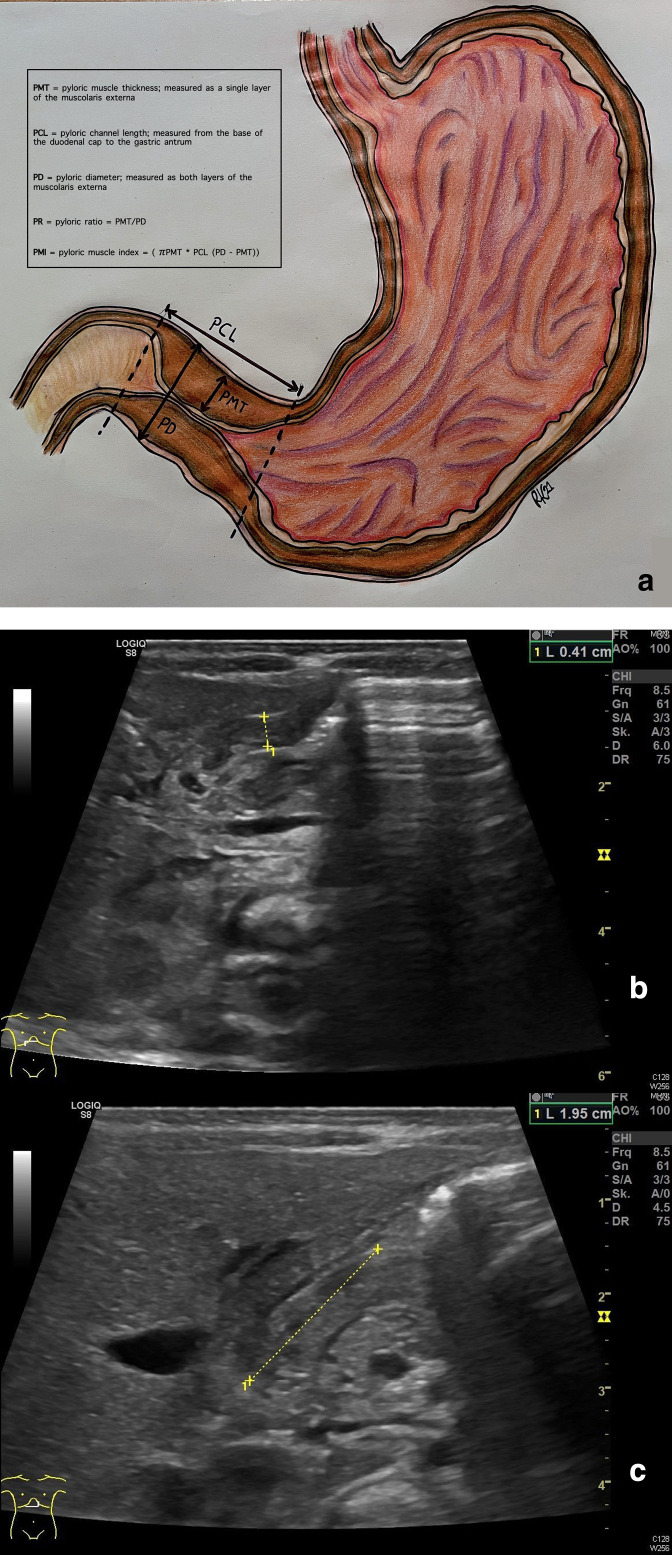
Of the 31 included studies on ultrasonography, 16 papers described the diagnostic accuracy of pyloric muscle thickness (PMT) (Table 2), 12 the pyloric channel length (PCL) and 6 the pyloric diameter (PD, Table 3) as unique parameters. Furthermore, 15 articles described sensitivity and specificity of ultrasonography in the diagnosis of hypertrophic pyloric stenosis based on combined measurements. (Table 4). The included studies used varying types of ultrasound systems. In most cases, a linear transducer was used with a frequency of 5 or 7.5 MHz, but other transducers and frequencies were described as well. Eight studies did not describe the type of transducer at all. In Figure 3, we have shown a schematic drawing and an ultrasound image of the pylorus illustrating the different measurements. Only two articles described the exact cursor placement for the measurement of PMT and PCL. 33,38 PMT measures included the thickness of one muscle layer and excluded the mucosa and lumen and PCL was measured from the base of the duodenal cap to the gastric antrum.
Table 2.
Sensitivity and specificity of ultrasonography in the diagnosis of infantile hypertrophic pyloric stenosis based on PMT ≥ 3 mm and PMT ≥ 4 mm
| Author | Yeara | Casesb | Proportion IHPS | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| O'Keeffe 32 | 1991 | 145 | 0.29 | 95.2 | 100 | 100 | 98.1 | 98.6 |
| Hernanz-Schulman 33 | 1994 | 150 | 0.44 | 100 | 100 | 100 | 100 | 100 |
| Hallam 34 | 1995 | 47 | 0.45 | 81 | 92.3 | 89.5 | 85.7 | 87.2 |
| Rohrschneider 35 | 1998 | 169 | 0.5 | 100 | 100 | 100 | 100 | 100 |
| Lowe 36 | 1999 | 84 | 0.41 | 100 | 100 | n/e | n/e | n/e |
| Forster 37 | 2007 | 187 | 0.47 | 91.9 | 85.1 | n/e | n/e | n/e |
| Alehossein 38 | 2009 | 444 | 0.17 | 100 | 100 | 100 | 100 | 100 |
| Malcom 30 | 2009 | 8 | 1 | 100 | n/e | n/e | n/e | n/e |
| Iqbal 39 | 2012 | 304 | 0.22 | 95.5 | 99.2 | 97 | 99.7 | 98.4 |
| Vinycomb 51 | 2021 | 284 | 0.5 | 97.8 | 94 | 94.6 | 97.7 | 96 |
| Tunell 40 | 1984 | 91 | 0.44 | 92.5 | 90.2 | 88.1 | 93.9 | 91.2 |
| Wilson 41 | 1984 | 50 | 0.58 | 93.1 | 90.5 | 93.1 | 90.5 | 92 |
| Cohen 42 | 1987 | 156 | 0.08 | 100 | n/e | n/e | n/e | n/e |
| Westra 43 | 1989 | 47 | 0.47 | 95.5 | 100 | 100 | 96.2 | 97.2 |
| Ito 44 44 | 2000 | 57 | 1 | 93 | n/e | n/e | n/e | n/e |
| Mullassery 26 26 | 2008 | 343 | 99.1 | 95.2 | 0 | 99.1 | 0 | 94.4 |
| Alehossein 38 38 | 2009 | 444 | 0.17 | 96 | 100 | 100 | 99.2 | 99.3 |
IHPS, infantile hypertrophic pyloric stenosis; NPV, negative-predictive value;PMT, pyloric muscle thickness; PPV, positive-predictive value.
Year published
Total number of cases included in the study. N/e means not estimable, because there was lack of sufficient data to calculate value and/or value was not reported in publication.
Table 3.
Sensitivity and specificity of ultrasonography in the diagnosis of infantile hypertrophic pyloric stenosis based on (A) PCL and (B) PD
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Yeara | Casesb | Proportion IHPS | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
| Hallam 34 | 1995 | 47 | 0.45 | PCL>13 mm. | 57.1 | 96.2 | 92.3 | 73.5 | 78.7 |
| Mullassery 36 | 2008 | 343 | 1 | PCL≥14 mm. | 99.6 | 0 | 98.7 | 0 | 98.3 |
| Rohrschneider 35 | 1998 | 169 | 0.5 | PCL≥15 mm. | 91.8 | 98.8 | 98.7 | 92.2 | 95.3 |
| Malcolm 30 | 2009 | 8 | 1 | PCL≥15 mm. | 100 | n/e | n/e | n/e | n/e |
| Iqbal 39 | 2012 | 304 | 0.22 | PCL≥15 mm. | 97 | 97 | 89 | 99.1 | 96.7 |
| Lowe 36 | 1999 | 84 | 0.4 | PCL≥14 mm | 84 | 100 | n/e | n/e | n/e |
| PCL≥15 mm | 92 | 97 | n/e | n/e | n/e | ||||
| PCL≥16 mm. | 98 | 97 | n/e | n/e | n/e | ||||
| PCL≥17 mm | 100 | 84.9 | n/e | n/e | n/e | ||||
| Vinycomb 51 | 2021 | 284 | 0.5 | PCL≥15 mm | 95.8 | 90.4 | 91.9 | 95 | 93.3 |
| PCL≥17 mm | 76.7 | 98.4 | 98.2 | 78.8 | 86.9 | ||||
| Forster 37 | 2007 | 87 | 0.47 | PCL≥16 mm. | 85.1 | 76.9 | n/e | n/e | n/e |
| PCL>17 mm. | 75.8 | 84.6 | n/e | n/e | n/e | ||||
| Ito 44 | 2000 | 57 | 1 | PCL≥18 mm. | 98 | n/e | n/e | n/e | n/e |
| Tunell 40 | 1984 | 91 | 0.44 | PCL>19 mm. | 95 | 100 | 100 | 96.2 | 97.8 |
| Westra 43 | 1989 | 47 | 0.47 | PCL≥19 mm. | 54.5 | 100 | 100 | 71.4 | 78.7 |
| Cohen 42 | 1987 | 156 | 0.07 | PCL≥20 mm. | 64 | n/e | n/e | n/e | n/e |
| B | |||||||||
| Author | Yeara | Casesb | Proportion HPS | Cut- off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Rohrschneider 35 | 1998 | 169 | 0.5 | PD≥11 mm. | n/e | n/e | n/e | n/e | 92 |
| Tunell 40 | 1984 | 91 | 0.44 | PD>13 mm. | 80 | 96.1 | 94.1 | 86 | 89 |
| Westra 43 | 1989 | 47 | 0.47 | PD≥13 mm. | 90.9 | 100 | 100 | 92.6 | 95.7 |
| Wilson 41 | 1984 | 50 | 0.58 | PD≥15 mm. | 51.7 | 100 | 100 | 60 | 72 |
| Cohen 42 | 1987 | 156 | 0.07 | PD≥15 mm. | 55 | n/e | n/e | n/e | n/e |
| Ito 44 | 2000 | 57 | 1 | PD≥15 mm. | 93 | n/e | n/e | n/e | n/e |
IHPS, infantile hypertrophic pyloric stenosis; NPV, negative-predictive value;PCL, pyloric canal length; PD, pyloric diameter; PPV, positive-predictive value.
Year published.
Total number of cases included in the study. N/e means not estimable, because there was lack of sufficient data to calculate value and/or value was not reported in publication.
Table 4.
Sensitivity and specificity of ultrasonography in the diagnosis of infantile hypertrophic pyloric stenosis based on combined measurements
| Author | Yeara | Casesb | Proportion IHPS | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Neilson 45 | 1994 | 147 | 0.46 | PMT > 2.5 mm or PCL>15 mm or PD>11 mm | 97.1 | 98.7 | 98.5 | 97.5 | 98.0 |
| Vinycomb 51 | 2021 | 284 | 0.50 | PMT ≥ 3 mm and PCL≥14.5 mm PMT ≥ 3 mm and PCL≥15 mm |
93.1 94.4 |
99.1 99.1 |
99.4 99.3 |
90.6 93.5 |
95.5 96.5 |
| Khatami 28 | 2009 | 84 | 1.00 | PMT > 3 mm and PCL>15 mm | 87.7 | n/e | n/e | n/e | n/e |
| Sivitz 31 | 2013 | 67 | 0.85 | PMT > 3 mm and PCL>17 mm | 100.0 | 100.00 | 100.0 | 100.0 | 100.0 |
| Niedzielski 46 | 2011 | 115 | 0.83 | PMT ≥ 3 mm and/or PCL≥17 mm | 97.9 | 100.0 | 100.0 | 90.5 | 98.3 |
| Frkovic 23 | 2001 | 9 | 1.00 | PMT ≥ 3 mm, PCL≥17 mm and PD≥13 mm with pyloric canal closed | 88.9 | n/e | n/e | n/e | n/e |
| dell'Agnola 12 | 1984 | 17 | 0.82 | PMT > 4 mm or PCL>13 mm or PD>9.9 mm | 100.0 | 100.00 | 100.0 | 100.0 | 100.0 |
| Rollins 15 | 1991 | 100 | 0.49 | PMT ≥ 4 mm and/or PD≥15 mm | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Misra 47 | 1997 | 65 | 0.95 | PMT > 4 mm and PCL > 16 mm | 87.1 | 0.00 | 94.7 | 0.00 | 83.1 |
| De Backer 16 | 1994 | 63 | 1.00 | PMT > 4 mm and/or PCL>16 mm | 60.3 | n/e | 100.0 | n/e | n/e |
| Forman 14 | 1990 | 44 | 0.50 | PMT > 4 mm. and/or PCL>16 mm | 88.9 | 100.0 | 100.0 | 90.0 | 94.4 |
| Boneti 48 | 2008 | 30 | 0.93 | PMT ≥ 4 mm and/or PCL>16 mm. | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Copeland 49 | 2009 | 32 | 0.78 | PMT ≥ 4 mm and/or PCL≥16 mm | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| McVay 50 | 2009 | 71 | 0.92 | PMT ≥ 4 mm and/or PCL≥16 mm | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Mollitt 13 | 1987 | 54 | 0.58 | PMT > 4 mm MWT and/or PCL>17 mm and/or PD>13 mm | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
IHPS, infantile hypertrophic pyloric stenosis; NPV, negative-predictive value; PCL, pyloric canal length; PD, pyloric diameter; PMT, pyloric muscle thickness; PPV, positive-predictive value.
Year published
Total number of cases included in the study. PMT stands for pyloric muscle thickness, PCL pyloric canal length and PD pyloric diameter. N/e means not estimable, because there was lack of sufficient data to calculate value and/or value was not reported in publication.
Figure 3.
Schematic drawing of the pylorus illustrating the different sonographic measurements (a) and ultrasound images of PMT (b) or PCL (c) measurement. PCL, pyloric canal length; PD, pyloric diameter; PMT, pyloric muscle thickness; PR, pyloric ratio.
PMT
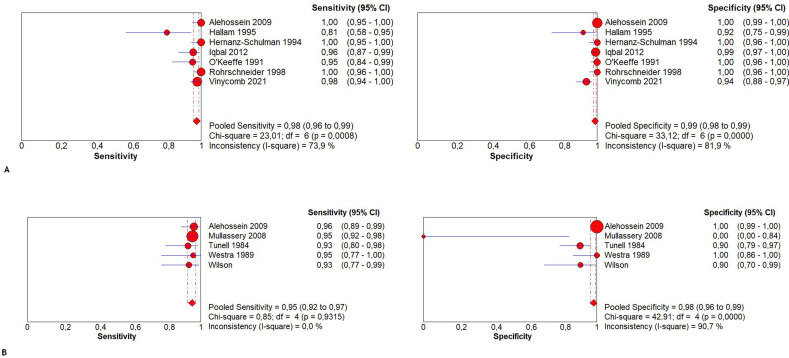
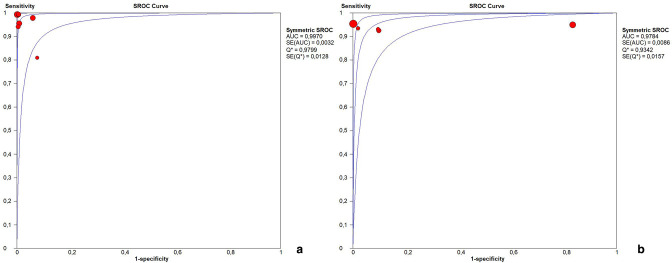
Cut-off levels used for PMT were 3 and 4 mm. One article described a cut-off of PMT ≥ 3.5 mm. 51 Based on PMT only, the sensitivity ranged from 76 to 100% and the specificity from 85–100%, depending on both the patient population and the cut off level chosen (Table 2). PPV and NPV ranged from 88.1 to 100% and 85.7 to 100% respectively with an accuracy ranging from 87.2 to 100%. Five studies were excluded from meta-analysis because the diagnostic or reference test results could not be extracted, calculated or obtained from the authors. 30,36,37,42,44 The pooled sensitivity of PMT ≥ 3 mm was 97.6% (95% CI 95.8–98.7%; χ2 = 23.01, p < 0.001; Ι2 = 73.9%) and the pooled specificity was 98.8% (95% CI 98.0–99.4%; χ2 = 31.12, p < 0.001; Ι2 = 81.9%) (Figure 4a). The AUC of the SROC was 0.997 (Figure 5a). The pooled sensitivity of PMT ≥ 4 mm was 94.0% (95% CI 92.0–96.0%; χ2 = 3.25, p 0.517; Ι2 = 0.0%) and the pooled specificity was 98.0% (95% CI 97.0–99.0%; χ2 = 36.80, p < 0.001; Ι2 = 89.1%) (Figure 4b). The AUC of the SROC was 0.966 (Figure 5b).
Figure 4.
Pooled sensitivity and specificity forest plots including the 95% CI of (A) PMT ≥ 3 mm and (B) PMT ≥ 4 mm. CI, confidence interval; PMT, pyloric muscle thickness.
Figure 5.
Summary receiver operating characteristic curve of (A) PMT ≥ 3 mm and (B) PMT ≥ 4 mm. PMT, pyloric muscle thickness.
PCL
Cut-off levels used for PCL ranged widely from >13 mm to ≥20 mm (Table 3). Based on PCL only, the sensitivity ranged from 54.5 to 100% and the specificity from 76.9 to 100%, this also depended on both the patient population and the cut-off level chosen. PPV and NPV could be determined in a limited number of studies and ranged from 89.0 to 100% and 71.4 to 99.1% respectively with an accuracy ranging from 78.7 to 98.3%. 34–36,39,40,43,51 Due to the large differences in cut-off values, no meta-analysis could be conducted.
PD
Cut-off levels used for PD were ranging widely from ≥11 mm to ≥15 mm (Table 3). Based on PCL only, the sensitivity ranged from 51.7 to 93.0% and the specificity from 96.1 to 100%, depending on both patient population and cut- off level chosen. PPV and NPV ranged from 94.1 to 100% and 60.0 to 92.6% respectively with an accuracy ranging from 72.0 to 95.7%. Due to the large differences in cut-off values, no meta-analysis could be conducted.
Combined measurements
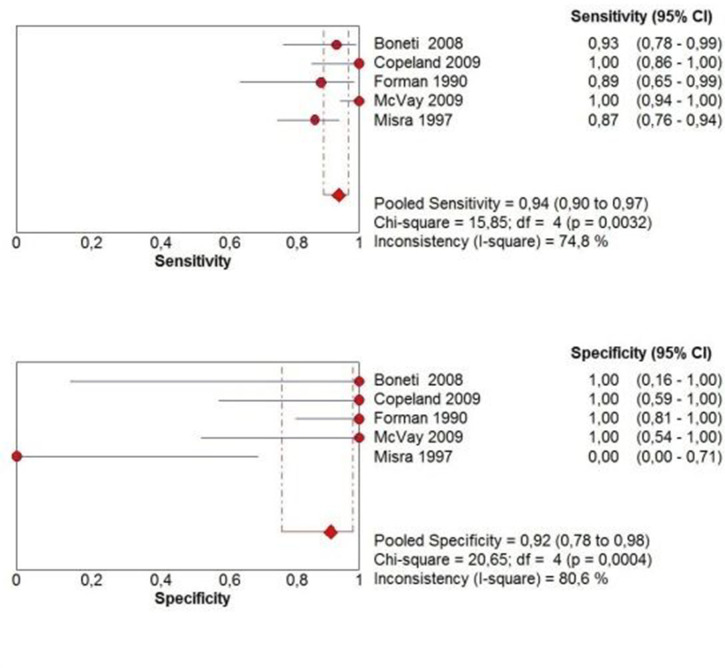
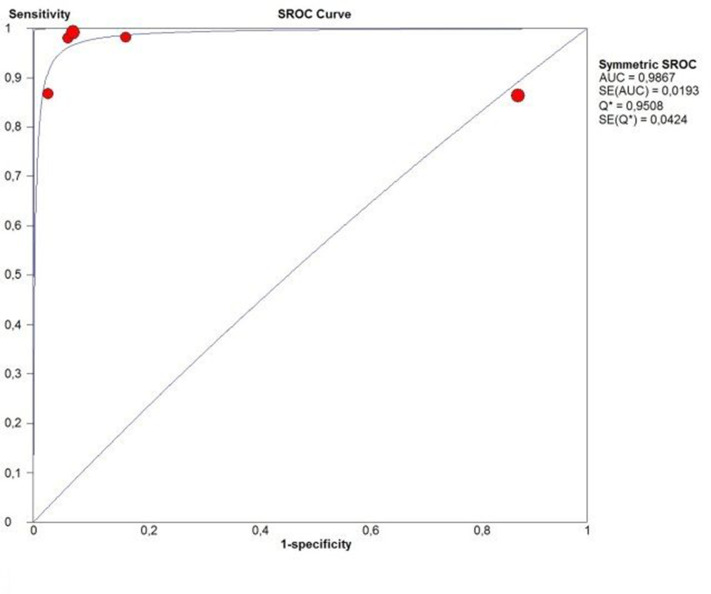
Table 4 shows the studies which used combined measurements. Those studies combined different cut-off values of PMT, PCL and/or PD. Dependent of patient population, combination and chosen cut-off levels, the sensitivity ranged from 60.3 to 100% and the specificity from 0.0 to 100%. PPV and NPV ranged from 94.7 to 100% and 0.0 to 100% respectively with an accuracy ranging from 83.1 to 100%. Most often used combined measurement was PMT > 4 mm and/or PCL > 16 mm. 14,16,47–50 Therefore, we conducted a meta-analysis of this combination. Two studies were excluded because the diagnostic or reference test results could not be extracted, calculated or obtained from the authors. The pooled sensitivity of PMT > 4 mm and/or PCL > 16 mm was 94.0% (95% CI 89.8–96.9%; χ2 = 15.85, p 0.003; Ι2 = 74.8%) and the pooled specificity was 91.7% (95% CI 77.5–98.2%; χ2 = 20.65, p < 0.001; Ι2 = 80.6%) (Figure 6). The AUC of the SROC was 0.981 (Figure 7).
Figure 6.
Pooled sensitivity and specificity forest plots including the 95% CI of a combination of PMT ≥ 4 mm and/or PCL ≥ 16 mm. CI, confidence interval; PCL, pyloric canal length; PMT, pyloric muscle thickness.
Figure 7.
SROC of a combination of PMT ≥ 4 mm and/ or PCL ≥ 16 mm. PCL, pyloric canal length; PMT, pyloric muscle thickness; SROC, summary receiver operating characteristic curve.
Pyloric ratio and pyloric muscle index
Only one paper used the pyloric ratio (PR), which is calculated by the PMT divided by PD. 36 They compared different cut-offs varying from 0.26 to 0.29 and suggested to use a cut-off of ≥0.27 (sensitivity of 96.0% and specificity of 93.9%). Another paper used the pyloric muscle index as described by Carver et al (πPMT*PCL (PD-PMT) with a cut-off value of>0.46 and found a sensitivity of 98.7% and specificity of 100.0%. 20,52
Discussion
We performed this systematic review to evaluate the accuracy of diagnostic strategies for diagnosing IHPS and to develop an evidence-based guideline for diagnosing IHPS to improve quality of care. In this study, we found a wide range in sensitivity of palpation of the hypertrophied pyloric muscle or ‘olive’ (10%–93%). An important finding was that the sensitivity seems to decrease over the years. This is in line with the results of Vinycomb et al who performed a trend analysis on the number of palpated tumors observed between 2005 and 2015 and found a significant trend. 53 It is thought that this decrease is caused by earlier presentation and/ or less experience of the medical staff. 18 Furthermore, we found different parameters and corresponding cut-off values for ultrasonography which could be used for diagnosing IHPS. Most used parameters were PMT (≥3 mm or ≥4 mm) or a combination of PMT (≥4 mm) and/or PCL (≥16 mm). Pooled sensitivity and specificity for both PMT solely or in combination with PCL were high. However, PMT ≥ 3 mm is just a little more accurate than PMT ≥ 4 mm or the combination of PMT ≥ 4 mm and/or PCL ≥ 16 mm and is therefore preferable. Although, the results should be interpreted with caution due to high heterogeneity. Unfortunately, we could not obtain pooled data for PCL solely or PD because cut-off values differed largely between the included studies. Future studies to investigate the diagnostic accuracy of these parameters are therefore recommended. Likewise, new combinations of parameters could be studied to aim for maximum accuracy. In literature, ancillary findings as antral nipple sign or double track sign are described as well, but insufficient evidence was found to evaluate the usability and accuracy. 42,54
Only two studies mentioned the placement of the cursor during ultrasonography. They both stated to use the standard manner, which included one muscle layer without submucosa and lumen for PMT measurement and the base of the duodenal cap to the gastric antrum for PCL measurement. It seems possible that others authors considered cursor placement as standard practice and therefore did not elucidate cursor placement. However, a recent study showed that in more than half of patient scans, the placement of the internal cursor during PMT measurements included components of the pylorus up to the submucosa, muscularis mucosa, mucosa and in a few instances even the luminal mucosal folds. 55 Furthermore, they found a moderate interobserver agreement of 66% between two pediatric radiologists, indicating operator variability for placement of the internal cursor. We advise to standardize cursor placement at least at an institutional level. It has become common practice in most institutions to use the hypoechoic muscularis externa (single layer) as a sonographical landmark for PMT measurement, the base of the duodenal cap and the gastric antrum for PCL measurement and both layers of the muscularis externa for PD measurement. We suggest to include this practice into local protocols not only to meet the high diagnostic standards in pediatric radiology but also to ensure comparability of future research on this topic. Since, a deviation of a millimeter may have major consequences we suggest to conduct 2–3 measurements and take the average. Furthermore, the cut-off values used to determine if the infant is diagnosed with pyloric stenosis are mostly based on the term born infant. Some authors demonstrated that sonographic measurements correlated with the weight and age of infants, suggesting that small and premature infants may not fulfill the criteria while having IHPS. 27,56 However, others state that measurements are not affected by weight at presentation and corrected gestational age or that although age, weight and pyloric thickness are associated they do not have impact on the diagnostic criteria for IHPS. 39,57 We think that it should be borne in mind that infants with low (birth)weight and age might not meet the diagnostic criteria and suggest in these cases repeated sonography after a couple of days.
In this review, we primarily focused on palpation and ultrasonography. The use of abdominal radiographs is only described in historic literature and nowadays, there is general consensus that the use of abdominal radiographs should be considered as obsolete in the diagnosis of patients presenting with acute abdominal complaints. 58 Except one study of 14 infants, we did not find any publications of sufficient quality presenting adequate data to calculate sensitivity and/or specificity of an upper gastrointestinal (UGI) study for the diagnosis of IHPS. 47 Although the ACR guideline mentions that contrast UGI studies are excellent for diagnosing obstructive causes of vomiting in young infants, they also note the limitation of the use of ionizing radiation. 6 It is potentially harmful for the infant and obsolete in the light of a radiation free alternative meeting the diagnostic standards needed. Therefore, fluoroscopy should only be considered in those patients where ultrasound is non-diagnostic. No relevant publications related to the use of CT or MRI in the diagnosis of IHPS were found. In our clinical experience, these advanced imaging techniques should have no role in the diagnostic process of patients suspected of IHPS.
The recommendations arising from this systematic review of the literature are subject to some limitations. The level of evidence of the included studies was low to moderate. It is in the nature of case–control studies that the results of the individual studies included are influenced by a certain degree of bias. The ultrasound technology may have been evolved over the years, but we were unable to analyze the potential relation between technical advances and diagnostic accuracy. Furthermore, the author’s main concern is the assumption that false-positive imaging results may be underreported. The gold-standard for positive imaging in IHPS is the intraoperative judgement by the surgeon during the laparoscopy or laparotomy for suspected IHPS. The binary classification of IHPS (“yes or no”) represents a highly subjective measure and must be regarded as a major source of bias for our results. It is not clear from most series what happened to the patients that were intraoperatively regarded as having “no IHPS” or “early IHPS”. Certainly, every pediatric surgeon pursues the intention to avoid re-operations which may have influenced the numbers of pyloromyotomy.
Based on this literature study, we support the current approach and have shown that ultrasonography by an experienced ultrasonographer or (pediatric) radiologist is a valid method to diagnose IHPS. We advise to use PMT ≥ 3 mm to confirm the diagnosis of IHPS. If ultrasound is positive for IHPS in infants with non-bilious, projectile vomiting, the patient should be sent to the operating theatre. In case of a negative ultrasound, further work-up should be done to exclude other causes of IHPS. Further work-up may consist of clinical follow-up or repeated ultrasonography. Optionally contrast UGI series could be considered. However, in this review no sufficient evidence was found to substantiate this. Furthermore, pediatricians and pediatric surgeons should be aware that palpation of a pyloric ‘olive’ has limited sensitivity and that this appears to be worsening over time.
Contributor Information
Fenne AIM van den Bunder, Email: f.a.vandenbunder@amsterdamumc.nl.
Joep PM Derikx, Email: j.derikx@amsterdamumc.nl.
Rim Kiblawi, Email: Kiblawi.Rim@mh-hannover.de.
Rick R van Rijn, Email: r.r.vanrijn@amsterdamumc.nl.
Jens Dingemann, Email: Dingemann.Jens@mh-hannover.de.
REFERENCES
- 1. Jobson M, Hall NJ. Contemporary management of pyloric stenosis. Semin Pediatr Surg 2016; 25: 219–24. doi: 10.1053/j.sempedsurg.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 2. Peters B, Oomen MWN, Bakx R, Benninga MA. Advances in infantile hypertrophic pyloric stenosis. Expert Rev Gastroenterol Hepatol 2014; 8: 533–41. doi: 10.1586/17474124.2014.903799 [DOI] [PubMed] [Google Scholar]
- 3. Zhu J, Zhu T, Lin Z, Qu Y, Mu D. Perinatal risk factors for infantile hypertrophic pyloric stenosis: a meta-analysis. J Pediatr Surg 2017; 52: 1389–97. doi: 10.1016/j.jpedsurg.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 4. Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg 2007; 16: 27–33. doi: 10.1053/j.sempedsurg.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 5. Pandya S, Heiss K. Pyloric stenosis in pediatric surgery: an evidence-based review. Surg Clin North Am 2012; 92: 527–39. doi: 10.1016/j.suc.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 6. Raske ME, Dempsey ME, Dillman JR, Dory CE, Garber M, Hayes LL, et al. ACR appropriateness criteria vomiting in infants up to 3 months of age. J Am Coll Radiol 2015; 12: 915–22. doi: 10.1016/j.jacr.2015.05.023 [DOI] [PubMed] [Google Scholar]
- 7. Glatstein M, Carbell G, Boddu SK, Bernardini A, Scolnik D. The changing clinical presentation of hypertrophic pyloric stenosis: the experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila) 2011; 50: 192–95. doi: 10.1177/0009922810384846 [DOI] [PubMed] [Google Scholar]
- 8. Teele RL, Smith EH. Ultrasound in the diagnosis of idiopathic hypertrophic pyloric stenosis. N Engl J Med 1977; 296: 1149–50. doi: 10.1056/NEJM197705192962006 [DOI] [PubMed] [Google Scholar]
- 9. Cremin BJ, Klein A. Infantile pyloric stenosis: a 10-year survey. S Afr Med J 1968; 42: 1056–60. [PubMed] [Google Scholar]
- 10. Macdessi J, Oates RK. Clinical diagnosis of pyloric stenosis: a declining art. BMJ 1993; 306: 553–55. doi: 10.1136/bmj.306.6877.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breaux CW, Georgeson KE, Royal SA, Curnow AJ. Changing patterns in the diagnosis of hypertrophic pyloric stenosis. Pediatrics 1988; 81: 213–17. doi: 10.1542/peds.81.2.213 [DOI] [PubMed] [Google Scholar]
- 12. Tomaselli V, Colombo C, Fagnani AM. Reliability of ultrasound for the diagnosis of hypertrophic pyloric stenosis. J Pediatr Gastroenterol Nutr 1984; 3: 539–44. doi: 10.1097/00005176-198409000-00011 [DOI] [PubMed] [Google Scholar]
- 13. Mollitt DL, Golladay ES, Williamson S, Seibert JJ, Sutterfield SL. Ultrasonography in the diagnosis of pyloric stenosis. South Med J 1987; 80: 47–50. doi: 10.1097/00007611-198701000-00012 [DOI] [PubMed] [Google Scholar]
- 14. Forman HP, Leonidas JC, Kronfeld GD. A rational approach to the diagnosis of hypertrophic pyloric stenosis: do the results match the claims? J Pediatr Surg 1990; 25: 262–66. doi: 10.1016/0022-3468(90)90436-d [DOI] [PubMed] [Google Scholar]
- 15. Rollins MD, Shields MD, Quinn RJ, Wooldridge MA. Value of ultrasound in differentiating causes of persistent vomiting in infants. Gut 1991; 32: 612–14. doi: 10.1136/gut.32.6.612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Backer A, Bové T, Vandenplas Y, Peeters S, Deconinck P. Contribution of endoscopy to early diagnosis of hypertrophic pyloric stenosis. J Pediatr Gastroenterol Nutr 1994; 18: 78–81. doi: 10.1097/00005176-199401000-00013 [DOI] [PubMed] [Google Scholar]
- 17. Muramori K, Nagasaki A, Kawanami T. Ultrasonographic serial measurements of the morphologic resolution of the pylorus after ramstedt pyloromyotomy for infantile hypertrophic pyloric stenosis. J Ultrasound Med 2007; 26: 1681–87. doi: 10.7863/jum.2007.26.12.1681 [DOI] [PubMed] [Google Scholar]
- 18. Shaoul R, Enav B, Steiner Z, Mogilner J, Jaffe M. Clinical presentation of pyloric stenosis: the change is in our hands. Isr Med Assoc J 2004; 6: 134–37. [PubMed] [Google Scholar]
- 19. Ozsvath RR, Poustchi-Amin M, Leonidas JC, Elkowitz SS. Pyloric volume: an important factor in the surgeon’s ability to palpate the pyloric “olive” in hypertrophic pyloric stenosis. Pediatr Radiol 1997; 27: 175–77. doi: 10.1007/s002470050094 [DOI] [PubMed] [Google Scholar]
- 20. Godbole P, Sprigg A, Dickson JA, Lin PC. Ultrasound compared with clinical examination in infantile hypertrophic pyloric stenosis. Arch Dis Child 1996; 75: 335–37. doi: 10.1136/adc.75.4.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White MC, Langer JC, Don S, DeBaun MR. Sensitivity and cost minimization analysis of radiology versus olive palpation for the diagnosis of hypertrophic pyloric stenosis. J Pediatr Surg 1998; 33: 913–17. doi: 10.1016/s0022-3468(98)90673-x [DOI] [PubMed] [Google Scholar]
- 22. Gotley LM, Blanch A, Kimble R, Frawley K, Acworth JP. Pyloric stenosis: a retrospective study of an australian population. Emerg Med Australas 2009; 21: 407–13. doi: 10.1111/j.1742-6723.2009.01218.x [DOI] [PubMed] [Google Scholar]
- 23. Frković M, Kuhar M, Perhoč Ž, Barbarić-Babić V, Molnar M, Vuković Jurica. Diagnostic imaging of hypertrophic pyloric stenosis. Radiol Oncol 2001; 35: 11–16. [Google Scholar]
- 24. Bakal U, Sarac M, Aydin M, Tartar T, Kazez A. Recent changes in the features of hypertrophic pyloric stenosis. Pediatr Int 2016; 58: 369–71. doi: 10.1111/ped.12860 [DOI] [PubMed] [Google Scholar]
- 25. Helton KJ, Strife JL, Warner BW, Byczkowski TL, Donovan EF. The impact of a clinical guideline on imaging children with hypertrophic pyloric stenosis. Pediatr Radiol 2004; 34: 733–36. doi: 10.1007/s00247-004-1255-z [DOI] [PubMed] [Google Scholar]
- 26. Mullassery D, Mallappa S, Shariff R, Craigie RJ, Losty PD, Kenny SE, et al. Negative exploration for pyloric stenosis--is it preventable? BMC Pediatr 2008; 8: 37. doi: 10.1186/1471-2431-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leaphart CL, Borland K, Kane TD, Hackam DJ. Hypertrophic pyloric stenosis in newborns younger than 21 days: remodeling the path of surgical intervention. J Pediatr Surg 2008; 43: 998–1001. doi: 10.1016/j.jpedsurg.2008.02.022 [DOI] [PubMed] [Google Scholar]
- 28. Khatami A, Ghoroubi J, Imanzadeh F, Attaran F, Mehrafarin M, Palpation SMRO. Sonography and barium study in the diagnosis of hypertrophic pyloric stenosis: decline in physicians. Art Iran J Radiol 2009; 6: 87–91. [Google Scholar]
- 29. Glatstein M, Carbell G, Boddu SK, Bernardini A, Scolnik D. The changing clinical presentation of hypertrophic pyloric stenosis: the experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila) 2011; 50: 192–95. doi: 10.1177/0009922810384846 [DOI] [PubMed] [Google Scholar]
- 30. Malcom GE, Raio CC, Del Rios M, Blaivas M, Tsung JW. Feasibility of emergency physician diagnosis of hypertrophic pyloric stenosis using point-of-care ultrasound: a multi-center case series. J Emerg Med 2009; 37: 283–86. doi: 10.1016/j.jemermed.2007.11.053 [DOI] [PubMed] [Google Scholar]
- 31. Sivitz AB, Tejani C, Cohen SG. Evaluation of hypertrophic pyloric stenosis by pediatric emergency physician sonography. Acad Emerg Med 2013; 20: 646–51. doi: 10.1111/acem.12163 [DOI] [PubMed] [Google Scholar]
- 32. O’Keeffe FN, Stansberry SD, Swischuk LE, Hayden CK Jr. Antropyloric muscle thickness at US in infants: what is normal? Radiology 1991; 178: 827–30. doi: 10.1148/radiology.178.3.1994426 [DOI] [PubMed] [Google Scholar]
- 33. Hernanz-Schulman M. Pyloric stenosis: role of imaging. Pediatr Radiol 2009; 39 Suppl 2: S134–9. doi: 10.1007/s00247-008-1106-4 [DOI] [PubMed] [Google Scholar]
- 34. Hallam D, Hansen B, Bødker B, Klintorp S, Pedersen JF. Pyloric size in normal infants and in infants suspected of having hypertrophic pyloric stenosis. Acta Radiol 1995; 36: 261–64. doi: 10.1177/028418519503600309 [DOI] [PubMed] [Google Scholar]
- 35. Rohrschneider WK, Mittnacht H, Darge K, Tröger J. Pyloric muscle in asymptomatic infants: sonographic evaluation and discrimination from idiopathic hypertrophic pyloric stenosis. Pediatr Radiol 1998; 28: 429–34. doi: 10.1007/s002470050377 [DOI] [PubMed] [Google Scholar]
- 36. Lowe LH, Banks WJ, Shyr Y. Pyloric ratio: efficacy in the diagnosis of hypertrophic pyloric stenosis. J Ultrasound Med 1999; 18: 773–77. doi: 10.7863/jum.1999.18.11.773 [DOI] [PubMed] [Google Scholar]
- 37. Forster N, Haddad RL, Choroomi S, Dilley AV, Pereira J. Use of ultrasound in 187 infants with suspected infantile hypertrophic pyloric stenosis. Australas Radiol 2007; 51: 560–63. doi: 10.1111/j.1440-1673.2007.01872.x [DOI] [PubMed] [Google Scholar]
- 38. Alehossein . The validity of ultrasound diagnosing hypertrophic pyloric stenosis. Pak K Med Sci 2009. [Google Scholar]
- 39. Iqbal CW, Rivard DC, Mortellaro VE, Sharp SW, St Peter SD. Evaluation of ultrasonographic parameters in the diagnosis of pyloric stenosis relative to patient age and size. J Pediatr Surg 2012; 47: 1542–47. doi: 10.1016/j.jpedsurg.2012.03.068 [DOI] [PubMed] [Google Scholar]
- 40. Tunell WP, Wilson DA. Pyloric stenosis: diagnosis by real time sonography, the pyloric muscle length method. J Pediatr Surg 1984; 19: 795–99. doi: 10.1016/s0022-3468(84)80371-1 [DOI] [PubMed] [Google Scholar]
- 41. Wilson DA, Vanhoutte JJ. The reliable sonographic diagnosis of hypertrophic pyloric stenosis. J Clin Ultrasound 1984; 12: 201–4. doi: 10.1002/jcu.1870120406 [DOI] [PubMed] [Google Scholar]
- 42. Cohen HL, Schechter S, Mestel AL, Eaton DH, Haller JO. Ultrasonic “double track” sign in hypertrophic pyloric stenosis. J Ultrasound Med 1987; 6: 139–43. doi: 10.7863/jum.1987.6.3.139 [DOI] [PubMed] [Google Scholar]
- 43. Westra SJ, de Groot CJ, Smits NJ, Staalman CR. Hypertrophic pyloric stenosis: use of the pyloric volume measurement in early US diagnosis. Radiology 1989; 172: 615–19. doi: 10.1148/radiology.172.3.2672088 [DOI] [PubMed] [Google Scholar]
- 44. Ito S, Tamura K, Nagae I, Yagyu M, Tanabe Y, Aoki T, et al. Ultrasonographic diagnosis criteria using scoring for hypertrophic pyloric stenosis. J Pediatr Surg 2000; 35: 1714–18. doi: 10.1053/jpsu.2000.19220 [DOI] [PubMed] [Google Scholar]
- 45. Neilson D, Hollman AS. The ultrasonic diagnosis of infantile hypertrophic pyloric stenosis: technique and accuracy. Clin Radiol 1994; 49: 246–47. doi: 10.1016/s0009-9260(05)81849-5 [DOI] [PubMed] [Google Scholar]
- 46. Niedzielski J, Kobielski A, Sokal J, Krakós M. Accuracy of sonographic criteria in the decision for surgical treatment in infantile hypertrophic pyloric stenosis. Arch Med Sci 2011; 7: 508–11. doi: 10.5114/aoms.2011.23419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Misra D, Akhter A, Potts SR, Brown S, Boston VE. Pyloric stenosis: is over-reliance on ultrasound scans leading to negative explorations? Eur J Pediatr Surg 1997; 7: 328–30. doi: 10.1055/s-2008-1071185 [DOI] [PubMed] [Google Scholar]
- 48. Boneti C, McVay MR, Kokoska ER, Jackson RJ, Smith SD. Ultrasound as a diagnostic tool used by surgeons in pyloric stenosis. J Pediatr Surg 2008; 43: 87–91. doi: 10.1016/j.jpedsurg.2007.09.027 [DOI] [PubMed] [Google Scholar]
- 49. Copeland DR, Cosper GH, McMahon LE, Boneti C, Little DC, Dassinger MS, et al. Return of the surgeon in the diagnosis of pyloric stenosis. J Pediatr Surg 2009; 44: 1189–92. doi: 10.1016/j.jpedsurg.2009.02.025 [DOI] [PubMed] [Google Scholar]
- 50. McVay MR, Copeland DR, McMahon LE, Cosper GH, McCallie TG, Kokoska ER, et al. Surgeon-performed ultrasound for diagnosis of pyloric stenosis is accurate, reproducible, and clinically valuable. J Pediatr Surg 2009; 44: 169–71. doi: 10.1016/j.jpedsurg.2008.10.028 [DOI] [PubMed] [Google Scholar]
- 51. Vinycomb T, Vanhaltren K, Pacilli M, Ditchfield M, Nataraja RM. Evaluating the validity of ultrasound in diagnosing hypertrophic pyloric stenosis: a cross-sectional diagnostic accuracy study. ANZ J Surg 2021; 91: 2507–13. doi: 10.1111/ans.17247 [DOI] [PubMed] [Google Scholar]
- 52. Carver RA, Okorie M, Steiner GM, Dickson JA. Infantile hypertrophic pyloric stenosis--diagnosis from the pyloric muscle index. Clin Radiol 1987; 38: 625–27. doi: 10.1016/s0009-9260(87)80342-2 [DOI] [PubMed] [Google Scholar]
- 53. Vinycomb TI, Laslett K, Gwini SM, Teague W, Nataraja RM. Presentation and outcomes in hypertrophic pyloric stenosis: an 11-year review. J Paediatr Child Health 2019; 55: 1183–87. doi: 10.1111/jpc.14372 [DOI] [PubMed] [Google Scholar]
- 54. Hernanz-Schulman M, Dinauer P, Ambrosino MM, Polk DB, Neblett WW. The antral nipple sign of pyloric mucosal prolapse: endoscopic correlation of a new sonographic observation in patients with pyloric stenosis. J Ultrasound Med 1995; 14: 283–87. doi: 10.7863/jum.1995.14.4.283 [DOI] [PubMed] [Google Scholar]
- 55. Calle-Toro JS, Kaplan SL, Andronikou S. Are we performing ultrasound measurements of the wall thickness in hypertrophic pyloric stenosis studies the same way? Pediatr Surg Int 2020; 36: 399–405. doi: 10.1007/s00383-019-04601-2 [DOI] [PubMed] [Google Scholar]
- 56. Haider N, Spicer R, Grier D. Ultrasound diagnosis of infantile hypertrophic pyloric stenosis: determinants of pyloric length and the effect of prematurity. Clin Radiol 2002; 57: 136–39. doi: 10.1053/crad.2001.0853 [DOI] [PubMed] [Google Scholar]
- 57. Cascio S, Steven M, Livingstone H, Young D, Carachi R. Hypertrophic pyloric stenosis in premature infants: evaluation of sonographic criteria and short-term outcomes. Pediatr Surg Int 2013; 29: 697–702. doi: 10.1007/s00383-013-3324-6 [DOI] [PubMed] [Google Scholar]
- 58. Riggs W, Long L. The value of the plain film roentgenogram in pyloric stenosis. Am J Roentgenol Radium Ther Nucl Med 1971; 112: 77–82. doi: 10.2214/ajr.112.1.77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.