Abstract
Objectives:
Splenic switch-off (SSO) is a validated indicator of adequate vasodilator stress unique to adenosine stress cardiac MR (CMR). Patients in atrial fibrillation (AF) may have a reduced adenosine response due to lower hyperaemic coronary flow reserve and may achieve SSO less frequently versus sinus rhythm (SR).
Methods:
1100 stress CMR studies were identified from a clinical CMR database (2016–2021). 70 patients in AF were propensity score matched to a SR group for age, sex, and body mass index. The adenosine dose administered, symptoms, heart-rate change and scan result were recorded. SSO was evaluated subjectively and semi-quantitatively via changes in splenic and myocardial signal intensity (SI) from rest to stress.
Results:
SSO occurred significantly less frequently in AF than SR (34/70 [49%] vs 53/70 [76%], p = 0.003). Semi-quantitative assessment supported this, with a smaller splenic SI difference between stress and rest in AF vs SR (median splenic stress:rest peak SI ratio 0.92 [IQR:0.61–1.11] vs 0.56 [IQR:0.45–0.75], p < 0.001). A heart-rate increase >10 bpm predicted visual SSO in SR but not AF. Fewer patients in AF than SR had inducible ischaemia (9/70 [13%] vs 17/69 [25%], p = 0.058). This difference was not driven by inducible ischaemia rates in patients who did not achieve SSO (6/36 [17%] AF vs 4/17 [24%] SR, p = 0.403).
Conclusions:
SSO occurs significantly less frequently with AF. This may risk the under diagnosis of inducible ischaemia and requires further assessment.
Advances in knowledge:
SSO, a validated marker of adequate stress in CMR, occurs significantly less frequently in the presence of AF, risking a suboptimal functional assessment of coronary disease.
Introduction
Vasodilator stress cardiac magnetic resonance (CMR) imaging is an important diagnostic tool to evaluate myocardial perfusion in the investigative work-up of suspected stable angina. Functional imaging has a class one indication in current European Society of Cardiology guidelines for the investigation of chronic coronary syndrome, either as the initial test or in the further assessment of coronary artery disease (CAD) of unclear significance identified via CT coronary angiography. 1 Similarly in the UK, National Institute for Health and Care Excellence guidelines recommend non-invasive functional imaging as the first-line investigation to assess the potential ischaemic burden of any patient with a non-diagnostic CT coronary angiogram or confirmed CAD. 2 Stress CMR is an excellent option to achieve this goal, having been shown to reduce the frequency of unnecessary angiography with no change in the occurrence of major adverse cardiovascular events, 3 therefore reducing risk for patients without compromising safety.
In stress CMR, a variety of vasodilator agents (e.g., adenosine, regadenoson, dipyridamole) can be used to achieve hyperaemia and thus induce ‘stress’ via coronary steal phenomenon. Among the various agents, adenosine is commonly used. Adenosine is administered at 140mcg/kg/min which induces coronary vasodilatation via the activation of A2A receptors. 4 Haemodynamic response (heart-rate (HR) increase over 10bpm) and the subjective presence of symptoms have traditionally been accepted as markers of stress adequacy, but neither are significantly related to adequate myocardial vasodilator response and coronary hyperaemia. 5,6
Adenosine infusion results in A1 A2B receptor mediated splenic vasoconstriction, 7 giving an attenuation of splenic perfusion, observed as reduced signal intensity on CMR, in the ‘stress’ state. This ‘splenic switch-off’ (SSO) has been validated as an additional objective marker of adequate stress response to adenosine and can be used to identify false-negative results in stress CMR. 8 This finding was not observed with other agents (regadenoson, dobutamine).
Atrial fibrillation (AF) is the most common arrhythmia, 9 and there is a high prevalence of CAD amongst AF patients (17–47%). 10 Anecdotally stress CMR is less reliable for the assessment of inducible ischaemia in AF patients and previous studies demonstrate a reduced diagnostic performance for the detection of coronary stenosis, 11 potentially due to a reduced hyperaemic coronary flow reserve. 12 Therefore, we hypothesised that patients in AF may achieve SSO less frequently than those in sinus rhythm (SR) and assessed this with a propensity matched study.
Methods
Population
1100 adenosine stress CMR studies were retrospectively identified from a prospectively maintained clinical CMR database over a 5-year period (2016–2021). Approval for retrospective analysis of CMRs acquired for routine clinical practice was granted by our institution’s Trust Audit Committee and informed written consent was not required.
Cardiac rhythm on 12-lead ECG performed immediately before CMR was recorded along with the patient’s body mass index (BMI) and adenosine protocol. Scans were excluded if: an alternative agent was used, patients were in atrial flutter, unable to identify rhythm, a duplicate patient (i.e., re-scanned patient), the spleen was inadequately visualised or there was an incomplete dataset (i.e., no rest images). This left a total study population of 916 patients (845 SR, 71 AF) as outlined in Figure 1.
Figure 1.
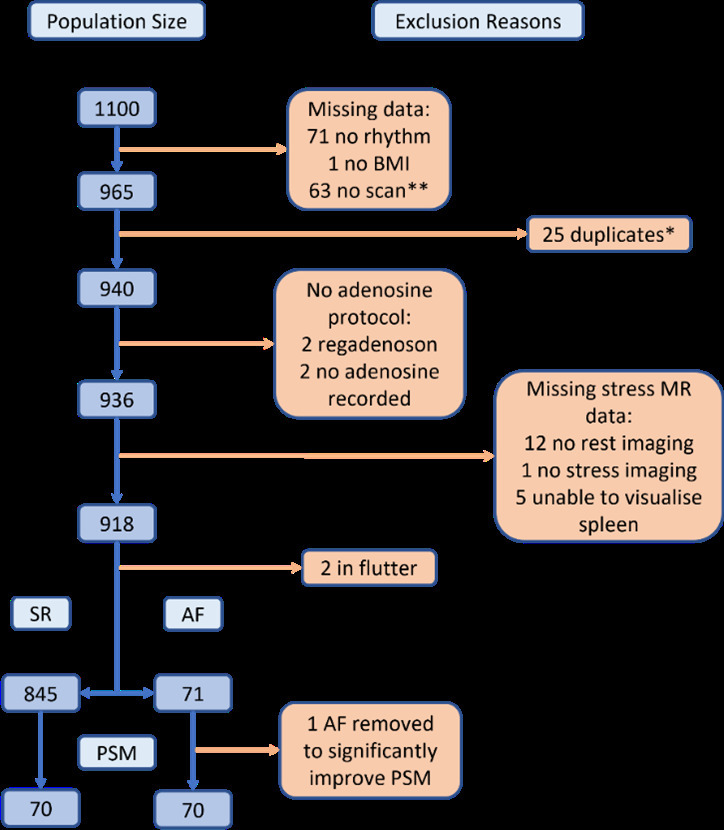
Study Flowchart. * Patients with greater than one scan during the study period. ** Breakdown of reasons for no scan: Claustrophobic (4), recent steroids (1), recent caffeine (4), abandoned (3), hypotensive (3), unable to tolerate symptoms (10), cancelled (1), low eGFR (2), asthma (1), body habitus (2), CMR not required (1), tachycardic (4), admitted for CP (1), bradycardic (2), dropped oxygen saturations (1), ECG rhythm (3), pacemaker (1), incorrectly coded (1), no reason given (18).
Propensity score matching
This population was poorly matched for age and sex between AF and SR groups (mean age: 72 ± 8 years vs 63 ± 12 years, p < 0.001; %male: 86 vs 62%, p < 0.001; BMI: 29.6 ± 5.0 vs 28.9 ± 5.3, p = 0.30). Therefore, propensity score matching (PSM) was performed to minimise the impact of these confounding variables. The data were randomised to avoid selection bias towards the earlier studies, before SR and AF groups were matched for age, sex, and BMI through the calculation of propensity scores. 1 AF patient outlier was excluded during this process to markedly reduce the probability of significant differences in matching, leaving a final cohort of 140 matched patients. Data were collected for comorbidities, chest pain presence and typicality, medication history, and mortality.
Stress CMR and visual splenic analysis
CMRs were performed at 1.5 T (Avanto Fit, Siemens, Germany). Adenosine was administered at 140 (micrograms/kg)/min and increased incrementally if the patient had no symptoms or inadequate heart rate response, with the contrast agent gadobutrol (Gadovist, Bayer Pharma AG, Germany) administered intravenously at 0.1 mmol/kg. First-pass perfusion imaging was performed every cardiac cycle by using a T 1-weighted saturation recovery gradient-echo sequence with fast low-angle shot readout with stress and after 15–20 min of recovery (i.e., at rest). Further acquisition protocol data are available in the electronic Supplementary Table 1.
For each stress CMR, baseline and maximal HR was recorded from documentation at the time of scan, and the difference calculated. The maximum dose of adenosine infusion was also noted, along with the presence of resulting symptoms (chest pain and dyspnoea). Intracardiac measurements, including left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume index, and left atrium volume index (LAVI), were recorded from documentation at the time of the scan. The presence of inducible ischaemia reported by level 3 CMR reader at the time of study was recorded, and the prevalence compared between patient groups. Performance of invasive coronary angiography and coronary intervention undertaken within the following 12 months was recorded for all patients.
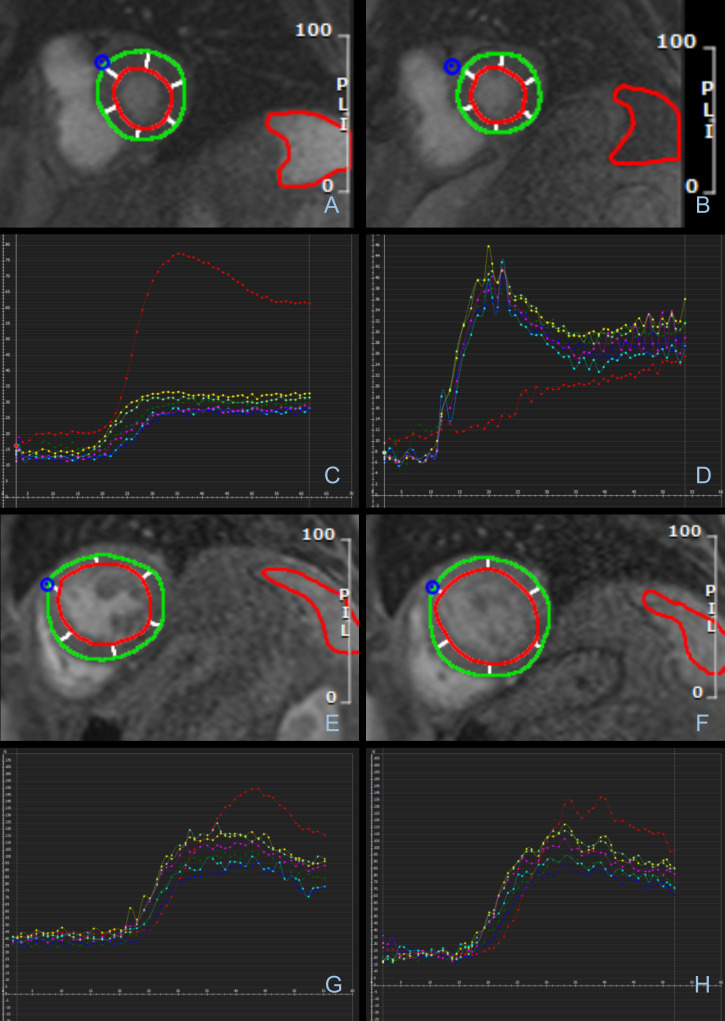
Splenic perfusion was visually assessed on all stress CMR images following the same criteria as previously reported, unblinded to rhythm. 8 SSO was recorded as either achieved (visually reduced splenic signal intensity at stress compared to rest), or not achieved (visually similar splenic signal intensity at stress and rest). Examples of both are provided in Figure 2.
Figure 2.
Splenic Switch-Off on Stress CMR. (A-D) Stress CMR images showing splenic switch-off in a 75-year-old male in sinus rhythm. Splenic signal intensity (SI) is clearly visually reduced at stress. This is confirmed in the corresponding graphical representation of tissue SI against time. Red line indicates splenic SI. Other lines each represent SI of a myocardial segment. (A) Rest image B) Stress image C) Rest graph D) Stress graph. (E-F) Stress CMR images showing failed splenic-switch off in a 68-year-old male with AF. (E) Rest maximum splenic SI F) Stress maximum splenic SI. (G-H) Graphical representation of tissue perfusion on stress CMR showing failed splenic switch-off in a 72-year-old male with AF. (G) Rest H) Stress.
Semi-Quantitative splenic analysis
Semi-quantitative analysis was performed on whichever basal, mid, or apical perfusion slices demonstrated both the myocardium and spleen optimally. Using dedicated post-processing tools (cvi42 v.5.13, Circle Cardiovascular Imaging Inc, Calgary), splenic and myocardial borders were contoured for each frame of imaging generating signal intensity curves. Maximal and minimal splenic and myocardial signal intensities were recorded. The mean signal intensity across all myocardial segments was used to average out any motion artefact and volume averaging of the myocardium and blood pool.
Peak signal intensity, for the spleen and myocardium at rest and stress, was defined as the difference between maximal and minimal signal intensity as per previous studies. 8 To allow comparison between studies, we calculated splenic and myocardial percentage drops, defined as the stress:rest peak signal intensity ratio.
To assess the potential for global hypoperfusion impacting study findings, we also calculated the spleen:myocardial peak signal intensity ratio at rest and stress, and finally the stress:rest ratio of spleen:myocardial peak signal intensity (Table 1).
Table 1.
Quantitative Calculations Example – 81-year-old female in SR
| Rest SIa | Stress SI | ||
|---|---|---|---|
| Myocardium | Minimum | 15.72 | 7.12 |
| Maximum | 40.69 | 44.23 | |
| Peakb | 24.97 (A) | 37.10 (B) | |
| Splenic | Minimum | 9.64 | 8.47 |
| Maximum | 86.86 | 43.40 | |
| Peak | 77.22 (C) | 34.92 (D) | |
| Calculation | Value | ||
| Spleen Stress:Rest | D/C | 0.45 | |
| Myocardial Stress:Rest | B/A | 1.49 | |
| Rest Spleen:Myocardium | C/A | 3.09 (E) | |
| Stress Spleen:Myocardium | D/B | 0.94 (F) | |
| Stress:Rest Spleen:Myocardium | F/E | 0.30 | |
SI = Signal Intensity.
Peak = Maximum – Minimum.
Invasive angiography and outcomes
Rates of invasive coronary angiography in the 12-month post-stress CMR were recorded, alongside subsequent myocardial infarction (MI), all-cause mortality, and further coronary imaging assessments out to 3-year post-imaging.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 27. Data were treated as paired in line with the literature for the management of propensity matched data, 13 and the dichotomous variable of visual SSO was assessed with a McNemar test with the null hypothesis of ‘marginal homogeneity’, i.e. that the frequency distribution of SSO is equal between the AF and SR groups. 14
For the semi-quantitative analysis, we calculated median stress:rest ratios in splenic and spleen:myocardial peak signal intensity for the SR and AF groups. Data were tested for normality with Shapiro-Wilk testing and non-parametric data were managed with Wilcoxon signed-rank testing. 15 A p-value of <0.05 was taken to be significant.
To identify predictors of SSO in each rhythm group, univariate followed by multivariate logistic regression analysis was undertaken. Significance was set at p < 0.25 and p < 0.05, respectively. This pre-selection strategy with a larger p-value allows for the reduction of the number of variables in the model whilst reducing the risk of excluding important variables. 16 Variables for which we propensity matched were included in the regression analysis. 17 To account for correlation between matched pairs, we used a repeated measures design when performing logistic regression on the combined cohort.
Inducible ischaemia rates between subgroups of heart rhythm and visual SSO were assessed with Fisher’s exact test (1-tailed), suitable for low sample sizes. 18
Results
The final study population (presented in Table 2) consisted of 70 patients in the AF group and 70 matched SR patients (mean age: 72 ± 8 years vs 72 ± 9 years, p = 0.96; %male 86 vs 84%, p = 0.81). In keeping with the PSM process the cohorts were well matched for age, sex and comorbidities, with between group differences including presence of chest pain, baseline HR and haemodynamic response to adenosine, maximum adenosine dose administered, LVEF and LAVI.
Table 2.
Patient Characteristics – CAD Risk Factors and Stress CMR Indication Details
| AF Group (N = 70) | SR Group (N = 70) | p-values | |
|---|---|---|---|
| Age – Median (IQR) | 73 (68–78) | 74 (67–79) | |
| Sex (%) | |||
| Male | 60 (85.7) | 59 (84.3) | |
| Female | 10 (14.3) | 11 (15.7) | |
| Diabetes (%) | 16 (22.9) | 15 (21.4) | 0.999 |
| Hypertension (%) | 38 (54.3) | 38 (54.3) | 0.999 |
| Smoking History (%) | |||
| Current Smoker | 3 (4.3) | 6 (8.6) | |
| Ex-smoker | 18 (25.7) | 18 (25.7) | |
| Non-smoker | 29 (41.4) | 37 (52.9) | |
| Missing Data | 20 (28.6) | 9 (12.9) | |
| Obesity (%)a | 27 (38.6) | 27 (38.6) | 0.999 |
| Dyslipidaemia (%) | 49 (70.0) | 54 (77.1) | 0.458 |
| Coronary Artery Disease (%) | 45 (64.3) | 46 (65.7) | 0.999 |
| Ischaemic Heart Disease (%) | 27 (38.6) | 22 (31.4) | 0.511 |
| Chest Pain (%) | 27 (38.6) | 42 (60.0) | 0.024* |
| Chest Pain Type (%)b | |||
| Typical | 13 (18.6) | 16 (22.9) | |
| Atypical | 5 (7.1) | 12 (17.1) | |
| Non-anginal | 9 (12.9) | 14 (20.0) | |
| Rate-control Drugs (%)c | 54 (77.1) | 42 (60.0) | 0.052 |
| Heart Rate - Median (IQR) Baseline Maximum Increase |
68 (61–74) 80 (72–88) 11 (8–19) |
61 (56–67) 80 (74–86) 19 (14–25) |
0.005* 0.698 <0.001* |
| Max. Adenosine Dose (%) 140 mcg/kg/min 180 mcg/kg/min 210 mcg/kg/min |
24 (34.3) 19 (27.1) 27 (38.6) |
42 (61.8) 17 (25.0) 9 (13.2) |
<0.001* |
| Intracardiac Measurements - Median (IQR) LVEF (%)d LV EDVIe LAVIf |
49 (39–54) 76 (65–100) 18 (14–21) |
55 (49–62) 71 (64–89) 11 (9–13) |
<0.001* 0.085 <0.001* |
| Symptoms During CMR (%) | 57 (82.6) | 63 (92.6) | 0.118 |
| Mortality (%)g | 6 (8.6) | 3 (4.3) | 0.453 |
Obesity defined as BMI>30.
NICE typical, atypical, and non-anginal definitions.1
Rate-control drugs recorded include β blockers, non-dihydropyridine calcium channel blockers, digoxin, and amiodarone.
Left ventricular ejection fraction
Left ventricular end-diastolic volume index (ml/m2)
Left atrium volume index (cm2/m2)
Between date of scan and study
Visual splenic analysis
SSO occurred significantly less frequently in AF than SR patients (49% vs 76%, X 2 (N = 70)=8.31, p = 0.003 (2-tailed)).
Semi-Quantitative analysis
The difference between splenic stress:rest peak signal intensity ratio in the AF cohort and SR cohort was statistically significant (median = 0.92 [IQR, 0.61–1.11] vs 0.56 [IQR, 0.45–0.75], Z = −3.99, p < 0.001), as presented in Table 3. This demonstrates a smaller difference in splenic perfusion between stress and rest in AF patients than those in SR.
Table 3.
Signal intensity ratio data
| AF Group (N = 70) | SR Group (N = 70) | p-values | |
|---|---|---|---|
| Spleen Stress:Rest | 0.92 [0.61–1.11]a | 0.56 [0.45–0.75] | <0.001 |
| Myocardial Stress:Rest | 1.43 [1.28–1.54] | 1.34 [1.15–1.50] | 0.144 |
| Rest Spleen:Myocardium | 1.35 [1.04–1.69] | 1.60 [1.34–1.90] | 0.006 |
| Stress Spleen:Myocardium | 0.79 [0.56–1.17] | 0.73 [0.56–0.94] | 0.119 |
| Stress:Rest Spleen:Myocardium | 0.64 [0.45–0.85] | 0.42 [0.33–0.60] | <0.001 |
All data are median [IQR].
The stress:rest ratio of spleen:myocardial peak signal intensity was also significantly higher in AF than SR patients (median = 0.64 [IQR, 0.45–0.85] vs 0.42 [IQR, 0.33–0.60], Z = −3.93, p < 0.001).
Predictors of visual splenic Switch-Off
In the AF group, multivariate analysis demonstrated that being female was a significant predictor of SSO (OR 7.67, 95th CI [1.28–45.90], p = 0.026).
In the SR group, increasing age was shown to be a significant predictor of SSO (OR 1.10, 95th CI [1.02–1.17], p = 0.010). A HR increase over 10bpm in response to adenosine was also shown to be a significant predictor (OR 13.06, 95th CI [1.65–103.35], p = 0.015) in addition to the absence of hypertension (OR 0.24, 95th CI [0.06–0.98], p = 0.046). This was in the context of a lower rate of AF patients achieving a > 10 bpm increment compared to the SR group despite significantly higher doses of adenosine used in the AF cohort (Table 2).
As a combined cohort, a HR increase over 10 bpm in response to adenosine was shown to be a significant predictor of SSO. Furthermore, being female, and the absence of hypertension were also predictors of SSO, as presented in Table 4.
Table 4.
Logistic regression analysis assessing factors impacting the presence of splenic switch-off in each cohort separately and when combined
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| N | OR (95% CI) | p-value | N | OR (95% CI) | p-value | ||
| AF | |||||||
| Age | 70 | 1.00 (0.94–1.06) | 0.962 | ||||
| Sex (F = 1) | 70 | 5.23 (1.02–26.74) | 0.047* | 69 | 7.67 (1.28–45.90) | 0.026** | |
| Hypertension | 70 | 0.57 (0.22–1.46) | 0.240* | 69 | 0.35 (0.11–1.15) | 0.084 | |
| Dyslipidaemia | 70 | 2.46 (0.84–7.14) | 0.099* | 69 | 4.88 (1.28–18.65) | 0.021** | |
| Haem. Responsee | 69 | 1.85 (0.70–5.13) | 0.236* | 69 | 1.59 (0.49–5.10) | 0.439 | |
| Symptoms | 69 | 2.22 (0.60–8.22) | 0.231* | 69 | 2.39 (0.51–11.24) | 0.271 | |
| Max. Adenosinef | 70 | 0.574 | |||||
| SR | |||||||
| Age | 70 | 1.07 (1.01–1.13) | 0.030* | 68 | 1.10 (1.02–1.17) | 0.010** | |
| Sex | 70 | 3.72 (0.44–31.45) | 0.228* | 68 | 9.30 (0.60–144.53) | 0.111 | |
| Hypertension | 70 | 0.40 (0.12–1.30) | 0.127* | 68 | 0.24 (0.06–0.98) | 0.046** | |
| Dyslipidaemia | 70 | 1.59 (0.46–5.48) | 0.462 | ||||
| Haem. Response | 68 | 3.62 (0.79–16.46) | 0.097* | 68 | 13.06 (1.65–103.35) | 0.015** | |
| Symptoms | 68 | 0.00 (0.00) | 0.999 | ||||
| Max. Adenosine | 68 | 0.352 | |||||
| All patients | |||||||
| Age | 140 | 1.03 (0.99–1.07) | 0.146* | 137 | 1.03 (0.99–1.08) | 0.148 | |
| Sex | 140 | 4.35 (1.20–15.72) | 0.025* | 137 | 7.15 (1.65–31.08) | 0.009** | |
| Hypertension | 140 | 0.52 (0.26–1.06) | 0.072* | 137 | 0.36 (0.15–0.84) | 0.018** | |
| Dyslipidaemia | 140 | 2.14 (0.99–4.62) | 0.054* | 137 | 3.03 (1.19–7.72) | 0.020** | |
| Haem. Response | 137 | 2.92 (1.28–6.70) | 0.012* | 137 | 3.07 (1.14–8.32) | 0.027** | |
| Symptoms | 137 | 1.53 (0.55–4.30) | 0.414 | ||||
| Max. Adenosine AF |
13,8140 | 0.30 (0.15–0.62) | 0.537 0.001* |
137 | 0.38 (0.17–0.85) | 0.018** | |
**p < 0.05.
*p < 0.25.
HR increase over 10bpm.
Maximum rate of adenosine infusion (mcg/kg/min).
Inducible ischaemia
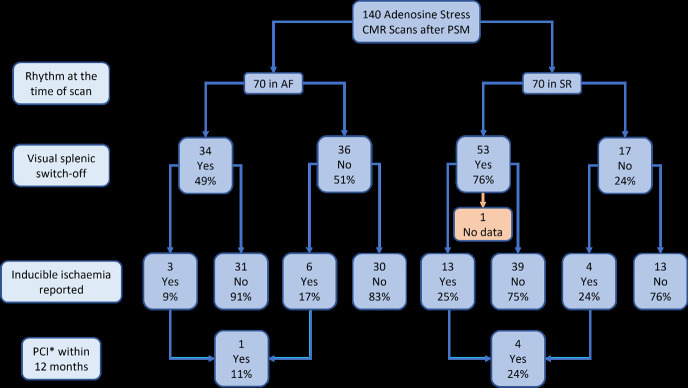
A lower proportion of AF patients demonstrated inducible ischaemia on adenosine stress CMR than SR patients (13% [9/70] vs 25% [17/69], p = 0.058), as seen in Figure 3.
Figure 3.
Inducible Ischaemia Frequency in Splenic Switch-Off. * Percutaneous coronary intervention.
Of those patients who did not achieve visual SSO, a non-significant lower proportion of AF patients had inducible ischemia than SR patients (17% [6/36] vs 24% [4/17], p = 0.403).
Invasive angiography and outcomes
In the AF cohort, 16% (11/70) patients underwent angiography within 12 months from their CMR, 78% (7/9) with inducible ischaemia and 71% (4/61) without. This compared with 11% (8/70) patients in the SR cohort, 41% (7/17) with inducible ischaemia and 2% (1/53) without. Of those with inducible ischaemia who underwent angiography, 14% (1/7) of AF patients had percutaneous coronary intervention (PCI) within the following 12 months compared to 57% (4/7) of SR patients. No patients without inducible ischaemia underwent PCI in either cohort.
After a median follow-up of 36 months (interquartile range 24–46), 10% (7/70) of patients in the AF cohort and 13% (9/70) in the SR cohorts had a subsequent assessment of coronary physiology (invasive or non-invasive). 31% (5/16) of those with repeat assessment did not achieve SSO at baseline stress CMR, and no new ischaemia was identified in either group.
Within the follow-up period post-CMR assessed, 3% (2/70) of patients in both the AF and the SR cohorts suffered a documented MI, both observed in those with SSO with event rate is too low to draw and conclusions. All-cause mortality was documented in 10% (7/70) in the AF group and 4% (3/70) in the SR group; however, there was no significant difference between those with SSO and those without (p = 0.8).
Discussion
In this single-centre retrospective propensity matched cohort study, we found that SSO is achieved significantly less frequently in patients with AF than those in SR. SSO has previously been validated as a marker of adequate stress, suggesting that a proportion of the AF population may not be achieving adequate physiological stress with current protocols.
Semi-quantitative analysis of splenic stress:rest peak signal intensity ratios supports the findings of visual assessment of SSO. The difference in splenic perfusion between stress and rest is 82% less in patients in AF than those in SR. This is corroborated by the lower spleen:myocardial signal intensity ratio between stress and rest in AF patients, which confirms that the difference is not a result of generalised hypoperfusion as the spleen dropped in signal intensity relative to the myocardium. A potential lack of adequate physiological stress risks an underdiagnosis of inducible ischaemia in patients with AF. However, whilst the presence of SSO was previously validated to represent adequate coronary adenosine-induced response, a recent study in fact demonstrated a low sensitivity for SSO prediction of coronary adenosine response based on comparative PET parameters. However, this study did not include any description of heart rhythm at the time of imaging, relevant given the clear distinction in SSO rates between SR and AF observed in our study cohorts, suggesting a larger, multicentre study is needed to further assess this. 19 Of note, in our study, the absence of SSO was not associated with an increase in adverse outcomes out to a median follow-up of 3 years.
A potential physiological mechanism for a reduced response to adenosine may relate to the increased expression of A2A receptors seen in patients with AF, 20 which when activated by endogenous adenosine leads to an increased intracellular release of calcium from the sarcoplasmic reticulum giving the effect of coronary smooth muscle vasoconstriction. 21,22 This may be the mechanism behind patients in AF having a raised coronary vascular resistance under hyperaemic conditions and a reduced hyperaemic coronary flow reserve, 12 hence limiting the potential response to exogenous adenosine administration.
The presence of SSO in published literature varies in frequency (72–90%), 8,19,23 which is comparable with our study population (76%). The median reduction in splenic signal intensity from rest to stress in our SR population (44% [IQR, 25–55%]) was lower than previously reported (median = 78% [IQR, 59–91%]). 8 However, the significant difference in the presence of SSO in AF patients remains clear.
Not all centres perform rest imaging, 7 and so SSO in these cases is less easily appreciated. Rest perfusion imaging should continue to be considered in the CMR protocol of all patients undergoing adenosine stress to enable objective assessment of splenic perfusion, particularly those in AF.
We assessed pre-existing comorbidities and physiological variables during the scan for their predictive value for SSO. For patients in SR, a HR increase over 10 bpm is a recognised predictor of SSO, but this widely accepted marker of adequate adenosine response did not have the same predictive value in our AF cohort. A potential confounder for this may be the expected increased use of rate-control medications in the AF population, although the difference in the numbers of patients on rate-control drugs between AF and SR groups did not reach statistical significance (p = 0.052). Additionally, higher doses of adenosine were required in the AF vs the SR cohort (Table 2). It is possible that AF patients received enhanced vasodilator stress by receiving higher doses of adenosine, meaning that the documented difference in rates of SSO may have been even larger if the standard 140 (micrograms/kg)/min for 3-min protocol was used for all patients. However, both the maximum adenosine dose infused and the presence of symptoms during the scan had no relation to SSO occurrence in either cohort, supporting the conclusion that they are not markers of adequate stress. It is therefore also plausible that a proportion of AF patients simply do not respond to adenosine regardless of the dose, in which case the dose of adenosine received would not have influenced the results. Previous studies that demonstrated a lower sensitivity and specificity for stress CMR in AF used a fixed dose adenosine infusion at 140(micrograms/kg)/min, suggesting that remaining at lower doses is unlikely to yield improved results. 11 Whilst patient sex had no significant predictive power for the presence of SSO in SR patients, for the AF group female patients were more likely to achieve SSO (OR = 7.67).
Our findings demonstrated that patients in AF had a significantly lower LVEF and a significantly larger LAVI than patients in SR, in line with expected clinical findings. 24 Future prospective studies assessing SSO may consider matching for these variables.
Inducible ischaemia was observed less frequently in patients in AF than those in SR (13% vs 25%), although this difference was not statistically significant. This difference was not overtly driven by a lack of SSO as there were similar rates of inducible ischaemia observed in both AF and SR patients who did not achieve SSO. Indeed, as discussed, there is some debate regarding the absence of SSO as a reliable indicator of a failed adenosine response. 19 This may be related to the lower frequency of chest pain observed in the AF cohort. However, overall rates of obstructive CAD may be expected to be higher in the presence of AF, 25 yet we observed a proportionately lower degree of inducible ischaemia in our AF population, reinforcing the need for the significance of lower rates of SSO in AF patients to be further assessed in a larger multi centre study.
Additionally, rates of inducible ischaemia were lower in AF patients with SSO than those without (9% vs 17%). However this statistic is limited by the low raw numbers and lack of distinguishing between truly negative inducible ischaemia and inadequate stress.
Of those with inducible ischaemia, less AF patients had PCI within the following 12 months compared to those in SR. This may again suggest the potential underdiagnosis of inducible ischaemia in patients with AF. However, a true appreciation of the sensitivity and specificity of stress CMR in AF patients would require a larger, prospective multicentre study. Whilst stress CMR is a well-validated tool for the assessment of functional significance of coronary lesions in a stable coronary disease population, only 0.75% of this population had any arrythmia. 3 Thus, whether it is a reliable test in the presence of AF is unclear. Indeed, previous research has shown that AF may impair the accuracy of functional imaging. 26 Although this assessed myocardial perfusion imaging only, the effect was in fact only seen in exams using physical stress and not vasodilators, impacted specificity only (with sensitivity preserved), and this study was limited by an anatomical rather than functional reference for the presence of significant CAD. This highlights the need for well-designed studies to interrogate the accuracy of stress imaging in AF further.
Limitations
This study was limited by its single-centre and retrospective nature, the requirement for PSM, as well as a relatively small sample size. However, whilst this limits its direct applicability to a wider population it does demonstrate a signal towards reduced SSO in the presence of AF, although whether this results in the underappreciation of ischaemia in the AF population requires further confirmatory studies. PSM also allowed for two well-matched groups for relevant variables. Rhythm factors such as the presence of bundle branch block and ectopic beats were not considered in the matching of SR and AF patients, the impact of which on SSO is not known. Technical factors in image acquisition may also impact the occurrence of splenic-switch off, however the same adenosine regimen criteria were used in both groups. The visual assessment of SSO was undertaken unblinded to the cardiac rhythm, however findings were consistent when compared with the semi-quantitative analysis suggesting this did not influence results. Relying on propensity matched data risks unmeasured confounding variables leading to biased results, 27 however we aimed to mitigate this by randomising the studies and undertaking an iterative process to produce a well-matched dataset.
Conclusion
In this retrospective propensity matched study, the presence of SSO (a validated marker of adequate stress) was seen significantly less frequently in patients with AF during adenosine stress CMR. This may be associated with the under diagnosis of inducible ischaemia in this population. Further studies need to address the question of whether SSO can indeed be used as a marker of adequate stress in AF patients, in particular whether its absence highlights patients without adenosine-induced coronary vasodilatation, and what adenosine protocol alterations may ensure adequate stress is achieved in patients with AF.
Footnotes
Acknowledgment: We thank the multidisciplinary cardiovascular magnetic resonance imaging team in the Department of Radiology at the Royal United Hospitals Bath NHS Foundation Trust.
Competing interests: Dr Rodrigues reports personal fees from NHSX outside of the submitted work and Dr Rodrigues and Dr Graby report personal fees from Sanofi outside of the submitted work. No other conflicts to disclose.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Adam Barrishi, Email: adam.barrishi@nhs.net.
John Graby, Email: john.graby@nhs.net.
Ali Khavandi, Email: ali.khavandi@nhs.net.
Amardeep Dastidar, Email: Amardeep.Dastidar@nbt.nhs.uk.
Jonathan CL Rodrigues, Email: j.rodrigues1@nhs.net.
REFERENCES
- 1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407–77. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 2. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. London: National Institute for Health and. 2016. Available from: https://www.nice.org.uk/guidance/cg95/chapter/Recommendations [PubMed] [Google Scholar]
- 3. Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S, Bucciarelli-Ducci C, et al. CE-MARC 2 Investigators. In: Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA. ; September 2016., pp. 1051–60. doi: 10.1001/jama.2016.12680 [DOI] [PubMed] [Google Scholar]
- 4. Einstein A, Fisher D, Gregory S, Hansen C, Messana S. Pharmacologic and Exercise Stress Tests. 2011. Available from: https://www.asnc.org/practicepoints
- 5. Brown LAE, Saunderson CED, Das A, Craven T, Levelt E, Knott KD, et al. A comparison of standard and high dose adenosine protocols in routine vasodilator stress cardiovascular magnetic resonance: dosage affects hyperaemic myocardial blood flow in patients with severe left ventricular systolic impairment. J Cardiovasc Magn Reson 2021; 23: 37. doi: 10.1186/s12968-021-00714-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moody WE, Arumugam P. Assessment of stress adequacy with adenosine: does the answer lie in the spleen? J Nucl Cardiol 2022; 29: 1215–18. doi: 10.1007/s12350-020-02485-7 [DOI] [PubMed] [Google Scholar]
- 7. Lymburner P, Webber M, Neill J, Strugnell W, Hamilton-Craig C. P443Simplified splenic switch off - an easy method for determining adequacy of vasodilation during adenosine stress CMR. European Heart Journal - Cardiovascular Imaging 2019; 20(Supplement_2). 10.1093/ehjci/jez118.030 [DOI] [Google Scholar]
- 8. Manisty C, Ripley DP, Herrey AS, Captur G, Wong TC, Petersen SE, et al. Splenic switch-off: A tool to assess stress adequacy in adenosine perfusion cardiac MR imaging. Radiology 2015; 276: 732–40. doi: 10.1148/radiol.2015142059 [DOI] [PubMed] [Google Scholar]
- 9. Kralev S, Schneider K, Lang S, Süselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One 2011; 6(9): e24964. doi: 10.1371/journal.pone.0024964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk-Kazberuk A, Malyszko J. Patients with atrial fibrillation and coronary artery disease - double trouble. Adv Med Sci 2018; 63: 30–35. doi: 10.1016/j.advms.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 11. Greulich S, Steubing H, Birkmeier S, Grün S, Bentz K, Sechtem U, et al. Impact of arrhythmia on diagnostic performance of adenosine stress CMR in patients with suspected or known coronary artery disease. J Cardiovasc Magn Reson 2015; 17: 94. doi: 10.1186/s12968-015-0195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kochiadakis GE, Kallergis EM. Impact of atrial fibrillation on coronary blood flow: A systematic review. J Atr Fibrillation 2012; 5(3): 458. doi: 10.4022/jafib.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med 2011; 30: 1292–1301. doi: 10.1002/sim.4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pembury Smith MQR, Ruxton GD. Effective use of the mcnemar test. Behav Ecol Sociobiol 2011; 74: 133. doi: 10.1007/s00265-020-02916-y [DOI] [Google Scholar]
- 15. Yap BW, Sim CH. Comparisons of various types of normality tests. Journal of Statistical Computation and Simulation 2011; 81: 2141–55. doi: 10.1080/00949655.2010.520163 [DOI] [Google Scholar]
- 16. Sperandei S. Understanding logistic regression analysis. Biochem Med (Zagreb) 2014; 24: 12–18. doi: 10.11613/BM.2014.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007; 15: 199–236. doi: 10.1093/pan/mpl013 [DOI] [Google Scholar]
- 18. Kim HY. Statistical notes for clinical researchers: chi-squared test and fisher’s exact test. Restor Dent Endod 2017; 42: 152–55. doi: 10.5395/rde.2017.42.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patriki D, von Felten E, Bakula A, Giannopoulos AA, Kamani CH, Schwyzer M, et al. Splenic switch-off as a predictor for coronary adenosine response: validation against 13N-ammonia during co-injection myocardial perfusion imaging on a hybrid PET/CMR scanner. J Cardiovasc Magn Reson 2021; 23: 3. doi: 10.1186/s12968-020-00696-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Godoy-Marín H, Duroux R, Jacobson KA, Soler C, Colino-Lage H, Jiménez-Sábado V, et al. Adenosine a2a receptors are upregulated in peripheral blood mononuclear cells from atrial fibrillation patients. Int J Mol Sci 2021; 22(7): 3467. doi: 10.3390/ijms22073467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llach A, Molina CE, Prat-Vidal C, Fernandes J, Casadó V, Ciruela F, et al. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur Heart J 2011; 32: 721–29. doi: 10.1093/eurheartj/ehq464 [DOI] [PubMed] [Google Scholar]
- 22. Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992; 256: 532–35. doi: 10.1126/science.1373909 [DOI] [PubMed] [Google Scholar]
- 23. Bakula A, Patriki D, von Felten E, Benetos G, Sustar A, Benz DC, et al. Splenic switch-off as a novel marker for adenosine response in nitrogen-13 ammonia PET myocardial perfusion imaging: cross-validation against CMR using a hybrid PET/MR device. J Nucl Cardiol 2022; 29: 1205–14. doi: 10.1007/s12350-020-02448-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wijesurendra RS, Liu A, Eichhorn C, Ariga R, Levelt E, Clarke WT, et al. Lone atrial fibrillation is associated with impaired left ventricular energetics that persists despite successful catheter ablation. Circulation 2016; 134: 1068–81. doi: 10.1161/CIRCULATIONAHA.116.022931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nucifora G, Schuijf JD, Tops LF, van Werkhoven JM, Kajander S, Jukema JW, et al. Prevalence of coronary artery disease assessed by multislice computed tomography coronary angiography in patients with paroxysmal or persistent atrial fibrillation. Circ Cardiovasc Imaging 2009; 2: 100–106. doi: 10.1161/CIRCIMAGING.108.795328 [DOI] [PubMed] [Google Scholar]
- 26. Gimelli A, Liga R, Startari U, Giorgetti A, Pieraccini L, Marzullo P. Evaluation of ischaemia in patients with atrial fibrillation: impact of stress protocol on myocardial perfusion imaging accuracy. Eur Heart J Cardiovasc Imaging 2015; 16: 781–87. doi: 10.1093/ehjci/jeu322 [DOI] [PubMed] [Google Scholar]
- 27. Nuttall GA, Houle TT. Liars, damn liars, and propensity scores. Anesthesiology 2008; 108: 3–4. doi: 10.1097/01.anes.0000296718.35703.20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.