Abstract
Objective:
To evaluate the long-term outcomes of covered stent placement in patients with gastroduodenal artery (GDA) stump hemorrhage after pancreaticoduodenectomy (PD) and to identify risk factors of stent failure.
Methods and materials:
Covered stent was placed in total of 21 patients for GDA stump hemorrhage after PD from September 2012 to March 2021. Technical and clinical success, complications, and stent patency were retrospectively evaluated. Nine relevant variables were analyzed to determine risk factors for stent failure.
Results:
In 20 of 21 patients (95.2%), the GDA stump was completely excluded with covered stent placement. Immediate hemostasis was achieved in the 20 patients and rebleeding from jejunal artery occurred in one patient which was successfully embolized one day after the stent placement. There was no procedure-related complication or early mortality (<30 days). During follow-up period (median 655.5 days), stent thrombosis was found on CT in 10 patients (50.0%, 10/20) without any laboratory or CT abnormalities. One thrombosed stent migrated into the jejunum 20 months after placement. The six-month, one-year, and two-year stent patency were 81.9%, 52.9%, and 37.8%, respectively (median 620 days). The recurrence of primary malignancy was associated with stent failure (HR 5.70; 95% CI 1.18–27.76, p = 0.03).
Conclusions:
Covered stent placement is an effective and safe management of postoperative GDA stump hemorrhage. Stent failure occurred frequently (50%) but did not cause liver ischemia. Stent failure was associated with recurrence of primary malignancy.
Advances in knowledge
1. Covered stent placement is an effective and safe management of postoperative GDA stump hemorrhage.
2. Stent failure occurred frequently (50%) but did not cause liver ischemia.
3. Stent failure was associated with recurrence of primary malignancy.
Introduction
Post-pancreaticoduodenectomy (PD) hemorrhage is a rare but fatal complication that accounts for 10–40% of postoperative mortality. 1,2 In such patients, successful surgical treatment is compromised due to extensive inflammatory changes caused by recent dissections. Therefore, endovascular treatment is considered as the first line treatment especially in cases of delayed hemorrhage (occurring 24 h after surgery) from the hepatic artery (HA). Transcatheter embolization and covered stent placement are the most common endovascular techniques. However, transcatheter embolization typically involves sacrificing the major HA, which frequently causes severe hepatic ischemia or infarction. 3,4 Therefore, covered stent placement preserving HA flow should be preferred if it is technically feasible. Previous studies have reported covered stent placement can achieve immediate hemostasis in 70.5–93.8% of patients. 5–11
Despite the growing evidence, studies to date are inadequate to evaluate effectiveness of covered stent for postoperative HA hemorrhage due to i) heterogeneous population with various surgeries and hemorrhage vessels (HA, splenic artery, mesenteric artery etc.), ii) small number of patients (less than 20), and iii) a focus on technical feasibility and early clinical outcomes rather than long-term patency. To our knowledge, there are only a few studies that investigated the long-term covered stent patency and delayed complications. 5,11 Therefore, the purpose of this study was to evaluate long-term patency of covered stents for postoperative gastroduodenal artery (GDA) stump hemorrhage and to identify risk factors of stent failure.
Methods and materials
Patients
Our institutional review board approved this retrospective study and waived the requirement for informed consent. We searched our institutional database from September 2012 to March 2021 and identified a total of 861 patients who underwent PD. Among these patients, postoperative hemorrhage was identified in 102 patients and 92 patients underwent endovascular treatment; 71 patients were excluded due to bleeding from vessels other than the GDA stump (n = 23) or underwent endovascular embolization (n = 48). Finally, 21 patients (16 males, 5 females, mean age 65.3, range 51–83) who underwent covered stent placement for GDA stump hemorrhage after PD were selected for the study.
Procedure
Written informed consent for the procedure was obtained from each patient. Conscious sedation (1–3 mg of midazolam and 50–100 µg of fentanyl) was administered with continuous monitoring of the patients’ heart rate, blood pressure, and oxygen saturation. After right common femoral artery access, celiac and superior mesenteric artery (SMA) angiograms were obtained using a 5 F angiographic catheter (RH; COOK). The HA anatomy and location of hemorrhage were identified. A 0.035-inch hydrophilic guidewire (Glidewire; Terumo) and angiographic catheter were manipulated to pass beyond the bleeding focus into peripheral HA as distal as possible. The guidewire was exchanged with a long stiff guidewire (260 cm) and the angiographic catheter was removed. When celiac artery was stenotic or tortuous, an 8 F guiding catheter (Shuttle; COOK) was placed to facilitate the advancement of covered stent. A self-expandable covered stent was placed along the guidewire and deployed to cover the GDA stump. The following covered stents were used: Viabahn (Gore Medical) with six or 7 mm in diameter and 2.5 or 5 cm in length and Combi (TaeWoong medical) (6 mm, 4 cm). In case of persistent contrast extravasation or pseudoaneurysm due to insufficient expansion of the stent, balloon dilatation was performed with a six or 7-mm balloon catheter (Mustang; Boston Scientific). A completion hepatic angiogram was obtained to confirm complete exclusion of the GDA stump and patent hepatic arterial flow. When bleeding was found in arteries other than the HA, embolization was performed with N-butyl cyanoacrylate (NBCA) in the same session.
Follow-up
After the procedure, all patients were observed with continuous vital sign monitoring in the intensive care unit for at least 24 h. Patients were transferred to the general ward if there were no signs of recurrent bleeding. Complete blood count (CBC) and liver function test (LFT) were performed daily, and contrast-enhanced CT was performed whenever postoperative complication including recurrent bleeding was suspected. Dual antiplatelet therapy with aspirin (100 mg) and clopidogrel (75 mg) was performed according to the judgment of individual physicians. Patients were discharged after normalization of vital signs and laboratory findings. Afterwards, they were followed up in the outpatient clinic every three months. Contrast-enhanced CT was performed at three-month intervals for one year and every six months thereafter.
Definition and analysis
Patient demographics, underlying diseases, type of surgery, onset and severity of hemorrhage, covered stent procedure, and findings on follow-up imaging were collected. Technical success was defined as angiographic resolution of hemorrhage with patent HA flow. Clinical success was defined as resolution of hemorrhagic symptoms (based on vital signs, laboratory test, and hemorrhagic drainage) without requiring additional angiographic or surgical intervention. 9 Procedure-related complications were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0). Postoperative pancreatic fistula was defined according to previously published guideline. 12
The duration of patency of covered stent was defined as the time from stent placement to failure. Nine variables were tested with Cox regression analyses to find risk factors for stent failure. Kaplan-Meier estimates were used for stent patency and patients’ survival. Data were considered censored for analyses if stents remained patent to the point of death or follow-up loss. Data were analyzed with Stata 14 (College Station). A difference with a p-value of less than 0.05 was considered statistically significant.
Results
Patients
Patients’ characteristics are summarized in Table 1. The most common underlying disease was common bile duct (CBD) cancer (n = 8, 38.1%), followed by ampulla of Vater cancer (n = 4, 19.0%). All patients underwent pylorus preserving PD (n = 16) or Whipple’s operation (n = 5). Four patients received concurrent surgeries including colectomy (n = 2), gastrectomy (n = 1), and left hemi-hepatectomy (n = 1). The mean onset of hemorrhage was 23.1 days (range 4–62 days) after surgery. Hematemesis (n = 9) and sentinel bleeding (n = 8) were common clinical manifestation. All patients underwent contrast-enhanced CT before the procedure which revealed contrast extravasation or pseudoaneurysm at GDA stump. Six patients (7.1%, 3/21) were hemodynamically unstable at the time of covered stent placement. Before the procedure, six patients (28.6%) underwent percutaneous catheter drainage (PCD) for pancreatic fistula.
Table 1.
Baseline characteristics of 21 patients with postoperative GDA stump hemorrhage
| Number (%)* | |
|---|---|
| Age (mean ± SD) | 65.3 ± 10.5 |
| Gender (M:F) | 16:5 |
| Underlying disease | |
| Common bile duct cancer | 8 (38.1%) |
| Ampulla of Vater cancer | 4 (19.0%) |
| Pancreas cancer | 3 (14.3%) |
| Duodenal cancer | 3 (14.3%) |
| Intraductal mucinous papillary neoplasm | 2 (9.5%) |
| Choledochal cyst | 1 (4.8%) |
| Surgical procedure | |
| PPPD | 16 (76.2%) |
| Whipple’s operation | 5 (23.8%) |
| Time Interval from surgery | 4–62 days (median 24) |
| Clinical manifestation* | |
| Hematemesis | 9 (42.9%) |
| Sentinel bleeding | 8 (38.1%) |
| Hypotension | 6 (28.6%) |
| Hematochezia/melena | 4 (19.0%) |
| Abdominal pain | 4 (19.0%) |
| Pancreatic fistula (Y:N)a | 11 (52.4%): 10 (47.6%) |
| * 10 patients had multiple symptoms |
Defined as grade B or C according to the definition of the International Study Group of Pancreatic Surgery (12)
Among 20 patients in whom covered stent was technically feasible
Procedure outcomes
A pseudoaneurysm and/or contrast extravasation at GDA stump was identified in all patients. The covered stent placement was technically successful in all but one patient (95.2%, 20/21). The diameter of the covered stent was 6 mm (n = 13) or 7 mm (n = 7). In one patient, the covered stent failed to advance to attempted location due to highly angulated celiac artery takeoff despite use of a guiding catheter. Transcatheter embolization was subsequently performed. No hepatic ischemic complication was found on follow-up CT. The covered stent was placed to cover the orifice of left HA in six patients. However, the left HA perfusion remained through interlobar collaterals from right HA on completion angiogram (Figure 1). Two patients required post-stent balloon dilatation. Pseudoaneurysm at left gastric artery was in one patient and was embolized with NBCA (n = 1). PCD was performed in six patients for pancreatic fistula (n = 5) and hemoperitoneum (n = 1) in the same session. After the procedure, seven patients went through antiplatelet therapy for 6 months.
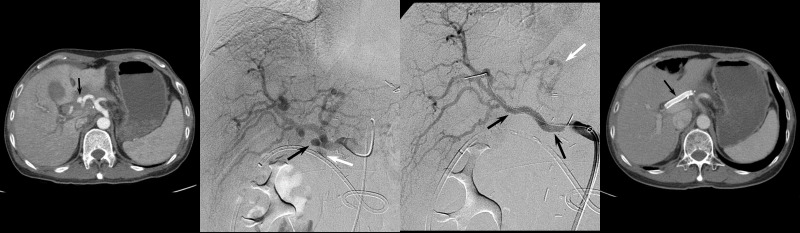
Figure 1.
A 55-year-old male who underwent pyloric preserving pancreaticoduodenectomy (PPPD) for ampulla of Vater cancer. (A) A contrast-enhanced CT obtained 15 days after PPPD shows a hepatic artery pseudoaneurysm (arrow). (B) Hepatic arteriography shows a pseudoaneurysm (black arrow) with rupture (white arrow) at gastroduodenal artery stump. PCD was placed for pancreatic fistula 7 days before. (C) A completion angiogram after covered stent placement shows complete exclusion of the pseudoaneurysm (black arrows). The orifice of left hepatic artery was covered by the stent, but opacified through interlobar collaterals (white arrow). (D) A follow-up CT taken 6 years later demonstrates good patency of the covered stent (black arrow).
Clinical outcomes
Clinical success was achieved in 19 of 20 patients who underwent stent placement (95.0%). One patient experienced recurrent hematochezia 2 days after covered stent placement. An emergency angiography revealed complete exclusion of the GDA pseudoaneurysm, but focal contrast extravasation at jejunal artery and therefore selective embolization was successfully performed with NBCA. All patients (20/20) recovered uneventfully and were discharged 4–49 days (median 11.5) after stent placement. Prolonged hospitalization (>2 weeks) was required in nine patients for indwelling PCD management placed before or during the procedure.
There was no major procedure-related complication more than CTCAE Grade 3. One patient who underwent left gastric artery embolization experienced subsegmental liver infarction in segment 3, which was asymptomatic (CTCAE Grade 1). A thrombosed stent migration into the jejunum was found on 10-month follow-up CT in one patient but was asymptomatic and therefore required no additional treatment (Figure 2).
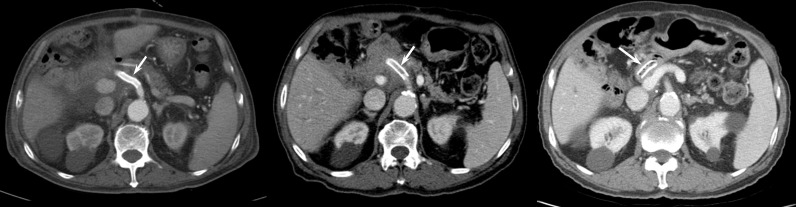
Figure 2.
A 83-year-old male who underwent covered stent placement for postoperative GDA pseudoaneurysm. (A) A contrast-enhanced CT taken 1 month after stent placement shows patent covered stent (white arrow). (B) Covered stent thrombosis was noted on 12-month follow-up CT (white arrow). (C) On 20-month follow-up CT, the thrombosed stent migrated into the jejunum (arrow), which was clinically asymptomatic.
The median follow-up duration was 655.5 days (range 41–2293 days). During follow-up, 10 patients (50%) experienced recurrence of the primary malignancy, and received chemotherapy (n = 7), radiation therapy (n = 3), and/or palliative surgery (n = 1). Additional intervention was required in nine patients: percutaneous biliary stent (n = 5) and PCD for liver abscess (n = 4) or ascites (n = 3). Five patients died 49–1449 days after covered stent placement (median 393 days). The causes of death were progression of recurrent malignancy (n = 4) and complications from brain metastasis operation (n = 1). Nine patients were lost during follow-up (three patients within one year, six referred to regional hospitals) and remaining six patients are still alive. The one, two, and four-year patients’ survival were 88.0%, 80.6%, and 43.0%, respectively (median 1449 days).
Covered stent patency and risk factor of failure
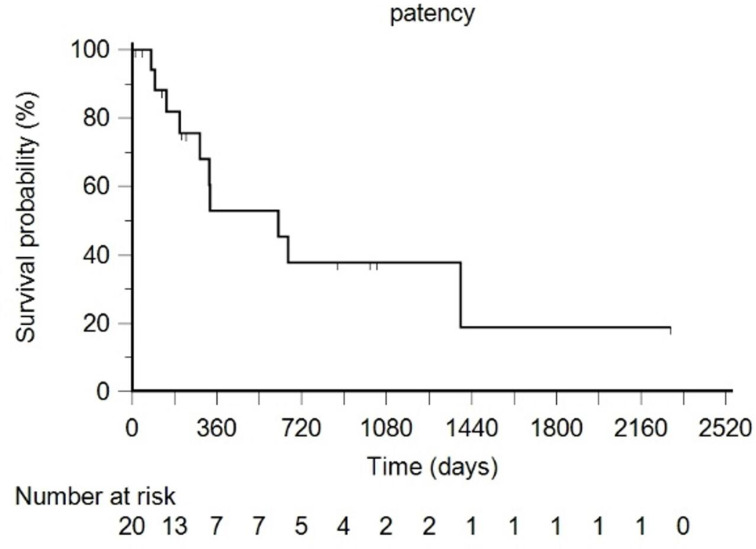
During the follow-up period, covered stent thrombosis occurred in 10 patients (80–1394 days, median 307). Seven patients (35.0%) developed stent failure within 1 year, while three (15.0%) developed late stent occlusion at an average of 891-day post-procedure. However, HA flow distal to the thrombosed covered stent was confirmed on CT in the 10 patients (Figure 3). All covered stent failure was asymptomatic. There was no evidence of liver ischemia or infarction on follow-up CT. The laboratory liver function test did not change more than 10% compared to the previous liver function test before stent failure. The six, 12-, and 24-month stent patency rates were 81.9%, 52.9%, and 37.8%, respectively (median 620 days) (Figure 4). Among the nine variables tested for potential risk factors of covered stent failure, Cox proportional hazard regression showed local tumor recurrence as the only significant factor for covered stent failure (Table 2).
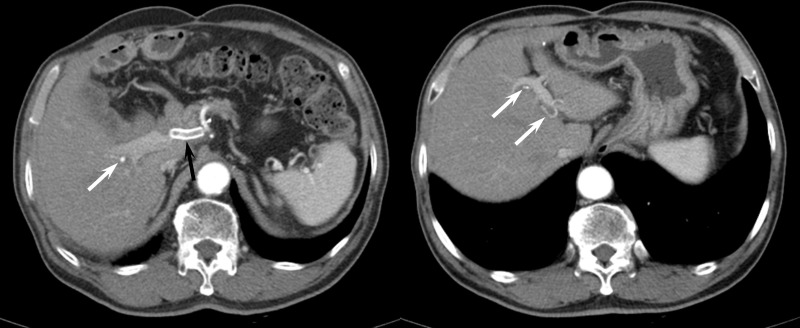
Figure 3.
A 71-year-old male who underwent covered stent placement for postoperative GDA pseudoaneurysm. (A) A contrast-enhanced CT obtained 22 months after stent placement shows thrombosed covered stent (black arrow). However, the right (white arrow) and left hepatic arterial flow (arrows in B) are maintained through collateral vessels.
Figure 4.
Stent patency. Solid line, Patency rate; dotted line, 95% confidence interval. Below, patients at risk.
Table 2.
Cox proportional hazard regression for predictors of stent failure
| variables | Hazard ratio* | p value |
|---|---|---|
| Age (<65 vs. >65) | 0.94 (0.27–3.26) | 0.92 |
| Gender (M vs F) | 0.81 (0.20–3.28) | 0.77 |
| Surgical procedure (PPPD vs Whipple) | 1.09 (0.28–4.33) | 0.89 |
| Time Interval from surgery (≤24 days:>24 days) | 0.76 (0.21–2.71) | 0.67 |
| Pancreatic fistula (Y vs N) | 0.86 (0.20–3.29) | 0.77 |
| Postoperative radiation therapy (Y vs N) | 1.23 (0.31–4.92) | 0.78 |
| Diameter of covered stent (6 mm vs 7 mm) | 0.48 (0.11–1.98) | 0.31 |
| Anticoagulation (Y:N) | 2.56 (0.51–12.81) | 0.25 |
| Local tumor recurrence (Y vs N) | 5.70 (1.18–27.76) | 0.03 |
* 95% confidence interval for each point estimate shown in parentheses
Discussion
Despite recent advances in surgical techniques and postoperative management, postoperative hemorrhage remains a fatal complication, and GDA stump is the most frequent focus of delayed hemorrhage. Previous studies have shown covered stents successfully achieve hemostasis in patients with GDA stump hemorrhage. 5–11 Likewise, in this study, all patients except one (95.0%) achieved immediate hemostasis after covered stent placement. The one patient with rebleeding from the jejunal artery was considered a clinical failure by definition, but initially treated GDA pseudoaneurysm was completely excluded on repeat angiography. Therefore, at present, covered stent placement seems to be theoretically the most desirable endovascular treatment that can reliably treat hemorrhage while preserving HA flow and thus avoid ischemic complications. The technical feasibility of this procedure has been reported in the range of 88–100%. 6,9–11 However, covered stent placement can be technically difficult or even impossible due to unsuitable HA anatomy, in which transcatheter embolization is frequently chosen by operator’s preference. 3 In this study, transcatheter embolization was more frequently performed (n = 48) than covered stent placement (n = 21). Therefore, the exact technical feasibility of this procedure might have been overestimated.
In this study, the only technical failure was due to highly angulated celiac artery takeoff. Although hepatic ischemic complication did not occur in this patient, sacrificing the major HA may cause severe hepatic ischemic complication in 14%–32% of patients. 3,4 When an antegrade approach was more likely to engage such a highly angulated celiac artery, a left transaxillary or transbrachial approach may facilitate the technical success of covered stent placement. 13
Although covered stent placement is known to be an effective treatment in post-PD hemorrhage, previous studies have focused on initial hemostasis and early complications, and studies on long-term outcomes such as stent patency and delayed complications are rare. The prior studies by Lim et al 11 and You et al 5 reported one-year patency rates of 69% (n = 17) and 84.2% (n = 19), respectively. Whereas Hassold et al 9 reported a relatively low one-year patency of 42% in 14 patients with postpancreatectomy hemorrhage. Our study reported 52.9% of one-year patency rate which was in between the previously reported values. Seven patients (35.0%) developed stent failure within one year, and the median stent patency was 620 days. Although data on long-term patency are still scarce and have a wide range, it is clear that low stent patency is a potential complication that should be clinically addressed. Frequently observed thrombosis in covered stents may be due to tortuous anatomy and stronger pulsation of the HA. Therefore, there is a clinical need for a softer and more conformable device.
In this study, local tumor recurrence was the only risk factor that showed statistically significant correlation with covered stent failure. Although covered stent is resistant to tumor invasion into the stent lumen, recurrent tumor can directly invade into or compress the celiac and/or common HA, which leads to compromised arterial flow through stent lumen and cause stent thrombosis. A previous study 5 suggested that a small diameter of the covered stent was associated with early thrombosis. On the contrary, another study claimed that oversizing is a possible cause of stent thrombosis. 9 However, we did not find difference in stent failure between 6 mm and 7 mm stent, which were the most commonly used stent diameters in previous studies. The association between anticoagulation and stent failure can be debatable. An adequate anticoagulation must be applied with caution since the patients undergoing the procedure has had experienced life-threatening hemorrhage and because bleeding risk is high during early postoperative period. For these reasons, anticoagulation was administered only in seven patients (35.0%) in this study. Although an association between anticoagulation and covered stent failure was not found in this study, it needs to be investigated in larger populations with meticulous monitoring of patient’s compliance.
While stent failure was common occurrence, it did not cause any clinical symptoms. There was no evidence of liver ischemia on CT and laboratory test. HA flow distal to covered stent was clearly demonstrated on follow-up CT in all patients with stent failure. In contrast to transcatheter embolization for postoperative HA hemorrhage which frequently leads to ischemic complication in the liver, 3 we believe that covered stent thrombosis process is gradual and therefore, formation of collateral circulation led to the maintenance of HA perfusion distal to the covered stent. Therefore, once acute hemorrhagic phase is bailed out, the covered stent failure is not likely to cause a serious problem, regardless of its timing (early or delayed).
We experienced an extravascular migration of thrombosed stent into the jejunum occurring 10 months after placement. Recently, Tipaldi et al 14 reported five similar cases of extravascular covered stent migration placed for hepatic and splenic artery pseudoaneurysm. The cause of this rare complication is not clear, but it is hypothesized that microenvironmental inflammatory state may have contributed to the loss of the wall integrity of the vessel. Although our patient was asymptomatic and did not require any further treatment, close monitoring should be done because stent migration can cause abdominal pain and gastrointestinal bleeding. 14
There are several major limitations in this study. First, the retrospective data collection from single institution may have resulted in selection bias of the patient cohort. Second, the number of patient population is small (n = 21), which was inevitable owing to paucity of cases. A multicenter study or meta-analysis is needed to confirm the results of this study. Third, as mentioned above, the attempt of covered stent was determined by operator’s discretion. Many patients received transcatheter HA embolization rather than covered stent placement. Since the group of patients to undergo the covered stent procedure is hand-selected by the operator, evaluating the success rate of the procedure is inevitably biased. Finally, the rate of follow-up loss was relatively high (9 of 21, 42.9%) mainly due to frequent patient transfer to regional hospitals for terminal care. Therefore, stent patency and the patients’ survival rates could be overestimated.
In conclusion, covered stent placement is an effective and safe management of postoperative GDA stump hemorrhage. It reliably provided immediate hemostasis in all patients in whom the procedure was technically feasible. Although stent failure occurred frequently (50%) with median patency of 620 days, it did not cause liver ischemia. The stent failure was associated with recurrence of primary malignancy.
Contributor Information
Hooney Min, Email: hooney.min@gmail.com.
Chang Jin Yoon, Email: yooncj1@gmail.com.
Jae Hwan Lee, Email: lzhwanmd@gmail.com.
Won Seok Choi, Email: kchoipro@gmail.com.
Joon Bum Yeo, Email: yeojb89@hotmail.com.
Yoo-Seok Yoon, Email: yoonys@snubh.org.
Jai Young Cho, Email: jycho@snubh.org.
Hae Won Lee, Email: lansh@snubh.org.
Jun Suh Lee, Email: rudestock@snubh.org.
REFERENCES
- 1. Kamada Y, Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, et al. Fatal arterial hemorrhage after pancreaticoduodenectomy: how do we simultaneously accomplish complete hemostasis and hepatic arterial flow? World J Hepatol 2021; 13: 483–503. doi: 10.4254/wjh.v13.i4.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biondetti P, Fumarola EM, Ierardi AM, Carrafiello G. Bleeding complications after pancreatic surgery: interventional radiology management. Gland Surg 2019; 8: 150–63. doi: 10.21037/gs.2019.01.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi WS, Yoon CJ, Lee JH, Yoon YS, Cho JY, Lee JS. Hepatic artery embolization for postoperative hemorrhage: importance of arterial collateral vessels and portal venous impairment. J Vasc Interv Radiol 2021; 32: 826–34. doi: 10.1016/j.jvir.2021.03.412 [DOI] [PubMed] [Google Scholar]
- 4. Hasegawa T, Ota H, Matsuura T, Seiji K, Mugikura S, Motoi F, et al. Endovascular treatment of hepatic artery pseudoaneurysm after pancreaticoduodenectomy: risk factors associated with mortality and complications. J Vasc Interv Radiol 2017; 28: 50–59. doi: 10.1016/j.jvir.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 5. You Y, Choi SH, Choi DW, Heo JS, Han IW, Han S, et al. Long-term clinical outcomes after endovascular management of ruptured pseudoaneurysm in patients undergoing pancreaticoduodenectomy. Ann Surg Treat Res 2019; 96: 237–49. doi: 10.4174/astr.2019.96.5.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui L, Kong L, Bai Y-H, Li X-H, Wang X-Q, Hao J-J, et al. Covered stent placement for hepatic artery pseudoaneurysm. Abdom Radiol (NY) 2020; 45: 3337–41. doi: 10.1007/s00261-020-02452-3 [DOI] [PubMed] [Google Scholar]
- 7. Hwang K, Lee JH, Hwang DW, Song KB, Kwon J, Gwon DI, et al. Clinical features and outcomes of endovascular treatment of latent pseudoaneurysmal bleeding after pancreaticoduodenectomy. ANZ J Surg 2020; 90: E148–53. doi: 10.1111/ans.16184 [DOI] [PubMed] [Google Scholar]
- 8. Gaudon C, Soussan J, Louis G, Moutardier V, Gregoire E, Vidal V. Late postpancreatectomy hemorrhage: predictive factors of morbidity and mortality after percutaneous endovascular treatment. Diagn Interv Imaging 2016; 97: 1071–77. doi: 10.1016/j.diii.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 9. Hassold N, Wolfschmidt F, Dierks A, Klein I, Bley T, Kickuth R. Effectiveness and outcome of endovascular therapy for late-onset postpancreatectomy hemorrhage using covered stents and embolization. J Vasc Surg 2016; 64: 1373–83. doi: 10.1016/j.jvs.2016.05.071 [DOI] [PubMed] [Google Scholar]
- 10. Pedersoli F, Isfort P, Keil S, Goerg F, Zimmermann M, Liebl M, et al. Stentgraft implantation for the treatment of postoperative hepatic artery pseudoaneurysm. Cardiovasc Intervent Radiol 2016; 39: 575–81. doi: 10.1007/s00270-015-1274-1 [DOI] [PubMed] [Google Scholar]
- 11. Lim SJ, Park KB, Hyun DH, Do YS, Park HS, Shin SW, et al. Stent graft placement for postsurgical hemorrhage from the hepatic artery: clinical outcome and CT findings. J Vasc Interv Radiol 2014; 25: 1539–48. doi: 10.1016/j.jvir.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 12. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017; 161: 584–91. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 13. Venturini M, Marra P, Colombo M, Panzeri M, Gusmini S, Sallemi C, et al. Endovascular repair of 40 visceral artery aneurysms and pseudoaneurysms with the viabahn stent-graft: technical aspects, clinical outcome and mid-term patency. Cardiovasc Intervent Radiol 2018; 41: 385–97. doi: 10.1007/s00270-017-1844-5 [DOI] [PubMed] [Google Scholar]
- 14. Tipaldi MA, Pisano A, Krokidis M, Laurino F, Corradini LG, Lucatelli P, et al. Extravascular migration of thrombosed covered stents after endovascular exclusion of splenic or hepatic artery aneurysms and pseudoaneurysms: an underestimated phenomenon. J Vasc Interv Radiol 2021; 32: 317–20. doi: 10.1016/j.jvir.2020.10.004 [DOI] [PubMed] [Google Scholar]