Abstract
Because Staphylococcus aureus strains contain multiple virulence factors, studying their pathogenic role by single-gene inactivation generated equivocal results. To circumvent this problem, we have expressed specific S. aureus genes in the less virulent organism Streptococcus gordonii and tested the recombinants for a gain of function both in vitro and in vivo. Clumping factor A (ClfA) and coagulase were investigated. Both gene products were expressed functionally and with similar kinetics during growth by streptococci and staphylococci. ClfA-positive S. gordonii was more adherent to platelet-fibrin clots mimicking cardiac vegetations in vitro and more infective in rats with experimental endocarditis (P < 0.05). Moreover, deleting clfA from clfA-positive streptococcal transformants restored both the low in vitro adherence and the low in vivo infectivity of the parent. Coagulase-positive transformants, on the other hand, were neither more adherent nor more infective than the parent. Furthermore, coagulase did not increase the pathogenicity of clfA-positive streptococci when both clfA and coa genes were simultaneously expressed in an artificial minioperon in streptococci. These results definitively attribute a role for ClfA, but not coagulase, in S. aureus endovascular infections. This gain-of-function strategy might help solve the role of individual factors in the complex the S. aureus-host relationship.
Staphylococcus aureus is extremely well equipped in both surface adhesins and secreted factors that mediate tissue colonization and infection (26). The expression of these factors is orchestrated by the global regulators agr (accessory gene regulator) (23), sar (staphylococcal accessory regulator), and maybe other determinants such as sigB (10, 24). Together, sar and agr promote the expression of adhesins during exponential growth and the secretion of soluble factors in the post-exponential growth phase in vitro (8). Moreover, they were shown to be critical for infectivity in experimental endocarditis and other models in vivo (5, 7, 18, 36). Thus, S. aureus pathogenesis depends not only on the intrinsic role of each individual factor but also on their regulated interplay in the host-parasite relationship.
In such an intricate context, it appeared difficult to analyze the pathogenic role of individual gene products by classical gene inactivation experiments. For instance, experiments with adhesin-defective mutants suggested that clumping factor A (ClfA) had only a marginal role in experimental endocarditis (33), whereas coagulase and fibronectin-binding proteins (FnBPs) A and B had no effect (1, 17, 33). This was in marked contrast with the visible coagulase activity in vitro and the fact that ClfA and FnBPs conferred powerful bacterial adherence to host proteins present in endovascular lesions (19, 31). Another puzzling observation was made with alpha-hemolysin (encoded by hla) (3). On the one hand, hla-defective mutants were less infectious than the parent in experimental endocarditis; on the other hand, hla overexpression on a multicopy plasmid also decreased infectivity (3). This indicated that the hla product could be detrimental either for the host or for the bacterium, depending on its level of expression. This further underlined the need for complementary strategies to better understand the complex S. aureus-host interaction.
Recent developments with the green fluorescence protein reporter system have helped determine the expression of sar by S. aureus in cardiac vegetations (9). Other strategies including in vivo expression technology (25, 29) and signature-tagged mutagenesis (21, 32, 42) became instrumental for detecting genes that were either overexpressed during infection or essential for bacterial survival at the infected site. However, although they should provide information on the dynamic of in situ gene expression and help reveal new targets for antistaphylococcal compounds, neither of these techniques directly addresses the pathophysiological role of specific determinants in infection.
Here we describe a method of gene transfer that might help to answer this question. S. aureus pathogenic genes were transferred and expressed in a less virulent surrogate bacterium, and the recombinants were tested for a gain of function. The recipient did not have the staphylococcal background yet could anchor surface proteins in a similar way (15, 34). This report presents the successful transfer and expression of the S. aureus clumping factor A (clfA) and coagulase (coa) genes, either alone or in tandem, in the recipient Streptococcus gordonii Challis. The impact of these two determinants on the ability of the surrogate bacterium to adhere to platelet fibrin clots mimicking cardiac vegetations in vitro and to produce experimental endocarditis in rats is analyzed.
MATERIALS AND METHODS
Microorganisms, plasmids, and reagents.
Bacteria and plasmids used are listed in Table 1. All organisms were grown at 37°C. Stocks were kept at −70°C in nutrient broth supplemented with 10% (vol/vol) glycerol. S. gordonii Challis (39) was grown in brain heart infusion (BHI; Difco Laboratories, Detroit, Mich. or on BHI supplemented with 3% blood; S. aureus Newman (12) was grown in tryptic soy broth (TSB; Difco) or on TSB-agar; and Escherichia coli was grown in Luria-Bertani (LB) broth or on LB-agar. Erythromycin was used at 5 mg/liter for streptococci and 500 mg/liter for E. coli; streptomycin was used at 200 mg/liter; and tetracycline was used at 2 mg/liter. Antibiotics and human fibrinogen (Sigma Chemical Co., St. Louis, Mo.), rabbit plasma (bioMérieux, Marcy l'Etoile, France), thrombin from (Diagnotec, Liestal, Switzerland), and DNA-modifying enzymes from (Gibco Life Technologies, Gaithersburg, Md.) were used according to the manufacturer's instructions.
TABLE 1.
Relevant bacterial isolates and plasmid constructs
| Bacterial isolate or plasmid | Relevant genotype | Phenotype or characteristics | Reference or source |
|---|---|---|---|
| S. aureus Newman | clfA coa | ClfA+ Coa+ | 12 |
| S. gordonii | |||
| Parent | clfA coa | ClfA− Coa− Ems | 28 |
| SMI 5 | Parent Ω pSMI 1 | ClfA+ Coa− Ems | This work |
| SMI 14 | SMI 5 ΔclfA | ClfA− Coa− Ems | This work |
| SMI 17 | SMI 5 ΔermB::tetM | ClfA+ Coa− Ems Tcr | This work |
| SMI 24 | SMI 17 Ω pSMI 7 | ClfA− Coa+ Emr Tcr | This work |
| SMI 28 | SMI 17 Ω pSMI 10 | ClfA+ Coa+ Emr Tcr | This work |
| Plasmids | |||
| pJDC9 | ermB | E. coli-Streptococcus suicide vector | 6 |
| pVA891 | ermB | E. coli-Streptococcus suicide vector | 27 |
| pSMI 1a | ermB, clfA | pJDC9::clfA (bp 284–3103) | This work |
| pSMI 2b | ermB, 5′-clfA | pJDC9::5′-clfA (bp 284–477) | This work |
| pSMI 7c | ermB, 5′-clfA-coa | pSMI 2::5′-clfA-coa | This work |
| pSMI 10d | ermB, 3′-clfA-coa | pVA891::3′-clfA-coa | This work |
Used to insert the clfA gene into the S. gordonii chromosome.
Used to delete a large 3′-end fragment (ca. 2 kb) from clfA in S. gordonii SMI 5.
Used to target the coa gene downstream of clfA in S. gordonii SMI 17.
Used to create a mini-clfA-coa operon by targeting coa downstream of clfA in S. gordonii SMI 17.
DNA preparation and genetic strategies.
DNA was prepared by published methods (30, 39). Plasmids were purified using a Qiagen plasmid midi kit (Qiagen GmbH, Hilden, Germany). Genetic transformation of S. gordonii Challis was performed as described elsewhere (39). PCR amplification was carried out with a GeneAmp PCR system 9700 (Perkin-Elmer, Norwalk, Conn.). The following S. gordonii recombinants were constructed.
(i) Construction of ClfA-positive S. gordonii.
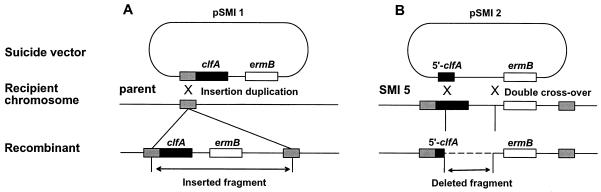
The S. aureus clfA gene (GenBank accession number Z18852) (31) was inserted into the streptococcal chromosome by insertion duplication, using the suicide vector pJDC9 (6) (Fig. 1A). A copy of clfA lacking its own promoter but carrying its ribosome-binding site (RBS) was amplified from S. aureus Newman using the forward primer P1 (nucleotides 284 to 306; CGGAATTCTTAAAAAGAGGGAATAAAATGAA), containing an EcoRI site (underlined), and the reverse primer P2 (nucleotides 3079 to 3103; CGCGGATCCTTATTTCTTATCTTTATTTTCTTTT), containing a BamHI site (underlined). The amplicon was ligated at the EcoRI/BamHI sites of the suicide vector pJDC9 (pSMI 1 [Table 1]) downstream of random streptococcal DNA fragments from an EcoRI chromosomal digest. The constructs were integrated by insertion-duplication into the streptococcal recipient (Fig. 1A). The transformants were selected for erythromycin resistance (Emr) and screened both for clfA acquisition by PCR and for clfA expression by the ability to clump in the presence of plasma. One Emr and ClfA-positive transformant was selected for further studies and called SMI 5 (Table 1).
FIG. 1.
Insertion and inactivation of clfA in the S. gordonii chromosome. (A) For insertion, a promoterless clfA copy (black boxes) containing both its RBS and its stop codon was ligated into the suicide vector pJDC9 (6) downstream of random pieces of S. gordonii chromosomal fragments (gray boxes). The constructs were transformed into parent streptococci and integrated by single-crossover homologous recombination. Recombinants were selected for Emr and screened for insertion and expression of clfA. (B) For clfA deletion, a 5′-end fragment of the gene was ligated into pJDC9, generating pSMI 2, and the construct used to transform the clfA-positive recombinant SMI 5. Deletion was obtained by double-crossover recombination as described elsewhere (38). The mutant SMI 14 (Table 1) contain a 2-kb internal deletion of clfA, as assessed both by PCR and by Southern blot.
(ii) Deletion of the clfA gene from SMI 5.
To inactivate the clfA gene in SMI 5 (Fig. 1B), a 5′-end clfA fragment (nucleotides 284 to 477) was produced using the forward primer P1 and the reverse primer P3 (nucleotides 457 to 477; CGCGGATCCGAATCATTACTTTTGCTTTCG) containing a BamHI site (underlined). The fragment was ligated into pJDC9, generating pSMI 2 (Table 1). pSMI 2 was transformed into SMI 5 and used to delete a large clfA segment by double-crossover recombination as described elsewhere (36) (Fig. 1B). Clumping-defective mutants were enriched by treating the transformed cultures with fibrinogen (10 mg/ml) followed by low-speed (400 rpm for 2 min) centrifugation. Nonaggregating bacteria remained in the supernatant. The loss of clfA was assessed both by PCR and by Southern blotting. One mutant, called SMI 14, contained a 2-kb deletion encompassing the two-thirds of the 3′ end of the gene.
(iii) Construction of coagulase-positive S. gordonii.
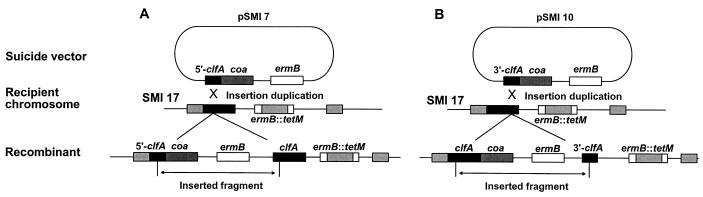
To express coa under the same promoter as clfA, the suicide vector pSMI 2 was modified to generate pSMI 7 (Fig. 5A and Table 1). In pSMI 7, the 5′-end clfA fragment (nucleotides 284 to 477) was followed by a promoterless copy of coa, carrying its RBS. The coa gene (GenBank accession number X17679) (37) was amplified from the S. aureus Newman chromosome, using the forward primer P5 (nucleotides 96 to 118; CGCGGATCCTGGAGGAATTAAAAAATTATAA), containing a BamHI site (underlined), and the reverse primer P6 (nucleotides 2004 to 2024); CGCGGATCCTTATTTTGTTACTCTAGGCCC), also containing a BamHI site. The BamHI site was used for appropriate ligation into pSMI 2. pSMI 7 was used to target coa into the clfA open reading frame (ORF) of a tetracycline-resistant (Tcr) version of SMI 5 (SMI 17; see below and Table 1). Insertion-duplication resulted in a clfA-coa transcriptional fusion as well as a silent promoterless remnant copy of clfA (Fig. 5A). One mutant, called SMI 24 (Table 1), was further studied.
FIG. 5.
Strategies used to create clfA-coa transcriptional fusion to express coa either alone (A) or together with clfA in an artificial minioperon (B). The ClfA-positive and Tcr mutant SMI 17 was used as a recipient. (A) The pSMI 7 suicide vector carrying clfA::coa fusion was transformed into SMI 17. Transformants were selected for Emr-Tcr double resistance and screened for both the acquisition of coagulase activity and the loss of clumping ability. (B) To generate a clfA-coa minioperon, the same promoterless coa amplicon was inserted downstream of a short 3′-end clfA stretch into the suicide vector pVA891 marker (27) (pSMI 10 [Table 1]). This allowed targeting the recombinant vector downstream of the SMI 17 clfA ORF, positioning coa just after the clfA stop codon. Transformants were selected for Emr-Tcr and screened for the coexpression of both clumping and coagulase activities.
To perform this experiment, we first changed the SMI 5 ermB to tetM. This allowed the utilization of a new Emr marker for further constructs. Allelic replacement of ermB by tetM was achieved by micro-homology PCR targeting (2). tetM was amplified from the transposon Tn916 (GenBank accession number U09422). The forward primer P7 (nucleotides 11801 to 11822 of Tn916; TTCT CAAAACTTTTTAACGAGTGAAAAAGTACTCAACCAAAATTGGAGAT TCCTTTACAA) encompassed nucleotides 133 to 172 of the ermB 5′ end (GenBank accession number M20334); underlined). The reverse primer P8 (nucleotides 14022 to 14042 of Tn916; CGTGTAACTTTCCAAATTTACAAAAGCGACTCATAGAATCTAAGTTATTTTATTGAACAT) encompassed nucleotides 343 to 379 of the ermB 3′ end (GenBank accession number M20335). The PCR fragment (2318 bp) was used to transform competent cells of SMI 5, generating SMI 17 (Table 1). PCR was used to confirm that all constructs had the expected structures.
(iv) Construction of a ClfA and coagulase-positive S. gordonii.
To create a minioperon containing both clfA and coa in streptococci, the coa gene was targeted downstream of the far 3′ end of clfA (Fig. 5B). A 3′-end fragment of clfA (nucleotides 2861 to 30103) was produced by using the forward primer P4 (nucleotides 2861 to 2881; CGGAATTCGACTCAGAAAGTGATTCAAAT) containing an EcoRI site and as the reverse primer P2 (see above). The fragment was subcloned at the EcoRI/BamHI site of the suicide vector pVA891 (27). A coa copy in the right (5′-3′) orientation was then ligated downstream of the clfA fragment, at the BamHI site, generating pSMI 10 (Table 1). pSMI 10 was used to transform SMI 17 (see above and Table 1). Insertion-duplication resulted in a clfA-coa transcriptional fusion. Since both ORFs were intact, they were both expressed. In addition, the transformants also contained an additional silent 3′-end replicate of clfA (Fig. 5B). One mutant, called SMI 28 (Table 1), was further studied.
PCR was used to confirm that all constructs had the expected structures.
Clumping activity, coagulase activity, and adherence to platelet-fibrin clots.
Ten-microliter aliquots of 50 mM sodium phosphate buffer (PBS) containing 1010 to 1011 CFU/ml from an overnight culture were mixed with equal volumes of rabbit plasma or fibrinogen (1 mg/ml). Clumping was assessed either visually, by the formation of instantaneous clumps when bacteria were suspended in plasma, or quantitatively as described elsewhere (20). Surface-bound ClfA was also determined by fluorescence-activated cell sorter (FACS) analysis, using purified F(ab′)2 fragments from anti-ClfA rabbit polyclonal immunoglobulin G (kindly provided by T. Foster, Dublin, Ireland). Bacteria were washed, suspended in PBS containing 10% fetal calf serum-antibody solution (with 10 mg of antibody/liter), and incubated for 2 h on ice. The cells were then washed twice with PBS, suspended in PBS–10% fetal calf serum-antibody solution containing F(ab′)2 fragments of a fluorescein isothiocyanate-labeled goat anti-rabbit antibody (TAGO, Inc., Burlingame, Calif.), and incubated for 1 h on ice before washing and FACS analysis.
Coagulase activity was assessed either in whole bacterial cultures (for qualitative tests) or in culture supernatants (for quantitative tests). Coagulase activity resulted in the formation of a firm plasma clot and thus was easy to distinguish from the ClfA activity, which induced macroscopic bacterial clumping at the bottom of the tubes without clot formation. For qualitative assessment, equal volumes (150 μl) of overnight cultures and rabbit plasma were mixed and incubated at 37°C. Coagulation was determined visually after 2, 4, and 24 h. For quantitative titration, the cultures were centrifuged three consecutive times (at 12,000 × g for 5 min) to remove the bacterial cells. The supernatants were then serially diluted with PBS, and equal volumes of rabbit plasma were added to the tubes. The reaction was allowed to proceed as above, and the coagulation titer was expressed as the reciprocal of the highest supernatant dilution triggering coagulation. Finally, cell-bound coagulase activity (4) was tested by washing the cells three times in 50 mM PBS, by centrifugation, before they were resuspended in the same buffer and tested for qualitatively coagulase activity as above.
Adherence to human platelet-fibrin clots was measured as described elsewhere (33).
Experimental endocarditis.
Sterile aortic vegetations were produced in rats as previously described (22). The ability of bacteria to produce endocarditis was determined in parallel for the test organisms by inoculating groups of animals with increasing inocula (103 to 105 CFU) from cultures in the exponential phase of growth. The rats were killed 12 h after bacterial challenge. The vegetations were dissected, weighed, and plated for quantitative cultures. Bacteria containing an antibiotic resistance marker were plated on both drug-containing and drug-free agar. Bacterial densities in the vegetations were expressed as mean log10 CFU per gram of tissue ± standard deviation (SD). The detection limit was ≥2 log10 CFU/g of vegetation.
Statistical evaluation.
The frequencies of valve infections were compared by the χ2 test with Yates correction. Mean vegetation bacterial densities and in vitro adherence ratios were compared by one-way analysis of variance (ANOVA), and pairwise differences between the means of groups were determined by t test using the Bonferroni correction. Differences between groups were considered significant when P was <0.05 using two-tailed significance levels.
RESULTS
Chromosomal integration and functional expression of the S. aureus clfA gene in S. gordonii.
The aim of the initial experiments was to achieve stable and functional expression of the staphylococcal clfA gene in S. gordonii. To ensure stability, the clfA gene was inserted into the streptococcal chromosome using the suicide vector pJDC9 (Emr) (6), containing a copy of the clfA gene as depicted in Fig. 1A. Transformants were selected for Emr and screened for both integration and expression of clfA by PCR and spontaneous clumping in plasma, respectively.
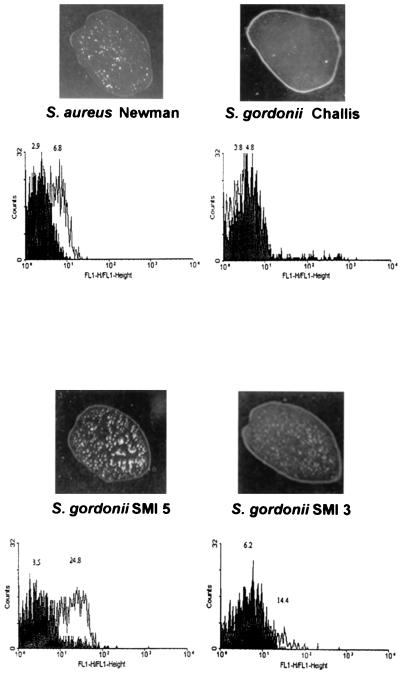
The rate of transformation to Emr was ca. 10−7. In a typical experiment, all of 13 Emr transformants carried a copy of the clfA gene, and 5 (38%) of them expressed ClfA to various degrees. Figure 2 indicates that bacterial clumping in the presence of plasma correlated with ClfA surface expression as determined by FACS analysis.
FIG. 2.
Macroscopic clumping and FACS analysis of S. aureus Newman and S. gordonii producing or not producing ClfA. Macroscopic clumping was assessed by mixing bacteria with 10 μl of rabbit plasma on a glass slide. FACS analysis was performed with both irrelevant antibodies (gray area) or purified anti-ClfA F(ab′)2 fragments (white area) as described in Materials and Methods. Clumping and FACS shifts were clearly observed both in S. aureus and in the strong ClfA-producing recombinant SMI 5. In contrast, no clumping or FACS shift was observed in the S. gordonii parent, and only moderate clumping and FACS shift were present in the low ClfA-producing recombinant SMI 3.
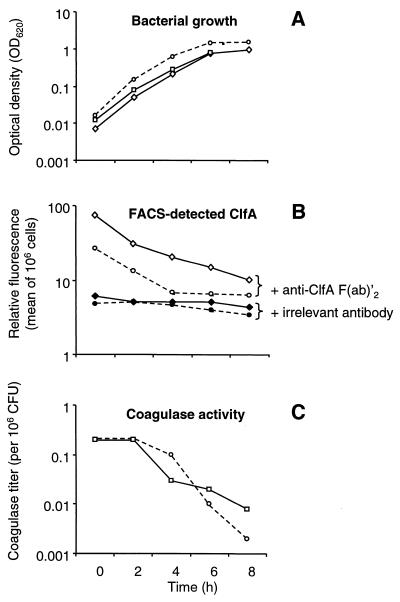
SMI 5 (Table 1) was selected for further experiments. In this organism, integration of heterologous DNA did not alter the growth rate in vitro, and both Emr and ClfA were stable for up to five consecutive passages (ca. 35 generation times) in drug-free medium. Table 2 and Fig. 3 indicate that expression of surface ClfA was comparable between SMI 5 and S. aureus Newman, both in terms of titer (Table 2) and as determined by FACS analysis (Fig. 3). Figure 3B indicates that the kinetics of ClfA expression were similar in S. aureus Newman and the ClfA-positive recombinant SMI 5. This was critical for the relevance of the model, as ClfA production by S. gordonii should mimic that of staphylococci.
TABLE 2.
Clumping and coagulase titers of S. aureus Newman and S. gordonii expressing or not expressing ClfA and coagulase
| Bacterial isolate | Clumping titera | Coagulase titerb |
|---|---|---|
| S. gordonii | ||
| Parent (ClfA− Coa−) | 0 | 0 |
| SMI 5 (ClfA+) | 2,048 | 0 |
| SMI 24 (ClfA− Coa+ | 0 | 64 |
| SMI 28 (ClfA+ Coa+) | 2,048 | 128 |
| S. aureus Newman (ClfA+ Coa+) | 1,024 | 64 |
Inverse of highest twofold dilution of a fibrinogen solution leading to visible clumping after addition of bacterial culture and overnight incubation at 4°C in a microtiter assay.
Inverse of highest twofold dilution of culture supernatant leading to plasma coagulation during incubation at 37°C.
FIG. 3.
Bacterial growth (A), production of ClfA (B), and production of coagulase titration (C) as a function of time for S. aureus Newman (dotted lines), the ClfA-positive recombinant S. gordonii SMI 5 (diamonds), and the coagulase-positive recombinant SMI 24 (squares). (A) Bacterial growth was followed by the optical density at 620 nm (OD620) of the cultures. At various times, samples were removed and processed either for FACS analysis (B) or for coagulase titration (C). FACS analysis was performed with either anti-ClfA (open symbols) or irrelevant (closed symbols) F(ab′)2 fragments, using bacterial samples containing 106 CFU/ml. Controls using coagulase-positive (but ClfA-negative) S. gordonii were negative by FACS and are not represented. Determination of coagulase titers is indicated by the highest twofold dilution of the culture supernatants triggering coagulation of rabbit plasma after 24 h of incubation at 37°C. Coagulase activity was expressed as a function of bacterial densities in the cultures by dividing the measured coagulation titer by the number of CFU (106) in the culture.
Finally, examination of parent and mutant cells by phase-contrast microscopy indicates that the two organisms formed similar short chains of 2 to 10 individuals. This was important for the preparation of bacterial inocula in further experiments. Indeed, variations in bacterial chaining may result in misinterpretation of viable counts when assessed by CFU enumeration of agar plates.
In vitro adherence and in vivo infectivity of ClfA-negative and ClfA-positive S. gordonii transformants.
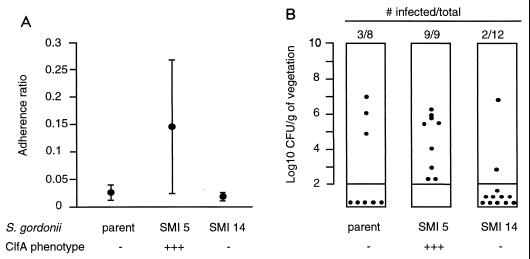
The clfA-positive SMI 5 recombinant was ≥5 times more adherent than the ClfA-negative parent in vitro (P < 0.05, compared by one-way ANOVA and by the t test using the Bonferroni correction) (Fig. 4A). Moreover, when the clfA determinant of SMI 5 was deleted (Fig. 1B), adherence of the ΔclfA construct SMI 14 (Table 1) had returned to that of the parent strain.
FIG. 4.
In vitro adherence (A) and in vivo infectivity (B) of the ClfA-negative parent S. gordonii, the ClfA-positive recombinant SMI 5, and its ClfA-negative (ΔclfA) mutant SMI 14. In vitro adherence was performed as described elsewhere (33). Data points indicate the mean ± SD adherence ratios of 12 to 24 individual determinations for each isolates. The adherence ratios of the ClfA-positive SMI 5 were significantly greater than that of either the parent or the ΔclfA mutant SMI 14 (P < 0.05, as determined by one-way ANOVA and pairwise comparisons using the t test with Bonferroni correction). For in vivo infection, each dot represents the vegetation bacterial density in one rat killed 12 h after bacterial challenge. Densities of less than 2 log10 CFU/g of vegetation were below detection limit and are indicated below the columns. The SMI 5 transformant was significantly more infective than the two comparative isolates (P < 0.05, as determined by the χ2 test with Yates correction).
Parent and SMI 5 and ΔclfA SMI 14 mutant strains were further tested for the ability to infect rats with catheter-induced aortic vegetations (14, 33). Groups of animals were inoculated in parallel with similar numbers of CFU of each of the test organisms. Figure 4B depicts the frequency and severity of valve infection in rats challenged with 104 CFU, an inoculum that reproducibly infected 20 to 30% of animals challenged with the parent cells (33). In these experiments, the differences between the ClfA-positive mutant SMI 5 and either the ClfA-negative parent or the ClfA-negative mutant SMI 14 were statistically significant (P < 0.05, compared by the χ2 test with Yates correction). All ClfA-positive streptococci recovered from the valves had retained the Emr marker.
At a lower inoculum (103 CFU), none of nine animals challenged with the parent became infected and only two (22%) of the nine challenged with SMI 5 developed endocarditis (P > 0.05). At a higher inoculum (105 CFU), on the other hand, both the parent and the SMI 5 recombinant infected all rats (five of five and six of six, respectively). Thus, although ClfA production clearly increased infectivity, the difference was confined to a specific range of inoculum sizes.
Rats that developed endocarditis had similar vegetation bacterial densities at the time of sacrifice (5.79 ± 0.71 [n = 8], 5.18 ± 1.53 [n = 20], and 5.72 ± 1.03 [n = 10] log10 CFU/g of tissue [mean ± SD] for S. gordonii [ClfA− Coa−], SMI5 [ClfA+ Coa−], and SMI 28 [ClfA+ Coa+], respectively), irrespective of the infecting organisms. This suggested that once in the vegetation milieu, parent and SMI 5 cells grew at similar rates. Therefore, ClfA was likely to operate at an early step of infection, e.g., during attachment and colonization of the damaged valves.
In vitro adherence and in vivo infectivity of coagulase-positive S. gordonii recombinants.
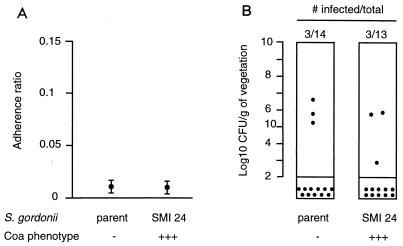
A shuttle system was developed to deliver the coa gene under the same promoter as clfA (Fig. 5A). This was important to avoid additional genetic alterations in the mutants. One stable coagulase-positive and clfA-negative transformant (SMI 24 [Tables 1 and 2]) was characterized. Production of coagulase in the culture supernatant was similar to that of S. aureus Newman in terms of both titers and kinetics (Table 2 and Fig. 3C). Moreover, like S. aureus, SMI 24 produced both secreted and surface-bound coagulase (4) (data not presented). However, coagulase increased neither in vitro adherence nor in vivo infectivity in rats killed at 12 h (Fig. 6). Moreover, in one experiment testing the effect of coagulase on later infection, i.e., in animals killed at 5 days, coagulase did not increase vegetation bacterial density over that of the parent cells. Paradoxically, it even tended to decrease it. At day 5, vegetation bacterial titers (mean ± SD CFU/gram of vegetations) were 8.27 ± 2.05 in seven rats inoculated with the parent, compared to 6.58 ± 2.04 in eight rats infected with the coagulase-positive mutant SMI 24 (P = 0.03, compared by the Student t test).
FIG. 6.
In vitro adherence (A) and in vivo infectivity (B) of the coagulase-negative parent S. gordonii and its coagulase-positive transformant SMI 24. The coagulase-positive SMI 24 transformant isolate was neither more adherent to platelet-fibrin matrices (P < 0.05) nor more infective rats with experimental endocarditis (P < 0.05) than the parent S. gordonii. Details are as for Fig. 4.
Infectivity of ClfA and coagulase double-positive transformants of S. gordonii.
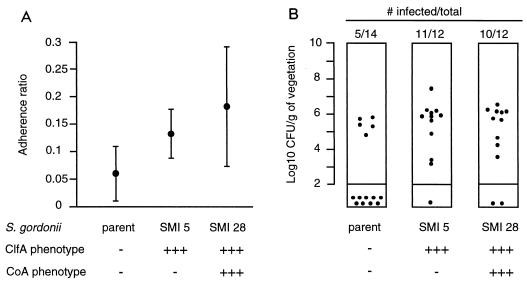
The final objective of these experiments was to test whether coexpression of ClfA and coagulase might affect pathogenesis. An artificial clfA-coa operon was constructed in S. gordonii as illustrated in Fig. 5B. Seven (50%) of fourteen transformants carrying the appropriate antibiotic markers (Emr-Tcr) expressed both clumping and coagulase activity. One stable transformant was analyzed for quantitative results (SMI 28 [Table 1]) and produced similar ClfA and coagulase titers similar to those for S. aureus (Table 2). Figure 7 indicates that the mutant was also significantly more adherent and infective than the parent S. gordonii (P < 0.05 for both). However, it was not more adherent or infective than SMI 5, expressing ClfA alone. Therefore, coexpression of ClfA and Coa did not increase the infectivity over that of single clfA-positive transformants.
FIG. 7.
In vitro adherence (A) and in vivo infectivity (B) of the parent S. gordonii, its ClfA+ transformant SMI 5, and its ClfA+ Coa+ derivative SMI 28 (Tables 1 and 2). Both SMI 5 and SMI 28 (Table 1 and 2) were significantly more adherent (P < 0.05) and infective (P < 0.05) than the parent strain but did not behave differently from each other (P < 0.05 for both in vitro adherence and in vivo infectivity), indicating that coagulase did not add to the pathological effect of ClfA. Details are as for Fig. 4.
DISCUSSION
The goal of this work was to develop a model of adoptive pathogenesis to study single or multiple staphylococcal pathogenic factors expressed in a surrogate bacterium lacking the whole S. aureus pathogenic background. The results indicated that both staphylococcal ClfA and coagulase could be functionally expressed by S. gordonii. Moreover, they clearly confirmed a role for ClfA, but not coagulase, in the pathogenesis of experimental S. aureus endocarditis in vivo (1, 33). Recombinant streptococci expressing ClfA had a gain in adherence to platelet-fibrin clots in vitro as well as in infectivity in vivo. Moreover, this positive effect was lost when the clfA insert was deleted from the streptococcal recombinants.
This was reminiscent of experiments with the clfA-inactivated S. aureus Newman, where deletion and complementation of the clfA gene resulted in marginal differences in the infective doses in rats with experimental endocarditis (33). In these experiments, the limited impact of clfA deletion was attributed to the redundancy of surface adhesins, including the recently described ClfB (35), FnBPs (16), and other determinants that might mediate adherence to endovascular lesions. Therefore, the potential contribution of ClfA could not clearly be defined. The present study on the other hand, provided a positive finding in this regard, demonstrating that expression of clfA by a naive and unrelated organism conferred a significant increase in both adherence and infectivity to the surrogate bacterium. Thus, the results of these two complementary approaches identify ClfA as one major staphylococcal factor mediating endovascular attachment and infection.
A second determinant that might be an important S. aureus pathogenic factor is coagulase (26, 37, 41). However, the exact role of coagulase in staphylococcal infection has not been established. Coagulase was shown to increase virulence in a murine model of mammary abscess (37) and pulmonary infection (41), but to have no effect on experimental endocarditis (1, 33). This lack of effect was further demonstrated in the present study. Recombinant S. gordonii produced both surface-bound and secreted coagulase at levels similar to those produced by S. aureus Newman. It was surprising that the impressive blood clotting triggered in vitro by either coagulase-producing staphylococci or recombinant streptococci did not result in some increased virulence in the vegetations. Moreover, in the present study this was true for animals killed early (at 12 h) or later (5 days) after inoculation, indicating that coagulase did not influence late infection either. Therefore, the role of this second determinant in endocarditis remains undetermined.
Finally, the concurrent expression of ClfA and coagulase in S. gordonii did not increase in vitro adherence and in vivo infectivity over that of single ClfA-positive recombinants. This was again reminiscent of the staphylococcal situation, where double deletion of the ClfA and coagulase determinants did not decrease infectivity more profoundly than in mutants carrying a single clfA deletion alone. In this study ClfA and coagulase were expressed in comparable amounts by S. aureus Newman and the surrogate streptococci. Moreover, the kinetics of production during bacterial growth indicated that the two factors were simultaneously present on the cell surface. Therefore, the lack of synergism between ClfA and coagulase in experimental endocarditis indicates that molecular cooperation implicating these determinants was not a pathogenic mechanism in the present model of infection.
In conclusion, the present study describes a genetic technique that may help dissect the role of putative gram-positive virulence factors by reconstituting them in a less pathogenic organism. In similar types of experiments, Salmonella genes transferred into E. coli helped to identify factors involved in both adherence and invasion of the host (11, 13). Thus, such an adoptive (gain-of-function) strategy may be an indispensable corollary of classical knockout mutagenesis, which always carries the risk of underestimating the effect of a given deletion on the expression of other pathogenic determinants or on nonspecific bacterial fitness. The present results indicate that individual and concurrent expression of two proteins that were thought to be important virulence determinants in staphylococci could be achieved in S. gordonii.
The genetic techniques demonstrate the feasibility of the concept and open the possibility for more sophistication. Expression of genes integrated into the chromosome may be more stable and reliable than determinants cloned in multicopy plasmids. On the other hand, the development of expression vectors and in bacteria less pathogenic than S. gordonii, such as lactococci (40), may allow more flexibility in gene cloning and expression. Considering the complexity of pathogenic gene regulation in S. aureus, adoptive pathogenesis might become a standard complementary test to identify the important factors.
ACKNOWLEDGMENTS
This work was supported by grants 3200-47099.96 and 3200-0458.95/2 from the Swiss National Funds for Scientific Research.
We thank Marlyse Giddey, Jacques Vouillamoz, and Patrice François for outstanding technical support.
REFERENCES
- 1.Baddour L M, Tayidi M M, Foster T J. Virulence of coagulase-deficient mutants of Staphylococcus aureus in experimental endocarditis. J Med Microbiol. 1994;41:259–263. doi: 10.1099/00222615-41-4-259. [DOI] [PubMed] [Google Scholar]
- 2.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer A S, Ramos M D, Menzies B E, Yeaman M R, Shen A J, Cheung A L. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun. 1997;65:4652–4660. doi: 10.1128/iai.65.11.4652-4660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden M K, Flock J I. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect Immun. 1989;57:2358–2363. doi: 10.1128/iai.57.8.2358-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth M C, Atkuri R V, Nanda S K, Iandolo J J, Gilmore M S. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig Ophthalmol Visual Sci. 1995;36:1828–1836. [PubMed] [Google Scholar]
- 6.Chen J D, Morrison D A. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene. 1988;64:155–164. doi: 10.1016/0378-1119(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 7.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar−lagr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Investig. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Nast C C, Bayer A S. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect Immun. 1998;66:5988–5993. doi: 10.1128/iai.66.12.5988-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung A L, Projan S J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crago A M, Koronakis V. Binding of extracellular matrix laminin to Escherichia coli expressing the Salmonella outer membrane proteins Rck and PagC. FEMS Microbiol Lett. 1999;176:495–501. doi: 10.1111/j.1574-6968.1999.tb13703.x. [DOI] [PubMed] [Google Scholar]
- 12.Duthie E S, Lorenz L L. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 13.Elsinghorst E A, Baron L S, Kopecko D J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entenza J M, Caldelari I, Glauser M P, Francioli P, Moreillon P. Importance of genotypic and phenotypic tolerance in the treatment of experimental endocarditis due to Streptococcus gordonii. J Infect Dis. 1997;175:70–76. doi: 10.1093/infdis/175.1.70. [DOI] [PubMed] [Google Scholar]
- 15.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 16.Flock J I, Froman G, Jonsson K, Guss B, Signas C, Nilsson B, Raucci G, Hook M, Wadstrom T, Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flock J I, Hienz S A, Heimdahl A, Schennings T. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect Immun. 1996;64:1876–1878. doi: 10.1128/iai.64.5.1876-1878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillaspy A F, Hickmon S G, Skinner R A, Thomas J R, Nelson C L, Smeltzer M S. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 20.Hawiger J, Hammond D K, Timmons S, Budzynski A Z. Interaction of human fibrinogen with staphylococci: presence of a binding region on normal and abnormal fibrinogen variants and fibrinogen derivatives. Blood. 1978;51:799–812. [PubMed] [Google Scholar]
- 21.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 22.Heraief E, Glauser M P, Freedman L R. Natural history of aortic-valve endocarditis in rats. Infect Immun. 1982;37:127–131. doi: 10.1128/iai.37.1.127-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornblum J, Kreiswirth B N, Projan S J, Ross H, Novick R P. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH; 1990. pp. 373–402. [Google Scholar]
- 24.Kullik I, Giachino P. The alternative sigma factor sigmaB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 25.Lowe A M, Beattie D T, Deresiewicz R L. Identification of novel staphylococcal virulence genes by in vivo expression technology. Mol Microbiol. 1998;27:967–976. doi: 10.1046/j.1365-2958.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 27.Macrina F L, Evans R P, Tobian J A, Hartley D L, Clewell D B, Jones K R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983;25:145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- 28.Macrina F L, Wood P H, Jones K R. Genetic transformation of Streptococcus sanguis (Challis) with cryptic plasmids from Streptococcus ferus. Infect Immun. 1980;28:692–699. doi: 10.1128/iai.28.3.692-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahan M J, Slauch J M, Mekalanos J J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 30.Marmur J. A procedure for isolation of desoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 31.McDevitt D, François P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 32.Mei J M, Nourbakhsh F, Ford C W, Holden D W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 33.Moreillon P, Entenza J M, Francioli P, McDevitt D, Foster T J, François P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in the pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni E D, Perkins S, Francois P, Vaudaux P, Hook M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson I M, Bremell T, Ryden C, Cheung A L, Tarkowski A. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect Immun. 1996;64:4438–4443. doi: 10.1128/iai.64.11.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phonimdaeng P, O'Reilly M, Nowlan P, Bramley A J, Foster T J. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990;4:393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 38.Pozzi G, Guild W R. Modes of integration of heterologous plasmid DNA into the chromosome of Streptococcus pneumoniae. J Bacteriol. 1985;161:909–912. doi: 10.1128/jb.161.3.909-912.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pozzi G, Musmanno R A, Lievens P M, Oggioni M R, Plevani P, Manganelli R. Method and parameters for genetic transformation of Streptococcus sanguis Challis. Res Microbiol. 1990;141:659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
- 40.Que Y-A, Haefliger J-A, Francioli P, Moreillon P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect Immun. 2000;68:3516–3522. doi: 10.1128/iai.68.6.3516-3522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawai T, Tomono K, Yanagihara K, Yamamoto Y, Kaku M, Hirakata Y, Koga H, Tashiro T, Kohno S. Role of coagulase in a murine model of hematogenous pulmonary infection induced by intravenous injection of Staphylococcus aureus enmeshed in agar beads. Infect Immun. 1997;65:466–471. doi: 10.1128/iai.65.2.466-471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwan W R, Coulter S N, Ng E Y, Langhorne M H, Ritchie H D, Brody L L, Westbrock-Wadman S, Bayer A S, Folger K R, Stover C K. Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect Immun. 1998;66:567–572. doi: 10.1128/iai.66.2.567-572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]