Abstract
Background
Charged particle beams from protons to carbon ions provide many significant physical benefits in radiation therapy. However, preclinical studies of charged particle therapy for prostate cancer are extremely limited. The aim of this study was to comprehensively investigate the biological effects of charged particles on prostate cancer from the perspective of in vitro studies.
Methods
We conducted a systematic review by searching EMBASE (OVID), Medline (OVID), and Web of Science databases to identify the publications assessing the radiobiological effects of charged particle irradiation on prostate cancer cells. The data of relative biological effectiveness (RBE), surviving fraction (SF), standard enhancement ratio (SER) and oxygen enhancement ratio (OER) were extracted.
Results
We found 12 studies met the eligible criteria. The relative biological effectiveness values of proton and carbon ion irradiation ranged from 0.94 to 1.52, and 1.67 to 3.7, respectively. Surviving fraction of 2 Gy were 0.17 ± 0.12, 0.55 ± 0.20 and 0.53 ± 0.16 in carbon ion, proton, and photon irradiation, respectively. PNKP inhibitor and gold nanoparticles were favorable sensitizing agents, while it was presented poorer performance in GANT61. The oxygen enhancement ratio values of photon and carbon ion irradiation were 2.32 ± 0.04, and 1.77 ± 0.13, respectively. Charged particle irradiation induced more G0-/G1- or G2-/M-phase arrest, more expression of γ-H2AX, more apoptosis, and lower motility and/or migration ability than photon irradiation.
Conclusions
Both carbon ion and proton irradiation have advantages over photon irradiation in radiobiological effects on prostate cancer cell lines. Carbon ion irradiation seems to have further advantages over proton irradiation.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-022-00942-2.
Keywords: Prostate cancer, In vitro, Charged particle irradiation, Systematic review
Introduction
Globally, there are estimated to be 1,414,259 new cases and 375,304 deaths from prostate cancer (PCa) in 2020, which is the second most frequent malignancy in males and the fifth leading cause of cancer mortality among men worldwide [1]. Radiation therapy (RT) has been widely used to treat PCa for many years. Given that PCa control is dose-dependent, intensity-modulated RT (IMRT), image-guided RT and brachytherapy can result in an adequate dose to the prostate or prostate bed while causing less gastrointestinal and genitourinary toxicity, especially in the rectum and bladder [2–4]. Charged particle therapy (CPT) for PCa gained increasing attention in the past decade because of its superior physical and biological properties. Several studies have shown a reduced radiation toxicity, lower risk of secondary primary tumor and an improved biochemical-free survival (also known as biochemical disease-free survival) compared with IMRT [5–14]. In these studies, despite the lack of high-quality randomized controlled trials, existing data suggested that CPT exhibited great potential for sparing normal tissue as well as excellent overall survival rate and local control rate [15]. CPT may become a promising approach to treat PCa someday in the years to come.
The physical properties of carbon ions and protons are quite similar. With the presence of depth-dose distribution, also known as the Bragg peak, CPT can give the maximum energy to the surroundings near the stop (cancer part) [16]. In addition to accommodate the tumor volume, a spread-out Bragg peak (SOBP) is also produced. Carbon ions exhibit less lateral scattering and longitudinal straggling than protons, but they have a tail of light fragments beyond the Bragg peak, and nuclear fragmentation causes a drop in dose in the plateau area [17]. As for the biological properties, due to the higher linear energy transfer (LET) and relative biological effectiveness (RBE) of carbon ions compared with protons and photons, they can directly cause complex damage to DNA molecules and cause clustered DNA double-strand breaks (DSBs), requiring multiple DNA repair pathways to resolve [18]. Additionally, as a result of this damage, the oxygen enhancement ratio (OER) of carbon ions is typically ranged between 1 and 2.5, depending on the LET value, whereas the OER of photons and protons is generally estimated to be as high as 3 [19].
Due to these particular features of charged particles, some in vitro studies have focused on the radiobiological effects of carbon ion and proton irradiation on PCa. However, most of these studies have different relevant parameters, such as charged particle type, cell line type, cell origin, energy, LET, SOBP, combination therapy and so forth. Therefore, we believe it is necessary to pool these studies for further analysis. In this paper, we conducted a systematic review based on published in vitro studies of carbon ion or proton irradiation for PCa to study its biological mechanisms regarding both traditional radiobiology and molecular level.
Materials and methods
This systematic review was performed in accordance with the guidelines proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Additional file 3 (PRISMA) 2020 statement [20]. As our review contained only in vitro studies and did not involve any human or animal studies, it did not meet the criteria for registration on PROSPERO website and therefore we were unable to register in.
Search strategy
The following electronic databases were used as our data sources: MEDLINE (Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review and Other Non-Indexed Citations, Daily and Versions(R) < 1946 to February 18, 2022 >), Embase (< 1974 to 2022 February 18 > , Ovid) and Web of Science databases (WOS, BIOSIS, KJD, RSCI, SCIELO). The following search terms were used: “heavy ion radiotherapy”, “heavy ion therapy”, “heavy ion radiation therapy”, “particle beam therapy”, “carbon ion therapy”, “carbon ion radiation therapy”, “carbon ion radiotherapy”, “carbon ion irradiation”, “proton therapy”, “proton radiation”, “proton irradiation”, “prostatic neoplasms”, “prostate cancer”, “prostatic cancer”, “prostate adenocarcinoma” and “prostatic adenocarcinoma”. Specific search strategies for each database were listed in Additional file 1. Furthermore, we searched Particle Irradiation Data Ensemble Version 3.2 (PIDE 3.2), a database including in vitro data for ions and cell lines established by GSI (https://www.gsi.de/bio-pide). We also screened all the references in the included studies to ensure no available publications were omitted.
Literature selection and criteria
We used the following selection criteria: (1) articles reporting in vitro studies of PCa cell lines irradiated by carbon ion or proton beam; (2) articles reporting at least one of these following outcomes: (i) cell clonogenic survival; (ii) DNA damage response and repair (DDR/R) (e.g., cell cycle checkpoints, DSB repair, or apoptosis); (iii) motility, migration or invasion; (iv) OER or standard enhancement ratio (SER) evaluating the effect of combination therapy on colony forming assay; (3) articles published in English. Articles not matched the selection criteria were excluded. Other exclusion criteria included the following: (1) using artificially modified cells lines; (2) review, editorial material, comment or conference/meeting abstract; (3) pilot studies and research projects; and (4) full text was not available.
After we imported the retrieved articles (as several RIS format files from different sources) into EndNoteX9 software, the duplicate publications were excluded automatically. Two trained investigators independently did the literature screening by reading the titles and abstracts, then evaluated potential full texts and determined eligibility. All conflicts were resolved by discussion with a senior investigator to achieve consensus.
Data extraction
After pilot testing our predefined data extraction forms, two trained investigators independently extracted relevant data from the studies. The main contents of them included general characteristics (first author, year of publication and country), irradiation information (particle type, particle accelerator facility or institution, initial energy, average LET, SOBP, dose rate, and dose group), cell line type and origin (human or animal), RBE, survival fraction (SF), SER of different combinations therapy and OER. All conflicts were resolved by discussion with a senior investigator to achieve consensus.
When the article failed to specify SF, then data were extracted from the survival curves in published plots using Web Plot Digitizer Version 4.5 (https://automeris.io/WebPlotDigitizer) to convert datapoints into numerical values, or calculated by following formula:
where D is the delivered dose, α and β are fitting constants representing the initial slope and the curvature of the survival curve if they were reported. When the article failed to specify SER, data were extracted in the same way, then determined the SER by calculating the ratio of doses at 10% survival level (SF = 0.1) in treated and control groups (namely, SER10, but abbreviated as SER in this article) [21].
Risk of bias assessment
As of today, there is still no accepted standard risk of bias assessment tool to refer to for in vitro studies. Our team has established these criteria by ourselves after referring to some acknowledged risk-of-bias tools [22–24]. In this review, we refined and updated the content over the last version (Additional file 2: Table S1). Two trained investigators independently assessed the risk of bias, and all conflicts were resolved by discussing with a senior investigator to achieve consensus.
Statistical analysis
All the parameters we extracted were conducted as descriptive statistics. The continuous data from RBE, SF, SER and OER were represented as the mean ± standard deviation or median with interquartile ranges. All analyses were done using GraphPad Prism software (version 9.3.0) and R statistical software (version 4.1.2).
Results
Search results
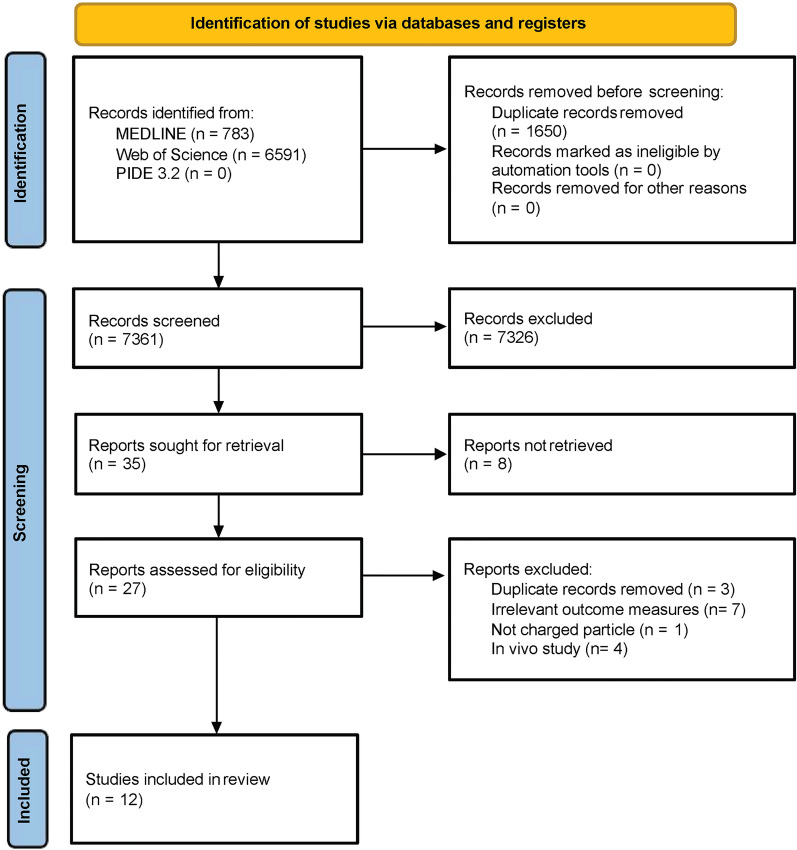
Our systematic search identified 7361 records were potentially eligible for review after 1650 duplication records were removed. 7326 records were excluded based on the screening of the titles and abstracts. Of the remaining 35 records, 8 failed to retrieve the full text because all of them were conference or meeting abstracts. Full texts of 27 records were read, ultimately, a total of 12 studies (7 for carbon ion irradiation, 4 for proton irradiation and 1 for both) met the inclusion criteria (Fig. 1).
Fig. 1.
PRISMA flow diagram of the systematic review. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Study characteristics
The included studies were published from 2011 to 2020, seven were on carbon ion irradiation [25–31], four on proton irradiation [32–35] and one on both [36]. These studies were completed in seven countries: four of them in Belgium [26–28, 36], three in China [30, 31, 33], and one each in India [25], the United Kingdom [32], Germany [29], Austria [34], and the United States of America [35]. Almost all of the cell lines used in these studies were of human origin: PC-3 [25–28, 31, 33, 36], DU-145 [32, 34, 35], LNCap [30], only one used rat origin RAT-1 cell line [29]. The studies varied in the initial energy, averaged LET, SOBP, dose groups, and dose rate used. It should be noted that only 4 studies reported the SOBP value [29, 32, 35, 36], and 1 used monoenergy [33], 1 used multi-energy but no SOBP value (carbon ion beam) [36], the other studies unspecified monoenergy or multi-energy. 3 studies combined with drugs, such as GLI antagonist (GANT61, inhibitor of GLI1/2), polynucleotide kinase/phosphatase inhibitor (PNKPi) and gold (Au) nanoparticles (GNPs) [25, 35, 36]. One study demonstrated the effect of acute oxygen depletion on cell survival for different types of radiation [29]. At last, 9 control groups were treated using X-ray [26–29, 31, 32, 34–36], and the other 3 control groups were not irradiated. The basic characteristics of the included studies are summarized in Table 1.
Table 1.
Overview of the different experimental parameters used in the studies
| Author (year) | Country | Charged particle | Accelerator facility or institution; location | Cell type | Cell origin | Initial energy | Average LET (keV/µm) | SOBP | Dose rate | Dose group | Combination therapy | Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang 2019 | China | Carbon Ion | HIRFL; Lanzhou, China | LNCaP | Human | 80 ~ 100 MeV/u | NA | NA* | 1 Gy/min | 0.5, 4 Gy | NA | No IR |
| Konings 2019 | Belgium |
Carbon Ion And Proton |
GANIL; Caen, France iThemba LABS; Cape Town, South Africa |
PC-3 | Human |
95 MeV/u 200 MeV |
73 3.96 |
NA† 50 mm |
NA |
0, 0.25, 0.5, 1, 2, 3, 4 Gy 0.25, 0.5, 2, 4, 6 Gy |
GANT61 | X ray |
| Srivastava 2018 | India | Carbon Ion | IUAC; New Delhi, India | PC-3 | Human | 7.08 MeV/u | NA | NA* | NA | 0, 2, 4 Gy | PNKPi | No IR |
| Butterworth 2012 | UK | Proton | MGH; Boston, USA | DU-145 | Human | 178 MeV | NA | 45 mm | NA | 0.5, 1, 2, 4, 6 Gy | NA | X ray |
| Khachonkham 2020 | Austria | Proton | NA | DU-145 | Human | 127.2–180.1 MeV | 1.9, 2.5, 4.1 (within SOBP); 1.0, 1.2 (before peak) | NA* | NA | 0, 0.5, 1, 2, 4, 6 Gy | NA | X ray |
| Tinganelli 2013 | Germany | Carbon Ion | GSI; Darmstadt, Germany | RAT-1 | Rodent | NA | 100, 150 | 10 mm | NA | NA | NA | X ray |
| Suetens 2016 | Belgium | Carbon Ion | GANIL; Caen, France | PC-3 | Human | 75 MeV/u | 33.7 | NA* | NA | 0, 0.5, 1, 2 Gy | NA | X ray |
| Polf 2011 | USA | Proton | NA | DU-145 | Human | 160 MeV | NA | 100 mm | NA | 0, 1, 2, 3, 4, 6 Gy | Gold nanoparticles | X ray |
| Suetens 2015 | Belgium | Carbon Ion | GANIL; Caen, France | PC-3 | Human | 75 MeV/u | 33.7 | NA* | NA | 0, 0.5, 2.0 Gy | NA | X ray |
| Chen 2020 | China | Proton (Microbeam) | NIRS; Chiba, Japan | PC-3 | Human | 3.4 MeV | 11.7 | monoenergy | NA | 0, 100, 250, 500 protons | NA | No IR |
| Wang 2020 | China | Carbon Ion | HIRFL; Lanzhou, China | PC-3 | Human | 81 MeV/u | 32.54 | NA* | 2 Gy/min | 0, 1, 2, 4, 6, 8 Gy | NA | X ray |
| Suetens 2014 | Belgium | Carbon Ion | GANIL; Caen, France | PC-3 | Human | 75 MeV/u | 33.7 | NA* | NA | 0, 0.5, 2.0 Gy | NA | X ray |
LET linear energy transfer, SOBP spread-out Bragg peak, IR irradiation, GANT61 GLI antagonist, PNKPi polynucleotide kinase/phosphatase inhibitor, NA not available
a Unspecifying monoenergy or multi-energy
b Multi-energy but without SOBP value
Risk of bias
There were five studies did not describe the method of cell counting, resulting in high risk of selection bias [27–30, 35]. All 12 studies reported the implementation process of experiment; but four studies did not describe the details of irradiation completely, resulting in moderate risk of performance bias [29, 30, 32, 35]. All 12 studies described the methods of measuring the results. 1 study did not repeat the data of experiment results, resulting in moderate risk of attrition bias [35], and 2 studies did not report whether the experiments were repeated [26, 33]. Only 1 study did not report the culture conditions of the cell and cell origin, resulting in a high and a moderate risk of cell-related bias [35]. In addition, one study failed to specify whether there was industry sponsoring involved [29]. These mentioned above results of the risk of bias assessment are shown in Fig. 2 and Additional file 2: Table S2.
Fig. 2.
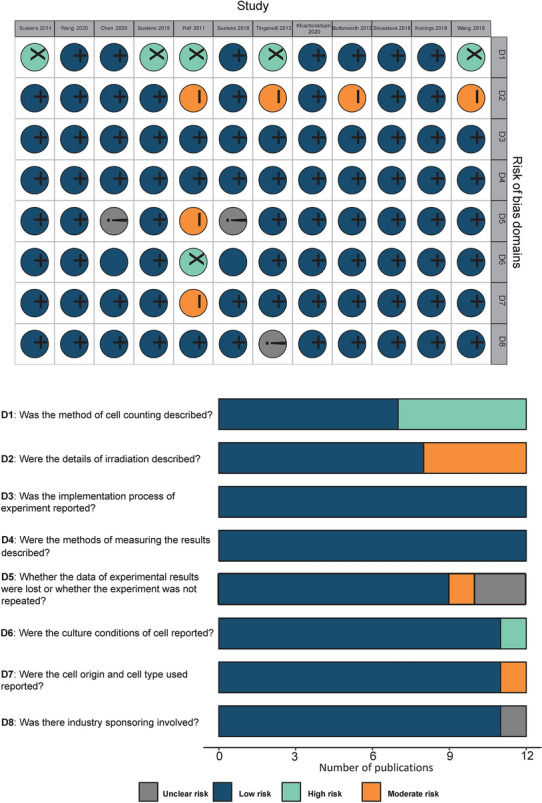
Results of the risk of bias assessment
RBE value
RBE value is a parameter that quantitatively expresses the difference in biological effects due to different types of irradiation, which is defined as the dose ratio between the reference photon radiation (usually as 250 kVp X-rays or Co-60 γ-rays) and the particle radiation that produces the same biological endpoint (usually as cell-killing) [37, 38]. In our review, 5 studies declared that they used linear-quadratic model to ascertain RBE values [28, 29, 34–36], the other 7 did not mention what model they used. In total, there were six studies reported 13 RBE values using three kinds of PCa cell lines. Among the 13 RBE values, 9 were proton irradiation (range 0.94–1.52) [26, 32, 34–36], and 4 were carbon ion irradiation (range 1.67–3.7) [28, 29, 36]. Tinganelli et al. reported RBE value for carbon ion irradiation was 2.8 ± 0.2 under normoxia and 3.7 ± 0.1 under anoxia in RAT-1 cell line [29]. Polf et al. reported that the RBE value for proton irradiation alone was 1.3 but could reach to 1.5 when combined with internalized GNPs [35] (Table 2 and Fig. 3).
Table 2.
RBE values carbon ion/proton in prostate cancer cell lines
| Author, year | RBE model | RBE value |
|---|---|---|
| Konings 2019 | L–Q | PC-3: 0.94 (proton) and 1.93 (carbon ion) |
| Butterworth 2012 | Not reported | DU-145: 1.1 |
| Khachonkham 2020 | L–Q | DU-145: 1.28 ± 0.25, 1.37 ± 0.17 and 1.52 ± 0.17 (within SOBP); 1.27 ± 0.27, 0.97 ± 0.4 (before peak)a |
| Tinganelli 2013 | L–Q | RAT-1: 2.8 ± 0.2 (oxic) and 3.7 ± 0.1 (anoxic) |
| Polf 2011 | L–Q | DU-145: 1.3 (untreated) and 1.5 (Au-treated) |
| Suetens 2015 | L–Q | PC-3: 1.67 |
RBE relative biological effectiveness, L–Q linear–quadratic
a Data were from RBE10, RBE2Gy and RBE4Gy were not shown in this table
Fig. 3.
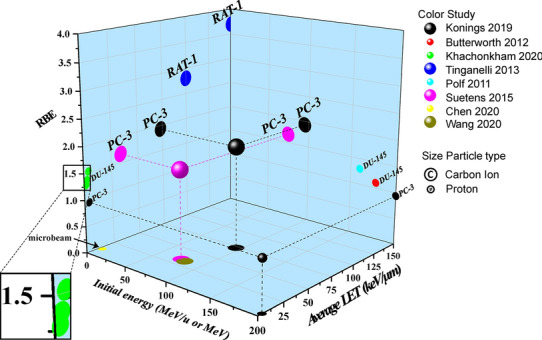
RBE, average LET, initial energy, particle type and cell line of the included studies. RBE relative biological effectiveness, LET linear energy transfer
Clonogenic Survival
According to the data given in the studies and prudential calculation conducted by ourselves, we obtained the SF values under different doses of carbon ion, proton, and photon irradiation from ten studies [25, 28–36] (Table 3). For example, SF2 means SF under 2 Gy irradiation. In this review, SF2 were 0.17 ± 0.12, 0.55 ± 0.20 and 0.53 ± 0.16 under carbon ion, proton, and photon irradiation, respectively. Figure 4 clearly indicates that carbon ion irradiation was more effective in clonogenic survival compared with X-rays or protons. The combinations of particle irradiation with drugs such as GANT61, PNKPi and GNPs also enhance the efficacy in cell killing, detailed information will be described in the SER section.
Table 3.
SF values of prostate cancer cells irradiated by carbon ion, proton and photon
| Carbon ion irradiation | Proton irradiation | Photon irradiation | |
|---|---|---|---|
| SF1 | 0.42 ± 0.16 | 0.77 ± 0.13 | 0.78 ± 0.15 |
| SF2 | 0.17 ± 0.12 | 0.55 ± 0.20 | 0.53 ± 0.16 |
| SF3 | 0.033 ± 0.017 | 0.21 ± 0.02 | 0.34 ± 0.18 |
| SF4 | 0.065 [0.038, 0.092] | 0.28 ± 0.19 | 0.31 ± 0.13 |
| SF5 | NA | NA | 0.16 [0.01, 0.21] |
| SF6 | NA | 0.035 ± 0.007 | 0.12 ± 0.06 |
| SF8 | NA | 0.007 ± 0.002 | 0.041 ± 0.025 |
SF surviving fraction, NA not available
Data were mean ± standard deviation or median with interquartile ranges
Fig. 4.
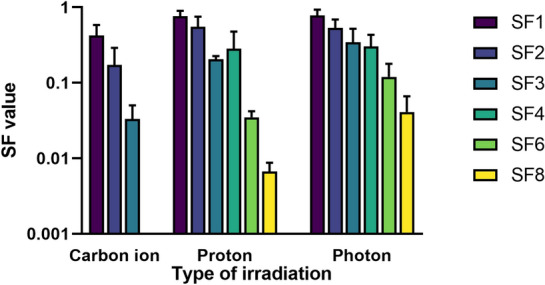
SF values of prostate cancer cells irradiated by carbon ion, proton and photon. SF surviving fraction
The effects of DDR/R
There were 5 studies reported the DDR/R induced by particle irradiation in total [25, 26, 30, 31, 33]. Wang et al. found that carbon ion irradiation induced cell cycle arrest at G0-/G1-phase via overexpression of miR-16-5p [30]. Three studies demonstrated that carbon ion irradiation, alone or combined with drug, can induce cell cycle arrest at G2-/M-phase at different levels [25, 26, 31]. 2 studies investigated the DDR/R in terms of apoptosis. Srivastava et al. proved that combined treatment of carbon ion beam and PNKPi further stimulated apoptosis on the basis of carbon ion irradiation alone through apoptotic body and nucleosomal DNA ladder formation [25]. The other one proved that carbon ion irradiation can lead to higher rates of apoptosis compared to X-ray [31]. Three studies investigated DSB repair. Chen et al. reported relative expression levels of γ-H2A histone family member X (γ-H2AX) were time dependent after proton irradiation [33]. Suetens et al. found dose-dependent increase in γ-H2AX foci numbers and foci occupancy after exposure to carbon ion irradiation [26]. Wang et al. proved that carbon ion irradiation can increase not only γ-H2AX foci numbers but also the foci lasting time and size compared with X-ray [31]. The details are summarized in Table 4.
Table 4.
DDR/R of prostate cancer cells after carbon ion/proton irradiation
| Author, year | Treatment | Outcome | Finds |
|---|---|---|---|
| Wang 2019 | Carbon ion irradiation | Cell cycle checkpoints | ↑miR‐16‐5p; G0-/G1-phase arrest |
| Srivastava 2018 | Carbon ion irradiation + PNKPi |
Cell cycle checkpoints Apoptosis |
G2-/M-phase arrest Inducing the apoptosis through apoptotic body and nucleosomal DNA ladder formation |
| Suetens 2016 | Carbon ion irradiation |
DSB repair Cell cycle checkpoints |
↑γ-H2AX foci numbers and foci occupancy Permanent G2-/M-phase arrest |
| Chen 2020 | Proton irradiation | DSB repair | ↑levels of γ-H2AX |
| Wang 2020 | Carbon ion irradiation |
DSB repair Cell cycle checkpoints Apoptosis |
↑γ-H2AX foci numbers, lasting time and size G2-/M-phase arrest The rates of apoptosis were 27.34% and 37.93% after 2 and 4 Gy carbon ion irradiation, respectively (versus 14.1% and 23.59% following 2 and 4 Gy X-ray irradiation, respectively) |
DDR/R DNA damage response and repair, PNKPi polynucleotide kinase/phosphatase inhibitor, DSB DNA double-strand break
↑, increase
Motility and migration ability
There were only 3 studies investigated the cell motility, migration or their related genes expression after charged particle irradiation. Konings et al. showed carbon ion irradiation displayed a stronger suppression effect regarding migration of PC-3 cells than X-rays and protons by down-regulating VEGFA [36]. Suetens et al. reported in two sequential articles that tumor motility-related genes such as CCDC88A, ROCK1, NEXN, FN1, MYH10 and MYH9 were downregulated after 2 Gy carbon ion irradiation; among them, CCDC88A, ROCK1, FN1, and MYH9 were also down-regulated after 0.5 Gy carbon ion irradiation [27, 28].
SER and OER
The SER values of combination therapy were reported in three studies, in which only one study reported the OER. Konings et al. proved that different types of irradiations combined with GANT61 can scarcely enhance the therapeutic effect; the SER values were 1.07, 0.98 and 1.09 for carbon ion, proton and photon irradiation, respectively [36]. Srivastava et al. concluded that the SER values of carbon ion irradiation combined with PNKPi increased with the concentration of the PNKPi in the range of 0.5 to 10 Μm [25]. Polf et al. demonstrated that proton irradiation combined with internalized gold nanoparticles can enhance the efficacy of therapy with a SER value of 1.15 [35]. Tinganelli et al. elaborated that the influence of acute hypoxia and irradiation on RAT-1 cells with a mean OER of 1.77 ± 0.13 for carbon ion and 2.32 ± 0.04 for photon irradiation [29]. The details are presented in Table 5 and Fig. 5.
Table 5.
SER values of combination therapy and OER
| Author, year | Combination therapy (Dose) | Cell line | SER | ||
|---|---|---|---|---|---|
| Carbon ion irradiation | Proton irradiation | Photon irradiation | |||
| Konings 2019 | GANT61 10 μM | PC-3 | 1.09 | 0.98 | 1.07 |
| Srivastava 2018 |
PNKPi 0.5 μM PNKPi 1.0 μM PNKPi 5 μM PNKPi 10 μM |
PC-3 PC-3 PC-3 PC-3 |
1.21 1.29 1.47 2.05 |
NA NA NA NA |
NA NA NA NA |
| Polf 2011 | Gold nanoparticles | DU-145 | 1.15 | ||
| OER | |||||
| Tinganelli 2013 | NA | RAT-1 | 1.77 ± 0.13 | NA | 2.32 ± 0.04 |
SER standard enhancement ratio, OER oxygen enhancement ratio, GANT61 GLI antagonist, PNKPi polynucleotide kinase/phosphatase inhibitor, NA not available
Fig. 5.
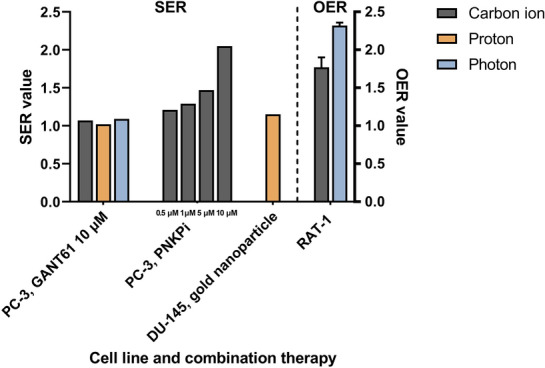
SER values of combination therapy and OER. SER standard enhancement ratio, OER oxygen enhancement ratio
Discussion
RBE value
LET is defined as the amount of energy transferred per track. Irradiation with high LET can cause more severe damage to cells, resulting in complicated DNA damage that is difficult to repair. The LET of a particle is influenced mainly by its charge and velocity. In general, more charge and less velocity can lead to a higher LET. That is the reason why carbon ions have a higher LET value than photons and protons. The RBE is an important benchmark that quantifies the difference in biological effects caused by the different LET. A higher RBE means more biological effects at equivalent doses. The most widely reported generic RBE value for protons is 1.1, which is based on a 10% survival rate, mainly used in clinical practice. Here, actually, RBE is a complex function, this value can be determined by various parameters. A growing number of evidences indicate that RBE varies with the LET with respect to given tissue depth, energy, particle type, the dose per fraction, oxygenation state, DNA repair status, cell cycle phase, the biological endpoint and the type of the tissue/cell (α/β ratio) [39, 40]. An in vitro study using the human prostate carcinoma cell line DU-145 irradiated by proton has shown a clear increase in experimental RBE values with LET, especially within the SOBP, with the highest value at the distal edge of the Bragg peak, and a significant decrease for higher doses [34]. The recognized RBE for carbon ion is generally estimated to be 2.5–3, however, values as high as 5 have also been reported [41]. Similarly, the RBE of carbon ion is also determined by these parameters. However, some of these parameters have significant differences in their effects on carbon ion and proton RBE. The RBE of proton with low LET increases slowly with LET value, but the RBE of carbon ion with high LET can reach a maximum at approximately 100–200 keV/μm and then decreases (overkill effect). Furthermore, the RBE of carbon ion is relatively less affected by oxygenation state than that of proton because the OER decreases as the LET increasing [16]. Significantly, the results of a most recent prospective randomized clinical trial revealed that local effect model (LEM) I and α/β = 2 Gy overestimated the RBE of carbon ions in PCa treatment, since RBE-weighted dose was strongly dependent on the α/β ratio as well as the RBE-model [42]. The study concluded that using LEM I with α/β = 4 Gy to adjust the biological dose calculation might be a more practical approach.
DNA damage and repair
The biological effect was induced by ionization events along the particle track, and these events can cause damage to DNA and other relevant biomolecules. Ionization events caused by photons (low LET irradiation) exhibit both a direct and indirect component. However, high LET-charged particle radiation primarily induced more clustered DNA damage by direct ionizations. The types of clustered DNA damage include chemically altered base lesions (oxidized purines or pyrimidines), abasic sites, intrastrand crosslinks, single-strand breaks (SSBs), and DSBs [43]. Clustered DNA damage is a serious impediment to effective repair mechanisms, with DSBs inside clustered lesions rejoining with slower kinetics and less thoroughly than frank DSBs, resulting in induction of genomic instability [44]. When DNA damage is detected, the corresponding DDR/R mechanism is triggered. The failure of cells to deal with cluster DNA lesions effectively has a significant influence on their normal function and survival. Complex lesions can lead to mutations, the loss of large parts of the genome, and even apoptotic cell death if they are unrepaired or misrepaired [45, 46].
DNA repair pathways in mammalian cells are as follows: base excision repair, nucleotide excision repair, mismatch repair, and the pathways responsible for the repair of DSBs, namely, homologous recombination (HR), classical non-homologous end joining (c-NHEJ), backup non-homologous end joining (b-NHEJ), and single-strand annealing [47]. The selection of the DNA DSB repair pathway is predominantly determined by radiation quality and potentially by DSB load. It is anticipated that the production of DNA lesions with variable degrees of complexity would concurrently activate several DNA repair mechanisms [48, 49]. In low LET irradiation-induced DSB, c-NHEJ is the main pathway in the G1- and early S-phases of the cell cycle, whereas both HR and c-NHEJ were activated in the late S- and G2-phases [50]. It is unclear if cells preferentially select a specific pathway to repair DSBs generated by high LET irradiation [51]. According to some studies, NHEJ is less effective at removing clustered DSBs caused by high LET irradiations than low LET irradiation [52–54]. Saha et al. indicated that the HR pathway may be preferable for the repair of clustered DNA damage caused by heavy charged particles [55]. Gerelchuluun et al. also found that compared to gamma rays and protons, the HR pathway seems to play a more important role in the repair of DSBs in carbon ions [56]. Interestingly, some studies showed that NHEJ may play a main role in carbon ion-induced damage repair, though the using of HR was increased [57–59]. Sridharan et al. indicated that a major complex of c-NHEJ has been implicated in the repair of high-LET radiation-induced clustered DSBs [60]. NHEJ inhibitors have been shown to be more effective than HR after carbon ion irradiation [61, 62]. There were also scholars assumed that NHEJ is essential for processing DNA DSB regardless of the irradiation quality, whereas the significance of HR repair increases when proton irradiation is used [63]. Soni et al. concluded that the choice of pathways may be dose-dependent; when HR becomes saturated under a high dose irradiation, NHEJ could be activated further [64]. This is a critical issue that should be addressed soon as it concerns whether NHEJ inhibitors or HR inhibitors should be used as a combination therapy for CPT.
The genetic background of cell lines may have distinct effects on radiation response for different radiation qualities, genetic defects in DNA repair and DDR genes in cancer cells are important factors [65]. HR-deficient cells and wild-type cells with small interfering RNA-downregulated RAD51 were significantly hypersensitive to proton irradiation, resulting in an elevated relative biological effectiveness compared to the relative biological effectiveness determined for wild-type cells. In contrast, the absence of nonhomologous end-joining did not result in hypersensitivity to proton exposure [66]. Andrea et al. also found human BRCA2-deficient ovarian cancer cells were hypersensitive to proton irradiation compared with photon irradiation [67]. Response to clustered DNA damage repair after particle irradiation is influenced by a transition of ataxia–telangiectasia mutated (ATM) and RAD3-related transition at lesion sites and switch from NHEJ to HR [68–70]. Besides, NHEJ deficiency is more essential than proton LET in determining cell survival. BRCA1 mutation-disrupted cells exhibited increased radiosensitivity for high-LET protons exclusively, whereas RAD51 depletion resulted in increased radiosensitivity for both photons and protons [71].
Histone H2AX, a variation of histone H2A, is one of the key proteins responsible for genome integrity monitoring [72]. H2AX becomes phosphorylated on Ser139 in response to DNA damage, which is defined as γ-H2AX, especially when the damage includes the induction of DSBs [73]. When cluster DNA damage occurs, the marker γ-H2AX can remain for long periods [51]. Ibanez et al. proved that γ-H2AX foci size is an accurate parameter for correlating the rejoining of DSBs induced by different LET radiations and radiosensitivity [74]. The studies included in our review also confirmed that the γ-H2AX level, γ-H2AX foci numbers, occupancy, lasting time and size increased after carbon ion and proton (11.7 keV/µm) irradiation in PC-3 cells [26, 31, 33]. Furthermore, the studies also found that carbon ion irradiation inhibited human PCa cell (LNCaP and PC-3) proliferation by inducing G0-/G1- and G2-/M-phase arrest [25, 26, 30, 31], and eventually resulted in more apoptosis compared to photon irradiation [25, 31].
Epigenetic regulation of DNA repair may also be reliant on radiation quality in addition to proteins directly engaged in DNA DSB rejoining [75–77]. Numerous studies have suggested that ubiquitination, methylation, and acetylation in DNA repair are important epigenetic pathways for targeting in particle therapy [78–82]. A previous study indicated that histone H2B ubiquitylation improves the repair of clustered DNA lesions, resulting in increased survival following exposure to high-LET radiation [79]. Targeting the ubiquitination pattern can thereby sensitize the tumor (high-LET) but not the normal tissue (low-LET) during heavy ion therapy, hence expanding the therapeutic window [77]. The pattern of DNA methylation in cells that survive being exposed to charged particles or X-rays seems to be very different [83]. In addition, histone deacetylase inhibitors appear to promote cell death more efficiently following proton or carbon ion irradiation than following X-ray exposure [81, 82]. All of this evidence seems to indicate that the radiation-induced epigenetic profile is influenced by radiation quality rather than LET alone.
Motility and migration ability
Although the literatures examining gene expression after exposure to charged particle irradiation on tumor cells are relatively limited, we can still obtain some valuable information about motility, migration or invasion. Fujita et al. found that carbon ion irradiation suppressed the migration and invasion of human pancreatic cells (MIAPaCa-2, BxPC-3 and AsPC-1) via the Rho/ROCK signaling pathway [84]. Akino et al. showed that carbon ion irradiation effectively suppressed migration and invasion of human non-small-cell lung cancer (NSCLC) cells (A549 and EBC-1) via down-regulating ANLN [85]. Likewise, proton irradiation has been shown in vitro and in vivo to repress pro-angiogenic gene expression as well as reduce cell motility genes [86, 87]. However, Maruta et al. believed that the motility of A549 cells was increased by carbon ion irradiation via the Rho/ROCK signaling pathway [88]. As mentioned earlier, motility and migration were suppressed in PC-3 cells after carbon ion irradiation by down-regulating several genes, and low expression of CCDC88A, FN1, NEXN and ROCK1 may be associated with better prognosis [26, 27]. Konings et al. focused on the effects of different radiation types on hedgehog (Hh) signaling pathway and target genes. They concluded carbon ion irradiation suppressed the migration of PC-3 cells more than both photon and proton by down-regulating VEGFA [36].
SER
We also noticed that there were a number of in vitro studies conducted with respect to different perspectives on biological effects of cancer cells induced by charged particles in combination with drugs, including chemotherapy, immunotherapy, nanoparticles and targeted therapy. In targeted therapy combination, many studies investigated a variety of typical targets or signaling pathways in different cancer cells: the combination of poly (ADP-ribose) polymerase (PARP) inhibitors and charged particle irradiation in NSCLC [89, 90], pancreatic cancer [89–91], glioblastoma (GBM) [92], and cervix carcinoma [92] cells; the combination of epidermal growth factor receptor (EGFR) and downstream mammalian target of rapamycin (mTOR) inhibitors and charged particle irradiation in chondrosarcoma [93], hepatocellular carcinoma [94], NSCLC [95], and head and neck squamous cell carcinoma (HNSCC) cells [96]; the combination of heat shock protein 90 (Hsp90) inhibitors and carbon ion irradiation in chondrosarcoma [97], NSCLC [98, 99] and cervix carcinoma [98, 99] cells; and the combination of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) inhibitors and charged particle irradiation in HNSCC [100], breast cancer [57], cervix carcinoma [57], NSCLC [101], and GBM cells [62]. In PCa, Hh inhibitor GANT61 failed to sensitize the cells to proton and carbon ion radiation, with SER values of 0.98 and 1.07; meanwhile, the migration of cancer cells was not inhibited by the combination of two particle irradiation and GANT61 compared with combined with X-ray [36]. Polynucleotide kinase/phosphatase (PNKP) is an enzyme that plays an important role in NHEJ. Srivastava et al. found that when carbon ion irradiation was combined with PNKPi, PC-3 cells experienced considerable apoptosis, and cell cycle arrest was also increased during the G2-/M-phase [25]. Besides, it was proven that metallic-based nano-agents (e.g., NPs, nanocauliflowers, and nanocrystals) expressed radiosensitizing and synergistic effects for radiotherapy. Many studies also demonstrated that nano-agents can be potent in combination with proton [102–107] and carbon ion [108–111] irradiation for the treatment of different malignancies both in vivo and in vitro. Polf et al. showed that the effectiveness of proton radiotherapy for the killing of prostate tumor cells (PC-3) was increased by approximately 15–20% (SER10 = 1.15, SER50 = 1.2) for those cells containing internalized GNPs [35].
OER
While there was only one included study that reported the OER of carbon ions, it is still necessary to discuss hypoxia. Hypoxia is very common in malignant solid tumors and is associated with malignancy and a poor prognosis. In radiobiology, oxygen-dependent indirect DNA damage is reduced when hypoxia occurs. A major proportion of this damage is from the production of reactive oxygen species (ROS) (the rest is from the radiolysis of water). When the partial pressure of oxygen (pO2) decreases, fewer oxygen molecules become available, impacting the generation of ROS, which can lead to increased radioresistance. Therefore, the concept of OER was proposed to quantify the radioresistance. The OER of carbon ions decreases with LET at the same oxygen concentration (under 21%) level as well as with oxygen concentration at the same LET value [112, 113]. Hypoxia-inducible factor-1α (HIF-1α; encoded by HIF1A) is a transcription factor that regulates several genes in response to hypoxic stimuli, including those involved in tumorigenesis and malignant progression, such as proliferation, metabolic changes, neoangiogenesis, invasion, metastasis, and treatment resistance [114–116]. Several studies demonstrated that carbon ion irradiation reduced HIFs expression in cancer stem cell (CSC) subpopulations of HNSCC cells (SQ20B-CSCs and FaDu-CSCs) and GBM cells (U251, GL15) compared to photon irradiation under hypoxia [117–119]. It was also validated in an in vivo experiment [120]. In general, carbon ion irradiation seems to have promising potential for reducing radioresistance caused by hypoxia, particularly in severely hypoxic malignancies, such as PCa and pancreatic cancer [112].
Limitations
The findings of this review should be interpreted within the context of its limitations. First, searching only English databases can lead to certain language biases. Furthermore, due to the scarcity of literature reporting radiobiological responses in PCa, as well as the diversification of outcome assessment tools, the validity of our results may be challenged. Lastly, the findings of in vitro studies do not necessarily agree exactly with in vivo studies, more in vivo data accumulation is required. Notwithstanding, we collected the most current information we could obtain, indicating the most recent evidence for charged particle irradiation on PCa in vitro. We hope this review could prompt further fundamental and clinical research regarding this matter.
Conclusions
To the best of our knowledge, this systematic review is the first study to pool the radiobiological effects of carbon ion and proton irradiation on PCa cell lines, including cell survival (as SF), DDR/R, motility and migration ability, SER and OER. In general, we believe it is plausible to conclude that both carbon ion and proton irradiation have advantages in radiobiological effect over photon irradiation on PCa cell lines. Combination therapy may enhance the gain ratio of CPT for PCa. Based on the information we have right now, carbon ion irradiation seems to have further advantages over proton irradiation.
Supplementary Information
Additional file 1. Specific search strategies for MEDLINE, Embase, and Web of Science.
Additional file 2: Table S1. Risk of bias scheme. Table S2. Results of the risk of bias assessment.
Additional file 3. PRISMA 2020 checklist.
Acknowledgements
Tian-Qi Du would like to express his sincere gratitude to his uncles and aunts for their longstanding support of his academic and personal life. All authors would like to acknowledge the reviewers for their valuable comments.
Abbreviations
- PCa
Prostate cancer
- RT
Radiation therapy
- IMRT
Intensity-modulated radiation therapy
- CPT
Charged particle therapy
- SOBP
Spread-out Bragg peak
- LET
Linear energy transfer
- RBE
Relative biological effectiveness
- DSBs
Double-strand breaks
- OER
Oxygen enhancement ratio
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- DDR/R
DNA damage response and repair
- SER
Standard enhancement ratio
- SF
Survival fraction
- GNPs
Gold (Au) nanoparticles
- γ-H2AX
γ-H2A histone family member X
- LEM
Local effect model
- SSBs
Single-strand breaks
- HR
Homologous recombination
- NHEJ
Homologous end joining
- NSCLC
Non-small-cell lung cancer
- GBM
Glioblastoma
- HNSCC
Head and neck squamous cell carcinoma
- PNKP
Polynucleotide kinase/phosphatase
- ROS
Reactive oxygen species
- CSC
Cancer stem cell
- HIF
Hypoxia-inducible factor
Author contributions
Study conception and design, investigation, results interpretation, writing (original draft preparation), TQD and RL; contribution to an oversight of the overall study, HL, YC, MT, QW, XW, ZL and SS; writing (review and editing), KY and JT; supervision, project administration, QZ and XW. All authors read and approved the final manuscript.
Funding
This study was supported by Science and Technology Plan Project of Chengguan District of Lanzhou (No.2020-2-2-5), the Talent Innovation and Venture Project of Lanzhou City (Grant nos. 2021-RC-125 and 2020-RC-113), the Key R&D Program of Science and Technology Department of Gansu Province (Grant No. 20YF8FA116), the 2021 “Hundred Cities and Hundred Parks” Action Project of Lanzhou National High-tech Zone, the authorized project of Lanzhou KejinTaiji Corporation, Ltd. (Grant No. BMP-B-02-002), and National Key R&D Program of China (No. 2022YFC2401500 and No. 2022YFC2401505). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Availability of data and materials
Research data are stored in an institutional repository and will be shared upon reasonable request to the author Tian-Qi Du (dutq21@lzu.edu.cn).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have no conflicts of interest to disclose regarding this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tian-Qi Du and Ruifeng Liu have contributed equally to this work and share first authorship
Contributor Information
Qiuning Zhang, Email: zhangqn@impcas.ac.cn.
Xiaohu Wang, Email: xhwang@impcas.ac.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Corkum M, Loblaw A, Hasan Y, Chung HT, Tseng CL, McGuffin M, et al. Prostate high dose-rate brachytherapy as monotherapy for prostate cancer: late toxicity and patient reported outcomes from a randomized phase II clinical trial. Radiother Oncol. 2021;156:160–165. doi: 10.1016/j.radonc.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Leite E, Ramos C, Ribeiro V, Salvajoli BP, Nahas WC, Salvajoli JV, et al. Hypofractionated radiation therapy to the prostate bed with intensity-modulated radiation therapy (IMRT): a phase 2 trial. Int J Radiat Oncol Biol Phys. 2021;109(5):1263–1270. doi: 10.1016/j.ijrobp.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Murray J, Griffin C, Gulliford S, Syndikus I, Staffurth J, Panades M, et al. A randomised assessment of image guided radiotherapy within a phase 3 trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2020;142:62–71. doi: 10.1016/j.radonc.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai M, Gergelis KR, Sir M, Whitaker TJ, Routman DM, Stish BJ, et al. Comparing bowel and urinary domains of patient-reported quality of life at the end of and 3 months post radiotherapy between intensity-modulated radiotherapy and proton beam therapy for clinically localized prostate cancer. Cancer Med. 2020;9(21):7925–7934. doi: 10.1002/cam4.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsky AR, Carmona R, Verma V, Santos P, Both S, Bekelman JE, et al. Comparative analysis of 5-year clinical outcomes and patterns of failure of proton beam therapy versus intensity modulated radiation therapy for prostate cancer in the postoperative setting. Pract Radiat Oncol. 2021;11(2):195. doi: 10.1016/j.prro.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Kubeš J, Haas A, Vondráček V, Andrlík M, Navrátil M, Sláviková S, et al. Ultrahypofractionated proton radiation therapy in the treatment of low and intermediate-risk prostate cancer-5-year outcomes. Int J Radiat Oncol Biol Phys. 2021;110(4):1090–1097. doi: 10.1016/j.ijrobp.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Patel SA, Jani AB, Gillespie TW, Patel PR, Godette KD, et al. Overall survival after treatment of localized prostate cancer with proton beam therapy, external-beam photon therapy, or brachytherapy. Clin Genitourin Cancer. 2021;19(3):255–66.e7. doi: 10.1016/j.clgc.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, et al. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol. 2019;20(5):674–685. doi: 10.1016/S1470-2045(18)30931-8. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Kasuya G, Ishikawa H, Nomoto A, Ono T, Nakajima M, et al. Long-term clinical outcomes after 12-fractionated carbon-ion radiotherapy for localized prostate cancer. Cancer Sci. 2021;112(9):3598–3606. doi: 10.1111/cas.15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi M, Demizu Y, Fujii O, Terashima K, Niwa Y, Daimon T, et al. Proton therapy for localized prostate cancer: long-term results from a single-center experience. Int J Radiat Oncol Biol Phys. 2021;109(4):964–974. doi: 10.1016/j.ijrobp.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Takakusagi Y, Katoh H, Kano K, Anno W, Tsuchida K, Mizoguchi N, et al. Preliminary result of carbon-ion radiotherapy using the spot scanning method for prostate cancer. Radiat Oncol. 2020;15(1):127. doi: 10.1186/s13014-020-01575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vapiwala N, Wong JK, Handorf E, Paly J, Grewal A, Tendulkar R, et al. A pooled toxicity analysis of moderately hypofractionated proton beam therapy and intensity modulated radiation therapy in early-stage prostate cancer patients. Int J Radiat Oncol Biol Phys. 2021;110(4):1082–1089. doi: 10.1016/j.ijrobp.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Li P, Yu Q, Wu S, Chen X, Zhang Q, et al. Preliminary exploration of clinical factors affecting acute toxicity and quality of life after carbon ion therapy for prostate cancer. Radiat Oncol. 2019;14(1):94. doi: 10.1186/s13014-019-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Li X, Yao L, Han X, Yan W, Liu Y, et al. Clinical efficacy and safety of proton and carbon ion radiotherapy for prostate cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:709530. doi: 10.3389/fonc.2021.709530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Fukumitsu N, Ishikawa H, Nakai K, Sakurai H. A critical review of radiation therapy: from particle beam therapy (proton, carbon, and BNCT) to beyond. J Pers Med. 2021;11(8):825. doi: 10.3390/jpm11080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinganelli W, Durante M. Carbon ion radiobiology. Cancers. 2020;12(10):3022. doi: 10.3390/cancers12103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorat Y, Timm S, Jakob B, Taucher-Scholz G, Rübe CE. Clustered double-strand breaks in heterochromatin perturb DNA repair after high linear energy transfer irradiation. Radiother Oncol. 2016;121(1):154–161. doi: 10.1016/j.radonc.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waissi W, Paix A, Nicol A, Noël G, Burckel H. Targeting DNA repair in combination with radiotherapy in pancreatic cancer: a systematic review of preclinical studies. Crit Rev Oncol Hematol. 2020;153:103060. doi: 10.1016/j.critrevonc.2020.103060. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. Syrcle’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Liu R, Zhang Q, Luo H, Chen J, Dong M, et al. Charged particle irradiation for pancreatic cancer: a systematic review of in vitro studies. Front Oncol. 2021;11:775597. doi: 10.3389/fonc.2021.775597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava P, Sarma A, Chaturvedi CM. Targeting DNA repair with PNKP inhibition sensitizes radioresistant prostate cancer cells to high LET radiation. PLoS ONE. 2018;13(1):e0190516. doi: 10.1371/journal.pone.0190516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suetens A, Konings K, Moreels M, Quintens R, Verslegers M, Soors E, et al. Higher initial DNA damage and persistent cell cycle arrest after carbon ion irradiation compared to X-irradiation in prostate and colon cancer cells. Front Oncol. 2016;6:87–87. doi: 10.3389/fonc.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suetens A, Moreels M, Quintens R, Chiriotti S, Tabury K, Michaux A, et al. Carbon ion irradiation of the human prostate cancer cell line PC3: a whole genome microarray study. Int J Oncol. 2014;44(4):1056–1072. doi: 10.3892/ijo.2014.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suetens A, Moreels M, Quintens R, Soors E, Buset J, Chiriotti S, et al. Dose- and time-dependent gene expression alterations in prostate and colon cancer cells after in vitro exposure to carbon ion and X-irradiation. J Radiat Res. 2015;56(1):11–21. doi: 10.1093/jrr/rru070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinganelli W, Ma N, Von Neubeck C, Maier A, Schicker C, Kraft-Weyrather W, et al. Influence of acute hypoxia and radiation quality on cell survival. J Radiat Res. 2013;54:23–30. doi: 10.1093/jrr/rrt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Mao A, Tang J, Zhang Q, Yan J, Wang Y, et al. microRNA-16-5p enhances radiosensitivity through modulating Cyclin D1/E1-pRb-E2F1 pathway in prostate cancer cells. J Cell Physiol. 2019;234(8):13182–13190. doi: 10.1002/jcp.27989. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Xiao Y, Yan J, Huang G, Zhang J, Di C, et al. Carbon ion irradiation-induced DNA damage evokes cell cycle arrest and apoptosis via the pRb/E2F1/c-Myc signaling pathway in p53-deficient prostate cancer PC-3 cells. Nucl Sci Tech. 2021;32(3):30. doi: 10.1007/s41365-021-00861-7. [DOI] [Google Scholar]
- 32.Butterworth KT, McGarry CK, Clasie B, Carabe-Fernandez A, Schuemann J, Depauw N, et al. Relative biological effectiveness (RBE) and out-of-field cell survival responses to passive scattering and pencil beam scanning proton beam deliveries. Phys Med BioL. 2012;57(20):6671–6680. doi: 10.1088/0031-9155/57/20/6671. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Yu Q, Wang X, Li P, Zhang Q, Fu S. DNA damage response in prostate cancer cells by proton microbeam irradiation. Transl Cancer Res. 2020;9(8):4811–4819. doi: 10.21037/tcr-19-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khachonkham S, Mara E, Gruber S, Preuer R, Kuess P, Dörr W, et al. RBE variation in prostate carcinoma cells in active scanning proton beams: in-vitro measurements in comparison with phenomenological models. Phys Med. 2020;77:187–193. doi: 10.1016/j.ejmp.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Polf JC, Bronk LF, Driessen WHP, Arap W, Pasqualini R, Gillin M. Enhanced relative biological effectiveness of proton radiotherapy in tumor cells with internalized gold nanoparticles. Appl Phys Lett. 2011;98(19):193702. doi: 10.1063/1.3589914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konings K, Vandevoorde C, Belmans N, Vermeesen R, Baselet B, Van Walleghem M, et al. The combination of particle irradiation with the hedgehog inhibitor GANT61 differently modulates the radiosensitivity and migration of cancer cells compared to X-Ray irradiation. Front Oncol. 2019;9:391. doi: 10.3389/fonc.2019.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fokas E, Kraft G, An H, Engenhart-Cabillic R. Ion beam radiobiology and cancer: time to update ourselves. Biochim Biophys Acta. 2009;1796(2):216–229. doi: 10.1016/j.bbcan.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Uzawa A, Ando K, Koike S, Furusawa Y, Matsumoto Y, Takai N, et al. Comparison of biological effectiveness of carbon-ion beams in Japan and Germany. Int J Radiat Oncol Biol Phys. 2009;73(5):1545–1551. doi: 10.1016/j.ijrobp.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Mohan R, Grosshans D. Proton therapy—present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willers H, Allen A, Grosshans D, McMahon SJ, von Neubeck C, Wiese C, et al. Toward a variable RBE for proton beam therapy. Radiother Oncol. 2018;128(1):68–75. doi: 10.1016/j.radonc.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Weyrather WK, Debus J. Particle beams for cancer therapy. Clin Oncol. 2003;15(1):S23–S28. doi: 10.1053/clon.2002.0185. [DOI] [PubMed] [Google Scholar]
- 42.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104(6):1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 43.Blaisdell JO, Harrison L, Wallace SS. Base excision repair processing of radiation-induced clustered DNA lesions. Radiat Prot Dosimetry. 2001;97(1):25–31. doi: 10.1093/oxfordjournals.rpd.a006634. [DOI] [PubMed] [Google Scholar]
- 44.Asaithamby A, Uematsu N, Chatterjee A, Story MD, Burma S, Chen DJ. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat Res. 2008;169(4):437–446. doi: 10.1667/RR1165.1. [DOI] [PubMed] [Google Scholar]
- 45.Nickoloff JA, Sharma N, Taylor L. Clustered DNA double-strand breaks: biological effects and relevance to cancer radiotherapy. Genes. 2020;11(1):99. doi: 10.3390/genes11010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavlopoulou A, Asfa S, Gioukakis E, Mavragani IV, Nikitaki Z, Takan I, et al. In silico investigation of the biological implications of complex DNA damage with emphasis in cancer radiotherapy through a systems biology approach. Molecules. 2021;26(24):7602. doi: 10.3390/molecules26247602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikitaki Z, Velalopoulou A, Zanni V, Tremi I, Havaki S, Kokkoris M, et al. Key biological mechanisms involved in high-LET radiation therapies with a focus on DNA damage and repair. Expert Rev Mol Med. 2022;24:e15. doi: 10.1017/erm.2022.6. [DOI] [PubMed] [Google Scholar]
- 48.Sridharan DM, Asaithamby A, Bailey SM, Costes SV, Doetsch PW, Dynan WS, et al. Understanding cancer development processes after HZE-particle exposure: roles of ROS, DNA damage repair and inflammation. Radiat Res. 2015;183(1):1–26. doi: 10.1667/RR13804.1. [DOI] [PubMed] [Google Scholar]
- 49.Iliakis G, Mladenov E, Mladenova V. Necessities in the processing of DNA double strand breaks and their effects on genomic instability and cancer. Cancers. 2019;11(11):1671. doi: 10.3390/cancers11111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang H, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18(8):495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, et al. Carbon ion radiotherapy: a review of clinical experiences and preclinical research, with an emphasis on dna damage/repair. Cancers. 2017;9(6):66. doi: 10.3390/cancers9060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata A, Conrad S, Birraux J, Geuting V, Barton O, Ismail A, et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011;30(6):1079–1092. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yajima H, Fujisawa H, Nakajima NI, Hirakawa H, Jeggo PA, Okayasu R, et al. The complexity of DNA double strand breaks is a critical factor enhancing end-resection. DNA Repair. 2013;12(11):936–946. doi: 10.1016/j.dnarep.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Zafar F, Seidler SB, Kronenberg A, Schild D, Wiese C. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat Res. 2010;173(1):27–39. doi: 10.1667/RR1910.1. [DOI] [PubMed] [Google Scholar]
- 55.Saha J, Wilson P, Thieberger P, Lowenstein D, Wang M, Cucinotta FA. Biological characterization of low-energy ions with high-energy deposition on human cells. Radiat Res. 2014;182(3):282–291. doi: 10.1667/RR13747.1. [DOI] [PubMed] [Google Scholar]
- 56.Gerelchuluun A, Manabe E, Ishikawa T, Sun L, Itoh K, Sakae T, et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res. 2015;183(3):345–356. doi: 10.1667/RR13904.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X, Zhang X, Xie Y, Tanaka K, Wang B, Zhang H. DNA-PKcs inhibition sensitizes cancer cells to carbon-ion irradiation via telomere capping disruption. PLoS ONE. 2013;8(8):e72641. doi: 10.1371/journal.pone.0072641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi A, Kubo M, Ma H, Nakagawa A, Yoshida Y, Isono M, et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat Res. 2014;182(3):338–344. doi: 10.1667/RR13782.1. [DOI] [PubMed] [Google Scholar]
- 59.Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat Res. 2006;165(1):59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- 60.Sridharan DM, Whalen MK, Almendrala D, Cucinotta FA, Kawahara M, Yannone SM, et al. Increased Artemis levels confer radioresistance to both high and low LET radiation exposures. Radiat Oncol. 2012;7:96. doi: 10.1186/1748-717X-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma H, Takahashi A, Yoshida Y, Adachi A, Kanai T, Ohno T, et al. Combining carbon ion irradiation and non-homologous end-joining repair inhibitor NU7026 efficiently kills cancer cells. Radiat Oncol. 2015;10:225. doi: 10.1186/s13014-015-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Li P, Hirayama R, Niu Y, Liu X, Chen W, et al. Genistein sensitizes glioblastoma cells to carbon ions via inhibiting DNA-PKcs phosphorylation and subsequently repressing NHEJ and delaying HR repair pathways. Radiother Oncol. 2018;129(1):84–94. doi: 10.1016/j.radonc.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Szymonowicz K, Krysztofiak A, Linden JV, Kern A, Deycmar S, Oeck S, et al. Proton irradiation increases the necessity for homologous recombination repair along with the indispensability of non-homologous end joining. Cells. 2020;9(4):889. doi: 10.3390/cells9040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soni A, Murmann-Konda T, Siemann-Loekes M, Pantelias GE, Iliakis G. Chromosome breaks generated by low doses of ionizing radiation in G(2)-phase are processed exclusively by gene conversion. DNA Repair. 2020;89:102828. doi: 10.1016/j.dnarep.2020.102828. [DOI] [PubMed] [Google Scholar]
- 65.Bright SJ, Flint DB, Martinus D, Turner BX, Manandhar M, Kacem MB, et al. Targeted inhibition of DNA-PKcs, ATM, ATR, PARP, and Rad51 modulate response to X rays and protons. Radiat Res. 2022;198(4):336–346. doi: 10.1667/RADE-22-00040.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grosse N, Fontana AO, Hug EB, Lomax A, Coray A, Augsburger M, et al. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys. 2014;88(1):175–181. doi: 10.1016/j.ijrobp.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 67.Fontana AO, Augsburger MA, Grosse N, Guckenberger M, Lomax AJ, Sartori AA, et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol. 2015;116(3):374–380. doi: 10.1016/j.radonc.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Whalen MK, Gurai SK, Zahed-Kargaran H, Pluth JM. Specific ATM-mediated phosphorylation dependent on radiation quality. Radiat Res. 2008;170(3):353–364. doi: 10.1667/RR1354.1. [DOI] [PubMed] [Google Scholar]
- 69.Saha J, Wang M, Cucinotta FA. Investigation of switch from ATM to ATR signaling at the sites of DNA damage induced by low and high LET radiation. DNA Repair. 2013;12(12):1143–1151. doi: 10.1016/j.dnarep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Ray S, Cekanaviciute E, Lima IP, Sørensen BS, Costes SV. Comparing photon and charged particle therapy using DNA damage biomarkers. Int J Part Ther. 2018;5(1):15–24. doi: 10.14338/IJPT-18-00018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bright SJ, Flint DB, Chakraborty S, McFadden CH, Yoon DS, Bronk L, et al. Nonhomologous end joining is more important than proton linear energy transfer in dictating cell death. Int J Radiat Oncol Biol Phys. 2019;105(5):1119–1125. doi: 10.1016/j.ijrobp.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, et al. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114(3):359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- 73.Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002;158(4):486–492. doi: 10.1667/0033-7587. [DOI] [PubMed] [Google Scholar]
- 74.Ibañez IL, Bracalente C, Molinari BL, Palmieri MA, Policastro L, Kreiner AJ, et al. Induction and rejoining of DNA double strand breaks assessed by H2AX phosphorylation in melanoma cells irradiated with proton and lithium beams. Int J Radiat Oncol Biol Phys. 2009;74(4):1226–1235. doi: 10.1016/j.ijrobp.2009.02.070. [DOI] [PubMed] [Google Scholar]
- 75.Christmann M, Kaina B. Epigenetic regulation of DNA repair genes and implications for tumor therapy. Mutat Res Rev Mutat Res. 2019;780:15–28. doi: 10.1016/j.mrrev.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez A, O'Leary C, O'Byrne KJ, Burgess J, Richard DJ, Suraweera A. Epigenetic mechanisms in DNA double strand break repair: a clinical review. Front Mol Biosci. 2021;8:685440. doi: 10.3389/fmolb.2021.685440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Helm A, Fournier C, Durante M. Particle radiotherapy and molecular therapies: mechanisms and strategies towards clinical applications. Expert Rev Mol Med. 2022;24:e8. doi: 10.1017/erm.2022.2. [DOI] [PubMed] [Google Scholar]
- 78.Miousse IR, Kutanzi KR, Koturbash I. Effects of ionizing radiation on DNA methylation: from experimental biology to clinical applications. Int J Radiat Biol. 2017;93(5):457–469. doi: 10.1080/09553002.2017.1287454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carter RJ, Nickson CM, Thompson JM, Kacperek A, Hill MA, Parsons JL. Complex DNA damage induced by high linear energy transfer alpha-particles and protons triggers a specific cellular DNA damage response. Int J Radiat Oncol Biol Phys. 2018;100(3):776–784. doi: 10.1016/j.ijrobp.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jakob B, Dubiak-Szepietowska M, Janiel E, Schmidt A, Durante M, Taucher-Scholz G. Differential repair protein recruitment at sites of clustered and isolated DNA double-strand breaks produced by high-energy heavy ions. Sci Rep. 2020;10(1):1443. doi: 10.1038/s41598-020-58084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi C, Lee GH, Son A, Yoo GS, Yu JI, Park HC. Downregulation of Mcl-1 by panobinostat potentiates proton beam therapy in hepatocellular carcinoma cells. Cells. 2021;10(3):554. doi: 10.3390/cells10030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrari B, Roda E, Priori EC, De Luca F, Facoetti A, Ravera M, et al. A new platinum-based prodrug candidate for chemotherapy and its synergistic effect with hadrontherapy: novel strategy to treat glioblastoma. Front Neurosci. 2021;15:589906. doi: 10.3389/fnins.2021.589906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goetz W, Morgan MN, Baulch JE. The effect of radiation quality on genomic DNA methylation profiles in irradiated human cell lines. Radiat Res. 2011;175(5):575–587. doi: 10.1667/RR2390.1. [DOI] [PubMed] [Google Scholar]
- 84.Fujita M, Otsuka Y, Imadome K, Endo S, Yamada S, Imai T. Carbon-ion radiation enhances migration ability and invasiveness of the pancreatic cancer cell, PANC-1, in vitro. Cancer Sci. 2012;103(4):677–683. doi: 10.1111/j.1349-7006.2011.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akino Y, Teshima T, Kihara A, Kodera-Suzumoto Y, Inaoka M, Higashiyama S, et al. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2009;75(2):475–481. doi: 10.1016/j.ijrobp.2008.12.090. [DOI] [PubMed] [Google Scholar]
- 86.Girdhani S, Lamont C, Hahnfeldt P, Abdollahi A, Hlatky L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat Res. 2012;178(1):33–45. doi: 10.1667/rr2724.1. [DOI] [PubMed] [Google Scholar]
- 87.Narang H, Kumar A, Bhat N, Pandey BN, Ghosh A. Effect of proton and gamma irradiation on human lung carcinoma cells: gene expression, cell cycle, cell death, epithelial-mesenchymal transition and cancer-stem cell trait as biological end points. Mutat Res. 2015;780:35–46. doi: 10.1016/j.mrfmmm.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Murata K, Noda SE, Oike T, Takahashi A, Yoshida Y, Suzuki Y, et al. Increase in cell motility by carbon ion irradiation via the Rho signaling pathway and its inhibition by the ROCK inhibitor Y-27632 in lung adenocarcinoma A549 cells. J Radiat Res. 2014;55(4):658–664. doi: 10.1093/jrr/rru002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirai T, Saito S, Fujimori H, Matsushita K, Nishio T, Okayasu R, et al. Radiosensitization by PARP inhibition to proton beam irradiation in cancer cells. Biochem Biophys Res Commun. 2016;478(1):234–240. doi: 10.1016/j.bbrc.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 90.Wéra AC, Lobbens A, Stoyanov M, Lucas S, Michiels C. Radiation-induced synthetic lethality: combination of poly(ADP-ribose) polymerase and RAD51 inhibitors to sensitize cells to proton irradiation. Cell Cycle. 2019;18(15):1770–1783. doi: 10.1080/15384101.2019.1632640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirai T, Shirai H, Fujimori H, Okayasu R, Sasai K, Masutani M. Radiosensitization effect of poly(ADP-ribose) polymerase inhibition in cells exposed to low and high liner energy transfer radiation. Cancer Sci. 2012;103(6):1045–1050. doi: 10.1111/j.1349-7006.2012.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lesueur P, Chevalier F, El-Habr EA, Junier MP, Chneiweiss H, Castera L, et al. Radiosensitization effect of talazoparib, a parp inhibitor, on glioblastoma stem cells exposed to low and high linear energy transfer radiation. Sci Rep. 2018;8(1):3664. doi: 10.1038/s41598-018-22022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vares G, Ahire V, Sunada S, Ho Kim E, Sai S, Chevalier F, et al. A multimodal treatment of carbon ions irradiation, miRNA-34 and mTOR inhibitor specifically control high-grade chondrosarcoma cancer stem cells. Radiother Oncol. 2020;150:253–261. doi: 10.1016/j.radonc.2020.07.034. [DOI] [PubMed] [Google Scholar]
- 94.Dehne S, Fritz C, Rieken S, Baris D, Brons S, Haberer T, et al. Combination of photon and carbon ion irradiation with targeted therapy substances temsirolimus and gemcitabine in hepatocellular carcinoma cell lines. Front Oncol. 2017;7:35. doi: 10.3389/fonc.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park HJ, Oh JS, Chang JW, Hwang SG, Kim JS. Proton irradiation sensitizes radioresistant non-small cell lung cancer cells by modulating epidermal growth factor receptor-mediated dna repair. Anticancer Res. 2016;36(1):205–212. [PubMed] [Google Scholar]
- 96.Moncharmont C, Guy JB, Wozny AS, Gilormini M, Battiston-Montagne P, Ardail D, et al. Carbon ion irradiation withstands cancer stem cells' migration/invasion process in head and neck squamous cell carcinoma (HNSCC) Oncotarget. 2016;7(30):47738–47749. doi: 10.18632/oncotarget.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li HK, Matsumoto Y, Furusawa Y, Kamada T. PU-H71, a novel Hsp90 inhibitor, as a potential cancer-specific sensitizer to carbon-ion beam therapy. J Radiat Res. 2016;57(5):572–575. doi: 10.1093/jrr/rrw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee Y, Li HK, Masaoka A, Sunada S, Hirakawa H, Fujimori A, et al. The purine scaffold Hsp90 inhibitor PU-H71 sensitizes cancer cells to heavy ion radiation by inhibiting DNA repair by homologous recombination and non-homologous end joining. Radiother Oncol. 2016;121(1):162–168. doi: 10.1016/j.radonc.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee Y, Sunada S, Hirakawa H, Fujimori A, Nickoloff JA, Okayasu R. TAS-116, a novel Hsp90 inhibitor, selectively enhances radiosensitivity of human cancer cells to X-rays and carbon ion radiation. Mol Cancer Ther. 2017;16(1):16–24. doi: 10.1158/1535-7163.MCT-16-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vitti ET, Kacperek A, Parsons JL. Targeting DNA double-strand break repair enhances radiosensitivity of hpv-positive and hpv-negative head and neck squamous cell carcinoma to photons and protons. Cancers. 2020 doi: 10.3390/cancers12061490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang L, Liu Y, Sun C, Yang X, Yang Z, Ran J, et al. Inhibition of DNA-PKcs enhances radiosensitivity and increases the levels of ATM and ATR in NSCLC cells exposed to carbon ion irradiation. Oncol Lett. 2015;10(5):2856–2864. doi: 10.3892/ol.2015.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cunningham C, de Kock M, Engelbrecht M, Miles X, Slabbert J, Vandevoorde C. Radiosensitization effect of gold nanoparticles in proton therapy. Front Public Health. 2021;9:699822. doi: 10.3389/fpubh.2021.699822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeynes JC, Merchant MJ, Spindler A, Wera AC, Kirkby KJ. Investigation of gold nanoparticle radiosensitization mechanisms using a free radical scavenger and protons of different energies. Phys Med Biol. 2014;59(21):6431–6443. doi: 10.1088/0031-9155/59/21/6431. [DOI] [PubMed] [Google Scholar]
- 104.Klebowski B, Depciuch J, Stec M, Krzempek D, Komenda W, Baran J, et al. Fancy-shaped gold-platinum nanocauliflowers for improved proton irradiation effect on colon cancer cells. Int J Mol Sci. 2020;21(24):9610. doi: 10.3390/ijms21249610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li S, Bouchy S, Penninckx S, Marega R, Fichera O, Gallez B, et al. Antibody-functionalized gold nanoparticles as tumor-targeting radiosensitizers for proton therapy. Nanomedicine. 2019;14(3):317–333. doi: 10.2217/nnm-2018-0161. [DOI] [PubMed] [Google Scholar]
- 106.Penninckx S, Heuskin AC, Michiels C, Lucas S. The role of thioredoxin reductase in gold nanoparticle radiosensitization effects. Nanomedicine. 2018;13(22):2917–2937. doi: 10.2217/nnm-2018-0171. [DOI] [PubMed] [Google Scholar]
- 107.Sotiropoulos M, Henthorn NT, Warmenhoven JW, Mackay RI, Kirkby KJ, Merchant MJ. Modelling direct DNA damage for gold nanoparticle enhanced proton therapy. Nanoscale. 2017;9(46):18413–18422. doi: 10.1039/c7nr07310k. [DOI] [PubMed] [Google Scholar]
- 108.Li F, Li Z, Jin X, Liu Y, Li P, Shen Z, et al. Radiosensitizing effect of gadolinium oxide nanocrystals in NSCLC cells under carbon ion irradiation. Nanoscale Res Lett. 2019;14(1):328. doi: 10.1186/s11671-019-3152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y, Liu X, Jin X, He P, Zheng X, Dai Z, et al. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low- and high-LET radiations. Phys Med. 2015;31(3):210–218. doi: 10.1016/j.ejmp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 110.Porcel E, Tillement O, Lux F, Mowat P, Usami N, Kobayashi K, et al. Gadolinium-based nanoparticles to improve the hadrontherapy performances. Nanomedicine. 2014;10(8):1601–1608. doi: 10.1016/j.nano.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Wozny AS, Aloy MT, Alphonse G, Magné N, Janier M, Tillement O, et al. Gadolinium-based nanoparticles as sensitizing agents to carbon ions in head and neck tumor cells. Nanomedicine. 2017;13(8):2655–2660. doi: 10.1016/j.nano.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 112.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676. doi: 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tinganelli W, Durante M, Hirayama R, Krämer M, Maier A, Kraft-Weyrather W, et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci Rep. 2015;5:17016. doi: 10.1038/srep17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burroughs SK, Kaluz S, Wang D, Wang K, Van Meir EG, Wang B. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem. 2013;5(5):553–572. doi: 10.4155/fmc.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int. 2014;2014:409272. doi: 10.1155/2014/409272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mujcic H, Hill RP, Koritzinsky M, Wouters BG. Hypoxia signaling and the metastatic phenotype. Curr Mol Med. 2014;14(5):565–579. doi: 10.2174/1566524014666140603115831. [DOI] [PubMed] [Google Scholar]
- 117.Wozny AS, Lauret A, Battiston-Montagne P, Guy JB, Beuve M, Cunha M, et al. Differential pattern of HIF-1α expression in HNSCC cancer stem cells after carbon ion or photon irradiation: one molecular explanation of the oxygen effect. Br J Cancer. 2017;116(10):1340–1349. doi: 10.1038/bjc.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wozny AS, Vares G, Alphonse G, Lauret A, Monini C, Magné N, et al. ROS production and distribution: a new paradigm to explain the differential effects of X-ray and carbon ion irradiation on cancer stem cell migration and invasion. Cancers. 2019;11(4):468. doi: 10.3390/cancers11040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valable S, Gérault AN, Lambert G, Leblond MM, Anfray C, Toutain J, et al. Impact of hypoxia on carbon ion therapy in glioblastoma cells: modulation by LET and hypoxia-dependent genes. Cancers. 2020;12(8):2019. doi: 10.3390/cancers12082019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Subtil FS, Wilhelm J, Bill V, Westholt N, Rudolph S, Fischer J, et al. Carbon ion radiotherapy of human lung cancer attenuates HIF-1 signaling and acts with considerably enhanced therapeutic efficiency. FASEB J. 2014;28(3):1412–1421. doi: 10.1096/fj.13-242230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Specific search strategies for MEDLINE, Embase, and Web of Science.
Additional file 2: Table S1. Risk of bias scheme. Table S2. Results of the risk of bias assessment.
Additional file 3. PRISMA 2020 checklist.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the author Tian-Qi Du (dutq21@lzu.edu.cn).