Abstract
Purpose
High body mass index (BMI) may lead to improved immune-checkpoint blockade (ICB) outcomes in metastatic clear cell renal cell carcinoma (mccRCC). However, BMI is a crude body size measure. We investigated BMI and radiographically assessed body composition (BC) parameters association with mccRCC ICB outcomes.
Patients and methods
Retrospective study of ICB treated mccRCC patients. BMI and BC variables (skeletal muscle index (SMI), and multiple adiposity indexes) were determined using pre-treatment CT scans. We examined the associations between BMI and BC variables with ICB outcomes. Therapeutic responses per RECIST V1.1 were determined. We compared whole transcriptomic patterns with BC variables in a separate cohort of 62 primary tumor samples.
Results
205 mccRCC patients included in the cohort (74% were male, 71% were overweight/obese, and 53% were classified as low SMI). High BMI patients experienced longer overall survival (OS) than normal weight patients (unadjusted HR 0.66 (95% CI: 0.45–0.97); p=0.035). The only BC variable associated with OS was SMI (unadjusted HR comparing low vs. high SMI 1.65 (95% CI: 1.13–2.43); p=0.009). However, this OS association became non-significant after adjusting for IMDC score and line of therapy. No OS association was seen for adiposity and no BC variable was associated with progression-free survival or radiological responses. Tumors from patients with low SMI displayed increased angiogenic, inflammatory, and myeloid signals.
Conclusion
Our findings highlight the relevance of skeletal muscle in the BMI paradox. Future studies should investigate if addressing low skeletal muscle in metastatic patients treated with ICB can improve survival.
Keywords: BMI, Skeletal Muscle, Adiposity, Renal Cell Carcinoma, immune-checkpoint blockade, immunotherapy
Introduction
The treatment landscape of metastatic renal cell carcinoma (mRCC) is rapidly changing with several approved immune checkpoint blockade (ICB) agents alone and in combinations, including the Programmed Death Receptor 1 (PD-1) or its Ligand (PD-L1) and CTL-associated protein 4 (CTLA-4)1,2. As only a subset of patients treated with ICB agents achieve durable long-term benefit, identifying novel prognostic and predictive biomarkers of ICB clinical benefit remains a significant research priority. Although promising signals are emerging from studies incorporating next-generation sequencing tissue-based biomarkers, thus far, these have not been integrated into routine clinical care3. Currently, survival probability can be best estimated using the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk stratification model, a validated risk score classifying patients into favorable, intermediate, and poor risk based on clinical and laboratory variables4,5,6. Beyond its prognostic value, the IMDC risk score can also help to project therapeutic benefit from doublet immunotherapy2. Notably, approximately 60% of metastatic RCC fall within the intermediate IMDC risk group. There is an active effort to improve the sub-stratification of the intermediate risk group by incorporating prognostic clinical, laboratory and molecular data7,8.
High body mass index (BMI) is an established risk factor for the development of clear cell RCC (ccRCC)9,10 but exhibits a counterintuitive association with prognosis. Clinical studies report that ccRCC patients with higher BMIs experience longer overall and cancer-specific survival (OS) compared to patients with lower BMIs. This phenomenon, known as the ‘BMI paradox’, has been observed in the post-nephrectomy setting11,12 but also among patients with metastatic ccRCC treated with Vascular Endothelial Growth Factor Receptor (VEGFR) directed Tyrosine Kinase Inhibitors (TKI)13. In a recent retrospective analysis of 785 RCC patients treated with ICB from the IMDC database, Lalani et al. found that patients with BMI ≥25 kg/m2 (i.e., overweight or obese) experienced significantly better OS than patients with BMI <25kg/m2 (i.e., normal weight)14. The mechanisms underlying the BMI paradox have not been fully elucidated but may relate to obesity inducing a chronic inflammatory state15, which in turn may render obese patients more responsive to ICB. It is also possible that high leptin levels in obese patients could drive T-cell dysfunction leading to better outcomes on ICB16. We recently reported our findings correlating transcriptomic features and BMI in independent cohorts of early-stage and metastatic RCC. While there was an apparent upregulation of angiogenesis pathways in tumors of obese compared to normal weight patients, we found few differences with respect to immune-related pathways17. The perinephric tumor microenvironment, however, showed significantly higher expression of immunologic pathways by BMI. Together, these patterns suggest that the BMI paradox may be biologically driven, and that the significance of body composition for tumor biology extends beyond a mere ‘inflammatory state’.
It is well recognized that BMI is an imprecise body size measure as it does not distinguish between adipose or muscle tissues18. More specific body composition features, including cross-sectional areas of visceral and subcutaneous adipose tissues as well as skeletal muscle, can be derived from standard of care computed tomography (CT) scan at the third lumbar vertebrae (L3) level to estimate the skeletal muscle index (SMI) and several adiposity indexes such as the visceral adiposity index (VATI) and the subcutaneous adiposity index (SATI) 19,20. Poor skeletal muscle health has emerged as a relevant poor prognostic factor in localized ccRCC setting21, and metastatic ccRCC patients treated with targeted therapy22,23, and among metastatic RCC patients treated with ICB24. Recent systematic reviews and meta-analyses of mixed cancer patients treated with ICB suggest that low SMI at treatment start or skeletal muscle loss during treatment are associated with inferior survival and worse treatment response, but it does not appear to increase the rate of immune-related adverse events25,26. Whether these results are generalizable to metastatic ccRCC patients treated with ICB specifically is unclear. as relatively few such patients were included in these studies.
In this study, we investigated how body composition features were associated with OS, progression-free survival (PFS), and objective response rates (ORR) among metastatic ccRCC patients treated with ICB. Given prior literature, we hypothesized that low SMI would be associated with worse ICB clinical outcomes. In exploratory analyses, we compared gene expression profiles by SMI using primary tumor samples from a separate cohort of patients treated at our institution and profiled by The Cancer Genome Atlas (TCGA) with body composition interpreted from pre-surgical CT scans.
Methods:
Study Subjects
After institutional review board approval, eligible patients were identified from electronic medical records and databases at the Memorial Sloan Kettering Cancer Center (MSKCC). We identified 267 patients who began systemic ICB therapies between July 2011 to April 2020. Eligible patients had histological confirmation of clear cell histology with prior receipt of ccRCC-directed ICB -based therapy in the setting of radiographically evident disease for a minimum of 4 weeks and had CT imaging including L3 within 60 days of treatment start. Patients with ICB combination treatment that included VEGFR-TKIs were excluded. A total of 205 metastatic ccRCC patients met these criteria.
Patient characteristics including demographics, BMI, IMDC score (i.e., hemoglobin, neutrophil counts, serum calcium, platelet counts, Karnofsky performance status, time from first diagnosis to systemic therapy), and Neutrophil-to-Lymphocyte ratio (NLR) were abstracted from electronic medical records at the time of ICB start. Patients were categorized into favorable, intermediate, and poor risk as per IMDC scores4, while NLR was assessed both as continuous and categorical variables (≥3 for high NLR; <3 for normal NLR, cut-off based on previous reports27,28). Type of ICB drug class and line of ICB treatment were also abstracted.
Body Composition Analysis
BMI values at the time of ICB treatment start were calculated for all patients using the formula weight in kilograms divided by height in meters squared, and classified according to the World Health Organization (WHO) categories of normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to < 30 kg/m2), and obese (BMI ≥30 kg/m2)29. Only one patient was underweight (i.e., BMI <18.5 kg/m2) and was included in the normal weight category. Standard Hounsfield Unit thresholds for skeletal muscle (−29 to +150 HU) and adipose tissues (−150 to −50 HU) were used to derive radiographic body composition variables for each patient using SliceOMatic version 5.0 software (Tomovision). The continuous values of these variables were divided by height in meters squared to determine their SMI, VATI, and SATI, which served as the primary exposure variables30,31. SMI was categorized as low vs. high based on the sex-specific classification endorsed by the International Consensus of Cancer Cachexia (ICCC) (<55 cm2/m2 for men and <39 cm2/m2 for women)32. As no validated cut-points exist for VATI and SATI, these variables were dichotomized based on the cohort sex-specific medians (Low VATI: Males<55 cm2/m2, Females<33 cm2/m2; Low SATI: Males<57 cm2/m2, Females<88 cm2/m2).
Clinical outcome analysis and statistical endpoints
Study baseline was defined as the date of ICB treatment start. Clinical outcomes assessed included OS, PFS, and ORR. OS was defined as the time from starting ICB treatment until death; patients who were still alive or lost to follow up at the data cut-off were censored at the date of last follow-up. Radiological responses and progression were determined per the Response Evaluation Criteria in Solid Tumor (RECIST) 1.133 through individual review by an expert radiologist blinded to body composition categories. PFS was defined as the time from starting ICB treatment to the date of radiographic progression or death; patients who were alive and had not progressed at the time of analysis were censored at the date of their last scan; those who discontinued treatment for reasons other than progression were censored at the time treatment was last received. Best ORR was defined as the proportion of patients achieving complete response, or partial response.
Continuous variables are presented as median or mean +/− SD while categorical variables are presented as frequencies and percentages. Differences in variables by BMI, SATI or VATI category were compared using the Chi-square test for categorical variables and ANOVA or T-test for continuous variables. Time-to-event outcomes (OS and PFS) were estimated using the Kaplan-Meier (KM) method, and multivariable Cox proportional hazards regression models were used to estimate the hazard ratios (HR) and 95% confidence intervals (CI). Odds ratios (ORs) and 95% CI from logistic regression models estimate associations between body composition variables and ORR.
We first examined how BMI at ICB treatment start was associated with OS, PFS and ORR. Next, we examined how BMI was associated with each of the three radiographic body composition variables (SATI, VATI, SMI). Finally, we evaluated how each body composition variable was associated with OS, PFS and ORR. Multivariable models for OS and PFS were adjusted for IMDC and line of treatment. In exploratory analyses, we evaluated the joint associations of SMI and VATI in relation to OS and performed a subgroup analysis of SMI and OS among patients with intermediate IMDC risk.
All statistical tests were 2-tailed, and a p-value <0.05 was considered as statistically significant. All analyses were performed in R version 4.0.3 and SAS version 9.3 software The cut-off date for follow-up and survival status was October 1st, 2020.
TCGA Molecular Analyses
We conducted an exploratory analysis of gene expression patterns by SMI using a separate cohort of 62 ccRCC patients who underwent nephrectomy at MSKCC with primary tumors transcriptomically-profiled as part of the TCGA. Body composition features were derived from CT scans at the time of nephrectomy using the same approach described above. Publicly-available RNAseq data for the TCGA cohort, including tumor only were downloaded from the NIH Genomic Data Commons (https://gdc.cancer.gov). Methods for RNA extraction and processing for the TCGA are documented in TCGA, Nature, 2013. Differential expression analyses were performed using the R package “DESeq2”34. Gene set enrichment analyses (GSEA) were used to evaluate differences in Molecular Signatures Database (MSigDB) hallmark gene set collection35,36. Immune deconvolution using single-sample GSEA (ssGSEA) was utilized with previously published immune cell signatures37 to estimate the abundance of immune cell types, T-cell infiltration score (TIS), and Immune Infiltration Score (IIS). Infiltration levels for different immune cell types were quantified using the R package “gsva”38. The R package “estimate” was used to infer the fraction of stromal and immune cells (ImmuneScore) in tumor samples based on given gene expression profile in FPKM or normalized log2 transformed values39. ssGSEA scores for each individual immune cell type were used to calculate total T-cell Infiltration score (TIS) and Immune Infiltration Score (IIS). Ingenuity Pathway Analyses (IPA) (QIAGEN Inc.) were performed using differentially expressed genes (DEG) with the Ingenuity Knowledge Base as the reference set40. Filters used in picking up DEG genes for IPA included: mean expression > 10, fold change > 20%, and P value < 0.05. P-values were corrected for multiple testing (p-adjust) using the Benjamini-Hochberg method.
We derived an immune cytolytic score (CYT) based on the geometric mean expression of two key cytolytic effectors; granzyme A (GZAM) and perforin (PRF1)41. Previously published signatures of immune cell function were utilized to assess differences in T-cell function42 (Teff score: includes CD8A, EOMES, PRF1, IFNG, and CD274), myeloid expression (Myeloid score: includes IL6, CXCL1, CXCL2, CXCL3, CXCL8, PTGS2)42, ImmuneCheckpoint (includes CD274, CTLA4, HAVCR2, LAG3, PDCD1, PDCD1LG2, TIGIT), and Adenosine Gene Signature (AdenoSig: includes IL1β, PTGS2, and CXCL1, 2, 3, 5, 6, 8)43.
The data generated in this study are not publicly available due to patient privacy/lack of consent to sharing of personal health information but are available upon reasonable request from the corresponding author.
Results:
The baseline characteristics of the 205 metastatic ccRCC patients included in this analysis are summarized in Table 1. The cohort was predominantly male (74%), white (91%), with median age starting ICB treatment of 63 years (range: 40–90). Most patients (65%) were classified as IMDC intermediate risk (65%); 17% were considered favorable risk and 18% were considered poor risk. Thirty percent of the cohort had received ICB treatment in the first-line setting, and 39% presented with de novo metastatic disease. Most patients (65%) had received single agent ICB (Anti-PD1 or Anti-PDL1) compared to combination ICB treatment (35%), predominately combining Anti-PD1 with Anti-CTLA4 ICB directed therapy. Patients were classified by BMI as obese (36%), overweight (35%), or normal weight (29%). Fifty three percent of the cohort were classified as having low SMI (per sex-specific ICCC-defined thresholds quoted above). Patients were equally distributed across high and low VATI and SATI because they were dichotomized at gender-specific medians. Tables S1–4 shows cohort characteristics differences by BMI, SMI, VATI, and SATI. The only characteristic that differed by BMI was age where normal weight patients were slightly older (median 66 years) than those who classified as overweight or obese patients (median 62 years, p=0.04); BMI categories did not correlate with other clinical variables, including IMDC score or ECOG performance status (Table S1). Most patient characteristics differed, however, when categorizing patients by SMI (Table S2). Compared to patients with high SMI, low SMI patients were more likely to be older, male, present with worse ECOG performance status, and classified as poor IMDC group. Low SMI was also significantly associated with higher pre-treatment NLR values, both as a categorical and continuous variable (p-values < 0.001). Patient characteristics did not differ by VATI (all p-values>0.05; Table S3), while patients with low SATI were slightly older than high SATI patients (p=0.03; Table S4).
Table 1.
Baseline Patient Characteristics at ICB Initiation
| Patient Characteristic | n = 2051 |
|---|---|
| Age at start of ICB treatment in years [range] | 63 [40, 90] |
| Sex | |
| Male | 152 (74%) |
| Female | 53 (26%) |
| Race | |
| White | 171 (91%) |
| Asian | 7 (4%) |
| Black or African American | 8 (4%) |
| Other | 2 (1%) |
| ICB drug class | |
| AntiPD1 or AntiPDL1 | 134 (65%) |
| AntiPD1+AntiCTLA4 | 64 (31%) |
| AntiPD1+AntiPDL1 | 7 (4%) |
| Line of ICB treatment 1 | |
| 1st line | 61 (30%) |
| 2nd line | 87 (42%) |
| 3+ line | 57 (28%) |
| Presented with de novo metastatic disease | |
| Yes | 80 (39%) |
| No | 125 (61%) |
| IMDC score at start of ICB treatment | |
| Favorable | 35 (17%) |
| Intermediate | 133 (65%) |
| Poor | 37 (18%) |
| ECOG performance status at start of ICB treatment | |
| 0 | 96 (47%) |
| 1 | 93 (45%) |
| 2 | 16 (8%) |
| Body Mass Index (BMI) | |
| Obese (≥30 kg/m2) | 74 (36%) |
| Overweight (25–30 kg/m2) | 71 (35%) |
| Normal weight (<25 kg/m2) | 60 (29%) |
| Skeletal Muscle Index (SMI) | |
| High SMI | 96 (47%) |
| Low SMI | 109 (53%) |
Abbreviations: ICB=Immune Checkpoint Blockade; ECOG=Eastern Cooperative Oncology Group; IMDC=International Metastatic Renal Cell Carcinoma Database Consortium; PD-1=Programmed Death-1; PD-L1=Programmed Death-Ligand 1; CTLA-4=Cytotoxic T-Lymphocyte-Associated Protein-4; SMI=Skeletal Muscle Index; BMI=Body Mass Index.
94% of patients who received ICB in the ≥2nd line setting had previously received VEGFR TKI treatment.
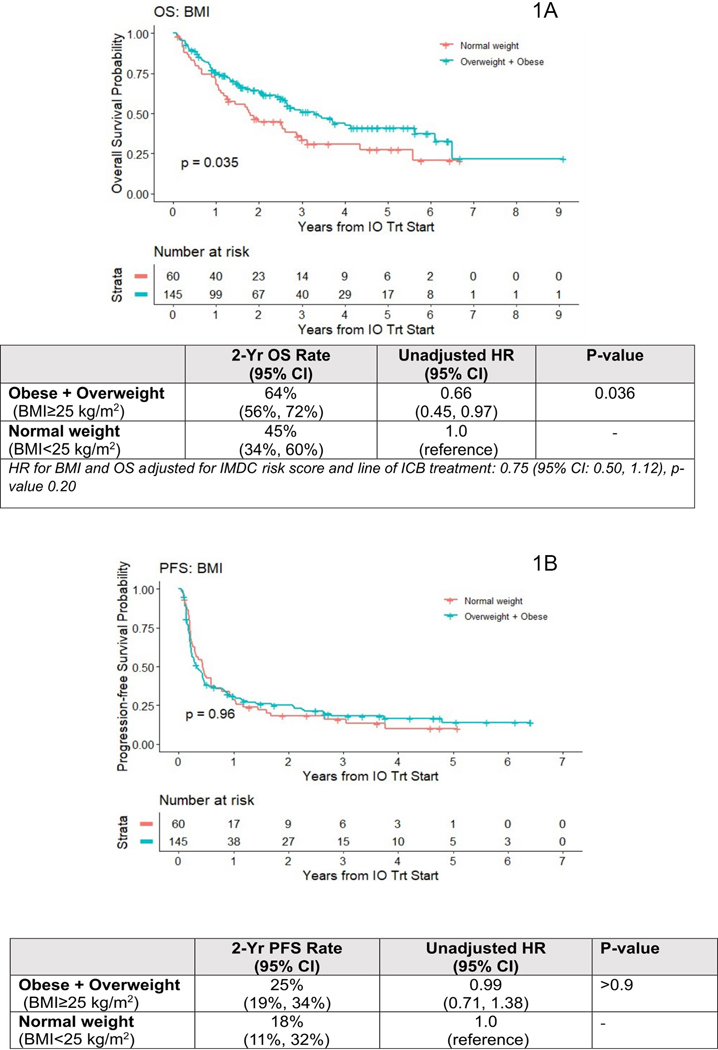
During a median follow-up time of 31.2 months (0.96–77.8 months) there were 110 deaths for OS analyses, and 165 PFS events. Figure 1A shows KM plots for BMI and OS. The log-rank test was significant (p=0.035) suggestive of survival advantage for overweight and obese patients at treatment start, compared to normal weight patients. The 2-year OS rate was 64% (95% CI: 56%−72%) for overweight or obese patients vs. 45% (95% CI: 34%−60%) for normal weight patients. The unadjusted HR comparing overweight or obese to normal weight patients was 0.66 (95% CI: 0.44–0.97); p=0.035). This OS association became non-significant after adjusting for IMDC scores and line of therapy (HR 0.75 (95% CI: 0.50–1.12); p=0.20). BMI category was neither associated with PFS (HR: 0.99 (95% CI 0.71–1.38, p>0.90) nor with ORR (OR: 1.04 (95% CI: 0.55–2.01; p=0.90)) (Figure 1B and Table 2, respectively).
Figure 1.
Kaplan Meier curve of OS among 205 metastatic ccRCC patients treated with ICB per BMI (1A). Kaplan Meier curve of PFS among 205 metastatic ccRCC patients treated with ICB per BMI (1B).
Abbreviations: OS=Overall survival. ccRCC=clear cell renal cell carcinoma. ICB= Immune-checkpoint blockade. PFS=Progression free survival
Table 2.
Odds Ratios and 95% Confidence Intervals for Body Composition Variables and Objective Response to ICB as defined by RECIST criteria, n=192
| CR n=8 (4%) | PR n=44 (23%) | SD ≥ 6 mo n=27 (14%) | SD <6 mo n=45 (23%) | PD n=68 (35%) | OR* (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|
| BMI | |||||||
| Obese + Overweight (BMI≥25 kg/m2) | 6 (4%) | 31 (22%) | 21 (15%) | 30 (21%) | 52 (37%) | 1.04 (0.55, 2.01) | 0.90 |
| Normal weight (BMI<25 kg/m2) | 2 (4%) | 13 (25%) | 6 (12%) | 15 (29%) | 16 (31%) | 1.0 (reference) | |
| SMI | |||||||
| High SMI | 4 (4%) | 23 (25%) | 11 (12%) | 23 (25%) | 31 (34%) | 1.0 (reference) | 0.97 |
| Low SMI | 4 (4%) | 21 (21%) | 16 (16%) | 22 (22%) | 37 (37%) | 0.99 (0.56, 1.76) | |
| VATI | |||||||
| High VATI | 5 (5%) | 19 (19%) | 17 (17%) | 25 (25%) | 33 (33%) | 0.97 (0.55, 1.74) | 0.94 |
| Low VATI | 3 (3%) | 25 (27%) | 10 (11%) | 20 (22%) | 35 (38%) | 1.0 (reference) | |
| SATI | |||||||
| Low SATI | 2 (2%) | 23 (23%) | 12 (12%) | 26 (27%) | 35 (36%) | 0.75 (0.42, 1.34) | 0.33 |
| High SATI | 6 (6%) | 21 (22%) | 15 (16%) | 19 (20%) | 33 (35%) | 1.0 (reference) | |
Abbreviations: ICB=Immune Checkpoint Blockade; OR=Odds Ratio; CI= Confidence Intervals; RECIST=Response Evaluation Criteria in Solid Tumors; CR=Complete Response; PR=Partial Response; SD=Stable Disease; PD=Progressive Disease; BMI=Body Mass Index; SMI=Skeletal Muscle Index; VATI=Visceral Adipose Tissue Index; SATI= Subcutaneous Adipose Tissue Index
Row percentages are shown.
OR compares CR+PR+SD for > 6 months vs. PD and SD <6 months
Table S5 shows how different body composition features were associated with BMI. As BMI increased, the proportion of patients classified as having low SMI decreased. Low SMI was observed in 82% of normal weight, 63% of overweight, and only 20% of obese patients (p<0.001). Considering that specific prior systemic treatments are potentially associated with muscle wasting44,45, we described prior treatment details in relation to SMI in Tables S6 and S7. The amount of time on prior treatment before ICB treatment start did not differ significantly between low vs. high SMI patients. Notably, the percentage of patients categorized as low SMI among those with limited prior treatment exposure (defined as <3 months) was the same (62%) as among those with more extensive prior therapy (defined as ≥2 years). However, the prevalence of low SMI increased as the number of lines of prior treatment increased (p=0.05; Table S7).
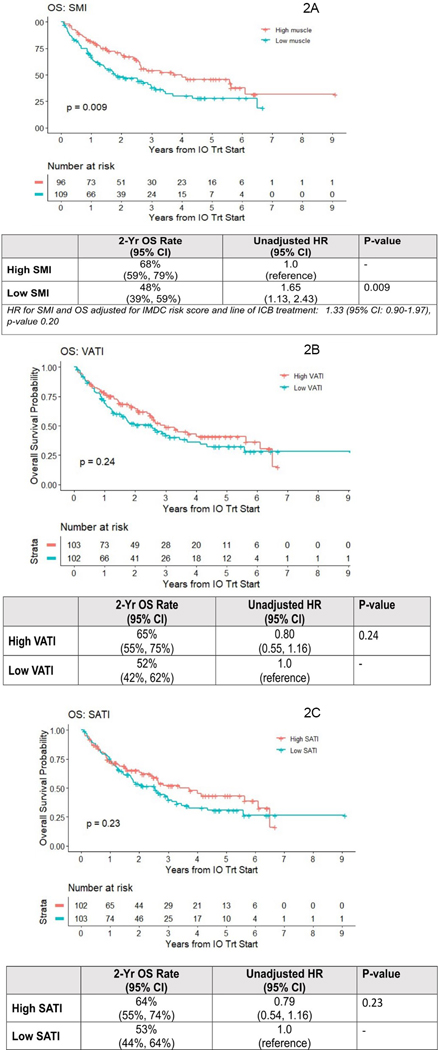
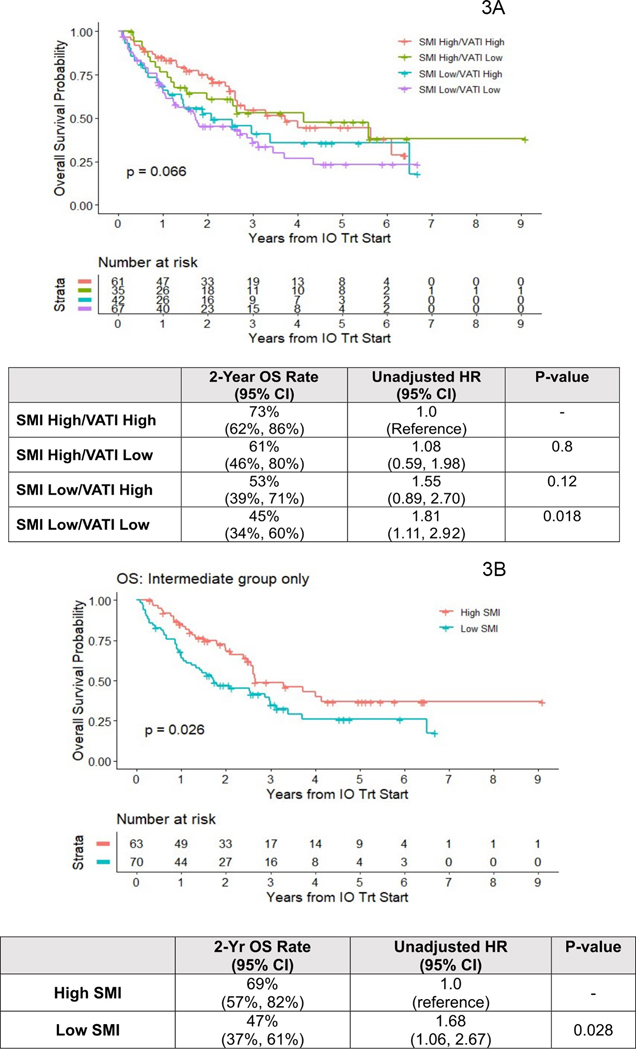
The only body composition variable significantly associated with OS was SMI (log-rank p-value=0.009) (Figures 2 A–C). The 2-year OS rate was lower for low SMI (48% (95% CI: 39%−59%)) compared high SMI patients (68% (95% CI: 59%−57%)). The unadjusted HR for death comparing low SMI to high SMI was HR 1.65 (95% CI: 1.13–2.43; p =0.009). Again, this became non-significant after adjustment for IMDC score and line of therapy HR 1.33 (95% CI: 0.90–1.97; p=0.20). The 2-year OS rates were numerically higher for high vs. low VATI and high vs low SATI categories. However, KM plots log-rank comparison was not significantly different for both VATI ((HR 0.80 (95% CI: 0.55–1.16); p=0.20) and SATI ((HR 0.79 (95% CI: 0.54–1.16); p=0.20). In an integrated analysis of muscle and adipose indices, we tested associations of OS with grouping defined on both SMI high/low and VATI high/low status (Figure 3A). Log-rank comparison suggested inferior survival for patients with low SMI, regardless of their VATI level (p=0.07), and worst for SMI low/VATI low group, where estimated 2-year OS was 45% (vs. 73% in SMI high/VATI high). Among intermediate IMDC risk patients (n=133) survival was significantly worse for low vs. high SMI (HR 1.68 (95% CI: 1.06–2.67)) (Figure 3B). There were no associations between any body composition variable with PFS (Figures S1 A–C) or ORR (Table 2).
Figure 2.
Kaplan Meier curves of Overall Survival (OS) among 205 metastatic ccRCC patients treated with ICB according to Skeletal Muscle Index (SMI) (2A), Visceral Adiposity Index (VATI) (2B), and Subcutaneous Adiposity Index (SATI) (2C).
Abbreviations: OS=Overall survival. ccRCC=clear cell renal cell carcinoma. ICB= Immune-checkpoint blockade. SMI=Skeletal Muscle Index. VATI=Visceral Adiposity Index. SATI=Subcutaneous Adiposity Index.
Figure 3.
Kaplan Meier curves of OS among metastatic ccRCC patients treated with ICB according to the joint effects of SMI and VATI for OS (3A), and SMI in the Intermediate IMDC group (3B).
Abbreviations: OS=Overall survival. ccRCC=clear cell renal cell carcinoma. ICB= Immune-checkpoint blockade. SMI=Skeletal Muscle Index. VATI=Visceral Adiposity Index. IMCD= International Metastatic RCC Database Consortium.
TCGA Analyses Results:
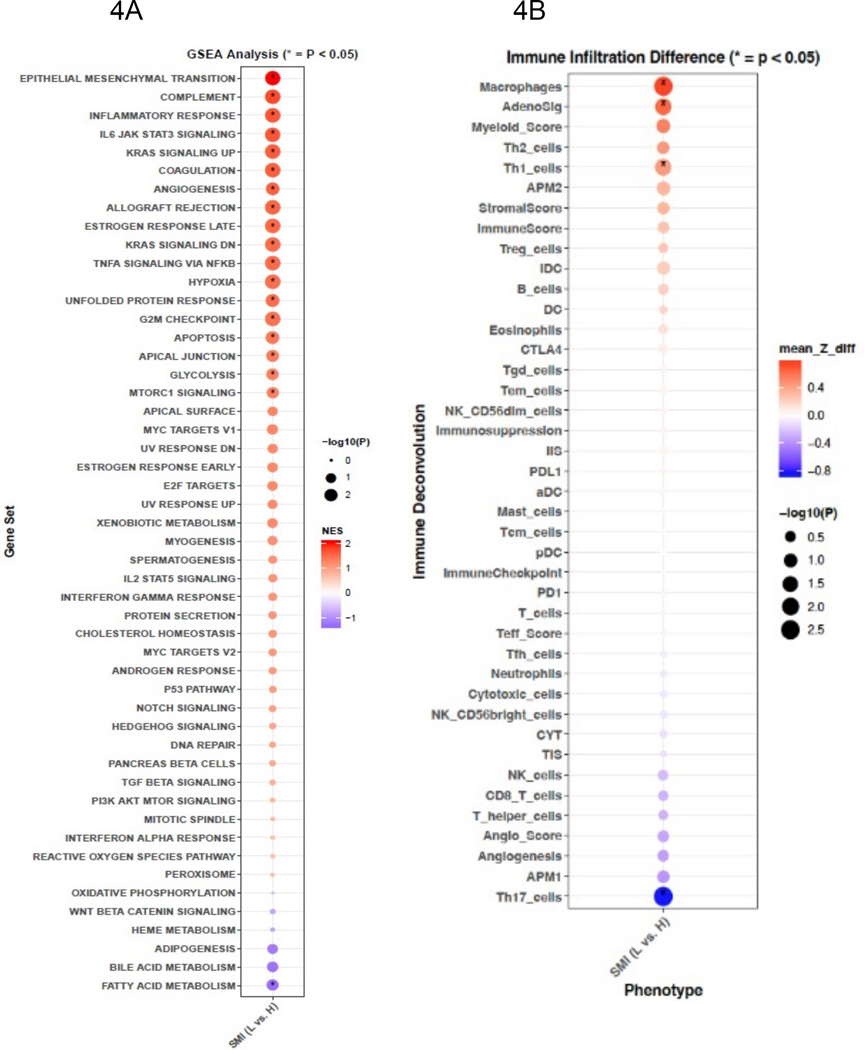
The TCGA cohort (n=62) was predominantly male (79%) and had localized disease (58%); 47% of patients we classified as low SMI according to pre-nephrectomy CT scans (Table S8) After confirming that low SMI was associated with inferior OS on univariate analysis (Figure S2; p-value=0.0004) we found that tumors of low SMI patients harbored increased expression of epithelial mesenchymal transition, inflammatory (inflammatory response, IL-6, TNF-alpha), and angiogenesis signaling programs (Figure 4A; adjusted p-values<0.05). Metabolically, tumors among low SMI patients had decreased fatty acid metabolism and increased glycolysis pathway expression (Figure 4A; adjusted p-values<0.05). Using immune deconvolution, we found no difference in total immune infiltration (per ImmuneScore), or immune checkpoints (CTLA4, PDL-1, PD1). However, tumors of low SMI patients demonstrated increased degrees of macrophage infiltration, higher AdenoSig scores, more Th1 cells and decreased Th17 cells (Figure 4B, p-adjusted <0.05). Ingenuity pathway analysis (Figure S3) demonstrated significant activation of the inflammatory IL-6 pathway.
Figure 4.
GSEA analysis of Hallmark gene sets comparing differences between low and high SMI. Enrichment scores are ranked and colored based on the NES and sized by the -log10 transformed value of the adjusted p-value. Colors signify directionality of expression changes, red=up and purple=down (4A). Differences in tumor immune infiltration by immune deconvolution between low vs. high skeletal muscle index (SMI) (low (L) vs. high (H)). Colors signify directionality of expression changes, red=up and purple=down (4B)
Abbreviations: GSEA=Gene-Set Enrichment Analysis. NES=Normalized Enrichment Score. SMI=Skeletal Muscle Index, L=Low vs H=High. mean Z_diff = mean Z-score difference.
Discussion:
We investigated multiple radiographically-assessed body composition features (i.e., SMI, VATI, SATI) in relation to clinical outcomes among metastatic ccRCC patients treated with ICB. In this large cohort of >200 patients with detailed clinical and radiographic annotation, we confirmed and extended the BMI paradox recently reported among ccRCC patients treated with ICB14,46. Low SMI emerged as a strong prognostic factor, with a more notable effect size than adiposity indices suggesting that the clinical benefit to ICB seen with higher BMI is possibly driven by differences in muscle rather than adiposity alone. The strong prognostic signal for SMI was apparent in patients with high as well as those with low adiposity, suggesting the prominent, independent role of skeletal muscle in systemic RCC biology. With further study, the integration of SMI into clinical risk scores may prove helpful in refining the IMDC intermediate patient classification. Over 41% of the overweight/obese patients in our cohort were classified as having low SMI, pointing out limitations of established vital sign measurements for evaluation of body composition. Over 80% of normal BMI patients had low SMI, suggesting that these patients do not have normal body size after all. Although we did not find that any body size variable was associated with PFS or ORR, our hypothesis-generating molecular findings suggest tumor transcriptomic differences in SMI that may have treatment implications.
Low SMI may be a marker of cancer cachexia, which is defined as the involuntary loss of skeletal muscle mass with or without accompanying loss of adipose tissue and can develop progressively from pre-cachexia to refractory cachexia during the disease course32. The degree of cachexia affecting our metastatic ccRCC patients with low SMI is not clear as we did not have data on pre- or post-treatment weight loss or functional status which are needed to accurately classify patients32. However, compared to patients with high SMI, those with low SMI were older, had a lower performance status, had received more lines of prior treatment, and higher IMDC risk scores, all of which could contribute to muscle catabolism and worse OS47. Patients with low SMI were also characterized by higher levels of the circulating inflammatory biomarker NLR at ICB treatment start which others have shown portends a worse prognosis in ICB treated patients. The HR for SMI and OS was attenuated slightly and lost its statistical significance after adjustment for IMDC risk score and line of treatment, which was expected given the strong inter-relationships between SMI and these variables48 (Tables S6 and S7). Indeed, prior studies demonstrate skeletal muscle loss on VEGF-TKIs44,45. While we found no association between the time on prior treatment before starting ICB and SMI in our study, the prevalence of low SMI increased as the number of lines of prior treatment increased (p=0.05).
A prior study from the TKI era demonstrated that the integration of low SMI to RCC risk stratification models improved its predictive performance22. We conducted a stratified analysis of SMI and OS among patients with intermediate IMDC risk score and found that low SMI was associated with inferior OS compared to high SMI (HR 1.68 (95% CI: 1.06–2.67, p= 0.03). This is worthy of further investigation as it suggests that the integration of SMI into the IMDC risk score may improve the predictive accuracy and sub-stratification of the intermediate risk IMDC group which represents the majority of metastatic ccRCC patients.
It has been hypothesized that the efficacy of ICB could be adversely affected by cachexia, but this was not supported by our study which demonstrated no adverse associations for PFS or ORR when comparing patients categorized by SMI. A recent pharmacokinetic analysis of clinical trial data of pembrolizumab-treated patients with melanoma and non-small cell lung cancer, reported that cachectic patients exhibited elevated pembrolizumab clearance and poor response, suggesting that cancer cachexia could change the pharmacokinetics enough contributing to resistance to pembrolizumab49. We found no associations between SMI and PFS or ORR with antitumor effects of ICB therapy but recognize that larger sample sizes and more accurate classification of cachexia may be needed. To determine whether SMI is a predictive biomarker that could be used to inform treatment selection, the interaction between SMI and treatment type (e.g., ICB vs. VEGF-TKI) should be investigated in a large-scale clinical trial that compares two treatment arms and ideally, is comprised of first-line treated patients.
The pathogenic mechanisms of cancer-induced wasting are complex and multi-factorial50. A recent study in ccRCC found that tumor-derived cytokines were associated with low skeletal muscle mass and poor survival51. In our molecular analyses, we found distinctive tumors features from patients with low SMI, in particular these tumors were characterized by increased anigogenic and IL-6 signaling with decreased fatty acid metabolism. Furthermore, immunogenomic features revealed no differences in total immune infiltration, or immune checkpoints (CTLA4, PDL-1, PD1) but a significant high myeloid signal, reflected by higher macrophage infiltration and higher AdenoSig scores. While this may suggest differences in the immune tumor microenvironment by SMI, we did not see such differences reflected in ORR or PFS in our metastatic patient cohort. Interestingly, clinical response to the adenosine 2A receptor antagonist ciforadenant have been associated with higher expression of AdenoSig scores43. With these findings in mind (in particular the higher angiogenesis and myeloid infiltration in low SMI), It would be of particular interest to assess how SMI category affects outcomes on ICB plus VEGF TKI combinations compared to doublet ICB combination, which are the current standards in the first line. Prospective evaluations would be helpful to further investigate these findings.
We regard our findings as hypothesis-generating which merit further study. Study strengths include the strict eligibility and outcome criteria with detailed clinical annotation including RECIST assessment of cross-sectional imaging. The analytic cohort was comprised of patients with clear cell histology who received ICB without the addition of VEGF-TKI combinations. Only contrast-free CT scans taken within two months of ICB start were interpreted for body composition52 and sex-specific body composition cut points were evaluated. Study limitations include its retrospective, observational nature and heterogeneous patient population with respect to the line of therapy. We could not consider the confounding effect of subsequent systemic therapies after ICB on OS outcomes, survival bias, or change in body composition during follow-up.
In conclusion, our study highlights the relevance of skeletal muscle in the BMI paradox observed in metastatic ccRCC patients treated with ICB. Low SMI patients experienced inferior survival regardless of adiposity although this was not significant in multivariable analysis. Exploratory molecular analyses suggest that low SMI patients harbor more aggressive tumor molecular profiles which may have implications for therapeutic strategies. Longitudinal studies that describe changes in body composition during ICB treatment are needed to further define the clinical relevance of muscle quantity and change over time in metastatic ccRCC patients. Additionally, studies are needed to assess how we can incorporate body composition measurements as an additional “vital sign” in the clinic to help assess prognosis and identify patients that may benefit from behavioral interventions to increase muscle mass.
Supplementary Material
Translational Relevance.
In this cohort of 205 patients with metastatic clear cell renal carcinoma treated with immune checkpoint blockade agents, we found that patients with low skeletal muscle index experienced inferior overall survival compared to patients with high skeletal muscle index. No adiposity-related body composition variable was associated with any clinical outcome. Exploratory molecular analyses suggested that patients with low skeletal muscle index harbor more aggressive tumors with higher expressions of angiogenic, inflammatory, and myeloid signals than patients with high skeletal muscle index. Our findings suggest that skeletal muscle as opposed to adiposity may be driving the obesity paradox observed in metastatic clear cell renal cell cancer patients treated with immune checkpoint blockade and potentially could inform on future therapeutic interventions in patients with low skeletal muscle mass.
Acknowledgements
National Institute of Health, National Cancer Institute, P30 CA008748
A. Knezevic, S. Petruzella, K. Weiss, C. Duzgol, J. Chaim, O. Akin, J. Scott, F. Kuo, R. Kotecha, A. Hakimi, C. Lee, R. Motzer, M. Voss, H. Furberg
National Institute of Health, National Cancer Institute CA R01 233885
A. Knezevic, S. Petruzella, O. Akin, M. Mourtzakis, F. Kuo, A. Hakimi, M. Voss, H. Furberg
We gratefully acknowledge the patients from MSK who allowed us to use their clinical data in this research project.
Yasser Ged reports advisory board consultation fees from Bristol Meyer Squid, Exelixis, and Aveo.
Ritesh Kotech reports advisory board consultation for Eisai and reports receiving institutional research funding from Pfizer and Takeda.
Chung-Han Lee reports receiving commercial research grants from BMS, Eisai, Exelixis, Pfizer, and Calithera, and is a consultant/advisory board member for Amgen, BMS, Exelixis, and Eisai.
Robert J, Motzer has received personal fees from Pfizer, Eisai, Exelixis, Novartis, Genentech/Roche, Merck, Incyte, and Eli Lilly; grants from Pfizer, Eisai, Novartis, Bristol-Myers Squibb, Genentech/Roche, and GlaxoSmithKline; and travel/accommodation expenses from Bristol-Myers Squibb.
Martin H. Voss has received grants from Bristol-Myers Squibb, Genentech/Roche, and Pfizer; has received nonfinancial support from AstraZeneca/Medimmune; has received personal fees from Novartis, Pfizer, Exelixis, Eisai, Corvus, Calithera, and Merck; has received travel/accommodation expenses from Eisai, Novartis, and Takeda; and has been a consultant/advisory board member for Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Natera, Novartis, OnQuality Pharmaceuticals, and Pfizer.
Footnotes
Conflict of interest:
The other authors made no disclosures.
References
- 1.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ged Y, Voss MH. Novel emerging biomarkers to immunotherapy in kidney cancer. Ther Adv Med Oncol. 2021;13:17588359211059367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. [DOI] [PubMed] [Google Scholar]
- 5.Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. The Lancet Oncology. 2015;16(3):293–300. [DOI] [PubMed] [Google Scholar]
- 6.Wells JC, Stukalin I, Norton C, et al. Third-line Targeted Therapy in Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2017;71(2):204–209. [DOI] [PubMed] [Google Scholar]
- 7.Chrom P, Stec R, Bodnar L, Szczylik C. Incorporating Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio in Place of Neutrophil Count and Platelet Count Improves Prognostic Accuracy of the International Metastatic Renal Cell Carcinoma Database Consortium Model. Cancer Res Treat. 2018;50(1):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Velasco G, Culhane AC, Fay AP, et al. Molecular Subtypes Improve Prognostic Value of International Metastatic Renal Cell Carcinoma Database Consortium Prognostic Model. The oncologist. 2017;22(3):286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow WH, Gridley G, Fraumeni JF Jr., Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343(18):1305–1311. [DOI] [PubMed] [Google Scholar]
- 10.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 11.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. Journal of the National Cancer Institute. 2013;105(24):1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. International journal of cancer. 2013;132(3):625–634. [DOI] [PubMed] [Google Scholar]
- 13.Albiges L, Hakimi AA, Xie W, et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J Clin Oncol. 2016;34(30):3655–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalani AA, Bakouny Z, Farah S, et al. Assessment of Immune Checkpoint Inhibitors and Genomic Alterations by Body Mass Index in Advanced Renal Cell Carcinoma. JAMA Oncol. 2021;7(5):773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators of inflammation. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. The Lancet Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez MC, Correia M, Heymsfield SB. A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care. 2017;20(5):314–321. [DOI] [PubMed] [Google Scholar]
- 19.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333–2338. [DOI] [PubMed] [Google Scholar]
- 21.Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased Skeletal Muscle Mass is Associated with an Increased Risk of Mortality after Radical Nephrectomy for Localized Renal Cell Cancer. The Journal of urology. 2016;195(2):270–276. [DOI] [PubMed] [Google Scholar]
- 22.Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. The Journal of urology. 2016;195(1):26–32. [DOI] [PubMed] [Google Scholar]
- 23.Vrieling A, Kampman E, Knijnenburg NC, et al. Body Composition in Relation to Clinical Outcomes in Renal Cell Cancer: A Systematic Review and Meta-analysis. European urology focus. 2018;4(3):420–434. [DOI] [PubMed] [Google Scholar]
- 24.Martini DJ, Olsen TA, Goyal S, et al. Body Composition Variables as Radiographic Biomarkers of Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Front Oncol. 2021;11:707050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Cao L, Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: A systematic review and meta-analysis. Int Immunopharmacol. 2020;88:106907. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Wang T, Tong G, Li X, You D, Cong M. Prognostic Impact of Sarcopenia on Clinical Outcomes in Malignancies Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:726257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015;5(4):e006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 30.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. American journal of epidemiology. 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 31.van der Kooy K, Leenen R, Seidell JC, Deurenberg P, Visser M. Abdominal diameters as indicators of visceral fat: comparison between magnetic resonance imaging and anthropometry. Br J Nutr. 1993;70(1):47–58. [DOI] [PubMed] [Google Scholar]
- 32.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology. 2011;12(5):489–495. [DOI] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 34.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. [DOI] [PubMed] [Google Scholar]
- 38.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshihara K, Shahmoradgoli M, Martinez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer A, Green J, Pollard J Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fong L, Hotson A, Powderly JD, et al. Adenosine 2A Receptor Blockade as an Immunotherapy for Treatment-Refractory Renal Cell Cancer. Cancer Discov. 2020;10(1):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28(6):1054–1060. [DOI] [PubMed] [Google Scholar]
- 45.Colomba E, Alves Costa Silva C, Le Teuff G, et al. Weight and skeletal muscle loss with cabozantinib in metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Giorgi U, Procopio G, Giannarelli D, et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(13):3839–3846. [DOI] [PubMed] [Google Scholar]
- 47.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 48.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner DC, Kondic AG, Anderson KM, et al. Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin Cancer Res. 2018;24(23):5841–5849. [DOI] [PubMed] [Google Scholar]
- 50.Peixoto da Silva S, Santos JMO, Costa ESMP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kays JK, Koniaris LG, Cooper CA, et al. The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma. Cancers (Basel). 2020;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paris MT, Furberg HF, Petruzella S, Akin O, Hotker AM, Mourtzakis M. Influence of Contrast Administration on Computed Tomography-Based Analysis of Visceral Adipose and Skeletal Muscle Tissue in Clear Cell Renal Cell Carcinoma. JPEN J Parenter Enteral Nutr. 2018;42(7):1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.