Abstract
The importance of CD40, CD80, and CD86 costimulatory molecules in anti-Leishmania immune responses has been established in murine models. A role for these costimulatory molecules in human anti-Leishmania immune responses was investigated in this study. Autologous macrophages and peripheral blood leukocytes (PBL) were prepared from peripheral blood mononuclear cells of Leishmania-naive donors and cultured with or without Leishmania major in various combinations. After 7 days of culture, high levels of CD40 and CD86 were expressed on macrophages in the presence or absence of L. major. When macrophages were cultured for an additional 7 days with PBL, expression of all three costimulatory molecules was detected. When L. major was present in these cultures, the expression of CD80, and to a lesser extent CD40, on macrophages was enhanced. Blockade of CD80, CD86, or both molecules (in the order of greatest effect) in cultures containing macrophages, PBL, and L. major significantly inhibited the production of gamma interferon, interleukin-5 (IL-5), and IL-12. Blockade of CD40-CD154 interactions also significantly inhibited production of these cytokines in response to L. major. Production of IL-10 was unaltered by the blockade of these costimulatory molecules. Thus, these data suggest that CD40, CD80, and CD86 expression and regulation may significantly impact anti-Leishmania immune responses in humans.
The outcome of experimental cutaneous infection with Leishmania major in mice is dependent on the strain of mouse and the predominating T-cell immune response that develops. Resistance is associated with Th1 responses and the appropriate production of interleukin-12 (IL-12) and gamma interferon (IFN-γ), leading to parasite destruction by macrophages (33, 34, 39, 48). Susceptibility is associated with Th2 responses, early IL-4 production, IL-12 insensitivity, and a failure of IFN-γ-induced macrophage activation (7, 24, 29, 30). Because costimulation of T cells by antigen-presenting cells (APCs; e.g., dendritic cells and macrophages) has been shown to influence the activation of T cells (18, 20), the influence of costimulatory molecule expression in mice on their resistance to Leishmania infection has been investigated.
Appropriate costimulation by CD40 may be essential for resistance in Leishmania-infected mice, but an essential role for B7 costimulation is in dispute. In CD40- or CD154-deficient mice, impaired production of IL-12 and IFN-γ is observed along with an increased mortality rate following Leishmania infection (6, 26, 45). In contrast, in CD28-deficient strains of mice, both Leishmania-resistant and Leishmania-susceptible phenotypes were maintained following infection (3), suggesting that B7-CD28 costimulation played a limited role. However, treatment of L. major-infected mice with CTLA-4Ig caused normally susceptible mice to develop protective Th1 responses (9), suggesting that B7 costimulation is essential for susceptibility. This inference was supported by results in the same study showing that CTLA-4Ig had no effect on the outcome of infection in normally resistant mice. More recently, blockade of CD86, but not CD80, in susceptible mice was shown to decrease parasite burdens and the production of Th2 cytokines following L. major infection (4). These results suggested that CD86, and not CD80, is critical for Th2 differentiation. However, other studies have indicated that B7 costimulation is required for the early development of both resistant and susceptible anti-Leishmania responses in mice, and that CD86 functions as the dominant costimulatory molecule in both cases (12). Thus, the role of B7 costimulation in experimental leishmaniasis is unclear.
With the exception of studies by Probst et al. (37), showing up-regulation of CD80 on leishmanial antigen-treated human macrophages and dendritic cells, little is known of the role that costimulation plays in experimental human responses to Leishmania infection. Based on what is known for mice, we hypothesized that human responses to L. major would be dependent on CD40 costimulation and influenced by both CD80 and CD86. To test these hypotheses, we used an in vitro cell priming system (IVS) for human peripheral blood mononuclear cells (PBMC) to investigate (i) the influence of L. major infection on the expression of these costimulatory molecules on macrophages in the presence or absence of peripheral blood leukocytes (PBL) and (ii) the influence of CD40, CD80, and CD86 costimulation on the production of Th1- and Th2-specific cytokines in response to L. major. We show that among these molecules, CD86 and CD40 play a critical role in the initiation of anti-Leishmania T-cell activation.
MATERIALS AND METHODS
Parasites.
L. major (isolate LV39, R/SU/59/P) parasites were grown on biphasic NNN medium (48) and passed through mice every 2 weeks to maintain virulence. Stationary metacyclic promastigotes were isolated by negative selection using peanut agglutinin as described previously (38).
Reagents.
The following reagents were used in cell cultures: human CTLA-4–Fc chimera (R&D Systems, Minneapolis, Minn.) at 10 μg/ml; neutralizing anti-human CD80 and CD86 monoclonal antibodies (MAbs) (mouse immunoglobulin G1 [IgG1] isotype; R&D Systems) at 2.5 μg/ml; neutralizing anti-CD154 MAb (mouse IgG1 isotype; PharMingen, San Diego, Calif.) at 10 μg/ml; and irrelevant isotype-matched antibodies from the same suppliers, used as controls at the same concentrations. The following reagents were used for flow cytometry: fluorescein isothiocyanate (FITC)-labeled anti-human CD14 (clone TUK4, mouse IgG2a; Caltag, Burlingame, Calif.); phycoerythrin (PE)-labeled anti-human CD80 (clone L307.4, mouse IgG1; PharMingen); PE- and FITC-labeled anti-human CD86 (clone 2331, mouse IgG1; PharMingen); CyChrome-labeled anti-human CD40 (clone 5C3, mouse IgG1; PharMingen); FITC-labeled anti human HLA DR, DP, and DQ (clone TU 39, mouse IgG2a; PharMingen); and appropriately labeled irrelevant isotype-matched control antibodies from the same suppliers.
IVS.
Human blood was obtained from healthy individuals at the Student Health Center of Colorado State University. Blood was collected from each donor at two time points (stages 1 and 2), 7 days apart.
From the first blood collection (stage 1), adherent cells (macrophages) were prepared to serve as APCs in stage 2. PBMC were obtained from heparinized venous blood by passage over a Ficoll-Hypaque gradient (16). PBMC were washed and resuspended at a concentration of 4 × 106 cells/ml in complete medium consisting of RPMI 1640 medium supplemented with 2 mM l-glutamine, penicillin (100 U/ml), gentamicin (100 μg/ml), and 10% heat-inactivated human AB serum (Pel-Freez, Clinical System, Brown Deer, Wis.). Routine testing of medium indicated lipopolysaccharide concentrations of <2 endotoxin units/ml in a Limulus amebocyte lysate assay (BioWhittaker Inc., Walkersville, Md.). These cells were plated in 24-well tissue culture plates (Costar; Corning Incorporated, Corning, N.Y.) at 1 ml/well and incubated for 2 h at 37°C and 5% CO2. Nonadherent cells were removed from the plates, and adherent cells were cultured at 37°C with 5% CO2 for an additional 6 days. Of the major histocompatibility complex (MHC) class II+ cells obtained with these methods, no CD19+ B cells were detected (data not shown). Further, no dendritic cells were identified among the adherent cells, based on a lack of CD1a expression on MHC class II+ cells as determined by flow cytometry or by morphology using light microscopy. Metacyclic L. major promastigotes were added to some cultures at a parasite/cell ratio of 5:1, and incubation was continued for an additional 24 h at 34°C with 5% CO2. Cultures were then washed to remove free parasites. These techniques routinely resulted in an approximate 90% infection rate.
In stage 2, primary stimulation of PBL was performed. From a second blood collection, both nonadherent cells (PBL) and adherent cells were obtained. Blood was collected from the same donor as in stage 1, and PBMC were plated at 4 × 106 cells/ml, 1 ml/well in 24-well tissue culture plates. After 2 h of culture at 37°C with 5% CO2, PBL were harvested and resuspended in complete medium at a concentration of 4 × 106 cells/ml. Adherent cells were cultured as described in stage 1 for use in stage 3. PBL (1 ml) were added to culture wells containing Leishmania-infected adherent cells obtained in stage 1 and cultured for an additional 7 days at 37°C with 5% CO2. Blocking reagents (anti-human CD80, anti-human CD86, and anti-human CD154, CTLA-4Ig, or controls) were present in some cultures during this 7-day incubation at concentrations of 2.5 to 10 μg/ml. After this incubation, culture supernatants and blast cells were harvested. Supernatants were stored at −20°C until assayed.
In stage 3, secondary stimulation of PBL blasts was performed. The blast cells obtained in stage 2 were washed and resuspended in complete medium at 4 × 106 cells/ml. These cells were restimulated by culturing (1 ml/well) for 48 h at 37°C with 5% CO2 with the autologous Leishmania-infected adherent cells obtained in stage 2. No blocking reagents were added to these cultures. Culture supernatants were collected and stored at −20°C until assayed.
Cytokine assays.
Concentrations of IFN-γ, IL-5, IL-10, and IL-12 in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) using commercial anticytokine antibody pairs (PharMingen) and a protocol provided by the manufacturer. Human recombinant IFN-γ, IL-5, IL-10, and IL-12 (PharMingen) were used to generate standard curves. The limits of detection for these assays were 10 pg/ml for IFN-γ, IL-10, and IL-12 and 2 pg/ml for IL-5.
Flow cytometry.
Monocytes and macrophages were analyzed for costimulatory molecule expression. Monocytes were obtained as fresh PBMC and were identified by expression of CD14. Macrophages were obtained from cultures of adherent cells (see “IVS” above) by using tissue culture cell scrapers (resultant cells were ≥90% viable by trypan blue exclusion) and were identified by their expression of MHC class II. Cells (5 × 105/sample) were prepared for analysis by resuspension in PAB (phosphate-buffered saline, 1% bovine serum albumin, 0.05% sodium azide) and blocked with mouse Ig (20 μg/ml) and 10% fetal bovine serum for 30 min on ice. Cells were then incubated with labeled antibodies or corresponding controls for an additional 30 min. Cells were fixed with 1% paraformaldehyde in phosphate-buffered saline and analyzed on a Coulter XL flow cytometer (Coulter Corp., Hialeah, Fla.).
Statistical analysis.
Comparison of cytokine levels between treatment groups and across multiple experiments was done by analysis of variance two-way ANOVA followed by Tukey all-ways comparison posthoc t tests. All tests were performed with Sigma-Stat software (SPSS Inc., Chicago, Ill.). Differences were considered significant at P < 0.05.
RESULTS
Expression of costimulatory molecules on monocytes/macrophages in the presence or absence of L. major and PBL.
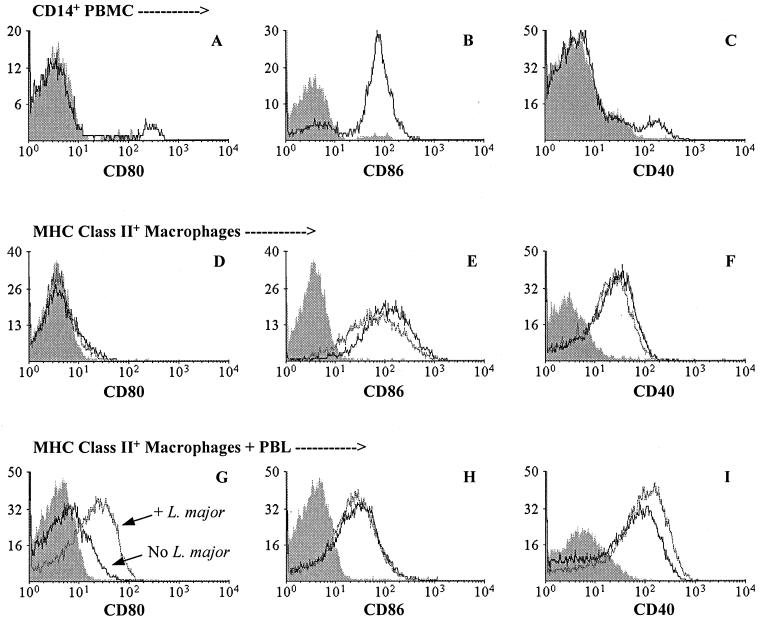
Freshly isolated CD14+ monocytes and cultured MHC class II+ macrophages were analyzed for expression of CD40, CD80, and CD86 molecules. As shown in Fig. 1A to C, each of these molecules was expressed on some CD14+ monocytes; whereas few monocytes expressed CD80 (2% [Fig. 1A]) and CD40 (4% [Fig. 1C]), 70% expressed CD86 (Fig. 1B). Expression of costimulatory molecules was also observed on some MHC class II+ macrophages after 7 days of culture (Fig. 1D to F). Few macrophages expressed CD80, and then only at low levels (Fig. 1D), whereas most (≥70%) expressed CD86 (Fig. 1E). The expression of both CD80 and CD86 on macrophages was similar to that observed on monocytes (see above). In contrast to monocytes, approximately half (53%) of the macrophages expressed CD40 (Fig. 1F). The presence of L. major during the last 24 h of culture did not alter the expression of CD80 or CD40 on macrophages (Fig. 1D and F). Compared with unexposed macrophages, the expression of CD86 on L. major-exposed macrophages was only slightly reduced, if changed at all (Fig. 1E), and only on macrophages from some donors (data not shown).
FIG. 1.
Expression of CD80, CD86, and CD40 on monocytes and macrophages. Three different populations of monocytes/macrophages were assessed for expression of CD80 (A, D, and G), CD86 (B, E, and H), and CD40 (C, F, and I) by flow cytometry before or after culture. (A to C) Expression on freshly isolated CD14+ PBMC. (D to F) Expression on MHC class II+ adherent cells (macrophages [see Materials and Methods]) for cells exposed (gray line) or not exposed (black line) to L. major during the last 24 h of 7-day cultures. Similar results were obtained for macrophages cultured for a total of 14 days. (G to I) Expression on MHC class II+ adherent cells exposed (gray line) or not exposed (black line) to L. major and cultured with PBL for an additional 7 days (14-day total culture). In all panels, shaded histograms represent nonspecific fluorescence of cells stained with an isotype control antibody. The data presented are representative of five individual donors.
Culture of macrophages with PBL altered the influence of L. major on costimulatory molecule expression (Fig. 1G to I). In the presence of PBL, more L. major-infected (44%) than uninfected (17%) macrophages expressed CD80 (Fig. 1G). A similar but less marked increase in the percentage of CD40-expressing macrophages was observed: 71% of infected macrophages and 53% of uninfected macrophages expressed CD40 (Fig. 1I). In contrast, no difference in the percentage of CD86 expression was observed between infected and uninfected macrophages (Fig. 1H).
Blockade of costimulatory molecules alters L. major-specific cytokine production.
Because costimulatory molecules can influence Th1 and Th2 cytokine production, and because L. major was shown to alter costimulatory molecule expression on macrophages cultured in the presence of PBL (see above), we examined the relative importance of CD40, CD80, and CD86 expression on the production of cytokines associated with Th1 and Th2 responses. PBL were stimulated with L. major-infected macrophages in (i) primary cultures with or without blocking reagents and (ii) secondary cultures without blocking reagents (stages 2 and 3 of IVS [see Materials and Methods]).
CD40-CD154 blockade.
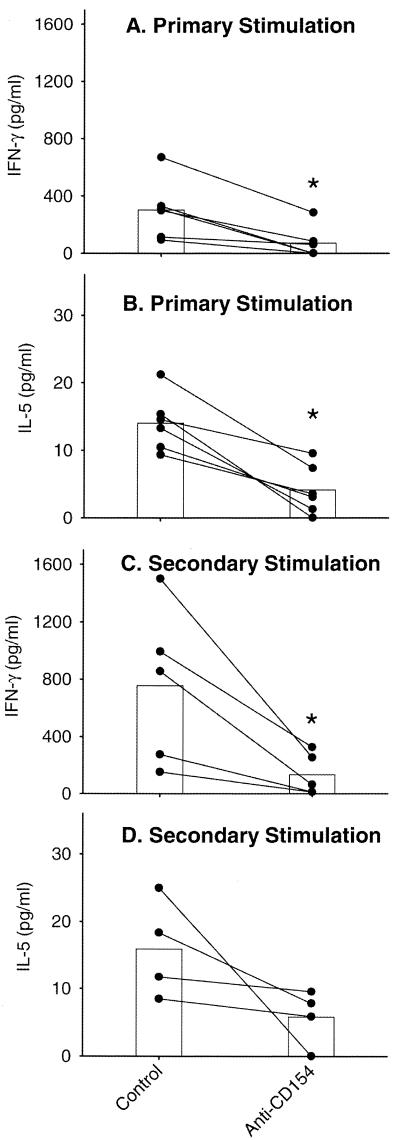
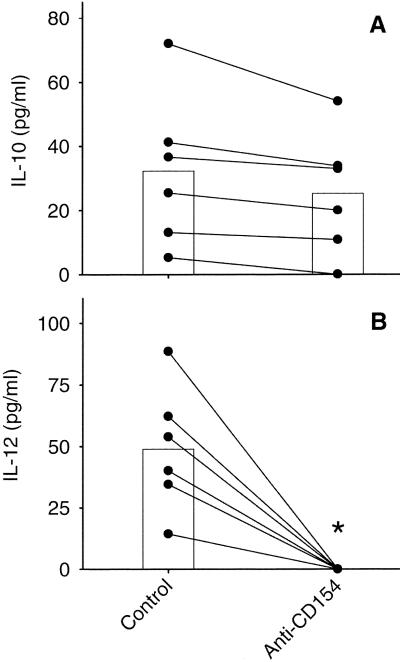
The influence of CD40 expression on L. major-specific cytokine production was examined by blocking CD40-CD154 interactions with anti-CD154. As shown in Fig. 2, the presence of anti-CD154 during primary stimulation of PBL resulted in significantly reduced IFN-γ (Fig. 2A) and IL-5 (Fig. 2B) levels compared to cultures containing isotype-matched control antibody. This difference in cytokine levels was also observed following blast cell restimulation even though no anti-CD154 was present in these cultures (Fig. 2C and D). Interestingly, no change in IL-10 levels was detected in primary (Fig. 3A) or secondary (data not shown) cultures as a result of anti-CD154 exposure, whereas anti-CD154 exposure reduced the amount of IL-12 to undetectable levels in both primary (Fig. 3B) and secondary (data not shown) cultures.
FIG. 2.
Blockade of CD40-CD154 interaction reduces IFN-γ and IL-5 production after primary and secondary stimulation. PBL were stimulated with autologous L. major-infected adherent cells (macrophages) in the presence or absence of anti-CD154 or an isotype control antibody (mouse IgG1). After 7 days, supernatants were harvested and concentrations of IFN-γ (A) and IL-5 (B) were determined by ELISA. The blast cells from these cultures were isolated and restimulated using autologous L. major-infected adherent cells. After 48 h, supernatants were harvested and concentrations of IFN-γ (C) and IL-5 (D) were determined. The data for individual donors (●) and means (bars) are presented with background cytokine levels subtracted. Background equaled the concentration of cytokine when PBL were stimulated with uninfected adherent cells and ranged <10 to 270 pg/ml for IFN-γ and <2.5 to 5.5 pg/ml for IL-5. The concentrations of IFN-γ and IL-5 observed in cultures treated with isotype control antibody were equivalent to those in cultures with no added antibody (data not shown). ∗, statistically different (P < 0.05).
FIG. 3.
Effects of CD40-CD154 blockade on IL-10 and IL-12 levels after primary stimulation. PBL were stimulated with autologous L. major-infected adherent cells (macrophages) in the presence or absence of anti-CD154 or an isotype control antibody (mouse IgG1). After 7 days, supernatants were harvested and concentrations of IL-10 (A) and IL-12 (B) were determined by ELISA. The data for individual donors (●) and means (bars) are presented with background cytokine levels subtracted. Background equaled the concentration of cytokine when PBL were stimulated with uninfected adherent cells and ranged 10 to 30 pg/ml for both IL-10 and IL-12. The concentrations of IL-10 and IL-12 observed in cultures treated with isotype control antibody were equivalent to those in cultures with no added antibody (data not shown). ∗, statistically different (P < 0.05).
CD80 and/or CD86 blockade.
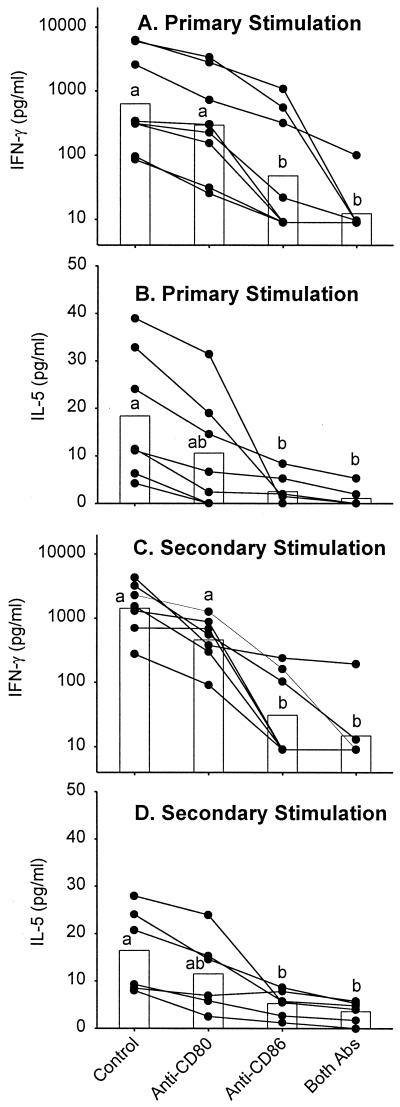
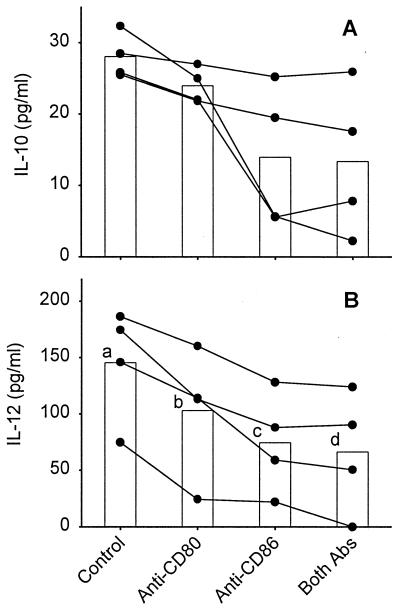
The influence of CD80 and CD86 expression on L. major-specific cytokine production was examined by blocking B7-CD28 and B7–CTLA-4 interactions with CTLA-4Ig. As shown in Table 1, the presence of CTLA-4Ig during primary stimulation of PBL resulted in the reduction of IFN-γ and IL-5 to below detectable levels. Cytokine levels in cultures containing control Ig were comparable to those seen in previous control cultures (see above) and cultures containing no control reagent (Table 1). The reduction of cytokine levels resulting from CTLA-4Ig exposure in primary cultures persisted in cultures of restimulated blast cells (Table 1). To investigate the contribution of individual B7 subtypes to cytokine production, we blocked CD80/CD86-CD28 and CD80/CD86–CTLA-4 interactions with anti-CD80 and anti-CD86 antibodies. As shown in Fig. 4, the presence of anti-CD80 during primary stimulation of PBL resulted in a slight but nonsignificant reduction of IFN-γ (Fig. 4A) and IL-5 (Fig. 4B) levels compared to cultures containing isotype-matched control antibody. The presence of anti-CD86 significantly reduced both IFN-γ and IL-5 levels, an effect that was greater than that observed for anti-CD80 exposure. The presence of both anti-CD80 and anti-CD86 during primary stimulation led to a significant and near-complete elimination of detectable cytokine levels (Fig. 4A and B, respectively), results similar to those obtained when CTLA-4Ig was used. Similar reductions of IFN-γ and IL-5 levels resulting from anti-CD80 and anti-CD86 exposure persisted in cultures of restimulated blast cells and were similarly statistically significant (Fig. 4C and D). No consistent effect of anti-CD80 or anti-CD86 on IL-10 levels was detected in primary (Fig. 5A) or secondary (data not shown) cultures. However, a slight but increasingly significant decrease in IL-12 levels resulting from exposure to anti-CD80, anti-CD86, or both antibodies, similar to that seen for IFN-γ and IL-5, was observed in both primary (Fig. 5B) and secondary (data not shown) cultures.
TABLE 1.
CTLA-4Ig blocks the production of cytokines in response to L. majora
| Treatment | 1st stimulation
|
2nd stimulation
|
||
|---|---|---|---|---|
| IFN-γ | IL-5 | IFN-γ | IL-5 | |
| L. major | 77 ± 10 | 14 ± 2 | 134 ± 11 | 18 ± 3 |
| L. major + CTLA-4Ig | <10 | <10 | <10 | <10 |
| L. major + control Ig | 90 ± 17 | 14 ± 1 | 142 ± 6 | 17 ± 2 |
PBL were stimulated with autologous L. major-infected adherent cells (macrophages) in the presence of CTLA-4Ig or control Ig. After 7 days (primary stimulation), supernatants and blast cells were harvested. Blast cells were restimulated using autologous L. major-infected adherent cells without additional CTLA-4Ig. Supernatants were harvested after 48 h (secondary stimulation). Cytokine concentrations in culture supernatants were determined by ELISA. Values are presented as mean ± standard error for three donors, with background cytokine levels subtracted. Cytokine levels for PBL cultured with uninfected adherent cells (background levels) ranged <10 to 35 pg/ml for IFN-γ and <2.0 to 5.5 pg/ml for IL-5.
FIG. 4.
B7 blockade reduces IFN-γ and IL-5 production after primary and secondary stimulation. PBL were stimulated with autologous L. major-infected adherent cells (macrophages) in the presence or absence of anti-CD80, anti-CD86, both antibodies, or an isotype control antibody (mouse IgG1). After 7 days, supernatants were harvested and concentrations of IFN-γ (A) and IL-5 (B) were determined by ELISA. The blast cells from these cultures were isolated and restimulated using autologous L. major-infected adherent cells. After 48 h, supernatants were harvested and concentrations of IFN-γ (C) and IL-5 (D) were determined. The data for individual donors (●) and means (bars [geometric for IFN-γ; arithmetic for IL-5]) are presented with background cytokine levels subtracted. Background equaled the concentration of cytokine when PBL were stimulated with uninfected macrophages and ranged from <10 to 270 pg/ml for IFN-γ or <2.0 to 5.5 pg/ml for IL-5. The concentrations of IFN-γ and IL-5 observed in cultures treated with isotype control antibody were equivalent to those in cultures with no added antibody (data not shown). Means with different letters are statistically different (P < 0.05).
FIG. 5.
Effects of B7 blockade on IL-10 and IL-12 levels after primary stimulation. PBL were stimulated with autologous L. major-infected adherent cells (macrophages) in the presence or absence of anti-CD80, anti-CD86, both antibodies, or an isotype control antibody (mouse IgG1). After 7 days, supernatants were harvested and concentrations of IL-10 (A) and IL-12 (B) were determined by ELISA. The data for individual donors (●) and means (bars) are presented with background cytokine levels subtracted. Background equaled the concentration of cytokine when PBL were stimulated with uninfected adherent cells and ranged from <10 to 30 pg/ml for both IL-10 and IL-12. The concentrations of IL-10 and IL-12 observed in cultures treated with isotype control antibody were equivalent to those in cultures with no added antibody (data not shown). Means with different letters are statistically different (P < 0.05).
DISCUSSION
The importance of various costimulatory molecules (e.g., CD40, CD80, and CD86) to the outcome of disease following Leishmania infection of mice has been examined. CD40 has been associated with resistance (6, 22, 26, 45), whereas the role of CD80 and CD86 is unclear (3, 4, 9, 12). Infection of mouse macrophages can alter costimulatory molecule expression, at least in vitro (27, 36, 41, 49). However, the impact on host resistance, if any, of this potential subversion of immune function is unclear. Although evidence suggests that dendritic cells play the major role in priming antileishmanial responses (31, 49), macrophages too are host cells for Leishmania and play the major role in killing these parasites (33, 34, 48). This parasitocidal activity is likely dependent, in part, on the macrophage's interaction with T cells (35). Little is known of the role that costimulation plays in directing anti-Leishmania responses in humans. Therefore, we undertook the study presented here to examine (i) the influence of L. major on costimulatory molecule expression on human monocyte-derived macrophages and (ii) the interactions of these macrophages with L. major-activated T cells.
Because costimulatory molecule expression has been shown to be modulated on nonhuman APCs following Leishmania infection in vitro (27, 36, 41, 49), we first determined if the expression of these molecules on cultured human macrophages was modulated following infection with L. major. CD86 expression was slightly decreased by L. major infection, but this effect was not observed with all donors, and we found no change in the expression of CD40 or CD80 (Fig. 1). Thus, no marked alteration of costimulatory molecule expression was observed on L. major-infected human macrophages. These results are similar to those for C57BL/6 mouse macrophages: no change in CD80 expression was observed following infection with L. donovani (41), and no change in CD40, CD80 or CD86 expression was observed following infection with L. major (49). However, Leishmania-induced changes in expression of costimulatory molecules has been observed on macrophages from BALB/c mice and dogs: decreased CD80 expression was observed following infection with L. donovani (27, 41) and L. infantum (36), respectively. From a comparison of the results shown here for humans, and shown by others in animal models, it seems reasonable to predict that the sensitivity of macrophages to modulation of costimulatory molecule expression following Leishmania infection may be dependent on the host's genetic background.
Because T cells may interact directly with infected macrophages through costimulatory molecules to promote parasite killing (35), we also examined the influence of T cells on the expression of costimulatory molecules by L. major-infected and uninfected macrophages. Infection with L. major had no effect on CD86 expression by macrophages cocultured with PBL (Fig. 1). In contrast, coculture with PBL led to an increase in CD40 and CD80 expression on L. major-infected macrophages (Fig. 1). These findings are significant because they demonstrate the feedback interactions between APCs and effector cells and suggest that the effects of L. major on macrophages, at least in part, require such interactions. These interactions may include indirect mechanisms, such as IFN-γ, which is known to induce expression of CD80 and to up-regulate expression of CD86 on monocytes (11, 14), or direct mechanisms such as stimulation of CD40 by CD154, which has been shown to up-regulate both CD40 and CD86 (28). A combination of direct and indirect mechanisms seems likely in this system.
This is the first report that live L. major modulates costimulatory molecule expression on human macrophages, but it is not the first such report regarding intracellular parasites. For example, Subauste et al. observed that infection of human monocytes with tachyzoites of Trypanosoma gondii caused a rapid induction of CD80 expression and up-regulation of CD86 (46). On the other hand, infection of mouse macrophages with L. donovani or Mycobacterium tuberculosis failed to up-regulate or actually decreased the expression of CD80 and CD86 (27, 40). These effects on costimulatory molecule expression may represent strategies used by pathogens to induce anergy or otherwise cause advantageous immune suppression. Interestingly, the results presented here contrast with those of Probst et al. (37), who cultured human macrophages (without PBL) in the presence of a purified Leishmania protein, LeIF, and showed an increase in both CD40 and CD80 expression. This difference in results may be due to confounding interactions between the multitude of potentially immunomodulatory factors released by live parasites versus a single purified protein, or a difference in the concentrations of LeIF present in the macrophage cultures.
We had predicted that primary human responses to L. major would be dependent on CD40 costimulation and influenced by CD80 and CD86 costimulation. These predictions were shown to be mostly true. Blocking CD40 or CD86 significantly reduced the production of IFN-γ, IL-12, and IL-5 following stimulation with L. major, whereas the only significant effect of blocking CD80 was a reduction in IL-12 production. Thus, of these three costimulatory molecules, CD40 and CD86 played the greatest role in initiating human in vitro anti-L. major responses. The relatively small influence of CD80 in this system is an interesting finding in light of the marked up-regulation of CD80 on macrophages cultured with PBL and L. major. The mechanism(s) behind this lack of effect is unclear, but a low sensitivity to CD80 costimulation may be a characteristic of PBMC, as suggested by others (2, 23).
Our prediction that CD40 would be essential to human in vitro responses to L. major was based on well-established pathways: (i) CD40 ligation leads to IL-12 production which leads to IFN-γ production and (ii) CD40 induces up-regulation of B7 molecules on APCs (for reviews, see references 15 and 18). Thus, our finding that blocking CD40 ligation reduced IL-12 and IFN-γ production is not unique to anti-Leishmania responses, but it is important to know that in human cells, as in mouse cells, L. major does not alter the predictability of this costimulatory pathway. The greater effect of CD40 blockade over B7 blockade could be explained, in part, by a double effect on cytokines and B7 expression when CD40 ligation was inhibited.
An unexpected observation was the reduction of IL-5 production in macrophage+L. major+PBL cultures following blockade of CD40 and CD86. IL-5 was used as an indicator of Th2 cell activity. The importance of CD40 to the development of Th1 responses has been well established, but the role of this costimulatory pathway in the development of Th2 responses is less well studied. The results of the studies presented here suggest that CD40, as well as CD86, can costimulate for both Th1 and Th2 responses. Similar findings in another model (44, 47) suggest that, at least for CD40, this may be a common phenomenon.
As shown here, IL-10 production was not significantly altered by blocking CD40 or the B7 molecules, unlike what was observed for IL-5, IL-12, and IFN-γ. The IL-10 levels measured in these studies were lower than those observed by others following mitogen or MAb stimulation (5, 10, 43) and were more in line with spontaneous production levels (5, 25). This can be explained by the use of metacyclic L. major in the study presented here. Metacyclic L. major has been shown to be a poor stimulator of IL-10 production from human PBMC, unlike the log-phase promastigote form of the parasite (42). Because blocking costimulation did not alter IL-10 levels, these results suggest that spontaneous or metacyclic L. major-stimulated IL-10 production is independent of the costimulatory molecules examined. Others have shown that IL-10 production can be modulated by IL-12 and IFN-γ (1, 8, 10, 15). Therefore, it was interesting that IL-10 levels were not changed in the face of markedly lower IL-12 and IFN-γ levels. These data suggest that spontaneous or metacyclic L. major-stimulated IL-10 production is also independent of modulation by IL-12 and IFN-γ. This possibility is supported for IL-12 by the results of Sartori et al. (42), who showed that IL-10 production by metacyclic L. major-infected human PBMC was unchanged by treatment with anti-IL-12 MAb.
In summary, we showed that L. major infection can influence the expression of some costimulatory molecules on human macrophages, with the greatest effect apparent in the presence of PBL. Further, we showed that CD40 and CD86 play critical roles in the initiation of human anti-Leishmania T-cell activation in vitro.
ACKNOWLEDGMENTS
We thank Robin Morris for technical assistance and Lamine Mbow critical review of the manuscript.
This work was supported by NIH grant AI-29955 and a fellowship (to C.I.B.) from CAPES/Fulbright (1997) and CAPES-Brasilia Brazil (1998).
REFERENCES
- 1.Akuffo H, Alexis A, Eidsmo L, Saed A, Nylen S, Maasho K. Natural killer cells in cross-regulation of IL-12 by IL-10 in Leishmania antigen-stimulated blood donor cells. Clin Exp Immunol. 1999;117:529–534. doi: 10.1046/j.1365-2249.1999.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashian G G, Braun C M, Huang S K, Kagey-Sobotka A, Lichtenstein L M, Essayan D M. Differential regulation of human, antigen-specific Th1 and Th2 responses by the B-7 homologues, CD80 and CD86. Am J Respir Cell Mol Biol. 1997;17:235–242. doi: 10.1165/ajrcmb.17.2.2739. [DOI] [PubMed] [Google Scholar]
- 3.Brown D R, Green J M, Moskowitz N H, Davis M, Thompson C B, Reiner S L. Limited role of CD28-mediated signals in T helper subset differentiation. J Exp Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown J A, Titus R G, Nabavi N, Glimcher L H. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174:1303–1308. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 5.Burden N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods. 1997;11:98–111. doi: 10.1006/meth.1996.0393. [DOI] [PubMed] [Google Scholar]
- 6.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 7.Chatelain R, Varkila K, Coffman R L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 8.Chomarat P, Rissoan M C, Banchereau J, Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med. 1993;177:523–527. doi: 10.1084/jem.177.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corry D B, Reiner S L, Linsley P S, Locksley R M. Differential effects of blockade of CD28–B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 10.Daftarian P M, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-α. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- 11.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 12.Elloso M M, Scott P. Expression and contribution of B7–1 (CD80) and B7–2 (CD86) in the early immune response to Leishmania major infection. J Immunol. 1999;162:6708–6715. [PubMed] [Google Scholar]
- 13.Ferlin W G, von der Weid T, Cottrez F, Ferrick D A, Coffman R L, Howard M C. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur J Immunol. 1998;28:525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Freedman A S, Freeman G J, Rhynhart K, Nadler L M. Selective induction of B7/BB-1 on interferon-γ-stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991;137:429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 15.Gately K K, Renzetti L M, Magram J, Stern A S, Gubler L A U, Presky D H. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 16.Goldrosen M H, Gannon P J, Lutz M, Holyoke E D. Isolation of human peripheral blood lymphocytes: modification of double discontinuous density gradient of Ficoll-Hypaque. J Immunol Methods. 1977;14:15–17. doi: 10.1016/s0022-1759(97)90015-6. [DOI] [PubMed] [Google Scholar]
- 17.Gomes N A, Barreto-de-Souza V, Wilson M E, DosReis G A. Unresponsive CD4+ T lymphocytes from Leishmania chagasi-infected mice increase cytokine production and mediate parasite killing after blockade of B7–1/CTLA-4 molecular pathway. J Infect Dis. 1998;178:1847–1851. doi: 10.1086/314520. [DOI] [PubMed] [Google Scholar]
- 18.Grewal I S, Flavell R A. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Gurunathan S, Irvine K R, Wu C Y, Cohen J I, Thomas E, Prussin C, Restifo N P, Seder R A. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 20.Harris N L, Ronchese F. The role of B7 costimulation in T-cell immunity. Immunol Cell Biol. 1999;77:304–311. doi: 10.1046/j.1440-1711.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 21.Heinzel F P, Maier R A., Jr Interleukin-4-independent acceleration of cutaneous leishmaniasis in susceptible BALB/c mice following treatment with anti-CTLA4 antibody. Infect Immun. 1999;67:6454–6460. doi: 10.1128/iai.67.12.6454-6460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzel F P, Rerko R M, Hujer A M. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell Immunol. 1998;184:129–142. doi: 10.1006/cimm.1998.1267. [DOI] [PubMed] [Google Scholar]
- 23.Jaffar Z H, Luminita S, Pandit A, Lordan J, Holgate T, Roberts K. Essential role for both CD80 and CD86 costimulation, but not CD40 interactions, in allergen-induced Th2 cytokine production from asthmatic bronchial tissue: role for αβ, but not γδ, T cells. J Immunol. 1999;163:6283–6291. [PubMed] [Google Scholar]
- 24.Jones D, Elloso M M, Showe L, Williams D, Trinchieri G, Scott P. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major. Infect Immun. 1998;66:3818–3824. doi: 10.1128/iai.66.8.3818-3824.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakumu S, Okumura A, Ishikawa T, Iwata K, Yano M, Yoshioka K. Production of interleukins 10 and 12 by peripheral blood mononuclear cells (PBMC) in chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;108:138–143. doi: 10.1046/j.1365-2249.1997.d01-987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaye P M, Rogers N J, Curry A J, Scott J C. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–2854. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- 28.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 29.Launois P, Ohteki T, Swihart K, MacDonald H R, Louis J A. In susceptible mice Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1−. Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 30.Launois P, Ohteki T, Swihart K, Milon G, Louis J A. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 31.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 32.Murphy M L, Engwerda C R, Gorak P M, Kaye P M. B7–2 blockade enhances T cell responses to Leishmania donovani. J Immunol. 1997;159:4460–4466. [PubMed] [Google Scholar]
- 33.Murray H W, Rubin B Y, Rothermel C D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that IFN-γ is the activating lymphokine. J Clin Investig. 1983;72:1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nacy C A, Fortier A H, Meltzer M S, Buchmeier N A, Schreiber R D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-γ and non-interferon lymphokines. J Immunol. 1985;135:3505–3511. [PubMed] [Google Scholar]
- 35.Nashleanas M, Scott P. Activated T cells induce macrophages to produce NO and control Leishmania major in the absence of tumor necrosis factor receptor p55. Infect Immun. 2000;68:1428–1434. doi: 10.1128/iai.68.3.1428-1434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinelli E, Rutten V P, Bruysters M, Moore P F, Ruitenberg E J. Compensation for decreased expression of B7 molecules on Leishmania infantum-infected canine macrophages results in restoration of parasite-specific T-cell proliferation and gamma interferon production. Infect Immun. 1999;67:237–243. doi: 10.1128/iai.67.1.237-243.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Probst P, Skeiky Y A, Steeves M, Gervassi A, Grabstein K H, Reed S G. A Leishmania protein that modulates interleukin (IL)-12, IL-10 and tumor necrosis factor-alpha production and expression of B7–1 in human monocyte-derived antigen-presenting cells. Eur J Immunol. 1997;27:2634–2642. doi: 10.1002/eji.1830271024. [DOI] [PubMed] [Google Scholar]
- 38.Sacks D L, Perkins P V. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 39.Sadick M D, Locksley R M, Tubbs C, Raff H V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-γ in response to Leishmania antigens in vitro. J Immunol. 1986;136:655–661. [PubMed] [Google Scholar]
- 40.Saha B, Das G, Vohra H, Ganguly N K, Mishra G C. Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the depression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–2624. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 41.Saha B, Das G, Vohra H, Ganguly N K, Mishra G C. Macrophage-T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on Leishmania-infected macrophages and its implication in the suppression of cell-mediated immunity. Eur J Immunol. 1995;25:2492–2498. doi: 10.1002/eji.1830250913. [DOI] [PubMed] [Google Scholar]
- 42.Sartori A, Oliveira M A P, Scott P, Trinchieri G. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J Immunol. 1997;159:2849–2857. [PubMed] [Google Scholar]
- 43.Schwarz M, Majdic O, Knapp W, Holter W. High-level IL-10 production by monoclonal antibody-stimulated human T cells. Immunology. 1995;86:364–371. [PMC free article] [PubMed] [Google Scholar]
- 44.Shi F D, He B, Li H, Matusevicius D, Link H, Ljunggren H G. Differential requirements for CD28 and CD40 ligand in the induction of experimental autoimmune myasthenia gravis. Eur J Immunol. 1998;28:3587–3593. doi: 10.1002/(SICI)1521-4141(199811)28:11<3587::AID-IMMU3587>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Soong L, Xu J C, Grewal I S, Kima P, Sun J, Longley Jr B J, Ruddle N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 46.Subauste C S, De Wall Malefyt R, Fuh F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J Immunol. 1998;160:1831–1840. [PubMed] [Google Scholar]
- 47.Taylor P A, Panoskaltsis-Mortari A, Noelle R J, Blazar B R. Analysis of the requirements for the induction of CD4+ T cell alloantigen hyporesponsiveness by ex vivo anti-CD40 ligand antibody. J Immunol. 2000;164:612–622. doi: 10.4049/jimmunol.164.2.612. [DOI] [PubMed] [Google Scholar]
- 48.Titus R G, Kelso A, Louis J A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984;55:157–165. [PMC free article] [PubMed] [Google Scholar]
- 49.von Stebut E, Belkaid Y, Jakob T, Sacks D L, Udey M C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]