Abstract
Background and aims:
We aimed to identify predictors of change in direct measures of coronary artery calcium (CAC) volume and density in South Asian participants.
Methods:
We used data from participants in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study with prevalent CAC and direct measures of CAC by serial computed tomography (CT) exams (2010–2013, 2016–2018). We examined the distribution of incident CAC volume and peak density, as well as progression and identified risk factors for progression of change in volume and density in multivariable models.
Results:
The study cohort consisted of 102 participants with incident CAC and 285 with CAC progression. CAC volume and density were highest, and incident CAC was most common in the left anterior descending artery (LAD). The greatest progression in volume was in the right coronary artery and the greatest change in density was in the left main. In linear regression models for CAC progression adjusted for baseline density, volume, risk factors, smoking (β +190.1, p = 0.02), baseline volume (β +0.24 per mm3, p < 0.01), and scan interval (β +0.15 per day, p = 0.01) were associated with change in total volume whereas Lp(a) (β +0.81 per mg/dL, p = 0.03), exercise (β +0.19 per 10 MET-min/week, p = 0.01), and baseline volume (β +0.15 per mm3, p < 0.01) and density (β −0.55 per unit, p < 0.01) were associated with change in total density.
Conclusions:
In this South Asian cohort, smoking was associated with CAC volume progression, while Lp(a) and exercise were associated with progression of peak CAC density.
Keywords: Coronary artery disease, Coronary artery calcium, South asians, lipoprotein(a)
1. Introduction
Coronary artery calcium (CAC) scoring is a powerful method for risk stratification for cardiovascular disease in asymptomatic individuals [1]. The standard method for quantification of CAC is the Agatston score, which weighs plaque area upward by a peak density factor. However, it has been shown that plaque density is inversely associated with cardiovascular events, and thus utilizing plaque volume (similar to plaque area) with adjustment for density improves predictive value [2]. Prior studies have derived a peak density factor (1–4) from the Agatston score [2], however, density may be measured directly as a continuous measure, which may improve predictive value [3]. However, there are limited studies involving direct CAC measures, particularly regarding the progression of these measures.
South Asians have higher risk of atherosclerotic cardiovascular disease and ischemic heart disease mortality than other ethnic groups [4]. South Asian men have been shown to have similar CAC burden and progression as White men, but greater than other ethnic groups, while South Asian women were similar to other ethnicities [5,6]. South Asians over age 60 have a higher prevalence of CAC than other ethnic groups, including White participants [7]. The association between South Asian ethnicity and CAC is particularly prominent in those with a family history of coronary artery disease [8]. South Asian individuals also have lower CAC volume than White individuals but higher density than White and non-White individuals, and a higher number of coronary vessels with CAC compared to non-White individuals [9]. In a prior study of cardiovascular risk factors, many factors were associated with CAC volume and density in opposite directions in non-South Asian populations [10]. However, risk factors for CAC incidence and progression among South Asians are not well studied, particularly as they relate to plaque volume and density.
We aimed to evaluate the distribution of incident CAC and CAC progression using direct volume and peak density measures, and to examine risk factors for incidence and progression of CAC volume and density in a population of South Asian individuals free of cardiovascular disease at baseline. For the findings presented in this study, density refers to the highest or peak density, in a given plaque.
2. Patients and methods
2.1. Study population
We used data from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study, a community-based longitudinal cohort of South Asian individuals recruited from the San Francisco Bay Area and the greater Chicago area. Exam 1 (baseline) included 906 participants and was conducted between 2010 and 2013; all surviving participants were invited for Exam 2 in 2015–2018 and 759 (83%) completed this exam. Study methods and recruitment have been published previously [11]. Briefly, eligible participants were ages 40–84 years, of South Asian ancestry, and able to speak, read and write in English, Hindi, or Urdu. MASALA was designed similarly to the Multi-Ethnic Study of Atherosclerosis (MESA) and excluded those with known cardiovascular disease or who had undergone any cardiovascular procedures [11]. The institutional review boards of the University of California, San Francisco (UCSF) and Northwestern University approved the protocol, and all participants gave written informed consent. The study protocol conforms to the Declaration of Helsinki.
2.2. MASALA cohort characterization
Trained bilingual research staff obtained demographic information, tobacco and alcohol use, physical activity, and medication use at both exams. Seated blood pressure was measured three times using an automated blood pressure monitor and the average of the last two readings used for analysis. Hypertension (HTN) was defined as self-reported treatment for HTN or a systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg. Participant weight was measured on a digital scale, height was measured using a stadiometer, and waist circumference was measured using a flexible tape measure tape at the site of maximum circumference. Fasting blood tests were obtained after a 12-h fast. Fasting plasma glucose was measured by the hexokinase method; total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C) were measured by enzymatic methods (Quest, San Jose, CA) and low-density lipoprotein cholesterol (LDL-C) was calculated [12]. Diabetes was defined if a participant was using a glucose-lowering medication, or had a fasting plasma glucose ≥126 mg/dl or post-challenge glucose of ≥200 mg/dL [13]. Lipoprotein(a) [Lp(a)] levels were measured by particle-enhanced immunonephelometry on a BNII nephelometer. Physical activity was assessed using the Typical Week’s Physical Activity Questionnaire [11].
2.3. Coronary artery calcium measurements
Cardiac-gated CT scans were performed at both clinical exams as previously described [11]. One site (UCSF) used a 16D scanner (Philips Medical Systems, Andover, MA) for the 200 participants seen between October 2010 and September 2011, and subsequently used a multi-detector CT Aquilon 64D CT (Toshiba Medical Systems, Tustin, CA) for the rest of Exam 1 and all of Exam 2. The second clinical site (Northwestern University) used a Sensation Cardiac 64 Scanner (Siemens Medical Solutions, Malvern, PA) for all exams. CAC measures have previously been shown to be reproducible across multiple CT scanners in MESA [14]. Participants were scanned in a supine position, superior to inferior, and 46 images were obtained with slice thickness of 3.0 mm. All CT scans were read at the Lundquist Research Institute, Torrance, CA, according to published methods [11]. Calcified plaque within coronary arteries was identified as attenuation >130 Hounsfield units (HU). CAC volume and peak density were measured for each of the four major coronary arteries, and a sum of the unadjusted scores was calculated.
The volume of each plaque in individual vessels was calculated. While prior studies have determined a categorical density factor (1–4) derived from the Agatston score [2], peak density was measured directly as a continuous measure. The density of CAC was determined by measuring the peak Hounsfield units of each plaque and the average peak density per vessel was calculated. Finally, total plaque volume and average peak density were calculated by summing the measures for the individual vessels.
2.4. Statistical analysis
Participants without any CAC at both exams or poor image quality were excluded. Participants who had cardiovascular events between exam 1 and exam 2 were not excluded as the study was designed to assess changes in CAC, not clinical events. We examined baseline CAC volume and density and change from exam 1 to 2, in total and by individual coronary vessel. We also performed sex-stratified analyses, among those with CAC progression (CAC>0 at both exams) and among those with incident CAC (CAC = 0 at Em 1, CAC>0 at Em 2). We performed multivariable linear regression to determine predictors of change in total CAC volume and total CAC peak density in the subsets with CAC progression and incident CAC. For those with incident CAC, change in volume and density was measured as the value for volume and density at exam 2 since the Agatston score (and thus volume and density) was 0 at Em 1. Linear models were adjusted for age, sex, baseline CAC volume and density, time between exams 1 and 2, hypertension, diabetes, current smoking, total cholesterol HDL-cholesterol (HDL-C), exercise, exam 2 statin use, Lp(a) level, and fasting insulin level for the analysis in the progression cohort. Other predictors, including BMI, waist circumference, waist-hip ratio, education level, family income, years in the United States and family history of myocardial infarction, were not included due to lack of statistical significance in univariate models. For the incident CAC volume and density analyses, linear regression models were adjusted for the same variables, except for exam 1 CAC volume and peak density. In sensitivity analysis, we performed linear regression using the models above in the subset of our study population, excluding those with any statin at exam 1 or exam 2. While change in CAC volume was not normally distributed, the sample size was sufficiently large to have normally distributed regression coefficients due to the central limit theorem. All analyses were conducted in SAS, version 9.4 (SAS Institute). A two-tailed p-value <0.05 was considered statistically significant.
3. Results
3.1. Population
There were 906 total participants in MASALA, 759 of whom also completed exam 2. A total of 701 participants had CAC measurements for both exam 1 and 2. We excluded 299 with CAC = 0 at both exams, 13 with CAC<5 at Em 2 (due to potential technical variability), and 2 participants with motion degraded images, resulting in a study sample of 387 participants. Median time between exam 1 and 2 was 4.78 [IQR 4.40,5.22] years. Characteristics of the cohort are shown in Table 1. India was the most common country of birth (86.3%), followed by other (7.0%), and Pakistan (3.4%). Participants at exam 2 had greater waist circumference, waist to hip ratio, SBP, DBP, HTN prevalence and fasting glucose. CAC Agatston scores were higher at exam 2 compared with exam 1. There were 102 participants with incident CAC. Participants with incident CAC had higher level of exercise, greater waist-hip ratio and greater fasting glucose at exam 2 (Supplemental Table 1). The remaining 285 participants with CAC at exam 1 were evaluated in the CAC progression analysis. Participants with CAC progression had similar baseline characteristics to the overall cohort. Notable exceptions included SBP and DBP, not significantly different between exams 1 and 2, while diabetes and statin use were significantly more prevalent at exam 2 than exam 1 (Supplemental Table 1).
Table 1.
Characteristics of the MASALA study participants, 2010–2013 and 2016–2018.
| Exam 1 n = 387 | Exam 2 n = 387 | p | |
|---|---|---|---|
| Sex, male | 286 (73.9) | – | |
| Age, years | 58.5 ± 9.0 | 62.8 ± 8.9 | <0.001 |
| Current smoker | 20 (5.2) | 17 (4.4) | 0.61 |
| Exercise, MET-min/week | 1050 (420–1890) | 1260 (443–2145) | 0.08 |
| BMI, kg/m2 | 25.9 ± 4.0 | 26.5 ± 4.2 | 0.05 |
| Waist circumference, cm | 94.3 ± 10.2 | 96.5 ± 10.6 | 0.003 |
| Waist-Hip ratio | 0.92 ± 0.07 | 0.94 ± 0.07 | <0.001 |
| Systolic blood pressure, mmHg | 128 ± 16 | 131 ± 17 | 0.007 |
| Diastolic blood pressure, mmHg | 75 ± 10 | 76 ± 9 | 0.03 |
| Hypertensiona | 205 (53.0) | 250 (64.6) | 0.001 |
| Diabetesb | 119 (30.7) | 144 (37.2) | 0.06 |
| LDL-cholesterol, mg/dL | 108 ± 35 | 105 ± 37 | 0.18 |
| HDL-cholesterol, mg/dL | 46 (39–56) | 47 (38–56) | 0.65 |
| Triglycerides, mg/dL | 121 (89–159) | 119 (85–161) | 0.59 |
| Lp(a), mg/dL | 16 (9–33) | – | |
| Fasting glucose, mg/dL | 106 ± 24 | 114 ± 24 | <0.001 |
| Fasting insulin, pmol/L | 60.5 (41.0–94.3) | ||
| Statin medication use | 144 (37.2) | 171 (44.2) | 0.05 |
| CAC Agatston Score: | |||
| 0 | 102 (26.4) | 0 | <0.001 |
| 1–100 | 155 (40.1) | 186 (48.1) | |
| 101–400 | 78 (20.2) | 100 (25.8) | |
| >400 | 52 (13.4) | 101 (26.1) | |
| Country of birth: | |||
| Bangladesh | 1 (0.3) | ||
| India | 334 (86.3) | ||
| Pakistan | 13 (3.4) | ||
| Sri Lanka | 5 (1.3) | ||
| United States | 7 (1.8) | ||
| Other | 27 (7.0) | ||
| Bachelor’s degree or higher | 348 (89.9) | ||
| Family income ≥ $75,000 | 280 (74.5) | ||
| Years lived in United States | 30.2 ± 11.1 | ||
| First degree relative with history of myocardial infarction | 188 (45.6) |
Values are presented as n (%), mean ± SD, or median (interquartile range), as appropriate. BMI = body mass index, HDL = high-density lipoprotein, LDL = low-density lipoprotein, Lp(a) = lipoprotein(a), MET = metabolic equivalent of task.
Hypertension was defined as systolic blood pressure ≥140 and/or diastolic blood pressure ≥90 mmHg and/or use of an anti-hypertensive medication.
Diabetes was defined as fasting glucose ≥126 mg/dL and/or 2-h post-challenge glucose ≥200 mg/dL and/or the use of an anti-diabetes medication.
3.2. Distribution of CAC volume and peak density
CAC volume and density were assessed in total across all 4 coronary arteries and by individual coronary vessel, in the whole cohort and by sex (Supplemental Table 2). Overall, the highest CAC volume and density were present in the left anterior descending artery (LAD). The greatest increase in volume was in the right coronary artery (RCA), and the greatest increase in density was in the left main artery (LM). CAC volume and density were generally higher in men than women, except in the LM, which was similar for both genders. Increase in CAC volume was greater in men (except for the LM, which was similar). Increase in CAC density was similar in the total assessment, LAD and RCA, and greater in men in the LM and in women in the left circumflex artery (LCx, Supplemental Table 2).
A similar analysis was performed only on those with baseline CAC for CAC progression (n = 285, Supplemental Table 3). CAC volume was generally higher for men than women but similar in LM and RCA. CAC volume progression was also higher in men than women and similar for LM. In contrast to the overall cohort, CAC density was higher in women than men overall and in RCA, and similar in the other arteries. Density change was higher in men than women (similar in LAD and LCx). The highest volume and density were present in LAD. Highest volume change was in RCA (Fig. 1), and highest density change was in LM (Fig. 2).
Fig. 1.
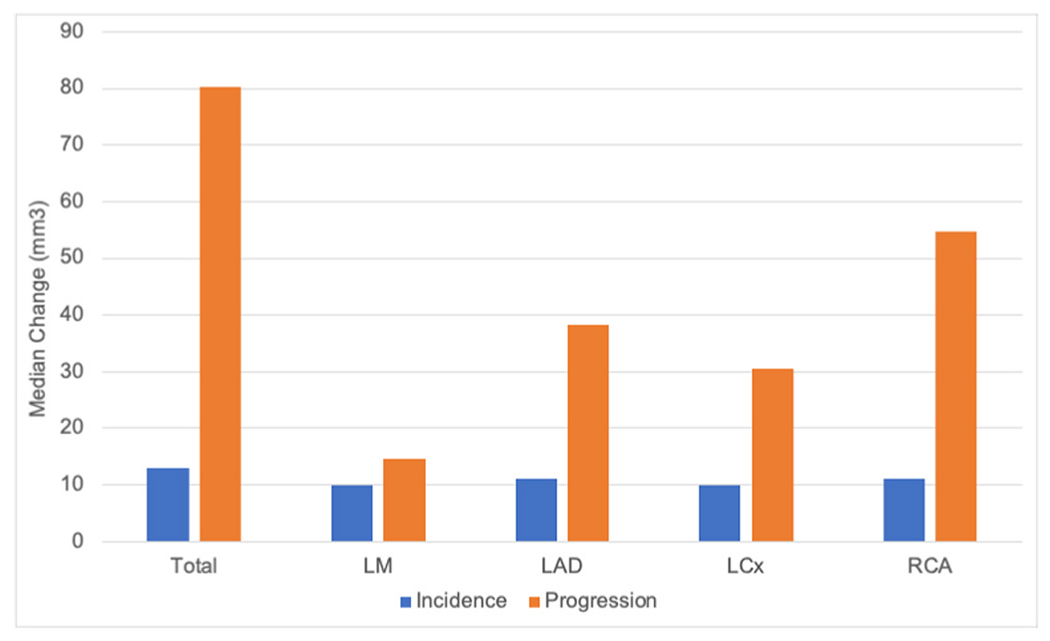
Median change in CAC volume by coronary artery.
LM = Left main artery, LAD = Left anterior descending artery, LCx = Left circumflex artery, RCA = Right coronary artery.
Fig. 2.
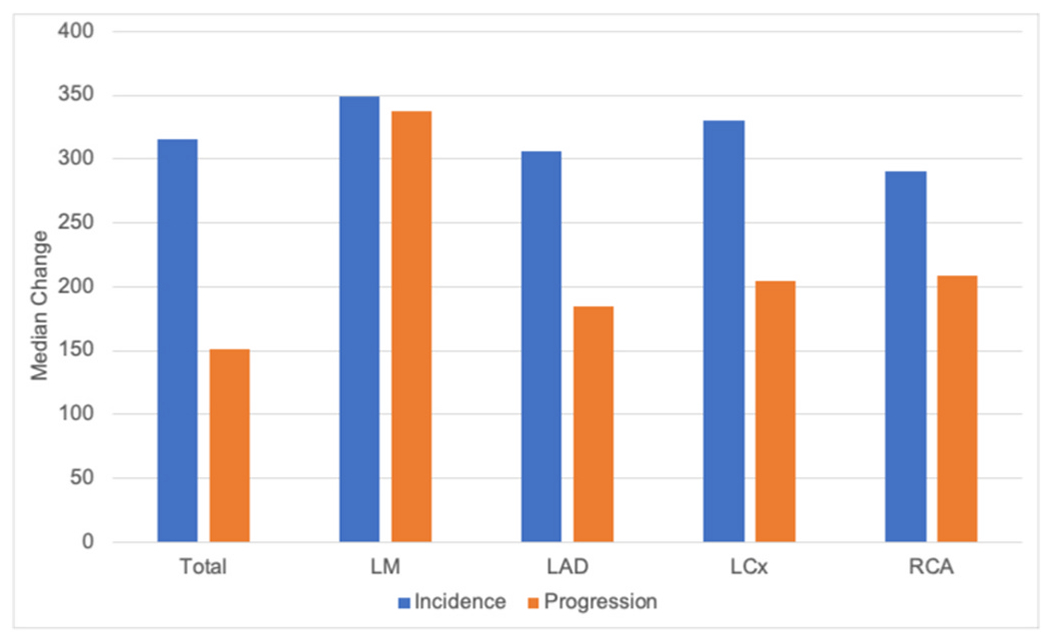
Median change in CAC density by coronary artery.
LM = Left main artery, LAD = Left anterior descending artery, LCx = Left circumflex artery, RCA = Right coronary artery.
Those with incident CAC were evaluated separately (Supplemental Table 4). In the total assessment and the individual coronary arteries (except for LM which had a low number of women), CAC volume was higher in men than women. CAC density, however, was higher in women overall (and in the LM and LCx), similar for women and men in LAD, and higher for men in RCA. Overall, incident CAC was most common in LAD for both men and women. The highest level of incident CAC volume was in LAD and RCA (Fig. 1), while the highest level of incident CAC density was in LM (Fig. 2).
3.3. Factors associated with CAC progression
Among those with baseline CAC (n = 285), smoking (β 190.09, 95% CI 34.91, 345.28, p = 0.02), time between exams (β 0.15 per day, 95% CI 0.04, 0.26, p = 0.01), and baseline CAC volume (β 0.24 per mm3, 95% CI 0.15, 0.34, p < 0.001, Table 2) were the only factors significantly associated with progression of CAC volume at exam 2. Baseline Lp(a) level (β 0.81 per mg/dL, 95% CI 0.08, 1.54, p = 0.03), exercise level (β 0.19 per 10 MET-min/week, 95% CI 0.05, 0.31, p = 0.01), time between exams (β 0.06 per day, 95% CI 0.01, 0.12, p = 0.02), and baseline CAC volume (β 0.15 per mm3, 95% CI 0.10, 0.20, p < 0.001) were positively associated with progression of CAC density at exam 2, while baseline CAC density (β −0.55 per unit of density, 95% CI −0.68, −0.43, p < 0.001) was inversely associated (Table 2). After excluding statin users in sensitivity analysis (n = 149), age (β 8.86 per year, 95% CI −3.74, 2.44, p = 0.003) and female sex (β −118.96, 95% CI −229.03, −8.89, p = 0.03) were significantly associated with change in CAC volume. Smoking (β 181.13, 95% CI −34.13, 396.40, p = 0.10) and Lp(a) (β 1.71 per mg/dL, 95% CI −0.27, 3.69, p = 0.09) were associated with borderline statistical significance. For density progression, Lp(a) was no longer associated, while exercise (β 0.16 per 10 MET-min/week, 95% CI −0.02, 0.35, p = 0.09) and diabetes (β −53.27, 95% CI −114.52, 7.98, p = 0.09) were associated with borderline statistical significance (Supplemental Table 5).
Table 2.
Association between risk factors and CAC volume and density progression among those with baseline CAC.
| Total change in CAC volume (n = 285) |
Total change in peak CAC density (n = 285) |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Age, years | 3.20 | −1.05, 7.45 | 0.14 | −0.91 | −3.02, 1.20 | 0.40 |
| Female sex | −61.62 | −148.72, 25.49 | 0.16 | −1.22 | −44.46, 42.02 | 0.96 |
| Days between exams | 0.15 | 0.04, 0.26 | 0.01 | 0.06 | 0.01, 0.12 | 0.02 |
| Hypertension | 6.38 | −69.08, 81.83 | 0.87 | 1.27 | −36.18, 38.73 | 0.95 |
| Diabetes | 14.74 | −62.23, 91.72 | 0.71 | −20.00 | −58.22, 18.21 | 0.30 |
| HDL, mg/dL | −2.22 | −5.29, 0.85 | 0.15 | 0.40 | −1.12, 1.93 | 0.60 |
| Total cholesterol, mg/dL | −0.31 | −1.30, 0.68 | 0.53 | −0.11 | −0.60, 0.38 | 0.65 |
| Current smoking | 190.09 | 34.91, 345.28 | 0.02 | 41.24 | −35.80, 118.27 | 0.29 |
| Exercise, 10 MET-min/week | 0.12 | −0.15, 0.38 | 0.38 | 0.19 | 0.05, 0.31 | 0.01 |
| Lp(a), mg/dL | 0.36 | −1.10, 1.83 | 0.63 | 0.81 | 0.08, 1.54 | 0.03 |
| Fasting insulin, pmol/L | −0.14 | −0.56, 0.27 | 0.50 | 0.17 | −0.03, 0.38 | 0.11 |
| Statin use | 24.30 | −48.44, 97.03 | 0.51 | −10.66 | −46.77, 25.45 | 0.56 |
| Baseline CAC volume, mm3 | 0.24 | 0.15, 0.34 | <0.001 | 0.15 | 0.10, 0.20 | <0.001 |
| Baseline CAC density | −0.12 | −0.36, 0.13 | 0.34 | −0.55 | −0.68, −0.43 | <0.001 |
Linear regression model adjusted for age, sex, exam 1 CAC volume and density, time between exam 1 and 2, hypertension, diabetes, current smoking, total cholesterol, HDL, exercise, exam 2 statin use, Lp(a), and fasting insulin. Abbreviations as per Table 1.
There were 6 participants who had a decrease in Agatston score from exam 1 to 2 (range −2 to −38). In a sensitivity analysis, we excluded these participants in the progression analysis and the results were not materially different from the primary analysis. Additionally, the Agatson score decrease was within the range of previously published interscan variability [14].
Among those with incident CAC (n = 102), total cholesterol (β 1.36 per mg/dL, p = 0.01) and statin use (β 86.99, p = 0.03) were the only factors significantly associated with CAC volume, while none of the variables assessed were significantly associated with CAC density (Table 3). After excluding statin users (n = 67), current smoking was associated with incident CAC volume (β 25.66, p = 0.003) and HDL was associated with borderline statistical significance (β −0.44 per mg/dL, p = 0.05). None of the variables assessed were associated with incident CAC density (Supplemental Table 6).
Table 3.
Association between risk factors and CAC volume and density among those with incident CAC.
| CAC volume (n = 102) |
Peak CAC density (n = 102) |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Age, years | −0.43 | −5.18, 4.32 | 0.86 | −1.73 | −5.94, 2.47 | 0.41 |
| Female sex | 18.42 | −70.03, 106.86 | 0.68 | 25.69 | −52.55, 103.02 | 0.52 |
| Days between exams | 0.01 | −0.11, 0.13 | 0.90 | 0.01 | −0.10, 0.11 | 0.90 |
| Hypertension | 29.87 | −44.66, 104.40 | 0.43 | 32.59 | −33.34, 98.51 | 0.33 |
| Diabetes | 12.61 | −70.62, 95.85 | 0.76 | 0.18 | −73.44, 73.82 | 1.00 |
| HDL, mg/dL | −0.87 | −3.91, 2.16 | 0.57 | −1.08 | −3.76, 1.61 | 0.43 |
| Total cholesterol, mg/dL | 1.36 | 0.37, 2.35 | 0.01 | 0.58 | −0.29, 1.46 | 0.19 |
| Current smoking | 53.84 | −87.03, 194.72 | 0.45 | 32.30 | −92.31, 156.91 | 0.61 |
| Exercise, 10 MET-min/week | 0.15 | −0.12, 0.42 | 0.27 | 0.15 | −0.09, 0.38 | 0.23 |
| Lp(a), mg/dL | −0.87 | −2.18, 0.45 | 0.19 | −0.65 | −1.81, 0.51 | 0.27 |
| Fasting insulin, pmol/L | −0.28 | −1.10, 0.54 | 0.50 | −0.20 | −0.92, 0.53 | 0.60 |
| Statin use | 86.99 | 7.73, 166.26 | 0.03 | 50.08 | −20.04, 120.19 | 0.16 |
Linear regression model adjusted for age, sex, time between exam 1 and 2, hypertension, diabetes, current smoking, total cholesterol, HDL, exercise, exam 2 statin use, Lp(a), and fasting insulin. Abbreviations as per Table 1.
4. Discussion
This analysis from the MASALA study describes the characteristics of CAC incidence and progression among asymptomatic South Asian individuals using direct measures of CAC volume and peak density. The highest CAC volume and density were present in LAD, while the greatest changes in volume and density were in RCA and LM, respectively. Incident CAC was also most common in the LAD. In general, CAC volume and density were higher in men than in women; however, when evaluating those with incident CAC and CAC progression separately, CAC density was higher in women. CAC volume progression was generally greater in men and density progression was similar by gender. Smoking was associated with CAC volume progression, while both Lp(a) and exercise were positively associated with CAC density progression. Total cholesterol and statin use were associated with incident CAC volume, while no factors tested were associated with incident CAC density. After exclusion of statin users, age and female sex were associated with CAC volume progression with a trend towards significance for Lp(a) and smoking; smoking was also significantly associated with CAC volume incidence.
Prior studies involving participants from the MASALA and MESA studies have shown that South Asian men have similar burden and progression of CAC as White men, but greater than other ethnic groups, while South Asian women were similar to other ethnicities [5,6]. In a symptomatic population, South Asians have been shown to have more extensive obstructive coronary disease, and higher CAC scores compared with White individuals [15]. South Asian men have been shown previously to have more proximal LAD stenosis than European men, with similar degrees of calcification, possibly due to narrower arteries [16]. Our study extends these results by demonstrating that the LAD is the most common location for incident CAC in South Asians and also has the greatest CAC volume and density. Given the significant myocardial territory supplied by the LAD, this may help explain why South Asians are at higher cardiovascular risk compared to other ethnic groups. In a prior study of MESA participants, the association between risk factors and prevalent CAC volume and density was identified and most risk factors were associated with volume and density in opposite directions [10]. Another prior cross-sectional study involving MASALA participants evaluated baseline risk factors associated with CAC volume and calculated density. It demonstrated that HDL-C was positively associated with CAC density, and waist circumference was inversely associated. BMI, hypertension, statin use, diabetes and insulin resistance were positively associated with CAC volume [17]. Our study expands upon this literature with unique results evaluating the association between risk factors for CAC progression in this population and the use of direct measures of both volume and density, which may have superior predictive value to the use of a calculated density factor [3]. Further studies are needed to evaluate whether modification of the identified risk factors affects CAC volume and density incidence/progression and the associated impact on prognosis.
Physical activity has previously been associated with higher Agatston scores. In a study of high-level endurance athletes, male athletes had higher prevalence of atherosclerosis and higher Agatston scores [18]. In another study, higher level of physical activity was associated with greater Agatston scores and faster progression of Agatston scores [19]. Though the underlying mechanisms for the association between physical activity and CAC are not fully understood, it is possible that this is due to increased plaque density, as density is known to be inversely related to adverse cardiovascular events [2]. Indeed, higher walking pace has previously been positively associated with CAC density [10]. In the present study, we demonstrated that exercise is positively associated with progression of CAC density, but not with CAC volume. While this study was in a South Asian cohort, these results may explain the underlying mechanism for physical activity and increased CAC as the Agatston score is weighted upward for density. It remains unclear how this relationship between exercise and plaque density relates to overall cardiovascular risk.
Lp(a) has previously been associated with higher Agatston scores in different populations [20–25], as well as increased progression of Agatston score [26,27] and plaque volume, but not density [28]. In a prior study of South Asian participants from the MASALA study, Lp(a) was not associated with the presence or degree of CAC using the Agatston score [29]. Here, we show that Lp(a) level is associated with change in CAC density, but not CAC volume among those who had progression of CAC. This is a novel observation as prior studies have shown an association with Lp(a) and plaque volume in different populations, and increased density is associated with lower cardiovascular risk in general [2]. In a prior study where CAC volume and density were assessed, South Asians had lower CAC volume but higher density than White individuals; CAC volume was similar to non-White individuals, but overall CAC density and RCA density were higher among South Asians [9]. Further studies are needed to assess whether CAC density is independently associated with a lower risk of incident CVD, as observed in other populations, given that Lp(a), which was paradoxically associated with increased density in this study, has been associated with increased CVD risk in other populations.
Statin use was associated with increased incident CAC volume. Statin use has previously been associated with greater progression of high-density plaque with decrease in volume of low-attenuation plaque [30]. Thus, this finding may be due to the progression of high-density plaque, as statin use was also associated with a trend towards increased density of plaque. These results may also be due to confounding by indication as those participants with prevalent CAC may have started on statins and may also be at increased risk for the progression of CAC volume due to increased CVD risk. Statin users were excluded as a sensitivity analysis, and there was a trend towards an association between Lp(a) and CAC volume progression, while Lp(a) was no longer associated with CAC density progression. Smoking was also associated with CAC volume incidence in addition to the previously observed association with CAC volume progression. These results must be interpreted with caution, given the significantly smaller sample size, but the association between Lp(a) and CAC density observed previously may be due to confounding by statin use.
This study has some limitations. The MASALA study is a relatively small cohort with participants from two geographical areas in the U.S. It is possible that with a greater sample size, or greater South Asian ancestral diversity, further relationships between risk factors and CAC incidence and progression may be elucidated. However, this is one of the largest studies with repeated CAC measures in South Asians to our knowledge. Additionally, the South Asian category in MASALA combines multiple different ethnic groups including Indians, Pakistanis and Bangladeshis, which may have different genetic predispositions and varying levels of risk due to social, cultural, and behavioral factors, while the vast majority of participants in this study were born in India. Exercise data was collected using a questionnaire, which may be subject to recall bias. Different CT scanners were used at the two sites and at different time points. However, CAC measures have been shown to be reproducible across multiple CT scanners in MESA [14]. Finally, as with any observational study, our findings are subject to residual confounding.
4.1. Conclusions
Overall, South Asians have the greatest coronary artery calcium burden in the LAD, and women had lower risk plaque characteristics than men. Smoking was associated with progression of CAC volume, while Lp(a) and exercise were associated with progression of CAC density. Among non-statin users, age and female sex were associated with CAC volume progression with a trend for Lp(a) and smoking, and smoking was also significantly associated with CAC volume incidence. Further studies are needed to evaluate how CAC volume and density predict incident CVD risk in the South Asian population.
Supplementary Material
Acknowledgments
The authors thank the study participants and other members of the MASALA study team.
Financial support
The MASALA study was supported by grants 1R01HL093009, 2R01HL093009, R01HL120725, K24HL112827, and at UCSF with grants UL1RR024131, UL1TR001872, and P30DK098722. HB was partially supported by the National Institutes of Health, Grant 5T32HL079891, as part of the UCSD Integrated Cardiovascular Epidemiology Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CRediT authorship contribution statement
Harpreet S. Bhatia: Conceptualization, Methodology, Writing – original draft. Feng Lin: Methodology, Data curation, Formal analysis, Writing – review & editing. Isac C. Thomas: Methodology, Writing – review & editing. Julie Denenberg: Methodology, Writing – review & editing. Namratha R. Kandula: Writing – review & editing. Matthew J. Budoff: Methodology, Writing – review & editing. Michael H. Criqui: Conceptualization, Methodology, Writing – review & editing. Alka M. Kanaya: Conceptualization, Methodology, Supervision, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2022.05.006.
References
- [1].Detrano R, Guerci AD, Carr JJ, et al. , Coronary calcium as a predictor of coronary events in four racial or ethnic groups, N. Engl. J. Med 358 (2008) 1336–1345. [DOI] [PubMed] [Google Scholar]
- [2].Criqui MH, Denenberg JO, Ix JH, et al. , Calcium density of coronary artery plaque and risk of incident cardiovascular events, JAMA 311 (2014) 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dzaye O, Razavi AC, Dardari ZA, et al. , Mean versus peak coronary calcium density on non-contrast CT: calcium scoring and ASCVD risk prediction, JACC Cardiovasc Imaging (2021), 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Volgman AS, Palaniappan LS, Aggarwal NT, et al. , Atherosclerotic cardiovascular disease in South Asians in the United States: Epidemiology, risk factors, and treatments: a scientific statement from the American heart association, Circulation 138 (2018) e1–e34. [DOI] [PubMed] [Google Scholar]
- [5].Kanaya AM, Kandula NR, Ewing SK, et al. , Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: the MASALA and MESA studies, Atherosclerosis 234 (2014) 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kanaya AM, Vittinghoff E, Lin F, et al. , Incidence and progression of coronary artery calcium in South Asians compared with 4 race/ethnic groups, J. Am. Heart Assoc 8 (2019), e011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hatwalkar A, Agrawal N, Reiss DS, Budoff MJ, Comparison of prevalence and severity of coronary calcium determined by electron beam tomography among various ethnic groups, Am. J. Cardiol 91 (2003) 1225–1227. [DOI] [PubMed] [Google Scholar]
- [8].Patel J, Al Rifai M, Cainzos-Achirica M, et al. , Family history of CHD is associated with severe CAC in South Asians: comparing the MASALA and MESA studies, JACC Cardiovasc Imaging 10 (2017) 958–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Al Rifai M, Kanaya AM, Kandula NR, et al. , Distribution of calcium volume, density, number, and type of coronary vessel with calcified plaque in South Asians in the US and other race/ethnic groups: the MASALA and MESA studies, Atherosclerosis 317 (2021) 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thomas IC, Shiau B, Denenberg JO, et al. , Association of cardiovascular disease risk factors with coronary artery calcium volume versus density, Heart 104 (2018) 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kanaya AM, Kandula N, Herrington D, et al. , Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description, Clin. Cardiol 36 (2013) 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Friedewald WTLR, Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin. Chem 18 (1972) 499–502. [PubMed] [Google Scholar]
- [13].Diagnosis ADA, And classification of diabetes mellitus, Diabetes Care 27 (Suppl 1) (2004) S5–S10. [DOI] [PubMed] [Google Scholar]
- [14].Budoff MJ, McClelland RL, Chung H, et al. , Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: the multi-ethnic study of atherosclerosis, Am. J. Roentgenol 192 (2009) 613–617. [DOI] [PubMed] [Google Scholar]
- [15].Koulaouzidis G, Nicoll R, Charisopoulou D, McArthur T, Jenkins PJ, Henein MY, Aggressive and diffuse coronary calcification in South Asian angina patients compared to Caucasians with similar risk factors, Int. J. Cardiol 167 (2013) 2472–2476. [DOI] [PubMed] [Google Scholar]
- [16].Tillin T, Dhutia H, Chambers J, et al. , South Asian men have different patterns of coronary artery disease when compared with European men, Int. J. Cardiol 129 (2008) 406–413. [DOI] [PubMed] [Google Scholar]
- [17].Rifai MA, Kanaya AM, Kandula NR, et al. , Association of coronary artery calcium density and volume with predicted atherosclerotic cardiovascular disease risk and cardiometabolic risk factors in South Asians: the Mediators of atherosclerosis in South Asians living in America (MASALA) study, Curr. Probl. Cardiol (2022) 101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Merghani A, Maestrini V, Rosmini S, et al. , Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile, Circulation 136 (2017) 126–137. [DOI] [PubMed] [Google Scholar]
- [19].Sung K-C, Hong YS, Lee J-Y, et al. , Physical Activity and the Progression of Coronary Artery Calcification, Heart, 2021. heartjnl-2021-319346. [DOI] [PubMed] [Google Scholar]
- [20].Chung YH, Lee BK, Kwon HM, et al. , Coronary calcification is associated with elevated serum lipoprotein (a) levels in asymptomatic men over the age of 45 years: a cross-sectional study of the Korean national health checkup data, Medicine (Baltim.) 100 (2021), e24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Verweij SL, de Ronde MWJ, Verbeek R, et al. , Elevated lipoprotein(a) levels are associated with coronary artery calcium scores in asymptomatic individuals with a family history of premature atherosclerotic cardiovascular disease, J. Clin. Lipidol 12 (2018) 597–603 e1. [DOI] [PubMed] [Google Scholar]
- [22].Jiang Y, Guo K, Chen M, Bao J, Shen C, Li Y, Serum lipoprotein(a) positively correlates with coronary artery calcification in low-risk Chinese han patients: a study from a single center, PLoS One 8 (2013), e71673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Greif M, Arnoldt T, von Ziegler F, et al. , Lipoprotein (a) is independently correlated with coronary artery calcification, Eur. J. Intern. Med 24 (2013) 75–79. [DOI] [PubMed] [Google Scholar]
- [24].Sharma A, Kasim M, Joshi PH, et al. , Abnormal lipoprotein(a) levels predict coronary artery calcification in Southeast Asians but not in Caucasians: use of noninvasive imaging for evaluation of an emerging risk factor, J. Cardiovasc Transl. Res 4 (2011) 470–476. [DOI] [PubMed] [Google Scholar]
- [25].Pechlivanis S, Mahabadi AA, Hoffmann P, et al. , Association between lipoprotein (a) (Lp(a)) levels and Lp(a) genetic variants with coronary artery calcification, BMC Med. Genet 21 (2020) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY, Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: the Multi-Ethnic Study of Atherosclerosis, J. Cardiovasc. Comput. Tomogr 15 (2021) 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cho JH, Lee DY, Lee ES, et al. , Increased risk of coronary artery calcification progression in subjects with high baseline Lp(a) levels: the Kangbuk Samsung Health Study, Int. J. Cardiol 222 (2016) 233–237. [DOI] [PubMed] [Google Scholar]
- [28].Ong KL, McClelland RL, Allison MA, et al. , Lipoprotein (a) and coronary artery calcification: prospective study assessing interactions with other risk factors, Metabolism 116 (2021) 154706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huffman MD, Kandula NR, Baldridge AS, Tsai MY, Prabhakaran D, Kanaya AM, Evaluating the potential association between lipoprotein(a) and atherosclerosis (from the Mediators of atherosclerosis among South Asians living in America cohort), Am. J. Cardiol 123 (2019) 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Rosendael AR, Van Den Hoogen IJ, Gianni U, et al. , Association of statin treatment with progression of coronary atherosclerotic plaque composition, JAMA Cardiology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


