Abstract
Introduction
Well trained, knowledgeable and competent pharmacists are indispensable in the fight against antimicrobial resistance (AMR), which is a current global public health problem. The aim of this work was to assess knowledge of antibiotics, antimicrobial resistance and antimicrobial stewardship of fifth year pharmacy students at three universities in Northern Nigeria.
Methods
A descriptive cross-sectional study that used a paper-based questionnaire to collect data from July to September 2021 was conducted. The questionnaire was self-administered and divided into four sections. The first section collected information about the demographic data of respondents, while section B explored their knowledge of antibiotics and AMR. Section C contained six questions assessing knowledge of various aspects of antimicrobial stewardship (AMS), while the final part assessed respondents’ preparedness to work with antibiotics and perceptions of their current knowledge of these concepts. Descriptive statistics were used to report the results obtained.
Results
A total of 164 questionnaires were retrieved. Majority of respondents were male (58.3%) and aged between 21 and 25 years (53.4%). Most of them had some knowledge of antibiotics and AMR, however several misconceptions with respect to these concepts were identified. Only 80 (48.8%) of respondents indicated that they knew what AMS was, although most of these students were correctly knowledgeable about the goals and scope of AMS and composition of the AMS team. Generally, less than half of respondents agreed that their current knowledge of antibiotics, AMR or AMS was adequate for their future careers, and over 90% of them agreed that they would like more education about these topics.
Conclusion
Many of the study’s’ respondents were somewhat knowledgeable about these concepts, although several knowledge gaps were also observed. Improving undergraduate pharmacy education with respect to these concepts is recommended.
Keywords: antibacterial agents, antibiotic resistance, antimicrobial stewardship, Nigeria, pharmacy students
Introduction
Despite the important role played by antimicrobials in the prevention and treatment of a wide range of disease conditions, they are some of the most abused and/ misused medications worldwide.1 This is one of the factors behind the increasing rates of Antimicrobial Resistance (AMR) globally, which can be said to occur when bacteria, viruses, fungi or other parasites change over time and become less responsive to antimicrobial agents.2 AMR has important implications not only for human wellbeing, but in other areas as well. Infections caused by resistant pathogens are more difficult to treat and are more likely to cause significant disabilities and/ death.2 In addition, because resistant pathogens cause prolonged illness; patients have longer hospital stays and need more expensive medicines for treatment, incurring larger costs to be borne by individuals, health systems or governments as the case maybe. Consequently, AMR has now been recognized as a global health and developmental threat,2 and concerted efforts are underway by individuals, international bodies and governments to try and tackle it.
One such measure to reduce AMR is through Antimicrobial Stewardship (AMS) programs. These programs generally include a wide range of interventions designed to promote the optimal use of antimicrobial agents and can improve patient outcomes and minimize treatment harms etc.3 Evidence suggests that when these programs are used in conjunction with other AMR reduction modalities, they have the potential to save lives, reduce healthcare associated costs and improve other clinical outcomes.3,4
Well trained, knowledgeable and competent healthcare professionals are indispensable in the fight against AMR. This is because many AMR reduction modalities (including AMS programs) must be carried out or spearheaded by these individuals. Pharmacists in particular have strategic roles to play with respect to AMS that include developing and managing antimicrobial usage guidelines; reviewing individual patient regimens to optimize therapy; educating healthcare staff and members of the public on the appropriate use of antimicrobials and monitoring and auditing outcomes.5 Despite this, surveys conducted all over the world that have assessed knowledge and/ practices with respect to rational antimicrobial use and antimicrobial resistance in healthcare professionals have reported varying levels of knowledge and /good practices.6–10
Similarly, several studies that have assessed awareness of AMR and related topics in university students studying health-related courses have equally identified several knowledge gaps with respect to these concepts.11–15 This suggests that the pre-service education with respect to these concepts that is provided to many potential healthcare professionals might be inadequate for their practice needs, and there have been calls from relevant stakeholders in recent times for curricular reviews.
Nigeria has a very high infectious disease burden caused by conditions including tuberculosis, respiratory infections and diarrhoeal diseases.16 There is also data suggesting that the resistance levels of many of the pathogens causing these conditions within the country is also very high, resulting in high infectious disease associated morbidity and mortality levels.16 The knowledge of antibiotics and related concepts among healthcare practitioners in the country is also reportedly low, with a recent nationwide survey of healthcare practitioners indicating that only about 50% of their respondents had good knowledge about AMR.10 Studies on undergraduate students studying health-related courses within the country have equally reported poor knowledge of antibiotics and related concepts.17–19 However, not much is known about pharmacy students’ knowledge of these concepts as only one of these studies17 specifically surveyed pharmacy students, and their study had a relatively small sample size of about 60 students. Thus, the aim of this study was to explore knowledge of antibiotics, antimicrobial resistance and antimicrobial stewardship in fifth year pharmacy students at selected universities in Northern Nigeria.
Methods
Study Design and Sites
The study was descriptive cross-sectional and used a structured paper-based questionnaire to collect data from fifth year pharmacy students at three Northern Nigerian universities from July to September 2021. Stratified random sampling was used to select three universities from the pool of eight universities currently offering either Bachelor of Pharmacy (BPharm) or Doctor of Pharmacy (PharmD) degrees in northern Nigeria. Consequently, one university was chosen from each of the three geopolitical regions that make up Northern Nigeria. Selected universities included Ahmadu Bello University Zaria, from the Northwest region, University of Jos from the North central region, and Gombe State University from the Northeast region.
Study Participants & Sample Size
Students were eligible to participate in the study if they fulfilled all the following inclusion criteria.
Were final year BPharm students.
Were enrolled in one of the selected universities.
Agreed to participate.
Could read and write English.
Students who did not fulfill all these criteria were excluded. Total population sampling was used for this study, consequently all the final year students at the selected universities (215 students at the time of the study) was the study’s sample size.
Study Instrument
The study instrument was a self – administered, structured, English language questionnaire (Appendix l). Questions on this instrument were adapted from questionnaires used in similar studies in other countries.12–15,20 After the instrument was designed, it was assessed for face and content validity by four Clinical pharmacy lecturers and found to be appropriate. The final draft was then pilot tested on 30 selected final year pharmacy students at a university not included in the study. No modifications were made after the pilot study as the respondents did not recommend any modifications.
The questionnaire was divided into four sections (A-D). Items in the first section collected information about the demographic data of respondents including their gender, age, university etc. Section B assessed respondents’ knowledge of antibiotics and antimicrobial resistance through the use of multiple-choice questions and “true” or “false” statements. Section C contained six questions that assessed respondents’ knowledge of various aspects of AMS. The final section assessed respondents’ preparedness to work with antibiotics and their perceptions of their current knowledge of antibiotics, AMR and AMS. This section contained 11 statements to be answered using a five-point Likert scale.
Data Collection
Study questionnaires were distributed to participants by their class representatives during usual lecture hours. Respondents were allowed 48–72 hours to fill and return the questionnaires to the class representatives, who then collated and returned them to the investigators.
Ethical Considerations
Ethical approval for the study was obtained from the Human Research Ethics Committee of Ahmadu Bello University Zaria (Approval number: ABUCUHSR/2020/UG/017). No data that could be used to identify respondents was collected, and all the information collected was securely handled and used only for study purposes. Informed consent was obtained from all participants and the study complied with all regulations set out in the approval letter.
Data Analysis
Data collected was coded and entered into a Microsoft Excel (2016) sheet and analyzed. Descriptive Statistics (frequencies and percentages) were used to report the results obtained. For items in the final section of the questionnaire, “strongly agreed” and “agreed” responses were grouped together and reported and vice versa.
Results
A total of 164 questionnaires were eventually retrieved out of the 215 distributed producing a 76.3% response rate.
Demographic Characteristics of Respondents
Study respondents ages ranged from 21 to over 30 years (Table 1), although the average age of respondents was 26 ± 3 years. Majority of them reported having taken at least one course centered on antibiotics, with 76.9% of them reporting that they had so far taken between two to four courses on antibiotics and related concepts.
Table 1.
Respondents’ Socio-Demographic Characteristics (n = 164)
| Characteristics | Variable | n (%) |
|---|---|---|
| Gender | Male | 95 (58.3) |
| Female | 68 (41.7) | |
| Age* | 21–25 years | 78 (53.4) |
| 26–30 years | 60 (41.1) | |
| Older than 30 years | 8 (5.5) | |
| Institution | University of Jos | 64 (39) |
| Ahmadu Bello University | 70 (42.7) | |
| Gombe State University | 30 (18.3) |
Note: *Values in this row sum up to less than the total because of missing values.
Respondents’ Knowledge of Antibiotics, Antimicrobial Resistance and Related Concepts
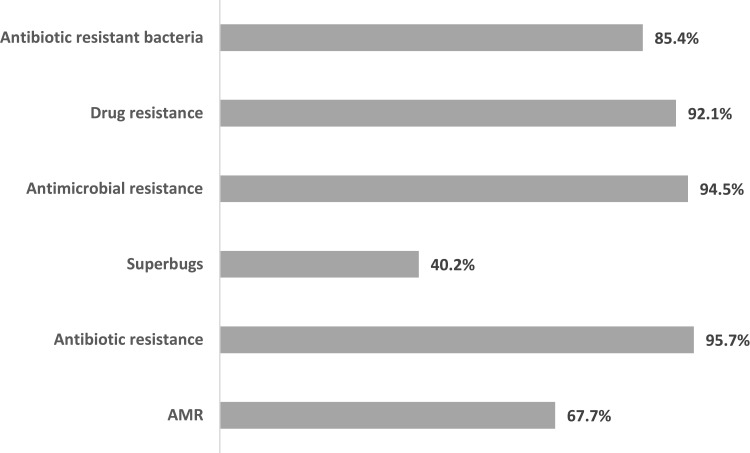
Majority of the respondents indicated that they were aware of antimicrobial resistance and most of the other terms assessed except for “superbugs” where the level of awareness was less than half the population (40.2%). Almost all the respondents were aware of antibiotic resistance (95.7%), antimicrobial resistance (94.5%) and drug resistance (92.1%). Antibiotic resistant bacteria and AMR had lower awareness rates of 85.4% and 67.7% respectively among the respondents (Figure 1).
Figure 1.
Respondents Awareness of Antimicrobial Resistance and Related Concepts.
When respondents were asked to fill in the blank spaces of the question “……. occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines”, 78.1% and 31.1% of them respectively selected “antimicrobial resistance” or “AMR” as one of their correct answers. However, only 23.8% of respondents were ostensibly able to correctly identify that both antimicrobial resistance and AMR referred to the same concept.
Similarly, when respondents were asked to fill in the blank spaces of the question “Vancomycin resistant enterococcus, methicillin resistant staphylococcus and multi-drug resistant mycobacterium tuberculosis are all examples of …………………”, around 50% selected “antibiotic resistant bacteria”, while 11.6% selected “superbugs” as one of their answers. Once again, only 5.5% of respondents realized that antibiotic resistant bacteria were synonymous to superbugs.
Study respondents identified an average of 5 out of the 12 indications provided as conditions requiring antibiotics for their treatment. The most common conditions identified by respondents were skin infections (91.5%), followed by urinary tract infections (89%). Fever (15.2%), malaria (21.3%) and HIV/AIDS (25%) were the least identified conditions by the respondents (Table 2).
Table 2.
Respondents’ Knowledge of Antibiotic Indications and Aspects of Antimicrobial Resistance
| Aspect Assessed | Options | n (%) |
|---|---|---|
| Antibiotic indications | HIV/AIDS | 41 (25) |
| Gonorrhea | 135 (82.3) | |
| Urinary Tract Infections | 146 (89) | |
| Diarrhea | 106 (64.6) | |
| Cold | 85 (51.8) | |
| Fever | 25 (15.2) | |
| Malaria | 35 (21.3) | |
| Measles | 64 (39) | |
| Skin infections | 150 (91.5) | |
| Sore throat | 102 (62.2) | |
| Practices that Promote Antimicrobial Resistance | Widespread/overuse of antimicrobials in humans | 135 (82.3) |
| Widespread/overuse of antimicrobials in animal livestock production | 103 (62.8) | |
| Use of broad-spectrum antimicrobials when narrow spectrum ones are available | 109 (66.5) | |
| Poor handwashing practices | 64 (39) | |
| Poor patient adherence to medication | 142 (86.6) | |
| Substandard quality of some antimicrobial drugs | 123 (75) | |
| Interventions to Combat Antimicrobial Resistance | Developing national and institutional antimicrobial usage policies | 149 (90.9) |
| Reducing antimicrobial use in humans | 105 (64) | |
| Reducing antimicrobial use in animal livestock | 92 (56.1) | |
| Establishing centers for national antimicrobial resistance surveillance | 142 (86.6) | |
| Educating health professionals and members of the public on rational antimicrobial use | 154 (93.9) | |
| Developing new antimicrobial drugs | 70 (42.7) |
With respect to respondents’ knowledge of practices that promote antimicrobial resistance (Table 2), more than half of them identified that antimicrobial resistance is promoted through poor patient adherence to medication (86.6%), overuse of antimicrobials in humans (82.3%) and substandard quality of some antimicrobial drugs (75%). Less than half of the population (39%) however thought that poor handwashing practices could promote AMR (Table 2).
Of the various intervention options listed in the questionnaire as useful in combating antimicrobial resistance, more than 90% of respondents thought that educating stakeholders and developing national and institutional antimicrobial usage policies would be useful (Table 2). Less than half the population (42.7%) thought that developing new antimicrobial drugs would help in combatting AMR (Table 2).
Majority of respondents correctly identified that there are many classes of antibiotics (98.8%) and that antibiotics can kill normal bacterial flora in the body (81.9%). Almost equal numbers of respondents answered both true and false to the statements that “better use of antibiotics will not have an impact on antimicrobial resistance” and “antimicrobial resistance is only a problem for people who use antibiotics” (Table 3). Finally, around 20% of respondents did not know whether intravenous antibiotic therapy was preferred over oral therapy for most patients (Table 3).
Table 3.
Respondents’ Knowledge of Antibiotics, Antimicrobial Resistance and Related Concepts (n = 164)
| Statement | True n (%) | False n (%) | I do Not Know n (%) | |
|---|---|---|---|---|
| 1 | There are many classes of antibiotics | 162 (98.8) | 2 (1.2) | 0 |
| 2 | Antibiotics can kill normal bacterial flora in the body* | 131 (81.9) | 21 (13.1) | 8 (5) |
| 3 | Better use of antibiotics will not have an impact on antimicrobial resistance* | 78 (48.5) | 80 (49.7) | 3 (1.8) |
| 4 | Prescribing broad spectrum antibiotics is always better even if there are narrower spectrum antibiotics that are effective | 21 (12.8) | 134 (81.7) | 9 (5.5) |
| 5 | Antibiotics should be used within the community for prophylaxis of infections like typhoid and pneumonia* | 45 (27.6) | 96 (58.9) | 22 (13.5) |
| 6 | Patients should stop taking their prescribed/recommended antibiotics as soon as they start to feel better | 12 (7.3) | 150 (91.5) | 2 (1.2) |
| 7 | Antibiotic resistant infections could make medical procedures like surgery, organ transplants and cancer treatment much more difficult | 114 (69.5) | 32 (19.5) | 18 (11) |
| 8 | Antibiotic resistance occurs when the human body becomes resistant to antibiotics, and they no longer work* | 125 (77.7) | 34 (21.1) | 2 (1.2) |
| 9 | Antimicrobial resistance is only a problem for people who use antibiotics* | 71 (44.4) | 80 (50) | 9 (5.6) |
| 10 | *Intravenous antibiotic therapy is preferred over oral therapy for most patients | 45 (28) | 83 (51.5) | 33 (20.5) |
Note: *Values in these rows sum up to less than the total because of missing values.
When asked whether they knew what antimicrobial stewardship was, 80 (48.8%) of respondents said yes, 57 (34.8%) said no while the rest were unsure. Majority of respondents who had heard about antimicrobial stewardship reported that they had first come across the concept in pharmacy school (80%) or other online/offline educational seminars organized by a variety of organizations or bodies (10%).
Majority of respondents who knew about AMS thought that choosing antimicrobials appropriately, selecting suitable dosing regimens and appropriate duration of therapy for antimicrobial drugs were within the scope of AMS (Table 4). Most of them also correctly identified the goals of AMS and roles for pharmacists with respect to AMS (Table 4).
Table 4.
Respondents’ Knowledge of Antimicrobial Stewardship Concepts
| Area Assessed | Options | n (%) |
|---|---|---|
| Scope of AMS | The study of antimicrobials | 42 (52.5) |
| Choosing antimicrobials appropriately | 75 (93.8) | |
| Selecting suitable dosing regimens for antimicrobials | 60 (75) | |
| Selecting suitable routes of administration for antimicrobials | 47 (58.8) | |
| Choosing appropriate duration of therapy for antimicrobial drugs | 54 (67.5) | |
| Goals of AMS | Increasing antimicrobial use | 11 (13.8) |
| Reducing hospital stay | 48 (60) | |
| Increasing duration of antimicrobial therapy to ensure maximum therapeutic efficacy | 22 (27.5) | |
| Increasing the use of broad-spectrum antibiotics | 11 (13.8) | |
| Reducing antimicrobial resistance | 76 (95) | |
| Minimizing toxicity and other adverse effects of antimicrobials | 65 (81.3) | |
| Members of the AMS team | Doctors | 79 (98.8) |
| Occupational therapists | 24 (30) | |
| Hospital pharmacists | 74 (92.5) | |
| Community pharmacists | 56 (70) | |
| Hospital cleaning staff | 17 (21.3) | |
| Nurses | 65 (81.3) | |
| Laboratory scientists | 56 (70) | |
| Roles for pharmacists with respect to AMS | Promoting optimal use of antimicrobial agents | 69 (86.3) |
| Prescribing antimicrobial agents over the counter | 22 (27.5) | |
| Educating other healthcare professionals about antimicrobials | 74 (92.5) | |
| Working with therapeutic committees to develop antimicrobial usage policies | 68 (85) | |
| Tracking and reporting antimicrobial consumption patterns | 66 (82.5) |
On whom they thought should be part of the AMS team, almost all the respondents (98.8%) believed medical doctors to be the most important members of the team followed by the hospital pharmacists (92.5%) and Nurses 81.3%. Although 70% of them thought that community pharmacists and laboratory scientists should be part of the team, only around 20% thought that hospital cleaning staff should be represented (Table 4).
Respondents’ Self-Assessment of Knowledge of Antimicrobials, Antimicrobial Resistance and Antimicrobial Stewardship and Preparedness to Work with Antibiotics
Majority of respondents agreed that they felt prepared with respect to recommending antimicrobials to patients (Table 5). Although only about 85 (52.2%) agreed that they knew the correct specimens to be collected from patients to identify various infections. Generally, less than half of them agreed that their knowledge of antibiotics, AMR or AMS was adequate for their future careers (Table 5). Over 90% of respondents also agreed that they would like more education on the appropriate use of antimicrobials, antimicrobial resistance and antimicrobial stewardship (Table 5).
Table 5.
Respondents’ Self-Assessment of Their Knowledge of Antimicrobials, Antimicrobial Resistance and Antimicrobial Stewardship
| Statement | Agreed n (%) | Neutral n (%) | Disagreed n (%) |
|---|---|---|---|
| I feel prepared to know whether to recommend an antimicrobial to a patient or not | 133 (81.1) | 25 (15.2) | 6 (3.7) |
| I feel prepared to know when to recommend that a patient start antimicrobial therapy | 134 (81.7) | 25 (15.2) | 5 (3.1) |
| I feel prepared to know how to select the best antimicrobial for a patient* | 109 (67.7) | 46 (28.6) | 6 (3.7) |
| I feel prepared to know which route of administration is best for a patients’ antimicrobial therapy. | 115 (70.1) | 37 (22.6) | 12 (7.3) |
| I know the correct and relevant specimens to be collected from patients that can be used to identify various infections* | 85 (52.2) | 60 (36.8) | 18 (11) |
| My current knowledge of antimicrobials is adequate for my future career as a pharmacist* | 74 (45.4) | 58 (35.6) | 31 (19) |
| My current knowledge of antimicrobial resistance is adequate for my future career as a pharmacist | 73 (44.5) | 63 (38.4) | 28 (17.1) |
| My current knowledge of antimicrobial stewardship is adequate for my future career as a pharmacist* | 53 (32.3) | 64 (39.8) | 44 (27.4) |
| I would like more education on the appropriate use of antimicrobials* | 146 (90.1) | 11 (6.8) | 5 (3.1) |
| I would like more education on antimicrobial resistance | 154 (93.9) | 8 (4.9) | 2 (1.2) |
| I would like more education on antimicrobial stewardship* | 149 (91.4) | 11 (6.8) | 3 (1.8) |
Note: *Values in these rows sum up to less than the total because of missing values.
Discussion
The aim of this study was to assess knowledge and perceptions of antibiotics, antimicrobial resistance and antimicrobial stewardship concepts among final year pharmacy students in selected northern Nigerian universities. Findings from the study revealed that majority of the respondents had some knowledge of the principles of rational antibiotic use and AMR. Only around half of them knew what AMS was, although most of these respondents were correctly knowledgeable about the goals, aims and scope of AMS. Finally, most of them felt prepared to work with antibiotics, although most of them admitted that they would like more education on antibiotics, AMR and related concepts.
Respondents in this study had some knowledge of rational antibiotic use. Most of them disagreed that broad spectrum antibiotics should be prescribed when there were effective narrow spectrum alternatives, similar to findings from an earlier study.15 Many of them also disagreed that people should stop taking antibiotics when they started feeling better, as reported in another study.14 However, when asked to identify conditions requiring the use of antibiotics, many respondents also selected infections including colds, sore throats and diarrhea. Many of these infections are self-limiting and caused by viruses, making antibiotics inappropriate for managing them. Several of our respondents also had misconceptions about appropriate indications for community antibiotic prophylaxis and intravenous antibiotic use. All these knowledge gaps have been previously identified as contributing to the inappropriate use of antibiotics in outpatient settings.21
Studies conducted by Lubwama et al15 and Sakeena et al13 all reported that majority of their respondents could identify causes of antimicrobial resistance, which is consistent with the results obtained from this study. Our respondents identified that the inappropriate use of antibiotics and inadequate duration of treatment with antibiotics could lead to resistance, just like respondents in the other studies.14,15 In addition, a lot of the study’s’ respondents could identify measures that could be used to combat antimicrobial resistance. Other studies,14,20 have also reported similar findings. Despite this, several of our respondents demonstrated significant knowledge gaps with respect to AMR. Many of them did not know that poor handwashing practices could promote AMR and did not realize that AMR occurs in pathogens not humans. Several of them also believed that AMR was only a problem for antibiotic users and that better antibiotic use would have no impact on AMR. Most of them were also not aware of the concept of “superbugs” which is consistent with findings from an earlier study carried out by Sakeena et al.13
Only around half of the respondents in this study were aware of AMS. However, majority of these respondents could correctly identify AMS’s goals, scope, objectives, the role of pharmacists with respect to AMS and members of the AMS team. Different findings were reported from a study by Ahmed et al,20 where most of their study population was also not aware of the concept of antimicrobial stewardship, likely due to its lack of inclusion in their curriculum. Another study conducted in South Africa by Burger et al however reported that most of their study population were aware of the AMS concept and could identify the goals of AMS.12 Majority of the respondents in this study that were knowledgeable about AMS also identified medical doctors as the most important member of the AMS team, followed by hospital pharmacists. This contrasts with other similar studies where respondents believed that hospital pharmacists should constitute and lead the AMS team followed by infectious disease physicians before others.12,20 Finally, respondents in this study were also able to correctly identify pharmacist roles in AMS programs including helping therapeutic committees in developing policies, encouraging the appropriate use of antimicrobials and the education of other health care professionals. Other respondents in similar studies were also similarly knowledgeable about pharmacist roles.12,20
Respondents’ feeling of preparedness to use antibiotics in this study was consistent with that reported in an earlier study.15 In addition, while the students had some knowledge of these concepts, majority of them agreed with the statements that they still needed more education and training on these concepts. Another study,20 has reported that pharmacy students would like more education and/training on these concepts.
Strengths of this study include the fairly large number of students included, and the fact that students from more than one institution were surveyed. Its limitations include the fact that not all the questionnaires distributed were eventually retrieved, although the overall response rate was over 70%. In addition, the possibility of respondents guessing or otherwise actively searching out the answers to the knowledge assessment questions cannot be totally ruled out since they were allowed to fill the questionnaires at their leisure.
This study was able to provide several insights and highlight specific knowledge gaps with respect to the students’ knowledge of antibiotics, AMR and AMS. These findings can be used to design or redesign relevant educational curricula for training or retraining pharmacy students or already trained pharmacists. Further research will however be necessary to discover whether these results hold true for students at other institutions, and whether there are significant differences in knowledge of these concepts between students of different institutions, and what might be the contributing factors to these differences.
Conclusion
Most of the study’s’ respondents had some knowledge of antibiotics, AMR and related concepts. Only around half of them indicated that they knew what AMS was, although most of these students were correctly knowledgeable about the goals and scope of AMS and composition of the AMS team. Generally, less than half of respondents agreed that their current knowledge of antibiotics, AMR or AMS was adequate for their future careers, and over 90% of them agreed that they would like more education about these topics. Improving undergraduate pharmacy education with respect to these concepts by correcting several of the knowledge gaps seen during this study is recommended.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Auta A, Hadi M, Oga E, et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect. 2019;78(1):8–18. doi: 10.1016/j.jinf.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Antimicrobial resistance. Who.int; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed December 5, 2021.
- 3.World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries. A Practical Toolkit. Geneva: World Health Organization; 2019:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani D, Varghese D, Stephens J, et al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8(1):35. doi: 10.1186/s13756-019-0471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garau J, Bassetti M. Role of pharmacists in antimicrobial stewardship programmes. Int J Clin Pharm. 2018;40(5):948–952. doi: 10.1007/s11096-018-0675-z [DOI] [PubMed] [Google Scholar]
- 6.ECDC. Survey of Healthcare Workers’ Knowledge, Attitudes and Behaviours on Antibiotics, Antibiotic Use and Antibiotic Resistance in the EU/EEA. Stockholm: European Centre for Disease Prevention and Control; 2019:14–60. [Google Scholar]
- 7.Hayat K, Rosenthal M, Gillani AH, et al. Perspective of Key Healthcare Professionals on Antimicrobial Resistance and Stewardship Programs: a Multicenter Cross-Sectional Study from Pakistan. Front Pharmacol. 2020;10:1520. doi: 10.3389/fphar.2019.01520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barchitta M, Sabbatucci M, Furiozzi F, et al. Knowledge, attitudes and behaviors on antibiotic use and resistance among healthcare workers in Italy, 2019: investigation by a clustering method. Antimicrob Resist Infect Control. 2021;10(1):134. doi: 10.1186/s13756-021-01002-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mufwambi W, Stingl J, Masimirembwa C, et al. Healthcare professionals’ knowledge of pharmacogenetics and attitudes towards antimicrobial utilization in Zambia: implications for a precision medicine approach to reducing antimicrobial resistance. Front Pharmacol. 2021;11:551522. doi: 10.3389/fphar.2020.551522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chukwu EE, Oladele DA, Enwuru CA, et al. Antimicrobial resistance awareness and antibiotic prescribing behavior among healthcare workers in Nigeria: a national survey. BMC Infect Dis. 2021;21(1):22. doi: 10.1186/s12879-020-05689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbo LM, Cosgrove SE, Pottinger PS, et al. Medical students’ perceptions and knowledge about antimicrobial stewardship: how are we educating our future prescribers? Clin Infect Dis. 2013;57(5):631–638. doi: 10.1093/cid/cit370 [DOI] [PubMed] [Google Scholar]
- 12.Burger M, Fourie J, Loots D, et al. Knowledge and perceptions of antimicrobial stewardship concepts among final year pharmacy students in pharmacy schools across South Africa. South Afr J Infect Dis. 2016;31(3):84–90. doi: 10.1080/23120053.2016.1192808 [DOI] [Google Scholar]
- 13.Sakeena MHF, Bennett AA, Jamshed S, et al. Investigating knowledge regarding antibiotics and antimicrobial resistance among pharmacy students in Sri Lankan universities. BMC Infect Dis. 2018;18(1):209. doi: 10.1186/s12879-018-3107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakeena MHF, Bennett AA, Carter SJ, et al. A comparative study regarding antibiotic consumption and knowledge of antimicrobial resistance among pharmacy students in Australia and Sri Lanka. PLoS One. 2019;14(3):e0213520. doi: 10.1371/journal.pone.0213520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubwama M, Onyuka J, Ayazika KT, et al. Knowledge, attitudes, and perceptions about antibiotic use and antimicrobial resistance among final year undergraduate medical and pharmacy students at three universities in East Africa. PLoS One. 2021;16(5):e0251301. doi: 10.1371/journal.pone.0251301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NCDC. Antimicrobial Use and Resistance in Nigeria: Situation Analysis and Recommendation. Abuja: Nigeria Center for Disease Control and Federal Ministries of Health, Agriculture and Environment; 2017:16–23. [Google Scholar]
- 17.Abubakar U, Sha’aban A, Mohammed M, et al. Knowledge and self-reported confidence in antimicrobial stewardship programme among final year pharmacy undergraduate students in Malaysia and Nigeria. Pharm Educ. 2021;21(1):298–305. doi: 10.46542/pe.2021.211.298305 [DOI] [Google Scholar]
- 18.Augie BM, van Zyl RL, McInerney PA, et al. Knowledge and perceptions about antibiotic resistance and prudent antibiotic prescribing among final year medical students in two African countries. Int J Pharm Pract. 2021;29(5):508–514. doi: 10.1093/ijpp/riab044 [DOI] [PubMed] [Google Scholar]
- 19.Akande-Sholabi W, Ajamu AT. Antimicrobial stewardship: assessment of knowledge, awareness of antimicrobial resistance and appropriate antibiotic use among healthcare students in a Nigerian University. BMC Med Educ. 2021;21(1):488. doi: 10.1186/s12909-021-02912-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed N, Abujheisha K, Balaha M. Pharmacy students’ knowledge and perceptions about antimicrobial stewardship. J Pharm Res Int. 2019;31(1):1–8. doi: 10.9734/jpri/2019/v31i130291 [DOI] [Google Scholar]
- 21.Lin L, Sun R, Yao T, et al. Factors influencing inappropriate use of antibiotics in outpatient and community settings in China: a mixed-methods systematic review. BMJ Glob Health. 2020;5(11):e003599. doi: 10.1136/bmjgh-2020-003599 [DOI] [PMC free article] [PubMed] [Google Scholar]