Abstract
Background
Deficiency vitamin D and hyperglycemia could be related to weakened innate immune response and aggravate the progression of tuberculosis (TB). This study hypothesized that DNA promoter methylation of the pivotal genes in the vitamin D metabolic pathway might be related to diabetes and tuberculosis co-morbidity (TB-DM) susceptibility.
Methods
A total of 50 TB-DM and 50 healthy subjects (HS) were included in the present study. Targeted bisulfite sequencing was applied to detect the methylation of the promoter regions of candidate genes in the vitamin D metabolic pathway (CYP24A1, CYP27B1, CYP2R1, DHCR7, and VDR) in whole blood.
Results
The overall methylation level of candidate genes in this study was lower in patients with TB-DM than HS, except for CYP2R1. The results of the ROC demonstrated the potential of CYP24A1, CYP27B1, DHCR7, and VDR promoter methylation as a biomarker for diagnosing TB-DM, with all the AUC above 0.7. In subgroup analysis, we found that lower circulating vitamin D is related to a low level of CYP24A1, CYP27B1, and DHCR7 promoter methylation in patients with TB-DM. With decreasing methylation level, risk of TB-DM was significantly increased (odds ratio, 95% CI 0.343, 0.144–0.821 for CYP24A1; 0.461, 0.275–0.773 for CYP27B1; 0.09, 0.015–0.530 for DHCR7; 0.006, 0.0003–0.115 for VDR). Besides, our results revealed that there was a significant correlation between DNA promoter methylation of selected genes in the vitamin D metabolic pathway and platelet indices in TB-DM. However, there was no correlation between DNA methylation of the four genes and fasting glucose and HbA1c.
Conclusion
Our results could suggest that the selected genes in the vitamin D metabolic pathway may be involved in the pathological process of TB-DM, but independent of the process of hyperglycemia to impaired immune responses to Mtb.
Keywords: tuberculosis, diabetes, epigenetics, DNA methylation, vitamin D
Introduction
There were about 5.8 million newly diagnosed TB patients worldwide, according to the 2021 global tuberculosis report.1 Low body mass index (BMI), diabetes, and smoking could be the risk factors for TB, and previous reports have established a strong relationship between diabetes and TB.2,3 Compared with TB only patients, patients with TB-DM are easier to have unfavorable treatment outcomes with a higher mortality rate. After screening 7043 articles and identifying 43 eligible studies, a study reported the prevalence of TB-DM patients in China: Northeast China (21.9%) among the four economic regions of China, followed by East Coast (8.3%), Western China (5.9%), and Central China (5.1%).4
Vitamin D is implicated in the regulation of cellular metabolism in the human body and is an essential regulator of both innate and adaptive immune responses. Previous studies have established that Vitamin D deficiency is related to impaired insulin secretion and function and anti-M. tuberculosis immune response, which could raise the risk of diabetes and TB.5,6 It has been found that Vitamin D may have a diverse effect on pancreatic β-cell and immune function and that vitamin D deficiency may cause insulin resistance and glucose intolerance.7 In chronic disease, vitamin D is used to restore health in organ systems, and vitamin D deficiency may increase the risk of disease. A previous study reported that vitamin D deficiency is associated with active TB (OR: 2.9, 95% CI: 1.3–6.5),8 so diabetes-related deficiency could lead to susceptibility to TB infection and vice versa.9
Epigenetics means functional modifications to the DNA without gene sequence alterations, primarily containing noncoding RNAs, DNA methylation, and histone modification.10 Emerging evidence indicates that epigenetics may have a vital impact on the occurrence and development of TB.11,12 Recently, based on microarray analysis, 1028 differentially methylated loci (DML) were found in TB patients versus health participants and 3747 DMLs in TB patients after vs before anti-TB treatment with the DNA methylation 450 K assay from Illumina.13 Furthermore, recent studies also found some abnormal DNA methylation sites, such as MRPS18B, RPTOR, RASGRP4, IL12B, IL12RB2, TYK2, IFNGR1, JAK1, JAK2, VDR, CYP24A1, CYP27A1, CG, CYP27B1 and so on.13–16 However, the knowledge of DNA methylation in TB or TB-DM is still lacking and should be explored.
Vitamin D can interact with epigenome in many aspects. Firstly, in the vitamin D metabolic pathway, VDR, CYP24A1, CYP27B1, and CYP2R1 have many CpG islands in their promoter regions and can be silenced with DNA methylation. Secondly, epigenetic regulation is more likely to occur in the genes that regulate 1.25-D3 levels and signaling, including CYP24A1, CYP27B1, and VDR. CpG islands span the promoters of CYP2R1, CYP24A1, and VDR. The CpG island, on the other hand, is located within the CYP27B1 gene locus. Consequently, the chromatin state can be changed from an open to a closed conformation, repressing transcription of these genes through DNA methylation and histone modifications within these regions.17 However, several genes of the vitamin D pathway have been reported to be epigenetically regulated in TB patients or diabetic patients, respectively.14,16,18 Nevertheless, the association of DHCR7 (encoding 7-dehydrocholesterol reductase enzyme for the synthesis of pre-vitamin D3), a key protein product of which involving in vitamin D and metabolism, with TB or TB-DM has not been reported. In addition, there is no research describing the relationship between DNA methylation patterns of the vitamin D metabolic pathways in TB-DM patients. Thus, we conducted the present case–control study in the Chinese Han population to investigate the impact of aberrant DNA methylation in the vitamin D metabolic pathways on TB-DM susceptibility and to evaluate their association with demographic, CT features, and laboratory indicators.
Materials and Methods
Study Design
This study performed the case–control design. From March 2022 to June 2022, we recruited 50 patients with TB-DM from Chongqing Public Health Medical Center, China. Our previous study has described the inclusion and exclusion criteria.2 In brief, inclusion criteria: age ≥18; antituberculosis therapy no more than one week before hospitalization; active TB. Exclusion criteria: using lipid-lower agents; active tuberculosis in this study should conform to at least one of the following standards: sputum or bronchial lavage fluid (BALF) smear positive, sputum or BALF bacterial culture positive, GeneXpert Mycobacterium tuberculosis/rifampicin resistance in sputum or BALF positive. Patients who had incomplete clinical data, history of tumor, rheumatoid arthritis, asthma, or coinfected with HIV and HBV were excluded. Fifty healthy subjects without TB or diabetes were recruited from the community population according to the rule of the same gender, similar personal history, and similar age (<3 years difference) to use a 1:1 matching case–control study. We collected 5 mL of fasting venous blood from all participants and collected the demographic data and laboratory parameters of participants by using Electronic Medical Records (EMR).
DNA Extraction
A whole blood genome DNA isolation kit (sparkjade, Shandong, China) was used to extract peripheral blood DNA based on the manufacturer’s guidelines. Nanodrop 2000 spectrophotometer was employed to determine the DNA quantity and to verify the DNA purity. Genomic DNA with OD26/OD280 between 1.8 and 2.0 and OD260/230 between 1.5 and 3.0 was used for subsequent analysis.
DNA Methylation
The MethylTargetTM method was used to determine the methylation levels of five candidate genes (CYP24A1, CYP27B1, CYP2R1, DHCR7, and VDR) and sequenced the CpG islands in the promoter’s region of the selected genes. In order to convert unmethylated cytosines to uracils, genomic DNA samples were treated with sodium thiocyanate following the manufacturer’s protocol (ZYMO, CA, USA). Bisulfite-converted DNA was amplified using primers designed to amplify the regions of interest (Table S1). The optimized primer sets were used for multiplex PCR. The methylation detection of five genes was made on the Illumina NextSeq platform according to the manufacturer’s protocols. Methylation FastTarget V4.1 (Genesky Biotechnologies Inc., CN) was used to analyze the methylation level in the promoter region of the five selected genes.
Vitamin D Classification
In the present study, we divided the serum 25-hydroxy vitamin D [25(OH)D] levels into three clinically relevant categories based on the Endocrinology Society Clinical Practice Guidelines.19 Vitamin D deficiency (VDD): 25(OH)D below 20 ng/mL; Vitamin D insufficiency (VDI): 25(OH)D between 21 and 29 ng/mL; Vitamin D sufficiency (VDS): 25(OH)D above 30 ng/mL.
Diabetes Status
Diabetes status in the study was classified as diabetes-poor control (HbA1c at or above 7%) or diabetes-good control (HbA1c under 7%).
Computed Tomography (CT) Features
Single cavity: CT imaging shows a solitary cavity; Multiple cavity: There are more than one cavity on the CT imaging; Air bronchial sign: the manifestation of air-filled bronchi being created visible by the opacification of surrounding alveoli. Most often, it happens when something other than air fills the alveoli because of a pathologic airspace/alveolar process (Figure 1).
Figure 1.
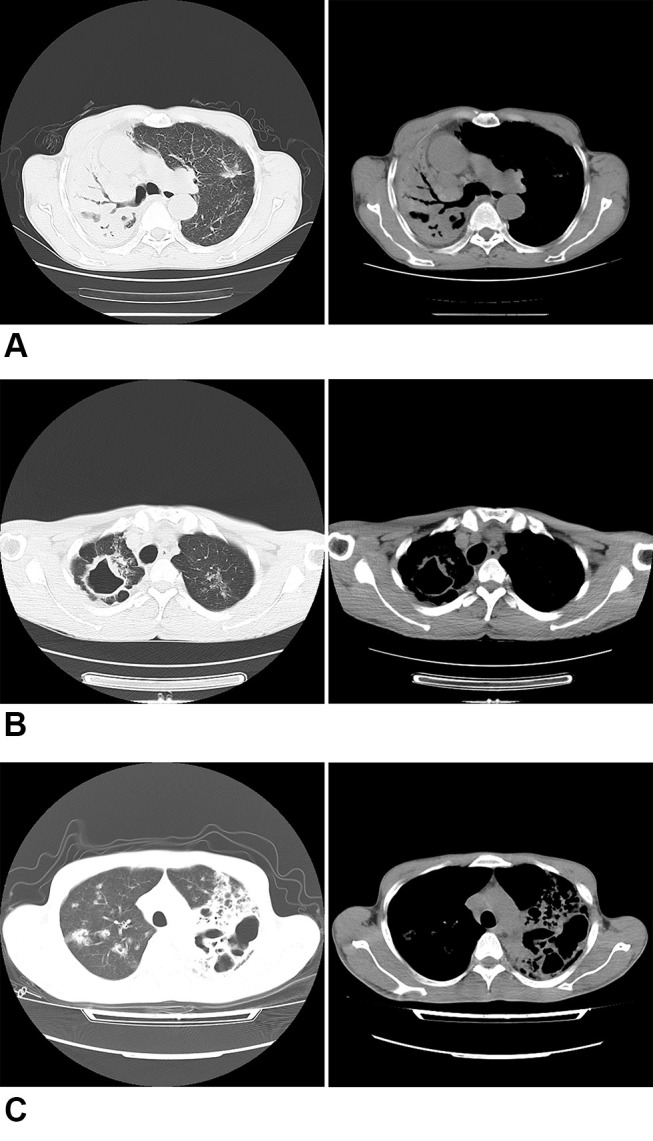
Chest radiograph sign in pulmonary window and mediastinal. (A) Air bronchial sign. (B) Single cavity. (C) Multiple cavities.
Statistical Analysis
A Shapiro test was used to test the normality of continuous data. A normally distributed dataset was analyzed by mean ± standard deviation and Student’s t-test, while nonnormally distributed data were analyzed using median and interquartile range (IQR) and Mann–Whitney U-tests. The Chi-square test was used to analyze categorical data in order to assess the diagnostic value of DNA methylation of genes, the sensitivity, specificity, and ROC were estimated. The association of selected gene methylation with TB-DM risk was explored by conditional logistic regression in the case–control study. A Spearman rank correlation coefficient analysis was performed to elevate whether the methylation of the candidate genes was correlated between them and other parameters associated with TB-DM.
All analyses were performed using RStudio (version 1.4.1717). A two-tailed P value <0.05 was considered statistically significant. Additionally, Benjamini–Hochberg (BH) method was performed to control the false discovery rate (FDR), and the corrected P value (FDR) <0.05 was considered as statistically significant in this study.
Results
Anthropometric and Biochemical Parameters of TB-DM
The anthropometric and biochemical parameters of participants are demonstrated in Table 1. There were no significant differences between age, thrombocytocrit (PCT), and triglyceride (TG) among the two groups. Compared with health objects, patients with TB-DM had a lower BMI, mean platelet volume (MPV), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TCHOL), systolic blood pressure systolic pressure (SBP), diastolic blood pressure (DBP), and serum 25(OH)D, but a higher value of platelet (PLT), platelet distribution width (PDW), fasting glucose (GLU).
Table 1.
General Characteristics and Clinical Parameters of Cases and Controls
| Variables | Case | Control | P value |
|---|---|---|---|
| N=50 | N=50 | ||
| Age (year) | 56.4 (11.1) | 55.8 (9.49) | 0.787 |
| Female, n (%) | 9 (18%) | 9 (18%) | |
| BMI (kg/m2) | 21.8 (2.58) | 24.3 (3.25) | <0.001 |
| PLT (× 109/L) | 287 [234;378] | 216 [191;250] | <0.001 |
| PCT (%) | 0.24 [0.22;0.29] | 0.24 [0.22;0.28] | 0.994 |
| PDW (fL) | 15.7 [15.4;16.1] | 13.2 [12.2;14.6] | <0.001 |
| MPV (fL) | 8.35 [7.80;9.28] | 10.9 [10.3;11.6] | <0.001 |
| TG (mg/dl) | 132 [100;163] | 105 [75.5;182] | 0.177 |
| HDL (mg/dl) | 32.1 [27.9;37.9] | 51.6 [47.2;61.8] | <0.001 |
| LDL (mg/dl) | 102 (26.0) | 133 (26.5) | <0.001 |
| TCHOL (mg/dl) | 157 (36.0) | 211 (32.9) | <0.001 |
| Sbp (mmHg) | 123 (17.1) | 137 (17.4) | <0.001 |
| Dbp (mmHg) | 75.9 (12.3) | 83.3 (9.62) | 0.001 |
| GLU (mg/dl) | 167 [121;201] | 98.8 [94.7;105] | <0.001 |
| 25(OH)D ng/mL | 24.3 [20.6;27.2] | 29.5 [26.2;33.1] | <0.001 |
| Overall methylation (%) | |||
| CYP24A1 | 3.29 [2.94;3.71] | 3.80 [3.49;4.01] | <0.001 |
| CYP2R1 | 0.67 [0.64;0.70] | 0.68 [0.64;0.72] | 0.270 |
| CYP27B1 | 3.84 [3.36;4.62] | 5.05 [4.53;5.87] | <0.001 |
| DHCR7 | 2.50 [2.35;2.68] | 2.77 [2.55;2.98] | <0.001 |
| VDR | 1.67 [1.48;1.83] | 2.05 [1.88;2.28] | <0.001 |
Notes: Normal variables are expressed as mean ± standard deviation (SD), and skewed variables are expressed as median (interquartile range). P < 0.05 was statistically significant.
Abbreviations: BMI, body mass index; PLT, platelet; PCT, thrombocytocrit; MPV, mean platelet volume; PDW, platelet distribution width; TG, triglyceride; HDL, high-density lipoprotein; LDT, low-density lipoprotein; TCHOL, total cholesterol; SBP, systolic blood pressure systolic pressure; DBP, diastolic blood pressure; GLU, fasting glucose.
Association of Methylation at Candidate Gene and TB-DM Risk
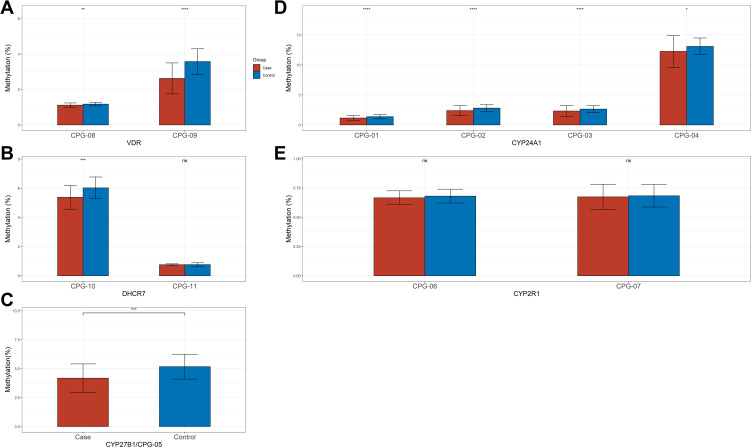
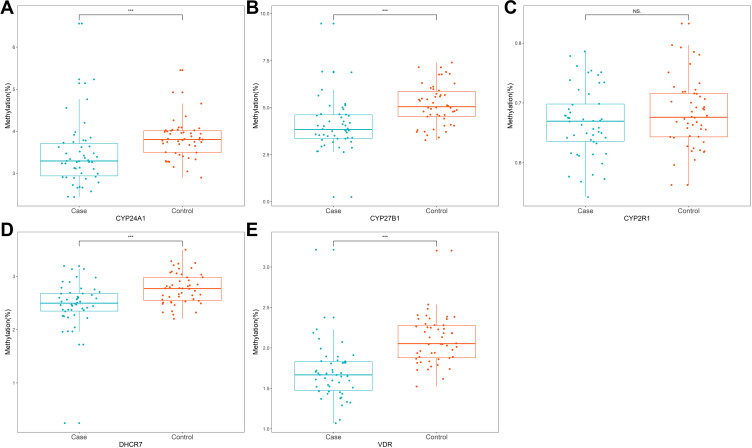
We sequenced 11 fragments and 198 CpGs sites in the promoter regions of the five selected genes. As shown in Figure 2, among all the CpGs sites of VDR, CYP27B1, and CYP24A1, the methylation level was significantly higher in the control group than in the case, as well as CpG-10 island of DHCR7. However, there was no significant difference in methylation level of 2 CpG islands of CYP27R1 between the two groups. The overall methylation level of CYP24A1, CYP27B1, DHCR7, and VDR promoter in the control group was significantly higher than that in the case group, except for CYP27R1 (Figure 3). Table 2 demonstrates the results of conditional logistic regression of the association of candidate gene methylation with TB-DM. After adjusting for age and BMI, the risk of TB-DM per 5% increase in methylation was reduced by 65.7% for CYP24A1 (OR, 95% CI 0.343, 0.144–0.821), 53.9% for CYP27B1 (0.461, 0.275–0.773), for DHCR7 91% (0.090, 0.015–0.530), and 99.994% (0.006, 0.0003–0.115), respectively.
Figure 2.
Methylation analyses of CpG islands of key genes promoter in patients with TB-DM and health subject. (A) Methylation analyses of two CpG islands of the VDR promoter in Peripheral blood mononuclear cells (PBMCs) from patients and Health subject. (B) Methylation analyses of two CpG islands of the DHCR7 promoter in Peripheral blood mononuclear cells (PBMCs) from patients and Health subject. (C) Methylation analyses of one CpG islands of the CYP27B1 promoter in Peripheral blood mononuclear cells (PBMCs) from patients and Health subject. (D) Methylation analyses of four CpG islands of the CYP27A1 promoter in Peripheral blood mononuclear cells (PBMCs) from patients and Health subject. (E) Methylation analyses of two CpG islands of the CYP2R1 promoter in Peripheral blood mononuclear cells (PBMCs) from patients and Health subject (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns refers to no significant).
Figure 3.
The overall methylation of candidate genes promoter in patients with TB-DM and health subject. (A) CYP24A1; (B) CYP27B1; (C) CYP2R1; (D) DHCR7; (E) VDR (***p < 0.001, and ns refers to no significant).
Table 2.
Association of DNA Methylation Level of Candidate Genes and Risk of TB-DM
| Genes | Crude Model | Adjusted Modela |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| CYP24A1 | 0.286 (0.117–0.699) | 0.343 (0.144–0.821) |
| CYP27B1 | 0.407 (0.245–0.679) | 0.461 (0.275–0.773) |
| DHCR7 | 0.062 (0.012–0.327) | 0.090 (0.015–0.530) |
| VDR | 0.004 (0.0002–0.074) | 0.006 (0.0003–0.115) |
Notes: aAdjusted for age and BMI.
Diagnostic Values of Selected Genes Methylation for TB-DM
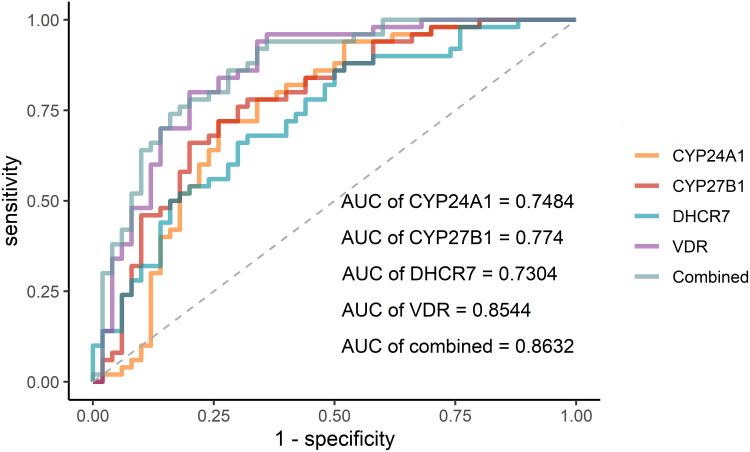
The ROC was conducted to assess the diagnostic values of four gene methylation for TB-DM (Figure 4). Among five ROC, the optimal AUC is the combination of four genes, 0.8632, with a specificity of 0.64, and a sensitivity of 0.94, following VDR, 0.8544, with a specificity of 0.64, and a sensitivity of 0.96. The detailed outcomes are shown in Table 3.
Figure 4.
The ROC curve of the methylation levels of candidate genes.
Table 3.
Diagnostic Values of Genes for TB-DM
| Gene | AUC | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|
| CYP24A1 | 0.7484 | 0.648–0.849 | 0.72 | 0.74 |
| CYP27B1 | 0.774 | 0.681–0.867 | 0.68 | 0.78 |
| DHCR7 | 0.7304 | 0.632–0.829 | 0.68 | 0.68 |
| VDR | 0.8544 | 0.778–0.931 | 0.64 | 0.96 |
| Combined | 0.8632 | 0.791–0.935 | 0.64 | 0.94 |
Subgroup Analysis in Patients with TB-DM
A subgroup analysis was conducted to explore the effects of vitamin D, diabetes status, and CT features on four selected gene methylation levels in patients with TB-DM, respectively. As shown in Table 4, after stratifying by vitamin D status, there were statistically significant between the three groups of vitamin D status for genes such as CYP24A1, CYP27B1, and DHCR7. Pairwise comparisons showed that there were significantly differences between VDD and VDS and between VDI and VDD for CYP24A1, respectively; there were significant differences between VDI and VDD for CYP27B1, as well as for DHCR7. However, there was no significant difference between serum 25(OH)D groups for VDR. Additionally, there were no statistical differences between diabetes status groups for all the genes, as well as three abnormal CT feature groups (single cavity, multiple cavities, and air bronchogram sign) (Tables S2–S5).
Table 4.
Methylation Levels of Genes in TB-DM According to Vitamin D Status
| Genes | VDS | VDI | VDD | P value | VDS vs VDI | VDS vs VDD | VDI vs VDD |
|---|---|---|---|---|---|---|---|
| N=7 | N=32 | N=11 | |||||
| CYP24A1 | 3.39 [3.17;3.99] | 3.47 [3.21;3.78] | 2.89 [2.62;3.05] | 0.001 | 0.971 | 0.019 | <0.001 |
| CYP27B1 | 3.76 [3.41;4.87] | 4.15 [3.53;4.71] | 3.18 [2.77;3.78] | 0.025 | 0.558 | 0.203 | 0.021 |
| DHCR7 | 2.46 [2.37;2.94] | 2.58 [2.43;2.69] | 2.26 [2.01;2.42] | 0.007 | 0.855 | 0.095 | 0.005 |
| VDR | 1.70 [1.58;1.81] | 1.67 [1.50;1.84] | 1.61 [1.46;1.69] | 0.466 | 1.000 | 0.417 | 0.417 |
Abbreviations: VDD, vitamin D deficiency; VDI, vitamin D insufficiency; VDS, vitamin D sufficiency.
Correlation of Methylation with Biochemical Parameters in TB-DM
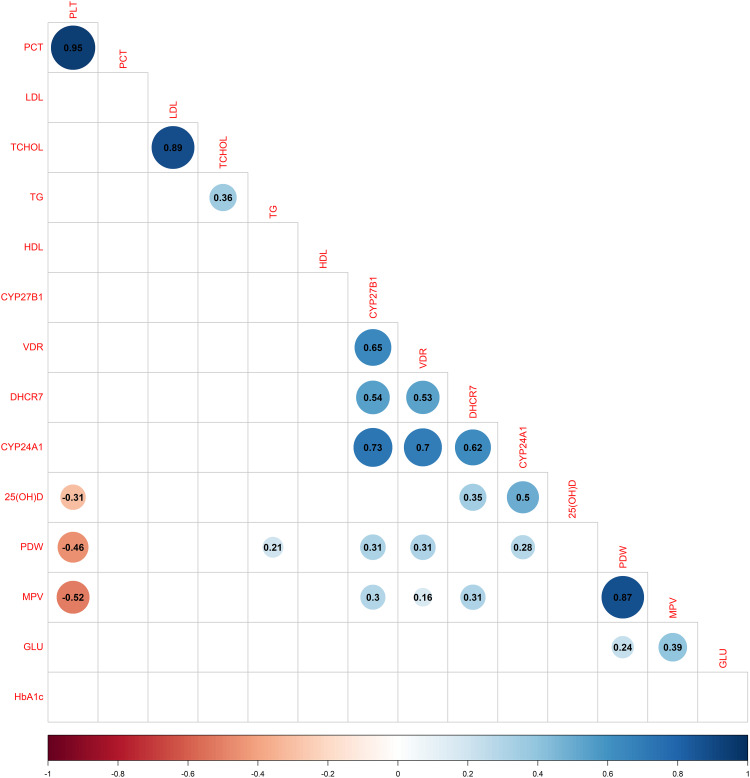
As shown in Figure 5 the methylation level of CYP24A1 in TB-DM was positively correlated with the PDW and serum 25(OH)D, respectively (rs = 0.28, P < 0.05; rs = 0.5, P < 0.05); the relationship between the methylation level of CYP27B1 and PDW and MPV was statistically significant, respectively (rs = 0.31, P < 0.05; rs = 0.3, P < 0.05); a positive correlation between the methylation level of DHCR7 and serum 25(OH)D and MPV was observed in TB-DM, respectively (rs = 0.35, P < 0.05; rs = 0.31, P < 0.05); a positively association of VDR and PDW and MPV was observed, respectively (rs = 0.31, P < 0.05; rs = 0.16, P < 0.05). All four DNA methylations of genes were not correlated with fasting glucose and HbA1c.
Figure 5.
Corrplot of studied correlation of methylation level of key genes in vitamin D metabolic pathway and with biochemical parameters in TB-DM. Blue colour represents positive correlation; red colour represents negative correlation; darker colors and larger shapes represent higher association. Blank represents no statistical difference.
Discussion
Previous studies have described the relationship between vitamin D deficiency, impaired insulin function, and anti-M. tuberculosis immune response, and proved that low-level vitamin D may be associated with an increased risk of diabetes and tuberculosis.6,20 Recently, the view of the immunomodulatory effects of vitamin D on the innate and adaptive immune system was supported by many scholars. Vitamin D has significant regulatory effects on insulin secretion and signaling. When vitamin D binds to the macrophage membrane Toll-like receptor, it activates 1-alpha hydroxylase and modulates the expression of the antimicrobial peptide Cathelicidin.6 Thus, the killing of M. tuberculosis ability of macrophages may be boosted through modulating the vitamin D metabolic pathway and inhibiting the activity of histone deacetylases.21
The current study demonstrated the association between DNA methylation of genes in vitamin D metabolic pathway and TB-DM. Compared with healthy participants, the overall DNA methylation levels of CYP24A1, CYP27B1, DHCR7, and VDR promoter in patients with TB-DM were significantly lower. Moreover, the ROC curve demonstrated that combination of the methylation status of the above four genes has an optimal diagnosis value of TB-DM.
Our finding showed significant hypomethylation patterns for vitamin D-related genes, such as CYP24A1, CYP27B1, and VDR promoter in TB-DM. Moreover, the methylation level of above genes was closely related to the risk of TB-DM in Chinese Han people, which is consistent with previous studies.14 However, Maruthai et al reported that children with active TB in an Indian hospital had a significantly elevated VDR DNA methylation level [Cases 75% (IQR, 50–75%); healthy control children 10% (IQR, 10–25%) (P < 0.0001)].16 The possible reason could be that age difference could affect the methylation patterns of VDR. Another possibility could be that there is an impact factor of ethnic discrepancy on methylation patterns of vitamin D, as Wang et al reported and our studies achieved similar conclusions among the Chinese Han population.14 DHCR7, involved in 25[OH]D synthesis within the skin, is engaged in the metabolic pathway of vitamin D that could be related to TB susceptibility.22,23 However, the DNA methylation of DHCR7 experiments has not been performed in TB or TB-DM. Our findings indicated that compared with healthy subjects, TB-DM patients have a significantly lower level of DNA methylation for DHCR7. Therefore, multicenter studies should investigate the role of DHCR7 in epigenetics on TB-DM to confirm prove this result.
The ROC curve was employed to investigate the diagnostic value of candidate genes. These results revealed that the four selected genes performed well, with all AUC above 0.7. Moreover, the combination of four genes demonstrated the optimal diagnostic value, with the AUC above 0.8. Therefore, the four genes in the vitamin D metabolic pathway could be functional as diagnostic tools for diagnosing TB-DM to some extent. Nevertheless, the real value of these gene methylations in clinical requires further testing.
A consistent subgroup analysis was performed based on the level of serum 25(OH)D in patients with TB-DM, considering the relationship between vitamin D and TB and diabetes comorbidity. We observed that lower circulating serum 25(OH)D is associated with low CYP24A1, CYP27B1, and DHCR7 promoter methylation. Thus, vitamin D could have a possible epigenetic effect on DNA methylation of the above three genes in patients with TB-DM, the underlying mechanism of which requires further exploration. In addition, the diabetes status did not affect CYP24A1, CYP27B1, DHCR7, and VDR methylation in patients with TB-DM, which could be related to our small sample size. However, we cannot exclude the possibility that DNA methylation of the above genes could not be involved in the hyperglycemic process to impaired immune responses to Mtb. Previous studies also reported that the DNA methylation of CYP24A1, CYP27B1, and VDR showed no significant differences in diabetic patients compared with the health control subjects.18,24,25 Cavitation is a severity result of pulmonary tuberculosis, which is related to poor prognosis, treatment relapse, higher infectious rates, and development of drug resistance.26 Our results demonstrated that the DNA methylation profile of all genes selected in patients with TB-DM was not related to three common abnormal CT features, single cavity, multiple cavities, and air bronchogram sign, referring to the methylation status of the candidate genes in the vitamin D metabolic pathway might not affect the prognosis and relapse of TB-DM.
There are reports that many diseases of the immune and hemopoietic system have close relationship with platelet indices.27 Previous studies showed a strong relationship between a low vitamin D level and a high MPV.28,29 Additionally, Xu et al established that both MPV and PCT were significantly reduced in tuberculosis and diabetes comorbidity patients compared with diabetes only individuals. In contrast, MPV was significantly higher in TB-DM patients than in TB only patients.30 MPV can mirror platelet size and the degree of the inflammation and can be conducted to assess the function of platelets. Among those TB patients, acute-phase reactants and proinflammatory cytokines affect megakaryocytes, reducing the platelet size. In addition, smaller platelets are released from the bone marrow, which could be the mechanism of the reduction of MPV.31 Tozkoparan et al observed significantly higher PDW, MPV, and plateletcrit values in the active TB group than in the control.32 Our results showed a significant correlation between DNA methylation status of selected genes within the vitamin D metabolic pathway, CYP24A1, CYP27B1, DHCR7, and VDR, and platelet indices in TB-DM. Therefore, the DNA methylation of the above genes could regulate platelet indices in tuberculosis immunopathogenesis. Besides, the study found no correlation between the DNA methylation of candidate gene promoter and fasting glucose and HbA1c, and it shows that variations in the DNA methylation profile of these genes associated with vitamin D do not predict the risk and progression of TB-DM development. Future research needs to investigate the molecular mechanism behind the DNA methylation of these candidate genes involved in the pathogenesis of TB-DM, considering molecular regulation and cellular function.
There are several limitations to our studies. First, the sample size in the present study is moderate and it is a single-center study. Thus, multicenter and large sample studies should be considered to verify the robustness of the findings from the present study. Second, we did not perform transcriptomic and translation experiments on the candidate genes in the study due to limited experimental conditions. Therefore, it is essential to study the relationship of DNA methylation, transcriptome, and translation level for vitamin D metabolic pathways in the future. Third, the MethylTargetTM method only has a limited number of probes in the candidate genomic regions of promoters. In the next step, we will identify more genes in the vitamin D metabolic pathway by employing other high throughput method, such as the 850k chip upgrade. This can provide a wealth of data on the DNA methylation regulation of the promoter and body region of genes. Fourth, no solid evidence from rigorous interventional trials exists to support vitamin D supplementation for people to cure or prevent TB. Although some specific randomized controlled trials (RCTs) showed the role of vitamin D supplementation on improving prognosis of TB,33,34 a recent RCT showed that compared with placebo among vitamin D-deficient participants, neither the risk of tuberculosis infection nor tuberculosis disease could not be lower by vitamin D supplementation.35 In the present study, we did not perform a longitudinal study to explore vitamin D supplementation to highlight the prognostic significance of these observations due to the cost. Even the basic of clinical interventional trials may be costly to implement. Hence, we expect other research will fill the gap in the future. Fifth, when we conducted to design our study, we considered to include TB only and DM only patients as control subjects. Previous study in China has proved that DNA methylation of vitamin D might not be involved in the pathogenesis of DM.24,25 Wang et al in their study demonstrated that methylation levels of key genes in the vitamin D metabolic pathway are related to the risk and prognosis of tuberculosis.14 Additionally, in the meantime, we are conducting this study, there is a rigorous anti-covid-19 battle going on in China, which disturbs our experimental program. We will make up this gap in a large and multicenter study in future.
Conclusions
In our study, serum 25(OH)D concentrations were significantly higher in healthy subjects than patients with TB-DM. People with low CYP24A1, CYP27B1, DHCR7, and VDR methylation level may increase the risk of TB-DM. These results indicate that the DNA methylation levels of CYP24A1, CYP27B1, DHCR7, and VDR genes in the vitamin D metabolic pathway could be involved in the pathological process of TB-DM. However, a correlation was not established between the above candidate gene methylation and the glucose and HbA1c levels. Thus, we suspected that DNA methylation of selected genes in the vitamin D metabolic pathway could not be involved in the hyperglycemic process to impaired immune responses to Mtb.
Funding Statement
There is no funding to report.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
This study was approved by the Ethics Committee of Chongqing Public Health Medical Center and the First Affiliated Hospital of Anhui Medical University. All participants included in the study signed informed consent, and all procedures have complied with the 1964 Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization. Global Tuberculosis Report. World Health Organization; 2021. [Google Scholar]
- 2.Chen Y, Peng A, Chen Y, et al. Association of TyG Index with CT features in patients with tuberculosis and diabetes mellitus. Infect Drug Resist. 2022;15:111–125. doi: 10.2147/IDR.S347089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngo MD, Bartlett S, Ronacher K. Diabetes-associated susceptibility to tuberculosis: contribution of hyperglycemia vs. Dyslipidemia. Microorganisms. 2021;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Q, Wang L, Long Q, Zhao Y, Abdullah AS. Systematic review and meta-analysis: prevalence of diabetes among patients with tuberculosis in China. Trop Med Int Health. 2021;26(12):1553–1559. doi: 10.1111/tmi.13686 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Chen Y-Q, Zhang Q. Association between vitamin D and insulin resistance in adults with latent tuberculosis infection: results from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. J Infect Public Health. 2022;15(8):930–935. doi: 10.1016/j.jiph.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Ma A, Schouten EG, Kok FJ. A double burden of tuberculosis and diabetes mellitus and the possible role of vitamin D deficiency. Clinical Nutrition. 2021;40(2):350–357. doi: 10.1016/j.clnu.2020.08.040 [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Chu A, Go VLW, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6 [DOI] [PubMed] [Google Scholar]
- 9.Handel AE, Ramagopalan SV. Tuberculosis and diabetes mellitus: is vitamin D the missing link? Lancet Infect Dis. 2010;10(9):596. doi: 10.1016/S1473-3099(10)70185-7 [DOI] [PubMed] [Google Scholar]
- 10.Chater-Diehl E, Goodman SJ, Cytrynbaum C, Turinsky AL, Choufani S, Weksberg R. Anatomy of DNA methylation signatures: emerging insights and applications. Am J Hum Genet. 2021;108(8):1359–1366. doi: 10.1016/j.ajhg.2021.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarashi S, Badi SA, Moshiri A, et al. The inter-talk between and the epigenetic mechanisms. Epigenomics. 2020;12(5):455–469. doi: 10.2217/epi-2019-0187 [DOI] [PubMed] [Google Scholar]
- 12.Sui J, Qiao W, Xiang X, Luo Y. Epigenetic changes in Mycobacterium tuberculosis and its host provide potential targets or biomarkers for drug discovery and clinical diagnosis. Pharmacol Res. 2022;179:106195. doi: 10.1016/j.phrs.2022.106195 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y-C, Hsiao -C-C, Chen T-W, et al. Whole Genome DNA methylation analysis of active pulmonary tuberculosis disease identifies novel epigenotypes: /// gene methylation and clinical phenotypes. Int J Mol Sci. 2020;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Kong W, He B, et al. Vitamin D and the promoter methylation of its metabolic pathway genes in association with the risk and prognosis of tuberculosis. Clin Epigenetics. 2018;10(1):118. doi: 10.1186/s13148-018-0552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiNardo AR, Rajapakshe K, Nishiguchi T, et al. DNA hypermethylation during tuberculosis dampens host immune responsiveness. J Clin Invest. 2020;130(6):3113–3123. doi: 10.1172/JCI134622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruthai K, Sankar S, Subramanian M. Methylation status of VDR gene and its association with vitamin D status and VDR gene expression in pediatric tuberculosis disease. Immunol Invest. 2022;51(1):73–87. doi: 10.1080/08820139.2020.1810702 [DOI] [PubMed] [Google Scholar]
- 17.Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Front Physiol. 2014;5:164. doi: 10.3389/fphys.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.S-c Y. Association Between Variation s of Polymorphism, Copy Number and Methylation in Vitamin D Metabolic Pathway Gene s and Type 2 Diabetes Mellitus. Zhengzhou: Zhengzhou University; 2018. [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 20.Abelha-Aleixo J, Fonseca R, Bernardo A, Mariz E, Costa L. Vitamin D - immunomodulatory actions and new potentialities. Acta Reumatol Port. 2014;39(4):355–356. [PubMed] [Google Scholar]
- 21.Kolloli A, Subbian S. Host-directed therapeutic strategies for tuberculosis. Front Med. 2017;4:171. doi: 10.3389/fmed.2017.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadykov M, Azizan A, Kozhamkulov U, et al. Association of genetic variations in the vitamin D pathway with susceptibility to tuberculosis in Kazakhstan. Mol Biol Rep. 2020;47(3):1659–1666. doi: 10.1007/s11033-020-05255-3 [DOI] [PubMed] [Google Scholar]
- 23.Zhang T-P, Chen -S-S, Zhang G-Y, Shi S-J, Wei L, H-M L. Association of vitamin D pathway genes polymorphisms with pulmonary tuberculosis susceptibility in a Chinese population. Genes Nutr. 2021;16(1):6. doi: 10.1186/s12263-021-00687-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S, Feng Y, Qu C, et al. Vitamin D receptor methylation attenuates the association between physical activity and type 2 diabetes mellitus: a case-control study. J Diabetes. 2022;14(2):97–103. doi: 10.1111/1753-0407.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang T, Huo Y, et al. Placenta expression of vitamin D and related genes in pregnant women with gestational diabetes mellitus. J Steroid Biochem Mol Biol. 2020;204:105754. doi: 10.1016/j.jsbmb.2020.105754 [DOI] [PubMed] [Google Scholar]
- 26.Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis. 2020;20(6):e117–e28. doi: 10.1016/S1473-3099(20)30148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed -A-AB, Elnady HM, Alhewaig HK, Moslem Hefny H, Khodery A. The mean platelet volume and plateletcrit as predictors of short-term outcome of acute ischemic stroke. Egypt J Neurol Psychiatr Neurosurg. 2019;55(1):4. doi: 10.1186/s41983-018-0035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cumhur Cure M, Cure E, Yuce S, Yazici T, Karakoyun I, Efe H. Mean platelet volume and vitamin D level. Ann Lab Med. 2014;34(2):98–103. doi: 10.3343/alm.2014.34.2.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park YC, Kim J, Seo MS, Hong SW, Cho ES, Kim J-K. Inverse relationship between vitamin D levels and platelet indices in Korean adults. Hematology. 2017;22(10):623–629. doi: 10.1080/10245332.2017.1318334 [DOI] [PubMed] [Google Scholar]
- 30.Xu F, Qu S, Wang L, Qin Y. Mean platelet volume (MPV): new diagnostic indices for co-morbidity of tuberculosis and diabetes mellitus. BMC Infect Dis. 2021;21(1):461. doi: 10.1186/s12879-021-06152-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7(2):157–161. doi: 10.1097/00001721-199603000-00011 [DOI] [PubMed] [Google Scholar]
- 32.Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K. Changes in platelet count and indices in pulmonary tuberculosis. Clin Chem Lab Med. 2007;45(8):1009–1013. doi: 10.1515/CCLM.2007.194 [DOI] [PubMed] [Google Scholar]
- 33.Mily A, Rekha RS, Kamal SMM, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS One. 2015;10(9):e0138340. doi: 10.1371/journal.pone.0138340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganmaa D, Uyanga B, Zhou X, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N Engl J Med. 2020;383(4):359–368. doi: 10.1056/NEJMoa1915176 [DOI] [PMC free article] [PubMed] [Google Scholar]