Abstract
Background
Hypertrophic cardiomyopathy in identical twins is rare. Cases of hypertrophic cardiomyopathy with homogenous and heterogeneous phenotypes have been described in the literature.
Case summary
We report a pair of monozygotic twins (Twin A and Twin B) with identical morphological expression of hypertrophic cardiomyopathy. On initial evaluation, both twins had resting left ventricular outflow tract obstruction, Grade II diastolic dysfunction, and New York Heart Association (NYHA) Class II symptoms, but they had a different clinical course afterward. Twin A progressed from NYHA Class II to Class III with a high left ventricular outflow tract pressure gradient that was unresponsive to medical treatment and required alcohol septal ablation. Twin B responded very well to medical treatment. Both patients had no risk factors for sudden cardiac death, and neither required an implantable cardioverter defibrillator.
Discussion
The morphology of hypertrophic cardiomyopathy has a strong genetic basis, but epigenetic factors may affect disease expression.
Keywords: Case report, Environmental, Genetic, Hypertrophic cardiomyopathy, Identical twins
Learning points.
The morphology in patients with hypertrophic cardiomyopathy has a strong genetic basis, but epigenetic factors may affect the phenotype and the clinical course.
The risk of sudden cardiac death in patients with hypertrophic cardiomyopathy may also be strongly impacted by genetics with a variable role of environmental factors.
Introduction
Hypertrophic cardiomyopathy is a relatively common inherited cardiomyopathy with global distribution,1 and it is the most common cause of sudden cardiac death in young adults.2 Most hypertrophic cardiomyopathy variants are caused by mutations in the myosin heavy chain 7 (MYH7) and myosin-binding protein C (MYBPC3) genes, but other genes have also been implicated in a minority of cases.3 The study of phenotype and clinical course in identical twins provides insight into the role of genetic and environmental factors in the expression of disease in patients with hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy in monozygotic twins is rare. It was first reported in 1972 by Littler,4 who described two sets of monozygotic and one set of dizygotic twins with hypertrophic cardiomyopathy. Here, we report a pair of monozygotic twins with identical hypertrophic cardiomyopathy but differing clinical courses.
Timeline
| Patient | Date | Description |
|---|---|---|
| Twin A | Index date | Patient seen for the first time after referral by patient’s primary cardiologist. Patient diagnosed with basal septal hypertrophic cardiomyopathy |
| Resting left ventricular outflow tract (LVOT) gradient 83 mmHg | ||
| Metoprolol succinate 50 mg twice daily, verapamil 120 mg daily, and disopyramide phosphate 100 mg twice daily started over a period of 1 month | ||
| 1–3 months | Metoprolol dose decreased and verapamil discontinued due to sinus bradycardia | |
| Disopyramide phosphate dose increased | ||
| 8 months | No resting LVOT obstruction | |
| Valsalva-induced gradient 60 mmHg | ||
| No further dosing adjustments | ||
| 24 months | Valsalva-induced gradient 76 mmHg | |
| Disopyramide phosphate increased to 600 mg in three divided doses | ||
| 32 months | Severe resting LVOT gradient of 108 mmHg | |
| Alcohol septal ablation recommended | ||
| 33 months | Patient had alcohol septal ablation | |
| No more resting or provocable LVOT obstruction | ||
| 40 months | Cardiac magnetic resonance imaging showed patchy delayed gadolinium enhancement of the basal septum | |
| 96 months | No resting or Valsalva-induced LVOT obstruction | |
| Twin B | Index date | After her twin sister’s diagnosis, patient seen for the first time. Patient diagnosed with basal septal variant of hypertrophic cardiomyopathy |
| Resting LVOT gradient of 146 mmHg | ||
| Metoprolol succinate 50 mg daily started | ||
| 1 month | Resting LVOT gradient improved to 100 mmHg. Metoprolol dose increased. | |
| 2 months | Resting LVOT gradient 90 mmHg | |
| Disopyramide phosphate 100 mg twice daily added | ||
| 4 months | Resting LVOT gradient stable at 90 mmHg. | |
| Disopyramide phosphate dose increased; verapamil 180 mg daily added. | ||
| 6 months | No more resting or provocable LVOT obstruction. | |
| 20 months | Cardiac magnetic resonance imaging shows patchy delayed gadolinium enhancement of the basal septum. | |
| 84 months | No resting or Valsalva-induced LVOT obstruction. |
Case summary
Twin A
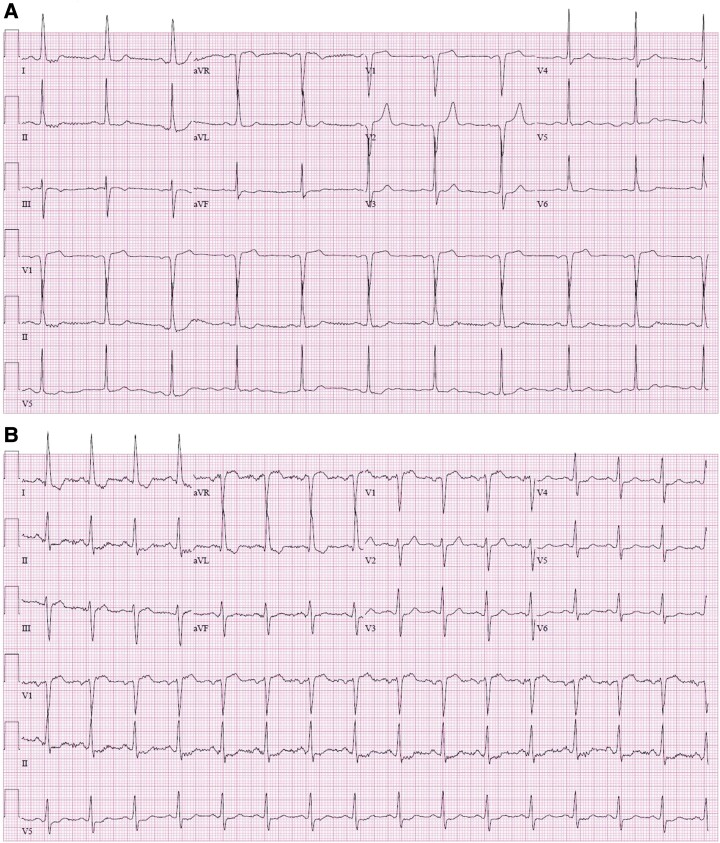
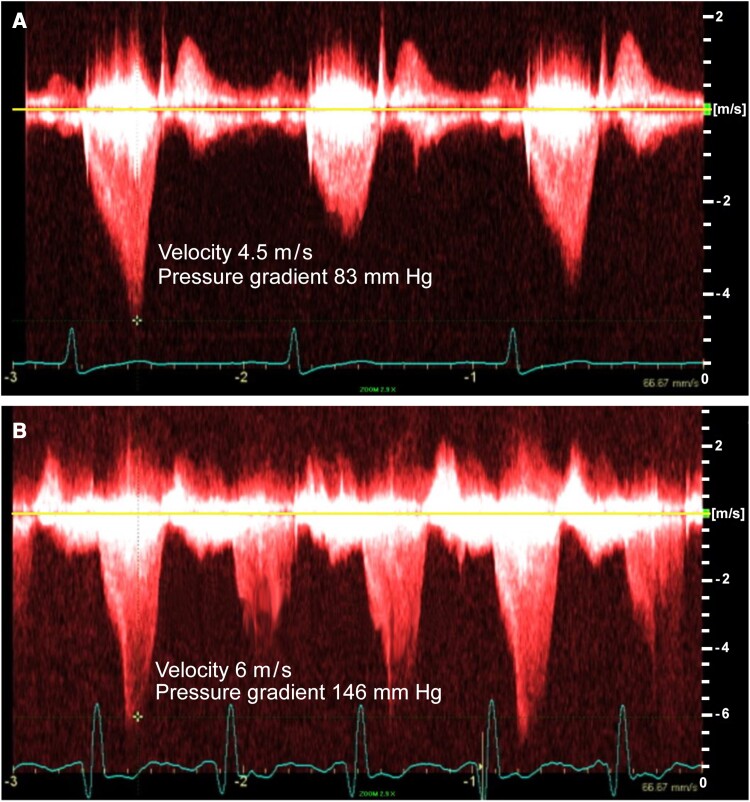
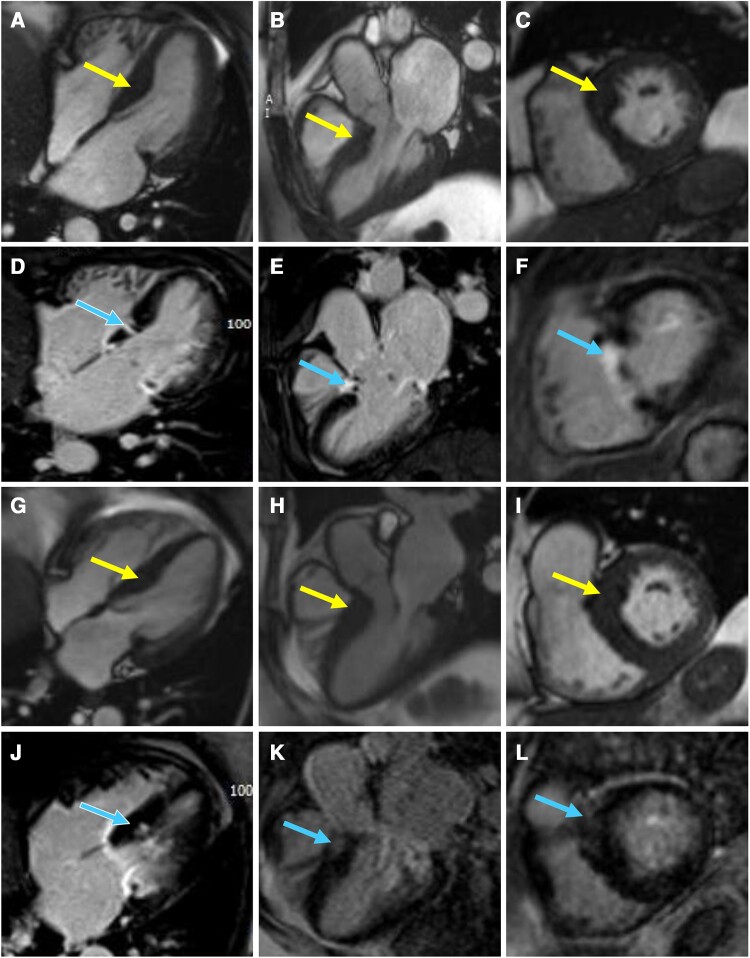
A 60-year-old non-Hispanic White woman with a past medical history of essential hypertension, Type 2 diabetes, morbid obesity, and obstructive sleep apnoea on continuous positive airway pressure was referred to our cardiomyopathy centre by her primary cardiologist. The patient had been diagnosed with basal septal hypertrophic cardiomyopathy at the age of 57 after undergoing an echocardiogram to evaluate a heart murmur. On initial presentation, the patient had New York Heart Association (NYHA) Class II symptoms. Examination revealed a body mass index of 34.5 kg/m2, a Grade 3/6 systolic ejection murmur in the resting position that increased to Grade 5/6 with the Valsalva manoeuvre, and S4. The electrocardiogram showed left ventricular hypertrophy according to the Sokolow–Lyon criteria5 (Figure 1A). Holter monitoring showed sinus bradycardia with a heart rate of 56 (range 47–81) b.p.m. with no episodes of non-sustained ventricular tachycardia or atrial fibrillation and no significant pauses. Echocardiographic features are shown in Table 1. The resting left ventricular outflow tract (LVOT) gradient was 83 mmHg (range 36–83 mmHg; Figure 2A). Cardiac magnetic resonance imaging showed basal septal hypertrophy and replacement fibrosis in the basal septum (Figure 3A–F; Supplementary material online, Videos S1–S3). The patient was started on metoprolol succinate 50 mg twice daily, verapamil 120 mg daily, and disopyramide phosphate 100 mg twice daily over a period of 1 month. She was followed up closely, and medical therapy was optimized for more than a year. Despite maximal tolerated medical therapy, she had a severe resting LVOT gradient (56 mmHg with inspiration, 108 mmHg with expiration). The patient’s symptoms also progressed to NYHA Class III. She underwent alcohol septal ablation with excellent results. She had NYHA Class II symptoms at her most recent follow-up visit, with no LVOT obstruction.
Figure 1.
Left ventricular hypertrophy (Sokolow–Lyon criteria) with R-wave amplitude in aVL >11 mm is seen on electrocardiography in Twin A (A) and Twin B (B).
Table 1.
Echocardiographic characteristics of the twins
| Characteristics | Twin A | Twin B |
|---|---|---|
| Maximum ventricular septal thickness (mm) | 25 | 20 |
| LVOT obstruction at rest | Yes | Yes |
| LVOT gradient resting (mmHg) | 83 | 146 |
| Left ventricular ejection fraction (%) | 77 | 72 |
| Mitral regurgitation | Mild | Mild |
| SAM | Yes | Yes |
| Global longitudinal strain (%) | −17 | −19 |
| Diastolic dysfunction grade | II | II |
| Left atrial volume index (mL/m2) | 51 | 47 |
LVOT, left ventricular outflow tract; SAM, systolic anterior motion of mitral valve leaflets.
Figure 2.
Continuous-wave Doppler showing a typical late peaking dagger-shaped appearance of Doppler velocity profile, with resting left ventricular outflow tract gradient of 83 mmHg for Twin A (A) and 146 mmHg for Twin B (B).
Figure 3.
Four-chamber (A, Supplementary material online, Video S1), three-chamber (B, Supplementary material online, Video S2), and basal short-axis cine steady-state free precession (SSFP) images (C, Supplementary material online, Video S3) of Twin A with corresponding delayed enhancement images (D–F) showing basal septal hypertrophy (arrows in A–C) with replacement fibrosis (arrows in D–F) in the basal septum consistent with infarct pattern after alcohol septal ablation. Four-chamber (G, Supplementary material online, Video S4), three-chamber (H, Supplementary material online, Video S5), and basal short-axis cine SSFP images (I, Supplementary material online, Video S6) of Twin B with corresponding delayed enhancement images (J–L) showing basal septal hypertrophy (arrows in G–I) with patchy mid myocardial replacement fibrosis (arrows in J–L) in the basal septum.
Twin B
Twin B, with a past medical history of hypertension, Type 2 diabetes, obesity, and obstructive sleep apnoea on continuous positive airway pressure, was diagnosed with basal septal hypertrophic cardiomyopathy at the age of 61 after a screening echocardiogram was recommended following her sister’s (Twin A) diagnosis. On initial evaluation, she had NYHA Class II symptoms. Examination showed a body mass index of 34 kg/m2 and a Grade 3/6 systolic murmur that increased to 4/6 intensity with the Valsalva manoeuvre. The electrocardiogram showed left ventricular hypertrophy by the Sokolow–Lyon voltage criteria in lead aVL, just like her twin sister’s electrocardiogram (Figure 1B). Holter monitoring showed normal sinus rhythm with a heart rate of 69 (range 48–115) beats per minute with no episodes of non-sustained ventricular tachycardia, atrial fibrillation, or significant pauses. Echocardiographic features are shown in Table 1. The resting LVOT gradient was 146 mmHg (range 60–146 mmHg; Figure 2B). Cardiac magnetic resonance imaging showed basal septal hypertrophy with mid myocardial replacement fibrosis (Figure 3G–L; Supplementary material online, Videos S4–S6). The patient is currently on metoprolol succinate 100 mg twice daily, verapamil 360 mg daily, and disopyramide phosphate 100 mg twice daily. Despite a severe resting gradient, she responded well to medical treatment, and the LVOT obstruction resolved completely. At her last follow-up visit, she had NYHA Class II symptoms.
Genetic testing that involves sequence analysis and deletion/duplication testing of 157 genes (Arrhythmia and Cardiomyopathy Comprehensive Panel, Invitae, San Francisco, CA, USA) showed a variant in the RAN guanine nucleotide release factor (RANGRF) gene, which is classified as a variant of unknown significance [c.52c > T (p. Leu18Phe)], in both patients. Neither patient required an implantable cardioverter defibrillator (ICD) owing to the absence of high-risk features of sudden cardiac death.1,2
Discussion
We present a unique set of monozygotic twins with hypertrophic cardiomyopathy. Although both patients had a morphologically identical pattern of basal septal hypertrophy, the clinical courses were different. Twin A required alcohol septal ablation due to LVOT obstruction and symptoms refractory to optimal medical treatment. However, Twin B responded very well to optimal medical treatment despite having a higher initial resting gradient than her sister. Neither patient had risk factors for sudden cardiac death or required an ICD.
A handful of cases of monozygotic twins with identical morphological appearance of hypertrophic cardiomyopathy are described in the literature, suggestive of the genetic basis of the disease. These cases are summarized in Table 2. However, identical twins with heterogeneous morphological phenotypes also have been described,12–16 suggesting the role of epigenetic factors and influences additional to the genomic code.
Table 2.
Summary of demographic and clinical features of identical twins with identical morphological phenotypes described in the literature
| Report | Age at diagnosis of first twin | Sex | Morphology | Risk factors for SCD | LVOT Gradient (mmHg) | Outcomes | ICD placement |
|---|---|---|---|---|---|---|---|
| Wylie et al.6 | 62 | Female | Asymmetrical septal hypertrophy | None | Unknown | Asymptomatic | None |
| Agirbasli et al.7 | 38 | Female | Severe asymmetrical septal hypertrophy with LVOT obstruction and SAM | None | 130 | Both responded well to beta-blockers | None |
| 170 | |||||||
| Maron et al.8 | 18 | Male | HCM confined to posterior septum | Family history of SCD due to HCM | <30 | Asymptomatic | Unknown |
| Zenovich et al.9 | 44 | Female | Mid-ventricular hypertrophy | Apical aneurysm in both twins | <30 | Asymptomatic | ICD reported in only one twin sister |
| Goh et al.10 | 62 | Male | Asymmetrical septal hypertrophy | Twin brother had SCD 2 months prior | <30 | Syncope with termination of malignant ventricular tachyarrhythmia by ICD shock | ICD in one twin brother |
| Maron et al.11 | 49 | Male | HCM confined to posterior inferior septum with LVOT obstruction | None | 85 | Both had paroxysmal atrial fibrillation and required septal myectomy with excellent response | None |
| 75 |
HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LVOT, left ventricular outflow tract obstruction; SAM, systolic anterior motion of the mitral valve; SCD, sudden cardiac death.
Our patients had morphologically identical phenotypes involving the basal septum but different clinical courses despite a similar risk profile, including similar degree of obesity, obstructive sleep apnoea with compliance with treatment, and type 2 diabetes mellitus. Twin A was refractory to optimal medical therapy and required alcohol septal ablation, whereas Twin B had an excellent response to optimal medical treatment. Similar findings also were seen in the monozygotic twin pair described by Littler4; in that case, one twin had a benign course, but the other had worsening symptoms with atrial fibrillation and congestive heart failure despite identical phenotypes.
Both twins lacked a pathogenic variant in known sarcomeric or non-sarcomeric hypertrophic cardiomyopathy–mimetic genes. A study by Harper et al.17 showed that single-nucleotide polymorphism inheritability indicated a strong polygenic influence for sarcomere-negative hypertrophic cardiomyopathy patients. It also showed that diastolic blood pressure is a modifiable risk factor in sarcomere-negative hypertrophic cardiomyopathy patients, as a 1 SD increase in diastolic blood pressure increases the risk of hypertrophic cardiomyopathy four-fold. Both twins were hypertensive but compliant with antihypertensives and with good control of both systolic and diastolic blood pressure.
Zenovich et al.9 described a pair of identical twin sisters who had identical cardiac morphology, including apical aneurysms. Implantable cardioverter defibrillator placement was reported in only one of the twins. Another interesting pair of monozygotic twins was reported by Goh et al.10 One patient survived a sudden cardiac arrest because he had had an ICD implanted a week after his twin brother’s fatal cardiac arrest 2 months prior. Our patients did not have any ventricular apical involvement and were at low risk for sudden cardiac death, so they did not require ICD placement.
Another interesting feature in our patients was that although both twins did not show marked R-wave or T-wave abnormalities in the precordial or frontal leads as is typically seen with hypertrophic cardiomyopathy, both exhibited only minor voltage criteria for left ventricular hypertrophy as described by Sokolow–Lyon, with R-wave amplitude in aVL >10 mm.
Supplementary Material
Acknowledgements
The authors thank the following from Aurora Cardiovascular and Thoracic Services: Jennifer Pfaff and Sarah Kennedy for editorial preparation of the manuscript and Brian Miller and Brian Schurrer for assistance with the figures.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written, informed consent for submission and publication of this case series including images and associated text has been obtained from the participants in line with COPE guidance. All participants were 18 years of age at the time of consent.
Funding: None declared.
Contributor Information
Muddasir Ashraf, Aurora Cardiovascular and Thoracic Services, Aurora Sinai/Aurora St. Luke’s Medical Centers, Advocate Aurora Health, 2801 W. Kinnickinnic River Parkway, Ste. 880, Milwaukee, WI 53215, USA.
Fatima Samad, Aurora Cardiovascular and Thoracic Services, Aurora Sinai/Aurora St. Luke’s Medical Centers, Advocate Aurora Health, 2801 W. Kinnickinnic River Parkway, Ste. 880, Milwaukee, WI 53215, USA.
Arshad Jahangir, Aurora Cardiovascular and Thoracic Services, Aurora Sinai/Aurora St. Luke’s Medical Centers, Advocate Aurora Health, 2801 W. Kinnickinnic River Parkway, Ste. 880, Milwaukee, WI 53215, USA.
Muhammad Fuad Jan, Aurora Cardiovascular and Thoracic Services, Aurora Sinai/Aurora St. Luke’s Medical Centers, Advocate Aurora Health, 2801 W. Kinnickinnic River Parkway, Ste. 880, Milwaukee, WI 53215, USA.
Patrycja Galazka, Aurora Cardiovascular and Thoracic Services, Aurora Sinai/Aurora St. Luke’s Medical Centers, Advocate Aurora Health, 2801 W. Kinnickinnic River Parkway, Ste. 880, Milwaukee, WI 53215, USA.
Abdul Jamil Tajik, Aurora Cardiovascular and Thoracic Services, Aurora Sinai/Aurora St. Luke’s Medical Centers, Advocate Aurora Health, 2801 W. Kinnickinnic River Parkway, Ste. 880, Milwaukee, WI 53215, USA.
Conclusion
The morphology of hypertrophic cardiomyopathy has a strong genetic basis, but epigenetic and non-genetic environmental factors may affect disease expression and progression.
Lead author biography
 Dr Muddasir Ashraf is a post-doctoral cardiovascular research fellow at Aurora St. Luke’s Medical Center in Milwaukee, WI, USA. He completed his internal medicine residency training at the University of Toledo Medical Center and his Master of Science in Clinical Investigation (MSCI) from the University of Iowa. His primary area of research interest is hypertrophic cardiomyopathy.
Dr Muddasir Ashraf is a post-doctoral cardiovascular research fellow at Aurora St. Luke’s Medical Center in Milwaukee, WI, USA. He completed his internal medicine residency training at the University of Toledo Medical Center and his Master of Science in Clinical Investigation (MSCI) from the University of Iowa. His primary area of research interest is hypertrophic cardiomyopathy.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
References
- 1. Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, et al. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:390–414. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Rowin EJ, Maron MS. Evolution of risk stratification and sudden death prevention in hypertrophic cardiomyopathy: twenty years with the implantable cardioverter-defibrillator. Heart Rhythm 2021;18:1012–1023. [DOI] [PubMed] [Google Scholar]
- 3. Walsh R, Thomson KL, Ware JS, Funke BH, Woodley J, McGuire KJ, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med 2017;19:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Littler WA. Twin studies in hypertrophic cardiomyopathy. Br Heart J 1972;34:1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–186. [DOI] [PubMed] [Google Scholar]
- 6. Wylie L, Ramage A, MacLeod DC. Hypertrophic cardiomyopathy with shared morphology in identical twins: a case report. Scott Med J 2002;47:64–65. [DOI] [PubMed] [Google Scholar]
- 7. Agirbasli M, Hamid R, Jennings HS III, Tiller GE. Situs inversus with hypertrophic cardiomyopathy in identical twins. Am J Med Genet 2000;91:327–330. [PubMed] [Google Scholar]
- 8. Maron BJ, Casey SA, Almquist AK. Hypertrophic cardiomyopathy in monozygotic twins. Circulation 2002;105:2229. [DOI] [PubMed] [Google Scholar]
- 9. Zenovich AG, Lesser JR, Hanna CA, Maron BJ. Identical twins with hypertrophic cardiomyopathy and apical aneurysm. Am J Cardiol 2006;97:1109. [DOI] [PubMed] [Google Scholar]
- 10. Goh CY, Asrar ul Haq M, Mutha V, van Gaal WJ. Synchronous cardiac arrest in monozygotic twins with hypertrophic cardiomyopathy—is sudden cardiac death genetically pre-programmed? BMC Cardiovasc Disord 2015;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maron BJ, Rowin EJ, Arkun K, Rastegar H, Larson AM, Maron MS, et al. Adult monozygotic twins with hypertrophic cardiomyopathy and identical disease expression and clinical course. Am J Cardiol 2020;127:135–138. [DOI] [PubMed] [Google Scholar]
- 12. Ko YL, Tang TK, Chen JJ, Hshieh YY, Wu CW, Lien WP. Idiopathic hypertrophic cardiomyopathy in identical twins. Morphological heterogeneity of the left ventricle. Chest 1992;102:783–785. [DOI] [PubMed] [Google Scholar]
- 13. Palka P, Lange A, Burstow DJ. Different presentation of hypertrophic cardiomyopathy in monozygotic twins. Heart 2003;89:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovacs A, Molnar A, Celeng C, Toth A, Vago H, Apor A, et al. Hypertrophic cardiomyopathy in a monozygotic twin pair: similarly different. Circ Cardiovasc Imaging 2016;9:e004794. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Li W, Han Y, Chen Y. Different clinical presentation and tissue characterization in a monozygotic twin pair with MYH7 mutation-related hypertrophic cardiomyopathy. Int Heart J 2019;60:477–481. [DOI] [PubMed] [Google Scholar]
- 16. Repetti GG, Kim Y, Pereira AC, Ingles J, Russell MW, Lakdawala NK, et al. Discordant clinical features of identical hypertrophic cardiomyopathy twins. Proc Natl Acad Sci USA 2021;118:e2021717118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harper AR, Goel A, Grace C, Thomson KL, Petersen SE, Xu X, et al. Common genetic variants and modifiable risk factors underpin hypertrophic cardiomyopathy susceptibility and expressivity. Nat Genet 2021;53:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.