Abstract
Purpose
Testicular cancer (TC) is the most common solid tumor in young adults. 95% of patients are cured, but they may experience late adverse effects (anxiety, fear of recurrence, and sexual dysfunction) with an impact on daily life. We attempted to assess Patient Reported Outcomes (PROMs), long-term sexual disorders, and difficulties in achieving fatherhood in a cohort of TC survivors, as well as their possible correlation with previous cancer treatments.
Methods
Different questionnaires, such as the Impact of Cancer (IOC) and the Body Image Scale (BIS), were used to investigate the distinct areas of the PROMs. International Index of Erectile Function (IIEF15) and the Premature Ejaculation Diagnostic Tool (PEDT) focused on sexuality and fertility. Patients were prospectively recruited between February 2020 and February 2022.
Results
144 participants completed all the questionnaires. Results showed a good QoL, a moderate fear of TC recurrence, a good satisfaction with their personal body image, low incidence of premature ejaculation and erectile dysfunction. 19.5% of patients who had a testicular implant reported general dissatisfaction. Only 18% of patients had unsuccessfully attempted fatherhood, while the majority had not yet tried, and 23.4% succeeded. A low percentage of patients used procedures assisted reproduction and adoption.
Conclusion
This trial supports the use of various questionnaires as a multifactorial tool capable of investigating all the aspects of long-term cancer survivorship. The assessment of medical and psychosocial sequelae is an essential part of patient care and is important for the development of a comprehensive care plan for TC survivors.
Keywords: testis cancer, testis cancer survivor, testis cancer PROMs, testis cancer fertility
Plain Language Summary
Why is This Study Needed?
Testicular cancer (TC) is the most common malignancy in young adult, with high cure rate: currently 95% of patients are cure and become cancer survivors. They may experience a wide range of late adverse events: to date there are a few data in literature about the severity of these issues and their impact on daily living, including sexual life and fatherhood.
What is the Key Problem/Issue/Question This Manuscript Addresses?
We tried to assess the patient reported outcomes (PROMs), long term sexual issues, difficulties in reaching fatherhood and in general global quality of life of TC survivors.
What is the Main Point of Your Study?
The assessment of the overall burden of medical and psychosocial comorbidities is an essential part of patients’ care, and it is important in order to develop comprehensive care plan of TC survivors.
What are Your Main Results and What Do They Mean?
Results showed a good QoL, with a moderate fear of TC recurrence, a good satisfaction with personal body image and low rates of premature ejaculation and erectile dysfunction. No significant differences were observed between these outcomes and chemotherapy other therapies received. The majority of the patients did not try to reach fatherhood yet while only few patients tried to reach it without success. These results support the use of various questionnaires as a multifactorial instrument that is able to investigate the most common aspects of long-term cancer survivorship.
Introduction
Testicular cancer (TC) is the most common solid tumor in young men aged between 14 and 40 years, with an estimated 25,000 new cases in Europe each year and a global incidence that has been steadily increasing.1,2
Therapeutic management consists of radical inguinal orchiectomy, cisplatin-based chemotherapy, radiotherapy, and, in selected cases, surgical resection of distant localizations. The efficacy of these treatments reflects an excellent 5-year survival rate, which is more than 95%.3
Patients who have been cured face potential short-term and long-term adverse effects. Short-term adverse effects occur during treatment and can be prevented with appropriate hydration, antiemetics, and corticosteroids.4
The most frequently reported long-term sequelae are cardiovascular diseases, peripheral neuropathy, second malignancies, hypogonadism, hearing impairment, and infertility; all these conditions can be associated with a deterioration in physical, social, and mental health, which determine the overall Quality of Life (QoL) or Patient Reported Outcomes (PROMs).4–6, Given the young age of TC patients, and long life expectancy, it is crucial to focus on late side effects.7
In the last decade, PROMs assessment tools have improved with the availability of questionnaires dedicated to the assessment of general aspects of PROMs relevant to the majority of cancer patients (EORTC QLQ-C30) and questionnaires specifically designed for TC patients (EORTC QLQ-TC26).8 The latter only applies to patients undergoing active treatment, whereas tools for TC survivors are lacking. The most utilized questionnaires are Brief Cancer Impact Assessment (BCIA), Cancer Problems in Living Scale (CPILS), Impact of Cancer (IOC, including Impact of Cancer version 2, IOCv2), Long-Term Quality of Life (LTQL), Quality of Life Cancer Survivors (QoL-CS), and Quality of Life in Adult Cancer Survivors (QLACS, SLDS), which normally investigate items concerning domains of cognitive, emotional, physical, social, spiritual, and sexual function.9 The Impact of Cancer (IOC) questionnaire is the only one that has been translated into Italian and validated. This survey consider factors such as health concerns and cancer’s adverse effect on social life, but disregards the sexual life domain. These elements identify cancer survivorship as a particular and unique condition in terms of PROMs.5
Addressing TC-specific topics is an important step toward a comprehensive assessment of symptoms and functioning in TC patients. Sexuality, body image and fertility are particularly relevant because the disease typically occurs at the peak of reproductive age, where we expect to find men pursuing stable relationships and a general desire for paternity.10,11 With respect to the latter, orchiectomy and chemotherapy can have a substantial impact on fertility.12,13 Many studies have demonstrated the presence of pre-surgery fertility issues, mainly related to the presence of common risk factors for both infertility and TC.14,15
TC patients may also eventually experience a lack of sexual desire and distorted body image, mainly related to the loss of a testicle and the newly shaped scrotum. Both these factors can contribute to a decrease in sexual activity and, therefore, the desire for fatherhood. A testicular prosthesis may help with the recovery of body image.16,17 Given all these potential impacts on fertility, all men should be counseled with regard to the insertion of a prosthesis and strongly encouraged to undertake semen cryopreservation prior to any type of treatment.18
All of these factors are largely neglected in the PROMs questionnaires and must therefore be investigated through the use of specific surveys, such as the International Index of Erectile Function (IIEF-15), the Premature Ejaculation Diagnostic Tool (PEDT), and the Body Image Scale (BIS), which address the multidimensional aspects of these issues.19–22
There is a paucity of data in the literature regarding the frequency with which TC patients attempt fatherhood after cancer treatments. These studies examine the correlation between previous cancer treatments and fatherhood.23,24 However, other non-medical issues, such as patients’ personal concerns about their own health and future, may be related to the failure to father a child but are much less investigated.25
This study primarily aims to assess the quality of life, long-term sexual issues, difficulties in achieving fatherhood, and possible links with previous cancer treatments in a cohort of long-term TC survivors. The secondary aims of the study were to assess the mode of procreation and the possible reasons why patients were unable to father a child.
Materials and Methods
Study Design and Population
This prospective observational study included patients aged 18 years or over without a maximum, able to complete five questionnaires, with histologically confirmed TC, no indication of active disease, and who had not received active treatment in the previous 2 years. Patients were recruited for this study between February 2020 and February 2022 during scheduled follow-up visits at the Istituto Oncologico Veneto. The patient’s visiting medical oncologist suggested participation in the trial and, consequently, the completion of an anonymous questionnaire. Clinical data were collected from electronic medical charts.
Questionnaires
We designed a specific paternity questionnaire (PQ) to evaluate and assess paternity status and social conditions. In addition, we used validated (also in Italian) questionnaires to investigate the QoL and sexual life of patients (Table 1). All the questionnaires were given in Italian.
Table 1.
Domains That Were Evaluated and Assessed in the Questionnaires
| Questionnaire | Domains | Score | Evaluation |
|---|---|---|---|
| IOC | 4 positive domains: Altruism/Empathy, Health Awareness, Meaning of cancer, and Positive Self-Evaluation 4 negative domains: Appearance Concerns, Body Change Concerns, Life Interferences, and Worry | 1–5 points for each question | The mean of the scores of domain-specific questions, followed by the mean of the positive and negative domains |
| IIEF15 | 4 domains of male sexual function: Erectile Function, Orgasmic Function, Sexual Desire, and Intercourse Satisfaction | 0–5 points for each question | The sum of the scores: normal if >25 |
| PEDT | 5 questions that examine the possible alteration of semen release | 0–4 points for each question | The sum of the scores: normal if <9 |
| BIS | 10 domains related to physical appearance and any changes potentially resulting from the disease or treatment | 0–4 points for each question | The sum of the scores: normality interval not determined |
| PQ | 4 domains: Achievement of Fatherhood before/after Treatment, Job, Relationship Status, Use of a Testicular Prosthesis and associated Patient Satisfaction | Yes/No | Descriptive evaluation |
PQ is just a simple questionnaire with multiple choice and open questions created to collect the data about social conditions, in particular relationship and fatherhood. In the questionnaire, patients were asked whether they had had children prior to diagnosis and whether they had fathered a child after treatment. We also investigated the means through which paternity was attained or the main reasons why it did not occur. Patients were asked if they had a stable job and a lasting relationship. Finally, patients were asked to report possible erection and fertility disorders before and after diagnosis, access to cryopreservation and fertility counseling as well as the use of a testicular prosthesis and their level of satisfaction with it (the questionnaire is available in English in Supplementary Material).
The Impact of Cancer scale (IOC). This is a special instrument specifically created, psychometrically tested and validated for long term cancer survivors. These people has some concerns, like work ability and fertility, that are different from those who experienced cancer more recently.26 This tool covers 8 different domains in which answers, with a score from 1 to 5, were grouped and analyzed: altruism/empathy, health awareness, meaning of cancer, positive self-evaluation, appearance concerns, body change concerns, life interferences, and worry. Answers were processed to produce results comparable to V2.0 a refinement of the original 81-item IOC scale.27 The positive impact summary score was then calculated by taking the mean of the scores of the first four items. The negative impact summary score is derived using the same methodology utilized for the last four items.
The International Index of Erectile Function (IIEF15) is a brief validated questionnaire consisting of a total of 15 questions. A score of 0–5 is awarded to each of the 15 questions that examine the 4 main domains of male sexual function: erectile function, orgasmic function, sexual desire, and intercourse satisfaction.19,28 We evaluate in particular the erectile function considering only the results of the five items examining this area.
The Premature Ejaculation Diagnostic Tool (PEDT) is a validated questionnaire able to diagnose presence or absence of premature ejaculation (PE) using DSM-IV-TR classification criteria. It consists of a total of 5 items. A score of 0–4 is given to each of the 5 questions that examine the possible alteration of semen release.20
The Body Image Scale (BIS) is a full validated 10-item self-rating scale developed to evaluate changes in the body image of cancer patients. It focuses on how cancer patients feel about their appearance and on changes in appearance due to cancer and/or treatment. A score of 0–4 is given to each of the questions and higher BIS score represents poorer body image.21,29
Statistical Analysis and Ethical Consideration
Key metrics were summarized by means of descriptive statistics (Chi-square, Fishers’ exact test, t-Student, Wilcoxon test, and logistic regression analysis). Data were analysed with “R” v4.0.5. The study was approved by the Ethics Committee of the Istituto Oncologico Veneto prior to the first patient’s enrolment. The study was conducted in accordance with the declaration of Helsinki.
Results
General
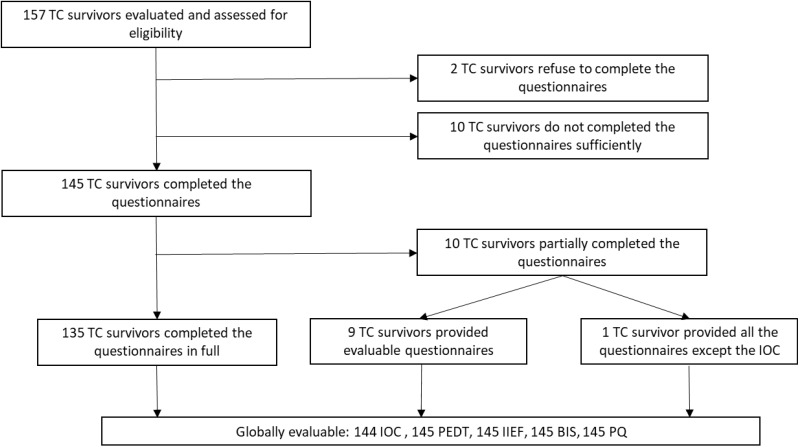
From February 2020 to February 2022, 157 TC survivors who met the inclusion criteria were asked to fill out the various questionnaires. Initially, all participants accepted, with the exception of 2 patients. Completing all questionnaires took approximately 30–45 minutes. Most patients filled out the questionnaires immediately but some preferred to fill it out at home and return it. Finally, 10 patients failed to complete or return the questionnaires, 135 completed all the questionnaires in full, and 10 patients completed the questionnaires in part. Of these, all but one of the questionnaires were sufficiently filled out to enable evaluation (Figure 1). The average age of the patients at the time of questionnaire completion was 41.1 years, while the average age at the time of diagnosis was 33.3 years. The median amount of time between diagnosis and questionnaire completion is approximately 6.0 years. In terms of disease type: 54.7% of patients had seminoma, 61.1% had stage I disease, and 72.6% received chemotherapy but 54.4% of these received it for adjuvant purposes. 20.4% required further treatment, mainly retroperitoneal lymphadenectomy. Nine patients had contralateral recurrence. The majority of patients were either married or cohabiting. Demographic and clinical characteristics are summarized in Table 2.
Figure 1.
Participants’ flow through the trial: from the total number of patients evaluable and eligible to the total number of questionnaires evaluable for each type of questionnaire.
Table 2.
Patients’ Demographic and Clinical Characteristics
| Median age at diagnosis | 33.3 years (IQR 29.0–39.9) |
| Median current age | 41.1 years (IQR 34.7–46.6) |
| Median time from diagnosis | 6.0 years (IQR 3.8–8.6) |
| Histology: | |
| Seminoma | 54.7% |
| Non-Seminoma | 45.3% |
| Stage: | |
| Localized | 61.1% |
| Lymph Nodes | 10.2% |
| Advanced | 28.7% |
| Tx: Adjuvant | |
| CT | 39.4% |
| 3–4 BEP | 33.1% |
| RPLND | 12.7% |
| RT | 4.4% |
| At least 2 Tx | 20.4% |
| Education: | |
| University | 16.6% |
| High School | 49.0% |
| Primary School | 4.5% |
| Unknown | 29.9% |
| Civil Status: | |
| Married or cohabiting | 75.8% |
| Widower | 0% |
| Divorced or separated | 1.9% |
| Never married or cohabited | 22.3% |
Abbreviations: Tx, therapy; CT, chemotherapy; BEP, Bleomycin, Etoposide, Cisplatin; RPLND, retroperitoneal lymph node dissection; RT, radiotherapy.
PROMs According to the IOC
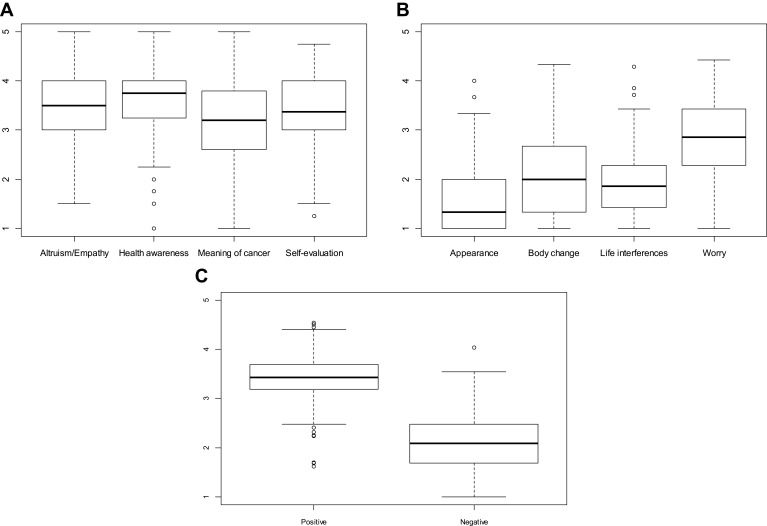
According to the IOC, TC survivors had significantly higher “Positive Summary Scale” scores than “Negative Summary Scale” scores (median 3.43, IQR 3.19–3.69 vs 2.08 IQR 1.69–2.48, p < 0.0001). The highest negative IOC scores were in the “worry” category (median 2.86 IQR 2.29–3.43), while the “Appearance”, “Body Change”, and “Life Interference” categories were less significant (median 1.33, IQR 1.00–2.00; 2.00 IQR 1.33–2.67; 1.86 IQR 1.43–2.29). With regard to positive IOC scores, “Altruism/Empathy”, “Health Awareness” “Meaning of Cancer” and “Positive Self-Evaluation” had high and similar scores (median 3.50, IQR 3.00–4.0; 3.75 IQR 3.25–4.00; 3.20 IQR 2.60–3.80; 3.76 IQR 3.00–4.00) (Figure 2). There was no correlation between the means of the two summary scales in relation to treatments received, social status and stage of the disease. Moreover, there were no correlations between positive or negative scores and age at diagnosis.
Figure 2.
Boxplots for positive scores (A) and negative scores (B) with the median scores and IQR for each category. A comparison of the median scores and IQR of the positive summary scale and the negative summary scale (C).
Paternity and Relationships
Forty patients (27.6%) had children prior to their diagnosis. There was a significant difference between the median age of patients with and without children at diagnosis (43.0 vs 31.0 years, p < 0.0001). After treatment, 34 patients had at least one child: 24 of whom were conceived naturally, 5 via assisted reproductive technology (ART), and 5 via adoption. Eleven patients resorted to ART, of whom 6 were unsuccessful. 129 (89.0%) patients had stable relationships post-diagnosis. Of the 111 childless patients: 44 (40.0%) claimed they were not ready for children, 21 (18.9%) had tried unsuccessfully, 22 (20.0%) stated they had no partner, 9 (8.4%) said their partner did not want children, 8 (7.4%) did not have the financial means, and 6 (5.3%) preferred not to resort to ART. The median age of patients who claimed they were not ready for children differed significantly from the mean age of patients who cited other reasons (28.0 years vs 33.0 years, p = 0.0017).
Sexual Dysfunction and Fertility
With regard to fertility, only 10 (6.9%) of patients were aware of issues before contracting the condition, whereas 43 (29.6%) reported concerns at the time of the test. Ninety-eight (67.6%) patients underwent a spermiogram after treatment: azoospermia and oligospermia were diagnosed in 11 and 6 cases, respectively. Cryopreservation and fertility counselling were proposed to 135 (93%) patients but 31 (23%) of them refused. Only 8 patients reported issues with sexual function prior to diagnosis. Low rates of dysfunction were found after therapy. The PEDT test showed that 18 (12.4%) patients had premature ejaculation issues, and that this problem was probable in 8 (5.5%) patients. The IIEF test confirmed a low rate of erectile dysfunction: 23 (15.9%) had severe dysfunction (although in the case of 23 of these 24 patients, the results were due to not having been sexually active in the previous 4 weeks) and 16 (11.0%) had mild or moderate dysfunction. No statistically significant differences were observed between the PEDT test and chemotherapy (yes vs no), other therapies (yes vs no), age (< 40 vs > 40), or social status (married or cohabiting vs others). The same result was recorded for the IIEF test.
Body Image Perception and Testicular Implants Satisfaction
The median BIS score was 3, and 106 (73.1%) patients scored < 8. Only 16 (11.0%) totaled > 14 and it was not possible to discern variations based on chemotherapy treatment (yes vs no), age, or social status. The BIS test varied significantly between patients who received other treatments (mainly RPLND) and those who received no treatment at all or only chemotherapy (mean 7.6 vs 4.9, p = 0.01). The insertion of a prosthesis was recommended to 135 (93.1%) patients, of whom 82 (60.7%) accepted. No complications were reported, but 16 (19.5%) were dissatisfied with the outcome and 24 (29.3%) would not have it repositioned. In contrast, only 5 (9.4%) patients who initially rejected the prosthesis would now have it implanted.
Discussion
Testicular cancer is the most common solid tumor in young adults.1,2 Due to the high treatment efficacy and cure of TC patients, who are usually young, late effects and long-term sequelae are of particular concern. Therefore, minimizing long-term toxicity for survivors is an important goal.3
Quality of life, fertility, and sexual functioning are other key issues for these patients. Data concerning these issues is currently limited. Different questionnaires were utilized in our study in order to capture the multidimensional aspects of PROMs pertinent to TC survivors. The IOC questionnaire is the only one that has been translated into Italian and also validated in patients with TC. However, it does not include sexual domains, which is an important area in young adults’ QoL and we, therefore, decided to use two specific questionnaires (IIEF15 and PEDT) to assess patients’ sexual functioning.5,8,9
In our population, with a median 6-year interval between treatment and questionnaire completion, the positive summary scale was significantly higher than the negative one. This means that these patients were able to retain a satisfactory global self-reported QoL in accordance with other data in the literature for cancer patients in general, including TC survivors.9,30 Concerning the negative domains, the “worry” category is significantly higher than the rest. It demonstrates that the fear of cancer recurrence continues to affect TC survivors several years after treatment. A similar conclusion may be drawn from other trials in which anxiety was found to be the most prevalent concern for TC survivors.4,30,31 This finding is even stronger in our cohort than in previous studies, but it may be attributable to the shorter period between treatments and questionnaire completion (6 versus 11.9 years reported in the work by Van Leeuwen et al).30
The body image assessment results indicated a good self-perception. The results of the BIS confirmed what had been found in these specific IOC domains. In fact, the specific test for body image (BIS) reports a very low total score, which is in line with that reported in the trial by Hopwood et al, in which the average score for the group of patients with testicular cancer was 7 only two years after diagnosis.21 In our trial, we also found a correlation between higher scores and prior treatments besides chemotherapy alone. This result was partially expected because retroperitoneal lymph node dissection, the most common alternative treatment, is highly invasive surgery with a consistent risk of retrograde ejaculation and other long-term complications that can justify a poorer body image perception.
Another observation arose from the items specifically designed to assess personal satisfaction with testicular prosthesis. Despite the near absence of relevant complications associated with the prosthesis, we found a higher global rate of regret of 29.3% than that reported in the literature.32 The majority of these patients were dissatisfied with the prosthesis’ consistency or size, yet some of the patients who would have preferred not to reposition the prosthesis expressed their satisfaction with it. It is not clear why the afterthought rate is so high in our sample, but it is evident that the patient’s willingness to have a prosthesis may change after surgery.
Fertility and sexual function are difficult items to measure. We observed that only about 12% of patients suffered from premature ejaculation and erectile dysfunction, and that there were no differences between men aged < 40 or > 40 years. There is a paucity of data in the literature, however, Verze et al demonstrate that the estimated prevalence of premature ejaculation in a healthy general population is approximately 18.0%,33 not so different compared to our cohort. However, sexual dysfunction may be a serious concern for long-term TC survivors, and it is known that treatment with chemotherapy plus radiotherapy is associated with disappointment and decreased sexual desire11,34 with the exception of erectile function which usually improve after one year from treatments.35 In our cohort, we did not find any association between IIEF and PEDT and previous treatment received, but the great majority of our patients received no treatment or just adjuvant chemotherapy. Two limitations must be considered in the interpretation of these data. The first is that the median time elapsed from treatments is about 6 years, and this long interval may be sufficient to allow the recovery of erectile function, and also sexual function in general, for most TC survivors as yet reported in literature by Capogrosso et al.36 The second is that the patients did not complete IIEF and PEDT questionnaires at the moment of the diagnosis so we were unable to determine if sexual impairment, although present in few TC survivors, is connected to the therapies or if the TC experience hinders the sexual desire. Finally, previous studies have indicated that adjuvant therapies do not affect sperm count.37 In our cohort, only 98 patients underwent sperm count after TC treatment. Although these data are self-reported and the time when the test was performed is not known, it is interesting to report that 11 were diagnosed with azoospermia but only 6 of them had previously received active oncological treatment while the other received only the orchifunicolectomy.
These data strongly suggest that sexual functioning and fatherhood are far more intricate issues that may be significantly influenced by other factors, such as social ones. In fact, after 2 years of treatment for TC, only 18% of patients attempted fatherhood without success. Most patients stated that they had no will to try, no stable partner, or their partner did not want to have children. Others reported that the search for paternity was hampered by a lack of financial resources or a refusal to undergo medically-assisted procreation. The majority of the patients performed sperm cryopreservation but only 11 out of 145 patients used it and resorted to ART, with 5 out of 11 cases having a successful outcome. Adoption provided another alternative to natural conception, but only 5 of our patients opted for it. These results demonstrated that alternative solutions to natural conception are rarely considered when compared to other data.38 This low rate could be attributed to the complicated nature of our country’s legislation, which makes access to these methods difficult and time-consuming, thereby discouraging less-determined couples.
To our knowledge, this is one of a few trials that investigate how the impediments to fatherhood were mostly social and economic and not only biological. However many limitations exists. The sample size is fairly low to make definitive conclusions, in particular for the relation between treatments received, stage of disease and QoL or fertility, but testis cancer is a rare disease and it is not easy to recruit a large number of survivors after many years from the diagnosis or to conduct multi-institutional trial. Moreover, the trial was conducted prospectively and almost all patients filled in the questionnaires, however, a selection bias could exist considering that those who follow a long follow-up in a cancer centre may differ from those who follow it in other centres or do not follow it at all. Moreover the majority of patients suspend the follow up after ten years, so it is probable that we lost a proportion of patients with a longer disease free interval and a better QoL and social condition. Another strong limitations is the fact that fatherhood is a really complex concept, and no validated questionnaires on this topic are available. We decided to create a questionnaire in which patients could report the problems that prevented them from having children, but it is also possible that we may not have covered all the aspects of their life. Similarly, PROMs are another multifaceted concept. Many questionnaires exist, but none are truly complete and capable of investigating all the domains that constitute QoL. Furthermore, the patients in our trial did not complete the questionnaires prior to TC treatments, making it impossible to compare QoL before and after the diagnosis of TC and to determine whether QoL has indeed deteriorated as a result of the treatments received.
On the other hand, our trial confirms the importance of gradually shifting the focus during follow-up of TC survivors from detection of tumor recurrence to identification of the treatment’s late effects and the promotion of a healthy lifestyle for improved wellbeing and the prevention of cardiovascular disease and secondary cancers, which are the most serious long-term toxicities.39,40 At the same time, it is fundamental to address the psychological and social aspects of the life of TC survivors. A survivorship care plan, in addition to routine oncological follow-up, can be implemented to provide adequate and specialized support, prevent the deterioration of Quality of Life, and identify the need for psychological interventions at an early stage.4,7,22,30,31,40
Conclusions
In our cohort of TC survivors, PROMs are favorable and low-grade sexual dysfunction is present. The majority of patients have not yet expressed the desire for paternity due to personal, couple-related, social, or economic reasons.
PROMs are a multifactorial issue, and various questionnaires should be used to investigate all the medical and non-medical aspects. Patients should be informed and educated in relation to late adverse events. We recommend that every cancer patient should receive an informative end-of-treatment summary report with a survivorship care plan.
Acknowledgment
This study was supported by the Benetton Rugby Team: we wish to take this opportunity to thank them for their kind and effective support. The abstract of this paper was presented at the 2021 Genitourinary Cancers Symposium as a Poster Presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Journal of Clinical Oncology 39, no. 6_suppl (February 20, 2021) 380-380.
URL: https://ascopubs.org/doi/abs/10.1200/JCO.2021.39.6_suppl.380
Funding Statement
This study was funded by Ricerca Corrente 2022, Ministero della Salute Italiano, for publication fee.
Availability of Supporting Data
All data presented in this paper are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
The study was approved by the Ethics Committee of the Istituto Oncologico Veneto prior to the first patient’s enrolment. All the patients signed an Informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Francesco Pierantoni reports personal fees from AstraZeneca, travel fees from Janssen, outside the submitted work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Znaor A, Skakkebæk NE, Rajpert‐De Meyts E, et al. Testicular cancer incidence predictions in Europe 2010–2035: a rising burden despite population ageing. Int J Cancer. 2020;147(3):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith ZL, Werntz RP, Eggener SE. Testicular cancer: epidemiology, diagnosis, and management. Med Clin North Am. 2018;102(2):251–264. doi: 10.1016/j.mcna.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Chovanec M, Lauritsen J, Bandak M, et al. Late adverse effects and quality of life in survivors of testicular germ cell tumour. Nat Rev Urol. 2021;18(4):227–245. doi: 10.1038/s41585-021-00440-w [DOI] [PubMed] [Google Scholar]
- 5.Muzzatti B, Gipponi K, Flaiban C, et al. The impact of cancer: an Italian descriptive study involving 500 long‐term cancer survivors. Eur J Cancer Care. 2019;28(3):e13007. doi: 10.1111/ecc.13007 [DOI] [PubMed] [Google Scholar]
- 6.Smith AB, Rutherford C, Butow P, et al. A systematic review of quantitative observational studies investigating psychological distress in testicular cancer survivors. Psycho Oncol. 2018;27(4):1129–1137. doi: 10.1002/pon.4596 [DOI] [PubMed] [Google Scholar]
- 7.Fung C, Dinh PC, Fossa SD, Travis LB. Testicular cancer survivorship. J Natl Compr Canc Netw. 2019;17(12):1557–1568. doi: 10.6004/jnccn.2019.7369 [DOI] [PubMed] [Google Scholar]
- 8.Holzner B, Efficace F, Basso U, et al. Cross-cultural development of an EORTC questionnaire to assess health-related quality of life in patients with testicular cancer: the EORTC QLQ-TC26. Qual Life Res. 2013;22(2):369–378. doi: 10.1007/s11136-012-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreira H, Williams R, Dempsey H, Stanway S, Smeeth L, Bhaskaran K. Quality of life and mental health in breast cancer survivors compared with non-cancer controls: a study of patient-reported outcomes in the United Kingdom. J Cancer Surviv. 2021;15(4):564–575. doi: 10.1007/s11764-020-00950-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brydøy M, Fosså SD, Klepp O, et al. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97(21):1580–1588. doi: 10.1093/jnci/dji339 [DOI] [PubMed] [Google Scholar]
- 11.Chovanec M, Vasilkova L, Petrikova L, et al. Long-term sexual functioning in germ-cell tumor survivors. BMC Cancer. 2020;20(1):1–10. doi: 10.1186/s12885-020-07301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril. 2013;100(3):673–680. doi: 10.1016/j.fertnstert.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 13.Petersen PM, Skakkebaek NE, Rorth M, Giwercman A. Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol. 1999;161(3):822–826. doi: 10.1016/S0022-5347(01)61781-6 [DOI] [PubMed] [Google Scholar]
- 14.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016;96(1):55–97. doi: 10.1152/physrev.00017.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169(4):351–356. doi: 10.1001/archinternmed.2008.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Incrocci L, Bosch JL, Slob AK. Testicular prostheses: body image and sexual functioning. BJU Int. 1999;84(9):1043–1045. doi: 10.1046/j.1464-410x.1999.00347.x [DOI] [PubMed] [Google Scholar]
- 17.Chapple A, McPherson A. The decision to have a prosthesis: a qualitative study of men with testicular cancer. Psycho‐Oncology. 2004;13(9):654–664. doi: 10.1002/pon.787 [DOI] [PubMed] [Google Scholar]
- 18.Zilberman D, Winkler H, Kleinmann N, Ramon J, Mor Y. Testicular prosthesis insertion following testicular loss or atrophy during early childhood–technical aspects and evaluation of patient satisfaction. J Pediat Urol. 2007;3(6):461–465. doi: 10.1016/j.jpurol.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the international index of erectile function. Urology. 1999;54(2):346–351. doi: 10.1016/S0090-4295(99)00099-0 [DOI] [PubMed] [Google Scholar]
- 20.Symonds T, Perelman MA, Althof S, et al. Development and validation of a premature ejaculation diagnostic tool. Eur Urol. 2007;52(2):565–573. doi: 10.1016/j.eururo.2007.01.028 [DOI] [PubMed] [Google Scholar]
- 21.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37(2):189–197. doi: 10.1016/S0959-8049(00)00353-1 [DOI] [PubMed] [Google Scholar]
- 22.Honecker F, Aparicio J, Berney D, et al. ESMO consensus conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29(8):1658–1686. doi: 10.1093/annonc/mdy217 [DOI] [PubMed] [Google Scholar]
- 23.Brydøy M, Fosså SD, Klepp O, et al. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin-based chemotherapy. Eur Urol. 2010;58(1):134–141. doi: 10.1016/j.eururo.2010.03.041 [DOI] [PubMed] [Google Scholar]
- 24.Spermon JR, Kiemeney LA, Meuleman EJ, Ramos L, Wetzels AM, Witjes JA. Fertility in men with testicular germ cell tumors. Fertil Steril. 2003;79:1543–1549. doi: 10.1016/S0015-0282(03)00335-2 [DOI] [PubMed] [Google Scholar]
- 25.Jonker‐Pool G, Basten JPV, Hoekstra HJ, et al. Sexual functioning after treatment for testicular cancer—review and meta-analysis of 36 empirical studies between 1975–2000. Arch Sex Behav. 2001;30(1):55–74. doi: 10.1023/A:1026468707362 [DOI] [PubMed] [Google Scholar]
- 26.Zebrack BJ, Ganz PA, Bernaards CA, Petersen L, Abraham L. Assessing the impact of cancer: development of a new instrument for long-term survivors. Psycho-Oncology. 2006;15:407–421. doi: 10.1002/pon.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crespi CM, Ganz PA, Petersen L, Smith SK. A procedure for obtaining impact of cancer version 2 scores using version 1 responses. Qual Life Res. 2013;22(1):103–109. doi: 10.1007/s11136-012-0127-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 29.Annunziata MA, Muzzatti B, Bomben F, Flaiban C, Piccinin M, Solfrini V. A contribution to the validation of the Italian version of the Body Image Scale (BIS). BMC Cancer. 2018;18(1):1–5. doi: 10.1186/s12885-018-5143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Leeuwen M, Kieffer JM, Efficace F, et al. International evaluation of the psychometrics of health-related quality of life questionnaires for use among long-term survivors of testicular and prostate cancer. Health Qual Life Outcomes. 2017;15(1):1–14. doi: 10.1186/s12955-017-0670-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rincones O, Smith A, Naher S, Mercieca-Bebber R, Stockler M. An updated systematic review of quantitative studies assessing anxiety, depression, fear of cancer recurrence or psychological distress in testicular cancer survivors. Cancer Manag Res. 2021;13:3803–3816. doi: 10.2147/CMAR.S198039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieckmann KP, Anheuser P, Schmidt S, et al. Testicular prostheses in patients with testicular cancer: acceptance rate and patient satisfaction. BMC Urol. 2015;15(1):1–7. doi: 10.1186/s12894-015-0010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verze P, Arcaniolo D, Palmieri A, et al. Premature ejaculation among Italian men: prevalence and clinical correlates from an observational, non-interventional, cross-sectional, epidemiological study (IPER). Sex Med. 2018;6(3):193–202. doi: 10.1016/j.esxm.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandak M, Lauritsen J, Johansen C, et al. Sexual function in a nationwide cohort of 2, 260 survivors of testicular cancer after 17 years of followup. J Urol. 2018;200(4):794. doi: 10.1016/j.juro.2018.04.077 [DOI] [PubMed] [Google Scholar]
- 35.Pallotti F, Petrozzi A, Cargnelutti F, et al. Long-term follow up of the erectile function of testicular cancer survivors. Front Endocrinol. 2019;10:196. doi: 10.3389/fendo.2019.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capogrosso P, Boeri L, Ferrari M, et al. Long-term recovery of normal sexual function in testicular cancer survivors. Asian J Androl. 2016;18(1):85–89. doi: 10.4103/1008-682X.149180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weibring K, Nord C, Ståhl O, et al. Sperm count in Swedish clinical stage I testicular cancer patients following adjuvant treatment. Ann Oncol. 2019;30(4):604–611. doi: 10.1093/annonc/mdz017 [DOI] [PubMed] [Google Scholar]
- 38.Paoli D, Pallotti F, Lenzi A, Lombardo F. Fatherhood and sperm DNA damage in testicular cancer patients. Front Endocrinol. 2018;9:506. doi: 10.3389/fendo.2018.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haugnes HS, Stephenson AJ, Feldman DR. Beyond stage I germ cell tumors: current status regarding treatment and long-term toxicities. Am Soc Clin Oncol Educ Book. 2014;34:e180–e190. doi: 10.14694/EdBook_AM.2014.34.e180 [DOI] [PubMed] [Google Scholar]
- 40.Banna GL, Nicolai N, Palmieri G, et al. Recommendations for surveillance and follow-up of men with testicular germ cell tumors: a multidisciplinary consensus conference by the Italian germ cell cancer group and the Associazione Italiana di oncologia medica. Crit Rev Oncol Hematol. 2019;137:154–164. doi: 10.1016/j.critrevonc.2019.03.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this paper are available from the corresponding author on reasonable request.