Abstract
Biofouling on the surface of implanted medical devices and biosensors severely hinders device functionality and drastically shortens device lifetime. Poly(ethylene glycol) and zwitterionic polymers are currently considered “gold standard” device coatings to reduce biofouling. To discover novel anti-biofouling materials, we created a combinatorial library of polyacrylamide-based copolymer hydrogels and screened their ability to prevent fouling from serum and platelet-rich plasma in a high-throughput parallel assay. We found certain non-intuitive copolymer compositions exhibit superior anti-biofouling properties over current gold standard materials, and employed machine learning to identify key molecular features underpinning their performance. For validation, we coated the surfaces of electrochemical biosensors with our hydrogels and evaluated their anti-biofouling performance in vitro and in vivo in rodent models. Our copolymer hydrogels preserved device function and enabled continuous measurements of a small-molecule drug in vivo better than gold standard coatings. The novel methodology we describe enables the discovery of anti-biofouling materials that can extend the lifetime of real-time in vivo sensing devices.
Keywords: anti-fouling, polymers, biosensors, devices, hydrogels
Graphical Abstract
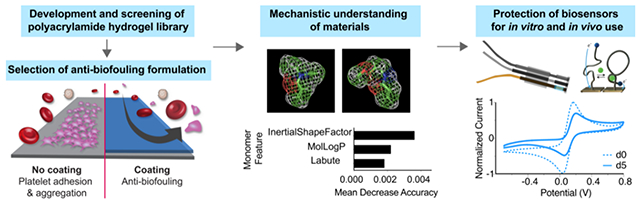
We identify hydrogel formulations that prevent protein and platelet adhesion in assays where “gold-standard” polymers exhibit significant platelet adhesion. By employing machine learning techniques to analyze molecular features of the polyacrylamide-based co-polymers, we identify features giving rise to this anti-biofouling performance. Coating electrochemical devices with our top-performing polyacrylamide-based hydrogels extends lifetime when implanted intravenously and enables in vivo real-time monitoring of analytes.
1. Introduction
Medical devices and biosensors improve the quality of life and have the potential to extend the lifespan of millions of people worldwide.1 In particular, technological advances in biosensing devices have enabled the real-time continuous monitoring of analytes and the capabilities for personalized medicine. Biosensors, which detect the presence or concentration of analytes, biomarkers, and other biological molecules, significantly reduce healthcare practitioner and patient burden. As many medication regimens for treatments to diseases such as diabetes and cancer rely on blood sample measurements, there is a growing desire from medical professionals and patients to monitor health markers and analytes continuously. There has been a shift to thus expand the capabilities of single-use biosensors, such as a pregnancy test or blood glucose finger pricks and driving towards real-time, continuous monitoring. The impact of continuous monitoring would be invaluable for dosing and tailoring medicine, thus enabling maximum benefit to patients and offering the potential to provide insights into the health of individuals. There is a great desire to develop both sensors and immunoassays that would allow for the continuous monitoring biomolecules and achieve proper therapeutic windows for diseases.2,3
Unfortunately, continuous monitoring has remained challenging due to poor sensor signal and limited lifetimes of sensors. After implantation in the body of a patient, biofouling—the non-specific adsorption of biomolecules—often occludes these devices and contributes to a reduction in their performance and lifetime. For devices that contact blood, the adhesion and aggregation of platelets from flowing blood significantly shorten functional device lifetimes.4,5 Once these devices foul, high-risk and costly invasive surgeries are often required to replace them, greatly increasing patient and hospital burden.
To address these challenges and bridge the gap between devices and long-last implants, devices are often coated with a soft material to mitigate biofouling. “Active” coatings such as drug-eluting6 or lubricant-infused polymer materials7 can be used to reduce adhesion and activation of platelets. While these approaches show promise in some applications, they offer only short-term benefits on account of the eventual depletion of the active molecules from the coating material,8–10 leading to major bleeding in patients near the end of the functional device lifetime.11 In contrast, “passive” coating strategies that modify the chemistry at the interface of the devices and bodily fluids are scalable, tunable, and inexpensive. The “gold standard” materials used for passive anti-biofouling coatings are poly(ethylene glycol) (PEG)12 and its derivatives,13–15 which form a tight hydration layer through hydrogen bonding with water that is hypothesized to contribute to anti-biofouling properties.16 Nonetheless, PEG has several critical drawbacks, including degradation through hydrolysis and auto-oxidation leading to the production of reactive oxygen species17–20 and reduced anti-fouling performance over time.21 In recent years, many alternatives to PEG have been developed,22 including zwitterionic polymers,23,24 fluoro- and acrylate-based polymers,25–27 and polyglycidols.28 Poly-zwitterionic materials in particular have shown promise as exceptional anti-biofouling coatings, though these materials may still face reduced stability in long-term applications due to the presence of hydrolysis-prone ester bonds.29–31 There is thus a critical need to develop materials that achieve superior stability and anti-biofouling performance relative to existing materials. Novel approaches that make use of high-performance assays to rigorously test the function of materials are needed to advance the discovery of such next-generation anti-biofouling materials, enabling the development of coatings that improve the performance of medical devices.
In this study, we used a high-throughput screening approach to evaluate biofouling of a library of 172 combinatorial copolymer hydrogel materials assembled from 11 distinct acrylamide-based monomers (Figure 1). Our results demonstrate that a subset of these materials exhibit remarkable anti-biofouling function compared to existing gold standard coatings. These polyacrylamide hydrogels exhibit tunable mechanical properties, and the simplicity of their synthesis makes them highly adaptable for biosensor coatings. While combinatorial screening approaches have been employed previously for materials discovery efforts32–34 aimed at mitigating marine or bacterial fouling,35–38 the foreign body response,34,39 or cell adhesion,40,41 we screen for the prevention of platelet adhesion, which is a critical initial process for biofouling in blood. We have rigorously assessed the anti-fouling behavior of our library of copolymer hydrogels using a high concentration of serum proteins and platelet-rich plasma over prolonged timeframes. Moreover, we have used machine learning techniques to elucidate the molecular features of these combinatorial copolymer hydrogels that give rise to their observed biofouling performance. We show that our top-performing copolymer hydrogel is superior to current gold standard materials in terms of enhancing the performance and lifetime of electrochemical biosensors in vitro and in vivo in multiple rodent models.
Figure 1. Polyacrylamide hydrogels as anti-biofouling device coatings.
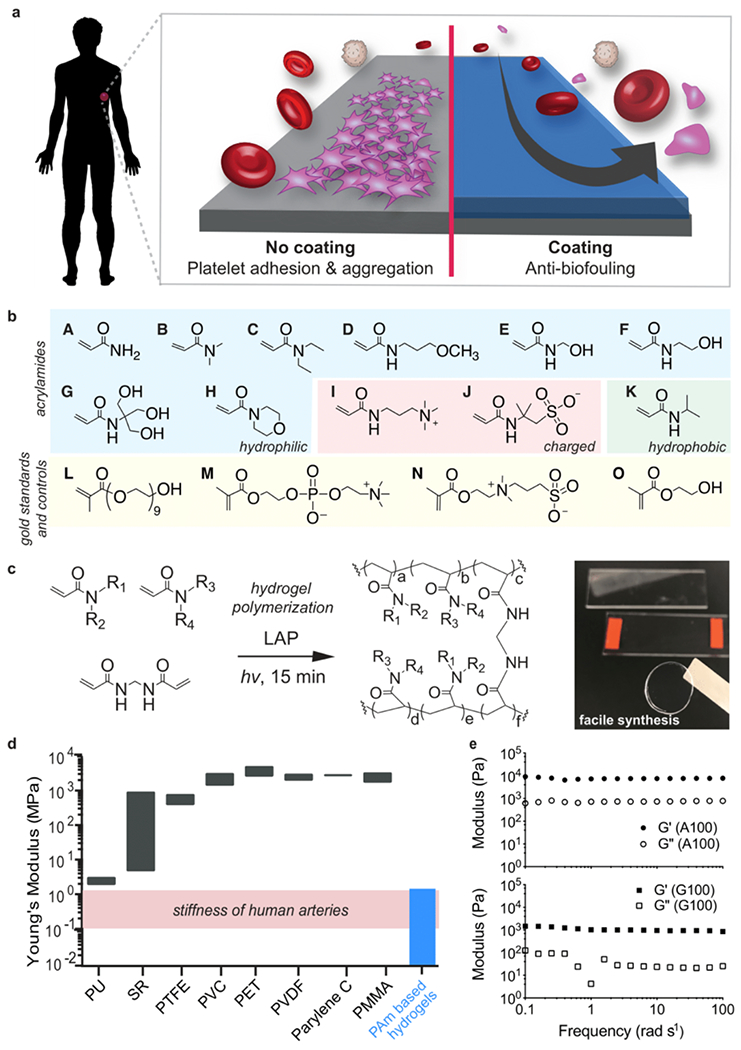
a. Coating substrates with anti-biofouling hydrogels can mitigate platelet adhesion and aggregation. b. Monomers employed for combinatorial hydrogel synthesis: acrylamide (A), dimethylacrylamide (B), diethylacrylamide (C), (3-methoxypropyl)acrylamide (D), hydroxymethylacrylamide (E), hydroxyethylacrylamide (F), [tris(hydroxymethyl)methyl]acrylamide (G), acryloylmorpholine (H), (acrylamidopropyl)trimethylammonium (I), 2-acrylamido-2-methyl-propane sulfonic acid (J), N-isopropylacrylamide (K), poly(ethylene glycol) methacrylate (L), 2-methacryloyloxyethyl phosphorylcholine (M), [2-(methacryloyloxy)ethyl]dimethyl(3-sulfopropyl)ammonium (N), 2-hydroxyethyl methacrylate (HEMA; O). c. Photopolymerization (λ = 350 nm) of polyacrylamide hydrogels using lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as a radical photoinitiator. d. Range of Young’s moduli for commercial polymers: polyurethane (PU), silicone rubbers (SR), polytetrafluoroethylene (PTFE), polyvinyl chloride (PVC), polyethylene terephthalate (PET), parylene C, and poly(methyl methacrylate) (PMMA). In this study, polyacrylamide copolymer hydrogels were fabricated with elastic moduli similar to human arteries.44,45 e. Oscillatory shear rheology of hydrogels formed with low (A100; top) and high (G100; bottom) molecular weight monomers indicates consistency in shear moduli amongst the hydrogels, regardless of composition.
2. Results
2.1. Design of a Combinatorial Library of Polyacrylamide Hydrogels for Anti-biofouling
Here we first sought to investigate the ability of a large library of chemically distinct polyacrylamide copolymer hydrogels to mitigate blood protein and platelet adhesion (Figure 1a). Several acrylamide-derived monomers have been widely utilized in biological settings (e.g., SDS-PAGE gels) and in biomedical applications (e.g., contact lenses, fillers, and drug delivery materials) and have previously demonstrated potential for use as inert materials in the human body.42,43 To maximize the scope of our evaluation of polyacrylamide materials, we selected 11 commercially-available acrylamide-derived monomers and fabricated a library of 172 polyacrylamide copolymer hydrogels comprising unique binary combinatorial mixtures (100:0, 75:25, 50:50, 25:75) of these monomers formulated at 20 wt% monomer (Figure 1b, 1c; Supplementary Tables 1 and 2) to generate an extensive combinatorial library of polyacrylamide-based hydrogel formulations.
Each of the hydrogels were prepared by photopolymerization of prepolymer solutions using lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as a radical photoinitiator and a simple LED (λ = 350 nm) light source. During synthesis, four copolymer formulations turned opaque on account of insolubility of the polymer chains in the aqueous media and were thus omitted from further evaluation. We synthesized our hydrogels at a monomer content of 20 wt% to generate a library of materials with stiffness values mimicking those of human vein or artery tissues (Figure 1d).44,45 As all monomers evaluated were acrylamide-derived, the reactivity ratios for copolymerization of each pair of monomers (r1r2 ≈ 1) were such that each copolymer hydrogel was expected to comprise a statistical incorporation of each monomer moiety throughout the hydrogel network.46 We further sought to ensure that subsequent assays would strictly screen for the impact of polymer chemistry on anti-biofouling performance, rather than mechanics. We performed oscillatory shear rheology on the homopolymer hydrogels comprising the two monomers with the most distinct molecular weights: acrylamide (A; Mw = 71.08 g/mol) and [tris(hydroxymethyl)methyl]-acrylamide (G; Mw = 175.18 g/mol). We selected these two formulations for more in-depth mechanical characterization in order to validate that the various members of our hydrogel library exhibited similar mechanical properties (Figure 1e). These studies indicated that even these highly distinct hydrogels exhibited similar shear storage and loss modulus values, and elastic modulus values similar to human veins and arteries.
2.2. Protein Adsorption and Platelet-Resistant Hydrogels
To rapidly evaluate the extent of biofouling on these hydrogels in a parallel fashion, we developed a high-throughput assay to screen platelet adhesion following incubation in protein serum. While the exact mechanism of biofouling has yet to be precisely elucidated, it is widely hypothesized that such fouling is initiated with the non-specific adsorption of proteins from the biological milieu.47 This undesirable binding of proteins to the surface of a biomaterial or device leads to the onset of platelet adhesion and aggregation that eventually causes thrombosis.47–50 While the majority of anti-biofouling assays appearing in the literature have focused on evaluating adsorption of model proteins at low concentrations (e.g., 1 mg/mL bovine serum albumin38 or 1 mg/mL fibrinogen51) onto materials of interest, these studies do not provide a realistic representation of the complex milieu of biomolecules in blood.52 Other studies have utilized more relevant mixtures of biological proteins (e.g., fibrinogen, bovine serum albumin, and lysozyme), or diluted or undiluted serum or plasma to evaluate biofouling of new materials, but only over short exposure timeframes ranging from 10–25 minutes.53,23 The ability to repel whole blood has also been studied; however, in these studies blood was applied to the biomaterial of interest for only a matter of seconds.54 Consequently, previous studies have not provided a sufficiently realistic representation of the complex engineering challenge imposed on anti-biofouling coatings in the body on account of low protein concentrations, insufficiently complex biological milieu, and short assay times.52
To identify anti-biofouling materials from our copolymer hydrogel library with translational potential, we therefore sought to subject these materials to severe fouling conditions for prolonged periods of time that would nevertheless provide a simple and high-throughput readout of fouling activity. We chose to use platelet counting to enable a straightforward and realistic metric for anti-biofouling performance of our copolymer hydrogels, as platelet adhesion causes occlusion and leads to thrombosis.6 In these assays, each hydrogel was first incubated in 50% fetal bovine serum (FBS) for 24 hr at 37 °C to introduce non-specific protein adsorption (Figure 2a) prior to incubation in rat platelet-rich plasma (PRP) for 1 hr at 37°C to introduce platelets that could be counted in an automated fashion. In the blood biofouling process, the adhesion of platelets recruits and instigates resulting platelet aggregation, thus leading to the likelihood of large platelet clumps and high variability of platelet counts. We chose a 1-hour incubation as our terminal timepoint to minimizing the variation seen with extremely high platelet counts (Figure 2b). While we anticipate subtle species-dependent effects on platelet adhesion on account of the use of both bovine serum and rat platelet-rich plasma, we assume that adsorption of rat proteins during the second incubation step sufficiently fosters rat platelet adhesion. Although we could not discern clear trends in the behaviors of the polyacrylamide copolymers based on their composition, hydrophobic monomers such as N-isopropylacrylamide (K100) or diethylacrylamide (C100) yielded high mean platelet counts (28,600 ± 11,000 platelets/cm2 and 9,870 ± 13 platelets/cm2, respectively). In contrast, a 50:50 copolymer of hydroxyethylacrylamide (F) and diethylacrylamide (C), denoted F50-C50, yielded the lowest platelet counts (82.0 ± 15 platelets/cm2) of all of the materials tested, significantly outperforming PEG (L; 4,810 ± 520 platelets/cm2; p < 0.0001), 2-methacryloyloxyethyl phosphorylcholine (M; 1,330 ±210 platelets/cm2; p = 0.0001), poly(2-hydroxyethyl methacrylate) (PHEMA; O; 9,560 ± 650 platelets/cm2; p < 0.0001), and [(methacryloyloxy)ethyl]-dimethyl(3-sulfopropyl)ammonium zwitterion (N; 15,900 ± 4,200 platelets/cm2; p = 0.0007) hydrogel formulations (mean ± standard error, unpaired t test) (Figure 2c, Figure S1). This approach to high-throughput screening of fouling with blood proteins and platelets therefore enabled identification of polyacrylamide-based copolymer hydrogel formulations with superior anti-biofouling properties in comparison to gold standard polymers. While protein adsorption has previously been investigated as a metric of anti-biofouling performance, we did not see trends correlating IgG and fibrinogen adsorption with platelet adhesion (Figure S2). This observation may indicate that other proteins and/or specific conformations of adsorbed proteins (rather than quantity alone) may play key roles in platelet adhesion. As protein layers evolve unpredictably over time and culture conditions of single proteins is insufficient and poorly defined for the growth of cells, such as embryonic stem cells and induced pluripotent stem cells,40 protein adsorption may offer limited utility as a metric correlating to platelet adhesion.55
Figure 2. Identification of anti-biofouling acrylamide-based copolymer combinations.
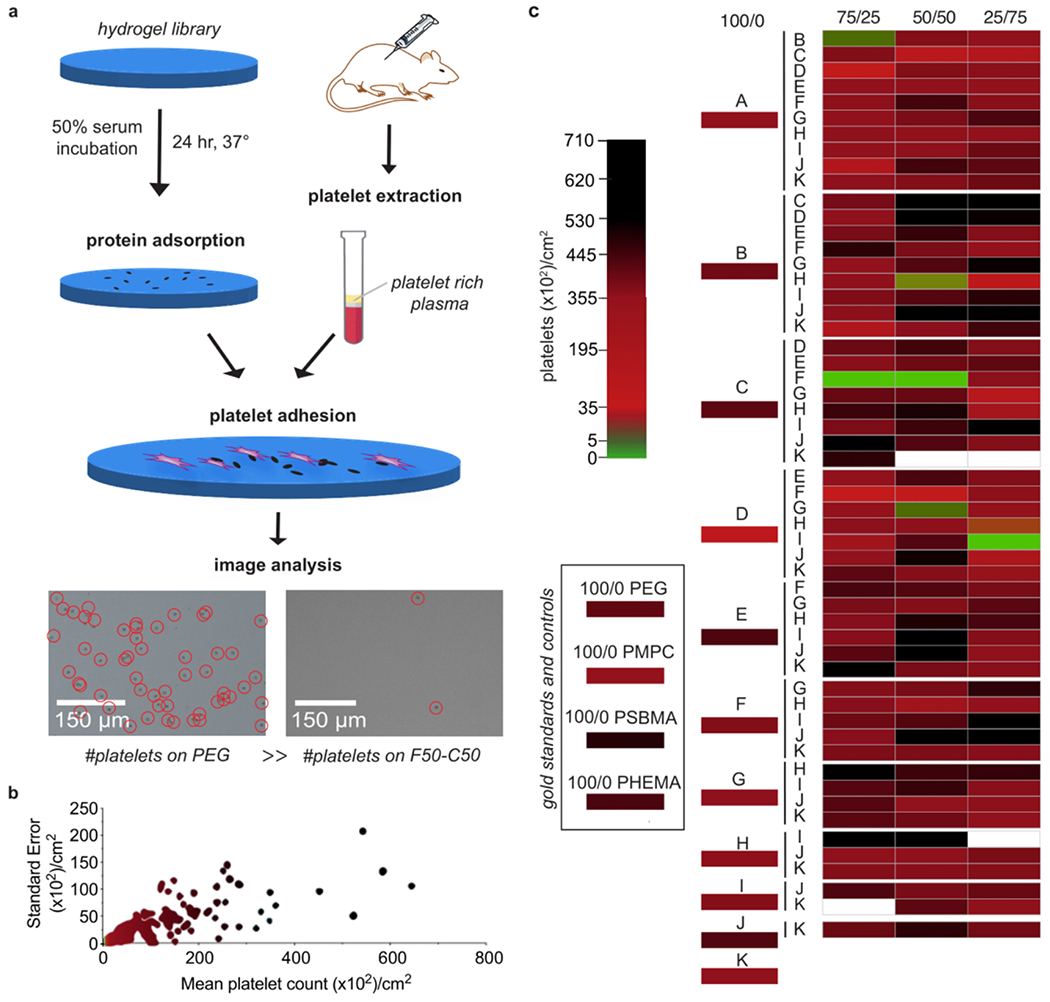
a. Hydrogel samples were first incubated in 50% fetal bovine serum for 24 hr at 37 °C to ensure extensive protein adsorption to the samples. Platelet rich plasma (PRP) obtained from centrifugation of rat blood was then incubated on the surface of the hydrogels for 1 hr at 37 °C. Several polyacrylamide copolymer hydrogels presented significantly less platelet adhesion than PEG, PHEMA, and polyzwitterionic hydrogel controls. b. Standard error versus mean of the platelet count distribution obtained for each copolymer hydrogel. c. Heat map of median platelet adhesion counts on copolymer hydrogel samples, ordered by the weight ratio of each monomer in the hydrogel formulation (100/0, 25/75, 50/50, 75/25). Colors were assigned to the medians obtained in n ≥ 3 tests.
We then performed scanning electron microscopy (SEM) imaging of treated hydrogel samples following lyophilization to observe platelet morphology. On the PEG hydrogel sample, aggregates of platelets were observed throughout the surface of the hydrogel, although their morphology was generally round, indicating that the platelets were not likely activated.56 Similarly, the PHEMA hydrogel exhibited aggregates of round platelets present across its surface.56 In contrast, polyzwitterionic materials contained fewer platelet aggregates, but the morphology of these platelets was indicative of activation—we observed that these platelets had morphed from spheroid form to exhibit bulbous protrusions on their surface. On the F50-C50 polyacrylamide hydrogels, we observed negligible platelet aggregation across the surface and little to no changes in platelet morphology (Figure S3).
2.3. Identifying Key Features of Monomers for Anti-Biofouling Properties
To aid in our understanding of the molecular features giving rise to the observed anti-biofouling properties of all of the materials we tested, we analyzed the physicochemical properties of each of the monomers and resulting hydrogels and the contribution of these features to fouling tendencies.57 In this study to identify such features, we accounted for the composition of the hydrogels, such as the monomers that comprised ~20wt%, and features of these monomers, including inherent physical and chemical properties, and also of the reactivity ratios, which affect the distribution of the monomers on the surface of the hydrogel. In addition, the use of platelet counts allowed for inputs that can link polymer behaviors to anti-biofouling performance, in contrast to protein adsorption assays that may not yield predictive models.40 The long-standing hypothesis regarding the anti-fouling mechanism of the gold standard polymers is the presence of a tight hydration layer between the material surface and water that forms a physical and energetic barrier to protein adsorption, determined often by the physicochemical properties of the materials and the surface packing.58 These properties include surface wettability and hydrophilicity, electrical neutrality, H-bonding, and prevalence of highly hydrated chemical groups. It has also been suggested that surface packing has a role in non-fouling properties, strongly correlated with the ability to form a hydration layer near the surface. Molecular simulations have also suggested that anti-biofouling properties may arise from steric repulsion of proteins and polymer surfaces - that the density of grafted polymer chains simply causes resistance to protein adhesion and obstructs their adsorption to the surface.16,59–61 Numerous other computational and molecular dynamics studies have shown that entropic contributions play a role in preventing biofouling,62,63 wherein reduction in ligand mobility would lead to an entropic penalty against protein adsorption.64 Penna et al. have identified the importance of ligand mobility and interfacial dynamics, highlighting a balance of hydration properties and a dynamic ligand layer that greatly affects entropic barriers associated with antifouling efficacy.65
Curiously, the compositions of the top-performing hydrogel formulations that yielded the lowest degree of platelet adhesion did not exhibit any intuitive trends, and so we utilized machine-learning analysis to identify mechanistic factors underpinning anti-biofouling activity. We first classified the copolymer hydrogels as “low platelet count” or “high platelet count” with a random forest model, which offers robustness to overfitting and the capability to evaluate relevance of data features via mean decrease of accuracy (MDA) (see Methods for technical details). We initially generated a simple feature set (Feature Set A) reflective of the polymer formulation, whereby each polymer was represented using one-hot encoding for the alphabet of monomers weighted by the molar ratios of the monomers in the respective copolymer hydrogels. Feature relevance analysis in combination with class-enrichment analysis (Figure 3a, Figure S4a) pointed to dimethylacrylamide (B), diethylacrylamide (C), and (acrylamidopropyl)trimethylammonium (I) being associated with heavy biofouling. In contrast, acrylamide (A), (3-methoxypropyl)acrylamide (D), and acryloylmorpholine (H) were prominent monomers in those hydrogel formulations exhibiting excellent anti-biofouling performance. As none of these monomers were in the top anti-biofouling hydrogel formulations, we determined that combinatorial formulations, rather than homopolymer formulations, are crucial in development of robust anti-biofouling coatings. This consideration motivated two approaches of weighing both the molecular features of each specific monomer and the ratio of each monomer present in a specific formulation. In this feature set, we increased resolution of the molecular features by generating vectors of physicochemical descriptors for the monomers, “inserting” these vectors into one-hot monomer encoding, and weighting by the molar ratios of the monomers in the corresponding copolymer hydrogels (Feature Set B). The f1 score (the overall measure of a model’s accuracy) indicated that these feature sets were suitable in data analysis (Table S3). Descriptors, in general, are numerical values that capture various characteristics of the molecules, both simple and complex. For example, a feature can be the number of proton donors, or the complexity measure of the molecular graph. The set of descriptors computed for each monomer included 19 values split between physicochemical descriptors (LabuteASA, TPSA, MolLogP, MolMR), molecular graph descriptors (BalabanJ, BertzCT, Chi0, HallKierAlpha), hydrogen-bonding descriptors (LipinskiHBA, LipinskiHBD), and 3D shape descriptors (FracCSP3, InertialShapeFactor, PMI1, PMI2, PMI3, RadGyration, SpherocityIndex, Asphericity, Eccentricity), all as implemented in the rdkit library66 (Table S4). Inspection of the feature analysis for the monomers weighted by their molar ratios helped to pinpoint relevant characteristics of the individual monomers. These features could then be expressed as descriptors (Figure 3b, Table S4, Figure S5). The top three features are from the physicochemical descriptor (MolLogP and LabuteASA) and 3D shape family (InertialShapeFactor). Overall, the most relevant features were found to be connectivity and shape descriptors: (i) InertialShapeFactor describes the mass distribution in the molecule (by three principal moments of inertia), (ii) MolLogP is an estimate of the octanol/water partition coefficient and captures the hydrophilicity/hydrophobicity of the molecule (an energetic characteristic), and (iii) LabuteASA describes the approximate surface area of the molecule. Surface area of the molecules plays a role in anti-biofouling performance because all monomers have a surface area that is accessible to other molecules (e.g. the solvent), and higher values of LabuteASA indicate that more surface is accessible (Figure 3c).
Figure 3. Feature relevance evaluated for monomers using trained random forest models.
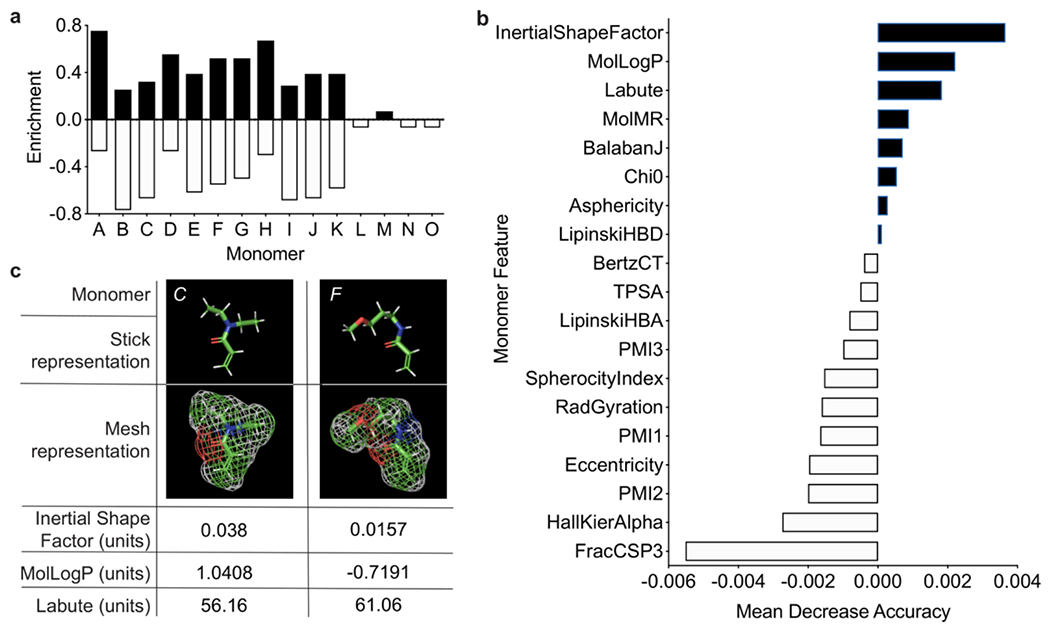
a. Monomer-specific class enrichment based on raw platelet adhesion data, indicating contribution of individual monomers to anti-biofouling performance. This does not account for the inherent features of the monomers, but simply quantifies the contribution of each specific monomer. b. Mean decrease of accuracy (MDA) of descriptor values evaluated with Feature Set B, summing the importance of the features across all monomers. Positive values (black) indicate that the feature is relevant to model performance, while negative (white) values do not inform the model. c. Descriptor values with the highest overall MDA for monomers in the copolymer hydrogel with the best anti-biofouling performance (F50-C50). Descriptors are obtained by transforming complex chemical information about the monomer into a number suitable for analysis.
In a second approach, we produced a simplified version of the descriptor-based feature set by summing up descriptor vectors of the monomers weighted by their molar ratios in corresponding copolymer hydrogels (Feature Set C), and our results indicated the importance of the 3D shape descriptor features (Figure S4b). Expanded analysis of the heterogeneity of the platelet count distribution also indicated the importance of a steric mode in the observed fouling (Figure S6). Overall, it appears that the relevant molecular features predominantly characterize properties associated with the steric aspect of the surface formation and the geometry of packing of the monomers.
2.4. Mechanical and Physical Properties of the Top Performing Hydrogel
The mismatch in mechanical properties between many implant materials and native tissues can lead to adverse events such as immune rejection, fibrotic responses, trauma, and thrombosis.67 Thus, it is crucial to match the mechanical properties of the biomaterial with surrounding tissue.68–70 Hydrogels in particular are widely tunable, and have mechanical similarity to biological tissue – in particular the human artery and vein – making them useful for implanted biosensors71 (Figure 1d, 1e and Figure S7). While other coating methods may incorporate polymers via either grafting-to or grafting-from approaches, these coatings are often only nanometers thick and have poor mechanical integrity, making them ill-suited to serve as a soft interface between implanted devices and the body.
We first assessed the mechanical properties of our top-performing hydrogel formulation and control hydrogels comprising PEG (20wt%), poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) (65wt%), poly(2-methacryloyloxyethyl phosphorylcholine) (PSBMA) (65wt%), and poly(2-hydroxyethyl methacrylate) (PHEMA) (~35wt%) using rheology (Figures S7, S8). These controls were synthesized using standard methods as described by literature to match the formulation as “gold-standard materials”.23,72 We found the shear rheological properties were comparable among each material at these formulations - at 1% strain, G’(F50-C50) = 27.0 ± 4.9 kPa, G’(PEG) = 66.7 ± 28.5 kPa, G’(PMPC) = 32.2 ± 9.4 kPa, G’(PCBMA) = 39.5 ± 12.8 kPa, and G’(PHEMA) = 41.8 ± 9.2 kPa. Moreover, we used fluorescence recovery after photobleaching (FRAP) spectroscopy to evaluate the mesh size of each of these hydrogel materials, which were found to be similar to each other and well below the size of albumin73 (Figure S7f). We also used interferometry-based nanoindentation, which enables examination of the mechanics of these materials under wet conditions (Figure S8a, 8c), as well as tensile testing (Figure S8b, 8c) to characterize the elastic modulus of our top-performing hydrogel formulation. While soft materials commonly used in the medical industry exhibit Young’s moduli values several orders of magnitude greater than that of human arteries, our F50-C50 hydrogel coating exhibits mechanical properties that are similar to human arteries,74 reducing the risk of chronic inflammation and potential failure of the implant.75 Due to the stability of polyacrylamide-based polymers, our lead candidate coating will inherently resist degradation and persist longer under biological conditions over control materials such as PEG. Under a variety of expedited degradation conditions, we observed improved stability of polyacrylamide-based hydrogels in comparison to PEG, PHEMA and zwitterionic polymer hydrogels, suggesting that these materials may be subject to biodegradation over time in the body, inherently limiting their anti-biofouling performance over extended timeframes (Figure S9).
2.5. Hydrogel Coating of Electrochemical Biosensors Extends Sensor Lifetime
In order to assess the usefulness of our new copolymer hydrogel material, we tested its anti-biofouling performance in protecting electrochemical biosensors in blood. Such biosensors generally offer a promising strategy for continuously detecting analytes in vivo, but their lifetimes are limited by biofouling.58,76 The degree of biofouling can be quantified using cyclic voltammetry (CV) to measure the anodic peak current. By applying a range of voltages through an electrode, electron transfer and current intensity may be monitored through the anodic peak. Changes in this peak can be used to assess interaction between the electrode surface and (FeCN6)4− ions in solution and the lifetime and quality of the signal. A larger reduction in signal indicates barriers to the electron transfer process and blocking of ions from diffusing toward the electrode surface due to biofouling.77
We prepared devices comprising a gold (Au) working electrode (WE), a silver/silver chloride (Ag/AgCl) reference electrode (RE), and a platinum (Pt) counter electrode (CE) (Figure 4a, 4b) with PEG and F50-C50 hydrogel coatings to assess the ability of the hydrogel coating to extend device lifetime. We first performed CV measurements in Na2EDTA-treated human whole blood spiked with 15 mM of ferrocyanide ions, (FeCN6)4−, to establish a baseline (Figure 4c). Then, devices were incubated in ferrocyanide-free human whole blood mixed with 50 mM CaCl2 to initiate expedited blood coagulation78,79 and to activate blood platelets (Figure S10). After 2 hr, probes were placed in the same ferrocyanide-spiked blood sample to record CV measurements (Figure 4d–f, Figure S11). As the addition of CaCl2 rapidly expedites blood clotting, 2 hr was ample time to observe significant differences in probe performance without full clotting of the blood medium.78 While anodic peak current was measured, the slight appearance of other peaks was observed with all probes, indicating a change in diffusion rate. The shift in potential often occurs due to the change of Cl− concentration in blood from the addition of CaCl2, as these concentrations affect the electroactive window.80,81 Considerable peak reduction was observed for the PEG-coated probe (50.8 ± 16%), whereas the F50-C50 hydrogel coating preserved current intensity (76.6 ± 21%) in these assays (Figure 4g). Uncoated, bare devices were also evaluated as controls (44.3 ± 9%) and did not significantly differ from PEG-coated probes (paired t-test, p = 0.931) (Figure S11, 12).
Figure 4. Hydrogel protection of an electrochemical device extends lifetime and signal quality over and PEG-coated devices.
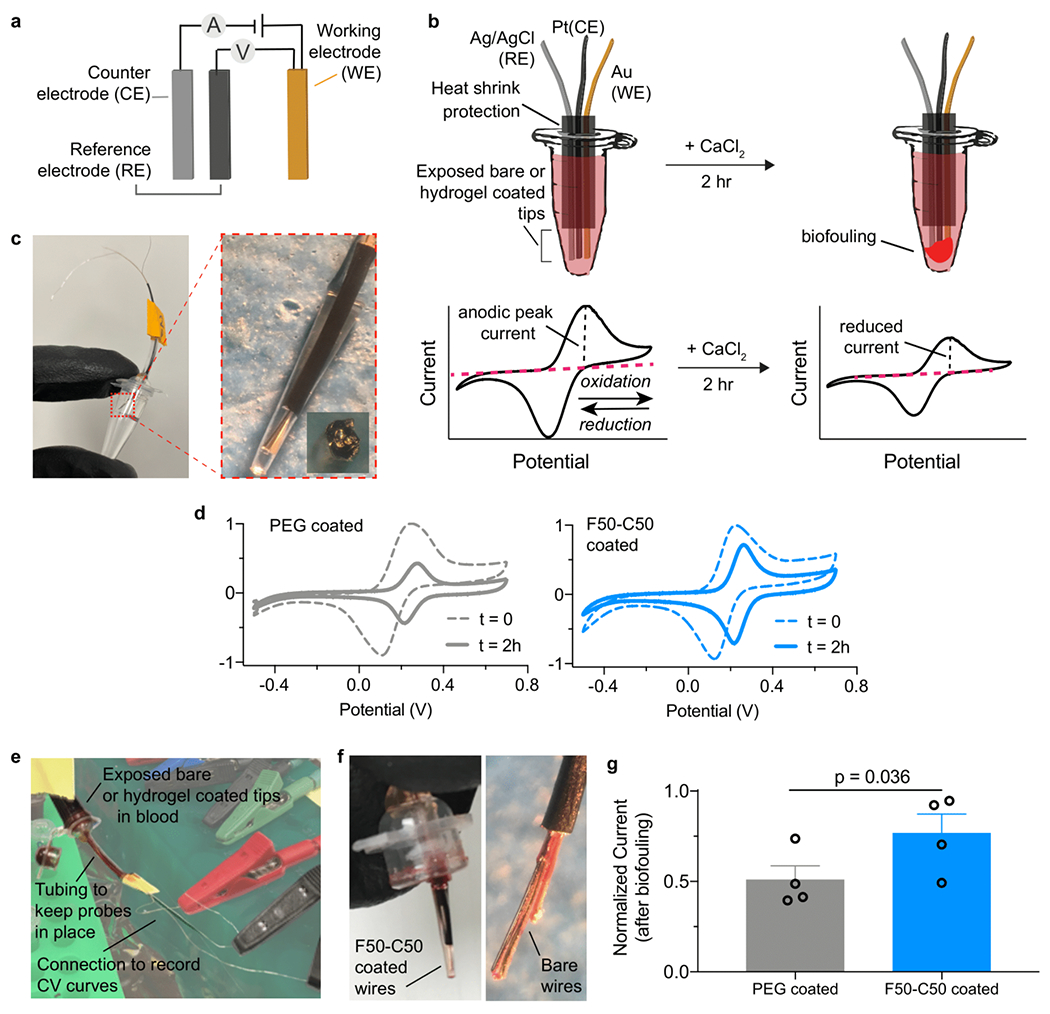
a. Affinity-based electrochemical sensors can be used to detect oxidation of ferrocyanide species (FeCN6)4−. b. In vitro assay to observe blood fouling on the device surface. Probes are inserted through the Eppendorf tube cap to prevent contact with side walls. Biofouling on the device reduces cyclic voltammetry (CV) oxidation and reduction peaks. c. Probes were coated with a visible layer of hydrogel to mitigate biofouling. d. CV curves were obtained by cycling electrodes between oxidation and reduction potentials of ferrocyanide. Representative graphs are shown before and after CaCl2 addition. e. In vitro assay setup. Tubes were inverted to ensure probes are completely immersed in blood. f. Devices show visible signs of fouling after 2 hr incubation. g. Normalized signal intensity, defined by anodic peak current from CV, of devices after biofouling. Data depict mean ± standard error, n =4. Significance was determined through a paired t-test.
Given that our F50-C50 copolymer hydrogel clearly outperformed PEG in a blood contacting setting in vitro, we proceeded to test the performance of these two materials in vivo. To assess these materials, we selected an area of implantation that would have direct blood contact. We first sought to evaluate the fouling of sensing probes implanted into the femoralal vein of a rat for 5 days (Figure S13a). This implantation site provided the best model for direct blood contact while the implantation time was the longest duration in which we were able to ensure device retrieval. Beyond 5 days, we experienced sutures failures and devices were lost. These device bundles comprised two WEs (one PEG-coated and one F50-C50-coated) and one RE (Figure S13b, c), and exhibited a diameter of ~500 μm after the hydrogel coating. CV was performed in a (FeCN6)4−-spiked blood sample in vitro to determine the anodic current intensity before and after in vivo incubation (a fresh Pt probe was included in the measurements for comparison). After 5 days, the sensor was recovered from the femoral vein (Figure S13d, e) and the hydrogel coatings visibly remained intact on the electrode surface (Figures S13f, S14). We observed significant signal degradation from bare (81 ± 27% reduction) or PEG-coated (83 ± 23% reduction) probes at the end-point of the study (Figure S15–16). Indeed, two thirds of these probes were completely non-functional after five days implanted in vivo. In contrast, we could obtain clear signals with notably less signal degradation (24 ± 36% reduction) from all probes coated with our F50-C50 hydrogel (Figure S15–16). The results of this study indicated that F50-C50-coated probes performed much more reliably than bare and PEG-coated probes.
2.6. Hydrogel Coating Enables Real-Time Measurement In Vivo via DNA Aptamer Biosensors
Based on the in vivo pilot study described above, we evaluated these coatings on real-time biosensors that utilize structure-switching aptamers to continuously measure specific analytes in vivo.82–85 Briefly, we engineer the aptamers to undergo reversible structure-switching upon target binding, and we conjugate electroactive reporters (e.g., methylene blue, MB) at the distal end of the aptamer (Figure 5a). We continuously measure the electron transfer between the reporter and the electrode using CV, which enables us to monitor the concentration of the analyte in real-time in a reagentless manner in real-time. We and other groups have used such sensors to continuously measure the systemic concentrations of small-molecule drugs in vivo85,86 and even control their levels using closed-loop feedback control,87 but biofouling of the surfaces of these devices limits their signal quality, and ultimately, their lifetime.88 We therefore sought to test whether our top-performing hydrogel coating could improve the performance of aptamer-functionalized electrodes in vivo. As a model system we employed aminoglycoside aptamers that bind kanamycin, which is a widely used small-molecule antibiotic drug (MW = 484.5 Da; diameter ~ 1 nm).89 To reduce the diffusion time of the drug to the probe surface, we reduced the thickness of the hydrogel coating by dip-coating the electrodes with precursor solutions for F50-C50 and PEG hydrogels and immediately cross-linked by photopolymerization (Figure S17). The mesh size of the resulting hydrogel coating was determined to be 2.33 ± 0.07 nm using FRAP spectroscopy, thus allowing free transport of kanamycin. We performed three experiments with this system. We initially characterized platelet adhesion on probes following incubation in Na2EDTA-treated human whole blood for three days at room temperature. We observed significantly less platelet adhesion on F50-C50-coated probes than PEG-coated or bare probes (Figure S18). Next, aptamer probes were inserted into a circulating blood flowing system for 6 and 12 days (Figure S19a). At the end of each assay, the probes were analyzed by SEM, which showed that the F50-C50 hydrogel coatings had noticeably less platelet adhesion after both time-points than control probes (Figure S19b).
Figure 5. F50-C50 hydrogel coating of a DNA aptamer biosensor enables continuous real-time monitoring of kanamycin in vivo.
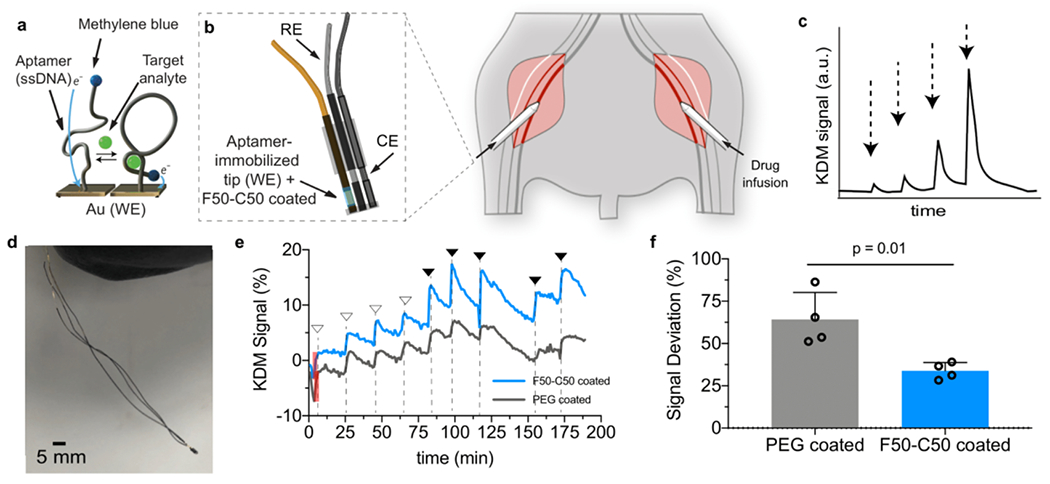
a. DNA aptamers that bind kanamycin with high specificity were functionalized onto the tip of Au probes. b. The probe was inserted into the femoral vein of a rat through a catheter. Kanamycin was infused into the other femoral vein. c. The kanamycin signal response should exhibit fast kinetics and dose-dependence. d. Image of the aptamer probe device. e. Representative graph of real-time in vivo sensing of kanamycin after administration of varying concentrations of the drug by injection into the contralateral femoral vein. Filled arrows represent a 200 μL injection of 200 mM kanamycin and empty arrows indicate a 100 μL injection of 200 mM kanamycin. Shaded red box represents peak intensity of initial baseline signal. f. Signal intensities as indicated by peak heights were measured and normalized to baseline signal of each probe. Deviation from the baseline signal between PEG- and F50-C50-coated sensors showed the ability of the F50-C50 hydrogel to maintain higher signal. Data represents mean ± standard deviation, n = 4, unpaired t-test.
Finally, to test the functional efficacy of these hydrogel coatings in vivo to monitor the analyte kanamycin in real-time, we tested our aptamer devices in the femoral vein of a rat as described above (Figure 5b). Kanamycin (0.1 mL or 0.2 mL at 200 mM) was administered intravenously into the contralateral femoral vein at different time-points while the rat was under anesthesia. Due to the required duration of the rat under continuous anesthesia, we were limited inherently by the assay time, to roughly 3.5 hours. Because of this assay limitation, we chose to dip coat the aptamer probes with hydrogel coating, which allowed for a thinner coating and thus reduced diffusion time of analyte to aptamer surface, enabling more rapid response times and increasing number of measurements in this real-time test. Aptamer activity was measured using square-wave voltammetry (SWV) at 400 and 60 Hz, and we used the kinetic differential measurement technique to compensate for drift as described previously86 (Figure 5c, d). After establishing equilibrium over the course of 60-120 minutes after implantation, we successfully recorded the real-time drug concentration in vivo for the first time with F50-C50-coated devices (Figure 5e, Figure S20). Signal peaks in response to kanamycin administration were determined at various time points over the course of roughly 200 minutes following incubation, and normalized to initial peak intensity, due to sensor-to-sensor variations. In these assays, PEG-coated sensors had an average deviation from original signal of 64.2 ± 16.1%, while the enhanced anti-biofouling capabilities of F50-C50 coatings resulted in significantly less deviation, 33.8 ± 4.9%, from the original signal (Figure 5f) (mean ± standard deviation; n = 4, data was analyzed via unpaired t-test where p = 0.01).
3. Discussion
In this work we developed a library of 172 polyacrylamide copolymer hydrogels – the most extensive library of this type generated to date – to discover anti-biofouling coatings for biomedical devices that outperform current gold standard materials. Machine-learning analysis of high-throughput, parallel platelet fouling assays suggested that the relevant mechanisms underlying the observed anti-biofouling properties of these materials arise from steric and inertial shape properties of the monomers used in these hydrogels. We also demonstrated that the top-performing formulation exhibits mechanical properties resembling those of human arteries. Finally, we validated the utility of this hydrogel formulation for improving the performance of biosensor devices both in vitro and in vivo.
To the best of our knowledge, only a small subset of polyacrylamide polymers have been explored previously as candidates for anti-biofouling coatings, including those derived from acrylamide,53,90 hydroxyethylacrylamide,51,91 (3-methoxypropyl)-acrylamide and N-isopropyl-acrylamide.92 We sought to explore a wide variety of commercially-available acrylamide monomers, as well as combinations of these monomers, on account of the ability to screen a large amount of chemical space with materials that have been demonstrated to be amendable to adhesion onto a variety of surfaces. While our platelet screening assay employed the use of both bovine serum and rat platelet-rich plasma, which may consequently lead to subtle species-dependent effects of protein adsorption and platelet adhesion, this method allowed us to screen our large polyacrylamide library in a high throughput manner. Though we did not evaluate in depth the adhesion of our hydrogel coatings to the device surfaces, these materials are amendable to a variety of adhesion chemistries.93 Polyacrylamide-based polymers are highly stable over long time-frames under physiological conditions, thereby enabling their use with medical devices requiring long functional lifetimes.94,95 While PEG and zwitterionic polymers are considered gold standard materials for anti-fouling coatings, our platelet screening assay identified numerous polyacrylamide copolymer hydrogel formulations exhibiting superior anti-biofouling performance, highlighting the promise of polyacrylamide copolymer hydrogels in these applications. Based on the successes of these polyacrylamide-based copolymer hydrogels, we believe that evaluation of copolymer hydrogels based on PEG and zwitterionic polymers will be important and may also yield next-generation coatings with superior anti-biofouling performance.
Our selected formulation for the L100 PEG hydrogel (PEG diacrylate Mn 780 and PEG acrylate 480) was chosen so as to design a PEG hydrogel that did not meaningfully differ in stiffness from the other hydrogels in our library at the same polymer content. As other PEG monomers have also been used historically for anti-biofouling studies, namely PEG diacrylates and PEG dimethyacrylates of various molecular weights, we evaluated three alternative formulations: (i) PEGDMA740 (15wt%) plus PEGMA550 (5wt%), (ii) PEGDA10K (20wt%), and (iii) PEGDMA10K (20wt%). Each of these formulations comprised 20wt% solids and 1wt% photoinitiator. We found that these formulations exhibited shear storage modulus values ranging over two orders of magnitude (Figure S21) and resembling values determined for the library of polyacrylamide-based hydrogels (Figure 1e). Further, we found that these alternative PEG hydrogels exhibited higher IgG and fibrinogen adsorption than the primary L100 PEG control (Figure S22a), while platelet adhesion was similar for all formulations evaluated (Figure S22b). For this reason, we were confident in our use of L100 as a representative PEG formulation in all of our subsequent experiments.
The anti-biofouling behavior of the various copolymer formulations was evaluated using machine learning algorithms with several different feature sets to elucidate the molecular features that give rise to the observed behavior. The features that were common amongst the top performing hydrogel formulations (Feature Set B) were heavily represented by connectivity and shape descriptors. Coupled with other studies57,64,65 indicating that ligand mobility makes an entropic contribution to anti-fouling performance, this work provides critical insight into the molecular mechanisms underpinning anti-biofouling behaviors and potentially enabling the rational design of next generation coatings. Recently, Rostam et al. developed a (meth)acrylate and (meth)acrylamide polymer library to modulate the foreign body response to implanted materials and made use of machine learning approaches to analyze important chemistry descriptors driving macrophage phenotype and implant fouling. While this study made use of arrays of neat polymers instead of hydrogels, steric (and electronic factors) were also attributed to feature importance.34 We sought to eliminate surface topography as a contributor to differences in biological performance in the PAAm library by polymerizing the hydrogels between glass slides. Although SEM images revealed that the various hydrogel formulations evaluated exhibit different levels of activation of platelets (Figure S3), the differences in hydrogel surface topography that were apparent in SEM are attributable to the sample processing, wherein hydrogels were lyophilized prior to imaging. To evaluate the true topology of the hydrated hydrogel surfaces, we performed optical microscopy on a subset of materials, including our top-performing polyacrylamide formulation, homopolymer hydrogels that comprise the top formulation, a subset of various polyacrylamide hydrogels across the library, and control materials. Optical microscopy images (Figure S23a–g) revealed that all of the materials evaluated are relatively flat, with << 3 μm variation in their height profile, and have negligible surface roughness (Table S5). As the topography and surface roughness of the hydrogels are similar across all materials evaluated, we believe surface topography is not an important variable within the hydrogel library.
When the top-performing polyacrylamide copolymer hydrogel was used as a coating on an electrochemical biosensor device, we observed little reduction in signal in vitro in the presence of whole blood. The mechanism behind this improved signal could be attributed to two critical factors that are enhanced by the hydrogel. First, the surface of a bare electrode is exposed to a broad spectrum of molecules, leading to broad peaks, while the pores of the hydrogel coating exclude molecules too large to diffuse through the matrix, reducing peak widths observed with hydrogel-coated probes. Second, the intensity of measured peaks is maintained throughout the duration of the assay with F50-C50 hydrogel-coated probes, suggesting that this formulation precludes biofouling58,96 while allowing molecules of interest to diffuse through the hydrogel matrix and bind reversibly to the surface to produce signal. In contrast, PEG-coated probes lose their ability to sense molecules over time, despite having a comparable mesh size to the F50-C50 hydrogel coating, suggesting that free diffusion of molecules through the coating is impaired by fouling. Indeed, other studies have suggested PEG coatings may not be suitable for use on biosensors of this type as they can hinder electron transfer.97
Through in vivo implantation studies, we observed that F50-C50-coated probes performed much more reliably than bare and PEG-coated probes. Indeed, only one probe from each of the control groups showed any response after five days implanted in the femoral vein of a rat on account of fouling in vivo. In contrast, all three F50-C50-coated probes exhibited clear signals with higher anodic currents (Figures S15). These studies suggest that the majority of signal loss likely occurs in the first few hours of blood exposure and changes relatively little out to 5 days following implantation. Similarly, PEG-coated DNA aptamer sensors showed twice as much deviation from their original signal relative to F50-C50-coated sensors within the first two hours after implantation in the femoral vein of a rat (Figure 5). In this timeframe alone, we notice significant improvement in current peaks with our polyacrylamide-based material. While future work will certainly be needed to evaluate the function of F50-C50-coated sensors over longer durations relevant for certain applications (e.g., catheter-bound sensors), these studies suggest that performance of F50-C50-coated sensors is rather stable through the first 5 days, thereby exhibiting promise for maintaining sensor function over longer time-frames. Overall, these data show that combinatorial screening of copolymer hydrogels offers an exciting avenue for the discovery of anti-biofouling coatings.
Although it may not be intuitively obvious from a materials design point-of view, we demonstrate that certain copolymer formulations can deliver considerably better anti-biofouling performance in comparison to gold standard materials than homopolymers. Considering the success of these polyacrylamide-based copolymer materials, it will be important to similarly evaluate the anti-biofouling performance of copolymer hydrogels comprising PEG and zwitterionic polymers. These observations highlight the value of coupling chemical design intuition with high-throughput screening approaches for discovery of new materials. We have demonstrated the applicability of these new hydrogel coatings to the surface of electrodes using facile synthetic approaches that are amenable to scalable manufacturing, although future studies must be conducted to optimize the adhesion strength of these hydrogels to device surfaces for longer-term biomedical use98,99 and to enhance the durability of the hydrogel materials.100 With further improvements, we believe this strategy of using combinations of acrylamide-based monomers may enable the discovery of anti-biofouling materials enabling long-term implantation of biosensor devices to continuously monitor chronic biomedical conditions.
4. Experimental Section
Materials and Reagents:
All reagents were purchased from Sigma-Aldrich and used as received, unless later specified. Au, Pt, and Ag wires were purchased from A-M Systems. EDTA-treated human whole blood for flowing in vitro measurements was purchased from BioIVT. Kanamycin monosulfate was ordered in USP grade from Gold BioTechnology.
Hydrogel Preparation:
Pre-polymer formulations containing 20 wt% acrylamide monomer, 1 wt% lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as photo-initiator, and 1 wt% methoxy-bisacrylamide were mixed and pipetted between two glass slides separated by a silicone spacer (0.25 mm ± 0.05 mm). Gels were cross-linked in a Luzchem photoreactor system with 8 W bulbs and an intensity of 25-40 W/m2 (LZC-4, hv = 350 nm, 15 min). Due to swelling of polyacrylamides in water, they were placed in 1x PBS for at least 24 hr before being punched with a 6 mm biopsy punch. PMPC, PSBMA, and PHEMA hydrogels were prepared as described previously72,101. Briefly, polyzwitterionic hydrogels were prepared with monomeric solutions in 1 M NaCl with 4% N,N’-methylenebisacrylamide (MBAm) cross-linker. A stock solution of 15% sodium metabisulfite and 40% ammonium persulfate was added to the prepolymer solution at ~1wt% and polymerization was initiated at 60 °C. PHEMA hydrogels were prepared in a solution of ethanol : ethylene glycol : water (1:1.5:1.5) with tetraethyleneglycol dimethacrylate as a cross-linker. A stock solution of 40% ammonium persulfate and 0.5 wt% TEMED was added to the prepolymer solution at 1.5 wt% and polymerization was initiated at 60°C. PEG hydrogels were prepared similarly with a prepolymer solution of 15 wt% PEG diacrylate (Mn 780102–104) and 5 wt% PEG acrylate (Mn 480). Prior work has shown that PEG molecular weight (Mn > 10K) affects anti-fouling performance when PEG is presented as brush polymers tethered to a surface. In addition, when molecular weight of PEG exceeds 40 kDa, issues arise such as potential immunogenicity and accumulation in tissues105. Here, we have used hydrogels to create a fully cross-linked network and, to our knowledge, have not seen effects of PEG molecular weight. Additional PEG hydrogels were synthesized with 20 wt% PEG10K diacrylate and 1 wt% LAP (PEGDA10K), 20 wt% PEG10K dimethacrylate and 1 wt% LAP (PEGDMA10K) and 15 wt% PEG740 dimethacrylate, 5 wt% PEG 550 methacrylate and 1 wt% LAP (PEGMA740_PEGMA550). Prepolymer solutions were mixed and pipetted between two glass slides separated by a silicone spacer (0.25 mm ± 0.05 mm). Gels were cross-linked in a Luzchem photoreactor system with 8 W bulbs and an intensity of 25-40 W/m2 (LZC-4, hv = 350 nm, 15 min). Hydrogels were placed in 1x PBS for at least 24 hr before being punched with a 6 mm biopsy punch.
Hydrogel Synthesis Modifications:
All 2-acrylamido-2-methyl-propane sulfonic acid (AMPSAm) formulations were made with slightly basic solution of 2:3 1 M NaOH:water. [tris(hydroxymethyl)methyl]acrylamide (tHMAm) formulations were made with 50:50 dimethylformamide:water as well as 100% N-isopropylacrylamide (NiPAm), 75% diethylacrylamide (DEAm)/25% NiPAm, and 25% hydroxymethylacrylamide (HMAm)/75% NiPAm. Acrylamidopropyl)trimethyl-ammonium (APTAC)-based formulations were used with 2-3x MBAm concentration to achieve comparable mechanical properties.
Platelet Adhesion Test:
Fresh whole rat blood was mixed in a 10:1 volume ratio of an acid citrate dextrose (ACD) anticoagulant buffer (containing 2.13% free citrate ion, BD Vacutainer Specialty Venous Blood Collection Tubes) for the preparation of platelet-rich plasma (PRP). PRP was obtained via centrifugation at 600 x g for 10 min at 10 °C. The platelets were counted using a Countess II Automated Cell Counter (ThermoFisher Scientific) and diluted to 2.5 x 106 platelets/mL in 1x PBS. 6 mm punches of hydrogels were placed in ultra-low adhesion 96-well plates and incubated for 24 hr at 37 °C. Gels were UV sterilized for 5 min prior to incubation with platelets. 100 μL of PRP was pipetted on top of the hydrogels. The plates were placed on a rotary shaker for 1 hr at room temperature. Platelets were rinsed once with 1x PBS and fixed with 4% paraformaldehyde (PFA). Cells were imaged with an EVOS XL Core Imaging System microscope (Life Technologies).
Platelet Detection in Images:
Platelets in the images are small round objects ~3-4 μm in diameter. Platelet images are of different color and can include noise such as gel chunks or dust. The noise typically exhibits sizes considerably smaller or larger than platelets. The platelets in the images were detected using a difference-of-Gaussian approach by blurring images using Gaussian kernels of a range of standard deviations in increasing order. Stacks of the differential images between two successively blurred images form a cube, and where blobs are local maxima of intensity. Blobs of noisy objects are avoided by tuning the range of standard deviations used in the process.
IgG and Fibrinogen Adhesion:
Hydrogel discs (n = 3 for each sample) were punched into a low-adhesion clear 96-well plate. To measure IgG adsorption to the surface of the hydrogels, 100 μL of 2 μg/mL Alexa Fluor 488-AffinityPure Rabbit Anti-Bovine IgG (Jackson ImmunoResearch) was added to each well; the plate was then sealed and placed on a shaker plate overnight. For fibrinogen adhesion, 100 μL of 5 μg/mL fibrinogen from human plasma conjugated to AF488 (Invitrogen) was added to each well; the plate was then sealed and placed on a shaker plate for 1 hour. Samples were then washed 5x with PBS, after which the gels were transferred to a black 96-well plate and fluorescence readings were taken (BioTek Synergy H1 microplate reader).
Fluorescence Recovery After Photobleaching (FRAP):
Hydrogel samples were loaded with 0.5 wt% FITC-dextran (4kDa). An Inverted Zeiss LSM 780 laser scanning confocal microscope (Germany) with a Plan-Apochromat 20X/0.8 M27 objective lens was used for FRAP analysis. We used pixel dwell time 1.58 μs. Samples were photobleached with 405-nm and 488-nm argon lasers set to 100% intensity. The samples were placed in a sterile 0.16-0.19 mm thick glass bottom μ-dish (MatTek). ZEN lite (Zeiss) software was used for all FRAP tests. To avoid any extra noise, the high voltage was limited to 700 V. Different tests (n = 5) were made at different locations of the sample. For each test, 10 control pre-bleaching images were captured per second, and we then bleached the spot with a pixel dwell time of 177.32 μs. 390 post-bleaching frames were recorded per second to form the recovery exponential curve. The pixel size was set to be 0.83 μm. The diffusion coefficient was calculated as described in the literature;106 D= γD(ω2/4τ1/2) where γD = 0.88 for circular beams, ω is the radius of the bleached ROI (12.5 μm), and τ1/2 is the half-time of the recovery. To estimate the mesh size (ξ) of our hydrogels, we used the obstruction theory of Amsden et al:.107
| (1) |
where D is diffusivity of the solute in the hydrogel, D0 is the diffusivity of the solute within the liquid in the hydrogel (saline-sodium citrate buffer), which is assumed to be the same as in pure water, rs is the radius of the solute (3.51 nm for FITC-dextran 4kDa), rf is the radius of the polymer chains within the hydrogel network (estimated as 0.65 nm108 for F50-C50 hydrogel, 0.51 nm for PEG109, 2.00 nm for pHEMA,110 1.00 nm for PMPC111,112 and 0.9 nm for PCBMA113). The diffusivity (D0) of a solute in a pure liquid was determined with the Stokes-Einstein equation to be 69.91 μm2/s using a solution viscosity of η = 0.89 x10−4 Pa s.
Scanning Electron Microscopy:
SEM images were acquired with an FEI Magellan 400 XHR microscope with a beam voltage of 1 kV. The sample was lyophilized prior to imaging, pressed onto silver paint and sputter-coated with Au:Pd (60:40) before imaging. When imaging hydrogels and adherent platelets, samples were fixed with 4% glutaraldehyde (GFA) for 5 minutes, then dehydrated with 10%, 30%, 50%, 70%, 90%, 100% ethanol solutions for 10 minutes each. Samples were then lyophilized, gently pressed onto silver paint and sputter-coated before imaging.
Performance Classification:
The relevant performance range of the antifouling polymer is platelet count under 100 units. This requirement naturally sets up the data analytic task as classification. From this point, antifouling candidates are considered as members of one of two performance classes. The positive class includes polymers with median platelet count below or equal to 100, and the negative class includes polymers with median platelet above 100 counts. Out of the characterized polymers, there were 76 members in the positive class and 91 members in the negative class. The slight class imbalance was considered irrelevant for practical purposes, and can be mitigated using standard techniques such as over- and under-sampling. The data was organized into three different feature sets (A, B, C). Feature Set A was reflective of the molar ratios of the monomers in the respective polymer. Feature Set B increased granularity by generating vectors of physical-chemical descriptors to the monomers and weighting these by the molar ratios of the monomer in the polymers. A simplified version was generated in Feature Set C, by summing up descriptor vectors of the monomers weighted by their molar ratios in the corresponding polymers. These classifiers (Table S3) showed highest performance with Feature Sets A and B.
Machine-Learning Data Analysis:
Classification was performed using a random forest (RF) model, as implemented in scikit-learn 0.20.2. As a model, RF offers robustness to overfitting and the capability to evaluate relevance of data features. RF model parameters were selected via cross-validated grid-search; optimized parameters included the number of estimators, maximum estimator depth, and maximum number of features used in the splitting procedure. Three-fold cross-validation was performed over the training set, which included 75% of the available observations. The model produced as a result of the cross-validated grid search was tested on the withheld test set that included 25% of the available observations. We selected MDA to evaluate feature relevance, which captures the drop in accuracy of the trained model under random permutation of the feature whose relevance is being evaluated with the rest of the features. Higher positive values of MDA are indicative of a larger drop in accuracy in the permutation test and point to higher impact of the respective feature on the model predictions. MDA values close to zero are indicative of minimal impact of the feature permutation on accuracy, and help identify irrelevant features. Negative MDA values point at features whose permutation increases model accuracy. Such features do not contribute meaningfully to the model performance. Model performance on the test set was evaluated using standard measures of accuracy in classification tasks, such as recall, accuracy, and f1-score.
Expanded Analysis of Platelet Homogeneity:
The coverage profile for each formulation was generated in the following manner: (i) partition the image of the polymer surface into square bins, (ii) count the platelet population in each bin, (iii) generate a population histogram for all images obtained for a given formulation, and (iv) normalize the histogram to the total number of population bins obtained for a given formulation. There are several general types of population histograms that one might expect to encounter (Figure S4a-d). We recognize that the difference between homogenous and heterogenous coverage modes is quantitative, not qualitative. In both cases, histograms correspond to distributions that have tails; the thickness of the tails distinguishes the two cases, where the tails fall off faster in homogenous cases than in heterogenous cases.
Using normalized population histograms as features, we performed anomaly detection analysis with the Isolation Forest algorithm.114 Each histogram received an anomaly score; negative values indicate outliers, and positive values indicate inliers. The notions of inliers and outliers are reflective of the structure of the dataset and do not have absolute meaning. The anomaly scores are visualized by reducing dimensionality of the dataset to two dimensions via t-SNE manifold embedding and encoding the anomaly score of the samples as the color (Figure S4i). Outlier analysis identifies histograms corresponding to homogenous coverage as the most normal. The change of the anomaly score towards the most abnormal system is associated with increased heterogeneity of the coverage (Figure S4j). Overall, there were 121 systems that bore signs of increasing heterogeneity of coverage, suggesting that the steric mode of fouling plays a meaningful role.
Rheological Characterization:
Oscillatory rheology measurements were performed with a TA Instruments AR-G2 rheometer. Amplitude sweeps were conducted at a frequency of 10 rad/s from 0.1-100%. Frequency sweeps were conducted at 0.1% strain from 0.1-100 rad/s. All tests were performed at 25 °C using an 8 mm parallel plate geometry.
Tensile Test:
Tensile strength measurements were performed with an Instron series 5560A with 100 N load cell. Tensile tests were conducted at 0.2 mm/s at room temperature.
Nanoindentation Test:
Young’s modulus measurements of the hydrogels were performed using a Piuma Nanoindenter (Optics11) with a probe from the same manufacturer with a stiffness of 38.8 N/m and a tip radius of 27.0 μm. Calibration was conducted as on glass under wet conditions as per the manufacturer’s instructions. Each sample was immersed in a sterile saline buffer solution (Opti-free - Replenish; Alcon) before the nanoindentation experiments. The indentation depth was fixed to be <1 μm in order to avoid bottom effects. At least 8 force curves were used to determine the local Young’s modulus of each sample, using the Optics11 Nanoindenter V2.0.27 software. The results are shown as mean ± standard error of the mean. A Hertzian contact model parameter was used for the fit of the curves assuming that the Poisson’s ratio of the samples is υ = 0.5.
Fabrication of Electrochemical Biosensors for In Vitro Assessment:
The working electrode (WE) was prepared with 75-μm diameter gold wire (PFA-coated). Ag/Ag Cl wires were prepared by incubating silver electrodes in bleach solution, rinsed vigorously with water, and dried. Pt wire was bundled with the Au electrode after bundling with Ag/AgCl wire to prevent shorting. The tip of the device was cut with a razor blade to expose bare gold and rebleached. No surface roughening was applied. To prevent wires from touching the side of the Eppendorf tube walls, they were propped in the middle of the tube. A hole was drilled in the cap of a 0.3 mL Eppendorf tube to fit 0.04” Tygon tubing. One inch of tubing was inserted into the drilled hole, and we applied epoxy and cured with UV light (365 nm) for 20 seconds. Wires were then inserted through the Tygon tubing into the tube.
Hydrogel Coatings on Electrochemical Biosensors for In Vitro Assays:
1 μL of prepolymer solution was pipetted into a 100 μL pipette tip. The probes were inserted and polymerized for 5 seconds was photo-cured at 365 nm. The pipette tip was removed, and the hydrogel coating was visible by eye. The hydrogel was then photo-cured for an additional 30 seconds.
In vitro Assays with Electrochemical Devices:
Bare, PEG-coated, and F50-C50-coated probes were incubated in EDTA-treated human whole blood at room temperature with 250 μL of 15 mM (FeCN6)4−- to record baseline CV data. This step was limited to 5 minutes to ensure a steady-state readout while minimizing biofouling. Probes were rinsed in 1x SSC buffer for 5 minutes to thoroughly remove any intrinsic fouling and transferred to 250 μL whole blood, followed by addition of CaCl2 to a final concentration of 50 mM. After 2 hr, probes were transferred to the original tube used for baseline measurement. The device was incubated for 5 minutes to allow the diffusion of (FeCN6)4− throughout the hydrogel. CV scanning was performed on the Au wire (potential range: −0.7–0.8 V, step size: 1 mV, scan rate: 0.1 V/sec).
Fabrication of Electrochemical Biosensors for In Vivo Assessment:
Bare gold was cut into ~10-cm wires. The tip (~1 mm) was bundled with black medical heat shrink tubing twice (ID 0.006” ± 0.001, Nordson Medical 103-0325). Another two layers of heat shrink were applied ~1-2 mm away, allowing exposure of ~1–2 mm of bare gold. Ag/AgCl wires were bundled likewise to prevent shorting. Two gold wires (WE) and one Ag/AgCl wire (RE) were bundled at the tip with clear shrink wrap (ID 0.02” ± 0.001, Nordson Medical 103-0249) and at the middle of the probe to provide mechanical strength when pushing through the catheter.
Hydrogel Coatings on Electrochemical Biosensors for In Vivo Assays:
For each device, one Au wire was left bare and one was coated with hydrogel. The prepolymer solution of hydrogel was pipetted into a short segment of tubing from a 26G catheter with inner diameter of 0.6 mm. The device was pushed through the tubing and aligned with one of the two bare gold wires. UV light (365 nm) was applied for 5 seconds and the tubing was gently removed. The hydrogel coating was visible on the entirety of one Au probe only, and UV light was applied for 25 more seconds to ensure complete photopolymerization. Probes were stored in SSC buffer and placed in 200 μL of EDTA-treated rat blood (BioIVT) with 15 mM (FeCN6)4− to record baseline data. Probes were incubated for 5 minutes to allow diffusion of (FeCN6)4−. CV scanning was performed on the Au wire (potential range: −0.7–0.8 V, step size: 1 mV, scan rate: 0.1 V/sec).
In Vivo Assays with Electrochemical Devices:
Live animal studies were performed with male Sprague–Dawley rats under Stanford APLAC protocol number 33226. All rats used in this work were purchased from Charles River Laboratories at a weight of 350-475 g. The rats were anesthetized using isoflurane gas (2.5%) during implantation procedures and monitored continuously. After a small incision to expose the femoral vein, a 24G catheter was inserted into the vein. The needle of the catheter was removed, and the catheter was clipped. The devices were inserted through the catheter and pushed far into the vein. The catheter was clamped, and the device was secured in place, after which the incision was sutured and the animal monitored during recovery and the duration of the 5 days. At the end of the experiments, animals were anesthetized using isoflurane gas and the devices were removed for immediate measurement in blood used for baseline sample. Rats were then euthanized by exsanguination while under general anesthesia.
Aptamer Device Fabrication and Functionalization:
The kanamycin aptamer probes were synthesized by Biosearch Technologies with the following sequence: 5′-HS-(CH2)6-GGGACTTGGTTTAGGTAATGAGTCCC-MB-3′. Probes were thiolated at the 5′ end with a 6-carbon linker for self-assembly onto the WE, and conjugated with a methylene blue (MB) redox label at the 3′ end with a 7-carbon linker for charge transfer measurements. The modified DNAs were purified through dual HPLC by the supplier. Upon receipt, the construct was dissolved and diluted to 100 μM using UltraPure water (Thermo Fisher Scientific) and frozen at −20 °C in individual aliquots of 1 μL until use. The WE was prepared with 8 cm pure Au wire (75 μm diameter) insulated with heat-shrinkable tubing (Nordson Medical, 103-0325) to define the aptamer immobilization surface. The exposed gold wire has a length of 1~2 mm with an overall surface area of 0.25~0.5 mm2. No surface roughening was applied. Before immobilizing the aptamer probes, the wire was rinsed with acetone, ethanol, and deionized water in a sonicator sequentially, followed by electrochemical cleaning. CV scanning on the gold wire was performed in 500 and 50 mM sulfuric acid solutions (potential range: −0.4–1.5 V, step size: 1 mV, scan rate: 0.1 V/sec), each with three scans. An aliquot of the DNA construct was thawed and reduced for 40 minutes with 2 μL 100 mM tris(2-carboxyethyl)phosphine at room temperature in the dark. The reduced DNA construct was diluted to 1 μM with deionized water, and a freshly cleaned gold electrode was immersed for 1 h at room temperature in dark. Next, the sensor was rinsed with deionized water for 3 min, followed by immersion in 6 mM 6-mercapto-1-hexanol in 1x SSC buffer for 2 hr at room temperature in the dark to passivate the remaining gold surface and remove nonspecifically adsorbed DNA. The sensor was rinsed with deionized water for another 3 min and stored in 1x SSC buffer at 4°C for 12 h before application of the hydrogel.
Electrochemical measurements were conducted using an EmStat Blue instrument (Palm Instruments BV) in square-wave voltammetry (SWV) mode. As only working and reference electrodes are employed in the device, the input connections for the reference and the counter electrode from the electrochemical analyzer were shorted. The WE was scanned in continuous succession with a scan period of 2 seconds, alternating between two SWV frequencies (400 and 60 Hz) at a constant SWV amplitude of 36 mV. Two frequencies were used in order to apply KDM for drift mitigation. As the MB redox peak was typically observed at about −350 mV in our setup, a potential range of −500 to −100 mV (with respect to the Ag/AgCl reference) was selected during the SWV scan. A custom peak-fitting script was created to fit the SWV measurements with a Gaussian curve on a hyperbolic baseline. Peak currents were then normalized to a baseline peak current to generate the signal gain. All reported gains were obtained via KDM, with the difference divided by the average of gains from the 400 and 60 Hz signals.
Hydrogel Coatings on Aptamer Devices:
Acrylamide monomers were purified through a basic alumina column. The hydrogel was applied to the sensing gold wire through capillary force, in which the sensor was dipped into the hydrogel prepolymer solution for 5 sec and removed immediately. The hydrogel was then photopolymerized by applying 365-nm UV light for 30 sec. No reduction in the MB peak current was observed after UV application. An Ag/AgCl reference electrode (75-μm-diameter Ag wire chlorinated in bleach overnight) was attached to the hydrogel-coated gold wire using heat shrinkable tubing. The final device was placed in 1x SSC buffer at 4 °C overnight before use.
In vitro Assessments of Aptamer Devices in Stationary and Flowing Blood:
Aptamer devices were incubated in EDTA-treated human whole blood (Bio-IVT) or inserted via a catheter into a closed-loop flowing blood set-up (Masterflex L/S Easy-Load II EW-77200-80 pump head at 1.5 mL/min) for 6 or 12 days at room temperature (Figure S19). Probes were then immersed in 2.5% GFA for 15 minutes and rinsed with PBS. Probes were then freeze-dried, lyophilized, and coated with Au:Pd for SEM imaging.
In vivo Assessment of Aptamer Devices:
Live animal studies were performed using Sprague–Dawley rats under Stanford APLAC protocol number 33226. Male rats were purchased from Charles River Laboratories at a weight of 300 - 350 g. The rats were anesthetized using isoflurane gas inhalation (2.5%) during the entirely of the experiment and drug infusions and monitored continuously. A small incision was made in both legs of the rat to expose both femoral veins. A 20G catheter was inserted into the left femoral vein for sensor probe insertion, and a 22G catheter was inserted into the right femoral vein for drug infusion. 0.1 - 0.3 mL of heparin (1000 U/mL, SAGENT Pharmaceuticals) were immediately infused through the catheter to prevent blood clotting. The needle of the 20G catheter was removed, catheter clipped, and the sensor was inserted deep into the vein, secured in place with a surgical suture, and allowed to equilibrate for 1-2 hours. A sequence of bolus injection was performed manually using a 5 mL syringe. In each administration of kanamycin through the sensor-free 22G catheter, either 100 or 200 μL of 200 mM kanamycin in PBS buffer was injected. At the end of the experiments, animals were euthanized by exsanguination while under general anesthesia.
Degradation Assay for Hydrogels:
Solutions of 12 M HCl, 12 M NaOH, 30% peroxide, and 1 mg/mL Amano Lipase PS were prepared (Sigma-Aldrich). Hydrogel discs were placed into one of each of these solutions at 50 °C for up to 72 hours. At specified time intervals, swollen hydrogel discs were removed from the solution and placed into deionized water for 24 hours to remove salts. Samples were then freeze-dried and lyophilized before mass was recorded. Weights were normalized to mass at time 0 to determine mass degradation.
Optical Microscopy and Roughness of Hydrogels:
Differential interference contrast (DIC) miscroscopy (10X) of the gel surfaces was performed with a Keyence VK-X250K/260K 3D laser scanning confocal microscope. We used a 408-nm violet laser and performed image analysis with VK Viewer and MultiFileAnalyzer software from Keyence. Surface roughness measurements used to quantify surface roughness measurements were defined and parameterized by ISO 25178 and measuring height profiles across the x-y plane of the hydrogel samples.
Statistical Analysis:
All values of significance were determined using a one-way ANOVA or student’s t-test with Prism GraphPad 8.4 software. As we noted drastic differences in clotting time between batches of blood, a paired t-test was used to determine significance between PEG-coated and F50-C50-coated probes in Figure 4. In Figure 5, an unpaired t-test was performed to compare performance of PEG-coated and F50-C50-coated sensors as there was one only group per leg of each animal (no pairing). In Figure S17 and Figure S22, a one-way ANOVA test was used as there were multiple groups being compared.
Supplementary Material
Acknowledgements
This work was supported in part by the NIDDK (R01DK119254). Doreen Chan is grateful for an award by the Department of Defense, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (ND-SEG) Fellowship, 32 CFR 168a with government support under FA9550-11-C-0028. Dr. Eneko Axpe is thankful for funding support from a Stanford Bio-X Interdisciplinary Initiative Program Round 8 (2016) Seed Grant. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 796557 INMARE. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation under award ECCS-1542152. We thank Grant Shao, Lab Operations Engineer at Stanford Nano Shared Facilities, for invaluable assistance in obtaining optical microscopy images and roughness measurements.
Footnotes
Ethical Statement
All animal procedures were performed according to Stanford APLAC approved protocols.
Code Availability
All code that supports the findings from this study is available on reasonable request.
Competing Interests
D.C., J.-C.C., E.A., H.T.S., and E.A.A are listed as authors on a provisional patent application describing the technology reported in this manuscript.
Contributor Information
Doreen Chan, Department of Chemistry, Stanford University, Stanford CA 94305, USA.
Jun-Chau Chien, Department of Electrical Engineering, Stanford University, Stanford CA 94305, USA.
Eneko Axpe, Department of Materials Science & Engineering, Stanford University, Stanford CA 94305, USA.
Louis Blankemeier, Department of Electrical Engineering, Stanford University, Stanford CA 94305, USA.
Samuel W. Baker, Department of Comparative Medicine, Stanford University, Stanford CA 94305, USA
Sarath Swaminathan, IBM Almaden Research Center, San Jose, CA 95120, USA.
Victoria A. Piunova, IBM Almaden Research Center, San Jose, CA 95120, USA
Dmitry Yu. Zubarev, IBM Almaden Research Center, San Jose, CA 95120, USA
Caitlin L. Maikawa, Department of Bioengineering, Stanford University, Stanford CA 94305, USA
Abigail K. Grosskopf, Department of Chemical Engineering, Stanford University, Stanford CA 94305, USA
Joseph L. Mann, Department of Materials Science & Engineering, Stanford University, Stanford CA 94305, USA
H. Tom Soh, Department of Electrical Engineering, Stanford University, Stanford CA 94305, USA; ChEM-H Institute, Stanford University, Stanford CA 94304, USA; Chan Zuckerberg Biohub, San Francisco, CA 94158, US.
Eric A. Appel, Department of Materials Science & Engineering, Stanford University, Stanford CA 94305, USA; Department of Bioengineering, Stanford University, Stanford CA 94305, USA; ChEM-H Institute, Stanford University, Stanford CA 94304, USA; Department of Pediatrics - Endocrinology, Stanford University School of Medicine, Stanford CA 94305, USA
Data Availability
All data supporting the results in this study are available within the article and its Supplementary Information. The broad range of raw datasets acquired and analyzed (or any subsets thereof), which would require contextual metadata for reuse, are available from the corresponding author upon reasonable request.
References
- 1.Harding JL & Reynolds MM Combating medical device fouling. Trends Biotechnol 32, 140–146, (2014). [DOI] [PubMed] [Google Scholar]
- 2.Poudineh M. et al. A fluorescence sandwich immunoassay for the real-time continuous detection of glucose and insulin in live animals. Nat Biomed Eng 5, 53–63, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo J-W et al. Real-time monitoring of drug pharmacokinetics within tumor tissue in live animals. Science Advances 8, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitt WG, Park K & Cooper SL Sequential Protein Adsorption and Thrombus Deposition on Polymeric Biomaterials. Journal of Colloid and Interface Science 111, 343–362, (1985). [Google Scholar]
- 5.Zhang T, Zhang X & Deng X Applications of protein resistant polymer and hydrogel coatings on biosensors and biomaterials. Annals of Biotechnology 2, 1–7, (2018). [Google Scholar]
- 6.Goudie MJ, Pant J & Handa H Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices. Sci Rep 7, 13623, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein AK, Wong TS, Belisle RA, Boggs EM & Aizenberg J Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc Natl Acad Sci U S A 109, 13182–13187, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie DC et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat Biotechnol 32, 1134–1140, (2014). [DOI] [PubMed] [Google Scholar]
- 9.MacCallum N. et al. Liquid-Infused Silicone As a Biofouling-Free Medical Material. ACS Biomaterials Science & Engineering 1, 43–51, (2014). [DOI] [PubMed] [Google Scholar]
- 10.Amini S. et al. Preventing mussel adhesion using lubricant-infused materials. Science 357, 668–673, (2017). [DOI] [PubMed] [Google Scholar]
- 11.Keefer LK Thwarting thrombus. Nature Materials 2, 357–358, (2003). [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Desai T & Ferrari M Proteins and cells on PEG immobilized silicon surfaces. Biomaterials 19, 953–960, (1998). [DOI] [PubMed] [Google Scholar]
- 13.Jo S & Park K Surface modification using silanated poly(ethylene glycol)s. Biomaterials 21, 605–616, (2000). [DOI] [PubMed] [Google Scholar]
- 14.Hawkins ML & Grunlan MA The protein resistance of silicones prepared with a PEO-silane amphiphile. Journal of Materials Chemistry 22, (2012). [Google Scholar]
- 15.Hawkins ML et al. Anti-protein and anti-bacterial behavior of amphiphilic silicones. Polym Chem 8, 5239–5251, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Li L, Zhao C & Zheng J Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 51, 5283–5293, (2010). [Google Scholar]
- 17.Herold DA, Keil K & Bruns DE Oxidation of Polyethylene Glycols by Alcohol Dehydrogenase. Biochemical Pharmacology 38, 73–76, (1989). [DOI] [PubMed] [Google Scholar]
- 18.Ostuni E. et al. Self-Assembled Monolayers That Resist the Adsorption of Proteins and the Adhesion of Bacterial and Mammalian Cells. Langmuir 17, 6336–6343, (2001). [Google Scholar]
- 19.Sung H-J et al. Poly(ethylene glycol) as a sensitive regulator of cell survival fate on polymeric biomaterials: the interplay of cell adhesion and pro-oxidant signaling mechanisms. Soft Matter 6, (2010). [Google Scholar]
- 20.Zustiak SP & Leach JB Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules 11, 1348–1357, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen M. et al. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: in vitro and in vivo studies. J Biomater Sci Polym Ed 13, 367–390, (2002). [DOI] [PubMed] [Google Scholar]
- 22.Ngo BKD & Grunlan MA Protein Resistant Polymeric Biomaterials. ACS Macro Letters 6, 992–1000, (2017). [DOI] [PubMed] [Google Scholar]
- 23.Jiang S & Cao Z Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater 22, 920–932, (2010). [DOI] [PubMed] [Google Scholar]
- 24.Laschewsky A Structures and Synthesis of Zwitterionic Polymers. Polymers 6, 1544–1601, (2014). [Google Scholar]
- 25.Vercellotti GM, Hammerschmidt DE, Craddock PR & Jacob HS Activation of Plasma Complement by Perfluorocarbon Artificial Blood: Probably Mechanism of Adverse Pulmonary Reactions in Treated Patients and Rationale for Corticosteroid Prophylaxis. Blood 59, 1299–1304, (1982). [PubMed] [Google Scholar]
- 26.Wang Z & Zuilhof H Antifouling Properties of Fluoropolymer Brushes toward Organic Polymers: The Influence of Composition, Thickness, Brush Architecture, and Annealing. Langmuir 32, 6571–6581, (2016). [DOI] [PubMed] [Google Scholar]
- 27.Chen PR, Wang TC, Chen ST, Chen HY & Tsai WB Development of Antifouling Hyperbranched Polyglycerol Layers on Hydroxyl Poly-p-xylylene Coatings. Langmuir 33, 14657–14662, (2017). [DOI] [PubMed] [Google Scholar]
- 28.Gam-Derouich S. et al. Highly hydrophilic surfaces from polyglycidol grafts with dual antifouling and specific protein recognition properties. Langmuir 27, 9285–9294, (2011). [DOI] [PubMed] [Google Scholar]
- 29.Davis KA & Anseth KS Controlled Release from Crosslinked Degradable Networks. Vol. 19 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Lin CC & Metters AT Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev 58, 1379–1408, (2006). [DOI] [PubMed] [Google Scholar]
- 31.Jo YS, Gantz J, Hubbell JA & Lutolf MP Tailoring hydrogel degradation and drug release via neighboring amino acid controlled esterhydrolysis. Soft Matter 5, 440–446, (2009). [Google Scholar]
- 32.Smith JR et al. Integration of Combinatorial Synthesis, Rapid Screening, and Computational Modeling in Biomaterials Development. Macromolecular Rapid Communications 25, 127–140, (2004). [Google Scholar]
- 33.Webster DC, Chisholm BJ & Stafslien SJ Mini-review: combinatorial approaches for the design of novel coating systems. Biofouling 23, 179–192, (2007). [DOI] [PubMed] [Google Scholar]
- 34.Rostam HM et al. Immune-Instructive Polymers Control Macrophage Phenotype and Modulate the Foreign Body ResponseIn Vivo. Matter 2, 1–18, (2020). [Google Scholar]
- 35.Fletcher JT, Finlay JA, Callow ME, Callow JA & Ghadiri MR A combinatorial approach to the discovery of biocidal six-residue cyclic D,L-alpha-peptides against the bacteria methicillin-resistant Staphylococcus aureus (MRSA) and E. coli and the biofouling algae Ulva linza and Navicula perminuta. Chemistry 13, 4008–4013, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieper RJ et al. Combinatorial approach to study the effect of acrylic polyol composition on the properties of crosslinked siloxane-polyurethane fouling-release coatings. Journal of Coatings Technology and Research 4, 453–461, (2007). [Google Scholar]
- 37.Hook AL et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat Biotechnol 30, 868–875, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu M. et al. Combinatorial synthesis with high throughput discovery of protein-resistant membrane surfaces. Biomaterials 34, 6133–6138, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vegas AJ et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol 34, 345–352, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater 9, 768–778, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Celiz AD et al. Chemically diverse polymer microarrays and high throughput surface characterisation: a method for discovery of materials for stem cell culture dagger Electronic supplementary information (ESI) available. See DOI: 10.1039/c4bm00054d Click here for additional data file. Biomater Sci 2, 1604–1611, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi TF et al. Cytotoxicity and altered c-myc gene expression by medical polyacrylamide hydrogel. J Biomed Mater Res A 78, 283–290, (2006). [DOI] [PubMed] [Google Scholar]
- 43.Sun JY et al. Highly stretchable and tough hydrogels. Nature 489, 133–136, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhosseini Hamedani B, Navidbakhsh M & Ahmadi Tafti H Comparison between mechanical properties of human saphenous vein and umbilical vein. BioMedical Engineering OnLine 11, 1–15, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khamdaeng T, Luo J, Vappou J, Terdtoon P & Konofagou EE Arterial stiffness identification of the human carotid artery using the stress-strain relationship in vivo. Ultrasonics 52, 402–411, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo J & Chen C A Method of Calculating Copolymerization Reactivity Ratios. Journal of Applied Polymer Science 26, 1117–1128, (1981). [Google Scholar]
- 47.Gifford R. et al. Protein interactions with subcutaneously implanted biosensors. Biomaterials 27, 2587–2598, (2006). [DOI] [PubMed] [Google Scholar]
- 48.Brash JL Hydrophobic Polymer Surfaces and their Interactions with Blood. Vol. 283 356–371 (The New York Academy of Sciences, 1977). [Google Scholar]
- 49.Vaddiraju S, Burgess DJ, Tomazos I, Jain FC & Papadimitrakopoulos F Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. Jounral of Diabetes Science and Technology 4, 1540–1562, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wörz A, Berchtold B, Moosmann K, Prucker O & Rühe J Protein-resistant polymer surfaces. Journal of Materials Chemistry 22, (2012). [Google Scholar]
- 51.Zhao C. et al. Dual functionality of antimicrobial and antifouling of poly(N-hydroxyethylacrylamide)/salicylate hydrogels. Langmuir 29, 1517–1524, (2013). [DOI] [PubMed] [Google Scholar]
- 52.Park K, Shim HS, Dewanjee MK & Eigler NL In vitro and in vivo studies of PEO-grafted blood-contacting cardiovascular prostheses. Journal of Biomaterials Science, Polymer Edition 11, 1121–1134, (2012). [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, Singh A, Lalani R & Liu L Ultralow fouling polyacrylamide on gold surfaces via surface-initiated atom transfer radical polymerization. Biomacromolecules 13, 1086–1092, (2012). [DOI] [PubMed] [Google Scholar]
- 54.Wong TS et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477, 443–447, (2011). [DOI] [PubMed] [Google Scholar]
- 55.Brash JL, Horbett TA, Latour RA & Tengvall P The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater 94, 11–24, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zilla P. et al. Scanning Electron Microscopy of Circulating Platelets Reveals New Aspects of Platelet Alteration During Cardiopulmonary Bypass Operations. Texas Heart Institute Journal 14, 13–21, (1987). [PMC free article] [PubMed] [Google Scholar]
- 57.Le TC, Penna M, Winkler DA & Yarovsky I Quantitative design rules for protein-resistant surface coatings using machine learning. Sci Rep 9, 265, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu N, Xu Z, Morrin A & Luo X Low fouling strategies for electrochemical biosensors targeting disease biomarkers. Analytical Methods 11, 702–711, (2019). [Google Scholar]
- 59.Jeon SI, Lee JH, Andrade JD & De Gennes PG Protein-Surface Interactions in the Presence of Polyethylene Oxide. Journal of Colloid and Interface Science 142, 149–158, (1991). [Google Scholar]
- 60.Szleifer I. Protein Adsorption on Surfaces with Grafted Polymers: A Theoretical Approach. Biophysical Journal 72, 595–612, (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang F & Szleifer I Controlled release of proteins from polymer-modified surfaces. Proc Natl Acad Sci U S A 103, 5769–5774, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrade JD & Hlady V Biopolymers/Non-Exclusion HPLC. Advances in Polymer Science. Vol. 79 (Springer, 1986). [Google Scholar]
- 63.Liu Y. et al. Computational Investigation of Antifouling Property of Polyacrylamide Brushes. Langmuir 36, 2757–2766, (2020). [DOI] [PubMed] [Google Scholar]
- 64.Penna M, Ley K, Maclaughlin S & Yarovsky I Surface heterogeneity: a friend or foe of protein adsorption - insights from theoretical simulations. Faraday Discuss 191, 435–464, (2016). [DOI] [PubMed] [Google Scholar]
- 65.Penna M. et al. Hydration and Dynamics of Ligands Determine the Antifouling Capacity of Functionalized Surfaces. The Journal of Physical Chemistry C 123, 30360–30372, (2019). [Google Scholar]
- 66.Todeschini R & Consonni V in Handbook of Chemoinformatics: From Data to Knowledge in 4 Volumes (ed Gasteiger J) Ch. VIII.2, 1004–1033 (WILEY-VCH Verlag GmbH & Co. KgaA, 2003). [Google Scholar]
- 67.Yu Y. et al. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv Mater 31, e1807101, (2019). [DOI] [PubMed] [Google Scholar]
- 68.Lee KY et al. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules 33, 4291–4294, (2000). [Google Scholar]
- 69.Jeong JW et al. Soft materials in neuroengineering for hard problems in neuroscience. Neuron 86, 175–186, (2015). [DOI] [PubMed] [Google Scholar]
- 70.Lacour SP, Courtine G & Guck J Materials and technologies for soft implantable neuroprostheses. Nature Reviews Materials 1, (2016). [Google Scholar]
- 71.Onuki Y, Bhardwaj U, Papadimitrakopoulos F & Burgess DJ A Review of the Biocompatibility of Implantable Devices: Current Challenges to Overcome Foreign Body Response. Journal of Diabetes Science and Technology 2, 1003–1015, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carr L, Cheng G, Xue H & Jiang S Engineering the polymer backbone to strengthen nonfouling sulfobetaine hydrogels. Langmuir 26, 14793–14798, (2010). [DOI] [PubMed] [Google Scholar]
- 73.Stopa B. et al. Albumin binds self-assembling dyes as specific polymolecular ligands. Int J Biol Macromol 40, 1–8, (2006). [DOI] [PubMed] [Google Scholar]
- 74.Teo AJT et al. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomaterials Science & Engineering 2, 454–472, (2016). [DOI] [PubMed] [Google Scholar]
- 75.Kozai TD et al. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 37, 25–39, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barfidokht A & Gooding JJ Approaches Toward Allowing Electroanalytical Devices to be Used in Biological Fluids. Electroanalysis 26, 1182–1196, (2014). [Google Scholar]
- 77.Labib M, Sargent EH & Kelley SO Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem Rev 116, 9001–9090, (2016). [DOI] [PubMed] [Google Scholar]
- 78.Maji D. et al. ClotChip: A Microfluidic Dielectric Sensor for Point-of-Care Assessment of Hemostasis. IEEE Trans. Biomed. Circuits Syst 11, 1459–1469, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toyoda T. et al. Direct activation of platelets by addition of CaCl2 leads coagulation of platelet-rich plasma. Int J Implant Dent 4, 23, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elgrishi N. et al. A Practical Beginner’s Guide to Cyclic Voltammetry. Journal of Chemical Education 95, 197–206, (2017). [Google Scholar]
- 81.Hoss U & Budiman ES Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology. Diabetes Technol Ther 19, S44–S50, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ellington AD In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822, (1990). [DOI] [PubMed] [Google Scholar]
- 83.Bock LC, Griffin LC, Latham JA, Vermaas EH & Toole JJ Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 355, 564–566, (1992). [DOI] [PubMed] [Google Scholar]
- 84.Song S, Wang L, Li J, Fan C & Zhao J Aptamer-based biosensors. TrAC Trends in Analytical Chemistry 27, 108–117, (2008). [Google Scholar]
- 85.Gold L SELEX: How It Happened and Where It will Go. J Mol Evol 81, 140–143, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferguson BS et al. Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Science Translational Medicine 5, 1–9, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mage PL et al. Closed-loop control of circulating drug levels in live animals. Nature Biomedical Engineering 1, (2017). [Google Scholar]
- 88.Arroyo-Currás N, Dauphin-Ducharme P, Scida K & Chávez JL From the beaker to the body: translational challenges for electrochemical, aptamer-based sensors. Analytical Methods 12, 1288–1310, (2020). [Google Scholar]
- 89.Lovering AM & Reeves DS in Antibiotic and Chemotherapy Ch. 12, 145–169 (Elsevier, 2011). [Google Scholar]
- 90.Yu H, Xu Z, Lei H, Hu M & Yang Q Photoinduced graft polymerization of acrylamide on polypropylene microporous membranes for the improvement of antifouling characteristics in a submerged membrane-bioreactor. Separation and Purification Technology 53, 119–125, (2007). [Google Scholar]
- 91.Zhao C. et al. Antifouling and biodegradable poly(N-hydroxyethyl acrylamide) (polyHEAA)-based nanogels. RSC Advances 3, (2013). [Google Scholar]
- 92.Mondal S & Wickramasinghe SR Photo-induced graft polymerization of N-isopropyl acrylamide on thin film composite membrane: Produced water treatment and antifouling properties. Separation and Purification Technology 90, 231–238, (2012). [Google Scholar]
- 93.Yang J, Bai R, Chen B & Suo Z Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Advanced Functional Materials 30, (2019). [Google Scholar]
- 94.Caulfield MJ, Qiao GG & Solomon DH Some Aspects of the Properties and Degradation of Polyacrylamides. Chemical Reviews 102, 3067–3084, (2002). [DOI] [PubMed] [Google Scholar]
- 95.Nishiyama N. Hydrolytic stability of methacrylamide in acidic aqueous solution. Biomaterials 25, 965–969, (2004). [DOI] [PubMed] [Google Scholar]
- 96.Norde W & Haynes CA (ed Horbett and Brash) Ch. 2, 26–40 (American Chemical society, 1995). [Google Scholar]
- 97.Campuzano S, Pedrero M, Yanez-Sedeno P & Pingarron JM Antifouling (Bio)materials for Electrochemical (Bio)sensing. Int J Mol Sci 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torres JR, Jay GD, Kim KS & Bothun GD Adhesion in hydrogel contacts. Proc Math Phys Eng Sci 472, 20150892, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang T, Yuk H, Lin S, Parada GA & Zhao X Tough and tunable adhesion of hydrogels: experiments and models. Acta Mechanica Sinica 33, 543–554, (2017). [Google Scholar]
- 100.Yi H, Lee SH, Seong M, Kwak MK & Jeong HE Bioinspired reversible hydrogel adhesives for wet and underwater surfaces. J Mater Chem B 6, 8064–8070, (2018). [DOI] [PubMed] [Google Scholar]
- 101.Zhang L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol 31, 553–556, (2013). [DOI] [PubMed] [Google Scholar]
- 102.Kolewe KW, Peyton SR & Schiffman JD Fewer Bacteria Adhere to Softer Hydrogels. ACS Appl Mater Interfaces 7, 19562–19569, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolewe KW, Dobosz KM, Emrick T, Nonnenmann SS & Schiffman JD Fouling-Resistant Hydrogels Prepared by the Swelling-Assisted Infusion and Polymerization of Dopamine. ACS Appl Bio Mater 1, 33–41, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yesildag C, Ouyang Z, Zhang Z & Lensen MC Micro-Patterning of PEG-Based Hydrogels With Gold Nanoparticles Using a Reactive Micro-Contact-Printing Approach. Front Chem 6, 667, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakala GP & Reches M Peptide-Based Approaches to Fight Biofouling. Advanced Materials Interfaces 5, (2018). [Google Scholar]
- 106.Xiong R. et al. Sizing nanomaterials in bio-fluids by cFRAP enables protein aggregation measurements and diagnosis of bio-barrier permeability. Nat Commun 7, 12982, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hadjiev NA & Amsden BG An assessment of the ability of the obstruction-scaling model to estimate solute diffusion coefficients in hydrogels. J Control Release 199, 10–16, (2015). [DOI] [PubMed] [Google Scholar]
- 108.Tong J & Anderson JL Partitioning and Diffusion of Proteins and Linear Polymers in Polyacrylamide Gels. Biophysical Journal 70, 1505–1513, (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hagel V, Haraszti T & Boehm H Diffusion and Interaction in PEG-DA hydrogels. Biointerfaces 8, 1–9, (2013). [DOI] [PubMed] [Google Scholar]
- 110.Liu DE et al. Macromolecule Sorption and Diffusion in HEMA/MAA Hydrogels. Industrial & Engineering Chemistry Research 52, 18109–18120, (2013). [Google Scholar]
- 111.Bengani P, Kou Y & Asatekin A Zwitterionic copolymer self-assembly for fouling resistant, high flux membranes with size-based small molecule selectivity. Journal of Membrane Science 493, 755–765, (2015). [Google Scholar]
- 112.Klein C, Iacovella CR, McCabe C & Cummings PT Tunable transition from hydration to monomer-supported lubrication in zwitterionic monolayers revealed by molecular dynamics simulation. Soft Matter 11, 3340–3346, (2015). [DOI] [PubMed] [Google Scholar]
- 113.Vousoughi P, Moghbeli MR & Nikkhah SJ Molecular Dynamics (MD) Simulation of Zwitterion-Functionalized PMMA with Hydrophilic and Antifouling Surface Characteristics. Macromolecular Research 27, 1200–1209, (2019). [Google Scholar]
- 114.Liu FT, Ting KM & Zhou Z-H in 2008 Eighth IEEE International Conference on Data Mining 413–422 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results in this study are available within the article and its Supplementary Information. The broad range of raw datasets acquired and analyzed (or any subsets thereof), which would require contextual metadata for reuse, are available from the corresponding author upon reasonable request.


