Abstract
The exponential rise of healthcare problems like human aging and road traffic accidents have developed an intrinsic challenge to biomedical sectors concerning the arrangement of patient-specific biomedical products. The additively manufactured implants and scaffolds have captured global attention over the last two decades concerning their printing quality and ease of manufacturing. However, the inherent challenges associated with additive manufacturing (AM) technologies, namely process selection, level of complexity, printing speed, resolution, biomaterial choice, and consumed energy, still pose several limitations on their use. Recently, the whole world has faced severe supply chain disruptions of personal protective equipment and basic medical facilities due to a respiratory disease known as the coronavirus (COVID-19). In this regard, local and global AM manufacturers have printed biomedical products to level the supply–demand equation. The potential of AM technologies for biomedical applications before, during, and post-COVID-19 pandemic alongwith its relation to the industry 4.0 (I4.0) concept is discussed herein. Moreover, additive manufacturing technologies are studied in this work concerning their working principle, classification, materials, processing variables, output responses, merits, challenges, and biomedical applications. Different factors affecting the sustainable performance in AM for biomedical applications are discussed with more focus on the comparative examination of consumed energy to determine which process is more sustainable. The recent advancements in the field like 4D printing and 5D printing are useful for the successful implementation of I4.0 to combat any future pandemic scenario. The potential of hybrid printing, multi-materials printing, and printing with smart materials, has been identified as hot research areas to produce scaffolds and implants in regenerative medicine, tissue engineering, and orthopedic implants.
Keywords: Additive manufacturing, Biomedical applications, 3D bioprinting, Energy consumption, COVID-19, Industry 4.0, Tissue engineering, Implants
Introduction
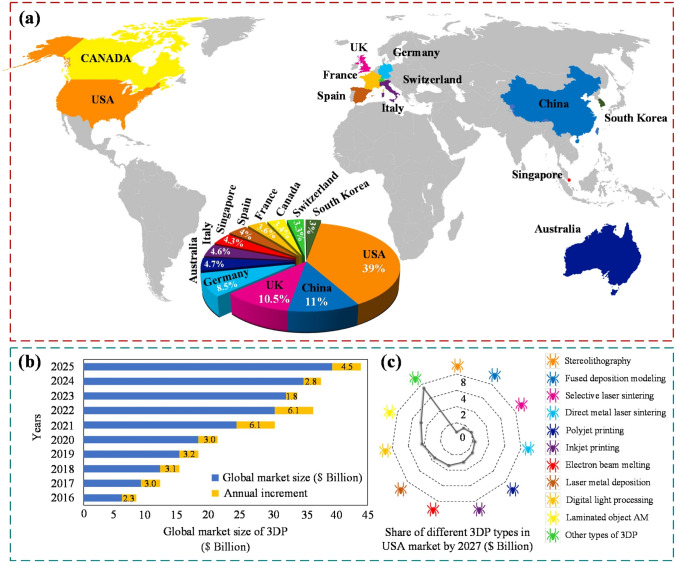
Additive manufacturing (AM) technologies are considered suitable manufacturing solutions for different industrial applications ranging from aerospace to biomedical sectors, even for industry 4.0. In the biomedical field, researchers are consistently working to fabricate functional parts to mimic the natural functioning of soft and hard tissues in the human body [1, 2]. Recently, the promising role of additive manufacturing to fabricate various biomedical products during the COVID-19 pandemic cannot be overlooked [3]. Furthermore, several works have been performed to reduce energy consumption and harmful carbon emissions in additively manufactured products to develop sustainable and environmentally responsive production [4]. An overview of different application prospects of AM throughout the globe is provided in Fig. 1.
Fig. 1.
Different applications’ prospects of additive manufacturing or 3D printing(3DP). a Global industrial rise of additive manufacturing in different countries, b influence of additive manufacturing on global market capture, c USA-based market share forecast in AM technologies by 2027 [5];
adopted with permission from Elsevier, Copyright 2021 (Lic. 5181740946721)
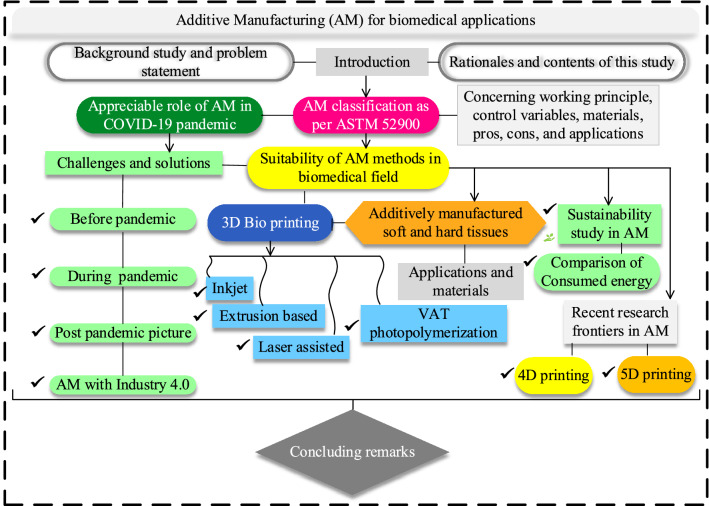
Increasing demand for implants and scaffolds has been evident recently because of road traffic accidents and human aging issues. It has arisen the need to produce extremely high-quality patient-specific implants to alleviate the repeating surgery, patient pain, and replacement of dysfunctional tissues. The conventional bone repairing techniques, like distraction osteogenesis and bone grafts, pose several limitations [6]. Additive manufacturing technologies are considered a good choice for fabricating various customized medical products with net or near-net shapes in a layer-wise manner. Typically, magnetic imaging or computed tomography (CT) scans are used to extract patient-specific geometries. The merits of additively manufactured biomedical products include minimal production time, mass customization, higher productivity, and minimal post-processing required [7]. Numerous other positive aspects include the reduction in surgery repetitiveness, improved implant fixation, recovery of the natural functioning of joint kinetics, assistance in bone ingrowth, improved cell proliferation, good mechanical properties, and the repair of osteochondral defects [8, 9]. The additively manufactured biomedical items are typically used in tissue/organ repair, drug delivery, clinical medicine, tissue engineering, and prosthetic implants that are specifically designed for the patient [7]. Thus, it can be argued that AM possesses a solid potential to produce a wide range of biomedical products. The nomenclature, including the acronyms and their complete text abbreviations, is mentioned in Table 1. This work's contents and sub-contents have been described in a flow chart as presented in Fig. 2.
Table 1.
Acronyms and their abbreviations
| Abbreviation | Description | Abbreviation | Description |
|---|---|---|---|
| AM | Additive manufacturing | VPP | VAT photopolymerization |
| 3DBP | 3D bioprinting | Ec | Energy consumption |
| FEF | Freeze-form extrusion fabrication | SEc | Specific energy consumption |
| DIW | Direct ink writing | Ea | Primary energy |
| SLA | Stereolithography | Eb | Secondary energy |
| PPE | Personal protective equipment | ||
| MJM | MultiJet modeling | USEIA | US energy information agency |
| RFP | Rapid freeze prototyping | ASTM | American standard of testing materials |
| SLM | Selective laser melting | PLA | Polylactic acid |
| DED | Directed energy deposition | Et | Total energy |
| MJ | Material jetting | MMC’s | Metal matrix composites |
| IJ 3DP | Inkjet 3D printing | NIOSH | National Institute of Occupational Safety and Health |
| EBM | Electron beam melting | EUA | Emergency use authorization |
| WHO | World Health Organization | LAB | Laser-assisted bioprinting |
| LENS | Laser-engineered net shaping | ECM | Extra-cellular matrix |
| ME | Material extrusion | DOD | Drop on demand |
| LOM | Laminated object manufacturing | Ti | Titanium |
| LIFT | Laser-induced forward transfer | TMC’s | Titanium matrix composites |
| GDP | Gross domestic produce | PEGDMA | Polyethylene glycol Dimethacrylate |
| BJ | Binder jetting | I4.0 | Industry 4.0 |
| Br | Build rate | CPAP | Continuous positive airways pressure |
| LDW | Laser direct writing | ABS | Acrylonitrile Butadiene Styrene |
| PEEK | Polyether ether ketone | PETE | Polyethylene |
| PVA | Polyvinyl acetate | PA | Polyamide |
| GelMA | Gelatin methacrylate | PLA | Polylactic acid |
Fig. 2.
Schematic flow chart of the current study including all the major contents and sub-contents to describe the work methodology
The World Health Organization (WHO) recognized COVID-19 as a pandemic on March 11, 2020, after the first verified case of the disease was reported in China in December 2019 [3, 10]. During this duration, COVID-19 rapidly spread in 188 countries, with 36.6 million infected patients and 1,063,000 global deaths (as investigated by Johns Hopkins University researchers) [3]. Due to the increased patient traffic, hospitals experienced supply chain disruptions for essential aid [11]. Specifically, patients' primary care was at risk in the early COVID-19 pandemic because the world faced shortages in primary healthcare products like personal protective equipment (PPE), which was a significant threat to patients and doctors. Healthcare systems were found to be in war footing condition to boost their supply of beds and trained workers to meet the rapid demands of medical supplies [12]. Also, the employees in local manufacturing companies were reduced due to the lockdown in many high-risk regions worldwide [13–15]. However, the response to this healthcare emergency was inefficient because the local manufacturers have not previously experienced this much pandemic disaster and had difficulty fulfilling the safety regulatory standards. The key challenge during the pandemic was alleviating the difficulties and increasing the global supply and demand production capacity [16]. Therefore, several corporations around the world installed additive manufacturing setups to produce different biomedical products like face shields, housings, manikins, swabs, N95 respirators, and surgical masks according to safety regulations.
The usual additively manufactured personal protective equipment includes face masks, valves, visors, safety goggles, isolation chambers and wards, N95 respirators, ventilators, continuous positive airways pressure (CPAP) machines, and ear savers. The highly prominent AM techniques since the COVID-19 pandemic to fabricate biomedical products include fused deposition modeling (FDM), stereolithography (SLA), selective laser sintering (SLS), binder jetting (BJ), and multi-jet modeling (MJM) [17]. The burden on conventional manufacturing technologies was reduced and the supply chain bottlenecks were resolved by AM technologies [18]. The safety regulatory authorities like the Food and Drug Administration (FDA), National Institute of Occupational Safety and Health (NIOSH), and WHO helped the manufacturers with emergency use authorization (EUA) and safety instructions. The pandemic developed the opportunity for AM technologies to fabricate robust and customized biomedical products to cope with shortages in supplies and demand [16, 18, 19]. Thus, AM possesses a key role in producing various biomedical products in response to emergencies.
The sustainable manufacturing system is the crucial goal of AM technologies which can be accomplished by minimizing the consumed energy. The estimation of consumed energy in AM necessarily involves the measurement of primary and secondary energy. The energy consumption of all AM technologies depends on their procedural requirements [20]. Thus, an eco-friendly production in AM demands minimum consumed energy and harmful emissions [4, 21].
This research study attempts to answer the following important questions: (a) how AM systems have helped before, during, and after the COVID-19 pandemic alongwith their conjunction with industry 4.0; (b) explain different ASTM-52900 standard-based additive manufacturing systems concerning their working principle, materials, control variables, pros, cons, and biomedical applications; (c) classify four major 3D bioprinting techniques based on their working principle, materials, control variables, pros, cons, and applications; (d) how are AM methods suitable to fabricate soft and hard tissues/implants along with their merits, challenges, and applications; (e) what are different factors affecting sustainability in additive manufacturing and which AM technologies consume more energy; (f) discuss recent research frontiers in AM (4D printing and 5D printing) for biomedical applications.
This work is composed of seven main sections followed by their sub-sections. First, there is an introduction section to describe the summary including the background and importance of the study with suitable rationales. Second, the nomenclature section is provided for all acronyms and their full abbreviations. The positive role of AM before, during, and post-COVID-19 pandemic alongwith the correlation of AM with industry 4.0, is expressed in Sect. 3. The fourth section reviews the additive manufacturing classification for biomedical applications based on ASTM 52,900. Major 3D bioprinting technologies like inkjet, micro-extrusion, laser assisted, and VAT photopolymerization, have been discussed in Sect. 5. Each AM technology briefly describes its working principle, nomenclature, biomaterials, essential variables, responses, pros and cons, and biomedical applications. The suitability of AM methods for soft and hard tissues/constructs is also discussed in sub-sections of Sect. 5. The sustainability study in AM for biomedical applications is explained in Sect. 6 with a key focus toward energy consumption to determine which process is sustainable. The research frontiers in the field involving 4D printing and 5D printing for biomedical applications have been explained in Sect. 7. The final section concludes the summary of the whole work along with some recent research frontiers in the field.
Nomenclature
See Table 1.
Appreciable role of AM against the COVID-19 pandemic
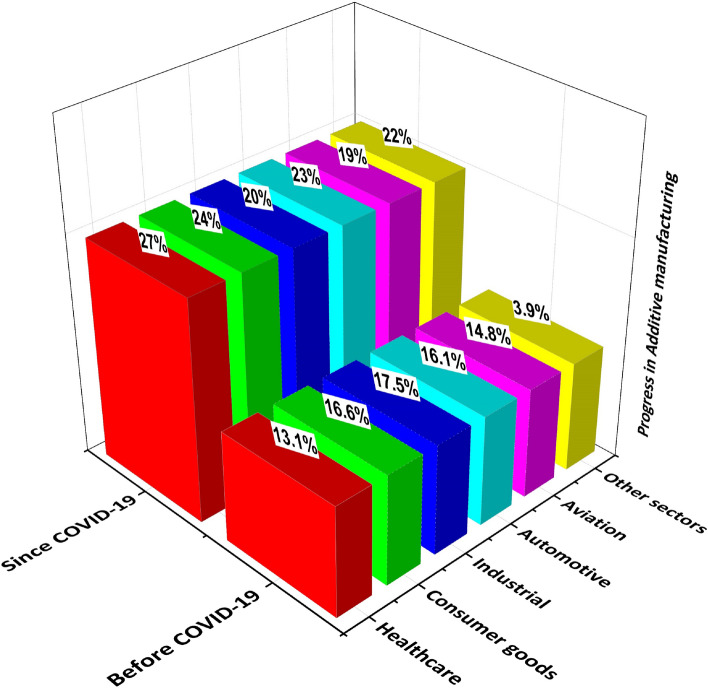
The additively manufactured medical products provided relief to the healthcare systems by alleviating the disruptions in global supplies and demands. The hygienic personal protective equipments, like face masks, face shields, valves, test swabs, visors, safety goggles, isolation chambers and wards, N95 respirators, ventilators, continuous positive airways pressure (CPAP) machines, surgery masks, ear savers, were additively manufactured [17]. Thus, the global rise of applications of AM technologies is evident in the recent era since the COVID-19 pandemic challenge, which is mainly discussed herein. The statistical significance of AM can be described by the % progress in various engineering sectors before and since the COVID-19 pandemic, as demonstrated in Fig. 3. The healthcare sector has progressed a lot among other industries to produce biomedical products during COVID-19, which indicates the supremacy of AM for biomedical applications [22, 23].
Fig. 3.
The demonstration of progress in additive manufacturing in various industrial sectors before and during the COVID-19 pandemic (inspired from [22, 23])
Challenges of AM for biomedical applications during the COVID-19 pandemic
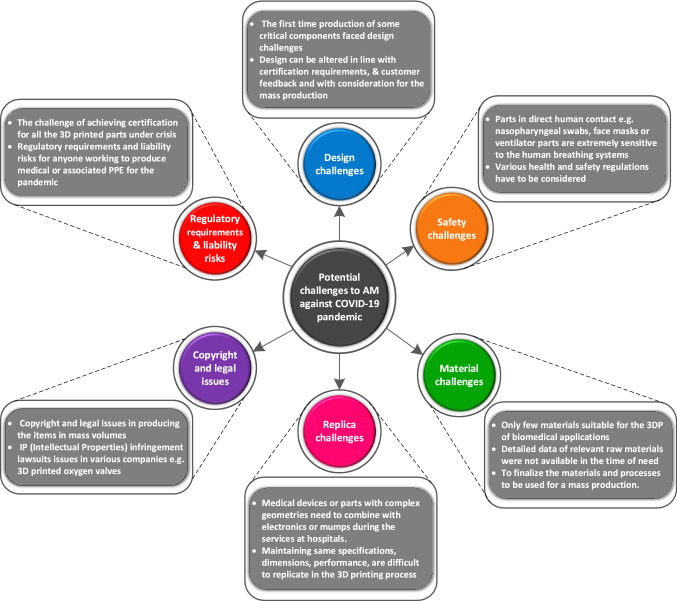
Additive manufacturing technologies are considered suitable solutions to fabricate soft and hard tissue/constructs. Since early 2020, the global challenge was to alleviate the severity of respiratory infection namely the COVID-19 pandemic among the people. The individual governments adopted several big decisions and harsh steps like social distancing, the necessary use of face masks, and restriction on travel to public places. This has exponentially increased the demand for biomedical products [3]. Consequently, conventional manufacturers faced a severe shortage of medical supplies for frontline healthcare workers and patients. Additive manufacturing technologies have positively contributed to fabricating various medical devices like personal protective equipment (PPE) to meet the shortages of local and global supplies [15, 24]. Thus, AM has sufficient potential to contribute positively during emergencies by incorporating mass customization in design and complexity [19]. However, several challenges in design, material, safety, copyright, replica, and regulatory requirements were faced by AM service providers. A schematic demonstration of several challenges being faced by the manufacturers in AM for biomedical applications is provided in Fig. 4.
Fig. 4.
A detailed demonstration of several challenges faced by AM for biomedical applications since the COVID-19 pandemic [5];
adopted with permission from Elsevier, Copyright 2021 (Lic. 5181740946721)
Promising AM techniques during the COVID-19 pandemic
Since the global supplies and demands were very high to manage the need for life-saving equipment, FDA approved EUA to meet the rising demand for ventilators. Several manufacturers like Isinnova, Lonati, Airwolf3D, 3D systems, and Materialize helped the healthcare community in fabricating AM ventilator parts [18, 19]. Numerous polymeric materials like polyether ether ketone (PEEK), polycarbonate (PC), polystyrene (PS), and high-density polyethylene (HDPE) were employed to fabricate PPE [25]. The most prominent AM techniques to fabricate COVID-19 pandemic-related biomedical products include fused deposition modeling (FDM), selective laser sintering (SLS), stereolithography (SLA), multi-jet fusion (MJF), and digital light processing (DLP). However, several criteria like biocompatibility, disinfection procedure, and non-toxicity were considered a challenge to select a suitable AM type [18].
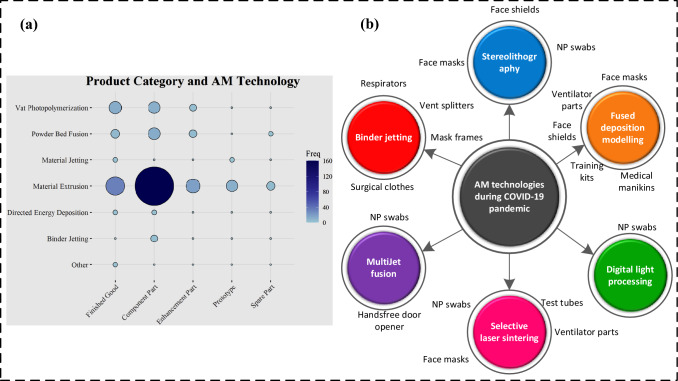
The fused deposition modeling performs working by melting and deposition. In the recent era, multi-head FDM printers print complex and intricate shapes. FDM resembles conventional manufacturing techniques like injection molding or extrusion except that no mold is required in it [26]. The upward or downward movement of the support structure happens after the previous layer is completed [27]. Selective laser sintering (SLS) is a PBF technique in which laser energy performs layer-by-layer sintering and melting. MultiJet fusion (MJF) employs the fusing agent (usually ink) to dispense the powder for infrared light absorption. Then ink areas are fused to perform bonding with the polymeric powder through high-power infrared energy [28]. During the pandemic, the key AM medical products mainly comprise swabs, face shields, masks, and ventilators. The contribution of AM technologies to fight against the COVID-19 pandemic by producing several biomedical products is illustrated in Fig. 5.
Fig. 5.
Schematic illustration to indicate the promising contribution of AM technologies in fighting against the COVID-19 pandemic, a usage frequency of various AM technologies during COVID-19, [25] (open access permission); b AM technologies alongwith their applications in the COVID-19 pandemic (inspired from [18])
Additively manufactured biomedical products since the COVID-19 pandemic
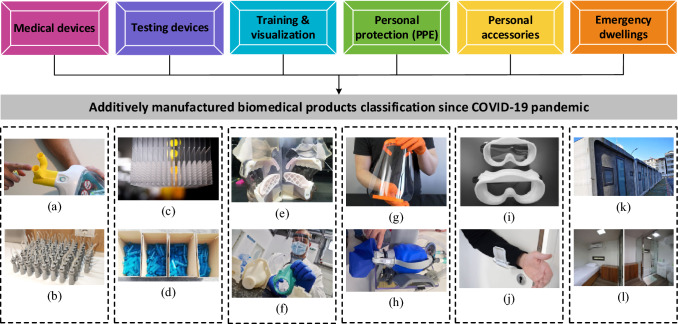
Several manufacturing and research organizations have developed their fabrication of face masks like Czech Technical University (CTU) [18]. The researchers from Maker Mask, Farsoon technologies, and Standard university helped to print the FDA-approved open-source designed face masks to cope with the unprecedented demands [29–31]. The personal safety of the frontline health workers was at risk because of the shortages in supplies of face shields to protect them against the infected persons. A face mask is supposed to cover the mouth, especially the nose to filter air and provide a sort of protection against the environmental situation. The N95 masks with suitable fitting were found useful to protect against harmful air contaminants. The name 95 in N95 masks indicates the ability to filter 95% of airborne particles. The manufacturers like Flashforge, Stratasys, and BCN3D assisted in fabricating the face shields mainly from polylactic acid (PLA) and polyethylene terephthalate glycol (PETG) through AM. Also, the AM-modified designs helped conventional manufacturers to enhance the production capacity of face shields [16, 29, 30]. Additive manufacturing also helped in producing various ventilator parts. The nasopharyngeal (NP) biocompatible swabs were also 3D printed to level the supplies and demands. Several manufacturing companies like Puritan medical products, Copan diagnostics, EnvisionTEC, and Formlabs printers started the production of NP swabs to fulfill the requirements during the COVID-19 pandemic [18, 32]. A detailed overview of various additively manufactured biomedical products is presented in Fig. 6.
Fig. 6.
Classification of additively manufactured biomedical products during the COVID-19 pandemic: a ventilator valves, b emergency respiration devices, c NP swabs, d test tubes, e manikins and bio-models, f training kits, g face shield, h respirator, i mask fitters, j door openers, k dwelling cabins, and l isolation wards; [5],
reprinted with permission from Elsevier, Copyright 2021 (Lic. 5181740946721)
Additive manufacturing also helped in overcoming the environmental hazards regarding medical waste of disposable products by fabricating recyclable products such as respirators and filters [33]. Additive manufactured emergency dwellings were employed for the isolation of infected patients to reduce rushing in hospitals [34]. These dwellings were urgently moved to the highly needed areas during the rushing time in hospitals. The healthcare workers were also trained with AM-fabricated life-sized medical manikins and prototypes to practice COVID-19 testing procedures [35]. Social media provided awareness to industries and users about the online CAD file repository websites like GitHub and GitLab, to promote AM. These platforms helped to overcome the communication barriers among different professionals and CAD data repositories for COVID-19-related PPE.
AM is a pre-requisite for implementing I4.0 to combat the COVID-19 pandemic
Additive manufacturing was found to have a strong potential in assisting against emergencies as COVID-19 is not the last disease. These situations indicate that AM exhibits a bright future by producing parts on demand with mass customization. It also helped a lot to develop sustainable solutions during supply chain redundancy and maintained the global economy. Therefore, the researchers are consistently working on technological advancements in AM techniques concerning materials and methods. The digital versatility and quick prototyping are boosted by integrating AM with industry 4.0 and the internet of things in the cyber-physical age to make it more flexible for mass customization and rapid response rate in future emergencies [37–39].
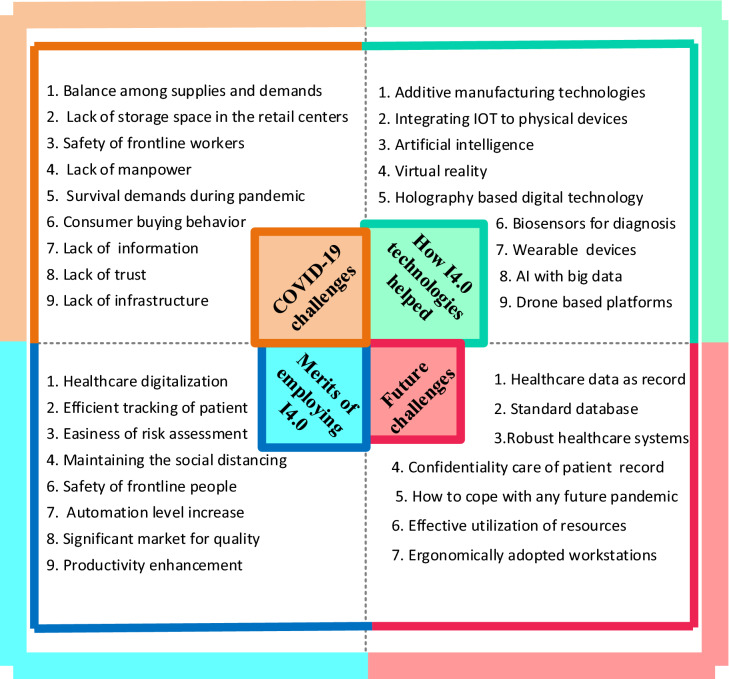
The positive role of industry 4.0 concerning the COVID-19 pandemic is multi-directional in which some of its merits include disease forecasting, diagnosis, and control [40]. The COVID-19 pandemic has also affected the sustainable development goals (SDG) of the United Nations which were approved in 2015. Specifically, the shortages of supplies in food and health facilities raised the global economic crisis. Therefore, the United Nations raised USD 2 billion to fight against COVID-19 and overcome the bottlenecks in the supply chain especially [41]. However, developing countries lacked skilled healthcare workers, funding, and necessary medical facilities. Therefore, the key variables to investigate the pandemic control included response rate, type of healthcare system, economy, availability of medical equipment, proper vaccination/treatment, research, education, communication, and manufacturing [42]. A detailed illustration of challenges during the COVID-19 pandemic, response to challenges by I4.0 technologies, merits, and future possible challenges have been presented in Fig. 7.
Fig. 7.
A detailed framework showing the COVID-19 pandemic challenges, industry 4.0 (I4.0)-assisted technologies, merits of implementing I4.0, and future challenges for digital transformation in manufacturing sectors through it (inspired from [38–40])
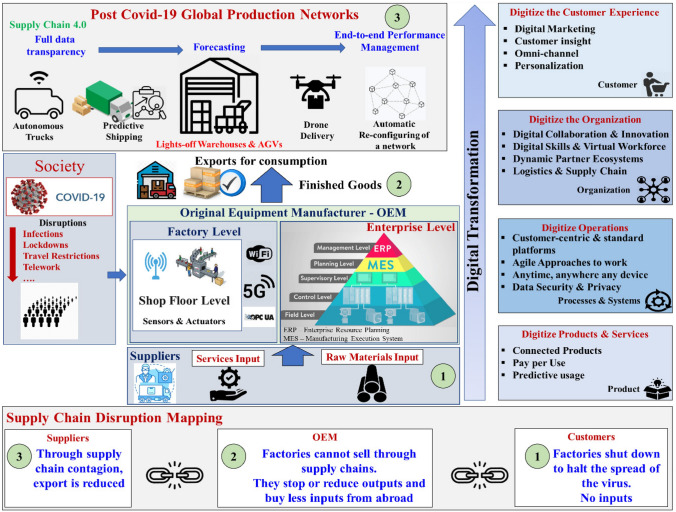
Considering all the aforementioned challenges in mind, industry 4.0 (also known as the fourth industrial revolution) helps to fulfill the needs of necessary medical devices by introducing several technologies. The symptoms of COVID-19 were detected by I4.0 technologies to track health disorders. The misinformation on several technical platforms can be identified through the digital technologies of I4.0 [43]. The digital versatility and quick prototyping are boosted by integrating AM with industry 4.0 and the internet of things in the cyber-physical age to make it more flexible for mass customization and rapid response rate in case of any future emergencies [15, 44]. An overview of detailed supply chain mapping concerning the COVID-19 pandemic with I4.0 is indicated in Fig. 8 which, in turn, helps in effective monitoring and control for any future pandemic.
Fig. 8.
A detailed framework to demonstrate the digital transformation in manufacturing sectors through industry 4.0 with supply chain mapping and post-COVID global production networks [37];
reprinted with permission (5397640532096), Elsevier 2022
Classification of AM technologies for biomedical applications
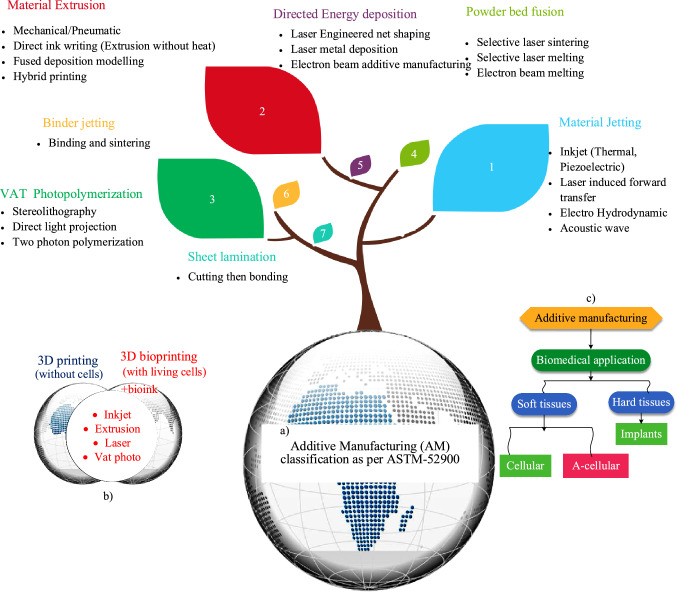
Additive manufacturing (AM) technologies are considered suitable for biomedical sectors even for industry 4.0 [1, 2]. AM technologies' classification was initially based on solid, liquid, and powder-based 14 technologies [45]. Calignano et al. [46] developed seven AM classification systems as per ASTM-52900. Lee et al. [47] categorized AM into inkjet, extrusion-based, laser-assisted, and VAT photopolymerization-based four 3D bioprinting techniques. The exploratory study of each classification concerning its working principle, raw materials, control variables, responses, merits, challenges, and biomedical applications, is mentioned in Table 2 and details have been discussed in onward sections herein. The classification tree of AM technologies concerning the ASTM 52,900 can be seen in Fig. 9.
Table 2.
Additive manufacturing techniques, along with their different useful prospects involving process, materials, control variables, merits, limitations, and biomedical applications
| AM technology | Process physics | Materials | Key variables | Merits | Challenges | Applications | References |
|---|---|---|---|---|---|---|---|
| Fused deposition modeling (FDM) | Melting and deposition | Polymers (PP,PC,ABS,PLA), composites | Layer thickness, temperature, speed, air gap, scan pattern | Economical, available, fast, wide material selection | Poor resolution (25 µm), inter-layer distortion, shrinkage stresses, poor roughness, and mechanical properties | Scaffolds, Biosensors, cartilage tissue engineering, PPE | [36, 49, 50] |
| Freeze-form extrusion (FEF) | Freeze drying (transfer of paste below its freezing temperature) | Low-viscosity materials, ceramic porous, and solid pastes (Al2O3, BaTiO3) | Extrusion pressure, nozzle diameter, table speed | No support, fast, hygienic, mass customization | More material wastage, sintering needed, binder removal required, under-filling | Soft tissues, casting patterns, biomedical scaffolds | [51–53] |
| Laminated object manufacturing (LOM) | Binding and cutting | Paper, ceramics, metal rolls and tapes, polymeric composite sheets | Laser power, orientation angle, sheet thickness, scan speed | No post-processing and support required, non-toxic, less cycle time | Only sheet material required, slow, poor surface integrity, more material waste | Intelligent structures, sensors, processors | [54–57] |
| Direct ink writing (DIW) | Extrusion without melting | Hydrogel, polymer, and ceramic matrix composite pastes (like graphene-based inks, Pluronic F-127) | Layer thickness, viscosity, extrusion pressure, nozzle diameter | Low cost, hygienic, resolution (15–400) µm, applicable to 4DP and 5DP | Liquid splashing, bulging, discontinued lines, difficult to print on rough surfaces | Nasal prosthesis, tissue repair, soft actuators, piezoelectric devices | [58, 59] |
| Stereolithography (SLA) | Curing of light-activated polymers | Light sensitive photopolymers, Hydrogels, ceramic suspensions (like SiO2, Al2O3, ZrO2) | Exposure time, scanning speed, layer thickness | Resolution (5–100 µm), no support, no nozzle clogging, mass customization | Slow, expensive, limited materials, poor mechanical properties, anisotropic | Soft robotics, tissue engineering, porous scaffolds | [25, 60, 61] |
| Rapid freeze prototyping (RFP) | Deposition and solidification | Biopolymers (alginate, Pluronic, Alginate + PLA, PLLA + chitosan) | Nozzle frequency, freezing temperature | Hygienic, cheap, Fast, less waste material | Pattern required, limited materials, less stability | Porous scaffolds, prototypes, casting patterns | [62–64] |
| Digital light processing (DLP) | Photopolymerize and project | Light-activated polymers, composites, resins, ceramic suspensions | Build orientation, exposure time, layer thickness | No material waste, better resolution and printing speed | Expensive, limited materials, support required, less printing control | Dental implants, drug delivery, biomedical devices | [65, 66] |
| MultiJet modelling (MJM) | Curing and deposition | Thermoplastic and photo-curable polymers (like acrylics, polyamide) | Build orientation, curing method | High speed, multi-color parts, good surface finish, cheap, | Warpage, limited materials, require support structure, poor mechanical properties, | Prototypes, casting patterns, soft tissues | [67, 68] |
| Inkjet 3D printing (i3DP) | Binding and sintering | Polymers, elastomers, ceramics, composites | Printing speed, layer thickness | Resolution (10-200 µm), fast, economical | High porosity, poor surface finish | Functionally graded materials, scaffolds | [69–71] |
| Selective laser sintering (SLS) | Partial melting | Polymers, ceramic, metallic, and composites | Scanning speed, laser power, layer thickness, scan strategy | Partial melting, high resolution (25-250 µm), fast, no additional support | Shrinkage, post-processing needed, limited surface finish | Lattice structures, oral drug delivery, biomedical Implants | [25, 72, 73] |
| Selective laser melting (SLM) | Full melting | Metal alloys (Ti, Al, CoCrMo, 316LSS, IN718) and their composites | Layer thickness, scan speed, hatch spacing, laser power | High resolution (25-250 µm), mass customization, fast | Required post-processing, porosity control, expensive tooling, and more power consumption | Prosthetic, load-bearing implants (hip, knee), cellular structures | [74–76] |
| Electron beam melting (EBM) | Full melting | Metal alloys (Ti, Al, CoCrMo, 316LSS, IN718) | Beam diameter and power, chamber pressure, layer thickness, exposure duration | Innovative heating and melting, stable patient-specific implants, metals only | Less hardness compared to SLM, powder preheating required, expensive tooling, limited product size, vacuum required | Patient-specific orthopaedic and orthodontic implants, cellular structures | [77–79] |
| Laser-engineered net shaping (LENS) | Melting | Hybrid, metallic powder (Ti and its alloys, Al, CoCrMo, 316LSS, IN718) | Beam power, powder feed rate, working distance, traverse speed | Damage parts repair, in situ applications, print dense parts, high strength | Uneven melting, post-processing needed, poor dimensional accuracy, poor resolution (30 µm), anisotropic | Orthopedic and dental implants, functionally graded structures | [80–82] |
Fig. 9.
a Tree diagram to demonstrate different prospects concerning AM classification, a AM systems according to ASTM-52900 standard, b an illustration to indicate the basic difference among 3D printing and 3D bioprinting techniques, c basic flowchart to indicate the additively manufactured soft and hard constructs for biomedical applications (inspired from [49])
Suitability of AM technologies in 3D bioprinting
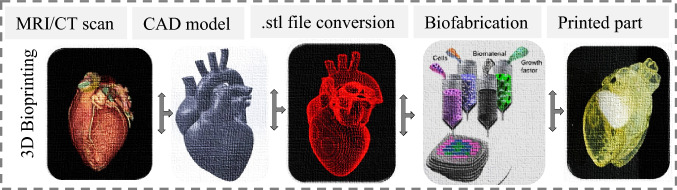
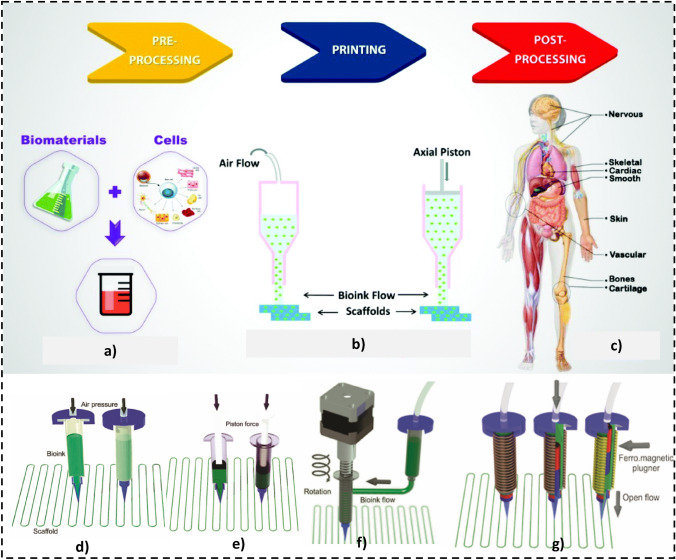
The major AM classification in 3D bioprinting technologies comprises four main types such as extrusion, droplet, resin, and laser-assisted systems. 3D bioprinting of soft constructs requires bioink, whose composition and environmental factors significantly influence the stability of the process [83]. The necessary details of each four main 3D bioprinting technologies have been explained in the onward sub-sections herein. Among the several merits, AM technologies are still not frequently used for mass production where patient-specific implants are required. Additive manufacturing for biomedical applications usually comprises soft tissues and hard constructs for clinical medicine, tissue engineering, and orthopedic implants. The basic process steps in 3D bioprinting have been indicated in Fig. 10.
Fig. 10.
Flow process to fabricate a tissue/organ in 3D bioprinting [84];
Copyright 2020 Elsevier
Classification of 3D bioprinting technologies
3D printing for tissues/constructs is also called 3D bioprinting (3DBP) if living cells are also used during implant fabrication, as indicated in Fig. 9b, which explores the difference between 3DP and 3DBP. Usually, the CT scan or magnetic resonance imaging of a living tissue/organ is taken, followed by its conversion into a CAD file in STL format, and a series of other steps are performed to fabricate an implant, as shown in Fig. 10. Soft tissue printing usually employs soft materials like shape memory polymers and composites to fabricate tissues, scaffolds, or implants. These are generally classified into four types: inkjet, micro-extrusion, laser-assisted, and VAT photopolymerization-based technologies, as demonstrated in Fig. 11. The choice of a certain 3D bioprinting technology can be governed by different performance assessment measures involving cell viability, speed, resolution, ink viscosity, merits, limitations, and processing cost per unit, as reported in Table 3.
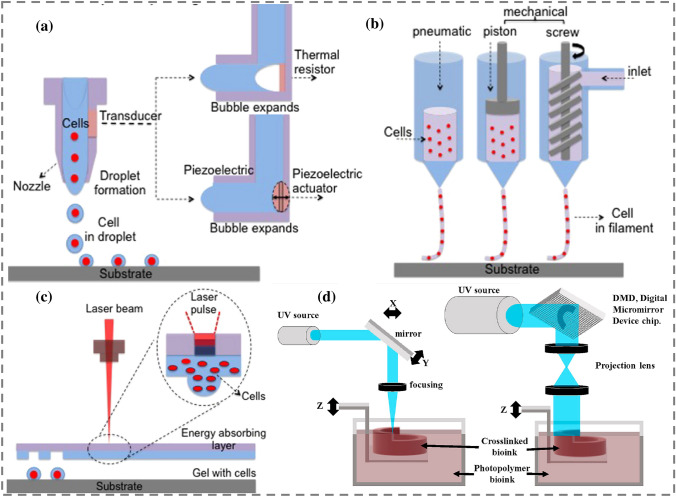
Fig. 11.
Classification schematics of 3D bioprinting technologies with different variations. a Inkjet (thermal and piezoelectric), b extrusion based (pneumatic, piston, screw), c laser assisted (laser-induced forward transfer), d VAT photopolymerization (stereolithography and digital light processing); [85, 86],
(5310950443686, Copyright 2020 Elsevier)
Table 3.
| Parameter | Inkjet (thermal, piezoelectric) | Extrusion (pneumatic, piston, screw) | Laser-assisted (LIFT) | VAT photopolymerization (SLA, DLP) |
|---|---|---|---|---|
| Cell viability | High (> 90%) | Medium (40–95%) | ≥ 95% | Low |
| Speed | High (1–10,000 droplet/sec) | Slow (10-50 µm/sec) | Medium fast (200–1600 mm/s) | High |
| Resolution (µm) | 50–300 | 50–250 | > 20 µm | 25–100 |
| viscosity (mPa/s) | 3.5–12 | 6–30 × 102 | 1–300 | Low |
| Cost | Low | Moderate | High | Moderate |
| Pros | Multiple cell deposition, mass-produced head | Wide materials selection, scalable | Nozzle free | Nozzle free, scalable printing |
| Cons | Limited choice and size of materials | Nozzle required; cells may damage during printing | Limited fabrication size and materials | Cytotoxicity of photo-initiator, limited materials |
Inkjet 3D bioprinting (IJ 3DBP)
Inkjet 3D bioprinting (IJ 3DBP) ejects tiny volume droplets of bioink by providing a pressure pulse in which each bioink droplet contains between 104 and 304 cells [85]. Bioink for scaffold and implant construction in IJ 3D bioprinting can be made from both natural and synthetic polymers like alginate, calcium chloride solution, bioceramics, polyethylene glycol dimethacrylate (PEGDMA), and bioglass. Bioink viscosity, temperature, and pressure pulse frequency are important factors in IJ 3D bioprinting [87]. The ink droplets are produced as drop-on-demand (DOD) and continuous methods (CIJ). The continuous IJ 3DBP depends on the natural tendency of a liquid stream to flow and undergo morphological change, producing continuous-discrete droplets [88]. The ink drop diameter ranges from 10 to 150 µm for continuous IJ 3DBP [88]. Drops are produced at a far faster rate by CIJ-based bioprinters over DOD systems. However, they are unsuitable for biological applications due to fluid inks and the risk of contamination during fluid recycling [85]. Droplet bioprinting was one of the first printing methods used in tissue and organ [89]. The drop-on-demand printing generates bioink droplets on the substrate as needed. DOD-based bioprinters can be employed for patterning and material deposition due to their minimal bioink waste and printing precision [90]. CIJ printing is mainly utilized for coding and marking applications with drop diameters of roughly 100 µm. DOD printing is primarily used for graphics and text printing with drop diameters of 20–50 μm [89, 91].
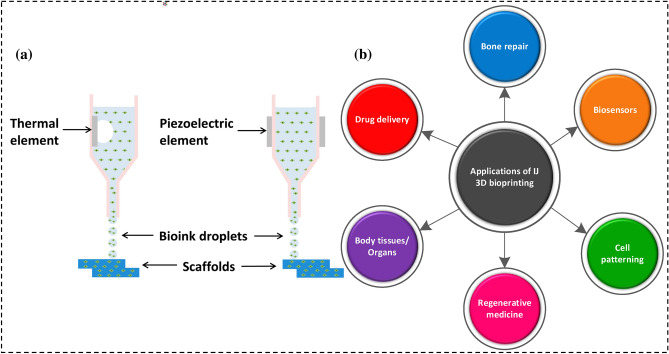
Ink deposition is often employed in thermal and piezoelectric IJ 3D bioprinting as shown in Figs. 11a and 12a. Thermal IJ bioprinting utilizes a heat source (100–300 °C) to create bubbles that produce building pressure, causing the droplet to eject [92]. Thermal IJ 3DBP is easy, efficient, and cost effective [71]. Thermal droplet 3D bioprinting causes pressure pulses to discharge droplets at the printer aperture by generating heat inside the bioink chamber [86]. However, frequent nozzle obstruction caused by bioink gelation and the formation of uneven-sized droplets interrupt the smooth printing. The involvement of temperature and shear stresses in the production of bioink drops may harm cell viability. According to several reports, a rise in the local temperature up to 300 °C owing to a short exposure of 2 µs has no effect on cells during the printing [93–95]. Thermal inkjet printing also employs a heated element to nucleate a bubble. The bubble causes pressure to build up within the print head, resulting in the discharge of a droplet. The thermal element has a temperature range of 100–300 °C. However, high temperatures may harm the cells in thermal IJ 3DBP [96]. The jetting method for droplet ejection by applying pressure is used in piezoelectric-based bioprinting. A piezoelectric crystal actuator generates sonic waves inside the bioink chamber, which releases the droplets via the printer nozzle [71, 93]. Mass modification in the piezoelectric element at low temperatures provides acoustic waves for bioink ejection. Highly viscous materials are difficult to handle using piezoelectric-based IJ 3D bioprinting [97]. The jetting behavior of linear polymeric materials in piezoelectric inkjet systems is dependent on the polymer's molecular weight and concentration. Shear rates in piezoelectric inkjet 3D bioprinting may range between 104 and 106 s−1 [98]. An illustration of key applications of inkjet 3D bioprinting is shown in Fig. 12. A summary of different works on IJ 3D bioprinting is shown in Table 3.
Fig. 12.
a An illustration showing the classification [92]
(Reproduced with permission from Kacarevic et al. 2018, Open access), and b various biomedical applications of inkjet 3D bioprinting
The advantages of IJ 3DBP include high printing speeds, low cost, higher resolution, more accurate cell positioning, quicker printing, 3D freeform manufacturing, heads for diverse materials, and increased cell survival [99]. The drawbacks include poor droplet directionality and inaccurate cell encapsulation owing to ink concentration. Drop-on-demand printheads are most commonly utilized in biomolecule-based applications [84]. Inkjet printers generally function in 1–20 mPa/s viscosity range [98]. The increase in viscosity affects the speed and accuracy of the ejected drop, and at high viscosities and drop rates, printing will fail if the ink cannot flow rapidly enough into the liquid chamber. The resolution of inkjet printing may reach up to 50 µm.
Surfactants can be used to modify surface tension in IJ 3D bioprinting. Shear stresses may also be induced in inkjet printing to damage cells (proteins and other biomacromolecules), resulting in manufactured cells with limited droplet functioning. Inkjet technology has recently been used in medicine and biomedical engineering applications such as drug screening, genomics, and biosensors [48, 100, 101].
Extrusion-based 3D bioprinting (E-3DBP)
The small nozzles are often used in extrusion-based 3D bioprinting (E-3DBP) using screw, piston, solenoid, or pneumatic pressure application techniques [83]. Figure 13 provides a basic overview of the process and several pressure application strategies in extrusion-based 3DBP. Micro-extrusion 3D bioprinting can be used to print desired biomaterial structures by spreading materials by nozzles or needles coupled to ink cartridges. For bioprinting cell-laden structures, cells are combined with bioink. Bioink is a material used to encapsulate cells to provide protection and a supportive extracellular matrix to help protect from the shocks that occur during printing [102].
Fig. 13.
A. Basic demonstration of extrusion 3D bioprinting. An illustration showing the basic steps including the a bioink mixing, b extrusion 3DBP, c extrusion 3D bioprinted tissues for biomedical applications
(Reproduced with permission from Askari et al. [107], Royal society of chemistry 2021), B. classification of extrusion 3DBP involving d pneumatic type, e piston driven, f screw driven, g solenoid based, Reprinted with permission from [105], Copyright (5323190293181) Elsevier 2022
The printing control variables like printing speed, dispensing pressure, and movement distance should be optimized for efficient manufacturing. The cell line and bioink characteristics significantly impact all printing parameters. The printability of a bioink is determined by its ease of printing with acceptable resolution and the retention of its structure after printing. The shape fidelity, resolution, biocompatibility, and cell-supporting capabilities are frequently used to define bioink printability [103, 104].
Extrusion-3DBP can produce biomaterials with different viscoelastic properties, like hydrogels with their composites [105]. A hybrid structure might be created with a multiple-cartridge system to dispense various bioinks and physiologically active ingredients. Controlling the syringe pump's actuation pressure in the nozzle is crucial for effective bioink deposition on the build platform. Extrusion 3D bioprinting success is determined by bioink properties like molecular weight, shear-thinning, biodegradation, and visco-elasticity [104, 106]. Dispensing pressure, bioink consistency, and nozzle diameter typically determine the shear stress experienced by the cells in the bioink, which affects cell survival. Air pressure, extrusion speed, temperature, collecting platform position, and type are all adjustable parameters that directly influence printing accuracy and resolution in E-3DBP.
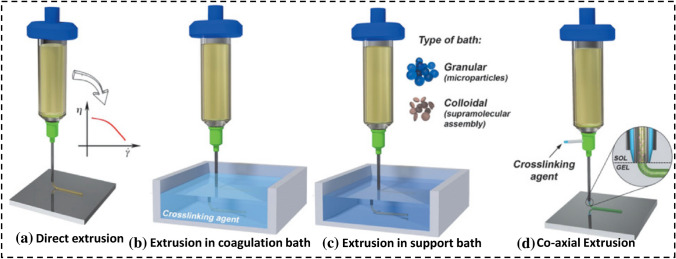
Extrusion-3DBP suffers from poor resolution and extended processing time. Other considerations include frequent nozzle obstruction and low resolution (200–1000 µm) [108]. The main challenges in E-3DBP include (1) bioink selection and process parameter optimization in tissue printing, (2) mechanical strength and bio-functionality of printed constructs, (3) vascularization of the target tissue, and (4) commercialization and mass-market challenges. These days, the integration of electrospinning and extrusion 3D bioprinting (hybrid printing) is an intriguing and growing aspect of 3D bioprinting [107]. Extrusion bioprinting is extremely versatile, making it excellent for producing scaffolds or prosthetic implants for tissue engineering [85, 106]. Different classes of extrusion 3DBP involving direct extrusion, in coagulation bath, in support bath, and co-axial extrusion, are demonstrated in Fig. 14.
Fig. 14.
a Different classes of extrusion bioprinting involving direct extrusion, coagulation bath-embedded printing, support bath-embedded printing, and co-axial extrusion, for printing fidelity and cells formation in tissue engineering [104, 106]
(5315690351444, Copyright 2020), John Wiley and Sons
Laser-assisted 3D bioprinting (LA-3DBP)
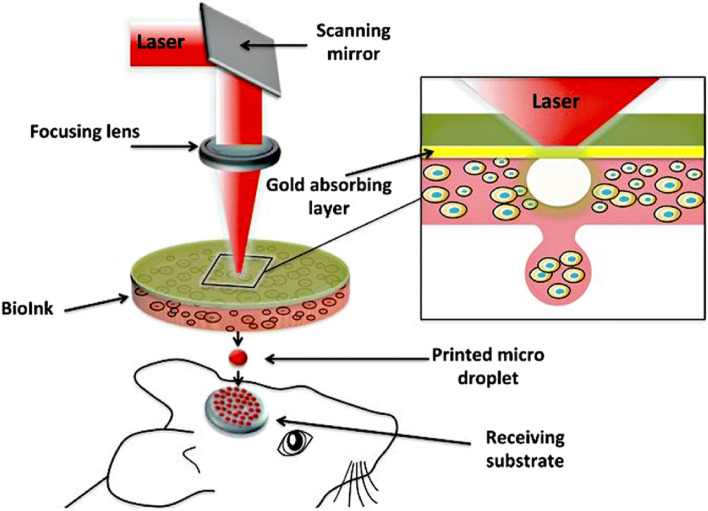
Laser-assisted 3D bioprinting (LA-3DBP) is usually known as laser-induced forward transfer (LIFT) which refers to the material transfer through pulsed laser from a source film to a substrate nearby or in contact with the film, enabling laser direct writing of patterns independent of the surface type or material form [109–111]. LIFT-based bioprinters are made up of three parts: (a) an energized pulsing laser, (b) a target or ribbon that acts as a biological material donor film, and (c) a receiving substrate to collect the printed material.[85, 112, 113]. The schematic demonstration of LA-3DBP is shown in Fig. 11c. This method uses a laser system to induce vaporization and a small droplet, as can be seen in Fig. 15 while printing circular droplets and calvaria defect treatment in the mouse.
Fig. 15.
Schematic of laser-induced forward transfer to indicate in situ printing to deposit biomaterial into the mouse for calvaria defect, reprinted with permission from (Keriquel et al. [122], Open Access), Springer 2019
The laser-induced forward transfer is less common than inkjet or micro-extrusion bioprinting, but its application for tissue and organ engineering is evolving these days. It can deposit cells at a density of up to 108 cells/ml with a resolution of a single cell per drop using a high-speed laser pulse [109, 110, 114].
LA-3DBP produces higher resolution patterns, and cell viability in the printed hydrogel is expected to be lower than with previous inkjet processes. The problems related to nozzle obstruction caused by cells or materials, which are important limits of traditional bioprinting technologies, do not occur with LIFT, which is nozzle free. Another advantage of LIFT is that it is compatible with a wide range of bioinks viscosities (1–300 mPa/s) and may be used to produce mammalian cells [92, 115].
LIFT can be employed in a variety of single-element materials, mostly metals with feature sizes often ranging between 10 and 100 µm. Instead, scalability, low cost, large area, and roll-to-roll compatibility are key challenges in LA-3DBP [116]. Polymers are extensively used in organic electrical devices as flexible substrates and semiconductors [28, 117]. LIFT may be used to make heterogeneous tissue structures with high cell densities, great accuracy, automation, reproducibility, high throughput, and a wide range of sizes that closely mimic their natural physiological counterparts [110]
The primary limitation of LIFT is the longer production time. LIFT has been successfully used to deposit biological materials such as cells, nucleic acids (DNA), and peptides using a 5 kHz laser pulse repetition rate.[102, 111, 118].
The selection of biomaterials for LIFT chosen should have quick gelation kinetics and working wavelength compatibility [119]. Other major challenges include gravity settling of cells in solution and long production durations [120]. In tissue engineering, LIFT has been utilized to produce cellularized skin constructions and deposit nanohydroxyapatite in a 3D mouse calvaria defect model [109, 121].
VAT photopolymerization 3D bioprinting (VPP-3DBP)
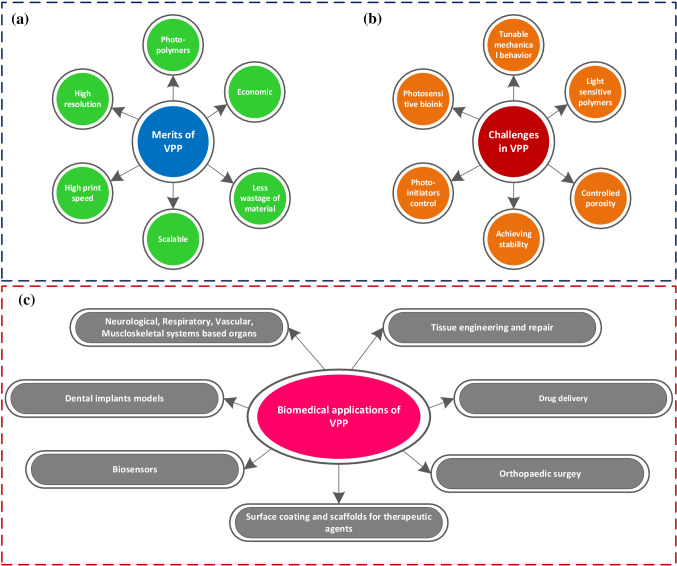
The first 3DP technology was VAT photopolymerization (VPP), which uses a light source to perform polymerization reactions in photosensitive materials [123]. VAT photopolymerization technology is an AM tool that enables polymerized material layers by exposing photosensitive materials to radiation/light in a controlled manner. Subsequent layers combine to form a 3D structure, though this process is limited to materials that polymerize when exposed to light. This approach can be used to process photopolymers and resins [123, 124]. Figure 16 depicts the benefits, difficulties, and applications of VAT photopolymerization.
Fig. 16.
A schematic visualization to indicate different a merits, b challenges, and c biomedical applications of Vat photopolymerization-based 3D bioprinting (inspired from [125, 132])
VPP methods are further classified based on the curing source as stereolithography (SLA), digital light processing (DLP), two-photon polymerization (2PP), continuous liquid interface processing (CLIP), and volumetric 3D printing [125]. The bulk of SLA photopolymer resins is composed of monomers, oligomers/binders, photoinitiators, and different additives. Monomers and oligomers are the primary components of photopolymer resin, which solidifies due to crosslinking [126, 127].
2PP has the highest resolution of any VAT polymerization-based printing method. The femtosecond (fs) laser pulses initiate polymerization in a highly restricted focus volume by two-photon absorption. As a result, 3D micro- and nano-scale structures are created. Although 2PP precision can approach 40 nm, its usefulness is restricted to the small size of printed components. CLIP is a popular method for producing dental implants quickly.
VAT photopolymerization has lately expanded its application into functional material printing, creating new hurdles for developing photopolymerizable functional resins and the associated curing process [128, 129]. The high-resolution printing capability makes VAT photopolymerization suitable for applications requiring stiff and accurate geometry [48]. However, it can also be used to print components that are not rigorously dimensioned, such as hydrogels. Hydrogel-based bioink in VPP can be useful to print both cell carrier and structural components [130].
Compared to E-3DBP and IJ-3DBP, VAT photopolymerization-based bioprinting has unique merits like high structural integrity and high printing resolution[123, 124]. Stereolithography applications in biomedical engineering are divided into four categories: patient-specific models and functional components, implantable devices, tissue engineering, and cell-laden hydrogels [123, 131]. The neurological, cardiovascular, musculoskeletal, and respiratory systems are now among the VAT photopolymerization-based 3D bioprinting applications [125]. Several interesting applications of VAT photopolymerization, such as the printing of instantaneously functional soft tissue engineering constructs, are still in their early phases [85] (Table 4).
Table 4.
Tabular data to describe the key details of each 3D bioprinting process principle, materials, control variables, responses, key findings, and target applications
| 3D bioprinting type | Working principle | Materials | Key control variables | Performance measures | Challenges | Target apps | References |
|---|---|---|---|---|---|---|---|
| Inkjet | Bioink droplet ejection by pressure pulse from 1–150pL | Both natural and synthetic biopolymers (like alginate, bioglass) | Bioink viscosity, temperature | Mechanical properties, biocompatibility, cell proliferation | Cell damage, limited viscosity (≤ 10 centipoise) | Biosensors, tissue engineering, drug delivery | [92, 133, 134] |
| Extrusion | Bioink ejection through the nozzle by pressure application | Bio-inert hydrogels, collagen, gelatin, | Printing speed, distance, temperature, nozzle diameter | Cell viability, proliferation rate, shape fidelity, mechanical properties | Low resolution, low speed, nozzle blockage | Prosthetic implants, tubular structures, skeletal muscles | [105, 135, 136] |
| Laser-assisted | A pulsed laser with a focusing mechanism for vaporization and printing microdroplets from bioink | All laser wavelength-compatible biomaterials with fast gelation kinetics | Printing speed, laser exposure duration, distance | Mechanical properties, cell biocompatibility, proliferation rate, | Protective thin film required, less cell viability, high cost, | Acellularized skin constructs, tissue engineering, multi-layered structures | [85, 137, 138] |
| VAT photopolymerization | Exposure of photosensitive materials to radiation/light | Photosensitive materials cell-laden hydrogels | Exposure duration, ink viscosity, | Biocompatibility, mechanical properties, | Limited size, costly, less multi-material printing capability | Functional parts, patient-specific implants, | [123, 125, 139] |
Materials and applications of additively manufactured biomedical products
Materials and applications of 3DP soft constructs
3D bioprinting has emerged as one of the fastest expanding areas in recent years, and its applications involve a series of steps like bioink preparation, 3DBP, and suitable post-processing for tissue regeneration, drug delivery, scaffolds, and other patient-specific implants [116]. The rapid use of hydrogels in 3DBP possibilities has aided in the creation of functional biological tissues, cartilage, skin, and artificial organs [140, 141]. The primary emphasis of the scientists is on the 3D bioprinting of biomaterials while preserving biocompatibility, biodegradability, and boosting growing productivity [142]. 3DBP technological advancements include the use of shape memory polymers like hydrogels, which are the best contender in biofabrication. Hydrogels are hydrophilic polymers and can hold a large amount of water. They are useful for biomedical applications due to their high elasticity. They respond quickly to external stimuli such as physical and chemical stimuli. Physical stimuli include temperature, intensity, electric and magnetic fields, solvent composition, and other physical stimuli, whereas chemical stimuli include pH, ion concentration, and degree of cross linking. To be categorized as a hydrogel, a material must contain 10% of its total water content [143]. The details of different natural and synthetic biopolymers have been mentioned in Table 5. Printable and biocompatible hydrogels are promising materials for 3D printing applications due to their high water content, porous structure, integration of bioactive compounds, and customizable mechanical characteristics and degradation rates. On the other hand, most typical hydrogel materials are brittle and mechanically weak, rendering them unsuitable for soft and elastic tissue applications. As a result, it is vital and fascinating to create printable, high-strength, elastic hydrogel materials for 3D printing in tissue repair and regeneration [144]. Hydrogels have a wide range of applications in wound dressing, drug delivery, and scaffolds, as shown in Fig. 17. Furthermore, different application procedures of AM medical products have been indicated in Fig. 18.
Table 5.
Summary of various additively manufactured natural and synthetic biopolymers along with their useful applications prospects [140, 147–149]
| Nomenclature of biopolymers | Name | Merits | Target tissues/organs | Tissue engineering applications |
|---|---|---|---|---|
| Synthetic | Polyvinyl acid (PVA) | Improved mechanical performance | Blood vessels, bone cartilage, heart, muscles, neural, skin | Scaffolds |
| Polyether ether ketone (PEEK) | Higher mechanical stability, biocompatible | Bone scaffolds | ||
| Acrylonitrile butadiene styrene (ABS) | Biocompatible, Cheaper | Prototypes for tissues/scaffolds | ||
| Polyethylene glycol (PEG) | Biocompatible | Drug delivery | ||
| Polylactic acid (PLA) | Higher mechanical strength | Scaffolds | ||
| Natural | Alginate | Biodegradable | Skin surgery | |
| Hydroxyapatite | Biocompatible | Bone replacement/repair | ||
| Hyaluronic acid | Improved cytotoxicity | Wounds recovery | ||
| Collagen | Improved biocompatibility | Skin implants | ||
| Silk fibroin | Near human skin tissue performance | Skin replacement | ||
| Gelatin | Biocompatible | Skin surgery |
Fig. 17.
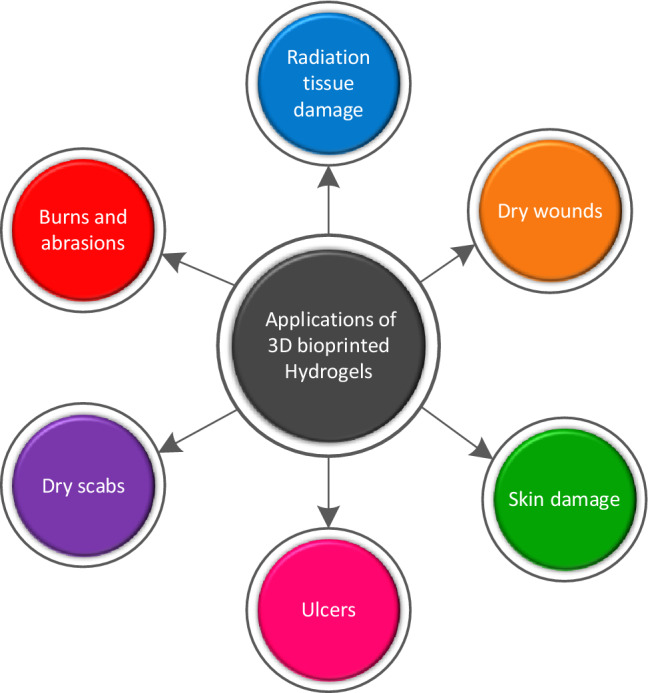
An overview of different applications of 3D bioprinted soft constructs(hydrogels) in the biomedical field; inspired from [145]
(Copyrights permission taken, Elsevier 2020)
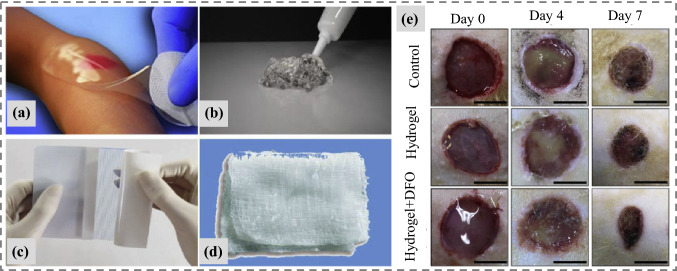
Fig. 18.
An overview of different application forms of 3D bioprinted hydrogels in wound dressing and healing. a Wound dressing with hydrogel sheet (neoheal), b burns and necrotic wounds treatment, c application of hydrogel film in wound recovery, d hydrogel impregnated wound gauze, e treatment and healing stages of diabetic skin using drug-loaded hydrogel, [145, 146]
(Copyrights permission taken, Elsevier 2020)
Applications and materials of additively manufactured hard constructs
Human aging is becoming a widespread problem these days, resulting in orthopedic concerns such as joint disease and bone fracture. People above the age of 60 are more affected by these said difficulties. These human disorders are predicted to affect 2.1 billion people globally by 2050 [150]. Furthermore, a spike in road accidents among young people throughout the world has highlighted the necessity for some appropriate ways to mitigate these difficulties. Therefore, the United Nations agenda of sustainable development goals (SDG-7) 2030 has also included this aspect to determine some suitable measures to cope with human aging issues [150, 151]. As a result, the fabrication of innovative materials with superior biomechanical, physical, and tribological characteristics using competent additive manufacturing techniques has emerged as the primary focus in biofabrication in the recent age [152]. Artificial implantation in affected patients can act as a substitute for fractured bones or disordered joints. However, there is a conscious need to improve the life and performance of these implants.
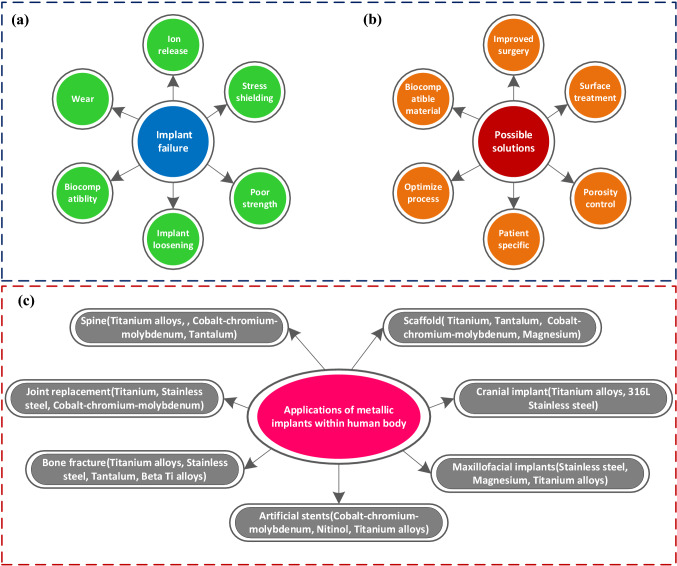
The desired biomedical implants are expected to improve biomechanical, physiological, and tribological performance [137]. Although, artificial implants cannot fully replace the natural functioning of body parts. However, the challenge to develop more novel materials with suitable performance characteristics and competitive manufacturing technology is the primary concern in recent biofabrication. The issues associated with the additively manufactured metallic implants, possible solutions, and applications are indicated in Fig. 19.
Fig. 19.
An overview of different issues associated with metal-based implants, possible solutions, and applications in the human body
Because of their good corrosion resistance, near bone elastic modulus, biocompatibility, low density, and good mechanical performance, biomedical Ti alloys are widely used to fabricate various artificial implants in orthopedic and orthodontic sectors [7, 161–163]. The chronic concerns of stress shielding (mismatch of stiffness between human bone and artificial implant), osteolysis (bone tissue loss), and chemical contamination, on the other hand, may have a major impact on the functional life of these implants [152]. Thus, additional improvement in their tribological and fatigue performance in orthopedic implants, particularly in load-bearing replacements (hip, knee), is required.
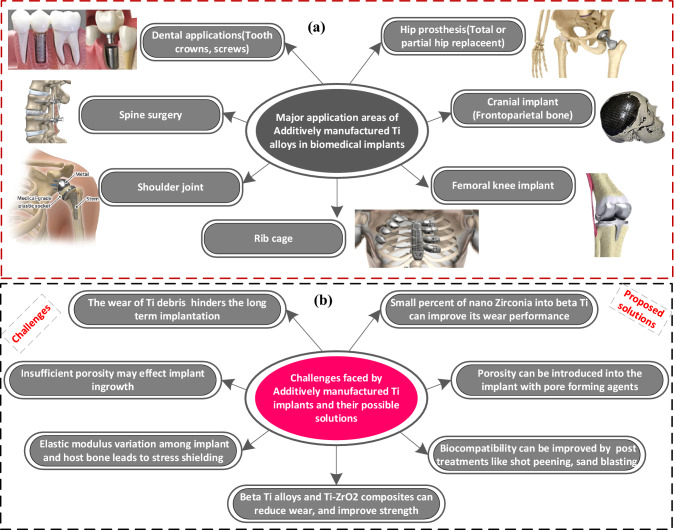
An overview of different works on metal-based hard tissue constructs for orthopaedic and orthodontic implants is reported in Table 6. A partial or total hip replacement is needed because of excessive load bearing in hip joints. The total hip replacement involves the replacement of the femoral bone, femor head/ball, and acetabular cup, whereas the partial hip replacement only replaces the ball of the hip joint [164–166]. Although, beta Ti alloys can be employed to improve the performance characteristics involving biocompatibility, fatigue strength, corrosion resistance, and mechanical properties in their Ti-based classification system. However, these beta Ti implants are still expected to possess minimal wear resistance which is a hindering factor in their long-term useful life. Also, little work has been investigated on metal matrix implants specifically Ti–ZrO2-based implants manufactured by selective laser melting. The merits, applications, and challenges of additively manufactured Ti implants are presented in Fig. 20, respectively.
Table 6.
Different works on AM of biomedical Ti alloys for orthopedic and orthodontic implants
| Author | 3DP type | Biomaterial | Control variables | Responses | Findings |
|---|---|---|---|---|---|
| Qin et al. [153] | SLM | Ti6Al4V | Energy density, scan strategy, spot size | Corrosion resistance, microstructural examination | Optimal corrosion potential was obtained from − 0.26 to 0.25 V |
| Hernandez et al. [154] | EBM | Ti-24Nb-4Zr-8Sn | Temperature, current, scan speed | Vicker hardness, surface morphology | The EBMed Ti2448 cellular structure produced more hardness (2.5GPa) than the precursor powder (2GPa) and SLMed (2.3GPa) |
| Yang et al. [155] | EBM | Ti6Al4V | Porosity, layer thickness | Recoverable strain, strength to modulus ratio, microscopic analysis | The recoverable strain was highly influenced by the cell shape, applied strain, and material characteristics, optimal structure: rhombic dodecahedron |
| Liu et al. [156] | EBM | Ti-24Nb-4Zr-8Sn | Layer thickness, voltage, beam power | Corrosion resistance, microstructural examination | The alkali-treated outer porous layer of hybrid oxides on EBMed Ti2448 enhanced the cell adhesion, which was further enhanced with type-1 collagen immobilization treatment |
| Yang et al. [157] | SLM | Ti-24Nb-4Zr-8Sn | Energy density | Mechanical performance (modulus, strength), micro-hardness, surface morphology | The hard-wraps-soft phenomenon during solidification in SLMed Ti2448 promotes strain hardening which prolongs the necking, thus improving its plasticity and yield strength |
| Liu et al. [9] | EBM | Ti-24Nb-4Zr-8Sn | Layer thickness (70 µm), spot size (200 µm), porosity (67–92%), strain rate (0.5 mm/min) | Compressive stress, Young’s modulus, fatigue strength, morphology | Ti2448 > Ti64 w.r.t fatigue strength, super-elasticity were linearly enhanced with the porosity |
| Liu et al. [158] | SLM, EBM | T Ti-24Nb-4Zr-8Sn | Melt pool characteristics, layer thickness (70 µm), spot size (200 µm), porosity (67–92%), strain rate (0.5 mm/min) | Compressive stress, Young’s modulus, fatigue strength, morphology | A comparative study in terms of compressive strength and elastic modulus: (SLM: 50 MPa, 0.95GPa) and EBM (45 MPa, 1.34GPa) |
| Liu et al. [159] | EBM | Ti-24Nb-4Zr-8Sn | Layer thickness (70 µm), spot size (200 µm), porosity (67–92%), strain rate (0.5 mm/min) | Compressive stress, Young’s modulus, fatigue strength, morphology | Ti2448 > Ti64 concerning strength-to-modulus ratio for biomedical applications |
| Cheng et al. [160] | SLM | Ti33Nb4Sn | Raster scanning strategy, laser wavelength, energy density, strut size | Compressive strength, elastic modulus, bending strength | The diamond structure was found with more compressive (76 MPa), and bending (127 MPa) strength, along with elastic modulus: (2.3 GPa) |
Fig. 20.
Different applications of additively manufactured Ti-based alloys, challenges, and proposed solutions for load-bearing applications
Sustainability assessment in AM for biomedical applications
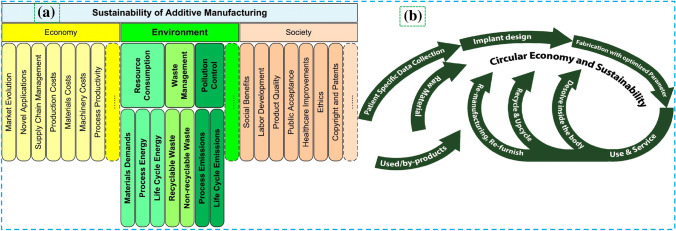
Manufacturing sustainability significantly influences the market's gross domestic product (GDP) [36]. The rationales of implementing AM for sustainable production are indicated in Fig. 21. It is equally important in 3D printing that which printing technology is more sustainable for biomedical applications concerning economic and environmental factors as illustrated in Fig. 22. The sustainability-based forecasting study inferred that additive manufacturing will significantly contribute to sustainable production with minimal harmful emissions by the year 2025 [182].
Fig. 21.
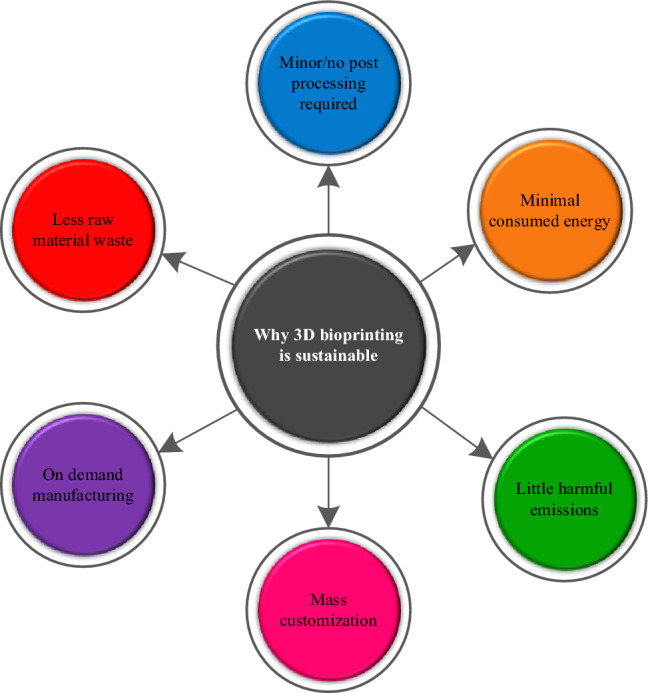
Merits of achieving sustainability in 3D bioprinting, inspired from [183]
Fig. 22.
a Different factors affecting the sustainability of AM based on economical, societal, and environmental aspects with process energy consumption included [184]; Copyright 2018 Elsevier, b sustainability assessment life cycle flowchart in additively manufactured biomedical implants coupled with circular economy factors in the context of biomedical engineering [185]; Copyright 2020 Elsevier
Energy consumption (Ec) bears prime importance [184] when process sustainability is questioned from different perspectives. A detailed overview concerning the revolution in AM concerning sustainable development, different perspectives of sustainability assessment, and the relationship of circular economy with sustainability for biomedical implants is indicated in Fig. 22. Since the development in additive manufacturing for biomedical applications is increasing day by day, it is crucial to determine which process consumes less energy and more sustainable.
Energy consumption (Ec) is recognized as one of the decision-making tools to analyze sustainability in manufacturing [4]. It usually comprises primary (required to alter material state) and secondary energy (required by supporting accessories). The consumed energy is usually measured in specific energy consumption (SEc) as kWh/kg of build part [4].
The energy consumption of different AM systems for various materials depends upon procedural needs. Faludi and Braunholz [186] evaluated the Ec of additive and subtractive techniques (FDM and CNC milling), resulting in equal SEc for ABS without considering environmental effects. An interaction between Ec and part geometry was examined by Dunaway and Harstvedt [187] using regression analysis. Clemon et al. [188], at different printing stages in FDM, inferred its direct influence on the performance quality of printed parts. Stefan Junk [189] performed an Ec comparison of two AM systems (inkjet 3D printing and FDM) and concluded the minimal energy consumed in inkjet 3D printing over FDM. Xu et al. [190] concluded that energy consumption is highly affected by slice layer thickness and build orientation in AM. Peng et al. [184] analyzed the general energy consumption in different AM systems and concluded the significant influence of resource utilization on energy consumed (Ec). The estimation of Ec is mentioned in Eq. 1.
| 1 |
where Ea and Eb are the primary and secondary energies. The variables and procedure for Ec estimation may vary, but the basics to calculate Ea and Eb are the same [4].
The primary energy for the SLM process (Ea) calculation is reported in Eq. 2.
| 2 |
where Pl denotes the laser power and t is laser exposure duration. This duration calculation is mentioned in Eq. 3.
| 3 |
where “Br” and “V” denote the build rate and volume of the fabricated part. The SLM's secondary energy (Eb) calculation is similar to FDM as reported by [4].
The specific energy consumption (SEC) for selective laser melting is calculated in Eq. 4.
| 4 |
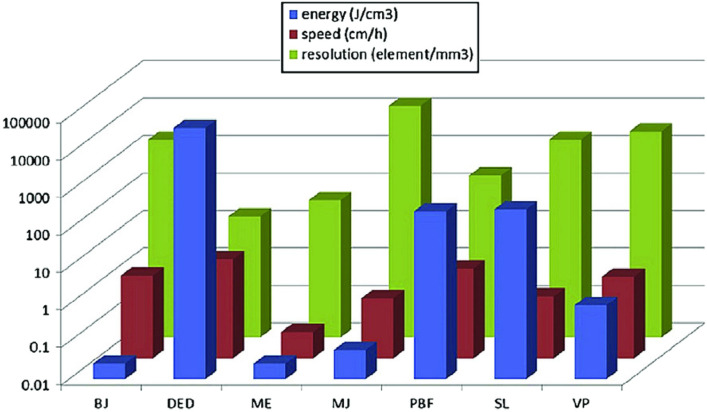
Similar estimations are used to measure Ec for other AM systems, but conditions may vary depending on the specific AM technique. A comparative study of SEc in ASTM-52900-based AM systems is reported in Table 7. The consumed energy may vary even for the same process and material. Therefore, it depends on several factors: material type, equipment model, part geometry, process variables such as layer thickness, required part quality, product volume, etc. Dermeik et al. [54] also performed a comparative study of consumed energy, printing resolution, and printing speed and concluded that DED-based 3DP consumes more energy at a higher speed compared to the other six AM technologies, as evident in Table 7 and Fig. 23. Furthermore, it was also inferred that the Ec of processing metals and their alloys is higher over polymers, ceramics, and composites. The printing resolution of material jetting (MJ) is higher compared to all other ASTM-based six techniques. The order of consumed energy in ASTM-based AM techniques is as material extrusion (ME) < binder jetting (BJ) < material jetting (MJ) < VAT photopolymerization (VPP) < powder bed fusion (PBF) < sheet lamination (SL) < directed energy deposition (DED).
Table 7.
Tabular representation of consumed energy in AM systems for different materials (inspired from Majeed et al. [4])
| Commercial AM systems | AM processes | Input material | Energy consumed (kWh/kg) | References |
|---|---|---|---|---|
| BJ | 3DP (Z printer) | ABS | 14.70–17.40 | [189] |
| PBF | SLM 250 | SS316 | 31 | [191] |
| DMLS | SS 17-4 PH | 94.17 | [192] | |
| EBM (Arcam A1) | Ti6Al4V | 17–50 | [191, 192] | |
| SLS (EOSINT P760) | PA 2200 | 36.50–39.80 | [193] | |
| DED | DMD (POM) | H13 tool steel | 833 | [194] |
| LENS (OPOMEC 750) | NiCr20Co18Ti | 292.22 | [195] | |
| ME | FDM 8000 | ABS | 23.08 | [196] |
| VPP | SLA 250 | Epoxy resin | 32.47 | [196] |
\
Fig. 23.
Comparative study of energy consumed(Ec) by different AM techniques (mentioned in blue) [54]; Copyright 2020 Elsevier
Recent research frontiers in AM for biomedical applications
4D printing in the medical field
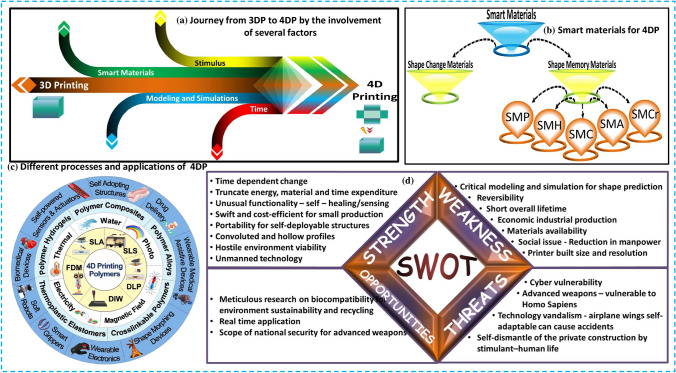
4D printing (4DP) renders tunable and sophisticated advanced materials capable of achieving many roles under controlled conditions like temperature, light, etc. These smart materials persist in the patient under in vivo conditions [135]. The primary distinction between 3DP and 4DP is that 3D printing yields a static production with no time factor [167–169]. 3DP employs metals, powders, thermoplastic polymeric materials, UV-curable resins, and other materials. In contrast, this 4D printing only utilizes temperature or humidity-responsive materials [112, 135, 170]. Medical implants, aviation, apparel, sustainable sources, automotive, soft robots, and smart actuators are among future applications of task-oriented additive manufacturing and smart materials [171, 172]. The 4DP process relies heavily on the continued development of smart and intelligent materials, which will remain the primary focus of future in-process research [167, 171, 173]. Although more material innovation studies will reveal this technology to light. Despite new material growth, it is necessary to investigate emerging printing strategies for specific tasks and structures. This technology will undoubtedly usher in new paradigms and explore new dimensions in all aspects of life. 4DP is expected to provide a manufacturing platform for tissue engineering, medical implantations, and organ development. Its benefits for biomedical products include 3D smart implants for bones such as the elbow, wrist, and ankle, the printing of intelligent multi-material organs, customizable smart multi-material printing, and the printing of organs that can grow within the living body and can be used to print body living organs such as skin, liver, heart, and kidney. 4D printing technology can be used to fabricate muscles and cardiovascular tissues, whose mechanical properties change dynamically as the patient moves [174–176]. Current progress is being made toward the development of more patient-specific and stimuli-responsive tissues, scaffolds, and implants. A detailed illustration including the basic process diagram, smart materials, processes, applications, and challenges in 4D printing has been indicated in Fig. 24.
Fig. 24.
A detailed overview of 4D printing involving the a key elements, b smart materials, c processes and applications, d pros, cons, and opportunities for future work, [167, 177]
(2019, 5323180563076), (2022, 5323181371124) Copyright Elsevier
5D printing in the medical field
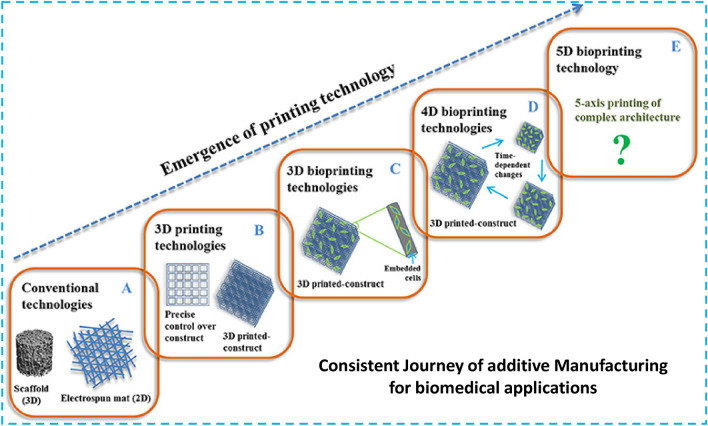
American universities first brought the theory of 5D printing in 2016 [178]. Mitsubishi Electric Research Labs are actively developing it through William Yerazunis, their high-ranking chief scientist [62, 178]. It is an emerging branch of additive manufacturing in which the print head, as well as the printable object, has five degrees of freedom, creating curved layers instead of flat ones. In this technique, the print part travels while the printer head prints, so the printing follows the curve path of the part being printed. The primary advantage of this technology is the ability to build a stronger part with a contoured layer. Etherea, based in Bengaluru, India, has been involved in 3D printing technology and devised the concept of building a 5D printer, and they were able to complete it successfully and win the CES award for best innovation [178, 179]. A 5DP prototype could be used to construct customized bone scaffolds for surgical applications. Since human bones are curved and not flat, artificial bones made with 5DP are required to provide excellent strength to these bone implants. This technology holds a lot of promise in terms of meeting this primary requirement. The development trend of AM for biomedical applications is shown in Fig. 25.
Fig. 25.
A detailed overview showing the consistent development in additive manufacturing toward 5D bioprinting [181], copyright permission taken
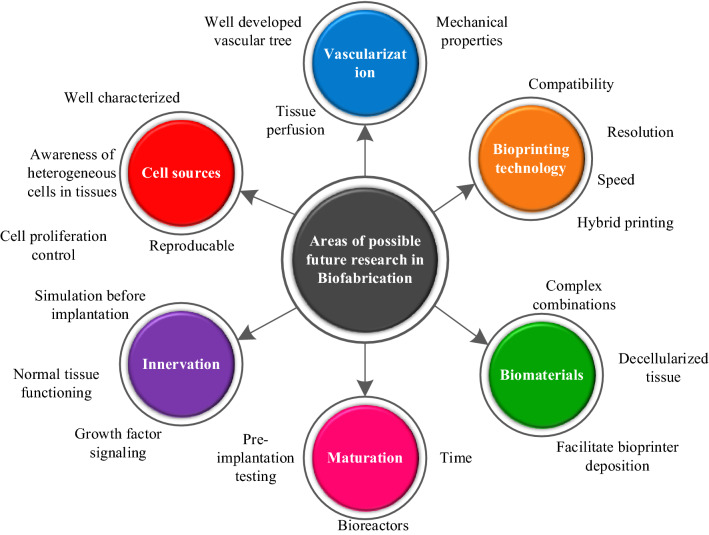
3D printing is ineffective for producing complex curved orthopedic implants because it uses flat layers. The results of the tests show that 5DP objects are 3–5 times stronger than 3D-printed objects. According to research, a 5D-printed cap is also four times stronger and can withstand four times the pressure of a 3D-printed cap [178]. Curved layers have material force in 5DP which makes them stronger after printing [180]. As a result, 5D printing requires less raw material to produce implants of the same strength as 3D printing. Orthopedic surgery necessitates complex, strong implants with curved surfaces. 5D printing is useful in preoperative treatment, teaching, and learning, as well as in printing these invasive medical implants based on the patient's actual surgery. As a consequence, 5D printing can easily create a complex and curved structure that requires a considerable amount of strength. The critical distinction between 3DP and 5DP is that stronger parts with a curved layer are generated in 5DP, whereas 3D printing develops a flat surface. The rest of the time, both processes rely on the same technologies, such as 3D CAD file input, 3D scanners, and 3D printing materials. In contrast, 4DP is markedly different from these two technologies. It makes use of reconfigurable materials that can change shape and function in reaction to time and temperature [135, 168, 178]. The emerging hotspots in the field concerning biomaterial, bioprinting, vascularization, maturation, and innervation with several challenging aspects can be seen in Fig. 26. Some hot research areas in biofabrication these days include AM of stimuli-responsive biomaterials, hybrid printing, and multi-materials printing for possible near-future studies.
Fig. 26.
An illustration to show different prospects for future work in additive manufacturing for biomedical applications
Concluding remarks
The recent focus of additive manufacturing (AM) technologies in the biomedical field are to produce patient-specific soft and hard constructs for implants and scaffolds. However, selecting suitable AM concerning material, technology, and control variables is a serious challenge. This work investigates the potential of AM technologies for biomedical applications before, during, and after the COVID-19 pandemic. The conclusions made from this research study include:
The discussion on the classification of AM technologies for biomedical applications concluded in several important aspects: (a) It is difficult to choose a single AM type for numerous biomedical applications because of stochastic challenges like compatibility, materials, resolution, and printing speed, (b) inkjet 3D bioprinting produces better resolution but highly viscous materials and feedstock cost are challenging, (c) highly viscous organic scaffolds can be economically fabricated with extrusion 3D bioprinting to promote the cell growth, except the slow speed and low printing resolution, (e) laser-assisted 3D bioprinting is nozzle free with the higher resolution to avoid the cell damage. However, several challenges like high cost, porosity control, and optimal parameters selection pose limitations on its use, (f) VAT photopolymerization 3D bioprinting is highly cell compatible with extreme printing resolution and speed. However, it is limited to photopolymers with the high cost and organic live tissue generation as challenges.
The selection of the suitable biomaterial for specific applications in AM is challenging without the proficient knowledge of key performance indicators like biocompatibility (in vivo, in vitro), osseointegration (for tissue regeneration), tribo-mechanical (elastic modulus, mechanical strength, wear resistance), physical (porosity, roughness), and chemical (corrosion resistance) properties. A wide range of additive manufactured natural (hydrogels like cellulose, collagen, gelatin, and hyaluronic acid) biomaterials find applications in drug delivery, wound healing, cosmetic surgery, and tissue engineering. The additively manufactured synthetic biomaterials for biomedical applications include metals (orthopaedic and orthodontic implants, artificial stents, fixations), polymers (tracheal tubes, artificial skin, breast implants, fixations), ceramics (implant coatings, hip, and dental implants), and their composites.
Concerning the additively manufactured hard constructs concerning metal alloys, the Ti–ZrO2 composites combine the superior properties of both bio-metal and bio-ceramic to produce patient-specific orthopedic implants. These metal matrix composites are expected to produce more biomechanical, physical, and tribological performance concerning useful/operational life, greater mechanical strength, high hardness, improved fracture toughness, higher fatigue strength, low wear, and greater corrosion resistance. A comprehensive investigation of the existing research indicated that the fabrication of orthopedic implants through the selective laser melting of Ti–ZrO2 composites is less significantly described with a strong research potential for orthopaedic implants.
The study of energy consumption in AM technologies indicates that metals and their alloys consume more energy than ceramics, polymers, and composites. The energy consumption in ASTM-52900-based AM seven techniques follows the order as “material extrusion (ME) < binder jetting (BJ) < material jetting (MJ) < VAT photopolymerization (VPP) < powder bed fusion (PBF) < sheet lamination (SL) < directed energy deposition (DED)”. The sustainability examination in AM as consumed energy was examined herein, whereas other environmental, economic, and social factors require further in-depth study.
The prominent additively manufactured biomedical products since the COVID-19 pandemic include emergency testing kits, swabs, dwellings, face masks, CPAP respirators, valves, fittings, and face shields. Since COVID is not the last disease, the integration of AM with industry 4.0 technologies helps to digitalize and monitor the diseases alongwith response to any future pandemic. Also, the mass customization in design and complexity of AM systems imparts a strong future potential in response to uncertain emergencies.
Recently, the terms like hybrid printing, multi-material printing, and printing using stimuli-responsive smart materials are considered hot research areas in the field. The usual applications of AM for biomedical applications include regenerative medicine, tissue engineering, scaffolds, biosensors, and load-bearing implants. This focus is shifted toward the use of smart functional materials like shape memory materials with timely response to external stimuli which is the key theme in 4D printing. The 5D printing for biomedical applications with five degrees of freedom is not fully explored yet, thus it needs further research.
Acknowledgements
Authors are thankful to the support provided by Northwestern Polytechnical University China to assist in completion of this research work.
Authors contribution
MR: writing—original draft, investigation, technical writing and validation, softwares for the analysis of data, visualization, resources, editing and review; YW, KI: supervision, validation; RTM, SZ, AA, MSK, TG, MMR, JS: editing and review.
Funding
The current study was funded by; The National Key Research and Development Program of China [Grant No. 2019QY(Y)0502]; The Key Research and Development Program of Shaanxi Province [Grant No. 2020ZDLSF04-07]; The National Natural Science Foundation of China [Grant No. 51905438]; The Fundamental Research Funds for the Central Universities [Grant No. 31020190502009]; The Innovation Platform of Bio fabrication [Grant No. 17SF0002]; and China postdoctoral Science Foundation [Grant No. 2020M673471].
Data availability
The data used to support the findings of this study is already contained within the article.
Declarations
Conflict of interest
Authors declare no conflict of interest or personal relationships that could have appeared to influence this work.
Data assessment
All the concerned data have already been contained in the paper.
Ethical approval
Authors confirm that they have abided by the publication ethics, state that this work is original, and have not been used for publication anywhere before.
Consent to publish
Authors give consent to journal regarding the publish of this work.
Footnotes
The original online version of this article was revised: funding not was not correct.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/19/2023
A Correction to this paper has been published: 10.1007/s40964-023-00405-y
Contributor Information
Mudassar Rehman, Email: mudassar@mail.nwpu.edu.cn.
Wang Yanen, Email: wangyanen@nwpu.edu.cn.
Ray Tahir Mushtaq, Email: tahirmushtaqray@mail.nwpu.edu.cn.
Kashif Ishfaq, Email: Kashifishfaq786@126.com.
Sadaf Zahoor, Email: sadafzahoor@uet.edu.pk.
Ammar Ahmed, Email: ammartahir@uet.edu.pk.
M. Saravana Kumar, Email: saravana312@gmail.com.
Thierno Gueyee, Email: alassdi12@gmail.com.
Md Mazedur Rahman, Email: mazed@mail.nwpu.edu.cn.
Jakia Sultana, Email: jakia@mail.nwpu.edu.cn.
References
- 1.da Silva LRR, Sales WF, dos Campos FAR, et al. A comprehensive review on additive manufacturing of medical devices. Prog Addit Manuf. 2021;6:517–553. doi: 10.1007/s40964-021-00188-0. [DOI] [Google Scholar]
- 2.Srivastava M, Rathee S. Additive manufacturing: recent trends, applications and future outlooks. Prog Addit Manuf. 2021 doi: 10.1007/s40964-021-00229-8. [DOI] [Google Scholar]
- 3.Longhitano GA, Nunes GB, Candido G, da Silva JVL. The role of 3D printing during COVID-19 pandemic: a review. Prog Addit Manuf. 2021;6:19–37. doi: 10.1007/s40964-020-00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majeed A, Ahmed A, Lv J, et al. A state-of-the-art review on energy consumption and quality characteristics in metal additive manufacturing processes. J Braz Soc Mech Sci Eng. 2020;42:1–25. doi: 10.1007/s40430-020-02323-4. [DOI] [Google Scholar]
- 5.Wang Y, Ahmed A, Azam A, et al. Applications of additive manufacturing (AM) in sustainable energy generation and battle against COVID-19 pandemic: the knowledge evolution of 3D printing. J Manuf Syst. 2021;60:709–733. doi: 10.1016/j.jmsy.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang D, Tare RS, Yang LY, et al. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–382. doi: 10.1016/j.biomaterials.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Kumar M, Chohan JS. The role of additive manufacturing for biomedical applications: a critical review. J Manuf Process. 2021;64:828–850. doi: 10.1016/j.jmapro.2021.02.022. [DOI] [Google Scholar]
- 8.Liu Y, Wang W, Zhang LC. Additive manufacturing techniques and their biomedical applications. Fam Med Community Heal. 2017;5:286–298. doi: 10.15212/FMCH.2017.0110. [DOI] [Google Scholar]
- 9.Liu YJ, Wang HL, Li SJ, et al. Compressive and fatigue behavior of beta-type titanium porous structures fabricated by electron beam melting. Acta Mater. 2017;126:58–66. doi: 10.1016/j.actamat.2016.12.052. [DOI] [Google Scholar]
- 10.Choong YYC, Tan HW, Patel DC, et al. The global rise of 3D printing during the COVID-19 pandemic. Nat Rev Mater. 2020;5:637–639. doi: 10.1038/s41578-020-00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Wang L, Fratini L, et al. Smart and resilient manufacturing in the wake of COVID-19. J Manuf Syst. 2021;60:707. doi: 10.1016/j.jmsy.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarfaoui M, Nachtane M, Goda I, et al. 3D printing to support the shortage in personal protective equipment caused by COVID-19 pandemic. Materials (Basel) 2020;13:3339. doi: 10.3390/ma13153339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallakpour S, Behranvand V, Hussain CM. Worldwide fight against COVID-19 using nanotechnology, polymer science, and 3D printing technology. Polym Bull. 2022 doi: 10.1007/s00289-021-04006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasha AD, Urbanic J, Hedrick B, et al. Leveraging advanced design and novel rapid manufacturing solutions to respond to the COVID19 pandemic. Comput Aided Des Appl. 2022;19:755–778. doi: 10.14733/cadaps.2022.755-778. [DOI] [Google Scholar]
- 15.Piotr KT, Strulak-Wojcikiewicz R, et al. Additive production management in COVID-19 pandemic. Eur Res Stud J. 2022;XXV:352–365. doi: 10.35808/ersj/2857. [DOI] [Google Scholar]
- 16.Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110:1162–1164. doi: 10.2105/AJPH.2020.305753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarfaoui M, Nachtane M, Goda I, et al. 3D printing to support the shortage in personal protective equipment caused by COVID-19 pandemic. Materials (Basel) 2020;13(15):3339. doi: 10.3390/ma13153339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tareq MS, Rahman T, Hossain M, Dorrington P. Additive manufacturing and the COVID-19 challenges: an in-depth study. J Manuf Syst. 2021;60:787–798. doi: 10.1016/j.jmsy.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunovjanek M, Wankmüller C. An analysis of the global additive manufacturing response to the COVID-19 pandemic. J Manuf Technol Manag. 2020;32:75–100. doi: 10.1108/JMTM-07-2020-0263. [DOI] [Google Scholar]
- 20.Mele M, Campana G. Sustainability-driven multi-objective evolutionary orienting in additive manufacturing. Sustain Prod Consum. 2020;23:138–147. doi: 10.1016/j.spc.2020.05.004. [DOI] [Google Scholar]
- 21.Peng T, Lv J, Majeed A, Liang X. An experimental investigation on energy-effective additive manufacturing of aluminum parts via process parameter selection. J Clean Prod. 2021;279:123609. doi: 10.1016/j.jclepro.2020.123609. [DOI] [Google Scholar]
- 22.Wohlers Associates (2012) Terry Wholer: “Wohler’s 2012 Report on Additive Manufacturing and 3D printing”,1-287. https://wohlersassociates.com/press-releases/wohlers-associates-publishes-2012-report/
- 23.Ekren BY, Stylos N, Zwiegelaar J et al (2022) Additive manufacturing integration in e-commerce supply chain network to improve resilience and competitiveness. Simul Model Pract Theory 102676
- 24.Advincula RC, Dizon JRC, Chen Q, et al. Additive manufacturing for COVID-19: devices, materials, prospects, and challenges. MRS Commun. 2020;10:413–427. doi: 10.1557/mrc.2020.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brighenti R, Cosma MP, Marsavina L, et al. Laser-based additively manufactured polymers: a review on processes and mechanical models. J Mater Sci. 2021;56:961–998. doi: 10.1007/s10853-020-05254-6. [DOI] [Google Scholar]
- 26.Chadha U, Abrol A, Vora NP, et al. Performance evaluation of 3D printing technologies: a review, recent advances, current challenges, and future directions. Berlin: Springer; 2022. [Google Scholar]
- 27.Chandra M, Shahab F, Kek V, Rajak S. Selection for additive manufacturing using hybrid MCDM technique considering sustainable concepts. Rapid Prototyp J. 2022 doi: 10.1108/RPJ-06-2021-0155. [DOI] [Google Scholar]
- 28.Ali MA, Hu C, Yttri EA, Panat R. Recent advances in 3D printing of biomedical sensing devices. Adv Funct Mater. 2022 doi: 10.1002/adfm.202107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nazir A, Azhar A, Nazir U, et al. The rise of 3D Printing entangled with smart computer aided design during COVID-19 era. J Manuf Syst. 2021;60:774–786. doi: 10.1016/j.jmsy.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel P, Gohil P. Role of additive manufacturing in medical application COVID-19 scenario: India case study. J Manuf Syst. 2020;60:811–822. doi: 10.1016/j.jmsy.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stansbury JW, Idacavage MJ. 3D printing with polymers: challenges among expanding options and opportunities. Dent Mater. 2015;32:54–64. doi: 10.1016/j.dental.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Arjunan A, Zahid S, Baroutaji A, Robinson J. 3D printed auxetic nasopharyngeal swabs for COVID-19 sample collection. J Mech Behav Biomed Mater. 2021;114:104175. doi: 10.1016/j.jmbbm.2020.104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irfan Ul Haq M, Khuroo S, Raina A, et al. 3D printing for development of medical equipment amidst coronavirus (COVID-19) pandemic—review and advancements. Res Biomed Eng. 2022;38:305–315. doi: 10.1007/s42600-020-00098-0. [DOI] [Google Scholar]
- 34.Celik HK, Kose O, Ulmeanu M, et al. Design and additive manufacturing of medical face shield for healthcare workers battling coronavirus (COVID-19) Int J Bioprint. 2020 doi: 10.18063/ijb.v6i4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop EG, Leigh SJ. Using Large-Scale Additive Manufacturing as a Bridge Manufacturing Process in Response to Shortages in Personal Protective Equipment during the COVID-19 Outbreak. Int J bioprinting. 2020;6(4):281. doi: 10.18063/ijb.v6i4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Mushtaq RT, Ahmed A, et al. Additive manufacturing is sustainable technology: citespace based bibliometric investigations of fused deposition modeling approach. Rapid Prototyp J. 2021 doi: 10.1108/RPJ-05-2021-0112. [DOI] [Google Scholar]
- 37.Stief P, Dantan J, Etienne A, Siadat A. Procedia CIRP. Oxford: Elsevier B.V; 2022. A Conceptual Framework for Industrial Transformation in the May Digital COVID-19 Pandemic Era A new methodology to analyze the functional and physical architecture of existing products assembly oriented product family identification; pp. 45–50. [Google Scholar]
- 38.Moosavi J, Bakhshi J, Martek I. The application of industry 4.0 technologies in pandemic management: literature review and case study. Healthc Anal. 2021;1:100008. doi: 10.1016/j.health.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudrapati R. Using industrial 4.0 technologies to combat the COVID-19 pandemic. Ann Med Surg. 2022;78:103811. doi: 10.1016/j.amsu.2022.103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paranitharan KP, Ebenezer G, Balaji V, et al. Application of industry 4.0 technology in containing Covid-19 spread and its challenges. Mater Today Proc. 2022 doi: 10.1016/j.matpr.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moosavi J, Fathollahi-Fard AM, Dulebenets MA. Supply chain disruption during the COVID-19 pandemic: recognizing potential disruption management strategies. Int J Disast Risk Reduct. 2022;75:102983. doi: 10.1016/j.ijdrr.2022.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filho WL, Brandli LL, Salvia AL, et al. COVID-19 and the UN sustainable development goals : threat to solidarity or an opportunity? Sustain. 2020;12:1–14. [Google Scholar]
- 43.Javaid M, Haleem A, Vaishya R, et al. Diabetes & metabolic syndrome: clinical research & reviews industry 4.0 technologies and their applications in fighting COVID-19 pandemic. Diabetes Metab Syndr Clin Res Rev. 2020;14:419–422. doi: 10.1016/j.dsx.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian X, Wu L, Gu D, et al. Roadmap for additive manufacturing: toward intellectualization and industrialization. Chin J Mech Eng Addit Manuf Front. 2022;1:100014. doi: 10.1016/j.cjmeam.2022.100014. [DOI] [Google Scholar]
- 45.Abdulhameed O, Al-Ahmari A, Ameen W, Mian SH. Additive manufacturing: challenges, trends, and applications. Adv Mech Eng. 2019;11:1687814018822880. doi: 10.1177/1687814018822880. [DOI] [Google Scholar]
- 46.Calignano F, Manfredi D, Ambrosio EP, et al. Overview on additive manufacturing technologies. Proc IEEE. 2017;105:593–612. doi: 10.1109/JPROC.2016.2625098. [DOI] [Google Scholar]
- 47.Lee JM, Sing SL, Zhou M, Yeong WY. 3D bioprinting processes: a perspective on classification and terminology. Int J Bioprint. 2018;4:51. doi: 10.18063/ijb.v4i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Schmid SR. Additive manufacturing for health: state of the art, gaps and needs, and recommendations. J Manuf Sci Eng Trans ASME. 2018;140:1–11. doi: 10.1115/1.4040430. [DOI] [Google Scholar]
- 49.Wasti S, Adhikari S. Use of biomaterials for 3D printing by fused deposition modeling technique: a review. Front Chem. 2020;8:1–14. doi: 10.3389/fchem.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akhoundi B, Saed AB. Manufacturing of polymer/metal composites by fused deposition modeling process with polyethylene. J Appl Polym Sci. 2019;48717:1–9. doi: 10.1002/app.48717. [DOI] [Google Scholar]
- 51.Deuser BK, Tang L, Landers RG, et al. Hybrid extrusion force-velocity control using freeze-form extrusion fabrication for functionally graded material parts. J Manuf Sci Eng Trans ASME. 2013;135:1–11. doi: 10.1115/1.4024534. [DOI] [Google Scholar]
- 52.Li M, Ghazanfari A, Li W, et al. Modeling and analysis of paste freezing in freeze-form extrusion fabrication of thin-wall parts via a lumped method. J Mater Process Technol. 2016;237:163–180. doi: 10.1016/j.jmatprotec.2016.05.027. [DOI] [Google Scholar]
- 53.Renteria A, Diaz JA, He B, et al. Particle size influence on material properties of BaTiO3 ceramics fabricated using freeze-form extrusion 3D printing. Mater Res Express. 2019 doi: 10.1088/2053-1591/ab4a36. [DOI] [Google Scholar]
- 54.Dermeik B, Travitzky N. Laminated object manufacturing of ceramic-based materials. Adv Eng Mater. 2020;22:2000256. doi: 10.1002/adem.202000256. [DOI] [Google Scholar]
- 55.Chang B, Li X, Parandoush P, et al. Additive manufacturing of continuous carbon fiber reinforced poly-ether-ether-ketone with ultrahigh mechanical properties. Polym Test. 2020;88:106563. doi: 10.1016/j.polymertesting.2020.106563. [DOI] [Google Scholar]
- 56.Helfesrieder N, Lechler A, Verl A. Method for generating manufacturable, topology-optimized parts for Laminated Layer Manufacturing. Procedia CIRP. 2020;93:38–43. doi: 10.1016/j.procir.2020.04.048. [DOI] [Google Scholar]
- 57.Liu Q, Yang C, Miao Q, et al. Experimental study on inert gas-assisted laser cut veneer based on lom. J Renew Mater. 2020;8:1681–1689. doi: 10.32604/jrm.2020.012031. [DOI] [Google Scholar]
- 58.Golcha U, Praveen AS, Belgin Paul DL. Direct ink writing of ceramics for bio medical applications—a review. IOP Conf Ser Mater Sci Eng. 2020 doi: 10.1088/1757-899X/912/3/032041. [DOI] [Google Scholar]
- 59.Guerra V, Wan C, McNally T. Fused deposition modelling (FDM) of composites of graphene nanoplatelets and polymers for high thermal conductivity: a mini-review. Funct Compos Mater. 2020;1:1–11. [Google Scholar]
- 60.Zakeri S, Vippola M, Levänen E. A comprehensive review of the photopolymerization of ceramic resins used in stereolithography. Addit Manuf. 2020 doi: 10.1016/j.addma.2020.101177. [DOI] [Google Scholar]
- 61.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 62.Foresti R, Rossi S, Pinelli S, et al. In-vivo vascular application via ultra-fast bioprinting for future 5D personalised nanomedicine. Sci Rep. 2020 doi: 10.1038/s41598-020-60196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Z, Lee KY, Wang X. Ultrasonic welding of dissimilar metals, AA6061 and Ti6Al4V. Int J Adv Manuf Technol. 2012;59:569–574. doi: 10.1007/s00170-011-3534-9. [DOI] [Google Scholar]
- 64.Foresti R, Rossi S, Pinelli S, et al. Highly-defined bioprinting of long-term vascularized scaffolds with Bio-Trap : complex geometry functionalization and process parameters with Computer Aided Tissue Engineering. Materialia. 2019 doi: 10.1016/j.mtla.2019.100560. [DOI] [Google Scholar]
- 65.Cao Y, Shi T, Jiao C, et al. Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing. Ceram Int. 2020;46:2300–2308. doi: 10.1016/j.ceramint.2019.09.219. [DOI] [Google Scholar]
- 66.Han C, Luo M, Zhang D. Optimization of varying-parameter drilling for multi-hole parts using metaheuristic algorithm coupled with self-adaptive penalty method. Appl Soft Comput. 2020;95:106489. doi: 10.1016/j.asoc.2020.106489. [DOI] [Google Scholar]
- 67.Ghomi ER, Khosravi F, Neisiany RE, et al. Future of additive manufacturing in healthcare erfan. Curr Opin Biomed Eng. 2020 doi: 10.1016/j.cobme.2020.100255. [DOI] [Google Scholar]
- 68.Marwah OMF, Sharif S, Ibrahim M, Idris MH. Collapsibility studies of MJM acrylate patterns for investment casting. Mater Eng Autom Control. 2013;330:839–842. doi: 10.4028/www.scientific.net/AMM.330.839. [DOI] [Google Scholar]
- 69.Mueller J, Shea K, Daraio C. Mechanical properties of parts fabricated with inkjet 3D printing through efficient experimental design. Mater Des. 2015;86:902–912. doi: 10.1016/j.matdes.2015.07.129. [DOI] [Google Scholar]
- 70.Sen K, Manchanda A, Mehta T, et al. Formulation design for inkjet-based 3D printed tablets. Int J Pharm. 2020;584:119430. doi: 10.1016/j.ijpharm.2020.119430. [DOI] [PubMed] [Google Scholar]
- 71.McCoul D, Rosset S, Schlatter S, Shea H. Inkjet 3D printing of UV and thermal cure silicone elastomers for dielectric elastomer actuators. Smart Mater Struct. 2017 doi: 10.1088/1361-665X/aa9695. [DOI] [Google Scholar]
- 72.Allahham N, Fina F, Marcuta C, et al. Selective laser sintering 3D printing of orally disintegrating printlets containing ondansetron. Pharmaceutics. 2020;12:1–13. doi: 10.3390/pharmaceutics12020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu SS, Li M, Wu JM, et al. Preparation of high-porosity Al2O3 ceramic foams via selective laser sintering of Al2O3 poly-hollow microspheres. Ceram Int. 2020;46:4240–4247. doi: 10.1016/j.ceramint.2019.10.144. [DOI] [Google Scholar]
- 74.Vrancken B, Thijs L, Kruth JP, et al. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014;68:150–158. doi: 10.1016/j.actamat.2014.01.018. [DOI] [Google Scholar]
- 75.Xie D, Zhao J, Liang H, et al. Assumption of constraining force to explain distortion in laser additive manufacturing. Materials (Basel) 2018;11:1–15. doi: 10.3390/ma11112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L-C, Wang J, Liu Y, et al. Additive manufacturing of titanium alloys. Materials science and engineering. Oxford: Elsevier; 2020. [Google Scholar]
- 77.Körner C. Additive manufacturing of metallic components by selective electron beam melting—a review. Int Mater Rev. 2016;61:361–377. doi: 10.1080/09506608.2016.1176289. [DOI] [Google Scholar]
- 78.Liu Y, Li S, Hou W, et al. Electron beam melted beta-type Ti–24Nb–4Zr–8Sn porous structures with high strength-to-modulus ratio. J Mater Sci Technol. 2016;32:505–508. doi: 10.1016/j.jmst.2016.03.020. [DOI] [Google Scholar]
- 79.Hauser M, King R, Wysk R, Harrysson O. Resource planning for direct fabrication of customized orthopedic implants using EBM technology. J Manuf Syst. 2021;60:500–511. doi: 10.1016/j.jmsy.2021.07.003. [DOI] [Google Scholar]
- 80.Eliaz N, Foucks N, Geva D, et al. Comparative quality control of titanium alloy Ti-6Al-4V, 17–4 PH stainless steel, and aluminum alloy 4047 either manufactured or repaired by laser engineered net shaping (LENS) Materials (Basel) 2020 doi: 10.3390/ma13184171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu H, Wang Z, Muránsky O, et al. The characterisation and formation of novel microstructural features in a Ti−Nb−Zr−Mo−Sn alloy manufactured by Laser Engineered Net Shaping (LENS) Addit Manuf. 2020 doi: 10.1016/j.addma.2020.101705. [DOI] [Google Scholar]
- 82.Xu K, Li B, Li S, et al. In situ observation for the fatigue crack growth mechanism of 316L stainless steel fabricated by laser engineered net shaping. Int J Fatigue. 2020;130:105272. doi: 10.1016/j.ijfatigue.2019.105272. [DOI] [Google Scholar]
- 83.Habib MA, Khoda B. Rheological analysis of bio-ink for 3D bio-printing processes. J Manuf Process. 2022;76:708–718. doi: 10.1016/j.jmapro.2022.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kengla C, Lee SJ, Yoo JJ, Atala A. 3-D bioprinting technologies for tissue engineering applications. 2. Oxford: Elsevier Ltd; 2020. [Google Scholar]
- 85.Matai I, Kaur G, Seyedsalehi A, Mcclinton A. Biomaterials Progress in 3D bioprinting technology for tissue organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 86.Arslan-Yildiz A, El Assal R, Chen P, et al. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication. 2016 doi: 10.1088/1758-5090/8/1/014103. [DOI] [PubMed] [Google Scholar]
- 87.Sheoran AJ, Chandra A, Kumar H. Recent advances in mechanical engineering. Oxford: Springer; 2021. Biomedical applications of additive manufacturing; pp. 553–566. [Google Scholar]
- 88.Li X, Liu B, Pei B, et al. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120:10793–10833. doi: 10.1021/acs.chemrev.0c00008. [DOI] [PubMed] [Google Scholar]
- 89.Derby B. Bioprinting: inkjet printing proteins and hybrid cell-containing materials and structures. J Mater Chem. 2008;18:5717–5721. doi: 10.1039/b807560c. [DOI] [Google Scholar]
- 90.Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res. 2010;40:395–414. doi: 10.1146/annurev-matsci-070909-104502. [DOI] [Google Scholar]
- 91.Ferguson J, Hudson NE, Warren BCH, Tomatarian A. Phase changes during elongational flow of polymer solutions. Nature. 1987;325:234–236. doi: 10.1038/325234a0. [DOI] [Google Scholar]
- 92.Kačarević ŽP, Rider PM, Alkildani S, et al. An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials (Basel) 2018;11:2199. doi: 10.3390/ma11112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering: bioprinting. Int Mater Rev. 2014;59:430–448. doi: 10.1179/1743280414Y.0000000040. [DOI] [Google Scholar]
- 94.Xu C, Chai W, Huang Y, Markwald RR. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioeng. 2012;109:3152–3160. doi: 10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- 95.Ihalainen P, Määttänen A, Sandler N. Printing technologies for biomolecule and cell-based applications. Int J Pharm. 2015;494:585–592. doi: 10.1016/j.ijpharm.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 96.Hutchings IM, Martin GD. Inkjet technology for digital fabrication. New York: Wiley; 2012. [Google Scholar]
- 97.Liu X, Gaihre B, George MN, et al. 3D bioprinting of oligo (poly [ethylene glycol] fumarate) for bone and nerve tissue engineering. J Biomed Mater Res Part A. 2021;109:6–17. doi: 10.1002/jbm.a.37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoath SD, Hutchings IM, Martin GD, et al. Links between ink rheology, drop-on-demand jet formation, and printability. J Imaging Sci Technol. 2009;53:41208. [Google Scholar]
- 99.Dhyani A, Singh N, Kumar V, Dhyani A. Applications of 3 dimensional (3D) printing in biomedical field. Int J Curr Res Rev. 2020;12:71–75. doi: 10.31782/IJCRR.2020.121918. [DOI] [Google Scholar]
- 100.Jammalamadaka U, Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater. 2018 doi: 10.3390/jfb9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seol Y-J, Kang H-W, Lee SJ, et al. Bioprinting technology and its applications. Eur J Cardio-Thoracic Surg. 2014;46:342–348. doi: 10.1093/ejcts/ezu148. [DOI] [PubMed] [Google Scholar]
- 102.Ji S, Guvendiren M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol. 2017;5:1–8. doi: 10.3389/fbioe.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu Z, Naghieh S, Xu C, et al. Printability in extrusion bioprinting. Biofabrication. 2021 doi: 10.1088/1758-5090/abe7ab. [DOI] [PubMed] [Google Scholar]
- 104.Panwar A, Tan LP. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules. 2016 doi: 10.3390/molecules21060685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tejada Jacob G, Passamai VE, Katz S, et al. Hydrogels for extrusion-based bioprinting: general considerations. Bioprinting. 2022;27:e00212. doi: 10.1016/j.bprint.2022.e00212. [DOI] [Google Scholar]
- 106.Cui X, Li J, Hartanto Y, Durham M, Junnan Tang H, Zhang GH, Lim K, Woodfield T. Advances in extrusion 3D bioprinting a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9:1901648. doi: 10.1002/adhm.201901648. [DOI] [PubMed] [Google Scholar]
- 107.Askari M, Afzali Naniz M, Kouhi M, et al. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci. 2021;9:535–573. doi: 10.1039/d0bm00973c. [DOI] [PubMed] [Google Scholar]
- 108.Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 109.Serra P, Piqué A. Laser-induced forward transfer: fundamentals and applications. Adv Mater Technol. 2019;4:1–33. doi: 10.1002/admt.201800099. [DOI] [Google Scholar]
- 110.Morales M, Munoz-Martin D, Marquez A, et al. Laser-induced forward transfer techniques and applications. Adv Laser Mater Process. 2018;2018:339–379. doi: 10.1016/B978-0-08-101252-9.00013-3. [DOI] [Google Scholar]
- 111.Ringeisen BR, Chrisey DB, Krizman DB et al (2002) Cell-by-cell construction of living tissue by ambient laser transfer. In: 2nd Annu int IEEE-embs spec top conf microtechnologies med boil—proc, pp 120–125. 10.1109/MMB.2002.1002277
- 112.Zhao W, Hu S, Shi Z, et al. 4D bioprinting: technological advances in biofabrication. J Mech Behav Biomed Mater. 2017;15:342–355. doi: 10.1002/mabi.201800441. [DOI] [Google Scholar]
- 113.Cui X, Li J, Hartanto Y, et al. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9:1–27. doi: 10.1002/adhm.201901648. [DOI] [PubMed] [Google Scholar]
- 114.Bil C, Massey K, Abdullah EJ. Wing morphing control with shape memory alloy actuators. J Intell Mater Syst Struct. 2013;24:879–898. doi: 10.1177/1045389X12471866. [DOI] [Google Scholar]
- 115.Zhu W, Ma X, Gou M, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016;40:103–112. doi: 10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017;51:1–20. doi: 10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 117.Koçak E, Yildiz A, Acartürk F. Three dimensional bioprinting technology: applications in pharmaceutical and biomedical area. Colloids Surf B Biointerf. 2020;197:111396. doi: 10.1016/j.colsurfb.2020.111396. [DOI] [PubMed] [Google Scholar]
- 118.Della G, Gandin A, Brigo L, et al. Polysaccharide hydrogels for multiscale 3D printing of pullulan scaffolds. Mater Des. 2019;165:107566. doi: 10.1016/j.matdes.2018.107566. [DOI] [Google Scholar]
- 119.Piqué A, Charipar KM. Laser-induced forward transfer applications in micro-engineering. Cham: Springer; 2020. [Google Scholar]
- 120.Muthaiah VMS, Indrakumar S, Suwas S, Chatterjee K. Surface engineering of additively manufactured titanium alloys for enhanced clinical performance of biomedical implants: a review of recent developments. Bioprinting. 2022;25:e00180. doi: 10.1016/j.bprint.2021.e00180. [DOI] [Google Scholar]
- 121.Piqué A, Charipar KM. In: Laser-Induced Forward Transfer Applications in Micro-engineering BT - Handbook of Laser Micro- and Nano-Engineering. Sugioka K, editor. Cham: Springer International Publishing; 2020. pp. 1–35. [Google Scholar]
- 122.Keriquel V, Oliveira H, Rémy M, et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci Rep. 2017;7:1778. doi: 10.1038/s41598-017-01914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang F, Zhu L, Li Z, et al. The recent development of vat photopolymerization: a review. Addit Manuf. 2021 doi: 10.1016/j.addma.2021.102423. [DOI] [Google Scholar]
- 124.Andreu A, Su PC, Kim JH, et al. 4D printing materials for vat photopolymerization. Addit Manuf. 2021;44:102024. doi: 10.1016/j.addma.2021.102024. [DOI] [Google Scholar]
- 125.Al Rashid A, Ahmed W, Khalid MY, Koç M. Vat photopolymerization of polymers and polymer composites: Processes and applications. Addit Manuf. 2021;47:102279. doi: 10.1016/j.addma.2021.102279. [DOI] [Google Scholar]
- 126.Alifui-Segbaya F, Varma S, Lieschke GJ, George R. Biocompatibility of photopolymers in 3D printing. 3D Print Addit Manuf. 2017;4:185–191. doi: 10.1089/3dp.2017.0064. [DOI] [Google Scholar]
- 127.Najmon JC, Raeisi S, Tovar A. Review of additive manufacturing technologies and applications in the aerospace industry. Oxford: Elsevier Inc.; 2019. [Google Scholar]
- 128.Turnbull G, Clarke J, Picard F, et al. 3D biofabrication for soft tissue and cartilage engineering. Med Eng Phys. 2020;82:13–39. doi: 10.1016/j.medengphy.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J, Hu Q, Wang S, et al. Digital light processing based three-dimensional printing for medical applications. Int J Bioprint. 2020;6:12–27. doi: 10.18063/ijb.v6i1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh SR. Advanced hydrogels for biomedical applications. Biomed J Sci Tech Res. 2018 doi: 10.26717/bjstr.2018.05.001144. [DOI] [Google Scholar]
- 131.Pagac M, Hajnys J, Ma QP, et al. A review of vat photopolymerization technology: materials, applications, challenges, and future trends of 3d printing. Polymers (Basel) 2021;13:1–20. doi: 10.3390/polym13040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Medellin A, Du W, Miao G, et al. Vat photopolymerization 3d printing of nanocomposites: a literature review. J Micro Nano-Manuf. 2019 doi: 10.1115/1.4044288. [DOI] [Google Scholar]
- 133.Pratap R, Mohit S, Dilbagh T, Davim PJP (2022) Proceedings of the international conference on industrial and manufacturing systems (CIMS-2020) optimization in industrial and manufacturing systems and applications
- 134.Dey M, Ozbolat IT. 3D bioprinting of cells, tissues and organs. Sci Rep. 2020;10:1–3. doi: 10.1038/s41598-020-70086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fu Z, Ouyang L, Xu R, et al. Responsive biomaterials for 3D bioprinting: a review. Mater Today. 2022;52:112–132. doi: 10.1016/j.mattod.2022.01.001. [DOI] [Google Scholar]
- 136.Shen Z, Su H, Liu H, et al. Directly fabricated Al2O3/GdAlO3 eutectic ceramic with large smooth surface by selective laser melting: rapid solidification behavior and thermal field simulation. J Eur Ceram Soc. 2022;42:1088–1101. doi: 10.1016/j.jeurceramsoc.2021.11.003. [DOI] [Google Scholar]
- 137.Davoodi E, Montazerian H, Mirhakimi AS, et al. Additively manufactured metallic biomaterials. Beijing: KeAi Communications Co. Ltd.; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pradhan S, Banda OA, Farino CJ, et al. Biofabrication strategies and engineered in vitro systems for vascular mechanobiology. Adv Healthc Mater. 2020;9:22–25. doi: 10.1002/adhm.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Appuhamillage GA, Chartrain N, Meenakshisundaram V, et al. 110th anniversary: vat photopolymerization-based additive manufacturing: current trends and future directions in materials design. Ind Eng Chem Res. 2019;58:15109–15118. doi: 10.1021/acs.iecr.9b02679. [DOI] [Google Scholar]
- 140.Feksa LR, Troian EA, Muller CD, et al. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2018;64:18–23. [Google Scholar]
- 141.Motealleh A, Kehr NS. Nanocomposite hydrogels and their applications in tissue engineering. Adv Healthc Mater. 2017 doi: 10.1002/adhm.201600938. [DOI] [PubMed] [Google Scholar]
- 142.Saroia J, Yanen W, Wei Q, et al. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Design Manuf. 2018 doi: 10.1007/s42242-018-0029-7. [DOI] [Google Scholar]
- 143.Van Tran V, Park D, Lee YC. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ Sci Pollut Res. 2018;25:24569–24599. doi: 10.1007/s11356-018-2605-y. [DOI] [PubMed] [Google Scholar]
- 144.Xu C, Dai G, Hong Y. Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater. 2019;95:50–59. doi: 10.1016/j.actbio.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aswathy SH, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6:e03719. doi: 10.1016/j.heliyon.2020.e03719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen H, Cheng R, Zhao X, et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019;11:1–12. doi: 10.1038/s41427-018-0103-9. [DOI] [Google Scholar]
- 147.Singh M, Jonnalagadda S. Advances in bioprinting using additive manufacturing. Eur J Pharm Sci. 2019 doi: 10.1016/j.ejps.2019.105167. [DOI] [PubMed] [Google Scholar]
- 148.Gull N, Khan SM, Islam A, Butt MTZ. Hydrogels used for biomedical applications. Bio Monomers Green Polym Compos Mater. 2019 doi: 10.1002/9781119301714.ch9. [DOI] [Google Scholar]
- 149.Liu S, Chen X, Zhang Y. Hydrogels and hydrogel composites for 3D and 4D printing applications. Oxford: Elsevier Inc.; 2019. [Google Scholar]
- 150.United Nations . Ageing, older persons and the 2030 agenda for sustainable development. New York: United Nations Publication; 2017. pp. 1–26. [Google Scholar]
- 151.United Nations (2019) World population ageing 2019. In: World population ageing 2019, p 64
- 152.Ishfaq K, Rehman M, Khan AR, Wang Y. A review on the performance characteristics, applications, challenges and possible solutions in electron beam melted Ti-based orthopaedic and orthodontic implants. Rapid Prototyp J. 2022;28:525–545. doi: 10.1108/RPJ-03-2021-0060. [DOI] [Google Scholar]
- 153.Qin P, Chen Y, Liu YJ, et al. Resemblance in corrosion behavior of selective laser melted and traditional monolithic β Ti-24Nb-4Zr-8Sn alloy. ACS Biomater Sci Eng. 2019;5:1141–1149. doi: 10.1021/acsbiomaterials.8b01341. [DOI] [PubMed] [Google Scholar]
- 154.Hernandez J, Li SJ, Martinez E, et al. Microstructures and hardness properties for β-phase Ti-24Nb-4Zr-7.9Sn alloy fabricated by electron beam melting. J Mater Sci Technol. 2013;29:1011–1017. doi: 10.1016/j.jmst.2013.08.023. [DOI] [Google Scholar]
- 155.Yang HX, Li SJ, Hou WT, et al. Recoverable strain in a new biomedical Ti-24Nb-4Zr-8Sn alloy with cellular structure fabricated by electron beam melting. Mater Technol. 2020;35:881–886. doi: 10.1080/10667857.2019.1709287. [DOI] [Google Scholar]
- 156.Liu CF, Li SJ, Hou WT, et al. Enhancing corrosion resistance and biocompatibility of interconnected porous β-type Ti-24Nb-4Zr-8Sn alloy scaffold through alkaline treatment and type I collagen immobilization. Appl Surf Sci. 2019;476:325–334. doi: 10.1016/j.apsusc.2019.01.084. [DOI] [Google Scholar]
- 157.Yang CL, Zhang ZJ, Li SJ, et al. Simultaneous improvement in strength and plasticity of Ti-24Nb-4Zr-8Sn manufactured by selective laser melting. Mater Des. 2018;157:52–59. doi: 10.1016/j.matdes.2018.07.036. [DOI] [Google Scholar]
- 158.Liu YJ, Li SJ, Wang HL, et al. Microstructure, defects and mechanical behavior of beta-type titanium porous structures manufactured by electron beam melting and selective laser melting. Acta Mater. 2016;113:56–67. doi: 10.1016/j.actamat.2016.04.029. [DOI] [Google Scholar]
- 159.Liu Y, Li S, Hou W, et al. Electron beam melted beta-type Ti-24Nb-4Zr-8Sn porous structures with high strength-to-modulus ratio. J Mater Sci Technol. 2016;32:505–508. doi: 10.1016/j.jmst.2016.03.020. [DOI] [Google Scholar]
- 160.Cheng X, Liu S, Chen C, et al. Microstructure and mechanical properties of additive manufactured porous Ti–33Nb–4Sn scaffolds for orthopaedic applications. J Mater Sci Mater Med. 2019;30:1–12. doi: 10.1007/s10856-019-6292-0. [DOI] [PubMed] [Google Scholar]
- 161.Nicolas-Silvente AI, Velasco-Ortega E, Ortiz-Garcia I, et al. Influence of the titanium implant surface treatment on the surface roughness and chemical composition. Materials (Basel) 2020;13:1–13. doi: 10.3390/ma13020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Çaha I, Alves AC, Rocha LA, Toptan F. A review on bio-functionalization of β-Ti alloys. Cham: Springer; 2020. [Google Scholar]
- 163.Kolli RP, Devaraj A. A review of metastable beta titanium alloys. Metals (Basel) 2018;8:1–41. doi: 10.3390/met8070506. [DOI] [Google Scholar]
- 164.Chua K, Khan I, Malhotra R, Zhu D. Additive manufacturing and 3D printing of metallic biomaterials. Eng Regen. 2021;2:288–299. doi: 10.1016/j.engreg.2021.11.002. [DOI] [Google Scholar]
- 165.Yang S, Xie F, Cui W, et al. A review of the clinical and engineering performance of dual-mobility cups for total hip arthroplasty. Am J Transl Res. 2021;13:9383–9394. [PMC free article] [PubMed] [Google Scholar]
- 166.Sercombe TB, Zhang LC, Li S, Hao Y. Additive manufacturing of cp-Ti, Ti-6Al-4V and Ti2448. Titan Med Dent Appl. 2018 doi: 10.1016/B978-0-12-812456-7.00014-7. [DOI] [Google Scholar]
- 167.Fu P, Li H, Gong J, et al. 4D printing of polymers: Techniques, materials, and prospects. Prog Polym Sci. 2022;126:101506. doi: 10.1016/j.progpolymsci.2022.101506. [DOI] [Google Scholar]
- 168.Subash A, Bkp D, Eng C. 4D printing of shape memory polymers. Eur Polym J. 2020;134:109771. doi: 10.1016/j.eurpolymj.2020.109771. [DOI] [Google Scholar]
- 169.Zafar MQ, Zhao H. 4D printing : future insight in additive manufacturing. Met Mater Int. 2020;26:564–585. doi: 10.1007/s12540-019-00441-w. [DOI] [Google Scholar]
- 170.Maniruzzaman M. 3D and 4D printing in biomedical. Oxford: Wiley; 2018. [Google Scholar]
- 171.Haleem A, Javaid M, Vaishya R. 4D printing and its applications in orthopaedics. J Clin Orthop Trauma. 2018;9:275–276. doi: 10.1016/j.jcot.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shan W, Chen Y, Hu M, et al. 4D printing of shape memory polymer via liquid crystal display (LCD) stereolithographic 3D printing. Mater Res Express. 2020 doi: 10.1088/2053-1591/abbd05. [DOI] [Google Scholar]
- 173.Wang Y, Adokoh CK, Narain R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin Drug Deliv. 2018;15:77–91. doi: 10.1080/17425247.2017.1360865. [DOI] [PubMed] [Google Scholar]
- 174.Hann SY, Cui H, Nowicki M, Zhang LG. 4D printing soft robotics for biomedical applications. Addit Manuf. 2020;36:101567. doi: 10.1016/j.addma.2020.101567. [DOI] [Google Scholar]
- 175.Chu H, Yang W, Sun L, et al. 4D printing: a review on recent progresses. Micromachines. 2020;11:796. doi: 10.3390/mi11090796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pan D, Zhang X, Hou X, et al. TiB nano-whiskers reinforced titanium matrix composites with novel nano-reticulated microstructure and high performance via composite powder by selective laser melting. Mater Sci Eng A. 2021;799:140137. doi: 10.1016/j.msea.2020.140137. [DOI] [Google Scholar]
- 177.Rastogi P, Kandasubramanian B. Breakthrough in the printing tactics for stimuli-responsive materials : 4D printing. Chem Eng J. 2019;366:264–304. doi: 10.1016/j.cej.2019.02.085. [DOI] [Google Scholar]
- 178.Haleem A, Javaid M, Vaishya R. 5D printing and its expected applications in orthopaedics. J Clin Orthop Trauma. 2019;10:809–810. doi: 10.1016/j.jcot.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ghazal AF, Zhang M, Mujumdar AS, Ghamry M. Progress in 4D/5D/6D printing of foods: applications and R&D opportunities. Crit Rev Food Sci Nutr. 2022 doi: 10.1080/10408398.2022.2045896. [DOI] [PubMed] [Google Scholar]
- 180.Reddy PR, Devi PA. Review on the advancements of additive manufacturing-4D and 5D printing. Int J Mech Prod Eng Res Dev. 2018;8:397–402. [Google Scholar]
- 181.Kumar A, Kargozar S, Baino F, Han SS. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: a review. Front Mater. 2019;6:313. doi: 10.3389/fmats.2019.00313. [DOI] [Google Scholar]
- 182.Gebler M, Uiterkamp AJMS, Visser C. A global sustainability perspective on 3D printing technologies. Energy Policy. 2014;74:158–167. doi: 10.1016/j.enpol.2014.08.033. [DOI] [Google Scholar]
- 183.Whenish R, Ramakrishna S, Jaiswal AK, Manivasagam G. A framework for the sustainability implications of 3D bioprinting through nature-inspired materials and structures. Bio-Design Manuf. 2022;5:412–423. doi: 10.1007/s42242-021-00168-x. [DOI] [Google Scholar]
- 184.Peng T, Kellens K, Tang R, et al. Sustainability of additive manufacturing: an overview on its energy demand and environmental impact. Addit Manuf. 2018;21:694–704. doi: 10.1016/j.addma.2018.04.022. [DOI] [Google Scholar]
- 185.Yadav D, Garg RK, Ahlawat A, Chhabra D. 3D printable biomaterials for orthopedic implants: solution for sustainable and circular economy. Resour Policy. 2020;68:101767. doi: 10.1016/j.resourpol.2020.101767. [DOI] [Google Scholar]
- 186.Faludi J, Braunholz C (2015) Does material choice drive sustainability of 3D printing ? Dartmouth Digit Commons
- 187.Dunaway D, Harstvedt JD (2017) A preliminary experimental study of additive manufacturing energy consumption. In: Proc ASME 2017 Int Des Eng Tech Conf Comput Inf Eng Conf, pp 1–8
- 188.Clemon, Lee, Anton Sudradjat, Maribel Jaquez AK, Rammah, Marwan DD (2016) Precision and energy usage for additive manufacturing. In: Proc ASME 2013 int mech eng congr expo, pp 1–8
- 189.Stefan Junk SC (2012) A practical approach to comparing energy effectiveness of rapid prototyping. In: Proc AEPR’12, 17th eur forum rapid prototyp manuf paris, Fr, pp 12–14
- 190.Xu X, Meteyer S, Perry N, Zhao YF. Energy consumption model of Binder-jetting additive manufacturing processes. Int J Prod Res. 2014 doi: 10.1080/00207543.2014.937013. [DOI] [Google Scholar]
- 191.Baumers M, Tuck C, Hague R et al (2010) A comparative study of metallic additive manufacturing power consumption. In: 2010 International solid freeform fabrication symposium. University of Texas at Austin
- 192.Baumers M, Tuck C, Bourell DL, et al. Sustainability of additive manufacturing: measuring the energy consumption of the laser sintering process. Proc Inst Mech Eng Part B J Eng Manuf. 2011;225:2228–2239. doi: 10.1177/0954405411406044. [DOI] [Google Scholar]
- 193.Kellens K, Yasa E, Dewulf W, Duflou JR. Environmental assessment of selective laser melting and selective laser sintering. Methodol. 2010;4(5):1–8. [Google Scholar]
- 194.Morrow WR, Qi H, Kim I, et al. Environmental aspects of laser-based and conventional tool and die manufacturing. J Clean Prod. 2007;15:932–943. doi: 10.1016/j.jclepro.2005.11.030. [DOI] [Google Scholar]
- 195.Wilson JM, Piya C, Shin YC, et al. Remanufacturing of turbine blades by laser direct deposition with its energy and environmental impact analysis. J Clean Prod. 2014;80:170–178. doi: 10.1016/j.jclepro.2014.05.084. [DOI] [Google Scholar]
- 196.Luo Y, Ji Z, Leu MC, Caudill R (1999) Environmental performance analysis of solid freedom fabrication processes. In: Proceedings of the 1999 IEEE international symposium on electronics and the environment (Cat. No. 99CH36357). IEEE, pp 1–6
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study is already contained within the article.