Abstract
Cardiovascular disease is the most common cause of death worldwide, especially beyond the age of 65, with the vast majority of morbidity and mortality due to myocardial infarction and stroke. Vascular pathology stems from a combination of genetic risk, environmental factors, and the biologic changes associated with aging. The pathogenesis underlying the development of vascular aging, and vascular calcification with aging in particular, is still not fully understood. Accumulating data suggests that genetic risk, likely compounded by epigenetic modifications, environmental factors, including diabetes mellitus and chronic kidney disease, and the plasticity of vascular smooth muscle cells to acquire an osteogenic phenotype are major determinants of age-associated vascular calcification. Understanding the molecular mechanisms underlying genetic and modifiable risk factors in regulating age-associated vascular pathology may inspire strategies to promote healthy vascular aging. This article summarizes current knowledge of concepts and mechanisms of age-associated vascular disease, with an emphasis on vascular calcification.
Introduction
Cardiovascular disease is the leading cause of death worldwide and increases with age, in large part due to the cumulative effects of risk factors such as hypertension, hyperlipidemia, diabetes, tobacco use, and sedentary behavior. However, with advancing age, even individuals without traditional risk factors gradually develop vascular pathology including arterial fibrosis, stiffness, and calcification, increasing the risk of serious cardiovascular events. The importance of understanding the interplay between vascular biology and aging, independent of traditional risk factors, is of utmost importance.
Isolating vascular aging as an independent biological variable is challenging for several reasons. First, vascular aging is often accompanied by one or more cardiovascular disease risk factors. Second, there is likely a synergistic effect of both the duration and number of cardiovascular risk factors that make it challenging to fully adjust for such variables. Although studies of aging exist, they are prone to survival bias in that only individuals who survived until older age can be studied if not enrolled earlier in life. Studies such as the Progression and Early detection of Subclinical Atherosclerosis (PESA)1 and the Asklepios Study2 were designed to circumnavigate these challenges in order to study the interactions of age and inflammation with cardiovascular hemodynamics and development of atherosclerosis. Studies of human longevity are also challenging due to the costly and time-consuming nature of studying an individual human over a lifespan, and long-lived individuals may have different genetic longevity variants and protein signatures.3–5
Improving our understanding of vascular aging and its role in cardiovascular disease progression, morbidity, and mortality is essential. The following review discusses what is currently known regarding the biology of vascular aging, clinical manifestations of age-associated vascular disease with a focus on calcification, the impact of genetic risk on vascular aging, and the environmental and molecular factors that may influence vascular aging and promote longevity (Figure 1).
Figure 1.
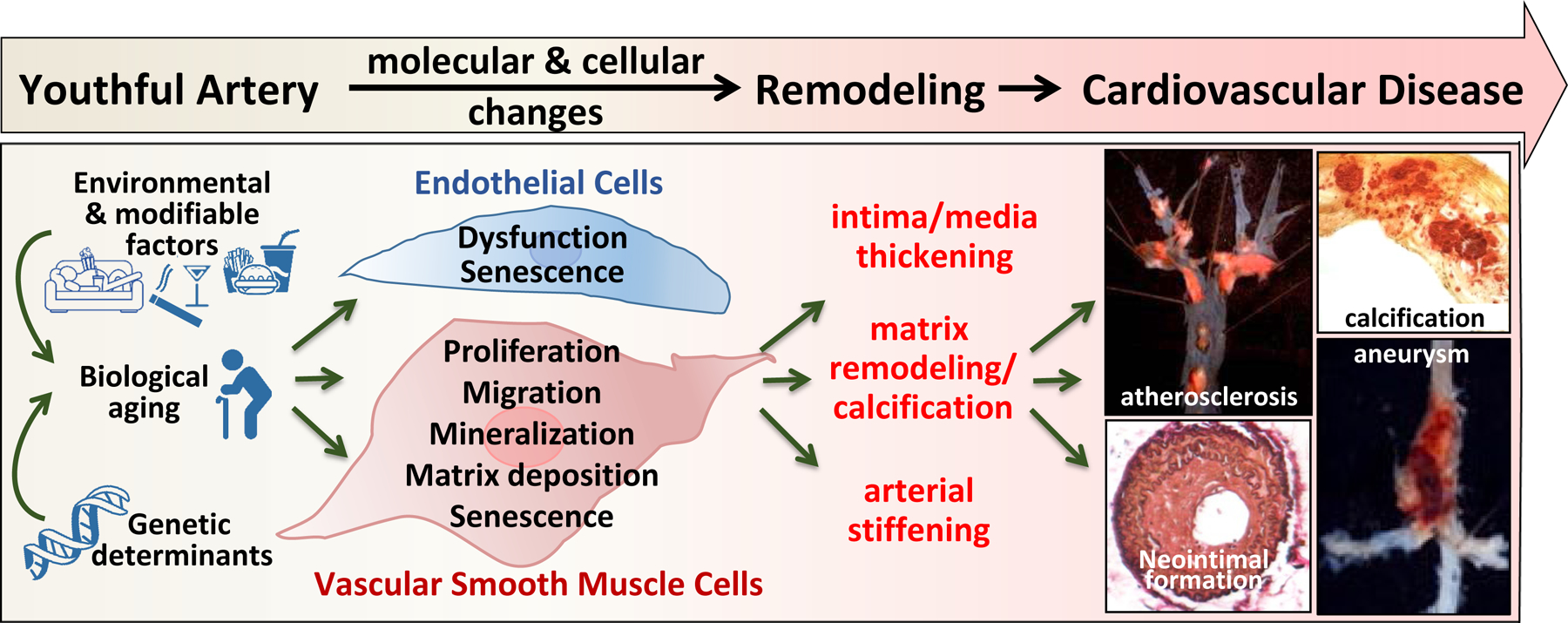
Vascular aging and aging-accelerated vascular disease. Genetic and environmental factors induced endothelial cell dysfunction and vascular smooth muscle cell phenotypic modulation that leads to vascular remodeling and development of cardiovascular disease.
Clinical manifestations of age-associated cardiovascular calcification
Arterial stiffening and calcification are characteristics of vascular aging, serve as important predictors of cardiovascular morbidity and mortality, and are exacerbated by cardiovascular disease risk factors and metabolic syndromes.6–9 Arterial calcification is closely associated with atherosclerotic plaque evolution, and the area of coronary artery calcification (CAC) quantified on noncontrast cardiac computed tomography (CT) has a direct relationship with histopathologic coronary plaque area.10 Autopsy studies have consistently shown a strong association between calcification of the coronary arteries and atherosclerosis.11 Calcification is often categorized as intimal, typically associated with atherosclerotic plaque, or medial, often a more diffuse arteriosclerotic process marked by vascular stiffening and associated with conditions such as chronic kidney disease and diabetes.
CAC volume and vulnerable plaques with a lipid-rich core, thin cap, or spotty or micro calcifications are associated with the risk of future atherosclerotic cardiovascular disease (ASCVD) events.12–15 However, within a given coronary artery, there is a wide variation between the degree of plaque calcification and severity of luminal stenosis on invasive coronary angiography due in part to individual variations in coronary artery remodeling.16
Noninvasive methods to evaluate coronary heart disease risk, such as exercise stress testing, typically only identify patients with advanced, obstructive atherosclerotic disease. This is of relevance as myocardial infarctions may occur when a non-obstructive atherosclerotic plaque ruptures.17 Thus, there has been great interest in characterizing atherosclerosis in its pre-flow limiting phase, so that intensified preventive strategies can be instituted. Measurement of CAC volume on CT imaging often improves the accuracy of cardiovascular risk assessment in intermediate risk adults and may help to determine which patients may benefit from initiation of or intensification of risk factor modification strategies such as lipid lowering, aspirin, or antihypertensive therapies.18–20
Prospective studies of CAC and incident cardiovascular disease risk from the Multi-Ethnic Study of Atherosclerosis and other observational cohorts show a very strong correlation between increased CAC and the risk of future cardiovascular disease events.21 CAC scores greater than the 75th percentile for age, sex, and ethnicity or more than 100 indicate an elevated 10-year ASCVD risk and should lead to more improved lifestyle habits and strong consideration of statin therapy in intermediate-risk adults.20 CAC percentiles based on age, sex, and ethnicity are better predictors of lifetime risk, whereas CAC scores provide the best estimate of absolute risk in the next decade.19
Traditionally, CAC scores have been used to determine the need for initiating statin therapy. However, evidence suggests that while high-intensity statin therapy lowers cardiovascular event risk, it paradoxically may modestly increase CAC and potentially stabilize existing atherosclerotic plaques.22, 23 While CAC volume has been associated with increased CVD risk, CAC density is inversely associated with CVD risk.24 Statin-induced atherosclerotic plaque calcification has been attributed to increased plaque alkaline phosphatase activity25 and disinhibition of the macrophage Rac (Ras-related C3 botulinum toxin substrate)–IL-1β (interleukin-1 beta) signaling axis.26
Emerging data has indicated that CAC scores can help in prioritizing the need for more intensive medications in higher risk individuals who have above average amounts of CAC for their age, gender, and ethnicity. These medications may include glucagon-like peptide 1 receptor agonists or sodium-glucose cotransporter 2 inhibitors in adults with diabetes or proprotein convertase subtilisin/kexin type 9 inhibitors in adults with suboptimal LDL-cholesterol lowering on maximally tolerated statin therapy. Cainzos-Achirica et al. have made a compelling case for measuring CAC to more accurately allocate medications and to use the CAC score to enrich study populations of primary prevention randomized controlled trials with participants at higher absolute risk of cardiovascular events.27 Conversely, a CAC score of zero is a powerful negative predictor of future cardiovascular events in older patients, such that it is reasonable to consider withholding statin therapy in the absence of other risk factors.28 Additional study of individuals who age without developing vascular calcification would be of interest.
Measurement of CAC is well-validated for risk stratification in middle to older-age adults. Incorporating CAC scores improves risk stratification for incident sudden cardiac death beyond traditional ASCVD risk factors in individuals with low-to-intermediate risk.29 Until recently, there was limited data in adults with an age less than 40. Javaid et al recently studied the prognostic importance of CAC in nearly 20,000 asymptomatic adults aged 30–45 years without known ASCVD. They found that any CAC in this age range placed females at >90th percentile (high lifetime risk). The presence of any CAC placed White males at the 90th percentile by age 34 and Black males by age 37.30
Extracoronary cardiovascular calcification, including aortic31, peripheral vascular32, and valvular, also predicts cardiovascular risk. Calcific aortic valve stenosis is the most common valvular heart disease in the Western world, and progressive fibrocalcific changes in the valve leaflets may lead to partial aortic outflow tract obstruction.33 However, aortic valve sclerosis (calcification and thickening of the aortic valve), even in the absence of hemodynamically significant obstruction of left ventricular outflow track, is independently associated with an increased risk of ASCVD events.34–36 Research is ongoing to determine if aortic valve calcium scoring using cardiac CT may be useful for risk stratification and to identify those at increased risk of developing significant aortic valve stenosis.37
A striking demonstration of accelerated vascular calcification in adults is calciphylaxis— a rare but devastating condition that is predominantly seen among patients with end-stage kidney disease (ESKD) who have typically been dialysis-dependent for over 2–3 years.38 The primary clinical manifestation of calciphylaxis is painful skin ulcers caused by cutaneous ischemia. These patients, almost universally, have diffuse extra-skeletal calcification. In addition to metabolic abnormalities of calcium and phosphate metabolism originating from the ESKD, over 40% of patients with calciphylaxis have diabetes mellitus, and as many as 30% have been exposed to warfarin prior to the development of calciphylaxis.39, 40
Warfarin, a vitamin K antagonist, may impair the gamma-carboxylation of a potent calcification inhibitor known as Matrix Gla Protein. This may further accelerate the process of vascular calcification among patients who are predisposed to it from their underlying comorbidities. At present, there is no approved treatment for calciphylaxis, although anecdotal reports of successful resolution of calciphylaxis lesions with treatments such as vitamin K supplementation and kidney transplantation provide potential insights into strategies to reduce calcification and eventually improve clinical outcomes.41, 42
The Biology of Vascular Aging
Vascular aging is a biological variable, conceptually distinct from chronological aging, whereby sequential and progressive changes in a cell or whole organism leads to an increased risk of dysfunction, disease, and death.43–45 Hallmarks of biological aging include cellular dysfunction and vulnerability to cell death, and many of these hallmarks also contribute to vascular dysfunction and calcification.46–48
Telomeres shorten with every cellular replication cycle leading to reduced proliferative capacity of cells.49, 50 The single strand ends of telomeres are protected to prevent the chromosomal ends from appearing as double-stranded DNA breaks, which otherwise trigger DNA damage responses.51 Breakdown of these telomere caps can lead to age-related vascular dysfunction, including increased cellular senescence, oxidative stress, and inflammation.52, 53
Senescent cells are not inert and may extrude chemical mediators that further propagate an inflammatory phenotype to neighboring cells.54 Vascular smooth muscle cells (SMCs) exhibit markers of senescence and calcify in response to uptake of endothelial-derived exosomes.55, 56 In addition, microparticles from older individuals’ senescent endothelial cells induce vascular SMC calcification57, and human vascular function in vivo inversely correlates with the presence of senescence markers in endothelial cells.58 In mice, senolytic drugs, which induce death of senescent cells, restore vascular function in aged mice.59
Accumulation of DNA damage, whether due to exogenous factors (such as ionizing radiation), replication errors, or impaired repair, contributes to cellular dysfunction in part due to the generation of reactive oxygen and nitrogen species and may also lead to cardiovascular calcification.60, 61 Increased oxidative stress is also a major factor promoting loss of vascular SMC contractility and increased osteogenic differentiation and calcification, characteristics of vascular aging.8, 62
Inflammageing, or the age-related increase in pro-inflammatory markers in the blood and tissues63, is likely both a biomarker of biological aging as well as cause of age-related cardiovascular pathology. Inflammageing may occur due to increased production of inflammatory mediators, such as from senescent cells, or due to impaired inflammatory resolution, 64, 65 as was recently reviewed in detail elsewhere as a target in atherosclerosis.66 That endothelial cells stimulated with tumor necrosis factor (TNF) α released microparticles containing bone morphogenetic protein 2 (BMP2), which in turn were phagocytosed by vascular SMCs and enhanced osteogenesis supports the role for inflammageing in promoting age-associated arterial calcification.67 BMP2 is also proinflammatory and induces endothelial activation, suggesting these local inflammatory perturbations could auto-feedback and escalate age-associated calcification.68 C-reactive protein has been implicated in promoting age-associated vascular SMC osteogenic transdifferentiation via the Fc fragment of IgG receptor IIa and the p38 mitogen-activated protein kinase pathway.69
Epigenetic marks on histones can dictate global gene expression patterns.44 These epigenetic modifications correlate with biological age and more accurately predict lifespan than chronological age.70–72 Epigenetic programming occurs during development and informs cellular phenotypes. Recent studies show that these developmental programs, or the loss of them, help to drive vascular cell dysfunction, including calcification and the loss of the contractile phenotype of vascular SMCs.73–79
Genetic risk and cardiovascular disease
While many traditional risk factors become more clinically relevant in middle age, one’s genetics are present from birth. Polygenic scores (PGS, also known as genetic risk scores or polygenic risk scores) build on results from genome-wide association studies (GWASs) to allow estimation of one’s cumulative genetic risk for a given endpoint.80 PGS enable identification of patients at high risk for common, complex diseases such as cardiovascular disease (CVD), much like carriers of a Mendelian mutation. PGS also allow for improved reclassification of patients with cardiovascular disease and early onset myocardial infarction and early onset coronary heart disease.81, 82
For example, in the United Kingdom (UK) Biobank, participants with a PGS for coronary artery disease (CAD) in the top 5% of the cohort’s PGS distribution have a greater than three-fold risk for CAD compared to the rest of the population.83 This is similar to the CAD risk conferred by mutations in genes causing familial hypercholesterolemia (FH), yet 20 times as many individuals fall into this polygenic high-risk category as carry an FH mutation.83, 84 Moreover, PGS have stronger risk stratification power in younger populations than older ones.85 PGS have been found to predict incident CAC86–88 and can be useful in predicting the optimal age for CAC screening.89 Favorable lifestyles mitigate the susceptibility to CAC even if genetic risk is elevated.90
The Finnish GeneRISK study, a web-based communication tool (KardioKompassi), aims to assess the clinical utility of PGS by providing personalized 10-year CVD risk to a prospective cohort of 7,342 individuals.91 After only 1.5 years, 71% of the participants were re-assessed, and genetic risk was found to motivate positive health behavior. In another study with a prospective observational cohort of 3,800 individuals, knowledge of having a high CAD PGS was associated with earlier initiation (52 years versus 65 years) and use of a lipid lowering therapy (42.4% versus 28.5%).92
The potential clinical utility of PGS is often quantified with the net reclassification index (NRI), the percent of patients who would be reclassified into a different risk category upon addition of the PGS to conventional CVD risk prediction models (Table 1). An important limitation is that PGSs have mostly been derived from populations of European genetic ancestry and are generally not available at large commercial labs for clinical use. However, the AHA recently issued a scientific statement with guidance for their use.93
Table 1.
Net Reclassification Index of Polygenic Risk Scores
| Publication | Score description | Outcome | Group (Ancestry) | # of samples | Continuous NRI (%) | Categorical NRI (%) |
|---|---|---|---|---|---|---|
| Elliot et al., JAMA 2020a200 | Lassosum | Incident CAD in UKBiobank | Events (EUR) | 6272 | 15.4 (13.0, 17.9) | 4.4 (3.5, 5.3) |
| Non-events (EUR) | 346,388 | 15.8 (15.5, 16.1) | −0.4 (−0.5, −0.4) | |||
| All (EUR) | 352,600 | 31.2 (28.7, 33.7) | 4.0 (2.1, 4.9) | |||
| All (EUR) | 4,168 | 1.8 (−0.2, 3.6) | ||||
| All (EUR) | 2,101 | 0.1 (−3.8, 7.6) | ||||
| Mars et al., Nature Medicine 202082 | LDpred from external GWAS | Incident CHD in FINRISK | Events (FIN) | 1,209 | 0.9 (−0.02, 2.0) | |
| Non-events (FIN) | 18,956 | 0.2 (−0.1, 0.5) | ||||
| All (FIN) | 20,165 | 1.1 (−0.1, 2.2) | ||||
| Hindy et al., ATVB, 2020201 | LDpred from Khera et al | Incident CAD in Malmö Diet and Cancer Study | Events (EUR) | 815 | 17.3 (8.8, 19.9) | |
| Non-events (EUR) | 4,870 | −0.9 (−1.8, −0.2) | ||||
| All (EUR) | 16.5 (7.6, 18.2) | |||||
| LDpred from Khera et al | Incident CAD in UKBiobank | Events (EUR) | 7,708 | 9.1 (7.7, 10.5) | ||
| Non-events (EUR) | 317,295 | −0.6 (−0.7, −0.6) | ||||
| All (EUR) | 325,003 | 8.5 (7.1, 9.8) | ||||
| Riveros-McKay, Circ Gen & Prec Med, 2021a202 | Novel PRS | Incident CAD in UKBiobank | Events | 4,122 | 5.97 (4.83–7.12) | |
| Non-events | 24,434 | −0.09 (−0.26, 0.08) | ||||
| All | 186,451 | 5.88 (4.73,7.04) | ||||
| Sun et al., PLOS Med, 2021b203 | metaPRS | Incident CVD in UKBiobank | Events (EUR) | 5680 | 10.2 (7.2, 13.2) | 0.3 (−0.7,1.2) |
| Non-events (EUR) | 300,974 | 12.6 (12.2,13.0) | 2.2 (1.8, 2.6) | |||
| Weale et al., American Journal of Cardiology, 2021204 | LDpred of custom GWAS summary statistics | Incident CVD combined across UKBiobank, MESA, ARIC | Events (EUR) | 2096 | 2.7 (1.17–4.22) | |
| Events (AFR) | 309 | 2.24 (0.39–4.08) | ||||
| Lu et al., European Heart Journal, 2022205 | Custom PRS of CAD and CAD-related traits in EAS and EUR | Incident CAD in China-PAR | Events (EAS) | 840 | 15.7 (7.7, 22.2) | 3.2 (0.9–5.8) |
| Non-events (EAS) | 32,859 | 10.1 (9.1, 11.1) | 0.3 (0.1–0.5) | |||
| All (EAS) | 33,699 | 25.8 (18.5, 32.5) | 3.5 (1.2–6.0) | |||
| Stienfeldt, et al. Lancet Digital Health, 2022 c206 | 6 PGS from PGS Catalog | Incident MACE in UKBiobank | Non-events (majority EUR) | 371,909 | 0.05 (0.03, 0.12) | |
| Events (majority EUR) | 23,790 | 1.12 (0.62,1.54) | ||||
| All (majority EUR) | 394,713 | 1.16 (0.66, 1.59) |
Net reclassification index (NRI) for ASCVD-Pooled Cohorts Equation (PCE) versus Pooled Cohorts Equation with polygenic score in several cohorts with various polygenic scores and primary outcomes used. Categorical NRI uses the 7.5% 10-year risk of ASCVD threshold unless otherwise noted.
NRI multiplied by 100 as pseudo percentage with range −200 to 200
Comparison made between conventional risk factors alone and with polygenic score. Categorical NRI using <5%, 5–7.5%, and ≥7.5% 10-year risk thresholds according to 2019 ACC/AHA guideline
Comparison made between a neural network CVD risk predictor with and without additional of polygenic score predictors. Categorical NRI using 10% risk thresholds.
Abbreviations: AFR African genetic ancestry, CAD Coronary Artery Disease, CHD Coronary Heart Disease, CVD Cardiovascular disease, EAS East Asian genetic ancestry, EUR European genetic ancestry, MACE major adverse cardiac event
Genetics underpinnings of cardiovascular calcification
In contrast to the use of polygenic scores based on common genetic variation to predict risk, certain gene defects are responsible for rare, Mendelian disorders of premature vascular pathology (Table 2). For example, rare diseases resulting in premature vascular calcification stem from abnormalities in the extracellular ATP metabolic pathway.94 ATP is released from cells under conditions of stress or death and can act in a paracrine manner through its cognate receptors or be metabolized to its constituent parts by a series of ectonucleotidases.95 Several ATP metabolites regulate vascular calcification.96 Calcium and inorganic phosphate are the building blocks of calcification, but an endogenous inhibitor of mineral nucleation is pyrophosphate, which is the product of the breakdown of ATP by ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1).96 In a murine model of Hutchinson-Gilford progeria syndrome with accelerated vascular aging, pyrophosphate treatment inhibited arterial calcification.97
Table 2.
Genetic Determinants of Vascular and Valvular Calcification
| Genomewide Significant Loci | |||
|---|---|---|---|
| Calcific Disorder | Gene/Locus (Lead Single Nucleotide Polymorphism) |
Gene/Locus description | Study |
| Coronary artery calcification |
PHACTR1/EDN1 (rs9349379; chr 6) |
phosphatase and actin regulator 1; endothelial cell survival; upregulates endothelin 1 (EDN1), a vasoconstrictor | O’Donnell et al., 2011109; Gupta et al., 2017110 |
|
9p21 (rs1333049; chr 9) |
CDKN2A/CDKN2B | O’Donnell et al., 2011109 | |
|
APOB (rs5742904; chr 2) |
apolipoprotein B | Natarajan et al., 2016111 | |
|
APOE (rs7412; chr 19) |
apolipoprotein E | Natarajan et al., 2016111 | |
| Abdominal aortic calcification |
HDAC9/TWIST1 (rs57301765; chr 7) |
histone deacetylase 9; modulator of osteogenic phenotype; promotes endothelial-to-mesenchymal transition twist family bHLH transcription factor 1 | Malhotra et al., 2019115; Lecce et al., 2021116; Nurnberg et al., 2020117 |
| Aortic valvular calcification |
LPA (rs10455872; chr 6) |
lipoprotein a; causal role for Lp(a) in AV calcification | Thanassoulis et al., 2013118; Helgadottir et al., 2018119 |
|
PALMD (rs7543130; chr 1) |
palmdelphin; also associated with congenital heart disease | Helgadottir et al., 2018119 | |
|
TEX41 (rs1830321; chr 2) |
testis expressed 41; also associated with congenital heart disease | Helgadottir et al., 2018119 | |
| Mitral valvular calcification |
IL1F9 (rs17659543; chr 2) |
interleukin 36 gamma; proinflammatory | Thanassoulis et al., 2013118 |
| Mendelian Disorders | |||
| Calcific Disorder | Gene(s) | Description | Study |
| Generalized arterial calcification of infancy (GACI) | ENPP1 or ABCC6 | ectonucleotide pyrophosphatase/phosphodiesterase 1; ATP binding cassette subfamily C member 6; purine and pyrophosphate metabolism | Rutsch et al., 200398 |
| Arterial Calcification due to Deficiency of CD73 (ACDC) | CD73 (aka NT5E) | Ecto-5’-nucleotidase or cluster of differentiation 73; purine metabolism | St. Hilaire et al., 2011100 |
| Pseudoxanthoma elasticum | ABCC6 | ATP binding cassette subfamily C member 6; purine and pyrophosphate metabolism | Jansen et al., 2013104 |
In generalized arterial calcification of infancy (GACI), mutations in ENPP1 lead to a deadly disease of extensive medial arterial calcification in large arteries, which presents in infancy, and it is the lack of local production of extracellular pyrophosphate that drives this devastating phenotype.98 The other product of ENPP1 is AMP, which is further metabolized to inorganic phosphate and adenosine by the ectonucleotidase CD73.96 Inactivating mutations in the gene encoding for CD73 lead to medial arterial calcifications in adulthood that phenocopy the pathologies seen in patients with diabetes and ESRD.99, 100 In this disease (termed Arterial Calcification due to Deficiency of CD73, ACDC), mechanistic studies have uncovered that the lack of adenosine signaling via the A2b adenosine receptor (A2bAR) drives the osteogenic transition of these SMCs.101 Calcified femoropopliteal arteries from patients with non-genetic forms of medial arterial calcification exhibit signatures of this rare disease, suggesting the mechanism that operates in this pathology.102, 103 Pseudoxanthoma elasticum is characterized by microvascular arterial calcification in childhood and is caused by mutations in the ATP binding cassette subfamily C member 6 (ABCC6); however, the factor being transported is debated.104, 105 Singleton-Merton syndrome is caused by a missense mutation in interferon-induced helicase C domain 1 (IFIH1)106, and Hutchinson-Gilford progeria syndrome is caused by a splice defect in lamin A (LMNA)107, 108; both disorders manifest with premature and extensive aortic and valvular calcification. The relationships of these genes to adult, age-related calcific disorders, however, remain uncertain.
GWAS have identified several seemingly unrelated genes implicated in CAC. The first GWAS for CAC identified two loci at 6p24 and 9p21.109 The former is nearest to the phosphatase and actin regulator 1 (PHACTR1) gene, which plays a role in endothelial cell survival. Targeted deletion at this locus increases the expression of nearby gene endothelin 1 (EDN1), a potent vasoconstrictor known to promote atherosclerosis.110 In an exome-wide association meta-analysis, protein-coding variants in apolipoprotein B (APOB) and apolipoprotein E (APOE) were also associated with CAC among patients without overt coronary heart disease, thus linking CAC, perhaps unsurprisingly, with lipid metabolism.111
More recently identified is the association between clonal hematopoiesis of indeterminate potential (CHIP) with CAC. CHIP carriers had 3.3 times higher CAC than non-carriers.112 Insufficiency of tet methylcytosine dioxygenase 2 (TET2), a gene commonly mutated in CHIP, exaggerated atherosclerosis in mice,112 which has been attributed to TET-2 deficient macrophages exhibiting an increase in Nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain containing (NLRP) 3 inflammasome-mediated IL-1β secretion.113 Finally, matrix gla protein (MGP) is considered to be one of the strongest endogenous inhibitors of vascular calcification, and putatively disruptive polymorphisms in MGP correlate strongly with subclinical CAC.114 These findings highlight the diversity of known cell types (e.g., endothelial, SMC, hematopoietic) and signaling pathways involved in CAC development.
GWAS have also identified an abdominal aortic calcification (AAC) risk locus on chromosome 7 in the intergenic region between histone deacetylase 9 (HDAC9) and twist family bHLH transcription factor 1 (TWIST1).115 Knockdown of HDAC9 reduced calcification, contractility, and RUNX2 expression of aortic SMCs induced to undergo osteogenic transformation.115 Conversely, overexpression of HDAC9 amplified RUNX2 expression and increased calcification.115 Analogously, Hdac9-null mice were protected from calcification and mortality compared to haploinsufficient and wild-type mice in a model of medial vascular calcification (MGP deficiency).115 HDAC9 also promotes endothelial-to-mesenchymal transition and unfavorable atherosclerotic plaque composition.116 In rat SMC calcification assays, TWIST1 knockdown increased calcification, and overexpression decreased calcification.117
Aortic valvular (AV) calcification has been associated with the lipoprotein a (LPA) locus on chromosome 6 at genome-wide significance, and minor alleles in this locus confer as much as a two-fold increase in the odds of AV calcification and an increased risk for aortic stenosis.118 Mendelian randomization analysis demonstrated a causal role for genetically determined Lp(a) levels in the development of AV calcification. In the same study, two variants near the proinflammatory gene, interleukin 36 gamma (IL1F9), were associated with mitral annular calcification at a genome-wide level of significance.
In a separate GWAS of calcific aortic stenosis, the association of AV disease with LPA was redemonstrated, and two additional risk loci near palmdelphin (PALMD) and testis expressed 41 (TEX41) were identified.119 These loci are also associated with bicuspid aortic valve and congenital septal defects, potentially implicating cardiac developmental pathways in calcific aortic valve disease.
Environmental and modifiable factors that accelerate calcification
There are several clinical conditions where vascular calcification is markedly accelerated, including metabolic abnormalities such as diabetes mellitus and chronic kidney disease (CKD).120 In these conditions, calcification and mortality do not track with chronological age, as observed in the general population. Instead, patients show accelerated cardiovascular mortality, such that young adults with ESKD requiring chronic dialysis have a risk of cardiovascular mortality similar to octogenarians.121, 122 Emerging evidence suggests that accelerated vascular aging may contribute to the development of vascular calcification and increased mortality in these patient groups.123–126
Using human tissue samples, several studies have documented the presence of DNA damage (gamma H2 histone family member X (gH2AX) and 8-oxo-2’-deoxyguanosine (8-oxo-Dg)) and senescence markers, such as the cell cycle regulators p21 and p16, in calcified arteries of patients with CKD and diabetes.126 Compelling evidence comes from studies of the arteries of children with CKD on dialysis, which showed elevated oxidative DNA damage and senescence in medial vascular SMCs.124 Numerous ex vivo and in vitro studies of SMCs have shown that a number of environmental factors contribute to oxidative stress and DNA damage in these disease states. These include elevated glucose and dysregulated mineral metabolism, with elevated phosphorus thought to be a driver of premature aging, as well as various uremic toxins and mitochondrial damage.120, 127–131
DNA damage signaling and cellular senescence drive a number of processes that lead to vascular SMC calcification, including osteogenic differentiation and cell death. Two key DNA damage signaling pathways upstream of vascular SMC osteogenic differentiation are ataxia telangiectasia mutated (ATM) and poly-ADP ribose polymerase (PARP), and blocking either of these pathways can alleviate mineralization both in vitro and in vivo in models that mimic the dysregulated mineral metabolism observed in CKD.77, 132
These signaling pathways exert their effects on osteogenic differentiation of SMCs in a number of ways, and many of them converge to the Runt-related transcription factor-2 (Runx2), the major transcription factor driving osteogenic phenotype transition.133–139 Oxidative stress-induced Runx2 upregulation plays an essential role in vascular SMC calcification, while SMC-specific Runx2 deletion protects from the development of vascular calcification in atherosclerosis and CKD.137–139 Multifaceted posttranslational modifications (PTMs) of Runx2, including phosphorylation, acetylation, ubiquitination, and O-GlcNAcylation, modulate Runx2 protein stability, cellular localization, and its interaction with other transcription factors and target genes that are critical for its osteogenic transcriptional activity.131
Recent studies have linked protein O-GlcNAcylation with Runx2 upregulation and SMC calcification in diabetes.140–143 In addition, Runx2 is a component of the DNA damage response (DDR). In response to elevated calcium and phosphate, Runx2 becomes PARylated, leading to the selective activation of its downstream osteogenic targets.139
Another mechanism whereby metabolic changes can influence vascular calcification include epigenetic modifications to DNA or histones. In many instances, these pathways also intersect with DNA damage signaling and senescence. Sirtuins, a family of histone deacetylases, play a role in regulating the DDR and senescence in vascular SMCs and hence osteogenic differentiation and calcification. Sirt1 is reduced in the vasculature of patients with diabetes mellitus, and its activation leads to efficient DNA repair and normalizes vascular SMC phenotype.144 Similarly, sirtuin 6 is reduced in the vessels of patients with CKD. Studies in vitro show that sirtuin 6acts to deactylate Runx2, leading to its nuclear export and degradation, thus preventing osteogenic differentiation.145
An additional feature of the persistent DNA damage and cellular senescence is activation of the senescence-associated secretory phenotype (SASP) and activation of innate immune signals by vascular SMC, including interleukin-6 (IL-6) and BMP2 as relevant to arteriosclerotic calcification.124, 146 The connections to osteogenic BMP-Msx-Wnt signaling are presented below.
Activation of the BMP2/Msx/Wnt signaling pathways increases SMC calcification in vitro and in vivo.147–150 Wnts are secreted, fatty acylated glycoproteins that signal through G-protein coupled receptors of the Frizzled (Fzd) family or via GPR124.151–153 Signaling is modulated by co-receptors including low density lipoprotein related proteins LRP5 and LRP6 and several transmembrane receptor tyrosine kinase-like proteins.154, 155 Wnts are fatty acylated and very hydrophobic -- associated with membranes, extracellular vesicles, and lipoprotein particles.156 The vertebrate genome encodes 19 Wnt ligands and 10 Fzd receptors with downstream signaling relays characterized as either canonical (requiring β–catenin) or noncanonical (calcium/NFAT, Jun kinase, planar cell polarity).157, 158 Alternative Wnt signaling through transcriptional coactivators YAP and TAZ resulting in osteogenic differentiation has also been described.157, 159
The first robust clue that Wnt signaling might be involved in vascular aging phenotypes came from the work of Mani and colleagues. They identified that a missense mutation in LRP6 (R611C) resulted in precocious osteoporosis and coronary artery disease in an Iranian kindred.160 This hypomorphic allele causes dysregulated signaling bias between canonical and noncanonical Wnt relays in vascular SMCs as necessary to stabilize phenotype.161 Consistent with this, others demonstrated that loss of SMC LRP6 increases noncanonical Wnt signals that activated SMC osteochondrogenic gene expression, and promoted vascular calcification, and arteriosclerotic stiffening in mice susceptible to atherosclerosis.162, 163
Interestingly, expression of non-canonical Wnt ligands is increased in calcific aortic valve disease and with cardiac fibrosis.164–167 Age-related mitochondrial dysfunction and ER stress bias towards non-canonical Wnt signaling as well.168, 169 These data suggest that development of LRP6 mimetics, or other strategies that restrain specific aspects of non-canonical Wnt signaling, may help prevent or mitigate progression of cardiovascular fibrocalcific disease processes with aging.170
A common theme in all of the age-associated cardiovascular “Wnt-opathies” is activation of innate immunity, a key feature of inflammation, and some features of cell senescence (vide infra).170–172 Pathogen- and senescence –associated programs elevate the expression of Wnt genes either directly or indirectly via TNF, IL1-β, or receptor for AGE (RAGE) ligands including oxylipids.170 Importantly, senescent cells that accrue in aging tissues actively contribute to the inflammatory phenotypes.173–176 A gene set containing numerous direct BMP/Wnt modulators (e.g., Bmp2, Wnt2, Wnt16, Dkk1, etc.) and targets of noncanonical Wnt action was shown to demarcate senescent cells in multiple tissues.174 However, the conflicting literature on the role of Wnt agonists in promoting or preventing cell senescence suggests that canonical-noncanonical signaling bias and duration of signal exposure deserves additional investigation.177, 178
Therapeutic considerations
There are no currently approved therapies specifically targeting prevention or promoting regression of vascular or valvular calcification for the general population at any age. Metformin is associated with reduced coronary calcification in animal and human studies179, 180; possible mechanisms included reduced osteoprotegerin181 production and decreased oxidative stress.182, 183 Senolytic combinations of desatinib and quercetin were shown to reduce vascular calcification in animal models, attributed to reduced oxidative stress.59, 184 Also in animal models, PARP inhibition with specific inhibitors or minocycline185 reduced vascular calcification, as has pyrophosphate administration.97 No therapy is available to treat valvular calcification except surgical and transcatheter interventions. Ample opportunities remain to apply known mechanisms of aging and calcification to clinical cardiovascular care.
Conclusions and perspective
In this review, we provide a high-level summary of the current knowledge of vascular aging, emphasizing the clinical manifestations, genetic diatheses, environmental risk factors, and emerging molecular mechanisms of cardiovascular calcification. Age-associated pathways critical to the development of vascular calcification are highlighted, including DNA damage repair and senescence signals, innate immunity, activating BMP2-Msx-Wnt pathways, and the Runx2 transcription factor. Arterial SMC phenotypic switching contributes significantly to vascular aging -- manifested as abnormal conduit vessel physiology and mechanical integrity due to arteriosclerotic calcification, fibrosis, matrix remodeling, and impaired contractile functions. Even though key discoveries have been made, much remains to be learned concerning the regulation of arteriosclerotic calcification and its relationship to the vascular SMC phenotype with aging.
For instance, both Runx2 and Msx2 directly reduce the expression of SMC contractile markers and promote the osteogenic phenotype, and Runx2 and Msx2 proteins interact to form a transcriptional complex.137, 186–188 On the other hand, O-GlcNAcylation via Ogt has also emerged as an important regulator for the master SMC transcription factors, including myocardin, serum response factor (SRF), and KLF4.141, 142 However, the reasons why activities of Runx2, Msx2, and Ogt in the SMC lineage – absolutely required for osteogenic differentiation and matrix deposition – can become dissociated from arterial matrix mineralization in some settings remains to be determined.189 Incorporation of multi-omics, systems biology, single cell sequencing, and computational studies are novel approaches for the identification of new pathways, candidate drug targets, and repurposing of old drugs to treat vascular and valvular calcification.187, 190, 191
Endothelial cell dysfunction, with or without the endothelial-mesenchymal transition, also impacts the SMC phenotype via juxtacrine/paracrine signals that controls osteogenic potential, and may be one such determinant.192, 193 Likewise, key components of the vascular extracellular matrix such as nitogen-2 also control SMC plasticity, and matricrine cues in cardiovascular aging are poorly characterized.194 Of note, in utero or childhood environmental exposures impair endothelial functions decades later in adulthood.195, 196
Therefore, a better understanding of the vascular epigenetic landscape that regulates vascular SMC phenotypic plasticity during health span and lifespan will be needed to mitigate age-associated vascular dysfunction. Finally, it has become abundantly apparent that duration of cardiometabolic insult exposure197 and sex significantly impact age-dependent responses, and women experience a much steeper increase in cardiovascular disease severity with age, later in life.198 Other age-related vasculopathies exhibit sex dimorphism as well, including aneurysmal remodeling, that is determined by sex chromosome content.199 Thus, additional studies are warranted to uncover in even greater detail the mechanisms controlling vascular SMC phenotypic stability vs. plasticity, phenotypic switching with osteogenic re-programming, and vascular mineralization as a function of environment, cardiometabolic insult, matricrine cues, (epi)genetics, age, and sex. Insights from these studies will afford novel targets and therapeutic strategies necessary to halt, or potentially reverse, processes of age-associated vascular calcification.
Highlights.
The pathogenesis underlying the development of vascular aging, and vascular calcification with aging in particular, is still not fully understood.
Genetic risk, likely compounded by epigenetic modifications, environmental factors, including diabetes mellitus and chronic kidney disease, and the plasticity of vascular smooth muscle cells to acquire an osteogenic phenotype are major determinants of age-associated vascular calcification.
Arterial SMC phenotypic switching contributes significantly to vascular aging -- manifested as abnormal conduit vessel physiology and mechanical integrity due to arteriosclerotic calcification, fibrosis, matrix remodeling, and impaired contractile functions.
Acknowledgments:
Funding sources:
R.M. was supported by the National Heart, Lung, and Blood Institute (R01HL142809), the American Heart Association (18TPA34230025), and the Wild Family Foundation.
E.A. is supported by research grants from the National Institutes of Health R01HL136431, R01HL141917 and R01HL147095.
C.S.H. holds grants from the National Institutes of Health (HL142932) and the American Heart Association (20IPA35260111).
B.N.W. is funded by the EU H2020 Research and Innovation programme INTERVENE, grant nr. 101016775.
N.R.S. National Institute on Aging 1K76AG064426-01A1
P.G. is supported by American Heart Association grant 20CDA35310455, National Institute on Aging grant K76AG064428, and Loan Repayment Program award L30AG060521
S.U.N. is supported by the National Institute of Biomedical Imaging and Bioengineering (1R01EB031813-01A1) and by the National Institute of Diabetes and Digestive and Kidney Diseases (1U01DK123818-01).
C.S. is supported by grants from the British Heart Foundation (RG/17/2/32808).
D.A.T. is supported by grants from the National Institutes of Health (HL069229-21), American Diabetes Association (1-18-IBS-224), and the Pak Center for Mineral Metabolism Research.
Y.C. is supported by grants from the National Institutes of Health (HL136165, HL146103 and HL158097) as well as United States Department of Veterans Affairs Basic Sciences R&D Service (BX005800 and BX004426).
Footnotes
Disclosures:
Sutton- Advisory: Philips, Abbott; Honoraria: Zoll, Abbott, Shockwave, Cordis.
Malhotra – SRA: Amgen, Bayer; Consultant: Renovocor, Third Pole, Myokardia (now BMS). Nigwekar- Consultant: Epizon Pharma, Laboratoris Sanifit, Inozyme Pharma; Grant support: Hope Pharma, Laboratoris Sanifit, Inozyme Pharma.
Goyal-Consultant: Sensorum Health. The other authors-none.
Contributor Information
Nadia R. Sutton, Division of Cardiovascular Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Rajeev Malhotra, Cardiology Division, Massachusetts General Hospital and Harvard Medical School, Boston, MA USA.
St. Cynthia Hilaire, Division of Cardiology, Departments of Medicine and Bioengineering, Pittsburgh Heart, Lung, and Blood Vascular Medicine Institute, University of Pittsburgh, 1744 BSTWR, 200 Lothrop St, Pittsburgh, PA, 15260 USA
Elena Aikawa, Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA USA
Roger S. Blumenthal, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease; Baltimore, MD
Grace Gackenbach, Division of Cardiovascular Medicine, Michigan Medicine, Ann Arbor, Michigan, USA.
Parag Goyal, Department of Medicine, Weill Cornell Medicine, New York, NY
Adam Johnson, Cardiology Division, Massachusetts General Hospital and Harvard Medical School, Boston, MA USA
Sagar U. Nigwekar, Division of Nephrology, Massachusetts General Hospital and Harvard Medical School, Boston, MA USA
Catherine M. Shanahan, School of Cardiovascular and Metabolic Medicine and Sciences, King’s College London, London, UK
Dwight A. Towler, Department of Medicine | Endocrine Division and Pak Center for Mineral Metabolism Research, UT Southwestern Medical Center, Dallas, TX USA
Brooke N. Wolford, K.G. Jebsen Center for Genetic Epidemiology, Department of Public Health and Nursing, Norwegian University of Science and Technology, Trondheim, Norway
Yabing Chen, Department of Pathology, University of Alabama at Birmingham and Research Department, Veterans Affairs Birmingham Medical Center, Birmingham, AL, USA.
References
- 1.Fernandez-Ortiz A, Jimenez-Borreguero LJ, Penalvo JL, Ordovas JM, Mocoroa A, Fernandez-Friera L, Laclaustra M, Garcia L, Molina J, Mendiguren JM, Lopez-Melgar B, de Vega VM, Alonso-Farto JC, Guallar E, Sillesen H, Rudd JH, Fayad ZA, Ibanez B, Sanz G, Fuster V. The progression and early detection of subclinical atherosclerosis (pesa) study: Rationale and design. Am Heart J 2013;166:990–998 [DOI] [PubMed] [Google Scholar]
- 2.Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, Van Damme P, Cassiman P, Langlois M, van Oostveldt P, Verdonck P, De Backer G, Gillebert TC, Asklepios I. Rationale, design, methods and baseline characteristics of the asklepios study. Eur J Cardiovasc Prev Rehabil 2007;14:179–191 [DOI] [PubMed] [Google Scholar]
- 3.Sebastiani P, Federico A, Morris M, Gurinovich A, Tanaka T, Chandler KB, Andersen SL, Denis G, Costello CE, Ferrucci L, Jennings L, Glass DJ, Monti S, Perls TT. Protein signatures of centenarians and their offspring suggest centenarians age slower than other humans. Aging Cell 2021;20:e13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, Glynn NW, Taylor CA, Sebastiani P, Perls TT, Mayeux R, Christensen K, Zmuda JM, Barral S, Lee JH, Simonsick EM, Walston JD, Yashin AI, Hadley E. Health and function of participants in the long life family study: A comparison with other cohorts. Aging (Albany NY) 2011;3:63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurinovich A, Song Z, Zhang W, Federico A, Monti S, Andersen SL, Jennings LL, Glass DJ, Barzilai N, Millman S, Perls TT, Sebastiani P. Effect of longevity genetic variants on the molecular aging rate. Geroscience 2021;43:1237–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–1327 [DOI] [PubMed] [Google Scholar]
- 7.Redheuil A, Wu CO, Kachenoura N, Ohyama Y, Yan RT, Bertoni AG, Hundley GW, Duprez DA, Jacobs DR, Daniels LB, Darwin C, Sibley C, Bluemke DA, Lima JAC. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident cv events: The mesa study. Journal of the American College of Cardiology 2014;64:2619–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Zhao X, Wu H. Arterial stiffness: A focus on vascular calcification and its link to bone mineralization. Arteriosclerosis, thrombosis, and vascular biology 2020;40:1078–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Chen Y. Cardiometabolic syndrome and vascular calcification. Cardiometab Syndr J 2022;2:1–21 [Google Scholar]
- 10.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157–2162 [DOI] [PubMed] [Google Scholar]
- 11.Rifkin RD, Parisi AF, Folland E. Coronary calcification in the diagnosis of coronary artery disease. The American journal of cardiology 1979;44:141–147 [DOI] [PubMed] [Google Scholar]
- 12.Hutcheson JD, Maldonado N, Aikawa E. Small entities with large impact: Microcalcifications and atherosclerotic plaque vulnerability. Current opinion in lipidology 2014;25:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, Quillard T, Libby P, Aikawa M, Weinbaum S, Aikawa E. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nature materials 2016;15:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary ct angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the romicat-ii trial. J Am Coll Cardiol 2014;64:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti A, De Paolis A, Grossman P, Dinh PA, Aikawa E, Weinbaum S, Cardoso L. The effect of plaque morphology, material composition and microcalcifications on the risk of cap rupture: A structural analysis of vulnerable atherosclerotic plaques. Frontiers in Cardiovascular Medicine 2022;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: What does it really mean? JACC. Cardiovascular imaging 2018;11:127–142 [DOI] [PubMed] [Google Scholar]
- 17.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866 [DOI] [PubMed] [Google Scholar]
- 18.Cainzos-Achirica M, Miedema MD, McEvoy JW, Al Rifai M, Greenland P, Dardari Z, Budoff M, Blumenthal RS, Yeboah J, Duprez DA, Mortensen MB, Dzaye O, Hong J, Nasir K, Blaha MJ. Coronary artery calcium for personalized allocation of aspirin in primary prevention of cardiovascular disease in 2019: The mesa study (multi-ethnic study of atherosclerosis). Circulation 2020;141:1541–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orringer CE, Blaha MJ, Blankstein R, Budoff MJ, Goldberg RB, Gill EA, Maki KC, Mehta L, Jacobson TA. The national lipid association scientific statement on coronary artery calcium scoring to guide preventive strategies for ascvd risk reduction. Journal of clinical lipidology 2021;15:33–60 [DOI] [PubMed] [Google Scholar]
- 20.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J, Ziaeian B. 2019 acc/aha guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol 2019;74:e177–e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, Folsom AR, Khera A, Ayers C, Mahabadi AA, Lehmann N, Jöckel KH, Moebus S, Carr JJ, Erbel R, Burke GL. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: Derivation in the mesa (multi-ethnic study of atherosclerosis) with validation in the hnr (heinz nixdorf recall) study and the dhs (dallas heart study). J Am Coll Cardiol 2015;66:1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 2015;65:1273–1282 [DOI] [PubMed] [Google Scholar]
- 23.Lee SE, Sung JM, Andreini D, Budoff MJ, Cademartiri F, Chinnaiyan K, Choi JH, Chun EJ, Conte E, Gottlieb I, Hadamitzky M, Kim YJ, Kumar A, Lee BK, Leipsic JA, Maffei E, Marques H, Pontone G, Raff G, Shin S, Stone PH, Samady H, Virmani R, Narula J, Berman DS, Shaw LJ, Bax JJ, Lin FY, Min JK, Chang HJ. Differential association between the progression of coronary artery calcium score and coronary plaque volume progression according to statins: The progression of atherosclerotic plaque determined by computed tomographic angiography imaging (paradigm) study. Eur Heart J Cardiovasc Imaging 2019;20:1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xian JZ, Lu M, Fong F, Qiao R, Patel NR, Abeydeera D, Iriana S, Demer LL, Tintut Y. Statin effects on vascular calcification: Microarchitectural changes in aortic calcium deposits in aged hyperlipidemic mice. Arterioscler Thromb Vasc Biol 2021;41:e185–e192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healy A, Berus JM, Christensen JL, Lee C, Mantsounga C, Dong W, Watts JP Jr., Assali M, Ceneri N, Nilson R, Neverson J, Wu WC, Choudhary G, Morrison AR. Statins disrupt macrophage rac1 regulation leading to increased atherosclerotic plaque calcification. Arterioscler Thromb Vasc Biol 2020;40:714–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cainzos-Achirica M, Bittencourt MS, Osei AD, Haque W, Bhatt DL, Blumenthal RS, Blankstein R, Ray KK, Blaha MJ, Nasir K. Coronary artery calcium to improve the efficiency of randomized controlled trials in primary cardiovascular prevention. JACC. Cardiovascular imaging 2021;14:1005–1016 [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS, Yeboah J. 2018 aha/acc/aacvpr/aapa/abc/acpm/ada/ags/apha/aspc/nla/pcna guideline on the management of blood cholesterol: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019;139:e1082–e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razavi AC, Uddin SMI, Dardari ZA, Berman DS, Budoff MJ, Miedema MD, Osei AD, Obisesan OH, Nasir K, Rozanski A, Rumberger JA, Shaw LJ, Sperling LS, Whelton SP, Mortensen MB, Blaha MJ, Dzaye O. Coronary artery calcium for risk stratification of sudden cardiac death: The coronary artery calcium consortium. JACC. Cardiovascular imaging 2022;15:1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javaid A, Dardari ZA, Mitchell JD, Whelton SP, Dzaye O, Lima JAC, Lloyd-Jones DM, Budoff M, Nasir K, Berman DS, Rumberger J, Miedema MD, Villines TC, Blaha MJ. Distribution of coronary artery calcium by age, sex, and race among patients 30–45 years old. J Am Coll Cardiol 2022;79:1873–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayalath RW, Mangan SH, Golledge J. Aortic calcification. Eur J Vasc Endovasc Surg 2005;30:476–488 [DOI] [PubMed] [Google Scholar]
- 32.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 2006;47:921–929 [DOI] [PubMed] [Google Scholar]
- 33.Aikawa E, Hutcheson JD. The developmental origin of calcific aortic stenosis. The New England journal of medicine 2022;386:1372–1374 [DOI] [PubMed] [Google Scholar]
- 34.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–147 [DOI] [PubMed] [Google Scholar]
- 35.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA, O’Brien KD. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging 2012;5:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen JL, Tan S, Chung HE, Ghosalkar DS, Qureshi R, Chu A, Yu W, Berus J, Shah NR, Wu WC, Chun H, Aikawa E, Choudhary G, Morrison AR. Aortic valve calcification predicts all-cause mortality independent of coronary calcification and severe stenosis. Atherosclerosis 2020;307:16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dzaye O, Whelton SP, Blaha MJ. Aortic valve calcium scoring on cardiac computed tomography: Ready for clinical use? Journal of cardiovascular computed tomography 2019;13:297–298 [DOI] [PubMed] [Google Scholar]
- 38.Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. The New England journal of medicine 2018;378:1704–1714 [DOI] [PubMed] [Google Scholar]
- 39.Nigwekar SU, Zhao S, Wenger J, Hymes JL, Maddux FW, Thadhani RI, Chan KE. A nationally representative study of calcific uremic arteriolopathy risk factors. Journal of the American Society of Nephrology : JASN 2016;27:3421–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy JT, El-Azhary RA, Patzelt MT, Weaver AL, Albright RC, Bridges AD, Claus PL, Davis MD, Dillon JJ, El-Zoghby ZM, Hickson LJ, Kumar R, McBane RD, McCarthy-Fruin KA, McEvoy MT, Pittelkow MR, Wetter DA, Williams AW. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clinic proceedings 2016;91:1384–1394 [DOI] [PubMed] [Google Scholar]
- 41.Nordheim E, Dahle DO, Syse IM, Åsberg A, Reisæter AV, Hartmann A. Resolution of calciphylaxis after urgent kidney transplantation in 3 patients with end-stage kidney failure. Transplantation direct 2016;2:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wajih Z, Singer R. Successful treatment of calciphylaxis with vitamin k in a patient on haemodialysis. Clinical kidney journal 2022;15:354–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging cell 2017;16:624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horvath S DNA methylation age of human tissues and cell types. Genome biology 2013;14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakrabarti A, Goldstein DR, Sutton NR. Age-associated arterial calcification: The current pursuit of aggravating and mitigating factors. Current opinion in lipidology 2020;31:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gems D, de Magalhães JP. The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing research reviews 2021;70:101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 2016;118:856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blasco MA. Telomere length, stem cells and aging. Nature chemical biology 2007;3:640–649 [DOI] [PubMed] [Google Scholar]
- 50.Olovnikov AM. Telomeres, telomerase, and aging: Origin of the theory. Experimental gerontology 1996;31:443–448 [DOI] [PubMed] [Google Scholar]
- 51.de Lange T Shelterin-mediated telomere protection. Annual review of genetics 2018;52:223–247 [DOI] [PubMed] [Google Scholar]
- 52.Morgan RG, Donato AJ, Walker AE. Telomere uncapping and vascular aging. American journal of physiology. Heart and circulatory physiology 2018;315:H1–h5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003;426:194–198 [DOI] [PubMed] [Google Scholar]
- 54.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. The Journal of clinical investigation 2013;123:966–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin X, Li S, Wang YJ, Wang Y, Zhong JY, He JY, Cui XJ, Zhan JK, Liu YS. Exosomal notch3 from high glucose-stimulated endothelial cells regulates vascular smooth muscle cells calcification/aging. Life sciences 2019;232:116582. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Zhan JK, Wang YJ, Lin X, Zhong JY, Wang Y, Tan P, He JY, Cui XJ, Chen YY, Huang W, Liu YS. Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell & bioscience 2019;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alique M, Ruiz-Torres MP, Bodega G, Noci MV, Troyano N, Bohorquez L, Luna C, Luque R, Carmona A, Carracedo J, Ramirez R. Microvesicles from the plasma of elderly subjects and from senescent endothelial cells promote vascular calcification. Aging (Albany NY) 2017;9:778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol 2017;313:H890–h895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016;15:973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meerman M, Driessen R, van Engeland NCA, Bergsma I, Steenhuijsen JLG, Kozono D, Aikawa E, Hjortnaes J, Bouten CVC. Radiation induces valvular interstitial cell calcific response in an in vitro model of calcific aortic valve disease. Frontiers in cardiovascular medicine 2021;8:687885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoeijmakers JH. DNA damage, aging, and cancer. The New England journal of medicine 2009;361:1475–1485 [DOI] [PubMed] [Google Scholar]
- 62.Chellan B, Sutton NR, Hofmann Bowman MA. S100/rage-mediated inflammation and modified cholesterol lipoproteins as mediators of osteoblastic differentiation of vascular smooth muscle cells. Frontiers in cardiovascular medicine 2018;5:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–254 [DOI] [PubMed] [Google Scholar]
- 64.Rymut N, Heinz J, Sadhu S, Hosseini Z, Riley CO, Marinello M, Maloney J, MacNamara KC, Spite M, Fredman G. Resolvin d1 promotes efferocytosis in aging by limiting senescent cell-induced mertk cleavage. FASEB J 2020;34:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadhu S, Decker C, Sansbury BE, Marinello M, Seyfried A, Howard J, Mori M, Hosseini Z, Arunachalam T, Finn AV, Lamar JM, Jourd’heuil D, Guo L, MacNamara KC, Spite M, Fredman G. Radiation-induced macrophage senescence impairs resolution programs and drives cardiovascular inflammation. J Immunol 2021;207:1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stojanovic SD, Fiedler J, Bauersachs J, Thum T, Sedding DG. Senescence-induced inflammation: An important player and key therapeutic target in atherosclerosis. Eur Heart J 2020;41:2983–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buendia P, Montes de Oca A, Madueno JA, Merino A, Martin-Malo A, Aljama P, Ramirez R, Rodriguez M, Carracedo J. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J 2015;29:173–181 [DOI] [PubMed] [Google Scholar]
- 68.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 2006;168:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henze LA, Luong TTD, Boehme B, Masyout J, Schneider MP, Brachs S, Lang F, Pieske B, Pasch A, Eckardt KU, Voelkl J, Alesutan I. Impact of c-reactive protein on osteo-/chondrogenic transdifferentiation and calcification of vascular smooth muscle cells. Aging (Albany NY) 2019;11:5445–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Guan W, Bressler J, Fornage M, Studenski S, Vandiver AR, Moore AZ, Tanaka T, Kiel DP, Liang L, Vokonas P, Schwartz J, Lunetta KL, Murabito JM, Bandinelli S, Hernandez DG, Melzer D, Nalls M, Pilling LC, Price TR, Singleton AB, Gieger C, Holle R, Kretschmer A, Kronenberg F, Kunze S, Linseisen J, Meisinger C, Rathmann W, Waldenberger M, Visscher PM, Shah S, Wray NR, McRae AF, Franco OH, Hofman A, Uitterlinden AG, Absher D, Assimes T, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Carty CL, LaCroix AZ, Reiner AP, Spector TD, Feinberg AP, Levy D, Baccarelli A, van Meurs J, Bell JT, Peters A, Deary IJ, Pankow JS, Ferrucci L, Horvath S. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging 2016;8:1844–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin MD, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli AA, Levy D, Visscher PM, Wray NR, Deary IJ. DNA methylation age of blood predicts all-cause mortality in later life. Genome biology 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a german case cohort. Clinical epigenetics 2016;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, Milano G, Brambilla F, Saccu C, Bianchi ME, Pompilio G, Capogrossi MC, Raucci A. Mir-34a promotes vascular smooth muscle cell calcification by downregulating sirt1 (sirtuin 1) and axl (axl receptor tyrosine kinase). Arteriosclerosis, thrombosis, and vascular biology 2018;38:2079–2090 [DOI] [PubMed] [Google Scholar]
- 74.Kwon DH, Eom GH, Ko JH, Shin S, Joung H, Choe N, Nam YS, Min HK, Kook T, Yoon S, Kang W, Kim YS, Kim HS, Choi H, Koh JT, Kim N, Ahn Y, Cho HJ, Lee IK, Park DH, Suk K, Seo SB, Wissing ER, Mendrysa SM, Nam KI, Kook H. Mdm2 e3 ligase-mediated ubiquitination and degradation of hdac1 in vascular calcification. Nature communications 2016;7:10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du Y, Gao C, Liu Z, Wang L, Liu B, He F, Zhang T, Wang Y, Wang X, Xu M, Luo GZ, Zhu Y, Xu Q, Wang X, Kong W. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by mir-29 repression mediates vascular smooth muscle calcification. Arteriosclerosis, thrombosis, and vascular biology 2012;32:2580–2588 [DOI] [PubMed] [Google Scholar]
- 76.Chao CT, Liu YP, Su SF, Yeh HY, Chen HY, Lee PJ, Chen WJ, Lee YM, Huang JW, Chiang CK, Hung KY, Chen HW. Circulating microrna-125b predicts the presence and progression of uremic vascular calcification. Arteriosclerosis, thrombosis, and vascular biology 2017;37:1402–1414 [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Xu W, An J, Liang M, Li Y, Zhang F, Tong Q, Huang K. Poly(adp-ribose) polymerase 1 accelerates vascular calcification by upregulating runx2. Nature communications 2019;10:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harman JL, Dobnikar L, Chappell J, Stokell BG, Dalby A, Foote K, Finigan A, Freire-Pritchett P, Taylor AL, Worssam MD, Madsen RR, Loche E, Uryga A, Bennett MR, Jørgensen HF. Epigenetic regulation of vascular smooth muscle cells by histone h3 lysine 9 dimethylation attenuates target gene-induction by inflammatory signaling. Arteriosclerosis, thrombosis, and vascular biology 2019;39:2289–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu M, Espinosa-Diez C, Mahan S, Du M, Nguyen AT, Hahn S, Chakraborty R, Straub AC, Martin KA, Owens GK, Gomez D. H3k4 di-methylation governs smooth muscle lineage identity and promotes vascular homeostasis by restraining plasticity. Developmental cell 2021;56:2765–2782.e2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kullo IJ, Lewis CM, Inouye M, Martin AR, Ripatti S, Chatterjee N. Polygenic scores in biomedical research. Nature reviews. Genetics 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isgut M, Sun J, Quyyumi AA, Gibson G. Highly elevated polygenic risk scores are better predictors of myocardial infarction risk early in life than later. Genome medicine 2021;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mars N, Koskela JT, Ripatti P, Kiiskinen TTJ, Havulinna AS, Lindbohm JV, Ahola-Olli A, Kurki M, Karjalainen J, Palta P, Neale BM, Daly M, Salomaa V, Palotie A, Widén E, Ripatti S. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nature medicine 2020;26:549–557 [DOI] [PubMed] [Google Scholar]
- 83.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nature genetics 2018;50:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, O’Dushlaine C, Leader JB, Lester Kirchner H, Lindbuchler DM, Barr ML, Giovanni MA, Ritchie MD, Overton JD, Reid JG, Metpally RP, Wardeh AH, Borecki IB, Yancopoulos GD, Baras A, Shuldiner AR, Gottesman O, Ledbetter DH, Carey DJ, Dewey FE, Murray MF. Genetic identification of familial hypercholesterolemia within a single u.S. Health care system. Science (New York, N.Y.) 2016;354 [DOI] [PubMed] [Google Scholar]
- 85.Jiang X, Holmes C, McVean G. The impact of age on genetic risk for common diseases. PLoS genetics 2021;17:e1009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thanassoulis G, Peloso GM, Pencina MJ, Hoffmann U, Fox CS, Cupples LA, Levy D, D’Agostino RB, Hwang SJ, O’Donnell CJ. A genetic risk score is associated with incident cardiovascular disease and coronary artery calcium: The framingham heart study. Circ Cardiovasc Genet 2012;5:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Natarajan P, Young R, Stitziel NO, Padmanabhan S, Baber U, Mehran R, Sartori S, Fuster V, Reilly DF, Butterworth A, Rader DJ, Ford I, Sattar N, Kathiresan S. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation 2017;135:2091–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salfati E, Nandkeolyar S, Fortmann SP, Sidney S, Hlatky MA, Quertermous T, Go AS, Iribarren C, Herrington DM, Goldstein BA, Assimes TL. Susceptibility loci for clinical coronary artery disease and subclinical coronary atherosclerosis throughout the life-course. Circ Cardiovasc Genet 2015;8:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Severance LM, Contijoch FJ, Carter H, Fan CC, Seibert TM, Dale AM, McVeigh ER. Using a genetic risk score to calculate the optimal age for an individual to undergo coronary artery calcium screening. J Cardiovasc Comput Tomogr 2019;13:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, Fuster V, Boerwinkle E, Melander O, Orho-Melander M, Ridker PM, Kathiresan S. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med 2016;375:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Widén E, Junna N, Ruotsalainen S, Surakka I, Mars N, Ripatti P, Partanen JJ, Aro J, Mustonen P, Tuomi T, Palotie A, Salomaa V, Kaprio J, Partanen J, Hotakainen K, Pöllänen P, Ripatti S. How communicating polygenic and clinical risk for atherosclerotic cardiovascular disease impacts health behavior: An observational follow-up study. Circulation. Genomic and precision medicine 2022;15:e003459. [DOI] [PubMed] [Google Scholar]
- 92.Muse ED, Chen SF, Liu S, Fernandez B, Schrader B, Molparia B, León AN, Lee R, Pubbi N, Mejia N, Ren C, El-Kalliny A, Prado Montes de Oca E, Aguilar H, Ghoshal A, Dias R, Evans D, Chen KY, Zhang Y, Wineinger NE, Spencer EG, Topol EJ, Torkamani A. Impact of polygenic risk communication: An observational mobile application-based coronary artery disease study. NPJ digital medicine 2022;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Sullivan JW, Raghavan S, Marquez-Luna C, Luzum JA, Damrauer SM, Ashley EA, O’Donnell CJ, Willer CJ, Natarajan P. Polygenic risk scores for cardiovascular disease: A scientific statement from the american heart association. Circulation 2022;146:e93–e118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rutsch F, Buers I, Nitschke Y. Hereditary disorders of cardiovascular calcification. Arteriosclerosis, thrombosis, and vascular biology 2021;41:35–47 [DOI] [PubMed] [Google Scholar]
- 95.Huang Z, Xie N, Illes P, Di Virgilio F, Ulrich H, Semyanov A, Verkhratsky A, Sperlagh B, Yu SG, Huang C, Tang Y. From purines to purinergic signalling: Molecular functions and human diseases. Signal transduction and targeted therapy 2021;6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minor M, Alcedo KP, Battaglia RA, Snider NT. Cell type- and tissue-specific functions of ecto-5’-nucleotidase (cd73). Am J Physiol Cell Physiol 2019;317:C1079–C1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acin-Perez R, Enriquez JA, Lopez-Otin C, Andres V. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of hutchinson-gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 2013;127:2442–2451 [DOI] [PubMed] [Google Scholar]
- 98.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P. Mutations in enpp1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 2003;34:379–381 [DOI] [PubMed] [Google Scholar]
- 99.St Hilaire C Medial arterial calcification: A significant and independent contributor of peripheral artery disease. Arteriosclerosis, thrombosis, and vascular biology 2022;42:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M. Nt5e mutations and arterial calcifications. N Engl J Med 2011;364:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin H, St Hilaire C, Huang Y, Yang D, Dmitrieva NI, Negro A, Schwartzbeck R, Liu Y, Yu Z, Walts A, Davaine JM, Lee DY, Donahue D, Hsu KS, Chen J, Cheng T, Gahl W, Chen G, Boehm M. Increased activity of tnap compensates for reduced adenosine production and promotes ectopic calcification in the genetic disease acdc. Science signaling 2016;9:ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sutton NR, Hofmann Bowman MA. Reining in peripheral arterial calcification. Arterioscler Thromb Vasc Biol 2020;40:1614–1616 [DOI] [PubMed] [Google Scholar]
- 103.Moorhead WJ 3rd, Chu CC, Cuevas RA, Callahan Jt, Wong R, Regan C, Boufford CK, Sur S, Liu M, Gomez D, MacTaggart JN, Kamenskiy A, Boehm M, St Hilaire C. Dysregulation of foxo1 (forkhead box o1 protein) drives calcification in arterial calcification due to deficiency of cd73 and is present in peripheral artery disease. Arterioscler Thromb Vasc Biol 2020;40:1680–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jansen RS, Küçükosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, Bergen AA, Gorgels TG, Borst P, van de Wetering K. Abcc6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proceedings of the National Academy of Sciences of the United States of America 2013;110:20206–20211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ziegler SG, Ferreira CR, MacFarlane EG, Riddle RC, Tomlinson RE, Chew EY, Martin L, Ma CT, Sergienko E, Pinkerton AB, Millán JL, Gahl WA, Dietz HC. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Science translational medicine 2017;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rutsch F, MacDougall M, Lu C, Buers I, Mamaeva O, Nitschke Y, Rice GI, Erlandsen H, Kehl HG, Thiele H, Nurnberg P, Hohne W, Crow YJ, Feigenbaum A, Hennekam RC. A specific ifih1 gain-of-function mutation causes singleton-merten syndrome. Am J Hum Genet 2015;96:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin a cause hutchinson-gilford progeria syndrome. Nature 2003;423:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Levy N. Lamin a truncation in hutchinson-gilford progeria. Science 2003;300:2055. [DOI] [PubMed] [Google Scholar]
- 109.O’Donnell CJ, Kavousi M, Smith AV, Kardia SL, Feitosa MF, Hwang SJ, Sun YV, Province MA, Aspelund T, Dehghan A, Hoffmann U, Bielak LF, Zhang Q, Eiriksdottir G, van Duijn CM, Fox CS, de Andrade M, Kraja AT, Sigurdsson S, Elias-Smale SE, Murabito JM, Launer LJ, van der Lugt A, Kathiresan S, Krestin GP, Herrington DM, Howard TD, Liu Y, Post W, Mitchell BD, O’Connell JR, Shen H, Shuldiner AR, Altshuler D, Elosua R, Salomaa V, Schwartz SM, Siscovick DS, Voight BF, Bis JC, Glazer NL, Psaty BM, Boerwinkle E, Heiss G, Blankenberg S, Zeller T, Wild PS, Schnabel RB, Schillert A, Ziegler A, Münzel TF, White CC, Rotter JI, Nalls M, Oudkerk M, Johnson AD, Newman AB, Uitterlinden AG, Massaro JM, Cunningham J, Harris TB, Hofman A, Peyser PA, Borecki IB, Cupples LA, Gudnason V, Witteman JC. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011;124:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gupta RM, Hadaya J, Trehan A, Zekavat SM, Roselli C, Klarin D, Emdin CA, Hilvering CRE, Bianchi V, Mueller C, Khera AV, Ryan RJH, Engreitz JM, Issner R, Shoresh N, Epstein CB, de Laat W, Brown JD, Schnabel RB, Bernstein BE, Kathiresan S. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell 2017;170:522–533.e515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Natarajan P, Bis JC, Bielak LF, Cox AJ, Dörr M, Feitosa MF, Franceschini N, Guo X, Hwang SJ, Isaacs A, Jhun MA, Kavousi M, Li-Gao R, Lyytikäinen LP, Marioni RE, Schminke U, Stitziel NO, Tada H, van Setten J, Smith AV, Vojinovic D, Yanek LR, Yao J, Yerges-Armstrong LM, Amin N, Baber U, Borecki IB, Carr JJ, Chen YI, Cupples LA, de Jong PA, de Koning H, de Vos BD, Demirkan A, Fuster V, Franco OH, Goodarzi MO, Harris TB, Heckbert SR, Heiss G, Hoffmann U, Hofman A, Išgum I, Jukema JW, Kähönen M, Kardia SL, Kral BG, Launer LJ, Massaro J, Mehran R, Mitchell BD, Mosley TH Jr., de Mutsert R, Newman AB, Nguyen KD, North KE, O’Connell JR, Oudkerk M, Pankow JS, Peloso GM, Post W, Province MA, Raffield LM, Raitakari OT, Reilly DF, Rivadeneira F, Rosendaal F, Sartori S, Taylor KD, Teumer A, Trompet S, Turner ST, Uitterlinden AG, Vaidya D, van der Lugt A, Völker U, Wardlaw JM, Wassel CL, Weiss S, Wojczynski MK, Becker DM, Becker LC, Boerwinkle E, Bowden DW, Deary IJ, Dehghan A, Felix SB, Gudnason V, Lehtimäki T, Mathias R, Mook-Kanamori DO, Psaty BM, Rader DJ, Rotter JI, Wilson JG, van Duijn CM, Völzke H, Kathiresan S, Peyser PA, O’Donnell CJ. Multiethnic exome-wide association study of subclinical atherosclerosis. Circ Cardiovasc Genet 2016;9:511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with tet2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cassidy-Bushrow AE, Bielak LF, Levin AM, Sheedy PF 2nd, Turner ST, Boerwinkle E, Lin X, Kardia SL, Peyser PA. Matrix gla protein gene polymorphism is associated with increased coronary artery calcification progression. Arteriosclerosis, thrombosis, and vascular biology 2013;33:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malhotra R, Mauer AC, Lino Cardenas CL, Guo X, Yao J, Zhang X, Wunderer F, Smith AV, Wong Q, Pechlivanis S, Hwang SJ, Wang J, Lu L, Nicholson CJ, Shelton G, Buswell MD, Barnes HJ, Sigurslid HH, Slocum C, Rourke CO, Rhee DK, Bagchi A, Nigwekar SU, Buys ES, Campbell CY, Harris T, Budoff M, Criqui MH, Rotter JI, Johnson AD, Song C, Franceschini N, Debette S, Hoffmann U, Kälsch H, Nöthen MM, Sigurdsson S, Freedman BI, Bowden DW, Jöckel KH, Moebus S, Erbel R, Feitosa MF, Gudnason V, Thanassoulis G, Zapol WM, Lindsay ME, Bloch DB, Post WS, O’Donnell CJ. Hdac9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nature genetics 2019;51:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lecce L, Xu Y, V’Gangula B, Chandel N, Pothula V, Caudrillier A, Santini MP, d’Escamard V, Ceholski DK, Gorski PA, Ma L, Koplev S, Bjørklund MM, Björkegren JL, Boehm M, Bentzon JF, Fuster V, Kim HW, Weintraub NL, Baker AH, Bernstein E, Kovacic JC. Histone deacetylase 9 promotes endothelial-mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. J Clin Invest 2021;131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nurnberg ST, Guerraty MA, Wirka RC, Rao HS, Pjanic M, Norton S, Serrano F, Perisic L, Elwyn S, Pluta J, Zhao W, Testa S, Park Y, Nguyen T, Ko YA, Wang T, Hedin U, Sinha S, Barash Y, Brown CD, Quertermous T, Rader DJ. Genomic profiling of human vascular cells identifies twist1 as a causal gene for common vascular diseases. PLoS Genet 2020;16:e1008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O’Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O’Donnell CJ, Post WS. Genetic associations with valvular calcification and aortic stenosis. The New England journal of medicine 2013;368:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Helgadottir A, Thorleifsson G, Gretarsdottir S, Stefansson OA, Tragante V, Thorolfsdottir RB, Jonsdottir I, Bjornsson T, Steinthorsdottir V, Verweij N, Nielsen JB, Zhou W, Folkersen L, Martinsson A, Heydarpour M, Prakash S, Oskarsson G, Gudbjartsson T, Geirsson A, Olafsson I, Sigurdsson EL, Almgren P, Melander O, Franco-Cereceda A, Hamsten A, Fritsche L, Lin M, Yang B, Hornsby W, Guo D, Brummett CM, Abecasis G, Mathis M, Milewicz D, Body SC, Eriksson P, Willer CJ, Hveem K, Newton-Cheh C, Smith JG, Danielsen R, Thorgeirsson G, Thorsteinsdottir U, Gudbjartsson DF, Holm H, Stefansson K. Genome-wide analysis yields new loci associating with aortic valve stenosis. Nat Commun 2018;9:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: Review and perspectives. European heart journal 2014;35:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation 1998;32:S112–119 [DOI] [PubMed] [Google Scholar]
- 122.Shroff R, Dégi A, Kerti A, Kis E, Cseprekál O, Tory K, Szabó AJ, Reusz GS. Cardiovascular risk assessment in children with chronic kidney disease. Pediatric nephrology (Berlin, Germany) 2013;28:875–884 [DOI] [PubMed] [Google Scholar]
- 123.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008;118:1748–1757 [DOI] [PubMed] [Google Scholar]
- 124.Sanchis P, Ho CY, Liu Y, Beltran LE, Ahmad S, Jacob AP, Furmanik M, Laycock J, Long DA, Shroff R, Shanahan CM. Arterial “inflammaging” drives vascular calcification in children on dialysis. Kidney international 2019;95:958–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luttropp K, Debowska M, Lukaszuk T, Bobrowski L, Carrero JJ, Qureshi AR, Stenvinkel P, Lindholm B, Waniewski J, Nordfors L. Genotypic and phenotypic predictors of inflammation in patients with chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2016;31:2033–2040 [DOI] [PubMed] [Google Scholar]
- 126.Bartoli-Leonard F, Wilkinson FL, Schiro A, Serracino Inglott F, Alexander MY, Weston R. Loss of sirt1 in diabetes accelerates DNA damage-induced vascular calcification. Cardiovascular research 2021;117:836–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirakawa Y, Jao TM, Inagi R. Pathophysiology and therapeutics of premature ageing in chronic kidney disease, with a focus on glycative stress. Clinical and experimental pharmacology & physiology 2017;44 Suppl 1:70–77 [DOI] [PubMed] [Google Scholar]
- 128.Komaba H, Fukagawa M. Phosphate-a poison for humans? Kidney international 2016;90:753–763 [DOI] [PubMed] [Google Scholar]
- 129.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: Emerging concepts. Hypertension (Dallas, Tex. : 1979) 2010;55:9–14 [DOI] [PubMed] [Google Scholar]
- 130.Lacolley P, Regnault V, Avolio AP. Smooth muscle cell and arterial aging: Basic and clinical aspects. Cardiovascular research 2018;114:513–528 [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Zhao X, Wu H. Transcriptional programming in arteriosclerotic disease: A multifaceted function of the runx2 (runt-related transcription factor 2). Arteriosclerosis, thrombosis, and vascular biology 2021;41:20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duer M, Cobb AM, Shanahan CM. DNA damage response: A molecular lynchpin in the pathobiology of arteriosclerotic calcification. Arteriosclerosis, thrombosis, and vascular biology 2020;40:e193–e202 [DOI] [PubMed] [Google Scholar]
- 133.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor runx2 by akt signaling. The Journal of biological chemistry 2008;283:15319–15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997;89:765–771 [DOI] [PubMed] [Google Scholar]
- 135.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997;89:755–764 [DOI] [PubMed] [Google Scholar]
- 136.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes & development 1999;13:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circulation research 2012;111:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin ME, Chen T, Leaf EM, Speer MY, Giachelli CM. Runx2 expression in smooth muscle cells is required for arterial medial calcification in mice. The American journal of pathology 2015;185:1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cobb AM, Yusoff S, Hayward R, Ahmad S, Sun M, Verhulst A, D’Haese PC, Shanahan CM. Runx2 (runt-related transcription factor 2) links the DNA damage response to osteogenic reprogramming and apoptosis of vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology 2021;41:1339–1357 [DOI] [PubMed] [Google Scholar]
- 140.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ, Chatham JC, Wu H, Chen Y. Activation of akt by o-linked n-acetylglucosamine induces vascular calcification in diabetes mellitus. Circulation research 2014;114:1094–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ni Z, Deng J, Potter CMF, Nowak WN, Gu W, Zhang Z, Chen T, Chen Q, Hu Y, Zhou B, Xu Q, Zhang L. Recipient c-kit lineage cells repopulate smooth muscle cells of transplant arteriosclerosis in mouse models. Circulation research 2019;125:223–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paredes F, Williams HC, Quintana RA, San Martin A. Mitochondrial protein poldip2 (polymerase delta interacting protein 2) controls vascular smooth muscle differentiated phenotype by o-linked glcnac (n-acetylglucosamine) transferase-dependent inhibition of a ubiquitin proteasome system. Circulation research 2020;126:41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Y, Zhao X, Wu H. Metabolic stress and cardiovascular disease in diabetes mellitus: The role of protein o-glcnac modification. Arteriosclerosis, thrombosis, and vascular biology 2019;39:1911–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bartoli-Leonard F, Zimmer J, Aikawa E. Innate and adaptive immunity: The understudied driving force of heart valve disease. Cardiovascular research 2021;117:2506–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li W, Feng W, Su X, Luo D, Li Z, Zhou Y, Zhu Y, Zhang M, Chen J, Liu B, Huang H. Sirt6 protects vascular smooth muscle cells from osteogenic transdifferentiation via runx2 in chronic kidney disease. The Journal of clinical investigation 2022;132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin a accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circulation research 2013;112:e99–109 [DOI] [PubMed] [Google Scholar]
- 147.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic msx2-wnt calcification cascade is regulated by tnf-alpha-dependent signals in diabetic ldlr−/− mice. Arteriosclerosis, thrombosis, and vascular biology 2007;27:2589–2596 [DOI] [PubMed] [Google Scholar]
- 148.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular bmp msx2 wnt signaling and oxidative stress in arterial calcification. Annals of the New York Academy of Sciences 2007;1117:40–50 [DOI] [PubMed] [Google Scholar]
- 149.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine wnt signals. The Journal of clinical investigation 2005;115:1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng SL, Behrmann A, Shao JS, Ramachandran B, Krchma K, Bello Arredondo Y, Kovacs A, Mead M, Maxson R, Towler DA. Targeted reduction of vascular msx1 and msx2 mitigates arteriosclerotic calcification and aortic stiffness in ldlr-deficient mice fed diabetogenic diets. Diabetes 2014;63:4326–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nile AH, Hannoush RN. Fatty acylation of wnt proteins. Nature chemical biology 2016;12:60–69 [DOI] [PubMed] [Google Scholar]
- 152.Wang Y, Chang H, Rattner A, Nathans J. Frizzled receptors in development and disease. Current topics in developmental biology 2016;117:113–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cho C, Smallwood PM, Nathans J. Reck and gpr124 are essential receptor cofactors for wnt7a/wnt7b-specific signaling in mammalian cns angiogenesis and blood-brain barrier regulation. Neuron 2017;95:1056–1073.e1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davidson G Lrps in wnt signalling. Handbook of experimental pharmacology 2021;269:45–73 [DOI] [PubMed] [Google Scholar]
- 155.Green J, Nusse R, van Amerongen R. The role of ryk and ror receptor tyrosine kinases in wnt signal transduction. Cold Spring Harbor perspectives in biology 2014;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Routledge D, Scholpp S. Mechanisms of intercellular wnt transport. Development (Cambridge, England) 2019;146 [DOI] [PubMed] [Google Scholar]
- 157.Albrecht LV, Tejeda-Muñoz N, De Robertis EM. Cell biology of canonical wnt signaling. Annual review of cell and developmental biology 2021;37:369–389 [DOI] [PubMed] [Google Scholar]
- 158.Abdolmaleki F, Ahmadpour-Yazdi H, Hayat SMG, Gheibi N, Johnston TP, Sahebkar A. Wnt network: A brief review of pathways and multifunctional components. Critical reviews in eukaryotic gene expression 2020;30:1–18 [DOI] [PubMed] [Google Scholar]
- 159.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative wnt signaling activates yap/taz. Cell 2015;162:780–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. Lrp6 mutation in a family with early coronary disease and metabolic risk factors. Science (New York, N.Y.) 2007;315:1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Srivastava R, Zhang J, Go GW, Narayanan A, Nottoli TP, Mani A. Impaired lrp6-tcf7l2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell reports 2015;13:746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, Krchma K, Bello Arredondo Y, Kovacs A, Kapoor K, Brill LM, Perera R, Williams BO, Towler DA. Vascular smooth muscle lrp6 limits arteriosclerotic calcification in diabetic ldlr−/− mice by restraining noncanonical wnt signals. Circulation research 2015;117:142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramachandran B, Stabley JN, Cheng SL, Behrmann AS, Gay A, Li L, Mead M, Kozlitina J, Lemoff A, Mirzaei H, Chen Z, Towler DA. A gtpase-activating protein-binding protein (g3bp1)/antiviral protein relay conveys arteriosclerotic wnt signals in aortic smooth muscle cells. The Journal of biological chemistry 2018;293:7942–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Albanese I, Yu B, Al-Kindi H, Barratt B, Ott L, Al-Refai M, de Varennes B, Shum-Tim D, Cerruti M, Gourgas O, Rhéaume E, Tardif JC, Schwertani A. Role of noncanonical wnt signaling pathway in human aortic valve calcification. Arteriosclerosis, thrombosis, and vascular biology 2017;37:543–552 [DOI] [PubMed] [Google Scholar]
- 165.Abraityte A, Vinge LE, Askevold ET, Lekva T, Michelsen AE, Ranheim T, Alfsnes K, Fiane A, Aakhus S, Lunde IG, Dahl CP, Aukrust P, Christensen G, Gullestad L, Yndestad A, Ueland T. Wnt5a is elevated in heart failure and affects cardiac fibroblast function. Journal of molecular medicine (Berlin, Germany) 2017;95:767–777 [DOI] [PubMed] [Google Scholar]
- 166.Działo E, Rudnik M, Koning RI, Czepiel M, Tkacz K, Baj-Krzyworzeka M, Distler O, Siedlar M, Kania G, Błyszczuk P. Wnt3a and wnt5a transported by exosomes activate wnt signaling pathways in human cardiac fibroblasts. International journal of molecular sciences 2019;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zou Y, Pan L, Shen Y, Wang X, Huang C, Wang H, Jin X, Yin C, Wang Y, Jia J, Qian J, Zou Y, Gong H, Ge J. Cardiac wnt5a and wnt11 promote fibrosis by the crosstalk of fzd5 and egfr signaling under pressure overload. Cell death & disease 2021;12:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McGuire PJ. Mitochondrial dysfunction and the aging immune system. Biology 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Costa R, Peruzzo R, Bachmann M, Montà GD, Vicario M, Santinon G, Mattarei A, Moro E, Quintana-Cabrera R, Scorrano L, Zeviani M, Vallese F, Zoratti M, Paradisi C, Argenton F, Brini M, Calì T, Dupont S, Szabò I, Leanza L. Impaired mitochondrial atp production downregulates wnt signaling via er stress induction. Cell reports 2019;28:1949–1960.e1946 [DOI] [PubMed] [Google Scholar]
- 170.Gay A, Towler DA. Wnt signaling in cardiovascular disease: Opportunities and challenges. Current opinion in lipidology 2017;28:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Albanese I, Khan K, Barratt B, Al-Kindi H, Schwertani A. Atherosclerotic calcification: Wnt is the hint. Journal of the American Heart Association 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Frontiers in immunology 2017;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Saul D, Kosinsky RL, Atkinson EJ, Doolittle ML, Zhang X, LeBrasseur NK, Pignolo RJ, Robbins PD, Niedernhofer LJ, Ikeno Y, Jurk D, Passos JF, Hickson LJ, Xue A, Monroe DG, Tchkonia T, Kirkland JL, Farr JN, Khosla S. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues 2021:2021.2012.2010.472095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Khosla S, Farr JN, Monroe DG. Cellular senescence and the skeleton: Pathophysiology and therapeutic implications. The Journal of clinical investigation 2022;132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL. Reducing senescent cell burden in aging and disease. Trends in molecular medicine 2020;26:630–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lehmann M, Hu Q, Hu Y, Hafner K, Costa R, van den Berg A, Königshoff M. Chronic wnt/β-catenin signaling induces cellular senescence in lung epithelial cells. Cellular signalling 2020;70:109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ye X, Zerlanko B, Kennedy A, Banumathy G, Zhang R, Adams PD. Downregulation of wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Molecular cell 2007;27:183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu Y, Wang Y, Weng T, Chen Z, Sun X, Wei J, Cai Z, Xiang M. Association between metformin use and coronary artery calcification in type 2 diabetic patients. J Diabetes Res 2019;2019:9484717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Goldberg RB, Aroda VR, Bluemke DA, Barrett-Connor E, Budoff M, Crandall JP, Dabelea D, Horton ES, Mather KJ, Orchard TJ, Schade D, Watson K, Temprosa M, Diabetes Prevention Program Research G. Effect of long-term metformin and lifestyle in the diabetes prevention program and its outcome study on coronary artery calcium. Circulation 2017;136:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schinzari F, Tesauro M, Bertoli A, Valentini A, Veneziani A, Campia U, Cardillo C. Calcification biomarkers and vascular dysfunction in obesity and type 2 diabetes: Influence of oral hypoglycemic agents. Am J Physiol Endocrinol Metab 2019;317:E658–E666 [DOI] [PubMed] [Google Scholar]
- 182.Cao X, Li H, Tao H, Wu N, Yu L, Zhang D, Lu X, Zhu J, Lu Z, Zhu Q. Metformin inhibits vascular calcification in female rat aortic smooth muscle cells via the ampk-enos-no pathway. Endocrinology 2013;154:3680–3689 [DOI] [PubMed] [Google Scholar]
- 183.Ma WQ, Sun XJ, Wang Y, Zhu Y, Han XQ, Liu NF. Restoring mitochondrial biogenesis with metformin attenuates beta-gp-induced phenotypic transformation of vsmcs into an osteogenic phenotype via inhibition of pdk4/oxidative stress-mediated apoptosis. Mol Cell Endocrinol 2019;479:39–53 [DOI] [PubMed] [Google Scholar]
- 184.Cui L, Li Z, Chang X, Cong G, Hao L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vascul Pharmacol 2017;88:21–29 [DOI] [PubMed] [Google Scholar]
- 185.Muller KH, Hayward R, Rajan R, Whitehead M, Cobb AM, Ahmad S, Sun M, Goldberga I, Li R, Bashtanova U, Puszkarska AM, Reid DG, Brooks RA, Skepper JN, Bordoloi J, Chow WY, Oschkinat H, Groombridge A, Scherman OA, Harrison JA, Verhulst A, D’Haese PC, Neven E, Needham LM, Lee SF, Shanahan CM, Duer MJ. Poly(adp-ribose) links the DNA damage response and biomineralization. Cell Rep 2019;27:3124–3138 e3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hayashi K, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced msx1 and msx2 inhibit myocardin-dependent smooth muscle gene transcription. Molecular and cellular biology 2006;26:9456–9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tanaka T, Sato H, Doi H, Yoshida CA, Shimizu T, Matsui H, Yamazaki M, Akiyama H, Kawai-Kowase K, Iso T, Komori T, Arai M, Kurabayashi M. Runx2 represses myocardin-mediated differentiation and facilitates osteogenic conversion of vascular smooth muscle cells. Molecular and cellular biology 2008;28:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shirakabe K, Terasawa K, Miyama K, Shibuya H, Nishida E. Regulation of the activity of the transcription factor runx2 by two homeobox proteins, msx2 and dlx5. Genes to cells : devoted to molecular & cellular mechanisms 2001;6:851–856 [DOI] [PubMed] [Google Scholar]
- 189.Raaz U, Schellinger IN, Chernogubova E, Warnecke C, Kayama Y, Penov K, Hennigs JK, Salomons F, Eken S, Emrich FC, Zheng WH, Adam M, Jagger A, Nakagami F, Toh R, Toyama K, Deng A, Buerke M, Maegdefessel L, Hasenfuß G, Spin JM, Tsao PS. Transcription factor runx2 promotes aortic fibrosis and stiffness in type 2 diabetes mellitus. Circulation research 2015;117:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, Higashi H, DeLaughter DM, Hutcheson JD, Vyas P, Pham T, Rogers MA, Sharma A, Seidman CE, Loscalzo J, Seidman JG, Aikawa M, Singh SA, Aikawa E. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 2018;138:377–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Iqbal F, Lupieri A, Aikawa M, Aikawa E. Harnessing single-cell rna sequencing to better understand how diseased cells behave the way they do in cardiovascular disease. Arterioscler Thromb Vasc Biol 2021;41:585–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lilly B We have contact: Endothelial cell-smooth muscle cell interactions. Physiology (Bethesda, Md.) 2014;29:234–241 [DOI] [PubMed] [Google Scholar]
- 193.Zhang L, Yao J, Yao Y, Boström KI. Contributions of the endothelium to vascular calcification. Frontiers in cell and developmental biology 2021;9:620882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mao C, Ma Z, Jia Y, Li W, Xie N, Zhao G, Ma B, Yu F, Sun J, Zhou Y, Cui Q, Fu Y, Kong W. Nidogen-2 maintains the contractile phenotype of vascular smooth muscle cells and prevents neointima formation via bridging jagged1-notch3 signaling. Circulation 2021;144:1244–1261 [DOI] [PubMed] [Google Scholar]
- 195.Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, Laitinen T, Taittonen L, Lehtimäki T, Jokinen E, Sun C, Viikari JS, Dwyer T, Raitakari OT. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: The cardiovascular risk in young finns study and the childhood determinants of adult health study. Arteriosclerosis, thrombosis, and vascular biology 2012;32:1024–1031 [DOI] [PubMed] [Google Scholar]
- 196.Zambrano E, Lomas-Soria C, Nathanielsz PW. Rodent studies of developmental programming and ageing mechanisms: Special issue: In utero and early life programming of ageing and disease. European journal of clinical investigation 2021;51:e13631. [DOI] [PubMed] [Google Scholar]
- 197.Sutton NR, Bouis D, Mann KM, Rashid IM, McCubbrey AL, Hyman MC, Goldstein DR, Mei A, Pinsky DJ. Cd73 promotes age-dependent accretion of atherosclerosis. Arterioscler Thromb Vasc Biol 2020;40:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ji H, Kwan AC, Chen MT, Ouyang D, Ebinger JE, Bell SP, Niiranen TJ, Bello NA, Cheng S. Sex differences in myocardial and vascular aging. Circulation research 2022;130:566–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Alsiraj Y, Thatcher SE, Blalock E, Fleenor B, Daugherty A, Cassis LA. Sex chromosome complement defines diffuse versus focal angiotensin ii-induced aortic pathology. Arteriosclerosis, thrombosis, and vascular biology 2018;38:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Elliott J, Bodinier B, Bond TA, Chadeau-Hyam M, Evangelou E, Moons KGM, Dehghan A, Muller DC, Elliott P, Tzoulaki I. Predictive accuracy of a polygenic risk score-enhanced prediction model vs a clinical risk score for coronary artery disease. Jama 2020;323:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hindy G, Aragam KG, Ng K, Chaffin M, Lotta LA, Baras A, Drake I, Orho-Melander M, Melander O, Kathiresan S, Khera AV. Genome-wide polygenic score, clinical risk factors, and long-term trajectories of coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology 2020;40:2738–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Riveros-Mckay F, Weale ME, Moore R, Selzam S, Krapohl E, Sivley RM, Tarran WA, Sørensen P, Lachapelle AS, Griffiths JA, Saffari A, Deanfield J, Spencer CCA, Hippisley-Cox J, Hunter DJ, O’Sullivan JW, Ashley EA, Plagnol V, Donnelly P. Integrated polygenic tool substantially enhances coronary artery disease prediction. Circulation. Genomic and precision medicine 2021;14:e003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sun L, Pennells L, Kaptoge S, Nelson CP, Ritchie SC, Abraham G, Arnold M, Bell S, Bolton T, Burgess S, Dudbridge F, Guo Q, Sofianopoulou E, Stevens D, Thompson JR, Butterworth AS, Wood A, Danesh J, Samani NJ, Inouye M, Di Angelantonio E. Polygenic risk scores in cardiovascular risk prediction: A cohort study and modelling analyses. PLoS medicine 2021;18:e1003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Weale ME, Riveros-Mckay F, Selzam S, Seth P, Moore R, Tarran WA, Gradovich E, Giner-Delgado C, Palmer D, Wells D, Saffari A, Sivley RM, Lachapelle AS, Wand H, Clarke SL, Knowles JW, O’Sullivan JW, Ashley EA, McVean G, Plagnol V, Donnelly P. Validation of an integrated risk tool, including polygenic risk score, for atherosclerotic cardiovascular disease in multiple ethnicities and ancestries. The American journal of cardiology 2021;148:157–164 [DOI] [PubMed] [Google Scholar]
- 205.Lu X, Liu Z, Cui Q, Liu F, Li J, Niu X, Shen C, Hu D, Huang K, Chen J, Xing X, Zhao Y, Lu F, Liu X, Cao J, Chen S, Ma H, Yu L, Wu X, Wu X, Li Y, Zhang H, Mo X, Zhao L, Huang J, Wang L, Wen W, Shu XO, Takeuchi F, Koh WP, Tai ES, Cheng CY, Wong TY, Chang X, Chan MY, Gao W, Zheng H, Chen K, Chen J, He J, Tang CS, Lam KSL, Tse HF, Cheung CYY, Takahashi A, Kubo M, Kato N, Terao C, Kamatani Y, Sham PC, Heng CK, Hu Z, Chen YE, Wu T, Shen H, Willer CJ, Gu D. A polygenic risk score improves risk stratification of coronary artery disease: A large-scale prospective chinese cohort study. European heart journal 2022;43:1702–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Steinfeldt J, Buergel T, Loock L, Kittner P, Ruyoga G, Zu Belzen JU, Sasse S, Strangalies H, Christmann L, Hollmann N, Wolf B, Ference B, Deanfield J, Landmesser U, Eils R. Neural network-based integration of polygenic and clinical information: Development and validation of a prediction model for 10-year risk of major adverse cardiac events in the uk biobank cohort. The Lancet. Digital health 2022;4:e84–e94 [DOI] [PubMed] [Google Scholar]


