Abstract
In lipopolysaccharide-stimulated blood from 71 late-stage borreliosis patients, the ex vivo cytokine release capacity of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) was reduced to 28% ± 5% and to 31% ± 5% (P ≤ 0.001), respectively, compared to that of 24 healthy controls. White blood cell counts were normal in both groups. To investigate direct interactions between the pathogen and the immune cells, blood from healthy controls was exposed in vitro to live or heat-killed Borrelia or to Borrelia lysate. Compared to the pattern induced by bacterial endotoxins, a reduced release of TNF-α and IFN-γ and an enhanced secretion of interleukin-10 and granulocyte colony-stimulating factor was found. In blood from 10 borreliosis patients stimulated with Borrelia lysate, TNF-α formation was decreased to 31% ± 14% and IFN-γ formation was decreased to 8% ± 3% (P ≤ 0.001) compared to the cytokine response of blood from healthy controls (n = 24). We propose to consider anti-inflammatory changes in the blood cytokine response capacity elicited by Borrelia as a condition that might favor the persistence of the spirochete.
Lyme disease is a multisystemic disease caused by the spirochete Borrelia burgdorferi sensu lato which is transmitted to humans by the bite of Ixodes ticks (33). In general, acute infections with B. burgdorferi are successfully treated with antibiotics. However, if they are left untreated, persistent infection may result and may eventually develop into chronic Lyme disease, manifesting in neurological and/or articular symptoms such as Lyme arthritis. It is still unclear how Borrelia infection can persist in an immunocompetent host. Several hypothesis have been put forward: (i) localization of the spirochetes in immunoprivileged sites such as intracellular compartments (11), as well as in the extracellular matrix (18), as a rationale for how the pathogen escapes the immune system; (ii) a high variation of surface antigens in B. burgdorferi (31), similar to Borrelia hermsii, which causes relapsing fever (30); this surface antigen modulation could explain how Borrelia evades the immune response; (iii) a shift in the T-helper-cell response as the cause of the treatment-resistant form of Lyme disease (22); (iv) a self-propagating induction of autoimmunity following infection with Borrelia to become a chronic disease, recently supported by the finding that the Borrelia outer surface protein A (OspA) is homologous to the human LFA-1 antigen (12); and (v) modulation of the host immune response by the pathogen in a way that enables survival of the pathogen.
Examples of the last of these hypotheses are known for viral (21), bacterial (1), and parasitic infections (27); this has led to the concept of microbial cytokine-inducing or -suppressing molecules named modulins (19, 20). The effects of Borrelia infection on the acquired immune response have been investigated extensively; the strain- and disease stage-specific production of antibodies (39), as well as the T-cell responses (23), have been analyzed in great detail. Although infection with Borrelia induces a prominent antibody response in the human host, no protective immunity is conferred, indicating that Borrelia-induced antibody production alone is not sufficient to eradicate the pathogen. Similarly, the Th1-type cytokine response alone is not able to protect against current infection (40).
In contrast to these variations in the specific immune response, only few data exist on the consequences of the innate immune response during the course of an infection with Borrelia. Recent findings indicated that host-generated factors, like an aberrant or exuberant immune response, may actually be responsible for the onset of the disease while Borrelia-generated components, such as outer surface proteins, may influence the infectivity and persistence of the spirochete in the host (2, 3). Only recently, it was observed that the anti-inflammatory cytokine interleukin-10 (IL-10) was induced in peripheral blood mononuclear cells by Borrelia antigen (10).
Since we were interested in investigating the influence of an ongoing Borrelia infection on the effector cells of the innate immune system, we chose the ex vivo-stimulated cytokine release from human whole blood as a convenient and simple surrogate approach to characterize changes in immune function due to the disease (4, 6, 7, 14, 16, 26). In the first part of a pilot study, we compared the lipopolysaccharide (LPS)-elicited cytokine release capacity of whole blood taken from late-stage borreliosis patients with that of blood from healthy volunteers. Since we observed that in blood from healthy donors a modulation of the cytokine response to Borrelia lysate occurred in comparison to the response to LPS, we also investigated in a second part the response of blood from borreliosis patients to Borrelia lysate. From the attenuated release of proinflammatory cytokines under such conditions, we conclude that also the status of the innate immune system might represent a critical determinant in the course of an infection with Borrelia.
(Parts of this paper were presented at the 7th International Conference on Lyme Borreliosis and Other Emerging Tick-Borne Diseases, Munich, June 1999, and at the Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, September 1999.)
MATERIALS AND METHODS
Patients and healthy controls.
The mean age of the 24 control subjects, 7 women and 17 men, was 29 years (range, 22 to 42 years). The mean age of the 71 patients with Lyme disease enrolled in this study, 33 women and 38 men, was 54 years (range, 15 to 84 years). Inclusion criteria for the patients were clinical symptoms indicative of late-stage Lyme disease (arthritis, neurological complications, and acrodermatitis chronica atrophicans), as judged by an experienced physician (D.H.). All patients gave informed consent. Of these 71 patients, 14 had not previously been treated with antibiotics against Borrelia and the other 57 had been treated once (32 patients) or at least twice (25 patients) with antibiotics. In all patients, symptoms of active Lyme disease as summarized in Table 1 were present at the time of the investigation. Infection with Borrelia sp. was confirmed by positive serologic test results (positive serum immunoglobulin M titer of ≥1:32 and/or immunoglobulin G titer of ≥1:256) and positive Western blot result with a minimum of two highly Borrelia-specific (22-, 31-, 34-, or 94-kDa) bands. From the patients, 10 (34 to 67 years, mean of 54 years) were randomly selected and their blood was tested for cytokine release induced by Borrelia lysate in comparison to that of the 24 healthy controls. With regard to ex vivo endotoxin stimulation, this patient subgroup did not behave statistically differently from the entire patient group.
TABLE 1.
Clinical and serological characteristics of borreliosis patients and healthy control subjects
| Group | n | No. seropositive | Rha factor | Age (yr)
|
No. with tick bite recall | No. with EMa recall | No. with ACA | No. with NC | No. with arthritis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | |||||||||
| Patients | 71 | 71 | 0 | 46.9 | 15–84 | 35 | 18 | 5 | 30 | 36 |
| Controls | 24 | 0 | 0 | 35.4 | 22–42 | 0 | 0 | 0 | 0 | 0 |
Rh factor, rheumatoid factor; EM, erythema migrans; ACA, acrodermatitis chronica atrophicans; NC, neurological complications (such as meningitis and/or neuropathy).
The controls were recruited from laboratory personnel after giving informed consent. All controls had no history of tick bites or borreliosis and tested negative in the Enzygnost Borreliosis ELISA (Dade Behring, Marburg, Germany).
Cultivation of B. burgdorferi.
All reagents used throughout the study were ultrapure and pyrogen free. B. burgdorferi sensu stricto (N40), Borrelia afzelii (VS461), and Borrelia garinii (PSth) were cultivated at 33°C in BSK-H medium (Sigma, Deisenhofen, Germany) supplemented with 10% normal rabbit serum. Addition of amphotericin B (5.5 μg/ml), fosfomycin (1060 μg/ml), and rifampin (30 μg/ml) (all from Sigma) inhibited fungal and microbial growth. All Borrelia strains were kindly provided by T. Kamradt (Berlin, Germany). The strains were passaged fewer than eight times after isolation from mice. For heat inactivation, 10 ml of a Borrelia culture grown to log phase (≥108/ml) was incubated for 5 min at 95°C and the viability of the remaining Borrelia cells was checked visually under the microscope.
Preparation of Borrelia lysate.
A Borrelia culture (300 ml) grown to late log phase was washed twice (20 min at 14°C and 10,000 × g) with pyrogen-free saline solution supplemented with 1 mM MgCl2. The cell pellet was resuspended in 7.5 ml of saline with 1 mM MgCl2, and aliquots of 2.5 ml were lysed by sonification (Branson Sonifier 250/450 with a 3-mm microtip; Branson, Schwäbisch-Gmünd, Germany). Sonification was carried out on ice at a power setting of 5 duty cycle 50% for 2 min, and the lysate was checked for absence of intact cells under the microscope. The protein concentration of the lysate was determined by the BSA protein assay (Pierce, Rockford, Ill.) as specified by the manufacturer, and the protein concentration in the lysate was adjusted to a final concentration of 1 mg/ml with pyrogen-free saline. The lysate preparation contained less than 0.03 endotoxin unit per 10 μg of protein, as assessed by the Limulus amoebocyte assay (BioWhittaker, Verviers, Belgium).
Whole-blood incubation.
Heparinized venous blood was freshly drawn from either healthy donors or patients with Lyme disease and diluted 1:5 in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 2.5 IU of heparin (Liquemin; Hoffmann LaRoche, Grenzach-Wyhlen, Germany) and incubated in the presence of different stimuli: live or heat-inactivated Borrelia, Borrelia lysate, or endotoxins (LPS) from Salmonella enterica serovar Abortusequi (Sigma), Escherichia coli (026-B6, Sigma), Klebsiella pneumoniae (RIBI, Hamilton, Mont.), Bordetella pertussis (List, Quadratech, Epsom, England), Vibrio cholerae (Sigma), Pseudomonas aeruginosa (Sigma), and S. enterica serovar Enteritidis (Sigma), or without a stimulus (control). After incubation for 24 h at 37°C in the presence of 5% CO2, the blood was resuspended and subsequently centrifuged at 16,000 × g for 2 min and the cell-free supernatant was frozen and stored at −80°C until cytokine levels were measured. The white blood cell count was determined by staining with Türk's solution. Furthermore, blood smears were made for differential leukocyte counts and stained by the method of Pappenheim (see reference 36a).
Cytokine measurement.
The concentrations of tumor necrosis factor alpha (TNF-α), IL-1β, gamma interferon (IFN-γ), granulocyte colony-stimulating factor (G-CSF), and IL-10 in the supernatants were measured by an in-house sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available antibody pairs and recombinant standards. Monoclonal antibody pairs against TNF-α, IL-1β, and IFN-γ were purchased from Endogen (Eching, Germany), and recombinant TNF-α (Bender, Vienna, Austria), IL-1β (Endogen), and IFN-γ (Thomae, Bieberach, Germany) were used as standards. Anti-G-CSF antibodies from R&D (Wiesbaden, Germany) and recombinant G-CSF from Amgen (Thousand Oaks, Calif.) were used. For the measurement of IL-10, monoclonal antibodies from R&D and standard from Pharmingen (Hamburg, Germany) were used.
Assays were carried out in flat-bottom, ultrasorbant 96-well plates (Greiner, Frickenhausen, Germany). The secondary biotinylated antibodies were detected with horseradish peroxidase-conjugated streptavidin (Dianova, Hamburg, Germany) and tetramethylbenzidine solution (Sigma) used as substrate.
Statistics.
Data are shown either as mean ± standard error of the mean (SEM) or as box-and-whiskers plots. Cytokine release was calculated per milliliter of blood, i.e., corrected for the dilution factor of 5 since 20% blood was used. Statistical analyses were performed by the two-tailed, nonparametric Mann-Whitney U test. For the comparison of parametric data, the two-tailed, paired Tukey test was used. All tests are options of Prism 3.0 (GraphPad, San Diego, Calif.). P values of ≤0.05, 0.01, and 0.001 were considered significant.
RESULTS
Comparison of ex vivo endotoxin-inducible cytokine release in whole blood from borreliosis patients and healthy controls.
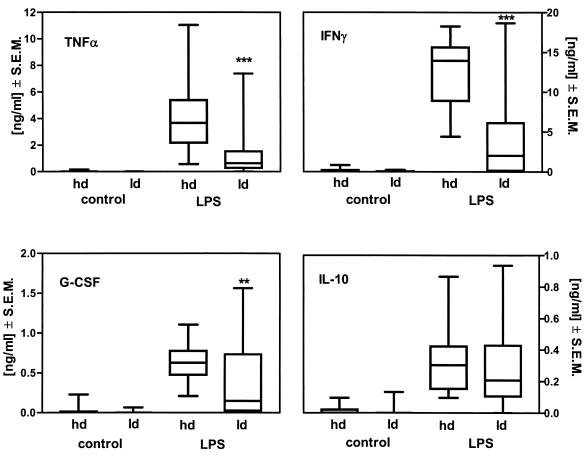
The stimulated whole-blood cytokine release capacity was taken as a surrogate marker to test the hypothesis that persistent Borrelia infection is associated with a modulation of the immune status. The ex vivo cytokine release capacity for TNF-α, IFN-γ, G-CSF, and IL-10 was measured in stimulated blood from 24 healthy donors and compared to that in blood from 71 patients who fulfilled the inclusion criteria for late-stage borreliosis. The data in Fig. 1 illustrate that the release of the cytokines TNF-α (−72% ± 5.2%; P ≤ 0.001), IFN-γ (−69% ± 5%; P ≤ 0.001), and G-CSF (−26% ± 16%; P ≤ 0.01) is attenuated in endotoxin-stimulated blood from borreliosis patients compared to that in blood from healthy controls. In contrast, the release of the anti-inflammatory cytokine IL-10 did not differ in the two groups. This finding suggests an association between the modulation of cytokine release capacity of blood and Borrelia infection.
FIG. 1.
Ex vivo endotoxin-inducible cytokine release capacity of blood from healthy donors or borreliosis patients. Whole blood from healthy donors (hd, n = 24) or borreliosis patients (ld, n = 71), diluted 1:5, was incubated in the presence of 100 ng of endotoxin from S. enterica serovar Abortusequi per ml for 24 h at 37°C. Cytokine levels in the cell-free supernatant were measured by ELISA. The data are depicted as box-and-whiskers plots (the box shows the median and the upper 75% and lower 25%, and the whiskers show the 95th percentile). P values of ≤0.01 and 0.001 were considered significant and are indicated by ∗∗ and ∗∗∗, respectively.
Since the study was carried out with 71 borreliosis patients of a general practitioner, leukocyte counts could not be immediately measured by flow cytometry. Therefore, we repeated our experiments with a subset of 14 patients and 10 healthy controls, counted 200 leukocytes per smear, and then normalized the cytokine release to the number of monocytes present in each sample. Also under this normalization, the expression levels of cytokines per number of mononuclear cells were similar to the results previously obtained with whole blood. Simultaneously, a possible influence of different patient and donor ages and regional settings were tested in this part of the study. The patient and control groups (n = 14 and 10, respectively) were age matched (mean, 46.9 ± 4.9 and 35.4 ± 4.4 years, respectively [not a significant difference]), and the incubations were carried out in parallel, i.e., in the same temporal, demographic, and geographic setting. As is shown in Table 2, the results of this second study corresponded to those obtained in the first study. In whole blood from borreliosis patients and from healthy controls stimulated ex vivo with 100 pg of LPS per ml, the release of the proinflammatory cytokines TNF-α and IFN-γ in the former group was uniformly lower than that in the controls. The release capacity of the anti-inflammatory cytokine IL-10 was slightly higher in the blood from borreliosis patients than in the control group; however, statistically there was no significant difference. In this small patient group, we also tested the cytokine release induced by further stimuli, i.e., 100 ng of staphylococcal enterotoxin B per ml, 10 μg of Borrelia lysate per ml, and a higher concentration of LPS (100 ng/ml). The results were uniform in that the release of TNF-α and IFN-γ in whole blood from borreliosis patients was always lower than the release in blood from healthy controls while the release of IL-10 in blood from the patients was insignificantly elevated compared to that in the control group (data not shown). We also tested if any measurable amounts of the cytokines TNF-α, IFN-γ, G-CSF, and IL-10 could be detected in plasma, as was described for TNF-α by Defosse and Johnson (5), but we found no significant difference in cytokine levels in plasma in the two groups: we detected 55 ± 35 pg of TNF-α/ml, 19 ± 8 pg of IFN-γ/ml, 134 ± 74 pg of G-CSF/ml, and 260 ± 157 pg of IL-10/ml in patient plasma and 3.2 ± 1.5 pg of TNF-α/ml, 11 ± 3 pg of IFN-γ/ml, 38 ± 36 pg of G-CSF/ml, and 246 ± 222 pg of IL-10/ml in plasma from healthy donors.
TABLE 2.
Cytokine release from blood from borreliosis patients and healthy controlsa
| Stimulus | % Release from blood ofb:
|
||||||
|---|---|---|---|---|---|---|---|
| TNF-α | TNF-α/106 monocytes | IFN-γ | G-CSF | G-CSF/106 monocytes | IL-10 | IL-10/106 monocytes | |
| LPS | −34.5 ± 21 | −42.9 ± 28 | −57 ± 30 | +96.2 ± 104 | +33.3 ± 42 | +85.2 ± 100 | +14.3 ± 25 |
| SEB | −20.4 ± 39 | −21.3 ± 43 | −5.5 ± 35 | −17.5 ± 75 | −46.2 ± 38 | +106 ± 113 | +111 ± 84 |
Blood was taken from 14 borreliosis patients and 10 healthy controls.
Blood cytokine release is shown as the percentage of the release from the healthy control group induced by 100 pg of S. enterica serovar Abortusequi LPS per ml or 100 ng of staphylococcal enterotoxin B (SEB) per ml.
Cytokine release induced by heat-killed or sonified Borrelia in whole blood from healthy donors.
The hypothesis that the presence of Borrelia or components of the bacterium directly induces modulations of the blood cytokine response was tested in vitro with blood from healthy donors. Both heat-inactivated Borrelia, incubated at a ratio of 10 Borrelia cells to 1 leukocyte, and a corresponding concentration of Borrelia lysate (10 μg of protein/ml) induced a significant release of TNF-α (1.6 ± 0.4 and 1.6 ± 0.5 ng/ml respectively [n = 4]). The extent of cytokine release induced by either 10 μg of protein/ml of Borrelia lysate (corresponding to approximately 2 × 107 sonified Borrelia cells) or the same number of heat-inactivated Borrelia cells was comparable for all cytokines measured, showing that Borrelia lysate from B. burgdorferi sensu stricto and heat-inactivated Borrelia are approximately equipotent stimuli. The different preparations of lysate from the three Borrelia species were equipotent with regard to the capacity to induce cytokine release in whole blood (e.g., 1.1 ± 0.3, 0.8 ± 0.2, and 1.2 ± 0.3 ng of TNF-α/ml induced by 10 μg of protein/ml of lysate from B. burgdorferi, B. garinii, and B. afzelii, respectively). This observation and the similarities of the cytokine pattern (data not shown) suggest that a highly conserved factor is responsible for cytokine induction by Borrelia species.
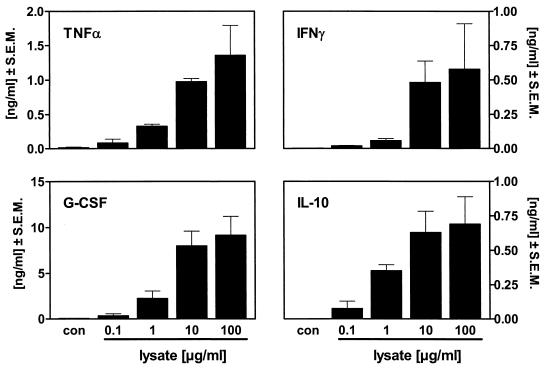
Borrelia lysate was used for the following experiments because it can be quantified by its protein content. The cytokine release induced by stimulating blood with Borrelia lysate from B. burgdorferi sensu stricto showed a concentration dependence at lysate protein concentrations ranging from 0.1 to 100 μg/ml (Fig. 2). The highest cytokine release for all cytokines tested was seen at the highest concentration tested, i.e., 100 μg of Borrelia lysate/ml, which was not toxic for the cells since no reduction in cytokine release was seen. Similar results were obtained using lysates from B. garinii and B. afzelii (data not shown).
FIG. 2.
Concentration dependence of blood cytokine release from healthy donors stimulated with Borrelia lysate. Human whole blood diluted 1:5 was incubated without a stimulus (con) or in the presence of Borrelia lysate (0.1 to 100 μg/ml) for 24 h at 37°C. Cytokine levels in the cell-free supernatant were measured by ELISA. Data are depicted as means ± SEM of results from four healthy donors.
Comparison of cytokine release induced by Borrelia lysate and by endotoxin in blood from healthy donors.
Endotoxin derived from gram-negative bacteria, i.e., LPS induces the release of a multitude of cytokines in blood in a concentration-dependent fashion. The potencies of endotoxins from different bacterial species vary considerably: the data in Table 3 demonstrate that for endotoxins from some E. coli or Salmonella species, picogram-per-milliliter concentrations suffice to induce cytokine release in blood, while nanogram-per-milliliter or migrogram-per-milliliter concentrations are required for other LPS species, e.g., P. aeruginosa and Bordetella pertussis. The Borrelia lysate concentrations which were needed to induce comparable amounts of cytokine release correspond approximately to those required for Bordetella pertussis endotoxin; i.e., these two stimuli have comparable low stimulatory activity. Borrelia lysates contain either highly active material or large amounts of less active components.
TABLE 3.
Threshold of IL-1β-induction in human whole blooda
| Stimulus | IL-1β induction threshold (pg/ml)b |
|---|---|
| Borrelia lysate | |
| B. burgdorferi | 1,000 |
| B. garinii | 1,000 |
| B. afzelii | 1,000 |
| LPS | |
| S. enterica serovar Enteritidis | 10 |
| K. pneumoniae | 10 |
| S. enterica serovar Abortusequi | 10 |
| E. coli O26:B6 | 1,000 |
| B. pertussis | 1,000 |
| P. aeruginosa | 10,000 |
| V. cholerae | 10,000 |
Human whole blood diluted 1:5 in RPMI was incubated in the presence of Borrelia lysate (0.1 ng/ml to 100 μg/ml) or various endotoxins (1 pg/ml to 10 μg/ml) in 10-fold serial dilutions for 24 h at 37°C.
The IL-1β level in the cell-free supernatant was measured by ELISA. Data represent the lowest concentration of the given stimulus where blood from all four healthy donors released significant amounts of IL-1β.
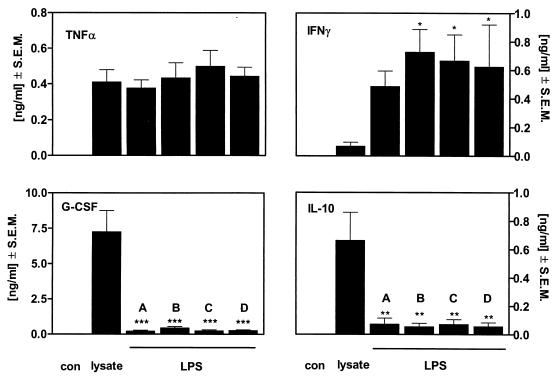
To compare the patterns of cytokine release induced by LPS and Borrelia lysate, the concentrations of endotoxin from four different LPS preparations were adjusted to induce the same levels of TNF-α release as seen with 10 μg of protein per ml of Borrelia lysate. The release of the cytokines TNF-α, IFN-γ, G-CSF, and IL-10 induced by endotoxins from S. enterica serovar Abortusequi (200 pg/ml), E. coli (10 ng/ml), K. pneumoniae (100 pg/ml), and S. enterica serovar Enteritidis (50 pg/ml) was uniform in blood from healthy volunteers (Fig. 3), suggesting that different endotoxins share a leukocyte activation principle. However, a pronounced difference was seen between the four LPS preparations and the Borrelia lysate: at concentrations which induced the same TNF-α release as 10 μg of protein per ml of Borrelia lysate, endotoxins induced much more IFN-γ than Borrelia lysate did. Instead, Borrelia lysate induced a 5- to 10-fold-higher release of the anti-inflammatory cytokines IL-10 and G-CSF than the LPS preparations did. The lysates from other Borrelia species, i.e., B. afzelii and B. garinii, induced the same cytokine pattern as did those from B. burgdorferi (data not shown). These findings show that LPS induces the release predominantly of the proinflammatory cytokine IFN-γ while Borrelia lysate is a stronger inducer of the anti-inflammatory cytokines IL-10 and G-CSF. Such an inverse cytokine induction pattern demonstrates that the immunostimulatory components of B. burgdorferi differ from those of endotoxins.
FIG. 3.
In vitro cytokine release capacity of blood from healthy donors stimulated with Borrelia lysate or LPS. Human whole blood diluted 1:5 was incubated without a stimulus (con) or with Borrelia lysate (10 μg/ml), endotoxin from S. enterica serovar Abortusequi (200 pg/ml) (A), endotoxin from E. coli (10 ng/ml) (B), endotoxin from K. pneumoniae (100 pg/ml) (C), or endotoxin from S. enterica serovar Enteritidis (50 pg/ml) (D) for 24 h at 37°C. Cytokines in the cell-free supernatant were measured by ELISA. Data are depicted as means ± SEM of results from four healthy donors. P values of 0.05, ≤0.01, and ≤0.001 versus lysate were considered significant and are indicated by ∗∗, and ∗∗∗, respectively.
Comparison of ex vivo cytokine release from borreliosis patients and healthy controls in response to Borrelia lysate.
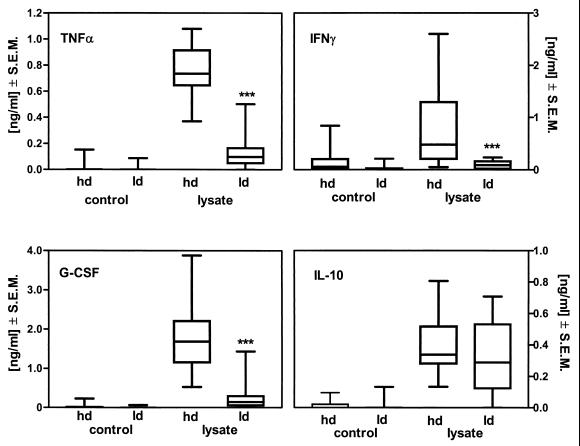
It was now of interest whether a similar immunomodulation by Borrelia might also be detected in blood from the patients. Therefore, the ex vivo cytokine response to Borrelia lysate of blood from a group of 10 borreliosis patients was compared with that of blood from 24 healthy controls. In blood from patients with Lyme disease, the cytokine release capacity for TNF-α (−61% ± 14.3%; P ≤ 0.001), IFN-γ (−92.0% ± 3.2%; P ≤ 0.001), and G-CSF (−84% ± 7.0%; P ≤ 0.001) in response to Borrelia lysate was significantly reduced compared to that in blood from healthy controls (Fig. 4). However, again, no difference between controls and patients was seen with regard to the release of the anti-inflammatory cytokine IL-10 in stimulated blood. These data indicate that ex vivo-stimulated blood from borreliosis patients responds differently to LPS as well as to Borrelia lysate from blood from healthy volunteers.
FIG. 4.
Ex vivo cytokine release capacity of blood from healthy donors or borreliosis patients stimulated with Borrelia lysate. Whole blood, either from healthy donors (hd, n = 24) or from patients with Lyme disease (ld, n = 10), diluted 1:5 was incubated with 10 μg of protein/ml of Borrelia lysate for 24 h at 37°C. Cytokine levels in the cell-free supernatant were measured by ELISA. Data are shown as box-and-whiskers plots. P values of ≤0.001 were considered significant and are indicated by ∗∗∗.
DISCUSSION
A possible interpretation of our results is that persistent infection with Borrelia spirochetes is associated with an attenuation of the release capacity of some cytokines, in particular the proinflammatory cytokines TNF-α and IFN-γ, in blood. Such an attenuation was seen not only with Borrelia-specific antigen but also after stimulation with the endotoxin of other gram-negative bacteria (LPS), thus indicating a more general underlying mechanism.
The cytokine release capacity of blood from patients and healthy controls can be used for comparative studies, and alterations have been detected in blood from patients with multiple sclerosis (6), malignant melanoma (7), rheumatoid arthritis (14), multiple myeloma (26), and human immunodeficiency virus infection (17) and children infected with enterohemorrhagic E. coli (38). Particularly because leukocyte counts are not affected in Borrelia infection (34, 37), it seemed appropriate to take a similar approach to characterize possible changes of the immune response in patients with persistent Borrelia infection.
There is no clear consensus on the accurate diagnosis of chronic infection, and it is not yet possible to separate the symptoms of persistent infection from possible sequelae of a successful eradication of the pathogen. Therefore we defined the following inclusion criteria both for the patient group and for the control group. The clinical diagnosis of persistent Lyme disease set by an experienced practitioner in an area of endemic infection plus well-established serologic test results were used. Furthermore, patients with other, non-borrelia-associated diseases were excluded. The healthy control group tested seronegative for Borrelia antibodies and had no history of tick bites or borreliosis.
Using these criteria, a significant attenuation of the proinflammatory cytokine response of blood was found in the patient group compared to the control group. Due to the exploratory character of the main study, a number of possible influences must be considered. Obviously, the ages of the patient and control groups differed, and cytokine release was not controlled for differences in leukocyte counts. Therefore, a separate control study was performed using healthy volunteers accompanying patients and personnel in the office of the same general practitioner as a parallel control group. This control experiment generated similar data to the main study, but the smaller number of volunteers was insufficient for it to reach statistical significance. Thus, an influence of an imbalance in patient and control group selection cannot be finally excluded.
Other explanations for the difference in cytokine release patterns between patients and healthy donors include the following: (i) interindividual differences in host response predispose patients in different degrees to persistent borreliosis; (ii) inflammatory disorders related to symptoms of borreliosis might have exhausted the inflammatory cytokine release; and (iii) counterregulatory anti-inflammatory mechanisms might attenuate immune responses even in the asymptomatic phase of disease. In the introduction, five different hypotheses were listed which could explain the persistence of B. burgdorferi sensu lato in the host. The first two, i.e., intracellular localization of the spirochetes in immunoprivileged sites and surface antigen modulation, should not affect the cytokine release capacity of leukocytes and therefore do not reflect our observations. We did not investigate a possible shift in the T-helper-cell response or a self-propagating autoimmune process. However, based on our data, we favor the possibility that a modulation of the host immune response is a mechanism that enables the survival of the spirochetes.
The immunostimulatory properties of Borrelia antigen have been investigated extensively in the past, with live or heat-killed pathogen or purified antigen preparations (24, 25). For our experimental approach, we needed a standardized Borrelia stimulus. Heat-inactivated and sonified Borrelia cells induced similar cytokine release patterns and displayed a similar concentration dependence. We also compared the immunostimulatory properties of three different Borrelia species (B. burgdorferi sensu stricto, B. garinii, and B. afzelii); however, we found no significant differences with regard to concentration dependence or pattern of cytokines induced. With a relatively high concentration of the lysate (at least 10 ng of protein/ml), a measurable cytokine release was induced that was quantitatively comparable to that produced by endotoxin from P. aeruginosa. Since in murine Borrelia infections, for example, accumulations of up to 105 spirochetes were found in different organs of infected mice (28), it is feasible that high concentrations of antigens are present at the site of infection that induce local cytokine formation.
Furthermore, Borrelia lysate- and LPS-induced cytokine release are qualitatively different in terms of the pattern of predominant cytokines released. Although Borrelia belongs to the group of gram-negative bacteria (33), it lacks the typical endotoxin LPS (15, 36). Instead, Borrelia expresses lipoproteins, e.g., outer surface proteins (Osp), which have the ability to induce the release of TNF-α (5), IL-1β (13), and IL-6 (35) when incubated with isolated leukocytes, which pointed toward a probably characteristic proinflammatory nature of Borrelia antigen (32). However, when we compared Borrelia lysate and LPS at concentrations which induced an equipotent TNF-α release, we found that Borrelia lysate induced greater amounts of the anti-inflammatory cytokines G-CSF and IL-10 than did LPS. Our results, which indicate that Borrelia induces an anti-inflammatory response, are corroborated by recent findings showing IL-10 induction in monocytes by Borrelia antigen (10).
We are well aware of the fact that OspA and further Borrelia-derived components are able to evoke a cytokine response from isolated peripheral blood monocytes (29, 9). We repeated and confirmed these experiments with our Borrelia lysate and observed complete inhibition of TNF-α release after neutralization of CD14, in analogy to previous work with other bacterial stimuli (8). However, under identical conditions, the cytokine response of whole blood to Borrelia was not attenuated (data not shown). This phenomenon is currently under further investigation. It implies that the cytokine pattern released from whole blood induced by Borrelia lysate is unrelated to these known CD14-mediated initiating mechanisms.
On the basis of published data and the study presented here, we propose to regard the attenuated release capacity of white blood cells for proinflammatory cytokines such as TNF-α and IFN-γ as a mechanism that weakens the immune response of borreliosis patients to circulating spirochetes. This might be due to a direct recognition of Borrelia components by immunocompetent cells or might be a consequence of an enhanced local production of the anti-inflammatory factor IL-10, as published by others (9). In any case, reconstitution of the immunocompetence of the patients represents an attractive target for supportive treatment to antibiosis in chronic Lyme disease.
ACKNOWLEDGMENTS
The excellent technical assistance of Margarete Kreuer-Ullmann and Gregor Pinski is gratefully acknowledged.
REFERENCES
- 1.Beuscher H U, Rodel F, Forsberg A, Rollinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown C R, Reiner S L. Clearance of Borrelia burgdorferi may not be required for resistance to experimental Lyme arthritis. Infect Immun. 1998;66:2065–2071. doi: 10.1128/iai.66.5.2065-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown C R, Reiner S L. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect Immun. 1999;67:1967–1973. doi: 10.1128/iai.67.4.1967-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chernoff A E, Granowitz E V, Shapiro L, Vannier E, Lonnemann G, Angel J B, Kennedy J S, Rabson A R, Wolff S M, Dinarello C A. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995;154:5492–5499. [PubMed] [Google Scholar]
- 5.Defosse D L, Johnson R C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dettke M, Scheidt P, Prange H, Kirchner H. Correlation between interferon production and clinical disease activity in patients with multiple sclerosis. J Clin Immunol. 1997;17:293–300. doi: 10.1023/a:1027374615106. [DOI] [PubMed] [Google Scholar]
- 7.Elsasser-Beile U, von Kleist S, Stahle W, Schurhammer-Fuhrmann C, Monting J S, Gallati H. Cytokine levels in whole blood cell cultures as parameters of the cellular immunologic activity in patients with malignant melanoma and basal cell carcinoma. Cancer. 1993;71:231–236. doi: 10.1002/1097-0142(19930101)71:1<231::aid-cncr2820710136>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Fan X, Stelter F, Menzel R, Jack R, Spreitzer I, Hartung T, Schutt C. Structures in Bacillus subtilis are recognized by CD14 in a lipopolysaccharide binding protein-dependent reaction. Infect Immun. 1999;67:2964–2968. doi: 10.1128/iai.67.6.2964-2968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giambartolomei G H, Dennis V A, Lasater B L, Philipp M T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giambartolomei G H, Dennis V A, Philipp M T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect Immun. 1998;66:2691–2697. doi: 10.1128/iai.66.6.2691-2697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girschick H J, Huppertz H I, Russmann H, Krenn V, Karch H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol Int. 1996;16:125–132. doi: 10.1007/BF01409985. [DOI] [PubMed] [Google Scholar]
- 12.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 13.Habicht G S, Beck G, Benach J L, Coleman J L, Leichtling K D. Lyme disease spirochetes induce human and murine interleukin 1 production. J Immunol. 1985;134:3147–3154. [PubMed] [Google Scholar]
- 14.Haddad A, Bienvenu J, Miossec P. Increased production of a Th2 cytokine profile by activated whole blood cells from rheumatoid arthritis patients. J Clin Immunol. 1998;18:399–403. doi: 10.1023/a:1023278606036. [DOI] [PubMed] [Google Scholar]
- 15.Hardy P H J, Levin J. Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc Soc Exp Biol Med. 1983;174:47–52. doi: 10.3181/00379727-174-41702. [DOI] [PubMed] [Google Scholar]
- 16.Hartung T, Doecke W D, Bundschuh D, Foote M A, Gantner F, Hermann C, Lenz A, Milwee S, Rich B, Simon B, Volk H D, von Aulock S, Wendel A. Effect of filgrastim treatment on inflammatory cytokines and lymphocyte functions. Clin Pharmacol Ther. 1999;66:415–424. doi: 10.1053/cp.1999.v66.a101210. [DOI] [PubMed] [Google Scholar]
- 17.Hartung T, Pitrak D L, Foote M, Shatzen E M, Verral S C, Wendel A. Filgrastim restores interleukin-2 production in blood from patients with advanced human immunodeficiency virus infection. J Infect Dis. 1998;178:686–692. doi: 10.1086/515338. [DOI] [PubMed] [Google Scholar]
- 18.Haupl T, Hahn G, Rittig M, Krause A, Schoerner C, Schonherr U, Kalden J R, Burmester G R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993;36:1621–1626. doi: 10.1002/art.1780361118. [DOI] [PubMed] [Google Scholar]
- 19.Henderson B, Poole S, Wilson M. Microbial/host interactions in health and disease: who controls the cytokine network? Immunopharmacology. 1996;35:1–21. doi: 10.1016/0162-3109(96)00144-0. [DOI] [PubMed] [Google Scholar]
- 20.Henderson B, Wilson M. Modulins: a new class of cytokine-inducing, pro-inflammatory bacterial virulence factor. Inflamm Res. 1995;44:187–197. doi: 10.1007/BF01782257. [DOI] [PubMed] [Google Scholar]
- 21.Hsu D H, de Waal Malefyt R, Fiorentino D F, Dang M N, Vieira P, de Vries J, Spits H, Mosmann T R, Moore K W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 22.Kamradt T, Krause A, Burmester G R. A role for T cells in the pathogenesis of treatment-resistant Lyme arthritis. Mol Med. 1995;1:486–490. [PMC free article] [PubMed] [Google Scholar]
- 23.Kamradt T, Lengl-Janssen B, Strauss A F, Bansal G, Steere A C. Dominant recognition of a Borrelia burgdorferi outer surface protein A peptide by T helper cells in patients with treatment-resistant Lyme arthritis. Infect Immun. 1996;64:1284–1289. doi: 10.1128/iai.64.4.1284-1289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause A, Burmester G R, Rensing A, Schoerner C, Schaible U E, Simon M M, Herzer P, Kramer M D, Wallich R. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. Complexity of humoral and cellular immune responses. J Clin Investig. 1992;90:1077–1084. doi: 10.1172/JCI115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller K, Herner E B, Stagg A, Bendtzen K, Woo P. Inflammatory cytokines and cytokine antagonists in whole blood cultures of patients with systemic juvenile chronic arthritis. Br J Rheumatol. 1998;37:562–569. doi: 10.1093/rheumatology/37.5.562. [DOI] [PubMed] [Google Scholar]
- 27.Osborne J, Devaney E. Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi. Infect Immun. 1999;67:1599–1605. doi: 10.1128/iai.67.4.1599-1605.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahl A, Kuhlbrandt U, Brune K, Rollinghoff M, Gessner A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol. 1999;37:1958–1963. doi: 10.1128/jcm.37.6.1958-1963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 30.Restrepo B I, Barbour A G. Antigen diversity in the bacterium B. hermsii through “somatic” mutations in rearranged vmp genes. Cell. 1994;78:867–876. doi: 10.1016/s0092-8674(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 31.Seiler K P, Weis J J. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 32.Sigal L H. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 33.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 34.Stiernstedt G, Eriksson G, Enfors W, Jorbeck H, Svenungsson B, Skoldenberg B, Granstrom M. Erythema chronicum migrans in Sweden: clinical manifestations and antibodies to Ixodes ricinus spirochete measured by indirect immunofluorescence and enzyme-linked immunosorbent assay. Scand J Infect Dis. 1986;18:217–224. doi: 10.3109/00365548609032330. [DOI] [PubMed] [Google Scholar]
- 35.Tai K F, Ma Y, Weis J J. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takayama K, Rothenberg R J, Barbour A G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987;55:2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Tolle A, Schaal E, Jahnke H D. Comparative studies of the differentiation of Pappenheim-Wright stained blood smears in the diagnosis of leukosis. Zentbl Vetmed Reihe B. 1966;13:62–67. . (In German.) [PubMed] [Google Scholar]
- 37.Weber K, Braun-Falco O. Blood picture changes in erythema chronicum migrans. Hautarzt. 1974;25:611–613. . (In German.) [PubMed] [Google Scholar]
- 38.Westerholt S, Hartung T, Tollens M, Gustrau A, Oberhoffer M, Karch H, Klare B, Pfeffer K, Emmrich P, Oberhoffer R. Inflammatory and immunological parameters in children with haemolytic uremic syndrome (hus) and gastroenteritis-pathophysiological and diagnostic clues. Cytokine. 2000;12:822–827. doi: 10.1006/cyto.1999.0624. [DOI] [PubMed] [Google Scholar]
- 39.Wilske B, Preac-Mursic V, Gobel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]