Abstract
Background
The worldwide outbreak of monkeypox has evidenced the usefulness of the dermatologic manifestations for its diagnosis.
Objective
To describe the histopathologic and immunohistochemical findings of monkeypox cutaneous lesions.
Methods
This is a retrospective histopathologic and immunohistochemical study of 20 patients with positive Monkeypox virus DNA polymerase chain reaction and immunohistochemical positivity for Vaccinia virus in cutaneous lesions. Four cases were also examined by electron microscopy.
Results
The most characteristic histopathologic findings consisted of full-thickness epidermal necrosis with hyperplasia and keratinocytic ballooning at the edges. In some cases, the outer root sheath of the hair follicle and the sebaceous gland epithelium were affected. Intraepithelial cytoplasmic inclusion bodies and scattered multinucleated keratinocytes were occasionally found. Immunohistochemically, strong positivity with anti-Vaccinia virus antibody was seen in the cytoplasm of ballooned keratinocytes. Electron microscopy study demonstrated numerous viral particles of monkeypox in affected keratinocytes.
Limitations
Small sample size. Electron microscopic study was only performed in 4 cases.
Conclusion
Epidermal necrosis and keratinocytic ballooning are the most constant histopathologic findings. Immunohistochemical positivity for Vaccinia virus was mostly detected in the cytoplasm of the ballooned keratinocytes. These findings support the usefulness of histopathologic and immunohistochemical studies of cutaneous lesions for diagnosis of monkeypox.
Key words: dermatopathology, electron microscopy, histopathology, immunohistochemistry, monkeypox, virology
Capsule Summary.
-
•
Histopathologic findings of cutaneous lesions support the diagnosis of monkeypox infection.
-
•
Immunohistochemical positivity for Vaccinia virus may differentiate the disease from other infections with similar histopathologic changes. Electron microscopy, if available, contributes to confirm the diagnosis.
Introduction
Monkeypox is a zoonotic disease caused by Monkeypox virus, which belongs to the genus Orthopoxvirus of the family Poxviridae, a large and diverse family of double-stranded DNA viruses that multiply in the cytoplasm of infected cells.1 , 2 It was first described in 1958, when cynomolgus monkeys shipped from Singapore to a Denmark research facility fell ill.3
In humans, the first confirmed case was described in 1970.4 Since then, several cases have been identified in many African countries. According to a systematic review conducted in 2022, the most affected country has been the Democratic Republic of Congo.5 There are 2 clades as follows: the Central African clade is responsible for the largest number of cases and has an estimated lethality of 10.6%; compared to the smaller West African clade with a lethality of 3.6%.5, 6, 7
The increase in cases can be explained mainly by the dramatic decline in herd immunity against smallpox since it was declared eradicated in 1980.8 Smallpox vaccination has been estimated to provide 80% to 85% cross-protection against monkeypox.9, 10, 11 The underlying immunological mechanisms seem to be diverse, with neutralizing cross-reactive antibodies among the principal components.12 , 13 Furthermore, it has been shown that monkeypox patients who had not received the Vaccinia virus-based vaccine showed more severe internal disease, pleomorphic cutaneous eruptions, and a higher mortality rate as compared to vaccinated individuals.14
Regarding monkeypox infection outside Africa, reported cases are scarce: 53 cases in the United States related to Gambian giant rats imported from Ghana in 2003,15 and a small amount of outbreaks (related to travelling to endemic countries) in the United Kingdom in 201816 and 2021,17 Israel in 2018,18 Singapore in 201919 and the United States in 2021.20 , 21 However, since May 2022 an unprecedented outbreak has been reported in multiple non-endemic countries, especially in the United States and Europe, which had already reached more than 80,000 cases by November 2022.22 , 23
Until the aforementioned outbreak, animal-human transmission had been considered the main source of infection.6 , 24 The major animal reservoir is found in rodents.25 Interhuman transmission has been found to be associated with respiratory droplets and contact with body fluids, skin lesions, or contaminated items.6 , 26 In the current outbreak, the observation that the majority of the cases have occurred among men who have sex with men27, 28, 29 can be explained by an early cluster,30 as the virus may spread through highly interconnected sexual networks.31
The incubation period is 5 to 21 days. The illness begins with fever, headache, asthenia, myalgia, and lymphadenopathy. Within 1 to 5 days after the onset of fever, skin lesions progressively appear: macules, papules, whitish pseudopustules with an umbilicated necrotic center, vesicles and pustules, followed by resolution with crusts and scabs. The initial location is generally related to the point of inoculation, with subsequent centrifugal spreading.14 , 22 , 27 , 28 , 32 A range of complications has been reported, such as secondary bacterial skin infections, pneumonia, keratitis, diarrhea, and encephalitis.14
The diagnosis can be confirmed via culture-based isolation or polymerase chain reaction for Monkeypox virus DNA from a patient specimen.33 Other diagnostic techniques include visualization of the virus on electron microscopy, immunohistochemical staining for Orthopoxvirus antigens, and serologic testing.14 , 32
Histopathologic features of cutaneous lesions of monkeypox infection have been rarely described. The aim of the present study, the largest of its kind to date, is to provide a detailed histopathologic and immunohistochemical description of cutaneous lesions caused by monkeypox infection.
Methods
Study design and patients
The study includes all patients examined between May and July of 2022 at the 3 participating hospitals from Madrid, Spain (Hospital Universitario Puerta de Hierro Majadahonda, Hospital Universitario Fundación Jiménez Díaz, and Hospital Universitario 12 de Octubre) with polymerase chain reaction-proven monkeypox infection in skin samples (vesicle/pustule fluid content and/or cutaneous biopsy), and immunohistochemical positivity for anti-Vaccinia virus antibody in the cutaneous biopsy specimen. Patients gave informed consent for biopsy.
This totals 20 patients. Clinical features of the first 8 cases (Supplementary Table I, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1) have been previously reported elsewhere.27
Sections of each case were stained with hematoxylin and eosin, and immunohistochemistry was performed with the use of a horseradish peroxidase-labeled polymer method, EnVision FLEX, high pH, and an automated staining system, Dako Omnis (Agilent Technologies Singapore International Pte Ltd), using commercially available antibodies to the following antigens: CD3 (polyclonal, RTU, Agilent Dako), CD4 (clone 4B12, dilution 1:50, Leica Biosystems Ltd), CD8 (clone C8/144B, RTU, Agilent Dako), CD20 (clone L26, RTU, Agilent Dako); CD30 (clone Ber-H2, RTU, Agilent Dako), CD79a (clone JCB117, RTU, Agilent Dako), and rabbit policlonal anti-Vaccinia virus antibody (clone ab35219, dilution 1:4000, Abcam). Immunohistochemical studies were semiquantitatively assesed as follows: +, less than 5%; ++, between 5% and 50%; and +++, over 50% of the inflammatory cells. In the stain for CD30, if less than 1% of cells were positive, a null value was recorded.
In 4 patients a transmission electron microscopy (TEM) study of the skin sample was also performed. Sections from the paraffin-embedded blocks were dewaxed using the Lighezan et al protocol,34 and then processed following conventional TEM protocol of postfixation with 1% osmium tetroxide aqueous solution at room temperature for 45 minutes. After, they were dehydratated with ketone, embedded in Spurr resin, and polymerized in an oven at 60 °C for 48 hours. Ultrathin sections were obtained with a diamond knife (Diatome ultra 45°) in a Leica ultramicrotome (Leica Reichert Ultracut S). The grids were observed in a JEOL JEM 1400 electron microscope at 80kv in the National Center of Electron Microscopy of Spain. A total of 200 viral particles were measured in length and width with image J FIJI program.
Ethics committee approval
All procedures were in accordance with the ethical standards of the Helsinki Declaration. On August 29, 2022, the study was approved by the research ethics committee of Hospital Universitario Puerta de Hierro Majadahonda.
Results
The clinical, histopathologic, and immunohistochemical findings of our patients are summarized (Supplementary Table I, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1).
Clinical features
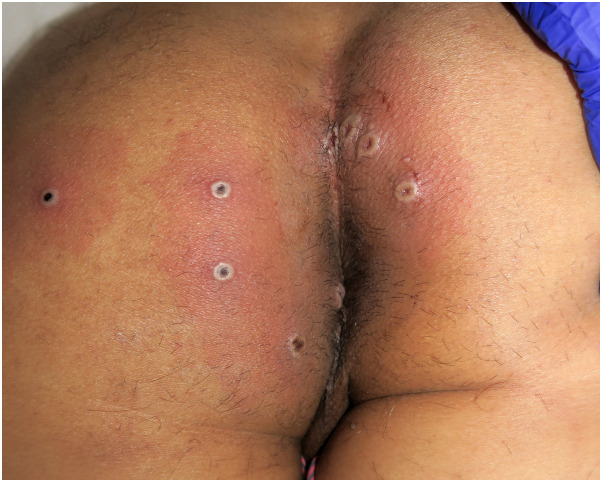
The 20 patients were males. The mean age was 40.5 (standard deviation: 10.86), ranging from 21 to 59 years. Generally the lesions appeared initially around the genital area, but in few days they spread in a centrifugal fashion to become generalized (Fig 1 , Supplementary Figs 6 to 8, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1). One patient showed the initial lesions around the mouth with associated involvement of oral mucosa, and another patient had lesions involving the conjunctiva of the lower right eyelid. Early cutaneous lesions mainly consisted of edematous whitish papules with marked umbilication and central crusting (pseudopustules) that evolved to crusts and erosions. Some lesions resolved with superficial atrophic scars. At consultation, most lesions were at the same stage resulting in a monomorphous exanthema.
Fig 1.
Pseudopustules on perianal skin.
Histopathologic features
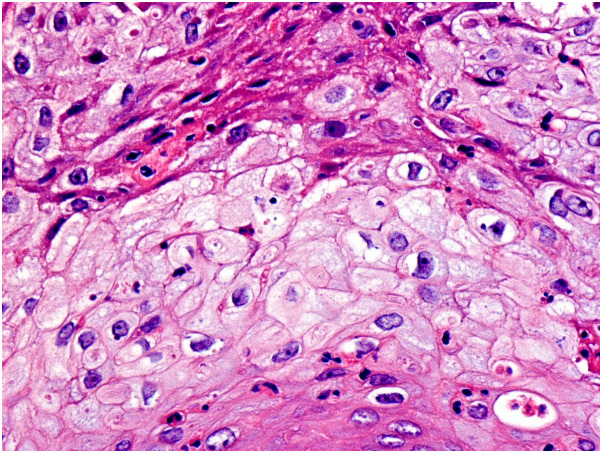
Histopathologic findings were similar in all cases. Lesional epidermis showed central necrosis with irregular hyperplasia at both sides of the erosion and exocytosis of neutrophils admixed with necrotic keratinocyte debris. At both sides of the epidermal necrosis, viable keratinocytes showed abundant pale cytoplasm indicative of ballooning (Supplementary Figs 9 to 11, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1). In some areas, swollen pale keratinocytes gave way to intraepidermal vesiculo-pustules. More advanced lesions showed full-thickness epidermal necrosis with a dense dermal infiltrate, mostly composed of small mature lymphocytes. Keratinocytes of the upper segment of the outer root sheath of the hair follicles also showed some degree of balloon cell change (Fig 2 , Supplementary Fig 12, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1). Adjacent sebaceous lobules exhibited variable degree of sebocyte necrosis. Eccrine units were spared both in their secretory and excretory segments.
Fig 2.
Balloon cell degeneration of the keratinocytes of the outer root sheath of the hair follicle. (Hematoxylin-eosin stain, original magnification ×400).
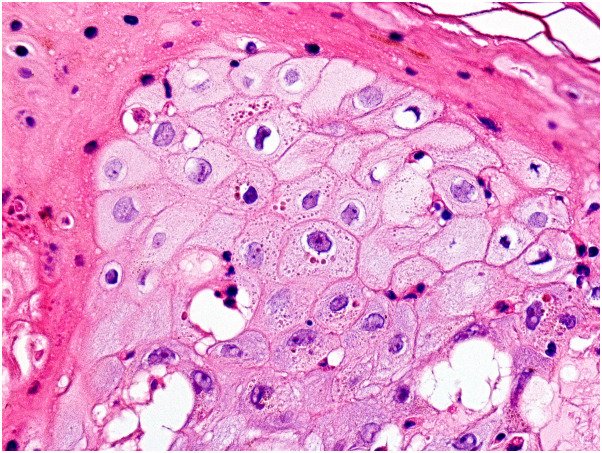
In early lesions, syncytial multinucleated cells with vesicular nuclei and prominent nucleoli were seen scattered within the areas of epidermal ballooning (Supplementary Figs 13 and 14, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1). A few atypical keratinocytes had large pyknotic nuclei with peripherally arranged chromatin resulting in a basophilic “halo”. Eosinophilic inclusion bodies, the so-called Guarnieri's inclusion bodies, were seen in the cytoplasm of some keratinocytes with balloon cell change, especially at the upper layers of lesional epidermis (Fig 3 ). One case showed squamous syringometaplasia involving eccrine ducts underneath necrotic epidermis, and another case featured mucin surrounded by multinucleated giant cells at the deep dermis as an incidental finding.
Fig 3.
Eosinophilic cytoplasmic inclusions (Guarnieri's inclusion bodies) in keratinocytes of the upper layers of the epidermis with balloon cell change. (Hematoxylin-eosin stain, original magnification ×400).
Immunohistochemical features
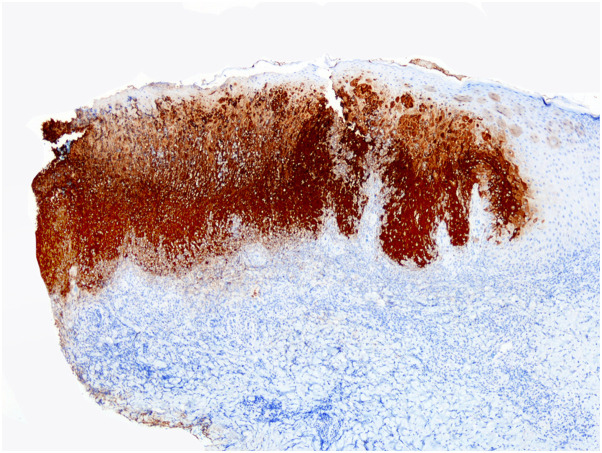
Immunohistochemically, viral antigen was detected most prominently in areas of keratinocytic ballooning at the sides of the necrotic epidermis, with diffuse cytoplasmic staining involving the full-thickness of the epidermis, as well as swollen pale keratinocytes of the upper outer root sheath of the hair follicles and necrotic sebocytes (Fig 4 , Supplementary Figs 15 to 18, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1). In 3 cases, the cytopathic changes and the immunohistochemical positivity predominated in the epithelium of the outer root sheath of the hair follicle. Scattered dermal dendritic cells also exhibited positivity with anti-Vaccinia virus antibody. The dermal inflammatory infiltrate was composed mostly of mature CD3+ T-cells and rare admixed CD20+ B-cells, with a higher proportion of CD8+ than CD4+ T cells (Supplementary Figs 19 to 30, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1).
Fig 4.
Immunostaining with anti-Vaccinia virus antibody, with immunohistochemical positivity in the cytoplasm of viable epidermal keratinocytes of lesional epidermis in sharp contrast with the negativity of the normal keratinocytes of the adjacent noninvolved epidermis. (Immunostaining with anti-Vaccinia virus antibody, original magnification ×20).
Electron microscopic features
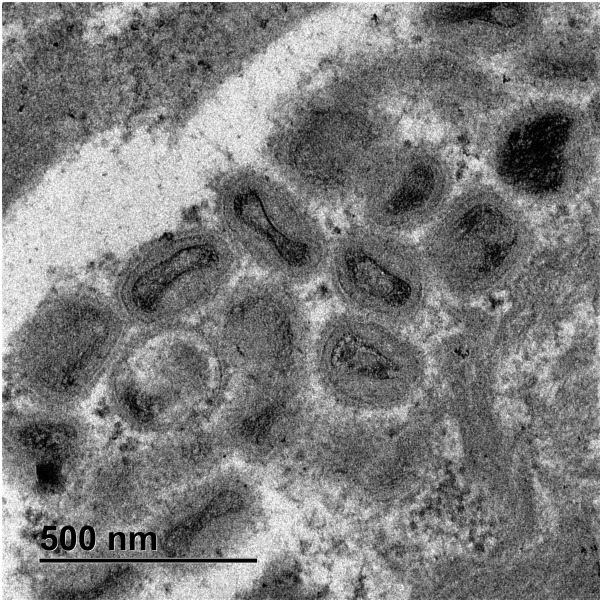
Samples observed in TEM showed mature extracellular viruses, among keratin fibers, with the outer envelope visible. The mature intracellular viruses were among the assembling virions found grouped together inside vesicles in the cytoplasm of keratinocytes. Some mature intracellular virus appeared with biconcave shaped central core, surrounded by lateral bodies and an external membrane with superficial tubules, and others appeared as brick-shaped particles (Fig 5 , Supplementary Fig 30, available via Mendeley at https://doi.org/10.17632/93pd9ymmb3.1). Immature virions still in formation appeared as round particles with no visible core. Groups of protein particles were also observed. Cytoplasmic viral particles were in different maturation phases, but the mature intracytoplasmic virus ranged 186 to 98 nm in length (median size 277.6 nm ± 38.65 standard deviation) and 130 to 273 nm in width (median size 198.38 nm ± 25.71 standard deviation).
Fig 5.
Electron microscopic findings. Some mature intracytoplasmic monkeypox with a biconcave shaped central core, lateral bodies and an external membrane with superficial tubules, and others appeared as brick-shaped particles. (Original magnification ×8000).
Discussion
There is a paucity of literature on the histopathologic findings of monkeypox in humans. The first description was made in 1985 by Stagles et al35 based on a postmortem study of a skin lesion of one of the first cases reported. The main findings were epidermal necrosis, multinucleated giant keratinocytes, keratinocytes with prominent nucleoli and eosinophilic cytoplasmic inclusions (Guarnieri's bodies) in the ductal eccrine epithelium, dermal edema, and perivascular inflammatory infiltrate. In addition, the electron microscopic study showed numerous mature and immature viral particles in the cytoplasm of the keratinocytes.
Later, in 2005, Bayer-Garner made an exhaustive histopathologic description of 3 skin samples from 2 cases of monkeypox.36 To the histopathologic findings previously reported by Stagles et al, Bayer-Garner added acanthosis, spongiosis, intra-epidermal bullae with degenerated keratinocytes in their center, and keratinocytes with an eosinophilic “ground glass” appearance of their nuclei. In his study, Bayer-Garner called attention additionally to the follicular involvement, the perivascular and perieccrine distribution of the inflammatory infiltrate, and the fact that necrosis of the keratinocytes appeared to be caused by apoptosis rather than oncosis (as previously described experimentally in monkeys).37 The degenerative epithelial changes result from a combination of hyperplasia of the spinous layer and ballooning degeneration. In this report, immunohistochemical staining with a rabbit anti-Vaccinia virus polyclonal antibody was positive in all involved keratinocytes, including those of the follicular and ductal eccrine epithelium, marking especially the Guarnieri's bodies. Finally, electron microscopy study disclosed a large number of intracytoplasmic mature and immature brick-shaped virions, with lateral bodies below the surface and thread-like structures over it (the latter identified by negative staining).
In the present outbreak, only 2 case reports have been published describing the histopathology of the cutaneous lesions, with similar findings to those previously mentioned, but no specific immunohistochemical study was performed.38 , 39
In our study, we found that characteristic histopathologic findings are mostly seen at both sides of the epidermal necrotic areas and they consist of balloon cell degeneration of epidermal and follicular outer root sheath keratinocytes, with some scattered intraepidermal syncytial multinucleated giant cells and occasional eosinophilic cytoplasmic inclusion bodies within the ballooned keratinocytes. Some keratinocytes with eosinophilic “ground glass” appearance within the central area of their nuclei were also found in a few cases, but definitely not to the extent seen in Herpesvirus cutaneous infections.40 Furthermore, immunohistochemistry with anti-Vaccinia virus antibody represents a reliable tool for confirmation of the diagnosis, demonstrating strong positivity in viable keratinocytes with balloon cell degeneration both in the epidermis and outer root sheath of the hair follicles of lesional skin. Scattered dendritic cells of the dermal infiltrate may also show immunoexpression for viral antigen. From ultrastructural point of view, our findings of mature and immature virions in the process of assembly within the cytoplasm are similar to those described by Moss.41 We found particles of different shapes, due to the virus orientation in the sectioned blocks. Ovoid-shaped virus with the biconcave shape of the core and the lateral bodies over the core also coincided with the findings reported by Moss41 and Bayer-Garner.36 All the keratinocytes affected showed viral vesicles at TEM and were settled in the same stratum that appeared positive in the immunostaining, as it was predicted.
Experimental studies have rarely been performed in animal models, such as mice42 and monkeys.37 Most remarkable is the description in monkeys of follicular and sebaceous gland involvement, whereas apocrine epithelium is spared, as well as the identification of the virus (disclosed by immunohistochemical staining with polyclonal rabbit anti-Vaccinia virus or mouse anti-Monkeypox virus antibodies) in dermal fibroblasts.37
Another aspect to consider is that the histopathologic changes occurring in Orthopoxvirus infections are almost indistinguishable between different species.39 , 43 However, it is possible to distinguish Orthopoxvirus from Herpesvirus histologically. Thus, viruses of the Herpesviridae family show cytopathic damage to keratinocytes consisting of pallor and ballooning degeneration, a “steel grey” appearance of the nuclei with chromatin margination, and eosinophilic nuclear inclusions surrounded by a clear “halo”.40 In contrast, eosinophilic inclusion bodies in Orthopoxvirus infections are in the cytoplasm of infected keratinocytes.35 , 36
A similar situation occurs in the electron microscopic study. Herpesvirus are very similar to each other, with a dense core and a hexagonal capsid surrounded by a multilayer proteic coat.44 Orthopoxvirus are also practically identical to each other, with brick-shaped or oval structures measuring 200 to 400 nm.6 , 45 Another decisive aspect in the differential diagnosis is the fact that while Herpesvirus replicate in the cell nucleus; Orthopoxvirus do so in the cytoplasm.1 , 2
To date, the only approved treatment for monkeypox is tecovirimat, an antiviral drug that interferes with the VP37 protein present on the surface of the virus, thus blocking the final steps in viral maturation and release from the infected cell.46, 47, 48 Other drugs under study are cidofovir, brincidofovir, ribavirin, and mycophenolic acid.47 In addition, according to the World Health Organization, smallpox vaccination is recommended within 4 days, after significant, unprotected exposure to a diseased animal or a confirmed human case.14 , 49, 50, 51
Conclusion
The so far unstoppable intercontinental outbreak of monkeypox, already declared as “public health emergency of international concern” by the World Health Organization,52 requires further knowledge not only in terms of treatment, but also in terms of diagnosis. In view of the wide differential diagnosis that some cases may elicit, and the importance of avoiding false negatives to establish a complete traceability of the outbreak and patient's contacts, procedures such as skin biopsy may be of great help.
In conclusion, dermatologists and dermatopathologists should be aware of the clinical and histopathologic features of the cutaneous lesions of monkeypox infection as they play a crucial role for specific and early diagnosis, and for adequate treatment of the present worldwide outbreak.
Conflicts of interest
None disclosed.
Acknowledgments
The patients in this manuscript have given informed consent to publication of their case details.
Footnotes
Funding sources: None.
IRB approval status: Reviewed and approved by the research ethics committee of Hospital Universitario Puerta de Hierro Majadahonda on August 29, 2022.
References
- 1.Hughes A.L., Irausquin S., Friedman R. The evolutionary biology of poxviruses. Infect Genet Evol. 2010;10(1):50–59. doi: 10.1016/j.meegid.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shchelkunov S.N., Totmenin A.V., Safronov P.F., et al. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Magnus P., Anderson E.K., Petersen K.B., et al. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 4.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 5.Bunge E.M., Hoet B., Chen L., et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alakunle E., Moens U., Nchinda G., et al. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10) doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen P.Y., Ajisegiri W.S., Costantino V., et al. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017-2020. Emerg Infect Dis. 2021;27(4):1007–1014. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine P.E., Jezek Z., Grab B., et al. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 10.Hammarlund E., Lewis M.W., Carter S.V., et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11(9):1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 11.Edghill-Smith Y., Golding H., Manischewitz J., et al. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 12.Gilchuk I., Gilchuk P., Sapparapu G., et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167(3):684–694.e9. doi: 10.1016/j.cell.2016.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss B. Smallpox vaccines: targets of protective immunity. Immunol Rev. 2011;239(1):8–26. doi: 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen E., Kantele A., Koopmans M., et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) Multistate outbreak of monkeypox--Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(23):537–540. [PubMed] [Google Scholar]
- 16.Vaughan A., Aarons E., Astbury J., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38):1800509. doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson G., Adamson J., Adler H., et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32):2100745. doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erez N., Achdout H., Milrot E., et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yong S.E.F., Ng O.T., Ho Z.J.M., et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao A.K., Schulte J., Chen T.H., et al. Monkeypox in a traveler returning from Nigeria - Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep. 2022;71(14):509–516. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costello V., Sowash M., Gaur A., et al. Imported monkeypox from international traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022;28(5):1002–1005. doi: 10.3201/eid2805.220292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention 2022 Monkeypox outbreak global map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 24.Durski K.N., McCollum A.M., Nakazawa Y., et al. Emergence of monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doty J.B., Malekani J.M., Kalemba L.N., et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses. 2017;9(10):283. doi: 10.3390/v9100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antinori A., Mazzotta V., Vita S., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22):2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Català A., Clavo-Escribano P., Riera-Monroig J., et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022;187(5):765–772. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]
- 28.Pinto-Pulido E.L., Fernández-Parrado M., Rodríguez-Cuadrado F.J. RF - Monkeypox: key concepts. Actas Dermosifiliogr. 2022 doi: 10.1016/j.ad.2022.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez B.S., Herrador B.R.G., Franco A.D., et al. Epidemiologic features and control measures during monkeypox outbreak, Spain, June 2022. Emerg Infect Dis. 2022;28(9):1847–1851. doi: 10.3201/eid2809.221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryer J.S., Freeman E.E., Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatological primer. J Am Acad Dermatol. 2022;87(5):1069–1074. doi: 10.1016/j.jaad.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupferschmidt K. Why the monkeypox outbreak is mostly affecting men who have sex with men. Science. 2022;376(6600):1364–1365. doi: 10.1126/science.add5497. [DOI] [PubMed] [Google Scholar]
- 32.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 33.Osadebe L., Hughes C.M., Shongo Lushima R., et al. Enhancing case definitions for surveillance of human monkeypox in the Democratic Republic of Congo. Plos Negl Trop Dis. 2017;11(9) doi: 10.1371/journal.pntd.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lighezan R., Baderca F., Alexa A., et al. The value of the reprocessing method of paraffin-embedded biopsies for transmission electron microscopy. Rom J Morphol Embryol. 2009;50(4):613–617. [PubMed] [Google Scholar]
- 35.Stagles M.J., Watson A.A., Boyd J.F., et al. The histopathology and electron microscopy of a human monkeypox lesion. Trans R Soc Trop Med Hyg. 1985;79(2):192–202. doi: 10.1016/0035-9203(85)90333-5. [DOI] [PubMed] [Google Scholar]
- 36.Bayer-Garner I.B. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32(1):28–34. doi: 10.1111/j.0303-6987.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 37.Zaucha G.M., Jahrling P.B., Geisbert T.W., et al. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81(12):1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maronese C.A., Beretta A., Avallone G., et al. Clinical, dermoscopic and histopathological findings in localized human monkeypox: a case from northern Italy. Br J Dermatol. 2022;187(5):822–823. doi: 10.1111/bjd.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berna-Rico E., Perna C., Azcarraga-Llobet C., et al. Monkeypox virus infection with a syphilitic-roseola-like rash and its histopathologic characterization during 2022 outbreak. J Eur Acad Dermatol Venereol. 2022 doi: 10.1111/jdv.18708. [DOI] [PubMed] [Google Scholar]
- 40.Khan Z.M., Cockrell C.L. In: Textbook of dermatopathology. Barnhill R.L., editor. McGraw-Hill Publishers; 1998. Cutaneous viral infections; p. 439. [Google Scholar]
- 41.Moss B. Membrane fusion during poxvirus entry. Semin Cell Dev Biol. 2016;60:89–96. doi: 10.1016/j.semcdb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osorio J.E., Iams K.P., Meteyer C.U., et al. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One. 2009;4(8) doi: 10.1371/journal.pone.0006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Embury-Hyatt C., Babiuk S., Manning L., et al. Pathology and viral antigen distribution following experimental infection of sheep and goats with capripoxvirus. J Comp Pathol. 2012;146(2-3):106–115. doi: 10.1016/j.jcpa.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blank H., Davis C., Collins C. Electron microscopy for the diagnosis of cutaneous viral infections. Br J Dermatol. 1970;83(Suppl):69–80. doi: 10.1111/j.1365-2133.1970.tb12866.x. [DOI] [PubMed] [Google Scholar]
- 45.Diven D.G. An overview of poxviruses. J Am Acad Dermatol. 2001;44(1):1–16. doi: 10.1067/mjd.2001.109302. [DOI] [PubMed] [Google Scholar]
- 46.European Medicines Agency . European Medicines Agency; 2022. Tecovirimat SIGA (Tecovirimat): An Overview of Tecovirimat SIGA and Why it is Authorised in the EU. [Google Scholar]
- 47.Rodríguez-Cuadrado F.J., Pinto-Pulido E.L., Fernández-Parrado M. RF - Potential treatments for monkeypox. Actas Dermosifiliogr. 2022 doi: 10.1016/j.ad.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizk J.G., Lippi G., Henry B.M., et al. Prevention and treatment of monkeypox. Drugs. 2022;82(9):957–963. doi: 10.1007/s40265-022-01742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization Vaccines and immunization for monkeypox: interim guidance. 2022. https://www.who.int/publications/i/item/WHO-MPX-Immunization-2022.2-eng
- 50.Rodríguez-Cuadrado F.J., Pinto-Pulido E.L., Fernández-Parrado M. Anti-Vaccinia immunoglobulin and post-exposure prophylaxis with Vaccinia-based vaccine for management of the monkeypox outbreak. Actas Dermosifiliogr. 2023;114(1):92–93. doi: 10.1016/j.ad.2022.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernández-Parrado M., Rodríguez-Cuadrado F.J., Pinto-Pulido E.L. Monkeypox vaccine: special considerations for dermatologic patients. J Eur Acad Dermatol Venereol. 2022 doi: 10.1111/jdv.18622. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization Second meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox. 2022. https://www.who.int/news/item/23-07-2022-second-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-regarding-the-multi-country-outbreak-of-monkeypox