Abstract
Background:
Proliferative lupus nephritis (PLN) is characterized by increased expression of inducible nitric oxide (NO) synthase (iNOS). Inhibition of iNOS with NG-monomethyl l-arginine (l-NMMA) abrogates renal disease in two models of murine PLN, but the mechanism of this effect is unknown. Reactive oxygen species have both direct and indirect pathogenic effects in inflammatory lesions and are therefore potentially an important therapeutic target in PLN. We hypothesized that inhibition of iNOS activity would reduce ROS production in murine PLN.
Methods:
A dose escalation of l-NMMA (0, 20, 100, and 500 mg/kg/day) was performed in New Zealand Black × New Zealand White F1 (NZB/W) mice with active renal disease. Twenty-four-hour urine nitrate + nitrite (NOx) was measured with a chemiluminescence NO analyzer. Twenty-four-hour urine 8-isoprostane F2α, (8-iso-PGF2α) was measured by gas chromatography–negative ion chemical ionization mass spectrometry. MRL-MpJFASlpr (MRL/lpr) and NZB/W mice were divided into three groups and given either l-NMMA, l-N6-iminoethyl-lysine (l-NIL), or distilled water for 2 weeks. Urine NOx and 8-iso-PGF2α were determined after 2 weeks.
Results:
l-NMMA reduced both urine NOx and 8-iso-PGF2α levels in a dose-dependent fashion in NZB/W and MRL/lpr mice. Urine NOx and 8-iso-PGF2α levels were highly correlated. Both specific (l-NIL) and nonspecific (l-NMMA) iNOS inhibition reduced urine NOx and 8-iso-PGF2α levels in both models of murine PLN.
Conclusion:
These findings suggest that iNOS activity is a major source of reactive oxidant stress in these models of murine PLN. Future studies will address the pathogenic role of reactive oxygen stress in PLN.
Keywords: nitric oxide synthase, isoprostanes, lupus nephritis
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease characterized by autoantibody production, immune complex deposition, and complement activation.1-3 When this process occurs in the kidney,4-6 it can lead to proliferative glomerulonephritis, fibrosis, and renal failure. Inducible nitric oxide synthase (iNOS) is upregulated in the murine models of lupus nephritis and is overexpressed in the glomerulus in human proliferative lupus nephritis.7-9 In MRL-MpJFASlpr (MRL/lpr) mice, dietary supplementation with l-arginine, a substrate for iNOS, accelerates renal fibrosis and shortens the life span.10 Pharmacologic inhibition of iNOS leads to significant improvement in murine lupus nephritis.11 These combined data suggest a pathogenic role for iNOS activity in murine lupus, but the mechanism of this effect is unknown.
Nitric oxide (NO) and superoxide (O−2), products of iNOS activity, rapidly react to form peroxynitrite (ONOO−), a potent reactive molecule capable of modifying proteins, deoxyribonucleic acid (DNA), and lipids. These modifications can alter enzyme activity, induce DNA mutations, and form biologically active lipid mediators.12-14 One such lipid mediator, 8-isoprostane F2α (8-iso-PGF2α), is formed via oxidation of arachidonate by reactive species such as peroxynitrite and can be measured in urine as a marker of systemic oxidative stress.15,16 Elevated levels of urine 8-iso-PGF2α have been described in SLE, but their source and significance are unknown.17-21 The following study was designed to determine the functional relationship between iNOS inhibition in murine SLE and systemic oxidative stress.
MATERIALS AND METHODS
Dose Escalation Trial for NG-Monomethyl l-Arginine
Mice
Female New Zealand Black/BlNJ F × New Zealand White/LacJ M F1 hybrid (NZB/W) mice were obtained from Jackson Laboratory (Bar Harbor, ME) at 8 weeks of age. They were housed in the Ralph H. Johnson Veterans Administration Medical Center (VAMC) animal facility at Charleston, South Carolina, in a pathogen-free environment. All protocols used were approved by the Institutional Animal Care and Use Committee. Four NZB/W mice at 36 weeks of age were used. They had an average proteinuria level of 1.7 mg/24 hours and a urine nitrate + nitrite (NOx) level of 1.3 μmol/24 hours, which are indicative of active disease.7 They were conditioned and treated as described below.
Treatment
Two days prior to the start of treatment and throughout treatment, all animals were placed on a nitrate- and nitrite-free diet (Zeigler Brothers, Gardners, PA). The mice were given increasing doses of NG-monomethyl l-arginine (l-NMMA) (0, 50, 100, and 500 mg/kg/d), with each dose being administered for 4 days. Twenty-four-hour urine collections were performed in metabolic cages after 4 days at each dose. All mice received treatment in distilled drinking water ad libitum throughout the 2-week duration of the experiment.
Urine NOx Analysis
Urine samples were collected as above in 1 mL of an antibiotic solution containing 25 μg/mL ampicillin, 50 μg/mL gentamicin, and 200 μg/mL chloramphenicol to prevent bacterial growth. The samples were centrifuged at 2,500g to remove debris and were filtered through a 0.22 μm ultrafree centrifugal filter (Millipore, Bedford, MA). NOx was assayed using a chemiluminescence detector (Sievers, Boulder, CO) with vanadium chloride at 95°C as a reducing agent and standard curves generated from nitrate (Sigma-Aldrich, St. Louis, MO), as has been reported.22-24 All samples were assayed in duplicate or triplicate.
8-Iso-PGF2α Analysis
Urine 8-iso-PGF2α levels were determined using validated immunoaffinity extraction–gas chromatography–negative ion chemical ionization–mass spectrometry (GC-NICI-MS) detection25 using modifications to the method of Tsikas and colleagues.26 Urine (1 mL) was centrifuged (5 minutes at 500g), and any precipitate was discarded. It was spiked with known amounts of 3,3,4,4-[2H4]-8-isoPGF2α (Cayman Chemical, Ann Arbor, MI; 1 ng in 10 μL methyl acetate) and then purified using an 8-iso-PGF2α immunoaffinity column (Cayman Chemical) according to the package insert. The eluate was esterified using 10% pentafluorobenzylbromide in acetonitrile and 10% diisopropylethylamine in acetonitrile at 40°C for 20 minutes and evaporated under nitrogen. Silyl ethers were formed by treating the residue with bis (trimethylsilyl)trifluoroacetamide at 40°C for 8 minutes.
The derivatized sample was analyzed by GC-NICI-MS using an Agilent model 5973N (Agilent, Wilmington, DE) GC-MS. A phenylmethylpolysiloxane column 30 m × 0.25 mm internal diameter × 0.25 μm film (DB-5MS, J&W Scientific, Folsom, CA) was held at 190°C for 2 minutes after injection (2 μL; pulsed splitless) and then ramped to 300°C at 20°C/min and held for 6 minues. The helium linear velocity was 50 cm/s. Selected ion monitoring of the debenzylated fragments ions 569 and 573 m/z for analyte and deuterium-labeled internal standard, respectively, were detected at 8.1 and 8.08 minutes after injection. There were no chemical interferences.
Effect of iNOS Inhibitor Therapy on Measures of Systemic NO Production (NOX) and Oxidant Stress (8-Iso PGF2α) in MRL/lpr Mice
Mice
Female MRL/lpr mice were obtained from Jackson Laboratory at 8 weeks of age. They were housed in the Ralph H. Johnson VAMC animal facility in a pathogen-free environment. All protocols used were approved by the Institutional Animal Care and Use Committee. The MRL/lpr mice were treated beginning at 18 weeks of age when urine NOx levels had reached 1.2 μmol/24 hours in the group, which is indicative of active disease.7
Treatment
Two days prior to the start of treatment and throughout treatment, all animals were placed on a nitrate- and nitrite-free diet (Zeigler Brothers). The mice were randomly assigned into three groups. The first group of six received 500 mg/kg/d of l-NMMA (Cyclops Biochemical Corporation, Salt Lake City, UT). The second group of four received 20 mg/kg/d of 1-N6-iminoethyl-lysine (l-NIL) (Cayman Chemical, Ann Arbor, MI). The third group of six received equal volumes of distilled water. The mice were treated for 2 weeks to ensure that any reductions in reactive oxygen species (ROS) were due to the direct effect of iNOS inhibition and not the downstream effects of the therapy on the inflammatory glomerular infiltrate, as can be seen with longer durations of therapy.27 Earlier studies in our own laboratory demonstrated that 12 weeks of iNOS inhibitory therapy is necessary to reduce proteinuria in MRL/lpr mice (data not shown).
NOx and Isoprostane Measurement
Collection of urine sample and measurement of NOx and 8-isoPGF2α were performed as described for the l-NMMA dose escalation study above.
Effect of iNOS Inhibitor Therapy on Measures of Systemic NO Production (NOx) and Oxidant Stress (8-Iso PGF2α in NZB/W Mice
Mice
NZB/W mice were purchased and housed as in the dose escalation experiment. The NZB/W mice were used at 40 weeks of age when the proteinuria level had reached 1.7 mg/24 hours and the NOx level had reached 1.3 μmol/24 hours. Previous studies in our laboratory have demonstrated that proteinuria increased to abnormal levels in parallel with increases in urine NOx levels.7
Treatment
Two days prior to the start of treatment, all animals were placed on a nitrate- and nitrite-free diet (Zeigler Brothers). The mice were randomly assigned into three groups. The first group of nine received 500 mg/kg/d of l-NMMA (Cyclops Biochemical Corporation). The second group of five received 20 mg/kg/d of l-NIL (Cayman Chemical). The third group of seven received equal volumes of distilled water. The mice were treated for 2 weeks to ensure that any reductions in ROS were due to the direct effect of iNOS inhibition and not the downstream effects of the therapy on the inflammatory glomerular infiltrate, as can be seen with longer durations of therapy.27 Earlier studies in our own laboratory demonstrated that 16 weeks of iNOS inhibitory therapy is necessary to reduce proteinuria in NZB/W mice (data not shown).
NOx and Isoprostane Measurement
Collection of urine sample and measurement of NOx and 8-isoPGF2α were performed as described for the l-NMMA dose escalation study above.
Histopathologic Glomerular Scoring
Glomerular scores of activity and damage were determined as described.28 After sacrifice, kidneys were removed and fixed with buffered 10% paraformaldehyde overnight and immobilized in paraffin. Blocks were sectioned and stained with hematoxylin and eosin. The slides were read and interpreted in a blinded fashion by an experienced pathologist. The kidneys were graded for inflammation, proliferation, crescent formation, and necrosis. Interstitial changes and vasculitis were also noted. Scores from 0 to 3 were assigned for each of the features and then added together to yield a final renal score. For example, glomerular inflammation was graded as follows: 0 = normal, 1 = few inflammatory cells, 2 = moderate inflammation, and 3 = severe inflammation. This method was used for both mouse models.
Statistical Analysis
Values in the figures are expressed as mean ± SEM of n observations, with n representing the number of animals. The unpaired Student’s t-test or analysis of variance was used to test for significance between groups. Associations between urine NOx and 8-iso-PGF2α levels were determined using a Pearson correlation, and p values of < .05 were considered significant.
RESULTS
Dose Escalation Trial of l-NMMA in NZB/W Mice
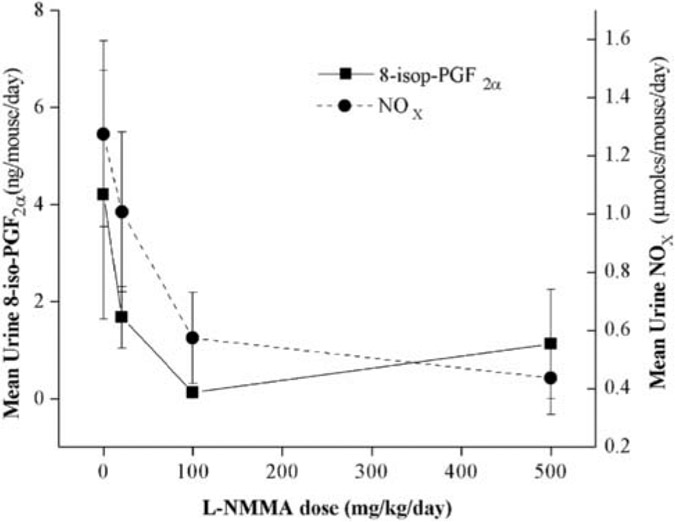
Four NZB/W mice with active disease (urine NOx > 1.3 μmol/24 hours) were administered increasing doses of l-NMMA (0, 20, 100, and 500 mg/kg/d) ad libitum in their drinking water while consuming a nitrate- and nitrite-free diet. Each dose was administered for 4 days, and urine was collected for 24 hours on the fourth day of treatment at each dose and prior to treatment. The urine samples were assayed for both NOx and 8-iso-PGF2α. Urine NOx as a marker of systemic NO production was reduced in a dose-dependent fashion, with a maximum effect at the 500 mg/kg/d dose. Urine 8-iso-PGF2α levels were similarly reduced with increasing l-NMMA dose in a manner that significantly correlated with urine NOx levels (Figure 1; r = .709, p = .002).
FIGURE 1.
Effect of increasing doses of NG-monomethyl l-arginine (l-NMMA) on urine nitrate + nitrite (NOx) and 8-isoprostane F2α (8-iso-PGF2α) levels in New Zealand Black × New Zealand White (NZB/W) mice. NZB/W mice with active disease (n = 4) were given increasing doses of l-NMMA (an inducible nitric oxide synthase inhibitor; 0, 50, 100, and 500 mg/kg/d). After 4 days at each dose, urine was collected and analyzed for NOx (a surrogate marker of systemic NO production) and 8-iso-PGF2α (a surrogate marker for systemic oxidant stress). l-NMMA reduced both NOx and 8-iso-PGF2α levels in a parallel, dose-dependent fashion. Urine NOx and 8-iso-PGF2α were significantly associated (r = .709, p = .002).
Effect of iNOS Inhibitor Therapy on Measures of Systemic NO Production and Oxidant Stress in MRL/lpr Mice
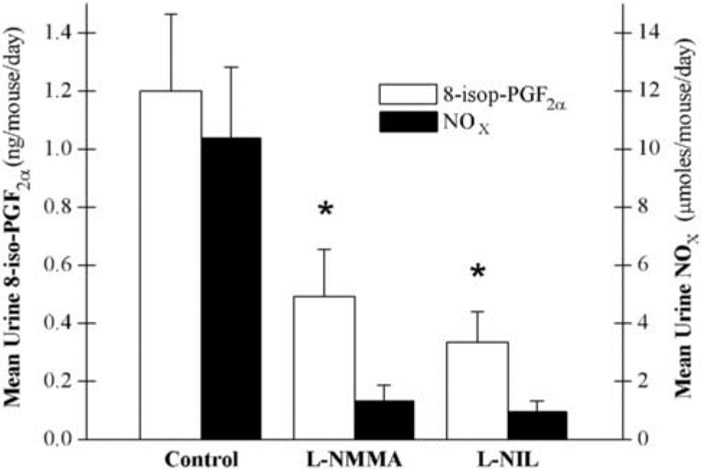
Twenty-week-old MRL/lpr mice with active disease (urine NOx > 1.2 μmol/24 hours) were administered either nonspecific (l-NMMA, 500 mg/kg/d, n = 6) or specific (l-NIL, 20 mg/kg/d, n = 4) iNOS inhibitors or distilled water (n = 6) ad libitum in their drinking water for 2 weeks. From 2 days prior to therapy and throughout therapy, mice were maintained on a nitrate- and nitrite-free diet. Urine was collected from all mice for 24 hours on day 14 of treatment and analyzed for NOx (a surrogate for systemic NO production) and 8-iso-PGF2α (a surrogate for systemic oxidant stress). Both urine NOx and urine 8-iso-PGF2α were significantly reduced by iNOS inhibitor therapy (Figure 2). Urine NOx and 8-iso-PGF2α for individual mice were significantly associated (r = .084, p = .0001). There was no statistically significant difference between the two iNOS inhibitor therapies.
FIGURE 2.
Effect of inducible nitric oxide synthase (iNOS) inhibitors on systemic nitrate + nitrite (NOx) levels and oxidant stress in MRL/lpr mice. Either a specific iNOS inhibitor (l-N6-iminoethyl-lysine [l-NIL], n = 4), a nonspecific iNOS inhibitor (NG-monomethyl l-arginine [l-NMMA], n = 6), or distilled water (n = 6) was administered for 2 weeks to MRL/lpr mice with active disease. Urine NOx and 8-isoprostane F2α (8-iso-PGF2α) levels were significantly lower in the combined treatment groups when compared with the control group. *p < .05.
No significant difference in tissue changes was observed between control and l-NMMA- and L-NIL-treated groups (5.8 ± 2.3, 6.8 ± 1.8 [p = .34 vs control] and 6.7 ± 3.5 [p = .54 vs control], respectively).
Effect of iNOS Inhibitor Therapy on Measures of Systemic NO Production and Oxidant Stress in NZB/W Mice
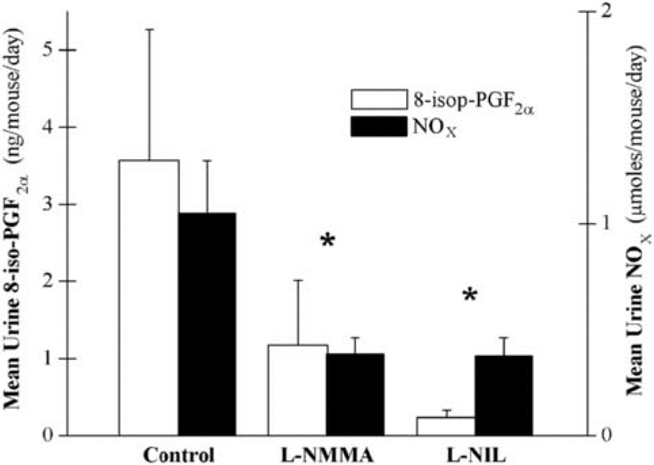
To ensure that the observations in MRL/lpr mice were not strain specific, we performed the same experiment as above in NZB/W mice with active disease (NOx > 1.3 μmol/24 hours). Mice were given the same doses of either l-NMMA (n = 9), l-NIL (n = 5), or distilled water (n = 7) as above for 2 weeks and were maintained on the nitrate- and nitrite-free diet. Urine was collected and analyzed as above. Both urine NOx and urine 8-iso-PGF2α were significantly reduced by iNOS inhibitor therapy (Figure 3). Urine NOx and 8-iso-PGF2α for individual mice were significantly associated in NZB/W mice as well (r = .660, p = .002). There was no statistically significant difference between the two iNOS inhibitor therapies.
FIGURE 3.
Effect of inducible nitric oxide synthase (iNOS) inhibitors on systemic nitrate + nitrite (NOx) levels and oxidant stress in New Zealand Black × New Zealand White (NZB/W) mice. Either a specific iNOS inhibitor (l-N6-iminoethyl-lysine [l-NIL], n = 5), a nonspecific iNOS inhibitor (NG-monomethyl l-arginine [l-NMMA], n = 9), or distilled water (n = 7) was administered for 2 weeks to NZB/W mice with active disease. Urine NOx and 8-isoprostane F2α (8-iso-PGF2α) levels were significantly lower in the combined treatment groups when compared with the control group. *p < .05.
No significant difference in tissue changes was observed between the control and treated groups (4.5 ± 4.9, 5.4 ± 3.3 [p = .84 vs control] and 4.0 ± 2.2 [p = .91 vs control], respectively).
DISCUSSION
These studies demonstrate that administration of competitive inhibitors of iNOS (l-NMMA and l-NIL) reduces markers of reactive oxidative stress in two murine models of lupus nephritis and strongly suggest that the majority of systemic oxidative stress in these models results from iNOS activity. The relative rapidity of ROS reduction by iNOS inhibitor therapy in this study suggests that it stems from a direct effect of the therapy on the iNOS enzyme rather than the known effects of more prolonged iNOS inhibitor therapy on cellular proliferation and infiltration in the glomerulus in these murine models.7,27 This notion is supported by our demonstration that this brief iNOS inhibitor therapy does not affect renal pathology in a significant fashion. This is consistent with earlier observations that acute pharmacologic inhibition of iNOS activity in the setting of infection29-31 or deletion of the iNOS gene in the absence of infection reduces measures of systemic oxidant stress.
This is the first report of reductions in systemic oxidant stress by reducing iNOS activity in models of autoimmune disease. These observations have important implications regarding the development of pharmacologic therapies for lupus nephritis. These observations logically lead to the hypothesis that the pathologic consequences of iNOS-mediated NO production in lupus nephritis would be reduced if it were isolated from the production of ROS. This is particularly important because development of arginine analogue–selective iNOS inhibitors for chronic use in humans has been delayed, likely owing to the difficulty with obtaining sufficient selectivity in this class of inhibitors. These data provide the rationale for a further hypothesis that potent antioxidants can reduce systemic oxidant stress and thus prevent the formation of ONOO− and oxidized lipids, such as 8-iso-PGF2α. There is a precedent in the literature for several potential mechanisms through which excessive ROS production leads to pathology in glomerulonephritis. The major clinical manifestations of glomerulonephritis in humans are the development of proteinuria and a reduction in glomerular blood flow. This is brought about by two general mechanisms. First, there is a reduction in the size and charge barrier of the glomerular basement membrane, partly via effacement of the foot processes, as in membranous nephritis. Oxygen radicals have been demonstrated to flatten the foot processes on the epithelial side of the basement membrane, leading to proteinuria.32 Second, inflammation and proliferation of resident and infiltrating inflammatory cells in the glomerulus lead to necrosis and/or obstruction of glomerular capillary tufts, leading to proteinuria and reduced glomerular blood flow. ROS are known to increase extracellular receptor kinase phosphorylation in mesangial cells, which leads to proliferation, whereas antioxidant therapy can reduce this effect.33
This study has several limitations. First, the treatment trials were not powered to detect a statistically significant effect of each iNOS inhibitor but rather the class of iNOS inhibitors combined. This limitation is tempered by the fact that both iNOS inhibitors had an equal effect on urine NOx and 8-iso-PGF2α levels and that the effect of iNOS inhibitors on 8-iso-PGF2α production was present in two models of lupus across three separate experiments. Second, this study does not eliminate the possibility that the arginine analogue class of competitive iNOS inhibitors reduces reactive oxidant stress via an alternative mechanism to inhibiting iNOS activity directly. For instance, members of this class of iNOS inhibitors are known to compete for l-arginine substrate entry into the cell via the y+ transporter system and thus limit NO production via reducing l-arginine substrate rather than by competitive inhibition of iNOS enzyme activity.34 This alternative activity of competitive iNOS inhibitors may, in fact, increase reactive oxygen stress because reduced l-arginine substrate availability can result in increased superoxide production by iNOS. It may be possible that a reduction in renal clearance owing to iNOS inhibitor therapy was responsible for the reduced urinary excretion of NOx and 8-iso PGF2α. However, studies in our laboratory indicated a 33% reduction in the glomerular filtration rate with l-NMMA compared with control and l-NIL therapy, both of which had no effect on the glomerular filtration rate (data not shown). The lack of a significant difference between l-NIL and l-NMMA therapy in the reduction of urine NOx and 8-iso PGF2α suggests that this may not be a major mechanism for the observed effect. Future studies should address these limitations and new hypotheses by determining the effect of an alternative class of iNOS inhibitors (dimerization inhibitors) on 8-iso-PGF2α production and the effect of superoxide dismutase mimetics on clinical disease activity. If the latter approach is effective, one could envision isolating the pathologic from the protective effects of NO production by selectively reducing the ROS generated by iNOS activity.
Acknowledgments
Supported by a research award from the Arthritis Foundation, Atlanta, GA, and grant numbers K08AR002193 and 5M01RR001070 from the National Institutes of Health, Bethesda, MD.
Contributor Information
Chinedu J. Njoku, Department of Medicine, Division of Rheumatology, Medical University of South Carolina, Charleston, SC
Kennerly S. Patrick, Department of Pharmaceutical Sciences, Medical University of South Carolina, Charleston, SC
Philip Ruiz, Jr, Department of Pathology, University of Miami, Miami, FL..
Jim C. Oates, Department of Medicine, Division of Rheumatology, Medical University of South Carolina, Charleston, SC
REFERENCES
- 1.Oates JC, Gilkeson GS. Mediators of injury in lupus nephritis. Curr Opin Rheumatol 2002;14:498–503. [DOI] [PubMed] [Google Scholar]
- 2.Amoura Z, Chabre H, Koutouzov S, et al. Nucleosome-restricted antibodies are detected before anti-dsDNA and/or antihistone antibodies in serum of MRL-Mp lpr/lpr and +/+ mice, and are present in kidney eluates of lupus mice with proteinuria. Arthritis Rheum 1994;37:1684–8. [DOI] [PubMed] [Google Scholar]
- 3.Fessel WJ. Systemic lupus erythematosus in the community. Incidence, prevalence, outcome, and first symptoms; the high prevalence in black women. Arch Intern Med 1974;134:1027–35. [PubMed] [Google Scholar]
- 4.Frostegard J. Autoimmunity, oxidized LDL and cardiovascular disease. Autoimmunol Rev 2002;1:233–7. [DOI] [PubMed] [Google Scholar]
- 5.Gescuk BD, Davis JC Jr. Novel therapeutic agents for systemic lupus erythematosus. Curr Opin Rheumatol 2002;14:515–21. [DOI] [PubMed] [Google Scholar]
- 6.Kant KS, Pollak VE, Weiss MA, et al. Glomerular thrombosis in systemic lupus erythematosus: prevalence and significance. Medicine (Baltimore) 1981;60:71–86. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg JB, Granger DL, Pisetsky DS, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L- arginine. J Exp Med 1994;179:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg JB. Nitric oxide synthase 2 and cyclooxygenase 2 interactions in inflammation. Immunol Res 2001;22:319–41. [DOI] [PubMed] [Google Scholar]
- 9.Wanchu A, Khullar M, Deodhar SD, et al. Nitric oxide synthesis is increased in patients with systemic lupus erythematosus. Rheumatol Int 1998;18:41–3. [DOI] [PubMed] [Google Scholar]
- 10.Peters H, Border WA, Ruckert M, et al. L-Arginine supplementation accelerates renal fibrosis and shortens life span in experimental lupus nephritis. Kidney Int 2003;63:1382–92. [DOI] [PubMed] [Google Scholar]
- 11.Cattell V Nitric oxide and glomerulonephritis. Semin Nephrol 1999;19:277–87. [PubMed] [Google Scholar]
- 12.Montuschi P, Ciabattoni G, Paredi P, et al. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med 1998;158(5 Pt 1):1524–7. [DOI] [PubMed] [Google Scholar]
- 13.Khan J, Brennan DM, Bradley N, et al. 3-Nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem J 1998;330(Pt 2):795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res 1997;36:1–21. [DOI] [PubMed] [Google Scholar]
- 15.Morrow JD, Minton TA, Roberts LJD. The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins 1992;44:155–63. [DOI] [PubMed] [Google Scholar]
- 16.Morrow JD, Roberts LJ II. The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol 1996;51:1–9. [DOI] [PubMed] [Google Scholar]
- 17.Alves JD, Grima B. Oxidative stress in systemic lupus erythematosus and antiphospholipid syndrome: a gateway to atherosclerosis. Curr Rheumatol Rep 2003;5:383–90. [DOI] [PubMed] [Google Scholar]
- 18.Kovacic P, Jacintho JD. Systemic lupus erythematosus and other autoimmune diseases from endogenous and exogenous agents: unifying theme of oxidative stress. Mini Rev Med Chem 2003;3:568–75. [DOI] [PubMed] [Google Scholar]
- 19.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol 2005;25:279–86. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Zackert WE, Yang JP, et al. Quantification of the major urinary metabolite of 15-F-2t-isoprostane (8-iso-PGF(2 alpha)) by a stable isotope dilution mass spectrometric assay. Anal Biochem 1999;269:326–31. [DOI] [PubMed] [Google Scholar]
- 21.Ames PR, Alves J, Murat I, et al. Oxidative stress in systemic lupus erythematosus and allied conditions with vascular involvement. Rheumatology (Oxford) 1999;38:529–34. [DOI] [PubMed] [Google Scholar]
- 22.Venkataraman S, Martin SM, Schafer FQ, Buettner GR. Detailed methods for the quantification of nitric oxide in aqueous solutions using either an oxygen monitor or EPR. Free Radic Biol Med 2000;29:580–5. [DOI] [PubMed] [Google Scholar]
- 23.Mayhan WG, Sharpe GM, Anding P. Agonist-induced release of nitric oxide during acute exposure to nicotine. Life Sci 1999;65:1829–37. [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Yen MH. Higher level of plasma nitric oxide in spontaneously hypertensive rats. Am J Hypertens 1999;12:476–82. [DOI] [PubMed] [Google Scholar]
- 25.Fam SS, Morrow JD. The isoprostanes: unique products of arachidonic acid oxidation—a review. Curr Med Chem 2003;10:1723–40. [DOI] [PubMed] [Google Scholar]
- 26.Tsikas D, Schwedhelm E, Suchy MT, et al. Divergence in urinary 8-iso-PGF(2alpha) (iPF(2alpha)-III, 15-F(2t)-IsoP) levels from gas chromatography-tandem mass spectrometry quantification after thin-layer chromatography and immunoaffinity column chromatography reveals heterogeneity of 8-iso-PGF(2alpha). Possible methodological, mechanistic and clinical implications. J Chromatogr B Analyt Technol Biomed Life Sci 2003;794:237–55. [DOI] [PubMed] [Google Scholar]
- 27.Oates JC, Ruiz P, Alexander A, et al. Effect of late modulation of nitric oxide production on murine lupus. Clin Immunol Immunopathol 1997;83:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly CM, Farrelly LW, Viti D, et al. Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase. Kidney Int 2002;61:839–46. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey KH, Sigar IM, Rana SV, et al. Inducible nitric oxide synthase regulates production of isoprostanes in vivo during chlamydial genital infection in mice. Infect Immun 2003;71:7183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marnett LJ, Wright TL, Crews BC, et al. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric oxide synthase. J Biol Chem 2000;275:13427–30. [DOI] [PubMed] [Google Scholar]
- 31.Matejovic M, Krouzecky A, Martinkova V, et al. Selective inducible nitric oxide synthase inhibition during long-term hyperdynamic porcine bacteremia. Shock 2004;21:458–65. [DOI] [PubMed] [Google Scholar]
- 32.Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: a model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol 1999;154:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budisavljevic MN, Hodge L, Barber K, et al. Oxidative stress in the pathogenesis of experimental mesangial proliferative glomerulonephritis. Am J Physiol Renal Physiol 2003;285:F1138–48. [DOI] [PubMed] [Google Scholar]
- 34.Miner SE, Al-Hesayen A, Kelly S, et al. L-Arginine transport in the human coronary and peripheral circulation. Circulation 2004;109:1278–83. [DOI] [PubMed] [Google Scholar]