Figure 4. IFT-A uses TPR domains for membrane attachment.
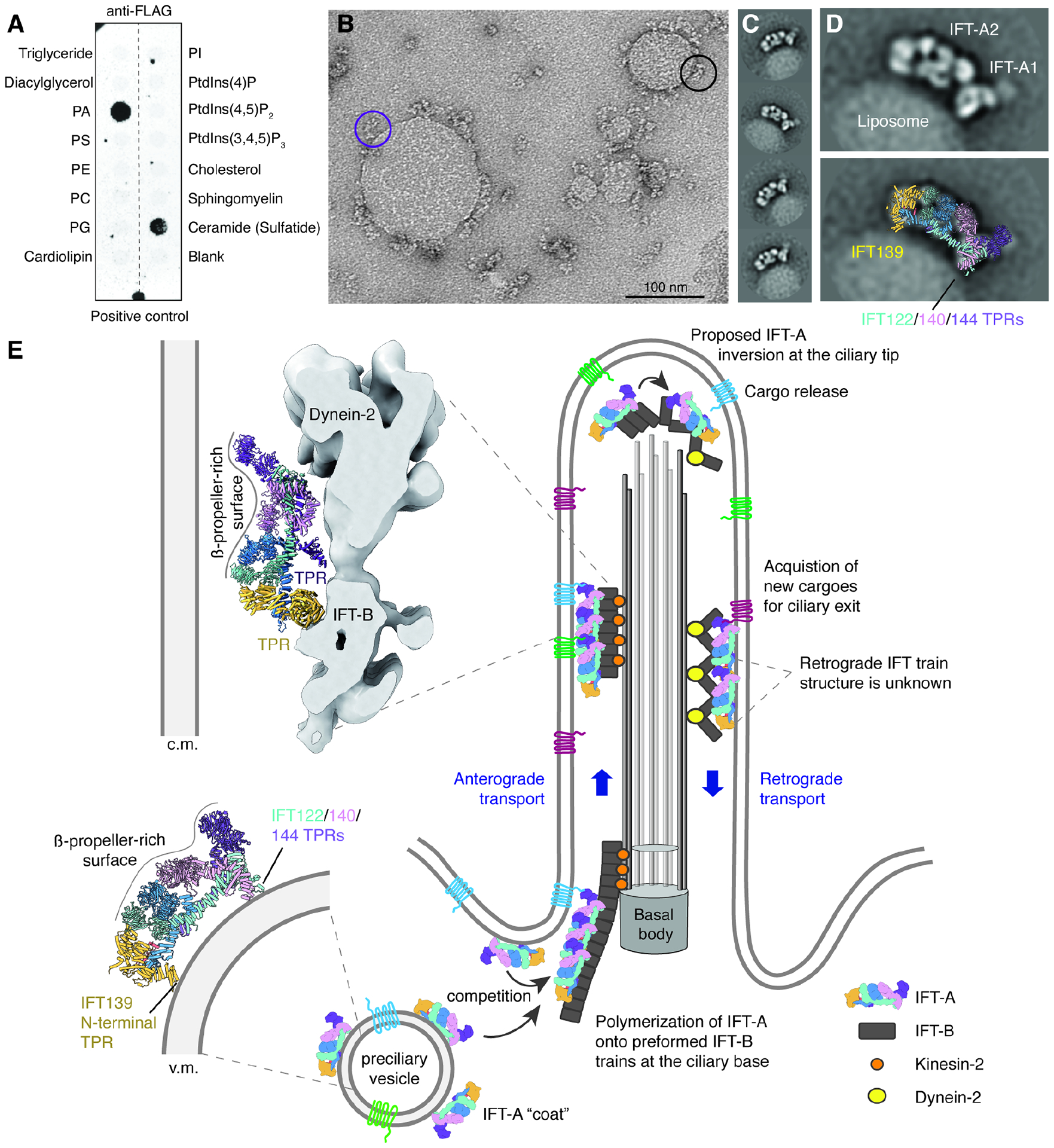
A. Results of a lipid-strip overlay assay showing that IFT-A has specificity for phosphatidic acid (PA) and 3-sulfogalactosylceramide. Identical results were obtained twice. Lipid abbreviations: PS, phosphatidylserine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PG, phosphatidylglycerol; PI, phosphatidylinositol.
B. A negative-stain electron micrograph showing PA-containing liposomes coated with IFT-A. The purple circle highlights an IFT-A complex tethered to the membrane only through the A1 subcomplex, whereas the black circle highlights an IFT-A complex tethered through its A1 and A2 subcomplexes.
C. Two-dimensional (2D) class averages showing a consistent arrangement on IFT-A on the liposome surface.
D. A 2D class average of a liposome-bound IFT-A (top) overlaid with the atomic model of IFT-A (bottom). Membrane contacts appear to involve the TPRs of IFT139 (yellow) and IFT122/140/144 (teal/pink/purple).
E. A hypothetical model for how IFT-A might convert between different orientations depending on whether it is associated with vesicles or in anterograde or retrograde trains. In this model, IFT-A binds vesicle (v.m) and ciliary membranes (c.m.) during retrograde transport through its TPR domains. These TPR domains are shielded from the membrane during anterograde transport by binding IFT-B.
