Abstract
Background:
There is a well documented relationship between cardiovascular risk factors and the development of brain injury, which can lead to cognitive dysfunction. Hypertension (HTN) is a condition increasing the risk of silent and symptomatic ischemic brain lesions. Although benefits of hypertension treatment are indisputable, the target blood pressure value where the possibility of tissue damage is most reduced remains under debate.
Method:
Our group performed a cross-sectional (n = 376) and longitudinal (n = 188) study of individuals without dementia or stroke (60% women n = 228, age 68.5 ± 7.4 years; men n = 148, age 70.7 ± 6.9 years). Participants were split into hypertensive (n = 169) and normotensive (n = 207) groups. MR images were obtained on a 3T system. Linear modeling was performed in hypertensive and normotensive cohorts to investigate the relationship between systolic (SBP) and diastolic (DBP) blood pressure, white matter lesion (WML), and brain volumes.
Results:
Participants in the hypertensive cohort showed a quadratic relationship between SBP and WML, with the lowest amounts of WML being measured in participants with readings at approximately 124 mmHg. Additionally, the hypertensive cohort also exhibited a quadratic relationship between DBP and mean hippocampal volume; participants with readings at approximately 77 mmHg showing the largest volumes. Longitudinally, all groups experienced WML growth, despite different BP trajectories, further suggesting that WML expansion may occur despite or because of BP reduction in individuals with compromised vascular system.
Conclusion:
Overall, our study suggests that in the hypertensive group there is a valley of mid-range blood pressures displaying less pathology in the brain.
Keywords: hypertension, magnetic resonance imaging, neuro-imaging, radiology, systolic and diastolic blood pressure, white matter lesions
INTRODUCTION
The impact of cardiovascular disease on the United States’ population cannot be overstated. One out of every three Americans is hypertensive, with 66% of adults aged 60 or older exhibiting above average blood pressure readings [1,2]. It was estimated in 2018 that 11 million individuals in the United States were living with undiagnosed hypertension (HTN), placing them at an increased risk for adverse cardiovascular events that can directly affect the central nervous system as well as cognition [1,2].
High blood pressure (BP) can cause damage and dysfunction to organ systems with perhaps the most significant of such being the brain and the heart [3–5]. Hypertensive patients develop atherosclerotic plaques within the large blood vessels supplying blood flow both into and out of the brain at an accelerated rate, increasing their risk of an ischemic event [6,7]. HTN can cause a remodeling of the brain microvasculature with vessels exhibiting rarefication and narrowing lumen [4,6,8]. Such changes in vessel architecture can have negative effects on cerebral blood flow (CBF) and autoregulation. Reduction in CBF has been shown to cause brain atrophy both in animal models [9] and in clinical population [10], and has been consistently linked with white matter damage [11,12]. White matter lesions (WML) are very common in HTN participants [13–15]. Indeed, several studies have confirmed that two major risk factors for WML development are increased age and high BP, with smoking, high cholesterol, and heart disease also being significant contributors to lesion formation [14,16,17].
Although benefits of HTN treatment are indisputable for major cardiovascular outcome, the target blood pressure for the brain remains under debate. While some argue for aggressive blood pressure reductions [18], others point to possible danger of this approach [19], as marked lowering of BP in longstanding HTN may cause relative hypoperfusion. This suggested to us the existence of an optimum BP range.
A recent metanalysis found a U-shape relationship between SBP and a risk of dementia in participants older than 75, as well as U-shape relationship between SBP and combined dementia and mortality across all age groups starting at 60 [20]. Our group has previously reported a quadratic (inverted U-shape) relationship between CBF and SBP in hypertensive patients, pointing to the optimum lying between 120 and 130 mmHg where CBF was the highest [21]. It was consistently shown that WML volumes are inversely related to global and local indices of CBF [22]. In this observational report, we investigated whether in hypertensive participants WML and atrophy, structural markers of HTN-related brain damage, show relationship with SBP, which is parallel to the one we observed for CBF and SBP: namely is there a quadratic association between them.
METHODS
The data of this study are available from the corresponding author upon request.
Participants recruitment
Study participants were recruited at the NYU School of Medicine, at the former Center for Brain Health. Out of 376 individuals described in this report, 369 (98%) were previously a part of a study assessing the relationship between BP and CBF. Here, we report on participants with quantitative assessment of white matter pathology. Baseline assessment was performed in a larger group of n = 376 participants, and n = 188 of them had follow-up examinations. The reasons for lack of follow-up are described in the Supplement. Participants were enrolled between November 2010 and November 2017. All participants signed Institutional Review Board (ethics committee)-approved consent forms and were free of neocortical stroke, brain tumor, life-long psychotic disorders and dementia at baseline. Information pertaining to recruitment, exclusion criteria, and participant flow is presented in the Supplement.
Clinical assessment
All participants underwent medical, psychiatric, and neurological assessments, blood tests, electrocardiogram (ECG), and magnetic resonance imaging (MRI) evaluation examinations at baseline, and the longitudinal group also at follow-up. Participants exhibiting dementia, based on a physician-administered interview using the Brief Cognitive Rating Scale, rating on the Global Deterioration Scale [23], and Clinical Dementia Rating were excluded [24]. Fasting blood was tested for complete blood count, liver function, as well as metabolic and lipid panel.
Blood pressure was taken once in a sitting position, after 5 min of rest. It was measured on the left upper arm using a manual sphygmomanometer. Body mass index (BMI) was calculated as weight/height (kg/m) [2].
Medication: We recoded the use of antihypertensive medications (angiotensin receptor blockers (ARBs), angiotensin converting enzyme inhibitors (ACE), beta-blockers, diuretics, and calcium channel blockers), statins and glucose lowering drugs.
Diabetes mellitus was defined as fasting glucose level of 126 mg/dl and higher and/or current use of glucose lowering medication [25]. Smoking status was defined as positive if participants was a current smoker or smoked within last 10 years.
Study groups
For consistency with our previous study hypertension was defined as current antihypertensive treatment or BP ≥140/90 mmHg [26]. One hundred and sixty-nine participants were classified as hypertensive; 136 participants were taking antihypertensive medication and 42 were not taking any antihypertensive medication with high BP recorded during their in-office visit. Normotension (NTN) was defined as BP <140/90 mmHg and no antihypertensive treatment.
For descriptive purpose and secondary analyses, we described hypertensive participants as untreated: no antihypertensive treatment and BP ≥140/90 mmHg; controlled: antihypertensive treatment and BP <140/90 mmHg, uncontrolled: antihypertensive treatment and BP ≥140/90 mmHg. We also divided our normotensive group into Stage 1 hypertension based on new guidelines: [27] no treatment and BP between 130–139/80–89 mmHg, and normotensive BP <130/80 mmHg.
MR imaging
All MR imaging was performed on the 3T system (Siemens, Erlangen, Germany). Parameters for Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) images were: repetition time (TR) = 2250 ms, echo time (TE) = 2.7 ms, inversion time (TI) = 900 ms, flip angle (FA) = 8°, slice thickness: 1.0 mm, field of view (FOV) = 200 mm, acquisition matrix = 256 × 192 × 124, reconstructed as 256 × 256 × 124. Axial fluid attenuation inversion recovery (FLAIR) images were acquired with TR/TE/TI 9000/99/2500 ms; FA 130°, slice thickness: 3.3 mm, field of view (FOV) 220 mm, matrix = 256 × 192 reconstructed as thirty 256 × 256 images.
MRI processing
White matter lesions were segmented on FLAIR MRI with freely available image analysis software FireVoxel (https://firevoxel.org/) using a previously validated workflow [28]. FLAIR images were first corrected for signal nonuniformity using the N3 algorithm. A 3D mask M was then created over the entire brain parenchyma, that is, the gray and white matter, with cerebrospinal fluid (CSF) excluded [29]. Voxels at the external surface S of M were then identified. Next was creation of set L, containing voxels in M with signal intensity 2.5 standard deviations above the mean FLAIR signal. Finally, L was filtered to remove (a) cortical voxels, those located within 3 mm of S, (b) small clusters of volume <12 mm3 (presumed to represent image noise), and (c) connected regions having >50% CSF border (presumed to represent the choroid plexus and the septum). The resulting mask represents our estimate of WML. Its volume VWML is presented as percentage of the total brain volume.
Gray matter (GM), white matter (WM) and CSF volumes were estimated using Statistical Parametric Mapping segmentation procedure (SPM, version 8, with ‘New-Segment’ extension) [30]. Total brain volume was the sum of GM and WM volumes. Intracranial volume (ICV) was the sum of all tissue types. Left and right hippocampal volumes were obtained with FreeSurfer version 6.0 [31], and averaged. They are referred to from now on as ‘mean hippocampal volume’. GM, WM and hippocampal volumes are presented as a fraction of the ICV. MRI data were acquired over the span of 9 years a 3T magnet that underwent hardware and software upgrades. We detected the inter-epoch variability in GM measurements. To avoid this fixed bias we z-scored GM values separately by epoch, recentered and rescaled them. Even though no other structural measure was affected, for consistency, we used rescaled values for hippocampal and WM volumes.
Statistics
Categorical variables were compared with χ2 tests. T-test, Mann–Whitney U-test, and analysis of covariance (ANCOVA) were used to compare group means for continuous variables, depending on the data distribution and the need to adjust for age and sex. Normality was tested with Shapiro–Wilk test.
Relationships between BP, WML volume and brain volumes (hippocampal, GM, WM) at baseline were examined with linear regression. Volumes were dependent variables; age, sex, body mass index, smoking, diabetes, systolic blood pressure and diastolic blood pressure were independent predictors. These relationships were tested in participants with and without hypertension separately. We present here F-statistics for the regression models, as well unstandardized (B) and standardized (β) coefficients for individual predictors. Again, we hypothesized that in the HTN group an optimal BP may exist [21], which minimizes WML volume and maximizes brain volumes. Thus, as before, we tested for the existence of the peak, by introducing both the linear BP and quadratic (BP [2]) terms into the regression models.
After a quadratic relationship between the volume and BP was confirmed, the critical BP at which volume reaches its maximum or minimum value was determined as:
To examine whether BP, WML and brain volumes changed over time, we used mixed models for repeated measures (MMRM), where BP or volume was a dependent variable and time, group (HTN vs. NTN), and group × time interaction were predictors. For models with WML or brain volumes age as added as a covariate. Both intercept and slope were treated as random.
To test whether baseline BP predicted change in WML and brain volumes, we created volume rates of change by regressing volume on time for each participant and tested whether baseline SBP and DBP was associated with volume slope. Baseline volumes, BMI, age, sex, smoking and diabetes status were initially included in the models, and retained only when significant. The relationships were tested in the normo- and hypertensive participants separately. For all the analyses, the most parsimonious model was chosen, defined as the model that included only significant or necessary (main effects when interaction was present) terms.
To test whether change in BP (independent variable) was related to change in volume (dependent) we used MMRM. WML or brain volumes were a dependent variable and BP (SBP or DBP), group (HTN vs. NTN), and group × BP interaction were predictors. Both intercept and slope were treated as random. SBP and DBP were predictors in separate models.
We performed supplementary analyses using pulse pressure (PP) as a predictor in all the models.
WML volumes were log transformed. We checked the linear models for violations of models assumptions. For all the analyses, the most parsimonious model was chosen, defined as the model that included only significant or necessary (main effects when interaction was present) terms. Statistical significance was defined as a P-value <0.05. SPSS (version 25, SPSS, Inc., Chicago, Illinois, USA) software was used for all analyses.
RESULTS
Our group of 376 participants consisted of HTN (n = 207) and NTN (n = 169) individuals. The characteristics of the groups are given in Table 1. Two hundred and twenty-eight (60.6%) were women (age, 68.5 ± 7.4 years (mean ± standard deviation); education, 16.7 ± 2.3 years); and 148 (39.4%) men (age, 70.7 ± 6.9 years; education, 17.0 ± 2.4 years). The majority of the participants were Caucasian (86%), 10% African American, 4% other races.
TABLE 1.
Baseline characteristics of the study group (n = 376)
| NTN (n = 207) | HTN (n = 169) | P | |
| Age (years) | 67.9 ± 7.2 | 71.1 ± 6.9 | <0.001 |
| Education (years) | 16.8 ± 2.2 | 16.8 ± 2.5 | 0.99 |
| Sex n (%female) | 138 (67%) | 90 (53%) | 0.008 |
| SBP (mmHg) | 117.3 ± 10.6 | 133.6 ± 17.8 | N/A |
| DBP (mmHg) | 71.1 ± 8.5 | 76.6 ± 11.2 | N/A |
| BMIa (kg/m2) | 25.5 ± 4.3 | 28.1 ± 6.1 | <0.001 |
| Total cholesterolb (mg/dl) | 200.5 ± 32.9 | 187.7 ± 35.2 | <0.001 |
| HDL cholesterolb (mg/dl) | 67.3 ± 18.7 | 60.8 ± 15.6 | 0.001 |
| LDL cholesterolc (mg/dl) | 114.6 ± 29.8 | 106.5 ± 29.5 | 0.01 |
| Triglyceridesb (mg/dl) | 93.1 ± 45.8 | 103.6 ± 47.0 | 0.009 |
| Glucosed (mg/dl) | 80.8 ± 12.7 | 88.1 ± 16.7 | <0.001 |
| Antihypertensive medication n (%) | N/A | 136 (81%) | N/A |
| Controlled HTN n (%) | N/A | 93 (55%) | N/A |
| Uncontrolled HTN n (%) | N/A | 43 (25%) | N/A |
| Untreated HTN n (%) | N/A | 33 (20%) | N/A |
| Statins n (%) | 43 (21%) | 82 (49%) | <0.001 |
| Glucose lowering medications n (%) | 2 (1%) | 14 (8%) | <0.001 |
| Diabetes mellitus n (%) | 2 (1%) | 14 (8%) | <0.001 |
| Smoking n (%) | 12 (6%) | 11 (7%) | 0.76 |
| WML volumee (% whole brain) | 0.59 ± 0. 039 | 0.67 ± 0. 043 | 0.15 |
| Gray matter volumef (% ICV) | 41.4 ± 0.24 | 40.5 ± 0.27 | 0.01 |
| White matter volumef (% ICV) | 29.0 ± 0.15 | 28.8 ± 0.17 | 0.23 |
| Hippocampal volumef (% ICV) | 0.263 ± 0.002 | 0.258 ± 0.002 | 0.04 |
Data is presented as mean ± standard deviation unless otherwise indicated. P-values come from t-test (total cholesterol, LDL) or Mann–Whitney U-test (age, education, BMI, HDL, triglycerides, glucose). For categorical variables, χ2 was used.
n = 371 (NTN = 204, HTN = 167)
n = 369 (NTN = 205, HTN = 164)
n = 363 (NTN = 200, HTN = 163)
n = 371 (NTN = 206, HTN = 165)
Values are presented as mean ± standard errors (SE), P-values from ANCOVA after accounting for age.
Hippocampal volume is a mean of left and right, values are presented as mean ± standard errors (SE), P-values from ANCOVA after accounting for age and gender. Since the residuals from GM model were not normally distributed, the values were reanalyzed using ranked ANCOVA. Results remained the same.
ICV, intracranial volume; HTN, hypertension; NTN, normotensive.
In the longitudinal group of 188 participants, 116 (61.7%) were women (age, 69.6 ± 7.5 years; education, 16.7 ± 2.1 years) and 72 (38.3%) men (age, 71.4 ± 6.1 years; education, 17.2 ± 2.3 years). The mean time of follow-up was 2.3 ± 0.70 years. The longitudinal group did not differ from the participants with only one examination in terms of sex, HTN prevalence, SBP, DBP, BMI, total cholesterol, LDL and HDL cholesterol, triglycerides, education and WML volume. Prevalence of smoking and diabetes, as well as the number of participants with normotension, controlled, uncontrolled and untreated hypertension were also not different between the two groups. However, participants with longitudinal observation were slightly older (age, 70.3 ± 7.0 vs. 68.4 ± 7.4 years, P = 0.005) and had lower glucose levels (glucose, 82.5 ± 16.0 vs. 85.6 ± 14 mg/dl) than the individuals with only one exam. The clinical characteristics, as well as baseline brain and WML volumes of HTN and NTN participants in the longitudinal subgroup are given in Table S1. WML volumes by five baseline study group are shown in Tables S2 and S3.
Baseline analyses
BP and white matter lesion volumes
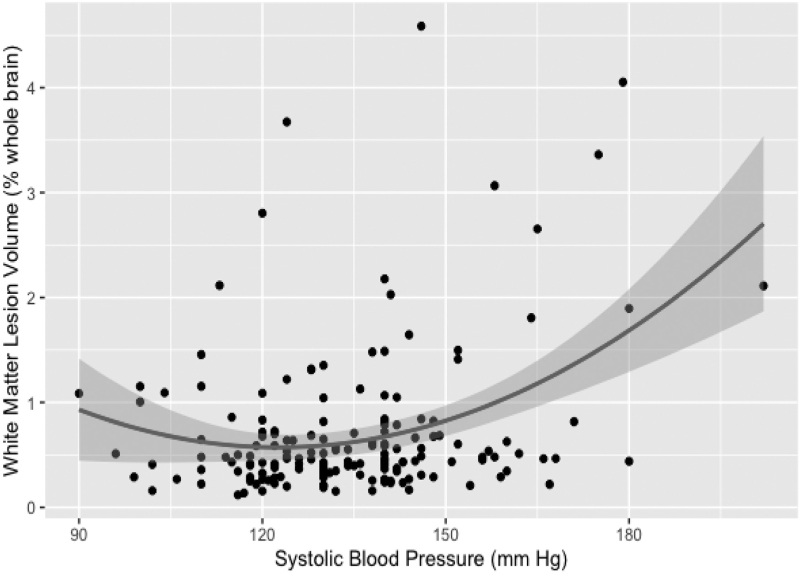
In the HTN group, SBP and quadratic SBP term were predictors of WML volume at baseline (regression model included age, F3,168 = 14.4, P < 0.001) (Table 2, Fig. 1). The results were similar with log-transformed data (F3,168 = 13.1, P < 0.001) (Table 3). The critical SBP value calculated from equation [2] was x = 124 mmHg.
TABLE 2.
Linear regression model predicting WML volume in the HTN group at baseline (raw data)
| Variable | Unstandardized coefficient B | Standardized β | P-value | |
| Main effects | Age | 0.029 | 0.28 | <0.001 |
| SBP | −0.07317 | −1.81 | 0.008 | |
| Quadratic term | SBP × SBP | 0.00030 | 2.10 | 0.002 |
Critical value (Eq. [2]): x = –(−0.07317/(2 × 0.0003)) = 122 mmHg.
HTN, hypertension; WML, white matter lesion.
FIGURE 1.
Quadratic relationship between systolic blood pressure readings and white matter lesion measurements in hypertensive participants.
TABLE 3.
Linear regression model predicting WML volume in the HTN group at baseline (log transformed data: log(WML volume))
| Variable | Unstandardized coefficient B | Standardized β | P-value | |
| Main effects | Age | 0.014 | 0.30 | <0.001 |
| SBP | −0.02741 | −1.57 | 0.023 | |
| Quadratic term | SBP × SBP | 0.00011 | 1.79 | 0.009 |
Critical value (Eq. [2]): x = –(−0.02741/(2 × 0.00011)) = 124.6 mmHg.
HTN, hypertension; WML, white matter lesion.
In the NTN group, neither SBP nor DBP were related to WML.
BP and brain volumes
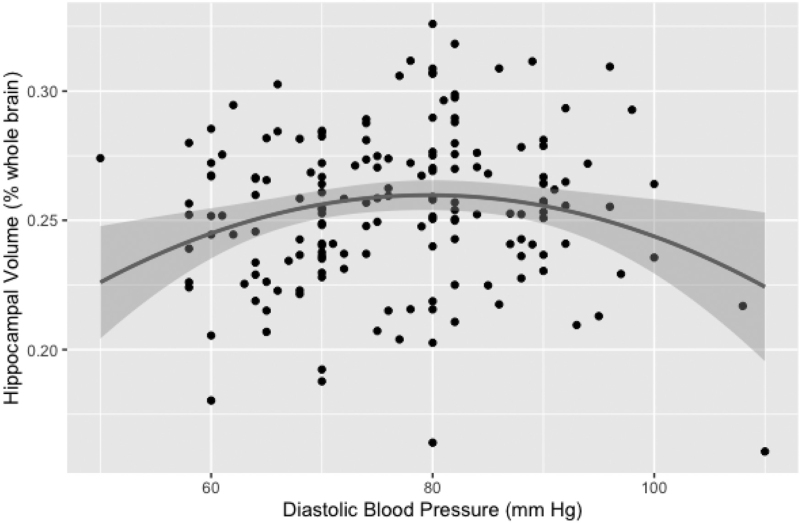
In HTN, the quadratic DBP term was a predictor of the mean hippocampal volume at baseline (the model included age and sex, F4,168 = 11.6, P < 0.001) (Table 4, Fig. 2). The critical DBP value calculated from equation [2] was x = 77 mmHg.
TABLE 4.
Model predicting mean hippocampal volume in the HTN group at baseline
| Variable | Unstandardized coefficient B | Standardized β | P-value | |
| Main effects | Age | −0.002 | −0.37 | <0.001 |
| Sex | 0.011 | 0.18 | 0.011 | |
| DBP | 0.00399 | 1.50 | 0.054 | |
| Quadratic term | DBP × DBP | −0.000026 | −1.55 | 0.046 |
Critical value (Eq. [2]): x = −(0.00399/(2 × (−0.000026))) = 77 mmHg.
HTN, hypertension; WML, white matter lesion.
FIGURE 2.
Relationship between diastolic blood pressure readings and mean hippocampal brain volume measurements in hypertensive participants.
GM was associated with SBP (β = −0.16, P = 0.02) and the model (F4,166 = 14.0, P < 0.001) included BMI, smoking and age.
In NTN, the mean hippocampal volume was inversely related to SBP (β = −0.17, P < 0.008), (regression model included age and sex, F3,206 = 22.4, P < 0.001). GM volume was inversely related to DBP (β = −0.15, P = 0.01), (the model included age and sex, F3,206 = 32.3, P < 0.001).
Longitudinal analyses
Changes in BP and volumes over time
SBP did not change significantly in the HTN or NTN group, but the interaction term was significant (P = 0.01) indicating divergent pattern of SBP dynamics (the estimate = −1.6, P = 0.07 for HTN and 1.4, P = 0.07 for NTN). Similarly, DBP patterns were different (P = 0.03 for interaction term) with a lack of change in the HTN participants (estimate −0.6, P = 0.31) and a significant increase in the NTN group (the estimate = 1.1, P = 0.03). In both groups, WML volumes increased with time, (the estimate = 0.025, P < 0.001) for HTN and (0.028, P < 0.001) for NTN. In both groups, brain volumes decreased with time. An estimate for the fixed effect of time for the mean hippocampal volumes was (−0.0044, P < 0.001) for HTN and (−0.0038, P < 0.001) among NTN participants. For GM, they were (−0.48, P < 0.001) and (−0.45, P < 0.001); and finally, for WM (−0.30, P < 0.001) and (−0.22, P = 0.001), for the HTN and NTN groups, respectively. Group × time interaction terms were not significant for models with brain or WML volumes, indicating that rates of change did not differ between groups. Figure S2 presents BP and WML changes in both groups.
Secondary analyses
Table S4 shows the stability of secondary group definition (untreated, uncontrolled and controlled HTN, Stage 1 HTN, and normotension). Table S5 presents the results from MMRM analyses of WML volumes and BP changes conducted with all five subgroups defined at baseline based on HTN control status. One can appreciate that WML volume increased significantly in untreated and controlled hypertension, as well as in normotensive participants.
Table S6 and Figure S3 shows that participants who at baseline were classified as untreated (n = 19) or uncontrolled hypertension (n = 20) and reclassified at the last visit as good outcome (improved BP control) experience the same increase in WML volumes as participants with bad outcome (BP remained untreated or uncontrolled).
Baseline BP and changes in white matter lesion and brain volumes
In the hypertensive group both baseline SBP (β = 0.33, P = 0.007) and DBP (β = −0.30, P = 0.008) were related to WML changes over time (entire model F4,83 = 10.5, P < 0.001, included also baseline WML volumes and smoking). Baseline BP was not related to changes in hippocampal, GM, or WM volumes.
In normotensive participants baseline BP was not related to change in any examined volumes.
Longitudinal changes in BP and changes in white matter lesion and brain volumes
In the HTN group, neither change in SBP nor DBP were associated with a change in WML, hippocampal, GM or WM volumes (Tables S7 and S8).
In the NTN group, both increase in SBP (an estimate for the fixed effect of SBP = 0.0023, P = 0.01) and DBP (an estimate for the fixed effect of DBP = 0.0035, P = 0.01) over time were related to the increase in WML volume. The significant interaction term indicated that these dynamics were meaningfully different from the HTN group. Moreover, in the normotensive group both increase in SBP (an estimate for the fixed effect of SBP = −0.0002, P = 0.03) and DBP (an estimate for the fixed effect of DBP = −0.0004, P = 0.01) over time were related to reduction in the mean hippocampal volume. However, only for SBP the interaction term was significant, indicating that these changes were different from HTN group.
Results for pulse pressure are presented in the Supplement p. 8–9.
DISCUSSION
Longitudinal observations of brain health in aging have continuously concluded that BP has a significant impact on the nervous system. It is well known that increased cardiovascular burden, as represented by an elevated systolic or diastolic BP, can substantially increase the risk of developing hemorrhagic and ischemic stroke [1,2], white matter lesions and atrophy [14,32], and cognitive dysfunction [33,34].
In our examination of a cohort of normotensive and hypertensive participants, we posit that adequate BP is vital for a healthy brain. We have previously observed that in hypertension, there appears to be a window of mid-range SBP around 125 mmHg which maximizes perfusion [21]. In the present study, we show that hypertensive participants with a similar SBP (124 mmHg) have lower WML volumes that participants with SBP further away from this optimum. This is a novel finding further supporting the notion of an ideal blood pressure range that promotes optimal brain function and minimizes damage. Opposite to our results, the SPRINT-Mind study found that the group where BP was lowered aggressively to less than 120 experienced smaller WML progression over time [18]. However our findings are consistent with a report showing that in hypertensive participants low BP was related to higher PWML volume [35] and an earlier study where an increase in WML volume over time was most pronounced in participants with the highest BP who also had the greatest BP fluctuations [36].
We also find that among hypertensive participants there was a quadratic relationship between the mean hippocampal volume at baseline and DBP, such as both the participants with low and high DBP had lower volume. This observation, although interesting, is not completely novel. Others have previously described a parallel U-shape association between brain atrophy and DBP, where both low and high concurrent DBP were related to greater cortical volume reduction [37]. The SPRINT MIND study showed that intensive BP treatment group experienced significantly greater hippocampal volume reduction [38]. It further confirms the hypothesis indicating the existence of a BP optimum, and reconciles reports showing that both high [39] and low BP [40] are related to lower hippocampal volumes.
Among normotensive participants relationships between BP and brain volumes were linear such as both higher SBP and DBP were related to lower brain volumes. It is congruent with previous reports and meta-analyses showing both BP components associated with volumes [3]. Notably, no quadratic relationship was observed in this group, further strengthening our initial assumption that optimum exists in the group with preexisting impairment of the vascular system.
Reduction in the volume of specific brain regions over time is another parameter indicative of abnormal aging and is often seen reported in HTN [3,41]. Our HTN group had more global and hippocampal atrophy at baseline (Table 1) than their normotensive counterparts. However, after adjustment for age, the WML volume did not differ between HTN and NTN groups. It is possible that substantial percentage (55%) of participants with controlled BP attenuated the differences. Comparisons across five groups showed that participants with untreated and uncontrolled hypertension tended to have higher WML volumes (Table S2).
In our hypertensive participants, higher baseline SBP and lower DBP were both independently related to the growth of WML over time. While the first finding is not surprising, we offer two possible explanations for the latter. First, as the mean arterial pressure, the major driver of perfusion pressure, depends mostly on DBP, the low DBP in HTN may indicate the propensity to hypoperfusion. Second, this association would be consistent with widening of pulse pressure, indicative of arterial stiffness. Indeed, an earlier analysis of the Lothian Birth Cohort 1936 showed that the sequence of DBP reduction, PP increase and rising internal carotid artery pulsatility index leads to WM damage [42]. Our supplemental analyses also confirm that baseline PP was related to WML growth.
It is worth noting that both normo and hypertensive groups experienced reductions in brain volumes and WML growth over time. This was present despite dissimilar trajectories of blood pressure: increases in the NTN and apparent lack of change in the HTN participants. A more fine-grained picture emerged from analyzing five groups (Table S5): WML lesions grew despite BP reductions in the untreated group and similar trend was observed among individuals with uncontrolled HTN, yet lesion volume increased concomitantly to BP increases in participants with controlled HTN and among normotensive participants. Even more interestingly we show that WML growth was similar in participants with baseline untreated/ uncontrolled HTN who at the last visit had disparate outcomes: improved or unchanged BP control (Table S6). It stands in opposition to the results of SPRINT-MIND trial where smaller increase in WML volume was observed in participants with more aggressive BP lowering [18]. We believe that the main reason for this discrepancy is that our groups were much smaller and follow-up time shorter than in SPRINT study. In addition, the difference in WML volume change between SPRINT groups amounted only to 0.5 cm3.
However, since BP load over time is unknown, it is also likely that in both groups BP was not controlled for a long time, and one group showed improvement only on the last measurement. Both groups could have also experienced similar, strong longitudinal BP fluctuation, which increase the probability of WML progression [36]. Then again, our results confirm that baseline WML volumes are good predictor of lesion progression, consistent with previous reports [43], and suggest that once the pathological process is set in motion, there is limited room for its modification. Such view has important implications for patient management, further strengthening the notion that early intervention (close monitoring of BP in normotensives and early treatment) would be most successful. Finally, the findings are also consistent with our hypothesis that in the setting of impaired cerebral flow regulation in participants with more severe form of HTN BP reductions are harmful.
Not surprisingly, in the light of the above considerations, only in the group of normotensive individuals, longitudinal increases of SBP and DBP were related to lesion growth and reduction is mean hippocampal volume. It is to be expected, as rising BP would contribute to brain deterioration. This finding is also in agreement with a previous report of community residing participants over the age of 75, where the 2-year change in ambulatory SBP was associated with the 2-year WML increase [44].
Our study suffered from a few limitations. First, only one measurement of blood pressure was taken at the time, instead of the average of three consecutive ones. It could have resulted in misclassification of cases. This was especially true for ‘untreated’: only 63% of participants defined at baseline as untreated were still classified as hypertensive at follow-up. However, high baseline WML volume in this group (Table S2) speaks against the possibility of misclassification. Almost everybody in the controlled and uncontrolled HTN groups remained hypertensive at follow-up, and 88% (91/104) of participants normotensive (<140/90) at baseline were normotensive at follow-up. Although, the method for BP measurement was not optimal it matched closely a common real-life practice. Second, the interval between assessment was short, and since WML progression is slow it might have precluded us from observing significant differences between groups. Analysis of participants WML volume did not include a differentiation of periventricular lesions from deep lesions. It is possible that the effects of hypertension of WML development could vary by lesion location. We did not perform manual segmentation of silent infractions. It may have artificially inflated lesion volumes, as some infarcts might have been erroneously classified as WML. Wang et al.[45] previously reported that including stroke lesions could bias WML volume estimation by as much as 20%. Although, in our case the error would have been likely smaller, since their study included patients recruited soon after presenting with stroke symptoms, while we excluded participants with overt infarcts, the misclassification cannot be excluded.
Only a half of participants had follow-up. Although participants studied only once and those with additional examinations did not differ in most of the characteristics, it still could have caused a bias. Another limitation was that our group was predominantly Caucasian, with high level of educational achievement thus generalizability to a more diverse cohort is uncertain. We did not have data on the duration of hypertension, which can modify the relationships we examined. Normotensive individuals were slightly (on average 3 years) but statistically significantly younger than hypertensive participants. It is plausible that had they been older, we might have seen stronger associations between BP and WML. A recent meta-analysis showed that the optimal blood pressure value changes depending on age [20]. We did not have a group large enough to address whether this is the case also for white matter lesions. Finally, since our study was observational, results cannot carry the same weight a these derived from clinical trial, and causal relations cannot be inferred.
In conclusion, we find that among hypertensive participants there was a quadratic relationship between BP and WML as well as mean hippocampal volume. The WML burden was the smallest when SBP was close to 124 mmHg. The mean hippocampal volume was the highest when DBP was in high 70-mmHg range. Such phenomena were not observed in the normotensive group, suggesting the existence of a BP optimum in hypertension. The current report extends our earlier findings showing that cerebral perfusion is maximized when systolic BP is in 120–130 mmHg range. Longitudinally, all groups experienced lesion growth, despite different BP trajectories, further suggesting the possibility that WML expansion may occur despite or because of BP reduction in individuals with compromised vascular system. Considering growing evidence showing nonlinear association between blood pressure an outcomes further clinical trials are needed.
ACKNOWLEDGEMENTS
A special thanks to Ke Xi for her help with the statistical modeling as well as assistance in the production of regression plots for this manuscript.
Sources of funding: Study funding comes from NIH grants HL111724, NS104364, AG022374, AG12101, AG08051, and Alzheimer's Association NIRG-09-132490.
Disclosures: None
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: ACE, angiotensin converting enzyme inhibitors; ANCOVa, analysis of covariance; ARBs, angiotensin receptor inhibitors; BP, blood pressure; CBF, cerebrospinal fluid; CSF, cerebral flood flow; ECG, electrocardiogram; FA, flip angle; FLAIR, fluid attenuation inversion recovery; FOV, field of view; GM, gray matter; HTN, hypertension; ICV, intracranial volume; MMRM, mixed model for repeated measures; MPRAGE, magnetization prepared rapid acquisition gradient echo; MRI, magnetic resonance imaging; NTN, normotensive; NYU, New York University; SPM, statistical parametric mapping; TE, echo time; TI, inversion time; TR, repetition time; WM, white matter; WML, white matter lesion
Supplemental digital content is available for this article.
REFERENCES
- 1.Ritchey MD, Gillespie C, Wozniak G, Shay CM, Thompson-Paul AM, Loustalot F, Hong Y. Potential need for expanded pharmacologic treatment and lifestyle modification services under the 2017 ACC/AHA Hypertension Guideline. J Clin Hypertens (Greenwich) 2018; 20:1377–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 3.Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens 2013; 31:1502–1516. [DOI] [PubMed] [Google Scholar]
- 4.Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, et al. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J Am Soc Hypertens 2013; 7:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popiołek L, Siga O, Dzieża-Grudnik A, Popiołek I, Moląg M, Królczyk J, et al. Personality traits and hypertension-mediated organ damage. Psychiatr Pol 2019; 53:1003–1020. [DOI] [PubMed] [Google Scholar]
- 6.Veglio F, Paglieri C, Rabbia F, Bisbocci D, Bergui M, Cerrato P. Hypertension and cerebrovascular damage. Atherosclerosis 2009; 205:331–341. [DOI] [PubMed] [Google Scholar]
- 7.Turin TC, Okamura T, Afzal AR, Rumana N, Watanabe M, Higashiyama A, et al. Hypertension and lifetime risk of stroke. J Hypertens 2016; 34:116–122. [DOI] [PubMed] [Google Scholar]
- 8.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev 2009; 8:61–70. [DOI] [PubMed] [Google Scholar]
- 9.Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci (Lond) 2017; 131:2451–2468. [DOI] [PubMed] [Google Scholar]
- 10.Alosco ML, Gunstad J, Xu X, Clark US, Labbe DR, Riskin-Jones HH, et al. The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J Am Soc Hypertens 2014; 8:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appelman APA, van der Graaf Y, Vincken KL, Tiehuis AM, Witkamp TD, Mali WPTM, et al. Total cerebral blood flow, white matter lesions and brain atrophy: the SMART-MR study. J Cereb Blood Flow Metab 2008; 28:633–639. [DOI] [PubMed] [Google Scholar]
- 12.Bastos-Leite AJ, Kuijer JPA, Rombouts SARB, Sanz-Arigita E, van Straaten EC, Gouw AA, et al. Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. AJNR Am J Neuroradiol 2008; 29:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging 2012; 33:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jochemsen HM, Muller M, Visseren FL, Scheltens P, Vincken KL, Mali WP, et al. Blood pressure and progression of brain atrophy: the SMART-MR study. JAMA Neurol 2013; 70:1046–1053. [DOI] [PubMed] [Google Scholar]
- 15.Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, Schmidt R. CT and MRI rating of white matter lesions. Cerebrovasc Dis 2002; 13: (Suppl 2): 31–36. [DOI] [PubMed] [Google Scholar]
- 16.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006; 37:1391–1398. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt R, Schmidt H, Haybaeck J, Loitfelder M, Weis S, Cavalieri M, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol 2011; 122:171–185. [DOI] [PubMed] [Google Scholar]
- 18.Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019; 322:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J-curve: pro side of the argument. Hypertension 2014; 63:29–36. [DOI] [PubMed] [Google Scholar]
- 20.van Dalen JW, Brayne C, Crane PK, Fratiglioni L, Larson EB, Lobo A, et al. Association of systolic blood pressure with dementia risk and the role of age, U-shaped associations, and mortality. JAMA Intern Med 2022; 182:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glodzik L, Rusinek H, Tsui W, Pirraglia E, Kim HJ, Deshpande A, et al. Different relationship between systolic blood pressure and cerebral perfusion in subjects with and without hypertension. Hypertension 2019; 73:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. Front Neurol 2021; 12:647848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisberg B, Sclan SG, Franssen EH, de Leon MJ, Kluger A, Torossian CL, et al. Clinical stages of normal aging and Alzheimer's disease: the GDS staging system. Neurosci Res Commun 1993; 13: (Suppl. 1): 551–554. [Google Scholar]
- 24.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 25.2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 26.Chobonian A, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 27.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 28.Glodzik L, Rusinek H, Li J, Zhou C, Tsui W, Mosconi L, et al. Reduced retention of Pittsburgh compound B in white matter lesions. Eur J Nucl Med Mol Imaging 2015; 42:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikheev A, Nevsky G, Govindan S, Grossman R, Rusinek H. Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J Magn Reson Imaging 2008; 27:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage 2000; 11:805–821. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355. [DOI] [PubMed] [Google Scholar]
- 32.van der Veen PH, Geerlings MI, Visseren FL, Nathoe HM, Mali WP, van der Graaf Y, et al. Hypertensive target organ damage and longitudinal changes in brain structure and function: the second manifestations of arterial disease-magnetic resonance study. Hypertension 2015; 66:1152–1158. [DOI] [PubMed] [Google Scholar]
- 33.Mossello E, Pieraccioli M, Nesti N, Bulgaresi M, Lorenzi C, Caleri V, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med 2015; 175:578–585. [DOI] [PubMed] [Google Scholar]
- 34.van Middelaar T, van Vught LA, van Gool WA, Simons EMF, van den Born BH, Moll van Charante EP, Richard E. Blood pressure-lowering interventions to prevent dementia: a systematic review and meta-analysis. J Hypertens 2018; 36:1780–1787. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Lee S, Suh SW, Bae JB, Han JH, Byun S, et al. Association of low blood pressure with white matter hyperintensities in elderly individuals with controlled hypertension. J Stroke 2020; 22:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010; 67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heijer T, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, Breteler MM. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging 2003; 24:307–313. [DOI] [PubMed] [Google Scholar]
- 38.Nasrallah IM, Gaussoin SA, Pomponio R, Dolui S, Erus G, Wright CB, et al. Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of alzheimer disease: secondary analysis of the SPRINT MIND Randomized Trial. JAMA Neurol 2021; 78:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005; 64:263–267. [DOI] [PubMed] [Google Scholar]
- 40.Foster-Dingley JC, van der Grond J, Moonen JE, van den Berg-Huijsmans AA, de Ruijter W, van Buchem MA, et al. Lower blood pressure is associated with smaller subcortical brain volumes in older persons. Am J Hypertens 2015; 28:1127–1133. [DOI] [PubMed] [Google Scholar]
- 41.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke 1999; 30:529–536. [DOI] [PubMed] [Google Scholar]
- 42.Aribisala BS, Morris Z, Eadie E, Thomas A, Gow A, Valdés Hernández MC, et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension 2014; 63:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown R, Low A, Markus HS. Rate of, and risk factors for, white matter hyperintensity growth: a systematic review and meta-analysis with implications for clinical trial design. J Neurol Neurosurg Psychiatry 2021; 92:1271–1277. [DOI] [PubMed] [Google Scholar]
- 44.Wolfson L, Wakefield DB, Moscufo N, Kaplan RF, Hall CB, Schmidt JA, et al. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol A 2013; 68:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Valdés Hernández MC, Doubal F, Chappell FM, Wardlaw JM. How much do focal infarcts distort white matter lesions and global cerebral atrophy measures? Cerebrovasc Dis 2012; 34:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.