Objective:
Evaluating the vascular function in HIV-infected compared with HIV uninfected with assessment of body composition, inflammation, and gut integrity markers.
Design:
A noninvasive test that measures the endothelial function.
Methods:
We included participants at least 18 years old, with peripheral arterial tonometry testing (EndoPAT2000) between 2014 and 2022. Persons with HIV (PWH) had documented infection, a stable ART regimen, and a viral load less than 400 copies/ml. We measured the vessel's function with the reactive hyperemia index (RHI) (normal >1.67) and Augmentation Index. Lower Augmentation Index reflect better arterial elasticity. We assessed markers of systemic inflammation, immune activation, and gut integrity. We used linear mixed models to estimate endothelial dysfunction with a significant P value less than 0.05.
Results:
Overall, 511 participants (296 HIV-infected; 215 HIV-uninfected controls) were included. Estimated RHI among PWH was 13% lower (P = 0.01) compared with persons without HIV. In nonwhite race, the estimated RHI was 9% lower (P = 0.001) than white race. For every 1% increase in BMI, we would expect RHI to increase 0.17% (P = 0.01). At the time of EndoPAT, the estimated RHI was 8% lower (P = 0.04) among protease inhibitor users compared with PWH who were not taking protease inhibitors. The estimated odds of abnormal RHI ≤1.67) is 1.56 times greater [95% confidence interval (CI) 1.05–2.31] in nonwhite race compared with white race, independent of HIV status [OR = 1.4 (95% CI 0.94–2.13)]. There was not enough evidence to suggest that inflammation, gut, or monocyte markers, current or nadir CD4+ cell count, or duration of HIV were associated with endothelial dysfunction.
Conclusion:
HIV, nonwhite race, and protease inhibitor use are independently associated with endothelial dysfunction.
Keywords: antiretroviral agents, cardiovascular diseases, endothelium, HIV, inflammation, racial groups
Introduction
The use of antiretroviral therapy (ART) increased the life expectancy for persons with HIV (PWH) [1]. However, this progress led to an increase in the prevalence of metabolic disease and cardiovascular disease (CVD) risk factors in PLWH compared with persons without HIV [2]. A longitudinal study from 1990 to 2015 concluded that HIV infection doubles the risk of developing cardiovascular diseases [3]. This increased risk could be attributed to an increase in atherosclerosis, as demonstrated in a meta-analysis [4]. To detect early signs of CVD in PWH, the scientific community developed and studied multiple predictive tools over the years [5].
In order to study early atherosclerosis and endothelial function in PWH, the latter being one of the earliest predictors of atherosclerosis [6], scientists used tests that detect changes in the structural and functional vascular-related markers like carotid intima–media thickness (IMT), flow-mediated vasodilation (FMD), and pulse wave velocity (PWV) [5]. FMD is the most studied method used for endothelial function assessment [7] but requires specially trained personnel and is highly operator-dependent with high variability, and therefore, could be poorly reproducible [8]. It was demonstrated that FMD results correlated with Peripheral Arterial Tonometry by EndoPAT, an FDA-cleared testing technique [9].
EndoPAT is a novel testing technique that is gaining traction [10] because of its accessibility and reliability after being tested in repeated measurements [11]. By assessing the digital blood flow-mediated dilation, the EndoPAT machine calculates the reactive hyperemia index (RHI) with a normal value being greater than 1.67 and its log transformation lnRHI with a matched cut of greater than 0.51. The machine also generates scores estimating the arterial stiffness called Augmentation Index and an adjusted Augmentation Index at a heart rate of 75 beats per minute (AI@75) to further normalize the results. The Augmentation Index score is inversely correlated to vessel elasticity and is provided relative to gender-matched, nonselective populations. Using PAT, a higher BMI was associated with endothelial dysfunction [12]; however, the influence of race and ethnicity on endothelial dysfunction is still under investigation [13].
Only one study, from our group, assessed vascular function in PWH compared with persons without HIV using the EndoPAT; it included 119 children and young adults and concluded a statistically significant worse vascular function in the prenatally HIV-infected subgroup [14]. Three other small studies used EndoPAT in PWH. One was to evaluate the effect of short-term aerobic training in seven participants [15] and another compared the EndoPAT to the myocardial perfusion reserve by 82-rubidium PET/CT in 48 PWH [16]. A third small study was conducted in Africa on 33 undernourished, HIV-infected adults starting ART [17]. Thus the need for a large analysis to evaluate the use of EndoPAT in PWH.
Our project aims to: compare the endothelial function in virologically suppressed adults living with HIV compared with uninfected controls; study the independent risk factors associated with endoPAT in HIV; assess the relationship between endothelial function and markers of systemic inflammation, immune activation, and gut integrity.
Method
Study design/population
This is a cross-sectional analysis evaluating endothelial function in adult PWH and persons without HIV, with concomitant assessment of HIV variables, body composition, and inflammatory markers. Data were collected at the Metabolic Research Center from participants who were evaluated and screened for potential entry into HIV metabolic studies, including endoPAT measurements, between 2014 and 2022. All studies were approved by the Institutional Review Board (IRB) of the University Hospitals Cleveland Medical Center in Cleveland, Ohio. We included data from participants 18 years or older, with an Endopat test at the time of the entry visit. In the PWH group, included participants had documented HIV infection, a stable ART regimen, and an HIV-1 RNA level of less than 400 copies/ml. Participants were instructed to fast for at least 12 h and refrain from caffeine, tobacco, exercise, vitamins, or medications that might affect the vascular tone for at least 4 h before undergoing EndoPAT testing and blood draws.
Written informed consent was obtained from all the participants in their respective IRB-approved studies, which included data collection, blood draw, and endoPAT measurement.
Study assessments
Medical history, demographics, and vitals
Participants were interviewed by trained healthcare professionals using standardized questionnaires about demographics, personal, and family medical history, including cardiovascular disease and substance use. PWH were interviewed and HIV-related data was confirmed from medical records, including ART regimen (type and duration), CD4+ T-cell counts, CD4+ T-cell nadir, and viral load. Vital signs including height, weight, and blood pressure were obtained by the staff conducting the visit.
Inflammation, monocyte activation, and gut integrity markers
Plasma from collected blood was stored at −80 °C and batched until processing without a prior thaw. Markers of systemic inflammation, monocyte activation, endothelial activation, and gut integrity were measured using ELISA. The markers of interest and their respective manufacturer were the following: soluble tumor necrosis factor receptors I and II (sTNFR-I and sTNFR-II), high-sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6) (R&D Systems, Minneapolis, Minnesota, USA), D-dimer (Diagnostica Stago, Parsippany, New Jersey, USA), oxidized low-density lipoprotein assays (Uppsala, Mercodia, Sweden), and the monocyte activation markers soluble CD14 and CD163 (R&D Systems). The endothelial activation marker soluble vascular cell adhesion molecule (VCAM) was measured by ELISA (R&D Systems). To assess gut-barrier integrity, we measured a marker of microbial translocation lipopolysaccharide-binding protein (LBP; Hycult Biotech Inc. Pennsylvania, USA) and a marker of acute intestinal injury [intestinal fatty acid-binding protein (IFAB) (R&D Systems)].
Body composition measures
Real-time measurements of lipid profiles, glucose, and insulin levels were obtained from blood sampled during the visit and run at the CLIA-certified local laboratory. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated [18].
EndoPAT measures
Under normal conditions, the endothelial cells trigger vasodilatation through the production of nitric oxide (NO) via the activity of endothelial nitric oxide synthase [19]. In the present study, indirect evaluation of this endothelial vasodilator function was performed noninvasively using postocclusive reactive hyperemia peripheral arterial tonometry (RH-PAT) (EndoPAT2000 device; Itamar Medical Ltd.; Caesarea, Israel) [20]. These endothelium-mediated changes in peripheral arterial tone were recorded using disposable plethysmographic probes on the finger of each hand. The finger probe imparts a uniform pressure field and measures pulsatile volume changes, by means of signals [21]. These signals are filtered, amplified, and stored for further analysis [9]. With the patient in a seated position and with both hands at the same level; hyperemia was induced by occluding the brachial artery (of the nondominant arm) using a blood pressure cuff. In parallel with that, the probes were placed on the same finger on both hands and continuous recording of blood volume responses from both hands was initiated. After a period of stabilization, the blood pressure cuff on the study arm was inflated to 60 mmHg above systolic pressure or 200 mmHg (whichever was higher) for 5 min. Then, the cuff was deflated to induce reactive hyperemia and assess PAT [22]. A RHI was generated from the change in the pulse wave amplitude (PWA) relative to baseline in the occluded arm and was corrected for corresponding changes in PWA relative to baseline in the contralateral, nonoccluded arm in order to minimize the influence of nonendothelial-dependent systemic effects [23–25]. A normal index is greater than 1.67 and an abnormal is 1.67 or less. The augmentation index is calculated from PAT pulses recorded at the baseline period and the result is further normalized to the heart rate of 75 beats per minute (AI 75). Lower augmentation index values reflect better arterial elasticity.
Statistical analysis
Participant characteristics were described using mean ± standard deviation (SD) or median (M) and interquartile range (IQR) for continuous variables and frequency (n) and percentage (%) for categorical variables. Differences between groups were computed using the antilog of either independent t test (Mann–Whitney U) or one-way ANOVA (Kruskal–Wallis) for continuous variables and chi-squared or Fisher's exact for categorical variables (Table 1). We used linear mixed models with random intercept to estimate measures of arterial stiffness conditional on demographics, family history of MI and HTN, risk factors for PWH and cardiovascular disease, inflammation, gut, and monocyte activation markers, and antiretroviral treatment at the time of EndoPAT (Table 2). Generalized linear mixed models with binomial distribution were used to estimate the likelihood of endothelial dysfunction (Table 3 and Fig. 1). All two-way interactions were assessed and log transformations were used to stabilize error variance. The inverse link function was used for continuous covariates to convert estimates to the data scale and interpreted as probability. When both the independent and dependent variables were log transformed, a one-unit change was interpreted as 1% percentage change. All analyses were conducted using SAS 9.4 (SAS Inc., Cary, North Carolina, USA) and P values less than alpha less than 0.05 were considered statistically significant.
Table 1.
Characteristics of participants by HIV status.
| HIV+ (n = 296) | HIV- (n = 215) | ||
| n (%) or median (IQR)/mean ± SD | P value | ||
| Age (years) | 48.48 (34.56–55.34) | 38.36 (29.20–50.45) | <0.0001 |
| Female sex | 74 (14.48) | 86 (16.63) | 0.0002 |
| Nonwhite racea | 197 (38.55) | 62 (11.99) | <0.0001 |
| BMI (kg/m2) | 26.4 (23.47–32.02) | 26.68 (23.09–30.58) | 0.71 |
| Current smoker | 157 (30.78) | 134 (25.97) | 0.02 |
| ARV duration (months) | 119.53 ± 80.87 | – | – |
| HIV duration (months) | 167.11 ± 104.91 | – | – |
| Family history of MI | 79 (15.46) | 53 (10.25) | 0.83 |
| Current CD4+ cell count | 711 (525–911) | – | – |
| Nadir CD4+ cell count | 222 (83.5–334) | – | – |
| HOMA-IR | 2.37 (1.25–3.75) | 1.98 (1.33–2.96) | 0.02 |
| Cholesterol (mg/dl) | 169 (143–192) | 171.00 (148.00–198.00) | 0.19 |
| LDL (mg/dl) | 90 (71–113) | 98.00 (78.00–121.00) | 0.01 |
| HDL (mg/dl) | 47 (39.9–58) | 49.00 (42.00–58.20) | 0.12 |
| Triglycerides (mg/dl) | 104 (76–162) | 95.00 (68.00–139.00) | 0.02 |
| Ox LDLb | 45.24 (35.29–58.47) | 43.58 (33.84–54.16) | 0.07 |
| Current NRTI | 268 (90.54) | – | – |
| Current NNRTI | 86 (29.05) | – | – |
| Current PI | 82 (27.70) | – | – |
| IL-6 (pg/ml) | 2.3 (1.39–3.53) | 2.33 (1.26–4.15) | 0.84 |
| VCAM (ng/ml) | 777.23 (640.15–936.55) | 768.11 (602.07–931.38) | 0.15 |
| hs-CRP (ng/ml) | 2233.39 (932.45–6062.38) | 2495.51 (915.95–5477.27) | 0.47 |
| D-dimer (ng/ml) | 329.63 (223.16–541.74) | 379.40 (234.74–629.09) | 0.41 |
| IFAB (pg/ml) | 2049.47 (1351.17–3527.09) | 1573.71 (1094.64–2372.43) | <0.0001 |
| LBP (ng/ml)b | 17.02 (11.92–25.47) | 17.73 (13.26–24.83) | 0.63 |
| sCD14 (ng/ml) | 1691.42 (1322.9–2138.64) | 1653.35 (1374.82–2036.00) | 0.47 |
| sCD163 (ng/ml) | 673.84 (480.37–1037.96) | 657.37 (459.85–999.93) | 0.29 |
ARV, antiretroviral; BMI, body mass index; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance; IFAB, intestinal fatty acid binding protein; IL-6, interleukin 6; IQR, interquartile range; LBP, lipopolysaccharide-binding protein; LDL, low-density lipoprotein; MI, myocardial infarction; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; Ox LDL, oxidized low-density lipoprotein; PI, protease inhibitor; sCD14; soluble CD14; sCD163, soluble SD163; SD, standard deviation; VCAM, vascular cell adhesion molecule.
Includes African American, Asian, Hispanic, and others.
Per 1000.
Table 2.
Independent associations with reactive hyperemia index.
| Estimate | P value | |
| HIV positive vs. HIV negative | − 0.13 | 0.01 |
| Age | 0.04 | 0.31 |
| Female vs. male | 0.0001 | 0.97 |
| Non-white vs. white | − 0.09 | 0.001 |
| BMI | 0.17 | 0.01 |
| Current smoker (yes vs. no) | 0.07 | 0.03 |
| ARV duration | − 0.0001 | 0.63 |
| HIV duration | 0.0002 | 0.54 |
| Family history of MI (yes vs. no) | 0.07 | 0.03 |
| Current CD4+ cell count | − 0.02 | 0.31 |
| Nadir CD4+ cell count | 0.01 | 0.82 |
| Current NRTI | 0.06 | 0.31 |
| Current NNRTI | 0.02 | 0.69 |
| Current PI | − 0.08 | 0.04 |
| IL-6 | 0.02 | 0.24 |
| VCAM | 0.1 | 0.01 |
| hs-CRP | 0.02 | 0.08 |
| D dimer | 0.02 | 0.23 |
| IFAB | − 0.04 | 0.09 |
| LBP | − 0.01 | 0.72 |
| sCD14 | 0.02 | 0.71 |
| sCD163 | 0.05 | 0.06 |
Bold font indicates statistical significance (P < 0.05). ARV, antiretroviral; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; IFAB, intestinal fatty acid binding protein; IL-6, interleukin 6; IQR, interquartile range; LBP, lipopolysaccharide-binding protein; MI, myocardial infarction; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitor.
Table 3.
Among HIV-positive, estimated odds of endothelial dysfunction.
| UOR and 95% CIs | P value | |
| Race | ||
| Nonwhite | 1.92 (1.17–3.18) | 0.01 |
| White | Reference | |
| Age | 0.52 (0.25–1.06) | 0.07 |
| Gender | ||
| Female | 0.8 (0.47–1.36) | 0.41 |
| Male | Reference | |
| BMI | 0.45 (0.16–1.23) | 0.12 |
| Current smoker | ||
| Yes | 1.09 (0.68–1.73) | 0.73 |
| No | Reference | |
| Family history of MI | ||
| Yes | 0.65 (0.39–1.1) | 0.11 |
| No | Reference | |
| Current CD4+ cell count | 1.26 (0.93–1.81) | 0.15 |
| Current PI | ||
| Yes | 1.88 (1.12–3.18) | 0.02 |
| No | Reference | |
Bold font indicates statistical significance (P < 0.05). BMI, body mass index; CI, confidence interval; MI, myocardial infection; PI, protease inhibitor; UOR, unadjusted odds ratio.
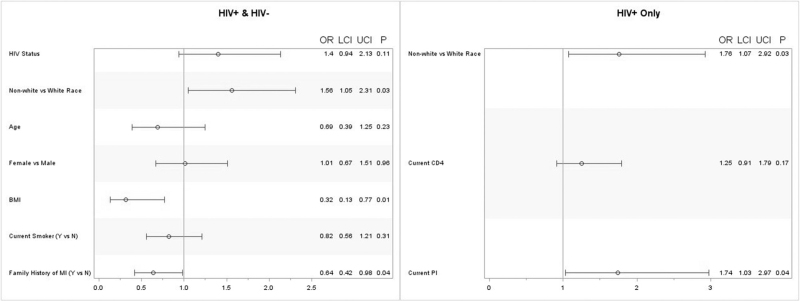
Fig. 1.
Estimated odds of endothelial dysfunction (RHI ≤ 1.67).
Adjusted odds ratio, 95% confidence intervals (CI) and P-value. BMI, body mass index; MI, myocardial infarction; PI, protease inhibitor.
Results
Characteristics
A total of 511 participants (296 HIV+ and 215 HIV− controls) were included in this study. Among the 296 PWH, 43.44% were men, 38.55% were nonwhite, and the median age was 48.48 years (IQR: 34.56–55.34). The median CD4+ cell count was 711.0 (IQR: 525.0–911.0), median nadir CD4+ cell count was 222.0 (IQR: 83.5–334.0), and median BMI was 26.4 kg/m2 (IQR: 23.47–32.02). There was a large proportion of cumulative ART use: nucleoside reverse transcriptase inhibitor (NRTI) (94.93%), protease inhibitor (83.45%), and non-nucleoside reverse transcriptase inhibitor (NNRTI) (84.12%). Persons without HIV were younger in age with a larger proportion of female sex and fewer nonwhite race (Table 1).
Among PWH, the median RHI was 1.67 (IQR: 1.42–2.06), median augmentation index at 75 bpm (AI@75) was 4.0 (IQR: −7.0 to 13.0), and 51.01% (n = 151) had RHI 1.67 or less. In persons without HIV, the median RHI was higher [1.77 (IQR: 1.47–2.25); P = 0.01], median AI@75 was lower [1.0 (IQR: −10 to 15.00); P = 0.14], and a smaller proportion (40.47%; P = 0.02) had abnormal RHI 1.67 or less.
Within PWH, differences in both RHI [Mnonwhite =1.61 (IQR: 1.39–1.99) vs. Mwhite =1.81 (IQR: 1.48–2.14); P = 0.004] and the proportion of abnormal RHI 1.67 or less [Mnonwhite =55.84% vs. Mwhite = 41.41%; P = 0.02] between nonwhite race (n = 197) and white race (n = 99) were observed. There was not enough evidence to suggest any differences in AI@75 [Mnonwhite =3.0 (IQR: −9.0 to 13.0) vs. Mwhite = 5.0 (IQR: −4.0 to 14.0); P = 0.06].
Independent associations with reactive hyperemia index
The estimated RHI among PWH was 13% lower (P = 0.01) compared with persons without HIV. In nonwhite race, the estimated RHI was 9% lower (P = 0.001) than white race. For every 1% increase in BMI, we would expect RHI to increase 0.17% (P = 0.01). At the time of EndoPAT, the estimated RHI was 8% lower (P = 0.04) among protease inhibitors antiretroviral therapy use compared with PWH who were taking NRTI or NNRTIs. There was not enough evidence to suggest that HIV disease factors (current or nadir CD4+ cell count, HIV-1 RNA levels, or ART history) influenced any expected increase or decrease in RHI. These differences were not attenuated in adjusted models (Table 2).
Endothelial dysfunction: relationship to race, inflammation, body composition, and antiretroviral therapy
Overall, the estimated odds of having endothelial dysfunction (RHI ≤ 1.67) is 1.56 times greater (95% CI: 1.05–2.31) in nonwhite race compared with white race, independent of HIV status [OR = 1.4 (95% CI: 0.94–2.13)]. For every one-unit increase in BMI, the odds of endothelial dysfunction decrease by 0.32 (95% CI: 0.13–0.77). Within PWH, the estimated odds of endothelial dysfunction in nonwhite race was 1.76 times (95% CI: 1.07–2.92) greater compared with white race. Compared with treatment with NRTI or NNRTIs at the time of EndoPat, the estimated odds of endothelial dysfunction is greater among protease inhibitor treatment [1.74 (95% CI: 1.03–2.97)] (Table 3 and Fig. 1). There was not enough evidence to suggest that inflammation, gut, or monocyte markers, HOMA-IR, cholesterol, LDL, HDL, Ox LDL, current or nadir CD4+ cell count, duration of HIV, or current treatment with NRTI or NNRTIs were associated with endothelial dysfunction.
Discussion
This is the first large study that compares endothelial function between adult PWH and persons without HIV using peripheral arterial tonometry. Our results suggest that PWH with good virologic suppression on ART have worse endothelial function than healthy controls. We also provide evidence of racial differences in endothelial function where nonwhite race is associated with worse endothelial function independently of HIV status.
Endothelial dysfunction in persons with HIV
Our results are compatible with our previous study using PAT in children and young adults with HIV infection, in which we reported; significant impairment of endothelial function [14]. We cannot compare our results with previous studies that used PAT in HIV because of differences in cohort characteristics as the small study conducted in Southern Africa included undernourished HIV-infected adults that were newly started on ART. This also applies to a study conducted on seven men living with HIV to evaluate the effect of short-term aerobic training. Furthermore, a study concluded that an abnormal myocardial flow reserve (<2.0) as assessed by 82-rubidium PET/CT was inversely correlated with RHI in a sample of 48 PWH, despite previous evidence of a correlation between coronary microvascular and endothelial dysfunction [26]. This further demonstrates the need for a large study using EndoPAT in PWH [16] Furthermore, using FMD to assess blood vessels, Solages et al.[27] surveyed 75 HIV-1-positive and 223 control individuals and found that PWH had a significantly impaired vascular function. This could be attributed to the effect of HIV proteins gp 120 (envelope glycoprotein), Tat (transactivator of viral replication), and Nef (Negative Factor) on the endothelium [28].
Augmentation index
When comparing the augmentation index between the groups, we did not find a statistically significant difference, in concordance with the results found in the study conducted on children and young adults [14]. The augmentation index reflects the arterial stiffness by measuring the percentage of the central pulse pressure to evaluate arterial elasticity [29]. Augmentation index has been correlated with cardiovascular risk [30], and despite its reliability and reproducibility [31], augmentation index could be influenced by physiologic changes at the time of the measurement, like heart rate and vasomotor tone [32].
Risk factors associated with endothelial dysfunction
As hypothesized, we found significant racial differences in RHI, even after covariate adjustment. Our results were similar to those of a study conducted on uninfected adults where both groups had a normal RHI (RHI > 1.67), but patients of black race tended to have a lower RHI with a difference of marginal statistical significance [13]. The racial difference in RHI could be attributed to an increased susceptibility to pro-inflammatory cytokines [33] and a lower endothelial nitrous oxide bioavailability in black race when compared with white race [34].
Protease inhibitor use at the time of EndoPAT testing was negatively associated with RHI and increases the likelihood of endothelial dysfunction, which suggests that protease inhibitor use is a risk factor for poor vascular function. The link between protease inhibitors and cardiovascular disease is well established [35]. This highlights the importance of testing that could detect vascular functional changes before established anatomic vessel changes/atherosclerosis. There were no racial differences in protease inhibitor use and protease inhibitor use did not attenuate the estimated racial differences in RHI or endothelial dysfunction. This reinforces our findings that protease inhibitor use increases risk of poor vascular function and may not explain the racial variability in RHI and endothelial dysfunction.
Endothelial dysfunction and inflammatory markers
To evaluate the relationship between endothelial function and markers of inflammation in HIV, we selected a priori a number of inflammatory markers previously shown to be associated with mortality and cardiovascular disease and other comorbidities in HIV such as IL-6, VCAM, hs-CRP, D-dimer, sCD14, and sCD163. We also included markers of gut integrity (LBP and IFAB) to assess whether intestinal impairment, which is known to happen in PWH [36] are associated with endothelial function or arterial elasticity measured by EndoPAT. In our study, there was no evidence that inflammation was associated with RHI or endothelial dysfunction. The lack of a relationship between endothelial function (measured by non endoPAT tests such as FMD) had been previously described in HIV [37,38].
Limitations
We acknowledge that our study has several limitations. Because of the cross-sectional nature of this study, we cannot establish causation between HIV status and vascular function nor between race and vascular function. Some populations were underrepresented in our sample, mainly women, which could prevent the generalizability of our results. Although we adjusted for the difference in age, sex, and race between the groups, possible residual confounding cannot be excluded. The peripheral arterial tonometry results could have been affected by unmeasured confounders, especially cardiovascular disease risk factors (hypertension, diabetes, and CAD). Due to the wide variation between the ART regimens and the small sample size for each drug, evaluation of the effect of each antiretroviral drug was not possible.
In conclusion, PWH had a significantly worse endothelial function compared with persons without HIV. The nonwhite group had statistically significant lower RHI than the white group independently of HIV status. Protease inhibitor use was associated with vascular dysfunction in all racial groups.
Acknowledgements
This work was supported by the Clinical and Translational Science Collaborative of Cleveland (UL1TR002548) and (R01DK121619) from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH Roadmap for Medical Research.
Author contributions. C.M., J.D., S.N.Z., and G.A.M. contributed to study concept and design. All authors contributed to acquisition of data. J.D. and G.A.M. contributed to analysis and interpretation of data. C.M., J.D., S.N.Z., and G.A.M. drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. J.D. contributed to statistical analysis. G.A.M. obtained funding. C.M. and S.N.Z., contributed to administrative, technical, or material support. G.A.M. supervised the study.
Disclaimer: G.A.M. has received grant support from ViiV, Tetraphase, Roche, Vanda, Astellas, and Genentech. She served as a scientific advisor for Gilead, Merck, ViiV/GSK, Theratechnologies, and Janssen. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. The contents are solely the responsibility of the authors and do not necessarily represent the official views of University Hospitals Cleveland Medical Center (UHCMC) or the National Institutes of Health (NIH).
Conflicts of interest
There are no conflicts of interest.
Footnotes
A list of other author contributors is provided in the Acknowledgment section.
References
- 1.Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 2017; 18:256–266. [DOI] [PubMed] [Google Scholar]
- 2.Savès M, Chêne G, Ducimetière P, Leport C, le Moal G, Amouyel P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003; 37:292–298. [DOI] [PubMed] [Google Scholar]
- 3.Shah ASV, Stelzle D, Ken Lee K, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure? Meta-analysis of the markers of arterial structure and function. Atherosclerosis 2015; 242:109–116. [DOI] [PubMed] [Google Scholar]
- 5.Achhra AC, Lyass A, Borowsky L, Bogorodskaya M, Plutzky J, Massaro JM, et al. Assessing cardiovascular risk in people living with HIV: current tools and limitations. Curr HIV/AIDS Rep 2021; 18:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol 1997; 80:11I–16I. [DOI] [PubMed] [Google Scholar]
- 7.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 8.Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vasc Med 2012; 17:79–84. [DOI] [PubMed] [Google Scholar]
- 9.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003; 146:168–174. [DOI] [PubMed] [Google Scholar]
- 10.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009; 19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen AS, Butt JH, Holm-Yildiz S, Karlsson W, Kruuse C. Validation of repeated endothelial function measurements using EndoPAT in stroke. Front Neurol 2017; 8:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konttinen J, Lindholm H, Sinisalo J, Kuosma E, Halonen J, Hopsu L, et al. Association between lowered endothelial function measured by peripheral arterial tonometry and cardio-metabolic risk factors - a cross-sectional study of Finnish municipal workers at risk of diabetes and cardiovascular disease. BMC Cardiovasc Disord 2013; 13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owei I, Umekwe N, Mohamed H, Ebenibo S, Wan J, Dagogo-Jack S. Ethnic disparities in endothelial function and its cardiometabolic correlates: the pathobiology of prediabetes in a biracial cohort study. Front Endocrinol (Lausanne) 2018; 9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirajlal-Fargo S, Sattar A, Kulkarni M, Bowman E, Funderburg N, McComsey GA. HIV positive youth who are perinatally infected have impaired endothelial function. AIDS 2017; 31:1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva CS, Deresz LF, de Castelli GM, Dorneles GP, Mignoni L, Dal Lago P. Short-term aerobic training improves heart rate variability in men living with HIV: a prepost pilot study. HIV Res Clin Pract 2020; 21:99–104. [DOI] [PubMed] [Google Scholar]
- 16.Ørbæk M, Hasbak P, Ripa RS, Kjær A, Lebech AM, Knudsen A. Comparison of the peripheral reactive hyperemia index with myocardial perfusion reserve by 82Rb PET/CT in HIV-infected patients. Diagnostics 2017; 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bestawros M, Chidumayo T, Blevins M, Canipe A, Bala J, Kelly P, et al. Increased systemic inflammation is associated with cardiac and vascular dysfunction over the first 12 weeks of antiretroviral therapy among undernourished, HIV-infected adults in Southern Africa. J AIDS Clin Res 2015; 6:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 19.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identifcation as nitric oxide (Nobel lecture). Angew Chem Int Ed Engl 1999; 38:1870–1880. [DOI] [PubMed] [Google Scholar]
- 20.Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp 2010; 15:2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henriksen O, Nielsen SL, Paaske W. Intrinsic regulation of blood flow in adipose tissue. Acta Physiol Scand 1976; 98:30–36. [DOI] [PubMed] [Google Scholar]
- 22. Itamar Medical Ltd. EndoPATTM2000 Device User Manual 2002. Available at: https://www.itamar-medical.com/wp-content/uploads/2019/07/OM1695214.pdf. [Accessed 26 August 2021] [Google Scholar]
- 23.Jedlickova L, Merkovska L, Jackova L, Janicko M, Fedacko J, Novakova B, et al. Effect of ivabradine on endothelial function in patients with stable angina pectoris: assessment with the Endo-PAT 2000 device. Adv Ther 2015; 32:962–970. [DOI] [PubMed] [Google Scholar]
- 24.Winderman R, Rabinowitz SS, Vaidy K, Schwarz SM. Measurement of microvascular function in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019; 68:662–668. [DOI] [PubMed] [Google Scholar]
- 25.Zhu GH, Sun XP, Li J, Liu RK, Yang Z, Hua Q. Association between serum uric acid level and endothelial dysfunction in elderly individuals with untreated mild hypertension. J Geriatr Cardiol 2020; 17:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004; 44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 27.Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, et al. Endothelial function in HIV-infected persons. Clin Infect Dis [Internet] 2006; 42:1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand AR, Rachel G, Parthasarathy D. HIV proteins and endothelial dysfunction: implications in cardiovascular disease. Front Cardiovasc Med 2018; 5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohara K, Tabara Y, Oshiumi A, Miyawaki Y, Kobayashi T, Miki T. Radial augmentation index: a useful and easily obtainable parameter for vascular aging. Am J Hypertens 2005; 18 (1 Pt 2):11–14. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Nakayama Y, Tsumura K, Yoshimaru K, Ueda H. Reflection in the arterial system and the risk of coronary heart disease. Am J Hypertens 2002; 15:405–409. [DOI] [PubMed] [Google Scholar]
- 31.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 2002; 17:543–551. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Keith JC, Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther 2008; 26:214–223. [DOI] [PubMed] [Google Scholar]
- 33.Brown MD, Feairheller DL. Are there race-dependent endothelial cell responses to exercise?. Exerc Sport Sci Rev 2013; 41:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760. [DOI] [PubMed] [Google Scholar]
- 35.Alvi RM, Neilan AM, Tariq N, Awadalla M, Afshar M, Banerji D, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV and heart failure. J Am Coll Cardiol 2018; 72:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steele AK, Lee EJ, Vestal B, Hecht D, Dong Z, Rapaport E, et al. Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PLoS One 2014; 9:e97171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein JH, Kime N, Korcarz CE, Ribaudo H, Currier JS, Delaney JC. Effects of HIV infection on arterial endothelial function. Arterioscler Thromb Vasc Biol 2021; 41:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dysangco A, Liu Z, Stein JH, Dubé MP, Gupta SK. HIV infection, antiretroviral therapy, and measures of endothelial function, inflammation, metabolism, and oxidative stress. PLoS One 2017; 12:e0183511. [DOI] [PMC free article] [PubMed] [Google Scholar]