Abstract
Aims
Pulmonary hypertension (PH) and pulmonary vascular disease (PVD) are common and associated with adverse outcomes in left heart disease (LHD). This study sought to characterize the pathophysiology of PVD across the spectrum of PH in LHD.
Methods and results
Patients with PH-LHD [mean pulmonary artery (PA) pressure >20 mmHg and PA wedge pressure (PAWP) ≥15 mmHg] and controls free of PH or LHD underwent invasive haemodynamic exercise testing with simultaneous echocardiography, expired air and blood gas analysis, and lung ultrasound in a prospective study. Patients with PH-LHD were divided into isolated post-capillary PH (IpcPH) and PVD [combined post- and pre-capillary PH (CpcPH)] based upon pulmonary vascular resistance (PVR <3.0 or ≥3.0 WU). As compared with controls (n = 69) and IpcPH-LHD (n = 55), participants with CpcPH-LHD (n = 40) displayed poorer left atrial function and more severe right ventricular (RV) dysfunction at rest. With exercise, patients with CpcPH-LHD displayed similar PAWP to IpcPH-LHD, but more severe RV–PA uncoupling, greater ventricular interaction, and more severe impairments in cardiac output, O2 delivery, and peak O2 consumption. Despite higher PVR, participants with CpcPH developed more severe lung congestion compared with both IpcPH-LHD and controls, which was associated lower arterial O2 tension, reduced alveolar ventilation, decreased pulmonary O2 diffusion, and greater ventilation-perfusion mismatch.
Conclusions
Pulmonary vascular disease in LHD is associated with a distinct pathophysiologic signature marked by greater exercise-induced lung congestion, arterial hypoxaemia, RV–PA uncoupling, ventricular interdependence, and impairment in O2 delivery, impairing aerobic capacity. Further study is required to identify novel treatments targeting the pulmonary vasculature in PH-LHD.
Keywords: Heart failure, Left heart disease, Pulmonary hypertension, Combined post- and pre-capillary pulmonary hypertension, Pulmonary vascular resistance, Exercise haemodynamics
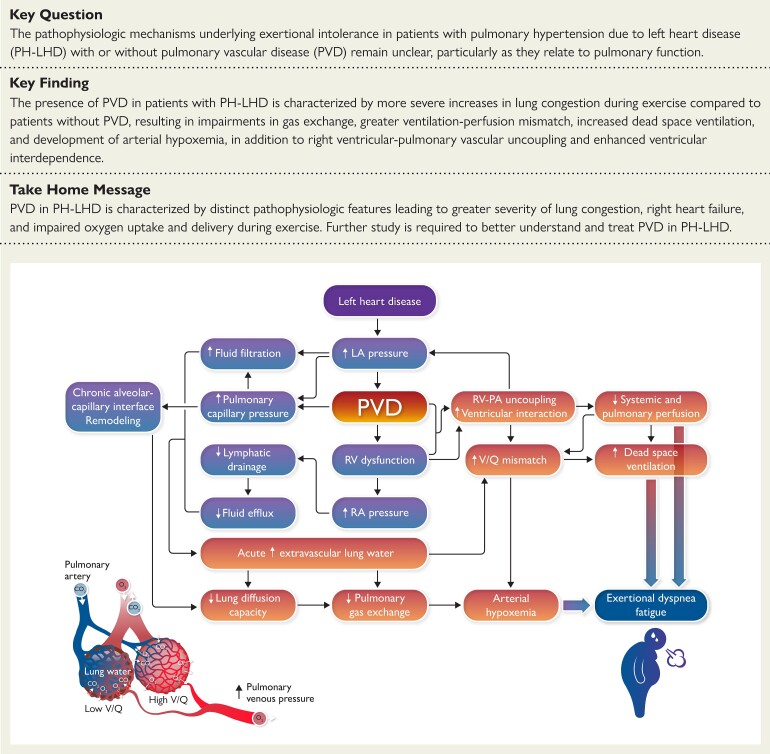
Structured Graphical Abstract
Structured Graphical Abstract.
Impact of pulmonary vascular disease on the heart and lungs in heart failure.
See the editorial comment for this article ‘Was Paul Wood wrong about precapillary pulmonary hypertension protecting against pulmonary congestion in left heart disease?’, by M.M. Hoeper and S. Rosenkranz, https://doi.org/10.1093/eurheartj/ehac176.
Introduction
Left heart disease (LHD) is the most common cause of pulmonary hypertension (PH).1–5 While PH-LHD is initially caused by passive transmission of high left atrial (LA) pressures, some patients develop pulmonary vascular disease (PVD), defined by an increase in pulmonary vascular resistance (PVR).6,7 Patients with PH-LHD and PVD display poorer outcomes.8–14 This is likely related in part to right ventricular (RV) dysfunction in this cohort,15–20 but the impact of PVD on the lungs is not well-described.
Diuretics and vasodilators reduce PVR in some patients with PH-LHD,8,21–23 impugning vasoconstriction and vascular oedema as mechanisms, but other patients develop vascular structural remodelling that coexists with vasoconstriction.2,7,10 This remodelling is often conceptualized as affecting small arterial vessels,10,24 but venous remodelling was described decades ago in mitral valve disease,2,25 and recent studies have revealed venous remodelling in non-valvular related forms of PH-LHD, including heart failure.7,26 This venous constriction could exaggerate pulmonary capillary hypertension out of proportion to the observed increase in LA pressure, worsening lung oedema. Structural and functional abnormalities in PVD also extend to the level of the pulmonary capillaries, which may further impair systemic O2 delivery during stress.27,28
We hypothesized that in addition to impairments in RV-pulmonary vascular coupling, patients with PVD would display abnormalities in the arterial, capillary, and venous components of the lung circulation during exercise that lead to greater increases in lung congestion and abnormalities in gas exchange. To test this hypothesis, we performed a prospective, simultaneous invasive/imaging study with expired gas and blood gas analysis at rest, and during exercise in patients with and without PVD.
Methods
Study population
The study cohort consisted of patients undergoing invasive haemodynamic exercise testing for the evaluation of dyspnoea at the Mayo Clinic, Rochester, MN, USA between February 2018 and May 2021 who agreed to participate in this prospective study. Pulmonary hypertension-left heart disease was defined by the presence of New York Heart Association (NYHA) Functional Class II–III dyspnoea, elevated mean pulmonary artery (PA) pressures at rest (>20 mmHg),29 and elevated PA wedge pressure (PAWP) at rest (≥15 mmHg), regardless of ejection fraction (EF).1 Individuals with LHD manifest exclusively by exercise-induced elevation in PAWP or PA pressure were not included. Participants with PH-LHD were then categorized as those without or with PVD based upon PVR, as isolated postcapillary PH (IpcPH, PVR <3.0 WU) or combined post- and pre-capillary PH (CpcPH; PVR ≥3.0 WU).1 Controls free of PH or LHD were included as an additional comparator group. These patients showed no evidence of myocardial or valve disease on echocardiogram, normal rest, and exercise PAWP (rest <15 mmHg, exercise <25 mmHg) and normal mean PA pressures (rest <20 mmHg, exercise <40 mmHg). All controls were found to have dyspnoea related to deconditioning and/or psychogenic aetiologies, as cardiac, pulmonary, and pulmonary vascular disorders had been excluded prior to or at the time of catheterization. In all patients, the probability for heart failure with preserved EF was determined according to the H2FPEF and HFA-PEFF scores.30,31
Patients with underlying lung disease (including both parenchymal and thromboembolic disease) were excluded, along with patients with pre-load failure, hypertrophic, infiltrative, or RV cardiomyopathies, constrictive pericarditis, high-output heart failure, and unstable coronary artery disease (see Supplementary material online, Figure S1). All participants provided written informed consent and the study was approved by the Mayo Clinic Institutional Review Board. All authors have read and agreed to the manuscript as written.
Haemodynamic evaluation
Participants underwent maximal-effort supine cycle ergometry testing with simultaneous expired gas analysis, echocardiography, blood sampling, and lung ultrasound, using methods we have previously described.32–34 Transducers were zeroed at mid-thorax, and all pressure tracings were digitized (240 Hz) and stored for offline analysis. At rest, right atrial pressure (RAP), PA pressures, and PAWP were measured at end-expiration from the mean of ≥3 beats using 2-Fr high fidelity micromanometer-tipped catheters advanced through the lumen of a 7-Fr fluid-filled catheter. During exercise, pressures were taken as the average over ≥3 respiratory cycles.35
PAWP position was confirmed by the appearance on fluoroscopy, characteristic pressure waveforms, and oximetry (saturation ≥94%). Because the focus of this analysis is on pulmonary vascular pressure loading downstream of the RV and PA, rather than left ventricular (LV) responses to exercise, PAWP was defined as the mean area under the pressure-time curve downstream of balloon occlusion, including A, C, and V waves, rather than mid-A wave.36 Left ventricular transmural pressure (LVTMP), which reflects LV pre-load independent of right heart filling pressure and external pericardial restraint, was estimated as PAWP-RAP.37,38
A 4–6 Fr radial arterial cannula was used to measure arterial blood pressure (BP) and enable arterial blood gases sampling. Arteriovenous O2 difference (AVO2 diff) was directly measured as the difference between systemic arterial O2 content (CaO2) and PA O2 content (CvO2) ( = saturation × haemoglobin×1.34). Cardiac output (QT) was then calculated using the direct Fick method [ = oxygen consumption (VO2)/AVO2 diff]. Convective O2 delivery (DO2) was calculated as QT × CaO2. Pulmonary vascular resistance [(mean PA−PAWP)/QT] and PA compliance [PAC = stroke volume/(PA systolic – diastolic pressure)] were calculated using standard equations.
After baseline data were acquired, haemodynamic assessment and expired gas analysis were performed during supine exercise, starting at 20 W for 5 min (to allow imaging during submaximal exercise), increasing in 20 W increments in 3 min stages to volitional exhaustion, at which time peak measurements were obtained. Symptoms of dyspnoea and fatigue during exercise were rated by subjects during each stage according to the Borg perceived dyspnoea (0–10) and fatigue scales (6–20).
Expired gas and blood gas analysis
Breath-by-breath VO2, carbon dioxide production , tidal volume (VT), minute ventilation (VE), and respiratory rate were measured throughout each study (MedGraphics, St Paul, MN, USA).39,40 Ventilatory efficiency was assessed by the slope of V̇E to V̇CO2, which was calculated from the slope of all V̇E and V̇CO2 data points during exercise.34 The degree of V/Q mismatch was estimated from the ratio of dead space ventilation to tidal volume (VD/VT) determined from the modified alveolar gas equation (higher values indicate greater dead space ventilation and higher V/Q),39,40 and by the physiologic pulmonary shunt fraction determined by the Berggren equation (higher values indicate greater physiologic right-to-left shunt and lower V/Q).41 Detailed methods for the derived blood gas and ideal alveolar equation parameters are described in Supplementary material online, Supplementary Methods.
Cardiac structure and function
Two-dimensional, M-mode, Doppler, and tissue Doppler echocardiography were performed by cardiologists according to the American Society of Echocardiography guidelines.42 Left ventricular ejection fraction (LVEF) was calculated using the Teichholz method. Heart failure with reduced EF was defined as EF <50%. Left ventricular diastolic function was assessed using the early diastolic mitral inflow velocity (E), early diastolic annular tissue velocity at septal and lateral annulus (e′), and the average E/e′ ratio. Speckle tracking strain analyses were performed using commercially available software (Image Arena, TomTec, Unterschleissheim, Germany) from resting images. Left atrial reservoir strain was measured taking the mean of three beats from the apical two-chamber and four-chamber views using the ECG R wave as fiducial point.43 Right atrial (RA) reservoir strain was measured from the four-chamber views.44 The average values of peak longitudinal systolic strain obtained from all segments of the free wall and septal wall of the right ventricle and only from the free wall were defined as RV global longitudinal strain (GLS) and RV-free wall longitudinal strain (FWLS), respectively.20
Echocardiographic data were also obtained simultaneously with invasive haemodynamic assessment at rest and during all stages of exercise.32,33 Right ventricular function was assessed using tricuspid annular plane systolic excursion (TAPSE), RV systolic tissue velocity at the lateral tricuspid annulus (RV s′), and RV fractional area change (FAC). Right ventricular–pulmonary artery coupling was assessed by the ratio of RV s′, TAPSE, and FAC to invasively measured systolic PA pressure as in prior studies.18,34 Ventricular interaction was assessed by the LV eccentricity index, whereby higher values indicate more septal flattening and greater ventricular interaction/pericardial restraint.38,45
Lung congestion imaging
Lung ultrasound was performed simultaneously at rest and during exercise to provide a quantitative measure of extravascular lung water (EVLW) as previously described.34 Lung ultrasound was performed using a phased array transducer using positions along the mid-axillary and mid-clavicular lines in the left third and fourth intercostal spaces. Because of time constraints for imaging of the lung and right heart in tandem, lung ultrasound was restricted to these four imaging windows with B-lines summed for the four windows at each stage. The total number of B-lines was calculated at rest and during all stages of exercise. The reproducibility of B-lines measurement was assessed in 20 randomly selected patients. Intra-observer agreement was evaluated after the same observer (K.O.) repeated the measurements 4 weeks later, and inter-observer agreement was tested by comparing the measurement made by another experienced reader (H.S.). The intraclass correlation coefficients for intra-observer and inter-observer agreement for B-lines at rest were 0.93 and 0.98 and B-lines with exercise were 0.86 and 0.96.
Pulmonary function test
Participants underwent assessment (when clinically indicated) of spirometry including assessment of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and the diffusing capacity of the lungs for carbon monoxide (DLCO) using the single-breath method.
Statistical analysis
Data are reported as mean and standard deviation (SD) for normally distributed data, median, and interquartile range (IQR) for non-normally distributed data, or number with relative frequency. Between-group differences were first compared by one-way ANOVA, the Kruskal–Wallis H test, or χ2 test, as appropriate. The Tukey honestly-significant-difference test or Steel–Dwass was then used to compare between two individual groups. Linear regression analyses and Pearson correlation coefficients were used to assess relationships between changes in variables of interest. A two-sided P-value of <0.05 was considered statistically significant. As the present study was focused on pathophysiology and mechanisms rather than treatment algorithms, no correction for multiple hypothesis testing was performed. All data were analysed using JMP14.0 (SAS Institute Inc., Cary, NC, USA).
Results
Participants with CpcPH were older and displayed higher prevalences of diabetes and atrial fibrillation, with more severe kidney dysfunction and higher N-terminal pro-B-type natriuretic peptide levels (Table 1). Participants with PH-LHD displayed lower LVEF, greater RV basal dilation, and LV mass index as compared with controls. Participants with CpcPH displayed lower septal and lateral e′, higher E/e′ ratio, more severely impaired LA and RA reservoir and booster strain, RV GLS, and RV FWLS, and greater LA volumes, and significant tricuspid regurgitation and mitral regurgitation compared with controls and IpcPH. Participants with available spirometry measurements did not differ from those without these data (see Supplementary material online, Table S1). Compared with controls, patients with PH-LHD displayed lower %predicted FVC and lower %predicted FEV1. Participants with CpcPH reduced pulmonary diffusion capacity assessed by DLCO, although the FEV1/FVC ratio was similar in all groups. Participants with PH-LHD displayed higher H2FPEF score and HFA PEFF score as compared with controls but has higher probability in CpcPH compared with IpcPH (Table 1, Supplementary material online, Figure S2).
Table 1.
Baseline characteristics
| Control subjects (n = 69) | IpcPH (n = 55) | CpcPH (n = 40) | P-value | |
|---|---|---|---|---|
| Age (years) | 60 ± 13 | 64 ± 11 | 71 ± 13*,** | 0.0002 |
| Female sex, n (%) | 41 (59%) | 30 (55%) | 24 (60%) | 0.8 |
| Body mass index (kg/m2) | 29.2 ± 5.7 | 34.8 ± 8.3* | 31.7 ± 8.4 | 0.0002 |
| Obesity | 29 (42%) | 39 (71%) | 19 (48%) | 0.004 |
| HFpEF/HFrEF/VHD | — | 46/6/3 | 26/8/6 | 0.1 |
| Comorbidities, n (%) | ||||
| Coronary disease | 18 (26%) | 17 (31%) | 10 (25%) | 0.8 |
| Diabetes mellitus | 7 (10%) | 16 (29%) | 18 (45%) | 0.0002 |
| Hypertension | 60 (87%) | 52 (95%) | 35 (88%) | 0.3 |
| Atrial fibrillation | 3 (4%) | 22 (40%) | 28 (70%) | <0.0001 |
| Medications, n (%) | ||||
| ACEI or ARB | 15 (22%) | 20 (36%) | 22 (55%) | 0.002 |
| β-Blocker | 22 (32%) | 23 (42%) | 29 (73%) | 0.0002 |
| Loop diuretic | 15 (22%) | 23 (42%) | 31 (78%) | <0.0001 |
| Laboratories | ||||
| Haemoglobin (g/dL) | 13.2 ± 1.6 | 12.9 ± 1.5 | 12.5 ± 1.9 | 0.1 |
| Estimated GFR (mL/min/1.73m2) | 69 ± 17 | 68 ± 20 | 53 ± 16*,** | <0.0001 |
| NT-proBNP (pg/mL) | 62 (39, 188) | 363 (90, 890)* | 1576 (939, 2766)*,** | <0.0001 |
| Cardiac structure and function | ||||
| LA volume index (mL/m2) | 26 (21, 34) | 34 (27, 48)* | 46 (36, 60)*,** | <0.0001 |
| LA reservoir strain (%) | 32.0 ± 8.7 | 22.6 ± 9.0* | 12.9 ± 6.5*,** | <0.0001 |
| LA booster strain (%) | 15.7 ± 5.7 | 11.4 ± 4.9* | 4.9 ± 3.1*,** | <0.0001 |
| LVEF (%) | 60 ± 6 | 58 ± 11 | 55 ± 14* | 0.03 |
| LV mass index (g/m2) | 81 ± 19 | 95 ± 31 | 102 ± 33* | 0.003 |
| Septal e′ (cm/s) | 7.3 ± 2.1 | 7.4 ± 2.6 | 5.3 ± 1.7*,** | <0.0001 |
| Lateral e′ (cm/s) | 10.0 ± 2.5 | 9.0 ± 3.0* | 7.3 ± 2.6*,** | <0.0001 |
| E/e′ ratio | 9 (8, 12) | 11 (9, 16)* | 18 (13, 25)*,** | <0.0001 |
| RV basal dimension (mm) | 35 ± 6 | 42 ± 9* | 45 ± 9* | <0.0001 |
| RV mid cavity dimension (mm) | 29 ± 10 | 33 ± 9 | 34 ± 9 | 0.04 |
| RV longitudinal dimension (mm) | 71 ± 11 | 76 ± 10 | 77 ± 11* | 0.02 |
| RV GLS (%) | 19.1 ± 3.5 | 16.3 ± 4.6* | 14.1 ± 3.4*,** | <0.0001 |
| RV FWLS (%) | 21.6 ± 5.0 | 20.8 ± 6.4 | 17.6 ± 5.0*,** | 0.007 |
| RA reservoir strain (%) | 36.4 ± 10.2 | 24.7 ± 13.0* | 12.1 ± 8.8*,** | <0.0001 |
| RA booster strain (%) | 17.4 ± 5.8 | 13.4 ± 5.2* | 6.3 ± 4.5*,** | <0.0001 |
| Moderate or greater TR, n (%) | 1 (1) | 5 (9) | 17 (43) | <0.0001 |
| Moderate or greater MR, n (%) | 0 (0) | 5 (9) | 8 (20) | 0.0009 |
| Pulmonary function test | ||||
| FVC (% predicted) (n = 81) | 98 ± 20 | 84 ± 15* | 78 ± 16* | <0.0001 |
| FEV1 (% predicted) (n = 81) | 95 ± 20 | 80 ± 16* | 72 ± 16* | <0.0001 |
| FEV1/FVC ratio (%) (n = 93) | 76 ± 7 | 74 ± 8 | 73 ± 7 | 0.2 |
| DLCO (mL/min*mmHg) (n = 91) | 20 ± 5 | 18 ± 5 | 12 ± 5*,** | <0.0001 |
| Probability of HFpEF | ||||
| H2FPEF score | 2 (1, 3) | 5 (3, 5)* | 7 (6, 8)*,** | <0.0001 |
| HFA-PEFF score | 2 (0, 2) | 4 (2, 6)* | 6 (5, 6)*,** | <0.0001 |
Data are mean ± SD, median (interquartile range), or n (%). Final column reflects overall group differences.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CpcPH, combined post- and pre-capillary pulmonary hypertension; DLCO, diffusing capacity for carbon monoxide; EF, ejection fraction; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; FWLS, free wall longitudinal strain; GFR, glomerular filtration rate; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IpcPH, isolated post-capillary pulmonary hypertension; LA, left atrial; LHD, left heart disease; LV, left ventricular; MR, mitral regurgitation; NT-pro BNP, N-terminal-pro-B-type natriuretic peptide; RA, right atrial; RV, right ventricular; TR, tricuspid regurgitation; VHD, valvular heart disease.
*P < 0.05 vs. controls, **P < 0.05 vs. IpcPH groups.
Haemodynamics
As compared with controls and IpcPH, participants with CpcPH displayed higher biventricular filling pressures, PA pressures, and (by definition) PVR, with lower PA compliance at rest (Table 2). Peak exercise workload achieved was lower in participants with CpcPH compared with IpcPH and controls (34 ± 25 and 53 ± 28 vs. 67 ± 40 W, P < 0.0001). During exercise, patients with IpcPH and CpcPH reached severe and similar elevation in PAWP that greatly exceeded controls, but RAP and PA pressures increased to a greater extent in CpcPH than both IpcPH and controls, along with higher PVR and lower PA compliance (Table 3). Systemic arterial pressures were higher at rest in IpcPH compared with controls, and systemic arterial pressures were lower in CpcPH than both IpcPH and controls during exercise.
Table 2.
Resting haemodynamics and integrated cardiopulmonary function
| Control subjects (n = 69) | IpcPH (n = 55) | CpcPH (n = 40) | P-value | |
|---|---|---|---|---|
| Vital signs | ||||
| Heart rate (b.p.m.) | 72 ± 12 | 76 ± 20 | 71 ± 15 | 0.2 |
| Systolic BP (mmHg) | 142 ± 22 | 156 ± 23* | 149 ± 28 | 0.009 |
| Mean BP (mmHg) | 95 ± 12 | 104 ± 13* | 97 ± 16 | 0.002 |
| Ventricular filling pressures | ||||
| RAP (mmHg) | 5 ± 2 | 12 ± 4* | 15 ± 5*,** | <0.0001 |
| PAWP (mmHg) | 8 ± 3 | 20 ± 3* | 23 ± 4*,** | <0.0001 |
| PAWP V wave (mmHg) | 10 ± 4 | 26 ± 6* | 34 ± 11*,** | <0.0001 |
| RAP/PAWP ratio | 0.67 ± 0.25 | 0.62 ± 0.18 | 0.66 ± 0.19 | 0.5 |
| LVTMP (mmHg) | 2 (1, 4) | 7 (4, 10)* | 8 (4, 11)* | <0.0001 |
| Pulmonary circulation | ||||
| PA systolic pressure (mmHg) | 25 ± 5 | 42 ± 10* | 67 ± 18*,** | <0.0001 |
| PA diastolic pressure (mmHg) | 10 ± 3 | 20 ± 5* | 27 ± 6*,** | <0.0001 |
| PA mean pressure (mmHg) | 16 ± 3 | 29 ± 6* | 44 ± 9*,** | <0.0001 |
| Diastolic pressure gradient (mmHg) | 2 (0, 4) | 1 (-2, 3) | 3 (0, 7) ,** | 0.002 |
| PVR (WU) | 1.5 (1.2, 2.0) | 1.6 (1.2, 2.1) | 4.8 (3.8, 7.5)*,** | <0.0001 |
| PAC (mL/mmHg) | 5.2 ± 1.8 | 4.0 ± 1.6* | 1.6 ± 0.7*,** | <0.0001 |
| Oxygen transport | ||||
| VO2 (mL/min/kg) | 2.7 ± 0.8 | 2.7 ± 0.6 | 2.5 ± 0.6 | 0.2 |
| QT (L/min) | 5.5 ± 1.4 | 5.9 ± 1.5 | 3.9 ± 1.2*,** | <0.0001 |
| AVO2 diff (mL/dL) | 4.1 ± 0.7 | 4.6 ± 1.0* | 5.6 ± 1.2*,** | <0.0001 |
| DO2 (mL/min) | 909 ± 256 | 926 ± 226 | 583 ± 191*,* | <0.0001 |
| VO2/DO2 | 0.25 ± 0.04 | 0.28 ± 0.05* | 0.38 ± 0.09*,* | <0.0001 |
| Pulmonary function and gas exchange | ||||
| VE (L/min) | 7.8 ± 3.8 | 8.4 ± 2.8 | 6.9 ± 1.9 | 0.1 |
| VT (mL) | 500 (389, 633) | 493 (409, 619) | 451 (378, 539) | 0.3 |
| Respiratory rate (/min) | 15 ± 5 | 16 ± 5 | 16 ± 5 | 0.4 |
| V̇A (L/min) | 4.7 ± 2.5 | 4.9 ± 1.6 | 3.9 ± 1.0 | 0.08 |
| VD/VT | 0.39 ± 0.09 | 0.41 ± 0.08 | 0.45 ± 0.08* | 0.02 |
| Physiologic shunt fraction | 0.05 (0, 0.10) | 0.08 (0, 0.16) | 0.09 (0.05, 0.14) | 0.3 |
| DLO2 (mL/min/mmHg) | 9 (5, 19) | 9 (7, 11) | 8 (6, 13) | 0.6 |
| PAO2 (mmHg) | 94 ± 18 | 97 ± 19 | 93 ± 16 | 0.6 |
| Arterial SaO2 (%) | 96 ± 2 | 95 ± 3* | 93 ± 5*,* | <0.0001 |
| Arterial pO2 (mmHg) | 79 ± 14 | 72 ± 12* | 67 ± 13* | 0.0001 |
| Arterial pCO2 (mmHg) | 37 ± 6 | 40 ± 5 | 40 ± 7 | 0.03 |
| PA SvO2 (%) | 72 ± 5 | 68 ± 5 | 58 ± 9*,* | <0.0001 |
| PA pO2 (mmHg) | 39 ± 7 | 36 ± 3 | 32 ± 5*,* | <0.0001 |
| PA pCO2 (mmHg) | 39 ± 5 | 42 ± 6 | 44 ± 6* | 0.0009 |
| Alveolar–arterial O2 gradient (mmHg) | 15 (2, 32) | 29 (18, 35)* | 29 (15, 35)* | 0.005 |
| Right heart function | ||||
| RV s′ (cm/s) | 11 ± 3 | 10 ± 3 | 8 ± 3*,** | <0.0001 |
| TAPSE (mm) | 20 ± 5 | 18 ± 5 | 14 ± 5*,** | <0.0001 |
| RV FAC (%) | 45 ± 7 | 42 ± 9 | 35 ± 9*,** | <0.0001 |
| RV s′/systolic PA (cm/s*mm Hg) | 0.47 ± 0.19 | 0.25 ± 0.10* | 0.12 ± 0.06*,** | <0.0001 |
| TAPSE/systolic PA (mm/mm Hg) | 0.82 ± 0.27 | 0.45 ± 0.16* | 0.22 ± 0.10*,** | <0.0001 |
| FAC/systolic PA (%/mm Hg) | 1.85 ± 0.49 | 1.06 ± 0.35* | 0.57 ± 0.25*,** | <0.0001 |
| Ventricular interaction | ||||
| Eccentricity index at end diastole | 1.06 ± 0.12 | 1.08 ± 0.15 | 1.12 ± 0.16 | 0.1 |
| Eccentricity index at end systole | 0.96 ± 0.09 | 1.01 ± 0.06 | 1.14 ± 0.18*,** | <0.0001 |
| Pulmonary congestion | ||||
| Number of B-lines | 0 (0–0) | 0 (0–2)* | 2 (0–5)* | <0.0001 |
Data are mean ± SD, median (interquartile range), or n (%). Final column reflects overall group differences.
AVO2 diff, arterial-venous O2 content difference; BP, blood pressure; CpcPH, combined post- and pre-capillary pulmonary hypertension; DLO2, estimated pulmonary diffusing capacity for oxygen; DO2, oxygen delivery; FAC, fractional area change; IpcPH, isolated post-capillary pulmonary hypertension; LHD, left heart disease; LVTMP, left ventricular transmural pressure; LV, left ventricular; PA, pulmonary artery; PAC, pulmonary artery compliance; PAWP, pulmonary artery wedge pressure; PAO2, alveolar oxygen tension; PA-aO2, alveolar-to-arterial O2 gradient; PVR, pulmonary vascular resistance; RAP, right atrial pressure; QT, cardiac output; RV, right ventricular; s′, systolic tissue doppler velocity; SaO2, arterial oxygen saturation; TAPSE, tricuspid annular plane systolic excursion; V̇A, alveolar ventilation; VD, pulmonary dead space; VE, minute ventilation; VT, tidal volume; , carbon dioxide volume; VO2, oxygen consumption volume.
*P < 0.05 vs. controls, **P < 0.05 vs. IpcPH groups.
Table 3.
Exercise haemodynamics and integrated cardiopulmonary function
| Control subjects (n = 69) | IpcPH (n = 55) | CpcPH (n = 40) | P-value | |
|---|---|---|---|---|
| Vital signs | ||||
| Heart rate (b.p.m.) | 114 ± 24 | 100 ± 22* | 86 ± 19*,** | <0.0001 |
| Systolic BP (mmHg) | 182 ± 26 | 179 ± 32 | 161 ± 35*,** | 0.008 |
| Mean BP (mmHg) | 114 ± 13 | 115 ± 18 | 103 ± 20*,** | 0.007 |
| Ventricular filling pressures | ||||
| RAP (mmHg) | 8 ± 4 | 20 ± 7* | 26 ± 7*,** | <0.0001 |
| PAWP (mmHg) | 15 ± 5 | 31 ± 7* | 29 ± 6* | <0.0001 |
| PAWP v wave (mmHg) | 18 ± 8 | 42 ± 11* | 42 ± 13* | <0.0001 |
| RAP/PAWP ratio | 0.57 ± 0.24 | 0.66 ± 0.22 | 0.92 ± 0.32*,** | <0.0001 |
| LVTMP (mmHg) | 6 (3, 9) | 10 (6, 16)* | 3 (-1, 9) ,** | <0.0001 |
| Pulmonary circulation | ||||
| PA systolic pressure (mmHg) | 41 ± 8 | 62 ± 12* | 86 ± 21*,** | <0.0001 |
| PA diastolic pressure (mmHg) | 17 ± 5 | 31 ± 7* | 37 ± 7*,** | <0.0001 |
| PA mean pressure (mmHg) | 28 ± 6 | 46 ± 9* | 57 ± 10*,** | <0.0001 |
| Diastolic pressure gradient (mmHg) | 3 (-1, 5) | 0 (-3, 5) | 6 (2, 11)*,** | <0.0001 |
| PVR (WU) | 1.3 (0.9, 1.7) | 1.7 (1.0, 2.5)* | 4.8 (3.4, 7.9)*,** | <0.0001 |
| PAC (mL/mmHg) | 4.2 ± 1.5 | 3.3 ± 1.3* | 1.5 ± 0.7*,** | <0.0001 |
| Exertional symptoms | ||||
| Borg effort (6–20) | 16.4 ± 2.5 | 15.8 ± 2.0 | 14.4 ± 2.9*,** | 0.0005 |
| Borg dyspnoea (0–10) | 7.2 ± 2.3 | 6.4 ± 1.8 | 5.9 ± 2.2* | 0.009 |
| Oxygen transport | ||||
| VO2 (mL/min/kg) | 12.4 ± 4.8 | 9.7 ± 3.5* | 6.9 ± 2.3*,** | <0.0001 |
| QT (L/min) | 10.2 ± 2.7 | 9.3 ± 2.8 | 5.4 ± 2.1*,** | <0.0001 |
| AVO2 diff (mL/dL) | 9.6 ± 1.8 | 10.4 ± 2.3 | 11.4 ± 2.4* | 0.0002 |
| DO2 (mL/min) | 1789 ± 567 | 1565 ± 474* | 861 ± 335*,* | <0.0001 |
| VO2/DO2 | 0.55 ± 0.09 | 0.61 ± 0.11* | 0.71 ± 0.09*,* | <0.0001 |
| Pulmonary function and gas exchange | ||||
| VE (L/min) | 37.4 ± 14.6 | 32.7 ± 9.9 | 22.6 ± 9.9*,* | <0.0001 |
| VT (mL) | 1165 (915-1452) | 965 (836-1205)* | 846 (594-976)* ,* | <0.0001 |
| Respiratory rate (/min) | 31 ± 9 | 33 ± 8 | 27 ± 8,** | 0.01 |
| V̇A (L/min) | 27.4 ± 12.6 | 22.9 ± 7.8 | 14.9 ± 8.1*,* | <0.0001 |
| VD/VT | 0.28 ± 0.09 | 0.31 ± 0.06* | 0.36 ± 0.09*,* | <0.0001 |
| V̇E/V̇CO2 slope | 33.5 ± 6.6 | 33.0 ± 6.1 | 39.0 ± 10.2*,* | 0.0008 |
| Physiologic shunt fraction | 0.03 (0, 0.05) | 0.05 (0.02, 0.09) | 0.08 (0.04, 0.12)* | 0.001 |
| DLO2 (mL/min/mmHg) | 41 (25, 65) | 23 (17, 34)* | 15 (10, 21)* ,* | <0.0001 |
| PAO2 (mmHg) | 107 ± 14 | 109 ± 14 | 108 ± 12 | 0.8 |
| Arterial and mixed venous blood composition | ||||
| Arterial SaO2 (%) | 96 ± 4 | 94 ± 4 | 93 ± 4* | 0.002 |
| Arterial pO2 (mmHg) | 84 ± 13 | 74 ± 14* | 66 ± 13*,* | <0.0001 |
| Arterial pCO2 (mmHg) | 36 ± 5 | 37 ± 5 | 37 ± 5 | 0.3 |
| PA SvO2 (%) | 43 ± 9 | 36 ± 11 | 27 ± 9*,* | <0.0001 |
| PA pO2 (mmHg) | 26 ± 4 | 25 ± 8 | 21 ± 4*,* | <0.0001 |
| PA pCO2 (mmHg) | 48 ± 8 | 50 ± 8 | 53 ± 7* | 0.03 |
| Alveolar–arterial O2 gradient (mmHg) | 23 ± 17 | 35 ± 20* | 41 ± 14* | <0.0001 |
| Right heart function | ||||
| RV s′ (cm/s) | 15 ± 4 | 13 ± 3* | 9 ± 3*,** | <0.0001 |
| TAPSE (mm) | 22 ± 5 | 21 ± 7 | 15 ± 5*,** | <0.0001 |
| RV FAC (%) | 50 ± 6 | 47 ± 9 | 38 ± 9*,** | <0.0001 |
| RV s′/systolic PA (cm/s*mmHg) | 0.38 ± 0.15 | 0.22 ± 0.08* | 0.11 ± 0.04*,** | <0.0001 |
| TAPSE/systolic PA (mm/mmHg) | 0.57 ± 0.18 | 0.34 ± 0.15* | 0.18 ± 0.08*,** | <0.0001 |
| FAC/systolic PA (%/mmHg) | 1.26 ± 0.28 | 0.78 ± 0.27* | 0.48 ± 0.17*,** | <0.0001 |
| Ventricular interaction | ||||
| Eccentricity index at end diastole | 1.04 ± 0.12 | 1.10 ± 0.12 | 1.25 ± 0.21*,** | <0.0001 |
| Eccentricity index at end systole | 0.98 ± 0.07 | 1.04 ± 0.08 | 1.36 ± 0.06*,** | <0.0001 |
| Pulmonary congestion | ||||
| Number of B-lines | 0 (0, 1) | 2 (0, 4)* | 7 (3, 9)* ,* | <0.0001 |
Data are mean ± SD, median (interquartile range), or n (%). Final column reflects overall group differences.
AVO2 diff, arterial–venous O2 content difference; BP, blood pressure; CO, cardiac output; CpcPH, combined post- and pre-capillary pulmonary hypertension; DLO2, estimated pulmonary diffusing capacity for oxygen; DO2, oxygen delivery; FAC, fractional area change; IpcPH, isolated post-capillary pulmonary hypertension; LHD, left heart disease; LVTMP, left ventricular transmural pressure; LV, left ventricular; PA, pulmonary artery; PAC, pulmonary artery compliance; PAWP, pulmonary artery wedge pressure; PAO2, alveolar oxygen tension; PA-aO2, alveolar-to-arterial O2 gradient; PVR, pulmonary vascular resistance; QT, cardiac output; RAP, right atrial pressure; RV, right ventricular; s′, systolic tissue Doppler velocity; SaO2, arterial oxygen saturation; TAPSE, tricuspid annular plane systolic excursion; V̇A, alveolar ventilation; VD, pulmonary dead space; VE, minute ventilation; VT, tidal volume; , carbon dioxide volume; VO2, oxygen consumption volume.
*P < 0.05 vs. controls, **P < 0.05 vs. IpcPH groups.
Oxygen transport
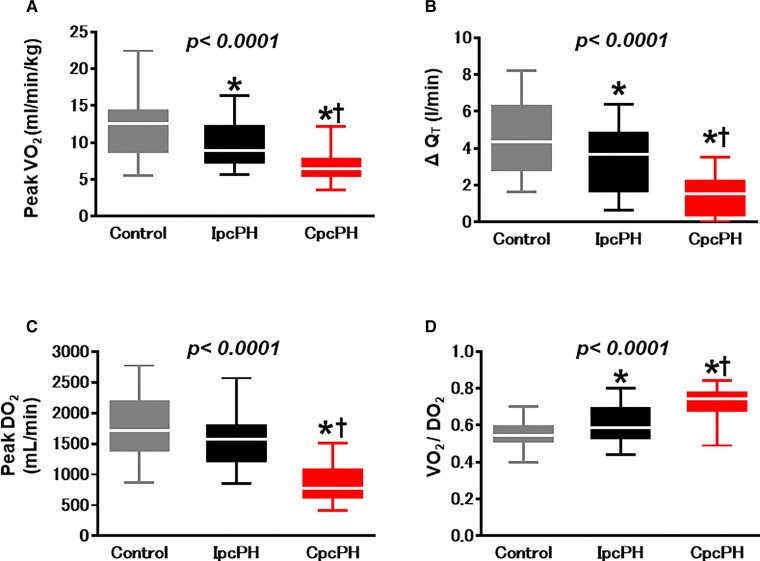
As compared with controls and IpcPH at rest, participants with CpcPH displayed similar VO2 but lower QT and DO2, resulting in higher AVO2 diff and VO2/DO2 ratio (Table 2). With exercise, individuals with CpcPH displayed markedly reduced peak VO2, which was related to impaired QT and DO2, with a higher VO2/DO2 ratio (Figure 1, Table 3).
Figure 1.
Compared with controls and participants with isolated post-capillary pulmonary hypertension, participants with combined post- and pre-capillary pulmonary hypertension displayed lower peak VO2 (A) and less increase in the cardiac output response to exercise (B). Oxygen delivery during exercise was lowest in combined post- and pre-capillary pulmonary hypertension (C), with the highest O2 extraction (D). QT, cardiac output; CpcPH, combined post-and pre-capillary pulmonary hypertension; IpcPH, isolated post-capillary pulmonary hypertension; VO2, oxygen consumption; DO2, oxygen delivery. *P < 0.05 vs. controls. †P < 0.05 vs. IpcPH groups.
Right ventricular reserve
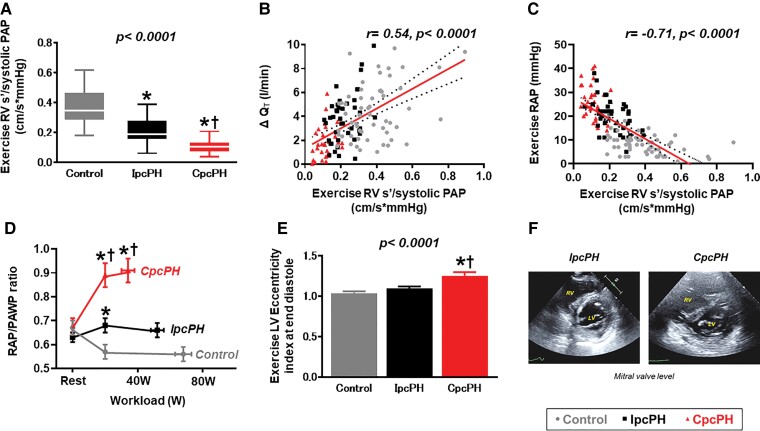
Right ventricular function was impaired in the CpcPH group compared with controls and IpcPH at rest (Table 2). During exercise, patients with CpcPH displayed even more profound RV–PA uncoupling than what was present at rest, manifest by lower absolute values of RV s′, TAPSE, and FAC, and lower ratios of RV s′, TAPSE, and FAC to systolic PA pressure (Table 3). Impairments in RV–PA coupling during exercise were associated with greater limitations in cardiac output reserve and greater increases in central venous pressure (Figure 2, Supplementary material online, Figure S3).
Figure 2.
Right ventricular–pulmonary artery coupling during exercise was worse in the combined post- and pre-capillary pulmonary hypertension group compared with other groups (A) and was associated with impairments in CO reserve (B) and greater elevation in central venous pressure (C). Pericardial restraint and diastolic ventricular interdependence are enhanced in combined post- and pre-capillary pulmonary hypertension, evidenced by higher right atrial pressure/pulmonary artery wedge pressure ratio (D) and a greater exercise left ventricular eccentricity index compared with isolated post-capillary pulmonary hypertension and controls (E, F). PA, pulmonary artery; PAP, pulmonary artery pressure; RAP, right atrial pressure; PAWP, pulmonary artery wedge pressure; RV, right ventricular; s′, systolic tissue doppler velocity; QT, cardiac output; CpcPH, combined post-and pre-capillary pulmonary hypertension; IpcPH, isolated post-capillary pulmonary hypertension; VO2, oxygen consumption; DO2, oxygen delivery. ‘r’ determined by Pearson’s correlation analysis. *P < 0.05 vs. controls. †P < 0.05 vs. IpcPH groups.
Ventricular interaction
At rest, participants with CpcPH displayed higher LV eccentricity index at end systole, with a trend for higher end diastolic eccentricity index (Table 2). During exercise, these differences were amplified, as patients with CpcPH displayed greater LV eccentricity index both at end systole and end diastole (i.e. more septal flattening), with a higher RAP/PAWP ratio, indicating greater dynamic ventricular interdependence (Figure 2). Greater ventricular interaction was further evidenced by reductions in LVTMP during exercise in CpcPH, indicating a decrease in LV pre-load despite marked elevation in PAWP, in contrast to increases in LVTMP during exercise in IpcPH and controls, indicating an increase in LV pre-load (Table 3, Supplementary material online, Figure S4).
Pulmonary function, lung congestion, and gas exchange
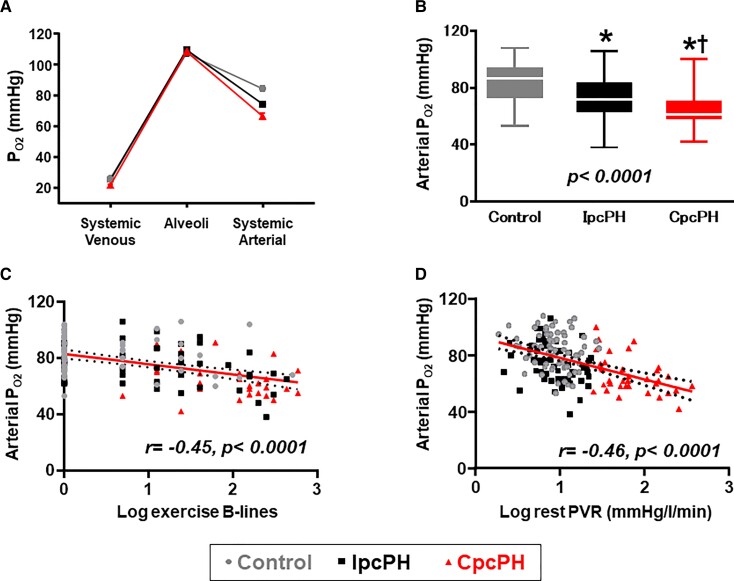
At rest there were no statistically significant differences between CpcPH, IpcPH, and controls in measures of minute ventilation, alveolar ventilation, physiologic shunt, lung diffusion, or alveolar pO2, but there was higher VD/VT, greater EVLW, and slightly lower arterial and venous pO2 in CpcPH (Table 2). Both IpcPH and CpcPH patients displayed higher alveolar-arterial O2 gradient than controls at rest.
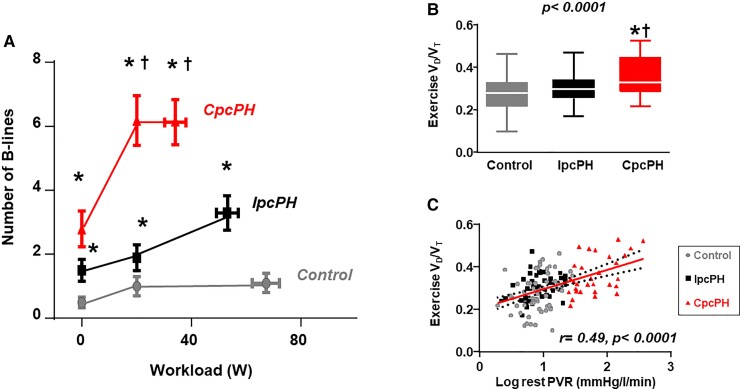
During exercise, patients with CpcPH developed greater increases in EVLW compared with both IpcPH and controls, reflected by increased appearance of B-line artefacts by lung ultrasound (Figure 3), even as PAWP during exercise was similar in CpcPH and IpcPH (Table 3). Participants with CpcPH displayed lower alveolar ventilation, increased dead space ventilation, higher physiologic shunt fraction, lower lung diffusion, lower mixed venous pO2, and increased slope, leading to a greater reduction in arterial O2 tension in CpcPH as compared with both IpcPH and controls (Figure 4). The increases in dead space ventilation and reductions in arterial pO2 during exercise were directly correlated with resting PVR, and arterial hypoxaemia worsened with greater increases in EVLW during exercise (Figure 4). Abnormalities in gas exchange, haemodynamics, and cardiac function in PVD at peak exercise were also observed at a common matched submaximal exercise workload (20 W) (see Supplementary material online, Table S2).
Figure 3.
Participants with combined post- and pre-capillary pulmonary hypertension-left heart disease display greater increases in extravascular lung water during exercise, indicated by increased B-line artefacts on lung ultrasound (A). This was coupled with increases in dead space ventilation (VD/VT ratio), which was directly correlated with resting pulmonary vascular resistance (B, C). EVLW, extravascular lung water; PVR; pulmonary vascular resistance, VD, pulmonary dead space; VT, tidal volume; QT, cardiac output; CpcPH, combined post-and pre-capillary pulmonary hypertension; IpcPH, isolated post-capillary pulmonary hypertension; VO2, oxygen consumption; DO2, oxygen delivery. ‘r’ determined by Pearson’s correlation analysis. *P < 0.05 vs. controls. †P < 0.05 vs. IpcPH groups.
Figure 4.
As compared with individuals with isolated post-capillary pulmonary hypertension and controls, individuals with combined post- and pre-capillary pulmonary hypertension displayed lower arterial pO2 with exercise despite similar alveolar O2 tension (A, B). Arterial pO2 during exercise decreased with greater increases in extravascular lung water during exercise (C) and higher resting pulmonary vascular resistance (D). PA, pulmonary artery; PAP, pulmonary artery pressure; RAP, right atrial pressure; PAWP, pulmonary artery wedge pressure; RV, right ventricular; s′, systolic tissue doppler velocity; QT, cardiac output; CpcPH, combined post-and pre-capillary pulmonary hypertension; IpcPH, isolated post-capillary pulmonary hypertension; VO2, oxygen consumption; DO2, oxygen delivery. ‘r’ determined by Pearson’s correlation analysis. *P < 0.05 vs. controls. †P < 0.05 vs. IpcPH groups.
Sensitivity analyses
Sensitivity analyses excluding patients with lung congestion at rest (B-lines) demonstrated similar results as in the overall population (see Supplementary material online, Tables S3–S5).
Discussion
The present study provides new insights into the pathophysiology of exercise intolerance in patients with PH-LHD and pulmonary vascular disease. Participants with CpcPH were more likely to display conditions associated with microvascular dysfunction, including diabetes and atrial fibrillation, as compared with IpcPH and controls. Participants with CpcPH displayed more deranged pulmonary vascular haemodynamics, impairments in RV–PA coupling, and greater ventricular interdependence leading to profound impairments in cardiac output during exercise. The most novel finding is that patients with CpcPH, who have historically been considered to be protected from lung congestion because of ostensible pre-capillary disease, in fact displayed greater increases in EVLW during exercise, which was associated with increased dead space ventilation, reduced alveolar ventilation, and greater physiologic shunting, ultimately leading to greater ventilation-perfusion mismatch. These abnormalities, in tandem with reductions in lung diffusing capacity and reduction in venous O2 content, compromised arterial O2 tension to a greater extent in CpcPH, further impairing convective O2 delivery (Structured Graphical Abstract). These data provide new pathophysiologic insights into the haemodynamic derangements during stress in LHD with PVD and point to an important and previously under-appreciated role of pulmonary abnormalities in CpcPH.
Left heart disease is the most common cause of PH in the community.1–5 Pulmonary hypertension in LHD first develops as a consequence of passive transmission of downstream LA hypertension, but sustained elevation LA pressure leads to pulmonary vascular remodelling and changes in pulmonary arterial tone leading to increases in PVR and reductions in PA compliance in 13–28% of patients with PH-LHD.1–4,6,7 Patients with this coexisting PVD display more severe right heart failure and increased mortality.8–14,19 Greater understanding of the pathophysiology underlying PVD in PH-LHD is critical to inform the design of novel therapies.
The right heart and ventricular interaction in pulmonary vascular disease
Right ventricular dysfunction at rest is common in PH-LHD and associated with adverse outcomes.15–18 Changes in RV–PA coupling during exercise may be even more important. In Group 1 PH, exercise stress reveals limitations in RV reserve resulting in acute RV dilatation.46 In patients with LHD in the absence of overt PVD, abnormalities in RV–PA coupling become manifest during exercise using imaging-based methods,32 as well as using invasive single-beat estimates of RV–PA coupling.47 Gorter et al.19 found that patients with CpcPH displayed more RV remodelling and dysfunction at rest, but cardiac imaging was not performed during exercise to directly evaluate RV–PA coupling or ventricular interaction.
The present study identified major deficits in the ability to enhance RV systolic function during exercise in CpcPH that were consistent across multiple indices, leading to dramatic limitations in RV–PA coupling.16,18 Impairments in RV–PA coupling were associated with impairments in cardiac output reserve, limiting convective O2 delivery to the tissues, and greater increases in central venous pressure. Thus, the present study confirms and extends earlier studies in Group 1 PH and IpcPH,32,46,47 showing that acute worsening of RV–PA uncoupling plays an even greater role in limiting functional reserve in CpcPH.
In tandem with LA dilation, RV and RA dilatation20 in PH-LHD increases total heart volume and augments pericardial restraint and ventricular interdependence.38,43 Here, we show that this dynamic ventricular interaction becomes much more dramatic during exertion in PVD, evidence by acute increases in LV eccentricity index along with higher RAP/PAWP ratio, indicating greater septal flattening, encroachment on LV filling. This is further evidenced by reduction in LVTMP, limiting the augmentation in LV pre-load, even in the face of marked elevation in pulmonary capillary pressures. These findings emphasize the importance of interventions to improve RV–PA uncoupling during stress to improve left heart filling and lung perfusion.
Pulmonary limitations in pulmonary vascular disease
The most conspicuous lung abnormality in CpcPH relates to its very operational clinical definition: an elevation in PVR.1 The notion that PVR is elevated because of pre-capillary disease in PH-LHD is firmly entrenched in the field. Indeed, Wood48 himself proposed that high PVR in PH-LHD ‘protects’ the lung from congestion, and the hypothesis that PVD effectively protects from left heart overload in PH-LHD persists in the literature.49 The current data argue against this paradigm, showing for the first time that lung congestion is in fact greater at rest in patients with LHD and PVD, and this congestion is exaggerated during exercise compared with patients with IpcPH, even as downstream PAWP was equivalent. What could explain this seemingly paradoxical finding?
In the normal lung circulation, roughly 40% of PVR resides downstream of the capillaries, in the pulmonary veins.50 Recent histopathologic studies have shown that venous remodelling is common in PH-LHD.7,26 If a substantial component of the increase in PVR in PH-LHD is due to venous disease, this would be expected to further pressurize the capillaries out of proportion to the increase in LA pressure, which could lead to greater increases in lung congestion, alterations in ventilation-perfusion matching, and lung diffusion abnormalities, as observed in the present study. While partitioning of PVR into arterial and venous resistance was not performed in the present study, the observation of greater, rather than less EVLW during exercise raises questions regarding the use of PVR to exclusively reflect ‘pre-capillary’ disease in patients with PH-LHD.
In addition to pulmonary venous remodelling,7,26 chronic exposure to LA hypertension causes capillary stress failure and structural remodelling in the alveolar–capillary interface.3 These changes protect the alveolar interstitium from oedema formation51 but this comes at the expense of an impairment in lung diffusion capacity.3 Impaired lung diffusion at rest and with exercise has been repeatedly shown in LHD,27,52,53 even as overt lung parenchymal disease is absent,28 which was also the case in the present study where patients with lung disease were excluded. Indeed, the presence of reduced lung diffusion capacity in PH-LHD is strongly associated with increased mortality.28 In the present study, we also observed that participants with CpcPH displayed greater reduction of DLCO, indicating how PVD also extends to the capillaries. This impairment is likely mediated by acute decreases in alveolar membrane conductance due to lung oedema, as in the present study, as well as the aforementioned chronic remodelling effects. Lung diffusion also varies with capillary blood volume, which may be reduced owing to vascular obliteration in chronic PVD. The individual contributions of membrane conductance and capillary blood volume cannot be assessed from these data but require further study.
There was also evidence for greater ventilation–perfusion (V/Q) mismatch in CpcPH in the present study, suggested by higher VD/VT and slope and a tendency for a greater physiologic shunt. As PVD progresses in LHD, lung perfusion is reduced in more diseased segments, resulting in a higher physiologic dead space (greater VD/VT) in those zones. Conversely, in segments developing increases in EVLW, there may be reductions in the surface area available for gas exchange, reducing V/Q ratio (higher physiologic shunt). Finally, the impairment in QT during exercise led to lower mixed venous pO2 owing to increased peripheral O2 extraction in the setting of reduced delivery. This mixed venous hypoxia further worsens arterial hypoxaemia, particularly when V/Q mismatch is increased.41
Limitations
Individuals participating in this study were referred for invasive testing at a tertiary referral centre, which may introduce bias. Furthermore, individuals in the control group had been referred for invasive cardiopulmonary exercise testing to evaluate the aetiology of dyspnoea, and these patients are thus more ill than healthy volunteers would be. However, this would only be expected to bias our results toward the null, as a truly normal comparator group would be expected to have better cardiopulmonary and exercise reserve. Importantly, in the absence of invasive testing, one cannot readily discern PH-LHD from controls, so the inclusion of this control group is scientifically necessary to test the study hypotheses. There were baseline differences between the groups, including in age, body mass index, and the prevalence of atrial fibrillation, but most of these differences are well-known and believed to be part of the underlying pathogenesis. Spirometry and lung diffusion capacity were missing in approximately half of the patients, but there were no baseline differences in patients with or without these data (see Supplementary material online, Table S1). Ideally, patients should undergo invasive haemodynamic exercise testing in optimized volume status, but in this study, many patients with PH displayed evidence of haemodynamic congestion even at rest. While baseline haemodynamic differences could certainly influence exertional changes, results were similar in a sensitivity analysis excluding patients with lung congestion at rest, and the present results mirror everyday clinical practice, where patients with PH-LHD are remain undertreated for congestion in the absence of invasive haemodynamic monitoring.54 Indeed, another implication of the present data is that the absence of specific therapies for CpcPH only magnifies the importance for aggressive diuresis in such patients, which may be facilitated through the use of implantable monitoring devices. The reversibility of PVR elevation was not assessed in this study, thus we cannot determine to what extent this was related to vasoconstriction, structural remodelling, or both.
Conclusions
Patients with PH-LHD and PVD display specific pathophysiological features during exercise that differ from and are more severe than what is observed in individuals with isolated LA hypertension, including more severe impairments in pulmonary vascular-right heart coupling, greater ventricular interdependence, and more severe pulmonary limitations. Despite the presence of an elevated PVR, these patients display greater lung congestion during exertion, which is coupled with increased dead space ventilation, lower alveolar ventilation, reduced lung diffusing capacity, abnormal ventilatory efficiency, and V/Q mismatching leading to hypoxaemia, which further limits O2 delivery during stress. Further study is required to identify the mechanisms of and therapies for pulmonary vascular disease to improve outcomes in people with LHD and coexisting PVD.
Supplementary Material
Acknowledgements
The authors thank the staff of the Mayo Clinic Earl Wood Catheterization Laboratory and the patients who agreed to participate in the research, allowing for this study to be completed.
Contributor Information
Kazunori Omote, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Hidemi Sorimachi, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Masaru Obokata, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Yogesh N V Reddy, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Frederik H Verbrugge, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA; Centre for Cardiovascular Diseases, University Hospital Brussels, Jette, Belgium; Biomedical Research Institute, Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium.
Massar Omar, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Hilary M DuBrock, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, USA.
Margaret M Redfield, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Barry A Borlaug, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
B.A.B. is supported by R01 HL128526 and U01 HL160226, both from the United States National Institutes of Health. H.S. is supported by a research fellowship from the Uehara Memorial Foundation, Japan. K.O. is supported by Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad and the JSPS Overseas Research Fellowships from the Japan Society for the Promotion of Science. F.H.V. is supported by a Fellowship of the Belgian American Educational Foundation (B.A.E.F.) and by the Special Research Fund (BOF) of Hasselt University (BOF19PD04).
References
- 1. Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017;69:1718–1734. [DOI] [PubMed] [Google Scholar]
- 3. Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered hemodynamics and end-organ damage in heart failure: impact on the lung and kidney. Circulation 2020;142:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reddy YNV, Borlaug BA. Pulmonary hypertension in left heart disease. Clin Chest Med 2021;42:39–58. [DOI] [PubMed] [Google Scholar]
- 5. Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, et al. Increasing incidence and prevalence of world health organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes 2018;11:e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 7. Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 2018;137:1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail 2011;4:644–650. [DOI] [PubMed] [Google Scholar]
- 9. Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail 2013;1:290–299. [DOI] [PubMed] [Google Scholar]
- 10. Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in ‘out-of-proportion’ pulmonary hypertension. Chest 2013;143:758–66. [DOI] [PubMed] [Google Scholar]
- 11. Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018;3:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caravita S, Dewachter C, Soranna D, D'Araujo SC, Khaldi A, Zambon A, et al. Haemodynamics to predict outcome in pulmonary hypertension due to left heart disease: a meta-analysis. Eur Respir J 2018;51:1702427. [DOI] [PubMed] [Google Scholar]
- 13. Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail 2018;11:e004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, et al. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur J Heart Fail 2018;20:248–255. [DOI] [PubMed] [Google Scholar]
- 15. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001;37:183–188. [DOI] [PubMed] [Google Scholar]
- 16. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013;305:H1373– H1381. [DOI] [PubMed] [Google Scholar]
- 17. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, et al. RV Contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017;10:1211–1221. [DOI] [PubMed] [Google Scholar]
- 19. Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghio S, Crimi G, Temporelli PL, Traversi E, La Rovere MT, Cannito A, et al. Haemodynamic effects of an acute vasodilator challenge in heart failure patients with reduced ejection fraction and different forms of post-capillary pulmonary hypertension. Eur J Heart Fail 2018;20:725–734. [DOI] [PubMed] [Google Scholar]
- 22. Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 2012;59:442–451. [DOI] [PubMed] [Google Scholar]
- 23. Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 2000;102:1718–1723. [DOI] [PubMed] [Google Scholar]
- 24. Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, Escribano P, et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 2005;7:1011–1016. [DOI] [PubMed] [Google Scholar]
- 25. Tandon HD, Kasturi J. Pulmonary vascular changes associated with isolated mitral stenosis in India. Br Heart J 1975;37:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leopold JA. Pulmonary venous remodeling in pulmonary hypertension: the veins take center stage. Circulation 2018;137:1811–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olson TP, Johnson BD, Borlaug BA. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail 2016;4:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail 2016;4:441–449. [DOI] [PubMed] [Google Scholar]
- 29. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reddy YNV, Carter RE, Obokata M, Redfield MM, Simple BBA. Evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 32. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017;50:1700578. [DOI] [PubMed] [Google Scholar]
- 36. Naeije R, Chin K. Differentiating precapillary from postcapillary pulmonary hypertension. Circulation 2019;140:712–714. [DOI] [PubMed] [Google Scholar]
- 37. Smiseth OA, Thompson CR, Ling H, Robinson M, Miyagishima RT. A potential clinical method for calculating transmural left ventricular filling pressure during positive end-expiratory pressure ventilation: an intraoperative study in humans. J Am Coll Cardiol 1996;27:155–160. [DOI] [PubMed] [Google Scholar]
- 38. Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail 2019;7:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 2017;19:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. West JB. State of the art: ventilation-perfusion relationships. Am Rev Respir Dis 1977;116:919–943. [DOI] [PubMed] [Google Scholar]
- 42. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 43. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol 2020;76:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gorter TM, van Melle JP, Rienstra M, Borlaug BA, Hummel YM, van Gelder IC, et al. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail 2018;24:177–185. [DOI] [PubMed] [Google Scholar]
- 45. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 2016;133:2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh I, Rahaghi FN, Naeije R, Oliveira RKF, Systrom DM, Waxman AB. Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: role of pulmonary vascular dysfunction. Chest 2019;156:933–943. [DOI] [PubMed] [Google Scholar]
- 48. Wood P. An appreciation of mitral stenosis: II. Investigations and results. Br Med J 1954;1:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Inampudi C, Silverman D, Simon MA, Leary PJ, Sharma K, Houston BA, et al. Pulmonary hypertension in the context of heart failure with preserved ejection fraction. Chest 2021;160:2232–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gaar KA Jr, Taylor AE, Owens LJ, Guyton AC. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am J Physiol 1967;213:910–914. [DOI] [PubMed] [Google Scholar]
- 51. Huang W, Kingsbury MP, Turner MA, Donnelly JL, Flores NA, Sheridan DJ. Capillary filtration is reduced in lungs adapted to chronic heart failure: morphological and haemodynamic correlates. Cardiovasc Res 2001;49:207–17. [DOI] [PubMed] [Google Scholar]
- 52. Agostoni P, Cattadori G, Bianchi M, Wasserman K. Exercise-induced pulmonary edema in heart failure. Circulation 2003;108:2666–2671. [DOI] [PubMed] [Google Scholar]
- 53. Olson LJ, Snyder EM, Beck KC, Johnson BD. Reduced rate of alveolar-capillary recruitment and fall of pulmonary diffusing capacity during exercise in patients with heart failure. J Card Fail 2006;12:299–306. [DOI] [PubMed] [Google Scholar]
- 54. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.