Abstract
This cross-sectional study examines the association between hyperuricemia and cardiovascular diseases (CVDs). Data from the Korean Genome and Epidemiology Study from 2004 to 2016 were analyzed. Among the 173,209 participants, we selected 11,453 patients with hyperuricemia and 152,255 controls (non-hyperuricemia). We obtained the history of CVDs (stroke and ischemic heart disease [IHD]) from all participants. Crude and adjusted odds ratios (aORs) (age, income group, body mass index, smoking, alcohol consumption, anthropometry data, and nutritional intake) for CVDs were analyzed using a logistic regression model. Participants with hyperuricemia reported a significantly higher prevalence of stroke (2.4% vs 1.3%) and IHD (5.6% vs 2.8%) than controls did (P < .001). Participants with hyperuricemia had a significantly higher aOR for CVD than the controls. The aOR of hyperuricemia for stroke was 1.22 (95% confidence interval = 1.07–1.39, P = .004). When analyzed by subgroup according to age and sex, this result was only persistent in women. The aOR of hyperuricemia for IHD was 1.45 (95% confidence interval = 1.33–1.59, P < .001). In the subgroup analyses, the results were similar, except in young men. Hyperuricemia was significantly associated with CVD in the Korean population.
Keywords: cardiovascular abnormalities, cohort studies, hyperuricemia, myocardial ischemia, stroke
1. Introduction
Hyperuricemia is caused by elevated uric acid in the blood,[1] and diagnoses have increased in the US over the past 20 years.[2] In Korea, the prevalence of gout has multiplied 4.4-fold within the last 15 years.[3] Asymptomatic hyperuricemia is related to multiple diseases, including coronary artery disease, chronic kidney disease, hypertension, and diabetes.[4] Reports show that elevated uric acid increases all-cause mortality (risk ratio [RR] 1.24, confidence interval [CI] 1.09–1.42) and cardiovascular mortality (RR 1.37, CI 1.19–1.57).[5] European guidelines on arterial hypertension state that uric acid can influence an individual’s cardiovascular risk.[6]
Cardiovascular disease (CVD) comprises coronary heart disease, heart failure, stroke, and hypertension,[7] and caused 17.9 million deaths globally in 2015.[8] In Korea, the ischemic heart disease (IHD) mortality rate and hospitalization rate have gradually risen in the last decade.[9,10] The percentage of people with >2 risk factors increases from 14.7% in 20 to 29-year-olds to 58.4% in those >70 years of age.[9] According to national data, cardiovascular risk factors, such as obesity, hypertension, diabetes mellitus, and dyslipidemia, have also increased.[11]
Hyperuricemia leads to CVD and chronic kidney disease by pathological induction of vascular smooth muscle cell proliferation and endothelial dysfunction, inducing inflammation.[12] There are conflicting results regarding the association between serum uric acid levels and CVD. In the Framingham Heart Study, uric acid was predictive of coronary heart disease in women, but it lost its significance after adjustment.[13] In contrast, the RR for coronary heart disease incidence in hyperuricemia was 1.21 (CI 1.07–1.36, P = .003) in 1 meta-analysis.[14] The pooled RR of stroke for the high-versus-low uric acid categories was 1.22 (CI 1.15–1.30) in another meta-analysis.[15] However, the heterogeneity (Q = 33.6, I2 = 67.3%) of previous meta-analyses,[14] the use of relatively old data, and the analysis of uric acid levels by grouping rather than by the hyperuricemia criteria have confounded meaningful interpretation.
Our hypothesis is that elevated uric acid levels are associated with CVD. Comorbid conditions and variations in anthropometry data could influence the association between hyperuricemia and CVD. This study investigated the association between hyperuricemia and CVD using national data through a cross-sectional study design. We matched hyperuricemia patients with control participants for age, sex, income, obesity, smoking, alcohol consumption, anthropometry data, and nutritional intake. Additionally, we performed a subgroup analysis based on age and sex.
2. Methods
2.1. Study population and data collection
The use of these data was approved by the ethics committee of Hallym University (2019-02-020). The requirement for written informed consent was waived by the Institutional Review Board. This prospective cohort study used data from the Korean Genome and Epidemiology Study (KoGES) from 2004 to 2016. A comprehensive description of these data was provided in a previous study.[16]
Among the KoGES Consortium, we included the KoGES health examinee (HEXA) data of urban residence participants aged ≥40 years. It consisted of baseline data from 2004 to 2013 and follow-up data from to 2012 to 2016.
2.2. Participant selection
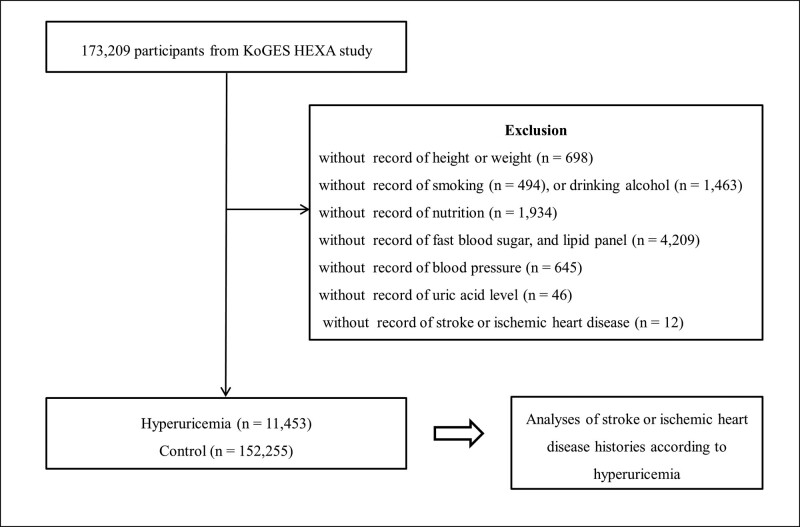
Participants who had no records of height or weight (n = 698), smoking history (n = 494), alcohol consumption habits (n = 1463), nutrition records (n = 1934), hypertension, diabetes mellitus, and hyperlipidemia histories (n = 125), fast blood sugar, lipid panels (n = 4209), uric acid measurement (n = 46), blood pressure (n = 645), stroke, or IHD (n = 12) were excluded from the pool of 173,209 participants. Finally, 11,453 participants with hyperuricemia and 152,255 control participants (non-hyperuricemia) were selected (Fig. 1). We then analyzed the history of cerebral stroke or IHD in participants with or without hyperuricemia.
Figure 1.
A schematic illustration of the participant selection process used in the present study. Out of 173,209 participants, 11,453 have hyperuricemia and 152,255 are controls (non-hyperuricemia group).
2.3. Survey
Trained interviewers asked participants about their prior histories of cerebral stroke (ischemic or hemorrhagic) and IHD (myocardial infarction or angina). We defined hyperuricemia as >7.0 mg/dL in men[2] and >6.0 mg/dL in women,[17] as outlined in previous studies. Systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), fasting blood sugar (mg/dL), total cholesterol (mg/dL), triglycerides (mg/dL), and high-density lipoprotein (HDL) cholesterol (mg/dL) were obtained from the health checkup data. Using health checkup data of weight and height, body mass index (BMI) was calculated in kg/m2. Smoking history was categorized as nonsmoker (<100 cigarettes throughout life), past smokers (quit for >1 year), and current smokers. Alcohol consumption was categorized as nondrinkers, past drinkers, and current drinkers. Participant nutritional intake (total calories [kcal/day], protein [g/day], fat [g/day], and carbohydrate [g/day]) was surveyed using a food-frequency questionnaire, which was validated in a previous study.[18] Income grouping was categorized into non-respondent, low-income (<$2000 per month), middle income ($2000–$3999 per month), and high income (≥$4000 per month) categories by household income.
2.4. Statistical analyses
Chi-square tests were used to compare the rates of sex, income group, smoking, alcohol consumption, and stroke and IHD history. To compare age, systolic blood pressure, diastolic blood pressure, fasting blood sugar, total cholesterol, triglycerides, HDL cholesterol, nutritional intake, and BMI, independent t tests were used.
A logistic regression model was used to analyze the odds ratio (OR) of hyperuricemia for stroke/IHD. Crude and adjusted models (age, income group, BMI, smoking, alcohol consumption, anthropometric data [systolic blood pressure, diastolic blood pressure, fasting blood sugar, total cholesterol, triglycerides, and HDL cholesterol], and nutritional intake [total calories, protein, fat, and carbohydrate]) were used. In the subgroup analyses according to age, the dividing point was determined by the median age (≤52 years and ≥53 years).
Two-tailed analyses were performed, and P values <.05 were considered significant. The results were statistically analyzed using SPSS (version 24.0; IBM, Armonk, NY).
3. Results
The general characteristics of hyperuricemia and control participants were not uniform (Table 1). The adjusted OR (aOR) of hyperuricemia for stroke was 1.22 (95% CI = 1.07–1.39, P = .004, Table 2). In subgroups based on age and sex, the results were persistent only in women. The aORs were 1.06 (95% CI = .71–1.58) in ≤52-year-old men; 2.00 (95% CI = 1.12–3.57) in ≤52-year-old women; 1.14 (95% CI = .95–1.37) in ≥53-year-old men; and 1.38 (95% CI = 1.09–1.75) in ≥53-year-old women.
Table 1.
General characteristics of participants.
| Characteristics | Total participants | P value | |
|---|---|---|---|
| Hyperuricemia | Control | ||
| Age (mean, SD, yr) | 55.0 (8.7) | 53.0 (8.3) | <.001* |
| Sex (n, %) | <.001* | ||
| Men | 7537 (65.8) | 48,454 (31.8) | |
| Women | 3916 (34.2) | 103,801 (68.2) | |
| BMI (mean, SD, kg/m2) | 25.5 (3.0) | 23.8 (2.9) | <.001* |
| Income (n, %) | <.001* | ||
| Missing, no response | 1990 (17.4) | 23,550 (15.5) | |
| Lowest | 3176 (27.7) | 41,607 (27.3) | |
| Middle | 3926 (34.3) | 55,271 (36.3) | |
| Highest | 2361 (20.6) | 31,827 (20.9) | |
| Smoking status (n, %) | <.001* | ||
| Non-smoker | 5620 (49.1) | 113,715 (74.7) | |
| Past smoker | 3415 (29.8) | 20,620 (13.5) | |
| Current smoker | 2418 (21.1) | 17,920 (11.8) | |
| Alcohol consumption (n, %) | <.001* | ||
| Non-drinker | 3894 (34.0) | 79,304 (52.1) | |
| Past drinker | 713 (6.2) | 5654 (3.7) | |
| Current drinker | 6846 (59.8) | 67,297 (44.2) | |
| Nutritional intake | |||
| Total calories (kcal/d) | 1786.2 (592.5) | 1756.8 (590.2) | <.001* |
| Protein (g/d) | 61.0 (27.2) | 59.8 (27.1) | <.001* |
| Fat (g/d) | 29.0 (18.8) | 28.1 (18.7) | <.001* |
| Carbohydrate (g/d) | 315.6 (96.5) | 312.0 (96.6) | <.001* |
| Anthropometry data | |||
| Systolic blood pressure (mm Hg) | 127.9 (15.6) | 122.3 (15.4) | <.001* |
| Diastolic blood pressure (mm Hg) | 79.7 (10.1) | 75.9 (10.0) | <.001* |
| Fasting blood sugar (mg/dL) | 99.6 (24.7) | 94.9 (21.4) | <.001* |
| Total cholesterol (mg/dL) | 204.2 (39.1) | 196.9 (24.7) | <.001* |
| Triglyceride (mg/dL) | 183.0 (127.9) | 122.7 (84.7) | <.001* |
| HDL-cholesterol (mg/dL) | 49.0 (12.0) | 54.4 (12.9) | <.001* |
| Stroke (n, %) | 275 (2.4) | 2021 (1.3) | <.001* |
| Ischemic heart disease (n, %) | 641 (5.6) | 4309 (2.8) | <.001* |
BMI = body mass index, HDL = high density lipoprotein, SD = standard deviation.
Chi-square test. Significance at P < .05.
Table 2.
Crude and adjusted odd ratios (95% confidence interval) for stroke in hyperuricemia and control groups.
| Characteristics | Odd ratios for stroke | |||
|---|---|---|---|---|
| Crude | P value | Adjusted† | P value | |
| Total participants (n = 163,708) | ||||
| Hyperuricemia | 1.83 (1.61–2.08) | <.001* | 1.22 (1.07–1.39) | .004* |
| Control | 1.00 | 1.00 | ||
| Age ≤52 yr, men (n = 25,631) | ||||
| Hyperuricemia | 1.11 (0.75–1.64) | .588 | 1.06 (0.71–1.58) | .766 |
| Control | 1.00 | 1.00 | ||
| Age ≤52 yr, women (n = 56,258) | ||||
| Hyperuricemia | 3.00 (1.71–5.26) | <.001* | 2.00 (1.12–3.57) | .019* |
| Control | 1.00 | 1.00 | ||
| Age ≥53 yr, men (n = 30,360) | ||||
| Hyperuricemia | 1.21 (1.02–1.45) | .034* | 1.14 (0.95–1.37) | .161 |
| Control | 1.00 | 1.00 | ||
| Age ≥53 yr, women (n = 51,459) | ||||
| Hyperuricemia | 1.76 (1.40–2.21) | <.001* | 1.38 (1.09–1.75) | .008* |
| Control | 1.00 | 1.00 | ||
Logistic regression model, significance at P < .05.
Models adjusted for age, income group, body mass index (BMI), smoking, alcohol consumption, anthropometry data (systolic blood pressure, diastolic blood pressure, fasting blood sugar, total cholesterol, triglyceride, and high density lipoprotein (HDL)-cholesterol), and nutritional intake (total calories, protein, fat, and carbohydrate).
The aOR of hyperuricemia for IHD was 1.45 (95% CI = 1.33–1.59, P < .001, Table 3). In subgroups based on age and sex, the results were consistent, except in young men. The aORs were 1.14 (.88–1.49) in ≤52-year-old men; 2.19 (1.46–3.29) in ≤52-year-old women; 1.47 (1.30–1.66) in ≥53-year-old men; and 1.53 (1.30–1.79) in ≥53-year-old women.
Table 3.
Crude and adjusted odd ratios (95% confidence interval) for ischemic heart disease in hyperuricemia and control groups.
| Characteristics | Odd ratios for ischemic heart disease | |||
|---|---|---|---|---|
| Crude | P value | Adjusted† | P value | |
| Total participants (n = 163,708) | ||||
| Hyperuricemia | 2.04 (1.87–2.22) | <.001* | 1.45 (1.33–1.59) | <.001* |
| Control | 1.00 | 1.00 | ||
| Age ≤52 yr, men (n = 25,631) | ||||
| Hyperuricemia | 1.16 (0.90–1.49) | .262 | 1.14 (0.88–1.49) | .315 |
| Control | 1.00 | 1.00 | ||
| Age ≤52 yr, women (n = 56,258) | ||||
| Hyperuricemia | 2.99 (2.02–4.43) | <.001* | 2.19 (1.46–3.29) | <.001* |
| Control | 1.00 | 1.00 | ||
| Age ≥53 yr, men (n = 30,360) | ||||
| Hyperuricemia | 1.44 (1.27–1.62) | <.001* | 1.47 (1.30–1.66) | <.001* |
| Control | 1.00 | 1.00 | ||
| Age ≥53 yr, women (n = 51,459) | ||||
| Hyperuricemia | 1.88 (1.61–2.19) | <.001* | 1.53 (1.30–1.79) | <.001* |
| Control | 1.00 | 1.00 | ||
Logistic regression model, significance at P < .05.
Models adjusted for age, income group, body mass index (BMI), smoking, alcohol consumption, anthropometry data (systolic blood pressure, diastolic blood pressure, fasting blood sugar, total cholesterol, triglyceride, and high density lipoprotein (HDL)-cholesterol), and nutritional intake (total calories, protein, fat, and carbohydrate).
4. Discussion
The association with CVD was larger in the hyperuricemia group than in the matched control group in the Korean population in this study. When grouped according to sex, the association between hyperuricemia and CVD was not evident in men after adjusting for other possible confounders. Hyperuricemia was strongly associated with stroke and IHD in women of all ages and associated with IHD only in older men. This study analyzed the largest number of subjects of any study published in the last decade and any study conducted in Korea. The anthropometric data used in this study included various laboratory results that may affect or be affected by CVD.
Using the terms “hyperuricemia,” “cardiovascular diseases,” “myocardial ischemia,” “ischemic heart disease,” and “stroke,” we explored PubMed and Embase and confined our search to English articles published before December 2021. There were 2 studies that investigated both cerebrovascular and coronary vascularization in hyperuricemia. A Taiwanese study also revealed an increased risk of stroke (RR 2.00 for men and 2.75 for women) and IHD (RR 2.45 for men and 3.96 for women) in patients with hyperuricemia.[19] However, the previous study only calculated RR, the control and hyperuricemic groups were not matched, and it was conducted on a rural population with relatively old data, so trends were difficult to demonstrate. A recent Italian study reported that the association between uric acid levels and CVD risk was observed only in men.[20] The highest quartile for uric acid level (uric acid >6.5 mg/dL) in men had an increased risk of CVD (hazard ratio [HR] 2.55 [1.41–4.62]) after adjustment. Because they calculated uric acid levels as quartiles, only men’s quartiles were close to the criteria for hyperuricemia. Furthermore, their study included a limited number of participants with moderate to high CVD risk. The advantage of our study was that it calculated the ORs of 2 different diseases using each criterion for hyperuricemia in men and women.
Patients with hyperuricemia had an increased aOR (1.22 [1.07–1.39]) of stroke, consistent with 2 meta-analyses (RR 1.22, and RR 1.41, respectively).[21,22] In the subgroup analysis, the results were duplicated only in the female participant groups in our study (ORs of 1.38–2.00), same as in a previous studies (HR 1.32 [1.00–1.73])[19] (OR 1.888 [1.244–2.864]).[23] The risk of hemorrhagic stroke for increased uric acid was statistically significant only in women in 1 meta-analysis (HR 1.19 [1.04–1.35]).[24] The authors suggested that women have a longer lifespan, greater vulnerability to depression and anxiety, and a higher stress level, which may cause differences. Additionally, key risk factors for stroke are more frequent in women, and the effects of diabetes mellitus (RR 2.28) and atrial fibrillation (RR 1.99) on stroke are stronger in women than in men.[25] In 1 meta-analysis, uric acid levels showed a J-shaped trend in men and a linear trend in women for the risk of stroke,[24] while stroke risk increased significantly from 6 mg/dL uric acid, which is similar to normal levels.[26] In men, it can be assumed that there is a compensatory mechanism for a certain amount of uric acid.
Hyperuricemia was associated with IHD (aOR 1.45 [1.33–1.59]) in this study, consistent with the risk of cardiovascular events (RR 1.35 [1.12–1.62])[27] and coronary heart disease (RR 1.34 [1.19–1.49]) in 1 meta-analysis.[28] In this study, the results were consistent in all age groups, but the risk of coronary heart disease increased only in women (RR 1.446 [1.323–1.581]) in another meta-analysis.[14] The authors suggested that differences in epidemiology and mortality may influence the results; the recurrence rate and mortality after the first event were higher in women. A recent cohort study also showed an independent correlation between hyperuricemia and coronary artery disease (OR 1.509 [1.106–2.057]) only in women.[29] In our study, the association between IHD and hyperuricemia was significantly high only in older men.
In a study of uric acid level and metabolic syndrome, men had higher cutoffs than women of all ages, which was close to hyperuricemia (6.5 mg/dL) in patients aged <50 years.[30] Based on these findings, we should focus on older men and women of all ages whose uric acid levels are within normal ranges. Additional studies are required to explain the practical role of age in adult men with hyperuricemia.
Accumulating evidence indicates that hyperuricemia may be an indicator or contribute to the pathogenesis of heart failure, coronary artery disease, chronic kidney disease, atrial fibrillation, hypertension, and cardiovascular death.[31] High uric acid inhibits insulin signaling and increases oxidative stress and insulin resistance in cardiomyocytes both in vitro and in vivo.[32] Hyperuricemia is associated with a larger myocardial infarction area, lower left ventricular ejection fraction, and higher atrial fibrillation.[33] Moreover, high uric acid induces cardiomyocyte mitophagy activation through the reactive oxygen species/CaMKIIδ/Parkin pathway axis, which is a pathogenic process of CVD.[34] Patients with hyperuricemia had a higher risk of CVD in 1 meta-analysis (standardized mean differences .264 [.161–.366]) and had increased carotid intima-media thickness compared to controls.[35] The possible mechanisms between uric acid and arterial stiffness include increased systemic inflammation and oxidative stress by hyperuricemia.[36]
Uric acid has 2 contrasting roles as both a pro-oxidant and an antioxidant. In experimental studies, hyperuricemia promotes the occurrence and development of CVD by regulating endoplasmic reticulum stress, insulin resistance, oxidative stress, and endothelial dysfunction.[37] Although uric acid acts as a scavenger of free radicals and singlet oxygen, high uric acid levels lead to endothelial dysfunction and maximize platelet adhesion,[38] potentially initiating a cascade of coagulation, stimulating thrombus formation and arterial occlusion, which progress to intracranial atherosclerosis.[39] Recent studies have suggested an association between uric acid and both hypertension and metabolic syndrome,[37] which can cause stroke. In a recent animal study, increased uric acid levels activated the myocyte enhancer factor-2C-dependent and nuclear factor-κB pathways by let-7c and generating thrombosis.[40]
Despite the large population database, this study had several limitations. First, data from the KoGES did not have all the records regarding potential confounders, including treatment of hyperuricemia, duration of disease, drug intake, and coronary angiography procedure; as such, the results should be interpreted with caution. Second, our results could be subjective or inaccurate compared to clinical data, as we used a questionnaire survey. However, the KoGES cohort study has been conducted consistently since 2004, and there is an advantage in terms of continuity. Above all, the fact that hyperuricemia was accurately diagnosed using blood test values of >160,000 people is a great advantage over any other study. Third, the causal relationship between hyperuricemia and CVD was not elucidated because of the cross-sectional study design. However, this study analyzed a large representative dataset of the general population in the country, resulting in strong statistical power. Lastly, our results might not be generalizable to younger people, as we only included participants >40 years of age. Despite these limitations, we demonstrated the association between hyperuricemia and CVDs, which differs according to age and sex. We found that hyperuricemia may be associated with CVD in women of all ages. An additional strength of this study was that we included anthropometric data and included a large number of asymptomatic low-risk participants.
In conclusion, this study demonstrated the association between hyperuricemia and CVD, suggesting that clinicians should consider treating asymptomatic hyperuricemia. This study broadens previous findings on the potential association between hyperuricemia and CVD by considering many confounders and using a large population-matched cohort. Our study presents a possible answer to whether the level of uric acid for hyperuricemia can act as a cutoff value for the occurrence of 2 types of CVD.
Acknowledgment
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
Conceptualization: Hyo Geun Choi.
Data curation: Hyo Geun Choi.
Formal analysis: Hyo Geun Choi, Jung Woo Lee.
Funding acquisition: Hyo Geun Choi, Jung Woo Lee.
Investigation: Hyo Geun Choi, Jung Woo Lee.
Methodology: Hyo Geun Choi.
Project administration: Hyo Geun Choi.
Resources: Hyo Geun Choi.
Software: Hyo Geun Choi.
Supervision: Hyo Geun Choi, Mi Jung Kwon, Joo-Hee Kim, Ji Hee Kim, Bong Cheol Kwon.
Validation: Hyo Geun Choi, Jung Woo Lee.
Visualization: Hyo Geun Choi, Sang Jun Lee, Jung Woo Lee.
Writing – original draft: Jung Woo Lee.
Writing – review & editing: Joo-Hee Kim, Mi Jung Kwon, Hyo Geun Choi, Sang Jun Lee, Sung-Woo Kim, Ji Hee Kim, Bong Cheol Kwon, Jung Woo Lee.
Abbreviations:
- aOR =
- adjusted odds ratio
- BMI =
- body mass index
- CI =
- confidence interval
- CVD =
- cardiovascular disease
- HDL =
- high-density lipoprotein
- HR =
- hazard ratio
- IHD =
- ischemic heart disease
- KoGES =
- Korean Genome and Epidemiology Study
- OR =
- odds ratio
- RR =
- risk ratio
J-HK and MJK contributed equally to this work.
This work was supported in part by a research grant (NRF-2021-R1C1C1004986,2021R1I1A1A01059476) from the National Research Foundation (NRF) of Korea, the Hallym University Research Fund (HURF 2019-31), and National Information Society Agency (NIA), and funded by the ministry of Science and ICT through the Lifelog Big Data Platform and Center Construction Project (No. 2022-Data-W18).
The study was conducted according to the guidelines of the Declaration of Helsinki. The use of these data was approved by the ethics committee of Hallym University (2019-02-020). The requirement for written informed consent was waived by the Institutional Review Board.
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
How to cite this article: Kim J-H, Kwon MJ, Choi HG, Lee SJ, Kim S-W, Kim JH, Kwon BC, Lee JW. The association between hyperuricemia and cardiovascular disease history: A cross-sectional study using KoGES HEXA data. Medicine 2022;101:51(e32338).
Contributor Information
Joo-Hee Kim, Email: kimjihee.ns@gmail.com.
Mi Jung Kwon, Email: bckwon@hallym.or.kr.
Hyo Geun Choi, Email: pupen@naver.com.
Sang Jun Lee, Email: berrybearlee@gmail.com.
Sung-Woo Kim, Email: kimjihee.ns@gmail.com.
Ji Hee Kim, Email: kimjihee.ns@gmail.com.
Bong Cheol Kwon, Email: bckwon@hallym.or.kr.
References
- [1].Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443–52. [DOI] [PubMed] [Google Scholar]
- [2].Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136–41. [DOI] [PubMed] [Google Scholar]
- [3].Kim Y, Kang J, Kim GT. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol. 2018;37:2529–38. [DOI] [PubMed] [Google Scholar]
- [4].Yip K, Cohen RE, Pillinger MH. Asymptomatic hyperuricemia: is it really asymptomatic? Curr Opin Rheumatol. 2020;32:71–9. [DOI] [PubMed] [Google Scholar]
- [5].Zhao G, Huang L, Song M, et al. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231:61–8. [DOI] [PubMed] [Google Scholar]
- [6].Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104. [DOI] [PubMed] [Google Scholar]
- [7].Okoth K, Wang J, Zemedikun D, et al. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: a retrospective matched cohort study. BJOG. 2021;128:1598–609. [DOI] [PubMed] [Google Scholar]
- [8].Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee HH, Cho SMJ, Lee H, et al. Korea Heart Disease Fact Sheet 2020: analysis of nationwide data. Korean Circ J. 2021;51:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang J-H, Lee B-H, Eum K-S, et al. Prevalence of gastrointestinal and cardiovascular risk in patients with degenerative lumbar spinal disease. Clin Orthop Surg. 2020;12:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rhee EJ. Prevalence and current management of cardiovascular risk factors in Korean Adults Based on Fact Sheets. Endocrinol Metab (Seoul). 2020;35:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yanai H, Adachi H, Hakoshima M, et al. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int J Mol Sci. 2021;22:9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. [DOI] [PubMed] [Google Scholar]
- [14].Braga F, Pasqualetti S, Ferraro S, et al. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016;54:7–15. [DOI] [PubMed] [Google Scholar]
- [15].Dong Y, Shi H, Chen X, et al. Serum uric acid and risk of stroke: a dose-response meta-analysis. J Clin Biochem Nutr. 2021;68:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim Y, Han BG. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol. 2017;46:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin KC, Lin HY, Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J Rheumatol. 2000;27:1045–50. [PubMed] [Google Scholar]
- [18].Ahn Y, Kwon E, Shim JE, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–41. [DOI] [PubMed] [Google Scholar]
- [19].Chien KL, Hsu HC, Sung FC, et al. Hyperuricemia as a risk factor on cardiovascular events in Taiwan: the Chin-Shan community cardiovascular cohort study. Atherosclerosis. 2005;183:147–55. [DOI] [PubMed] [Google Scholar]
- [20].Mannarino MR, Pirro M, Gigante B, et al. Association between uric acid, carotid intima-media thickness, and cardiovascular events: prospective results from the IMPROVE study. J Am Heart Assoc. 2021;10:e020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li M, Hou W, Zhang X, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis. 2014;232:265–70. [DOI] [PubMed] [Google Scholar]
- [22].Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun P, Chen M, Guo X, et al. Combined effect of hypertension and hyperuricemia on ischemic stroke in a rural Chinese population. BMC Public Health. 2021;21:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qiao T, Wu H, Peng W. The relationship between elevated serum uric acid and risk of stroke in adult: an updated and dose-response meta-analysis. Front Neurol. 2021;12:674398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cordonnier C, Sprigg N, Sandset EC, et al. Stroke in women - from evidence to inequalities. Nat Rev Neurol. 2017;13:521–32. [DOI] [PubMed] [Google Scholar]
- [26].Zhong C, Zhong X, Xu T, et al. Sex-specific relationship between serum uric acid and risk of stroke: a dose-response meta-analysis of prospective studies. J Am Heart Assoc. 2017;6:e005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao L, Cao L, Zhao TY, et al. Cardiovascular events in hyperuricemia population and a cardiovascular benefit-risk assessment of urate-lowering therapies: a systematic review and meta-analysis. Chin Med J (Engl). 2020;133:982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;62:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lan M, Liu B, He Q. Evaluation of the association between hyperuricemia and coronary artery disease: a STROBE-compliant article. Medicine (Baltim). 2018;97:e12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jeong J, Suh YJ. Association between serum uric acid and metabolic syndrome in Koreans. J Korean Med Sci. 2019;34:e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saito Y, Tanaka A, Node K, et al. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78:51–7. [DOI] [PubMed] [Google Scholar]
- [32].Zhi L, Yuzhang Z, Tianliang H, et al. High uric acid induces insulin resistance in cardiomyocytes in vitro and in vivo. PLoS One. 2016;11:e0147737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hajizadeh R, Ghaffari S, Salehi R, et al. Association of serum uric acid level with mortality and morbidity of patients with acute ST-elevation myocardial infarction. J Cardiovasc Thorac Res. 2016;8:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gao K, Li Y, Su Y, et al. High uric acid promotes mitophagy through the ROS/CaMKIIδ/Parkin pathway in cardiomyocytes in vitro and in vivo. Am J Transl Res. 2021;13:8754–65. [PMC free article] [PubMed] [Google Scholar]
- [35].Peng LH, He Y, Xu WD, et al. Carotid intima-media thickness in patients with hyperuricemia: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33:2967–77. [DOI] [PubMed] [Google Scholar]
- [36].Albu A, Para I, Porojan M. Uric acid and arterial stiffness. Ther Clin Risk Manag. 2020;16:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol. 2020;11:582680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Glantzounis GK, Tsimoyiannis EC, Kappas AM, et al. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–51. [DOI] [PubMed] [Google Scholar]
- [39].Tariq MA, Shamim SA, Rana KF, et al. Serum uric acid - Risk factor for acute ischemic stroke and poor outcomes. Cureus. 2019;11:e6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cheng X, Liu T, Ma L, et al. Prothrombotic effects of high uric acid in mice via activation of MEF2C-dependent NF-κB pathway by upregulating let-7c. Aging (Albany NY). 2020;12:17976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]