Background:
This systematic review and meta-analysis aimed to assess the association of hypernatremia with the outcomes of COVID-19 patients.
Methods:
We performed a systematic literature search on PubMed, Google Scholar, and Science Direct until October 2021 and found a total of 131 papers. With meticulous screening finally, 17 papers met the inclusion criteria. COVID-19 patients with sodium levels greater than the reference level were the study population and the outcome of interest was the poor outcome; such as mortality, mechanical ventilation, intensive care unit (ICU) admission, and prolonged hospital stay. The pooled estimate was calculated as the odds ratio (OR).
Results:
There were 19,032 patients with hypernatremia in the 17 studies included. An overall random effect meta-analysis showed that hypernatremia was associated with mortality (OR: 3.18 [1.61, 6.28], P < .0001, I2 = 91.99%), prolong hospitalization (OR: 1.97 [1.37, 2.83], P < .001, I2 = 0.00%) and Ventilation (OR: 5.40 [1.89, 15.42], P < .001, I2 = 77.35%), ICU admission (OR: 3.99 [0.89, 17.78], P = .07, I2 = 86.79%). Meta-regression analysis showed the association of age with the ICU outcome of hypernatremia patients. Whereas, other parameters like male, hypertension, chronic kidney disease, and diabetes mellitus did not significantly influence the odds ratio.
Conclusion:
Hypernatremia was markedly associated with poor outcomes in patients with COVID-19. Hence, a blood ionogram is warranted and special attention must be given to hypernatremia COVID-19 patients.
Keywords: coronavirus, hypernatremia, mortality, sodium
1. Introduction
The newly identified coronavirus illness (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has proven to be the worst global public health problem. Although the majority of COVID-19 patients have mild to moderate symptoms, a considerable proportion of patients have developed organ failure, necessitating critical care.[1] In this context, risk stratification for early identification of patients requiring critical care and those at high risk of death is paramount for efficient human and medical resource allocation. Various clinical and laboratory prognostic factors, that can effectively predict the severity and mortality of patients have been recognized in COVID-19. The clinical factors that are ascertained to have a positive influence on the increasing mortality among COVID-19 include the patients’ characteristics like the presence of diabetes mellitus, obesity, or ischemic heart disease, as well as older age, male sex, and, Black or Asian ethnicity.[2–5] On a similar note, elevated levels of white cell count, neutrophil count, C-reactive protein, urea, creatinine, transaminases, cardiac troponin I, and D-dimer, as well as low lymphocyte count and hypoalbuminemia, are some identified laboratory markers linked to an increased risk of COVID-19 associated death in the hospital.[6–8]
As per some earlier research, the changes in serum sodium, potassium, chloride, and calcium could be 1 of the closely related factors linked to COVID-19 and its severity.[9] Among these, dysnatremia has been commonly observed in hospitalized patients and is discerned as a potential risk factor for increased mortality, admission to medical critical care units, and a longer stay in the hospital among COVID-19 patients.[10,11]
Hypernatremia (defined as serum [Na+] > 145 meq/L) is caused by primary water deficit (with or without Na + loss) and frequently occurs because of inadequate access to water or impaired thirst mechanism. Recent studies have shown a more pronounced and much higher incidence of hypernatremia in COVID-19 patients, pointing to the fact that hypernatremia can be a manifestation of COVID-19, generally seen to be associated with adverse outcomes.[12–26] With a surge in such publications related to the prevalence and impact of hypernatremia in COVID-19 patients, our primary objective was to examine the association of elevated serum sodium with key clinical outcomes, including mortality, ventilation, intensive care unit (ICU) admission and prolonged hospital stay.
2. Materials and Methods
This meta-analysis is drafted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[27] This article is registered in PROSPERO with ID no. CRD42021270938.
2.1. Eligibility criteria
The studies that fulfilled the following criteria were included: Observational (prospective and retrospective) studies or randomized trials; COVID-19 patients; Hypernatremia and normonatremia; and outcomes including mortality/ need for intensive care unit/ prolong hospitalization/ requiring mechanical ventilation.
The following studies were excluded: Case reports; Case series; Conference and abstracts; Review articles; Letter to the editor.
2.2. Search strategy and study selection
We systematically conducted a literature search on PubMed, Google Scholar, and Science Direct with the keywords: “hypernatremia,” “high sodium,” “COVID-19,” “coronavirus disease,” and “SArs-Cov-2” from inception to October 2021. We found a total of 131 articles. The preliminary search strategy is provided in the Supplementary file (S1, Supplemental Digital Content, http://links.lww.com/MD/I250).
2.3. Data extraction
Studies retrieved from the electronic databases were initially exported to Mendeley version 1.19.8 reference manager software in compatible formats. Duplicate articles, it was screened first by the software and then manually. The title and abstracts of the studies remaining after removing the duplicates were screened independently by any of the 2 authors respectively (ABS, UHS, MA and SS). Two authors executed the full-text screening of the articles satisfying the eligibility criteria. Any disagreements between reviewers were discussed with the last author (SS).
The potential eligible studies retrieved after the full-text screening were followed up for the data extraction by 4 authors independently (ABS, UHS, MA and SS). The following data were extracted: the first author, year published, study design, setting, country, sample size, duration of follow-up, percentage of males, total cases, mean age, the cutoff value for hypernatremia, comorbidities, outcomes like admission in ICU, mortality, patients requiring intubation/ventilation, and prolong hospital stay. After extraction, data was verified by any of the 2 other authors (SS, AS, FC & SS).
2.4. Risk of bias assessment
Quality assessment was conducted using the newcastle-ottawa scale for observational studies.[28,29] Three parameters were checked for the quality of the article using this quality appraisal tool: selection, comparability and outcome. The step was executed by 2 authors (UHS and MA) independently. The mean score of the 2 authors was taken for the decision. Studies with the risk of bias were considered high (<5 stars), moderate (5–7) stars, or low risk of bias (≥8 stars).
2.5. Strategy for data synthesis
The data collected in Microsoft Excel version 2016 (Microsoft Corp., Redmond, WA) was exported, and analysis was conducted using the STATA software version 16 (StataCorp, College Station, TX). For the pooled studies, the Odds Ratio (OR) and 95% Confidence Interval (CI) were computed as the effect size of comparison among normonatremia and hypernatremia patients with various clinical outcomes like mortality, prolong hospitalization, invasive ventilation and ICU admission. Heterogeneity among the studies was examined using the Cochrane Q test I2 statistic (I2 < 25% considered as mild heterogeneity, if = 25–50% as moderate heterogeneity, and if > 50% as severe heterogeneity). A random-effect model was preferred for pooling the analysis. Statistical significance was considered with a 2-sided P-value <.1.
Association of other parameters like age, male, hypertension, chronic kidney disease, and diabetes mellitus, with the clinical outcomes and heterogeneity, were studied using meta-regression. Sensitivity analysis was done by continuously excluding each study to measure the impact of a single study on the overall heterogeneity of the studies. Finally, publication bias was assessed via visual inspection of funnel plot asymmetry along with Egger’s regression test and Begg’s test. A P-value of <.10 was marked as statistically significant for publication bias.
3. Results
3.1. Study selection and characteristics
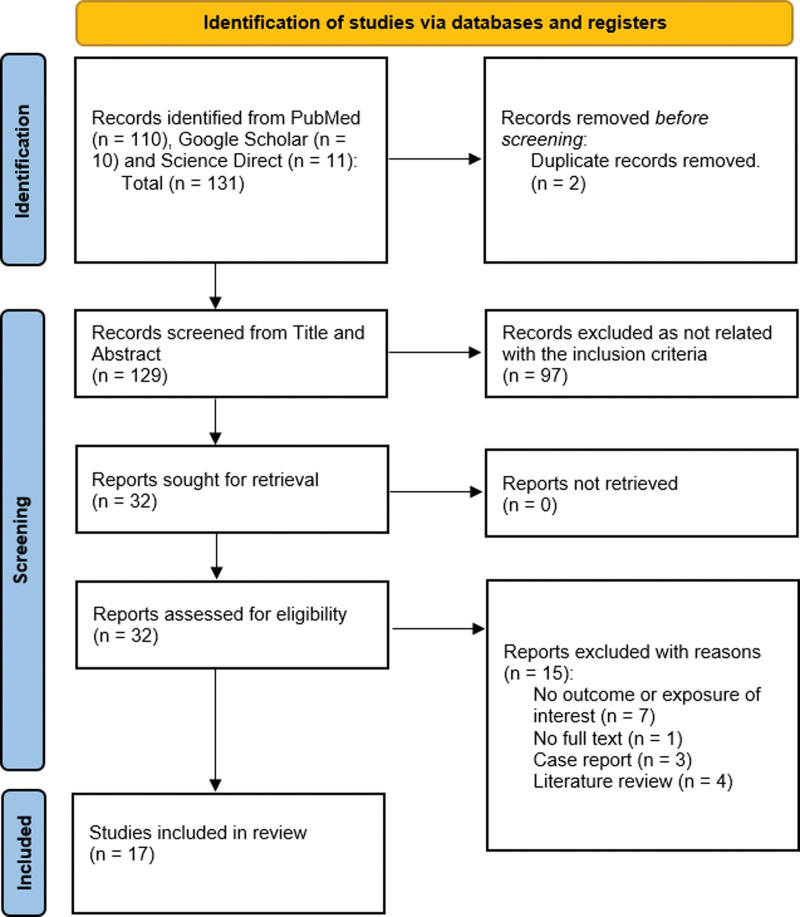
Three electronic databases were taken for the study: Pubmed, Science Direct, and Google Scholar. A total of 131 articles were sought initially and 2 duplicates were removed. Title and abstract screening were conducted making 32 article selections during this step. Finally, on full-text screening, 17 articles were included with a total of 19,032 patients for the meta-analysis. Figure 1. Zimmer et al were removed as it was a case series.[30]
Figure 1.
PRISMA flowchart for study selection.
Of which, pooled analysis for ICU admission was performed with 5 studies, mortality was performed with 13 studies, invasive ventilation was performed with 7 studies and prolong hospital stay with 4 studies as shown in Table 1. The incidence of hypernatremia was 11%, 95% CI: 10.69% to 12.17% with an overall poor outcome of 55.4%, 95% CI: 51.87% to 59.12% and mortality among 29.63%, 95% CI: 25.27% to 32.19% of hypernatremia patients.
Table 1.
Outcomes of COVID-19 patients.
| Author | Publication yr | ICU | Mortlity | Prolong | Hospital stay | Invasive | ventilation | Mortality | Prolong hospital stay | Invasive ventilation | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypernatremia | Normonatremia | Hypernatremia | Normonatremia | Hypernatremia | Normonatremia | Hypernatremia | normonatremia | HR (95%CI) | OR (95%CI) | OR (95%CI) | ||
| Wang et al | 2021 | – | – | – | – | 18/20 | 15/25 | – | – | – | – | – |
| Tzoulis et al | 2021 | – | – | – | – | – | – | – | – | 2.71 (1.28–5.76) | – | – |
| Trecarichi et al | 2020 | – | – | – | – | – | – | – | – | 9.12 (2.15–38.52) | – | – |
| Sjöström et al | 2021 | 53/61 | 77/239 | 20/61 | 29/236 | – | – | 53/61 | 47/236 | – | – | – |
| Sjöström et al | 2020 | – | – | 16/53 | 16/77 | – | – | – | – | – | – | – |
| Ruiz-Sanchez et al | 2020 | 40/174 | 555/35333 | 94/174 | 613/3533 | – | – | – | – | – | – | – |
| Maniero et al | 2021 | – | – | 12/37 | 10/87 | – | – | – | – | – | – | – |
| Maguire et al | 2021 | – | – | 9/58 | 7/203 | – | – | – | – | – | – | – |
| Asghar et al | 2020 | – | – | 13/121 | 46/76 | – | – | 20/36 | 31/124 | – | – | – |
| Atila et al | 2021 | 4/5 | 18/116 | 4/5 | 3/116 | 0/5 | 8/116 | 3/5 | 14/116 | – | – | – |
| Berni et al | 2021 | 1/19 | 59/274 | – | – | – | – | 1/19 | 62/274 | – | – | – |
| Duan et al | 2020 | – | – | – | – | – | – | – | – | – | – | 12.9 (2.8–58.7) |
| Hirsch et al | 2021 | – | – | – | – | – | – | – | – | 2.06 (1.57–2.70) | 1.91 (2.8–58.7) | – |
| Hu et al | 2021 | – | – | 5/30 | 69/1100 | – | – | 3/30 | 54/1100 | – | – | – |
| Longhitano et al | 2021 | – | – | 17/27 | 11/79 | – | – | 9/27 | 6/79 | – | – | – |
| Wu et al | 2020 | – | – | – | – | 0/59 | 1/66 | – | – | – | – | – |
| Sarvazad et al | 2020 | 4/4 | 12/32 | – | – | – | – | – | – | – | – | – |
ICU = intensive care unit, OR = odds ratio.
† “–“: Not studied.
In the articles included, studies were conducted within the year 2020 to 2021 with sample sizes ranging from 45 to 9946 patients. The mean age of patients was 63.77 and mean percentage of males was 58.57% and the cutoff value of hypernatremia varied from 138 to 147 mmol/L (Tables 1 and 2).
Table 2.
Characteristics of studies included.
| Author | Publication year | Country | Total population (n) | Age (mean ± SD) | Male (%) | Study design | Cutoff Na level |
|---|---|---|---|---|---|---|---|
| Wang et al[12] | 2021 | China | 45 | 64 ± 17.78 | 66.7 | Retrospective | ≥146 mmol/L |
| Tzoulis et al[13] | 2021 | UK | 488 | 68 ± 17.78 | 56.8 | Retrospective | ≥146 mmol/L |
| Trecarichi et al[14] | 2020 | Italy | 50 | 80 ± 12 | 57.1 | Retrospective | >145 mmol/L |
| Sjöström et al[31] | 2021 | Sweden | 406 | 59 ± 12.3 | 75 | Retrospective | ≥145 mmol/L |
| Sjöström et al[32] | 2020 | Sweden | 223 | 59 ± 2.37 | 79.37 | Retrospective | ≥145 mmol/L |
| Ruiz-Sanchez et al[15] | 2020 | Spain, Italy Cuba, Ecuador Germany, China, and Canada |
4664 | 66 ± 18.51 | 58 | Retrospective | >145 mmol/L |
| Maniero et al[16] | 2021 | UK | 124 | 83 ± 7.22 | 50.8 | Retrospective | NA |
| Maguire et al[17] | 2021 | UK | 261 | 66%>70 | 46 | Prospective | >146 mmol/L |
| Asghar et al[18] | 2020 | Pakistan | 373 | 52.78 ± 15.76 | 67.02 | Prospective | >145 mEq/L |
| Atila et al[19] | 2021 | Switzerland | Cases: 172 Control:849 |
59 ± 22.96 | 55 | Prospective | >147 mmol/L |
| Berni et al[20] | 2021 | Italy | 380 | 67.53 ± 15.48 | 61.57 | Prospective | >145 mEq/L |
| Duan et al[21] | 2020 | China | 348 | 44.8 ± 15 | 47.12 | Retrospective | >138 mmol/L |
| Hirsch et al[22] | 2021 | USA | 9946 | 71 ± 14.07 | 59.39 | Retrospective | >144 mEq/L |
| Hu et al[23] | 2021 | China | 1254 | 56 ± 55.5 | 51.1 | Retrospective | >145 mmol/L |
| Longhitano et al[24] | 2021 | Italy | 115 | 73 ± 60.74 | 55 | Prospective | >145 mmol/L |
| Wu et al[26] | 2020 | China | 125 | 55 | 52.8 | Retrospective | >145 mmol/l |
| Sarvazad et al[25] | 2020 | Iran | 58 | 56 | 57 | Prospective | >146 meq/L |
3.2. Study quality
Quality appraisal of all included studies was assessed using the Newcastle Ottawa scale. Out of 17 studies, 5 studies were of moderate quality that is moderate risk of bias, while the rest of the studies were of high quality that is low risk of bias. The details of the risk of bias assessment are illustrated in the Supplementary file (S2, Supplemental Digital Content, http://links.lww.com/MD/I251).
3.3. Meta-analysis
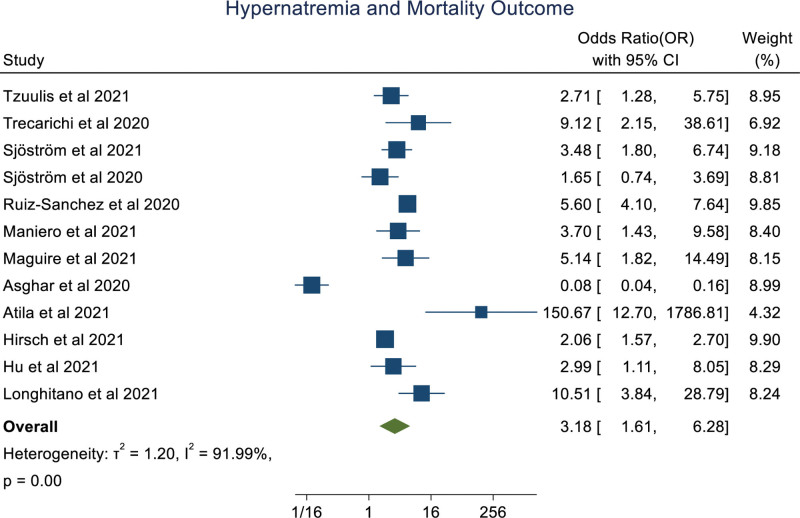
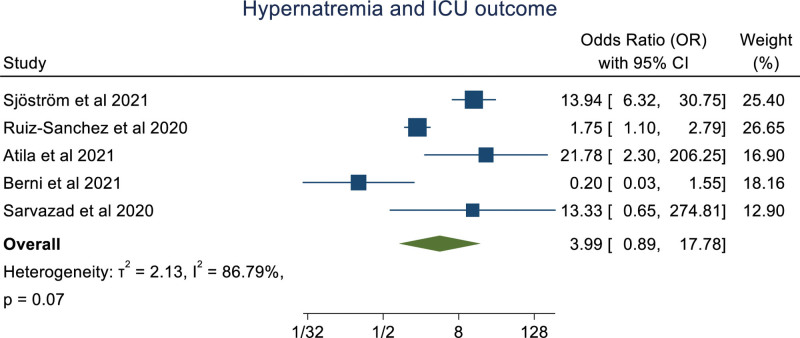
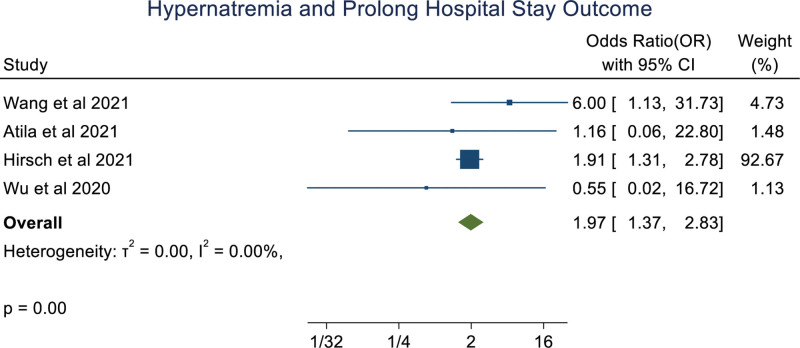
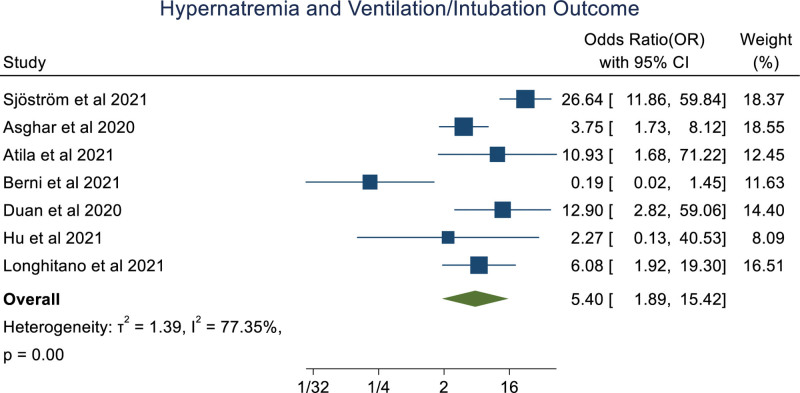
An overall random effect meta-analysis showed that hypernatremia was associated with mortality (OR: 3.18 [1.61, 6.28], P < .001, I2 = 91.99%), prolong hospitalization (OR: 1.97 [1.37, 2.83], P < .0001, I2 = 0.00%) and Ventilation (OR: 5.40 [1.89, 15.42], P < .001, I2 = 77.35%), ICU admission (OR: 3.99 [0.89, 17.78], P = .07, I2 = 86.79%; Figs. 2–5). Meta-regression analysis showed the association of age with the ICU outcome of hypernatremia patients. Whereas, other parameters like male, hypertension, chronic kidney disease, and diabetes mellitus didn’t significantly influence the odds ratio. (S3, Supplemental Digital Content, http://links.lww.com/MD/I252).
Figure 2.
Forestplot showing association of hypernatremia in COVID-19 patients with mortality.
Figure 5.
Forestplot showing association of hypernatremia in COVID-19 patients with ICU admission. ICU = intensive care unit.
Figure 3.
Forestplot showing association of hypernatremia in COVID-19 patients with with prolonged hospital stay.
Figure 4.
Forestplot showing association of hypernatremia in COVID-19 patients with ventilation.
3.4. Publication bias and sensitivity analysis
The funnel plot was qualitatively asymmetrical for clinical outcome of mortality with the Regression-based Egger test and Beggs test, with P values of .0024 and .0865, respectively. Whereas other clinical outcomes; including ICU, ventilation, and prolonged hospital stay didn’t show any publication bias (S4, Supplemental Digital Content, http://links.lww.com/MD/I253).
Even after omitting single studies, there were no statistical differences in the outcome. In the clinical outcomes of mortality, Omitting Atila et al and Asghar et al had pooled results of Odds Ratio: 3.674, 95% CI: 2.458 to 5.492, I2%= 73.25 (S4, Supplemental Digital Content, http://links.lww.com/MD/I253).
4. Discussion
The main finding of this meta-analysis is that COVID-19 patients with hypernatremia have unfavorable clinical outcomes. Overall, hypernatremia patients had associations with increased risk of mortality to 3 folds, prolong hospitalization to nearly 2 folds, the requirement for ventilation of 5 folds and ICU admission to nearly 4-fold.
Hypernatremia reflects a deficiency of total body water concerning total body sodium and is frequently associated with reduced extracellular fluid volume.[33] This idealizes hypovolemia that is dehydration as the driver of mortality in COVID-19. There are many plausible explanations for the pathophysiology behind hypernatremia in COVID-19 patients. Volume depletion could be explained by low oral intake due to anorexia or nausea, or increase in insensible fluid losses, or less likely, fluid losses due to diarrhea.[13] Insensible fluid loss is mainly from cutaneous that is due to constant pyrexia and respiratory that is increased respiratory rates or during intubation due to loss of fluid from mucous and the periciliary fluid by evaporation.[34,35] Dehydration occurring following acute respiratory distress (ARDS) treatment along with the virus disturbs the endocrine function, like Renin-angiotensin-aldosterone system, expecting to shift the electrolyte pattern to hypernatremia.[31] Contrary to physiological phenomena, despite the administration of diuretics (leading to sodium excretion) and despite the administration of free water, plasma sodium level remains raised. This depicts an unphysiological increase in renal tubular sodium reabsorption probably due to COVID-19 itself.[30] In addition, a study showed a significant decrease in hematocrit in both groups with hypernatremia, on the day of admission and the day of the peak sodium level. They proposed that COVID-19 had an association with the over-activation of the renin angiotensin aldosterone system.[31]
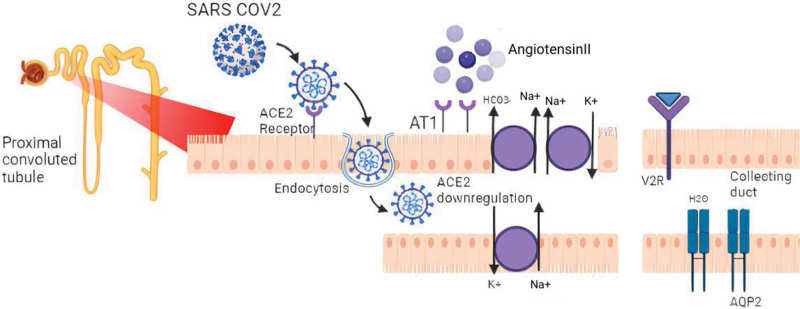
It is quite evident that the SARS-CoV-2 virus shows a tropism for renal cells. It binds to the angiotensin-converting enzyme 2 (ACE2) receptors expressed in the proximal tubules of the kidneys.[36,37] Identification of SARS-Cov-2 ribonucleic acid in the urine of COVID-19 patients depicts that the virus can infiltrate the tubular fluid whereby it may bind to proximal tubule ACE2 receptors.[38] After binding, SARS-Cov-2 enters the cells along with the membrane receptor which is functionally cleared away from the outer side of the membrane.[37] After endocytosis is completed, surface ACE2 is further downregulated ensuring angiotensin II accumulation. Further downregulation of ACE2 expression occurs due to Angiotensin II.[39] Resulting in sodium reabsorption by stimulating sodium-hydrogen exchange in the proximal convoluted tubule (Fig. 6). The role of genetics is also crucial. ACE2 and TMPRSS2 genes are found to be expressed in conjunction by podocytes and proximal convoluted tubular cells, preferably due to COVID-19.[40] Many systematic reviews and meta-analysis have delineated ACE2 and TMPRSS2 genes to increase the risk of susceptibility and severity of COVID-19.[41–43] This could lead to tubulopathy or even renal failure, which could explain the loss of free water or sodium retention for hypernatremia.
Figure 6.
Potential mechanism of hypernatremia. ACE 2 = angiotensin-converting enzyme 2, Ang II = angiotensin II, AT1 = angiotensin II receptor type 1, ADH = anti-diuretic hormone, V2R = vasopressin 2 receptor, AQP-2 = aquaporin 2.
However, ICU patients are at high risk of hypernatremia due to their inability to maintain free water balance due to sedation, intubation and fluid restrictions for various other reasons.[44] In addition, osmotic urea diuresis is a prevailing cause of hypernatremia in ICU patients.[45] Hence, during the evaluation of dysnatremia patients, solute-free water clearance should be considered deluding, and considering electrolyte-free water clearance, which explains the development of hypernatremia.[13,45] Amelioration of hyperinflammation using dexamethasone can also contribute to hypernatremia.[46] ICU-acquired hypernatremia is associated with increased in-hospital mortality.[47,48] Hence, it is crucially important that many studies should focus on the balance between dehydration prevention and concurrently avoiding pulmonary edema in COVID-19 patients who are admitted to the ICU.
Heterogeneity was due to different demographics of the study conducted with varying characteristics like age and comorbidities. Meta-regression showed an association of age with ICU admission. Other studies have also reported age being an important factor for ICU admission.[49–52] Moreover, elderly patients admitted to ICU have poor outcomes including a higher risk of mortality.[49–53] In critically ill patients, hypernatremia is a well-known prognostic marker.[24,47,48]
Dysnatremia could be considered as an indicator of upcoming bodily imbalance and enervation in a broad sense. Hence, with the consistency of our findings, an analysis of serum electrolyte levels (sodium) during the hospitalization of patients with COVID-19 is recommended. Elderly patients, those with severe lung disease and those with the risk of developing dysnatremia should be given special attention. Even correction of sodium imbalance in patients in ICU settings has been shown to improve survival rates.[54,55] Whilst, the role of prescription of low molecular weight heparin in hypernatremia patients seems crucial.[56] As hypernatremia dehydration brings a hypercoagulable state which risks the patient for venous thrombosis, and pulmonary embolism, mainly in ICU patients.[34,56] This is especially important since pulmonary embolism is evolving as 1 of the factors associated with mortality in COVID-19 patients. It is also recommended in COVID-19 patients with hypercoagulable states.[57]
However, there were limitations in our study. This study didn’t include the exact cause behind the hypernatremia in COVID-19 patients. Many studies were retrospective, hence more prospective studies are warranted to determine the exact pathophysiology. There might be many unknown cofounders associated which could influence the outcomes. Lastly, publication bias was observed with the clinical outcome of mortality in COVID-19 patients with hypernatremia. This might be due to more studies publishing only on mortality due to hypernatremia. Hence, the data should be studied cautiously.
5. Conclusion
Hypernatremia in COVID-19 patients has unfavorable outcomes like mortality, invasive ventilation, prolonged hospital stay, and ICU admission. Thus, a blood ionogram should be considered in hospitalized COVID-19 patients and special attention must be given to correct hypernatremia. More, prospective studies are required to know the mechanism and validate the findings.
Author contributions
Conceptualization: Abhigan Babu Shrestha.
Data curation: Abhigan Babu Shrestha, Unnat Hamal Sapkota, Manjil Aryal, Sajina Shrestha, Abdullah Salman, Faisal Chowdhury, Shumneva Shrestha.
Methodology: Abhigan Babu Shrestha.
Validation: Abhigan Babu Shrestha, Swati Chand, Sangharsha Thapa.
Software & formal analysis: Abhigan Babu Shrestha, Sangharsha Thapa.
Supervision: Vikash Jaiswal.
Writing – original draft: Abhigan Babu Shrestha, Unnat Hamal Sapkota, Sajina Shrestha.
Writing – review & editing: Swati Chand, Sangharsha Thapa, Sangam Shah.
Supplementary Material
Abbreviations:
- ACE2 =
- angiotension converting enzyme 2
- ICU =
- intensive care unit
- OR =
- odds ratio
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
Abhigan Babu Shrestha affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Shrestha AB, Sapkota UH, Shrestha S, Aryal M, Chand S, Thapa S, Chowdhury F, Salman A, Shrestha S, Shah S, Jaiswal V. Association of hypernatremia with outcomes of COVID-19 patients: A systematic review and meta-analysis. Medicine 2022;101:51(e32535).
Contributor Information
Unnat Hamal Sapkota, Email: hamalunnact@gmail.com.
Sajina Shrestha, Email: shumnevashrestha@gmail.com.
Manjil Aryal, Email: manjilaryal12@gmail.com.
Swati Chand, Email: Swatichand1046@gmail.com.
Sangharsha Thapa, Email: sanghars@buffalo.edu.
Faisal Chowdhury, Email: Faisalchwdhr@gmail.com.
Abdullah Salman, Email: salmanabdullah2404@gmail.com.
Shumneva Shrestha, Email: shumnevashrestha@gmail.com.
Sangam Shah, Email: sangam.shah.1997@gmail.com.
Vikash Jaiswal, Email: vikash29jaxy@gmail.com.
References
- [1].Lim MA, Pranata R, Huang I, et al. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7:2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. eClinicalMedicine. 2020;23:100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105:dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58:1131–4. [DOI] [PubMed] [Google Scholar]
- [8].Bannaga AS, Tabuso M, Farrugia A, et al. C-reactive protein and albumin association with mortality of hospitalised SARS-CoV-2 patients: a tertiary hospital experience. Clin Med (Lond). 2020;20:463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guan W, yi NZ, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wald R, Jaber BL, Price LL, et al. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170:294–302. [DOI] [PubMed] [Google Scholar]
- [11].Nair V, Niederman MS, Masani N, et al. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184–90. [DOI] [PubMed] [Google Scholar]
- [12].Wang P, Tan X, Li Q, et al. Extra-pulmonary complications of 45 critically ill patients with COVID-19 in Yichang, Hubei province, China: a single-centered, retrospective, observation study. Medicine (Baltim). 2021;100:e24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tzoulis P, Waung JA, Bagkeris E, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab. 2021;106:1637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Trecarichi EM, Mazzitelli M, Serapide F, et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci Rep. 2020;10:20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Front Endocrinol (Lausanne). 2020;11:599255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maniero C, Patel D, Pavithran A, et al. A retrospective cohort study of risk factors and outcomes in older patients admitted to an inner-city geriatric unit in London during first peak of COVID-19 pandemic. Ir J Med Sci. 2022;191:1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maguire D, Richards C, Woods M, et al. The systemic inflammatory response and clinicopathological characteristics in patients admitted to hospital with COVID-19 infection: comparison of 2 consecutive cohorts. PLoS One. 2021;16:e0251924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Asghar MS, Haider Kazmi SJ, Khan NA, et al. Role of biochemical markers in invasive ventilation of coronavirus disease 2019 patients: multinomial regression and survival analysis. Cureus. 2020;12:e10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Atila C, Sailer CO, Bassetti S, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. 2021;184:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berni A, Malandrino D, Corona G, et al. Serum sodium alterations in SARS CoV-2 (COVID-19) infection: impact on patient outcome. Eur J Endocrinol. 2021;185:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duan J, Wang X, Chi J, et al. Correlation between the variables collected at admission and progression to severe cases during hospitalization among patients with COVID-19 in Chongqing. J Med Virol. 2020;92:2616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hirsch JS, Uppal NN, Sharma P, et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. 2021;36:1135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hu W, Lv X, Li C, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern Emerg Med. 2021;16:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Longhitano E, Nardi C, Calabrese V, et al. Hypernatraemia and low eGFR at hospitalization in COVID-19 patients: a deadly combination. Clin Kidney J. 2021;14:2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sarvazad H, Cahngaripour SH, Eskandari Roozbahani N, et al. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect. 2020;38:100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu Y, Hou B, Liu J, et al. Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered, retrospective study. Front Med (Lausanne). 2020;7:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [29].Ottawa Hospital Research Institute. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [access date July 11, 2022].
- [30].Zimmer MA, Zink AK, Weißer CW, et al. Hypernatremia–A manifestation of COVID-19: a case series. A A Pract. 2020;14:e01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sjöström A, Rysz S, SjöSjströöström H, et al. Electrolyte and acid-base imbalance in severe COVID-19. Endocr Connect. 2021;10:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hypernatremia is common in patients with severe COVID-19 and indicates a poor prognosis.
- [33].Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471–503. [DOI] [PubMed] [Google Scholar]
- [34].Christ-Crain M, Hoorn EJ, Sherlock M, et al. ENDOCRINOLOGY IN THE TIME OF COVID-19-2021 UPDATES: the management of diabetes insipidus and hyponatraemia. Eur J Endocrinol. 2021;185:G35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lorente L, Lecuona M, Jiménez A, et al. Ventilator-associated pneumonia using a heated humidifier or a heat and moisture exchanger: a randomized controlled trial [ISRCTN88724583]. Crit Care. 2006;10:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20. [DOI] [PubMed] [Google Scholar]
- [37].Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peng L, Liu J, Xu W, et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020;92:1676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors–lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pan XW, Xu D, Zhang H, et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sari Motlagh R, Abufaraj M, Karakiewicz PI, et al. Association between SARS-CoV-2 infection and disease severity among prostate cancer patients on androgen deprivation therapy: a systematic review and meta-analysis. World J Urol. 2022;40:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ishak A, Mehendale M, AlRawashdeh MM, et al. The association of COVID-19 severity and susceptibility and genetic risk factors: a systematic review of the literature. Gene. 2022;836:146674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li J, Wang Y, Liu Y, et al. Polymorphisms and mutations of ACE2 and TMPRSS2 genes are associated with COVID-19: a systematic review. Eur J Med Res. 2022;27:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Arora SK. Hypernatremic disorders in the intensive care unit. J Intensive Care Med. 2013;28:37–45. [DOI] [PubMed] [Google Scholar]
- [45].Lindner G, Schwarz C, Funk GC. Osmotic diuresis due to urea as the cause of hypernatraemia in critically ill patients. Nephrol Dial Transplant. 2012;27:962–7. [DOI] [PubMed] [Google Scholar]
- [46].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care. 2013;28:216.e11–20. [DOI] [PubMed] [Google Scholar]
- [48].Olsen MH, Møller M, Romano S, et al. Association between ICU-acquired hypernatremia and in-hospital mortality: data from the medical information mart for intensive care III and the electronic ICU collaborative research database. Crit Care Explor. 2020;2:e0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yu W, Ash AS, Levinsky NG, et al. Intensive care unit use and mortality in the elderly. J Gen Intern Med. 2000;15:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fuchs L, Chronaki CE, Park S, et al. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive Care Med. 2012;38:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sadeghi A, Eslami P, Dooghaie Moghadam A, et al. COVID-19 and ICU admission associated predictive factors in Iranian patients. Caspian J Intern Med. 2020;11(Suppl 1):512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11:e044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gkoufa A, Maneta E, Ntoumas GN, et al. Elderly adults with COVID-19 admitted to intensive care unit: a narrative review. World J Crit Care Med. 2021;10:278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21:70–6. [DOI] [PubMed] [Google Scholar]
- [55].Saad G, Abdelkrim AB, El Abed YH, et al. Disorders of sodium balance in COVID-19 patients: two Tunisian patients report. Pan Afr Med J. 2021;39:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Miljic D, Miljic P, Doknic M, et al. Adipsic diabetes insipidus and venous thromboembolism (VTE): recommendations for addressing its hypercoagulability. Hormones (Athens). 2014;13:420–3. [DOI] [PubMed] [Google Scholar]
- [57].Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9:687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]