ABSTRACT
Immune checkpoint inhibitors have transformed the treatment of cancer. Nonetheless, multiple immune-related adverse events have been reported, including checkpoint inhibitor colitis. Severe colitis can be complicated by ileus, megacolon, intestinal perforation, and death. Current appropriate treatment includes steroids, followed by antitumor necrosis factor biologic therapy, infliximab. Alternatively, vedolizumab and fecal microbiota transplantation have reported efficacy for refractory cases. In this study, we present the first case report of a patient with steroid-refractory checkpoint inhibitor-induced colitis due to pembrolizumab for Stage IV anaplastic thyroid carcinoma successfully treated with ustekinumab after failure of infliximab, vedolizumab, and fecal microbiota transplantation. This may lead to a better understanding of treatment options for refractory checkpoint inhibitor colitis.
INTRODUCTION
Pembrolizumab is an immune checkpoint inhibitor or antineoplastic agent that inhibits programmed cell death protein 1 (PD1) activity by attaching to the PD1 receptor on T cells to prevent binding to PD1 ligands (PDL1 and PDL2).1 The most common gastrointestinal adverse effects include poor appetite, fatigue, nausea, stomatitis, diarrhea, and colitis.1 Adverse events are described based on the Common Terminology Criteria for Adverse Events, a nomenclature that grades the severity of these events from grade 1 through 5. Grade 1 is defined as asymptomatic or mild symptoms; grade 2 includes moderate symptoms; grade 3 involves severe or medically significant disease but not immediately life-threatening; and there is significant morbidity and mortality in grade 4 and grade 5.2
Severe checkpoint inhibitor colitis (CIC) is managed with glucocorticoids, followed by infliximab or vedolizumab infusion.3 Fecal microbiota transplantation (FMT) has been reported in case series for the treatment of refractory cases.4 We present a case report of refractory CIC treated with ustekinumab after treatment failure with the previously mentioned agents.
CASE REPORT
The patient was a 74-year-old man with a medical history significant for hypertension, hyperlipidemia, psoriatic arthritis, achalasia requiring multiple dilations, and Stage IV anaplastic thyroid carcinoma with total thyroidectomy on pembrolizumab, who presented to the emergency department because of abdominal pain shortly after his ninth pembrolizumab infusion. Symptoms were associated 10 bowel movements per day with blood and mucus, urgency, and weight loss of 20 pounds in 2 months. There was no history of other cancers nor inflammatory bowel disease (IBD). The patient also denied a family history of IBD or cancer. He was a former smoker (22 packs per year but quit when he was in his thirties). The patient had a history of Clostridium difficile infection treated with oral vancomycin and previous esophagogastroduodenoscopy and colonoscopy 2 years before the presentation, both of which were normal.
On admission, the patient was hemodynamically stable and in no acute distress. The physical examination was normal, except for dry mucous membranes. The abdomen was soft, nontender, and nondistended, and the rectal examination was negative for blood. Laboratory studies were significant for leukopenia (3.94 × 103/μL), thrombocytopenia (143 × 103/μL), and elevated fecal calprotectin levels (>2,000 μg/mg). Testing for C. difficile polymerase chain reaction and toxin was negative. Abdominal and pelvic computed tomography scan with contrast showed findings consistent with diffuse proctocolitis, and the gastroenterology service was consulted. The patient underwent sigmoidoscopy, which revealed diffuse severe inflammation characterized by congestion (edema), erythema, friability, mucus, and pseudomembranes (Figure 1). Pathology showed severely active chronic colitis. Therefore, he was started on steroids (intravenous [IV] methylprednisolone at 1–2 mg/kg/d). Pembrolizumab was discontinued because of the severity of colitis, which was determined as grade 4 Common Terminology Criteria for Adverse Events. Colon biopsies from the sigmoidoscopy also revealed cytomegalovirus inclusion bodies, and serum laboratory testing detected low-grade viremia. The patient was treated with IV ganciclovir and then switched to oral valganciclovir for 6 weeks, with subsequent valganciclovir prophylaxis for 1 year. Owing to the severity of colitis and lack of response to steroids, IV infliximab infusion was initiated with minimal improvement.
Figure 1.
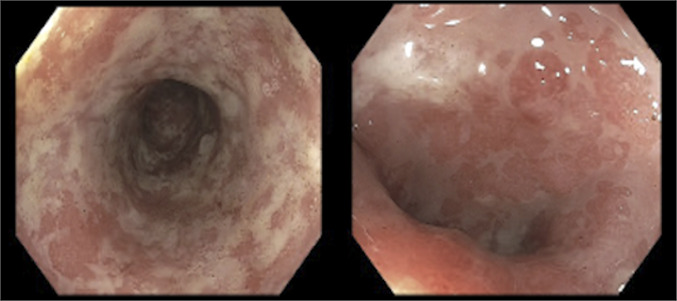
Sigmoidoscopy showed diffuse severe inflammation, erythema, mucus, and pseudomembranes on the descending colon.
The patient reported continued symptoms despite 3 infliximab infusions and hydrocortisone enemas twice a day. Therefore, vedolizumab was initiated with induction dosing, followed by maintenance dosing every 8 weeks and subsequently increased to every 4 weeks because the response to treatment was minimal. The patient still complained of lower abdominal pain related to multiple bowel movements, urgency with stool, and tenesmus. A repeat colonoscopy was performed, and the findings were similar to the previous examination showing severe active colitis. There was no evidence of C. difficile infection nor cytomegalovirus infection.
Owing to persistent severe checkpoint inhibitor-induced colitis with lack of response to vedolizumab, FMT was performed at a referral center. However, his symptoms continued, and subsequent fecal calprotectin levels remained elevated (>1,000 μg/mg).
Two months after FMT, a colonoscopy showed inflammation characterized by erosions, erythema, friability, granularity, loss of vascularity, mucus, and shallow ulcerations in a continuous and circumferential pattern in the entire colon with multiple polyps (Figure 2). These findings were unchanged when results were compared with previous examinations. Biopsies demonstrated severely active chronic colitis, prominent crypt epithelial apoptosis, and granulation tissue polyps.
Figure 2.
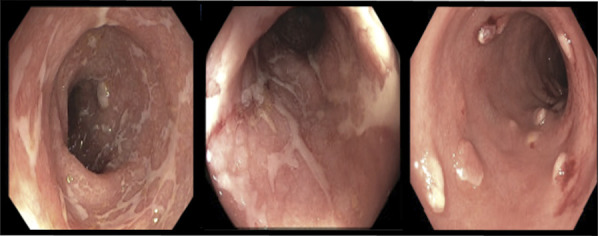
Colonoscopy revealed inflammation in a continuous and circumferential pattern throughout the colon with multiple polyps.
The patient still complained of persistent abdominal pain, urgency, and painful defecation secondary to more distal disease. Different treatment options were discussed with the patient, including colectomy; however, he elected to exhaust all medical treatment options. Therefore, decision was made to attempt treatment with ustekinumab, an agent that blocks the protein interleukins (ILs) 12 and 23. He received the initial IV infusion, followed by subcutaneous 90 μg injections. He also resumed prednisone 40 mg daily, and after 2 weeks, the dosage was tapered by 5 mg weekly.
His initial response to ustekinumab was substantial, with clinical symptom improvement of fecal urgency and frequency, as well as a significant decrease in fecal calprotectin from >1,000 μg/g (abnormal >120 μg/g) to 314 μg/g after 8 weeks and down to 135 μg/g after 6 months. Colonoscopy (Figure 3) after 6 months of ustekinumab therapy also showed significant improvement in colitis with only mildly erythematous mucosa seen in the descending and sigmoid colon along with nonbleeding internal and external hemorrhoids. Seven polyps less than 1 cm in size in the sigmoid colon were resected, and biopsies were obtained. Pathology reported mild chronic active colitis, and polyps were described as inflammatory pseudopolyps with ulceration. He has remained in clinical remission on maintenance dosing of 45 μg every 12 weeks.
Figure 3.
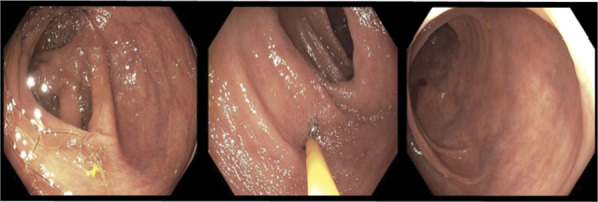
Colonoscopy observed a normal examined portion of the ascending and transverse colon. Sigmoid colon polyps were resected.
DISCUSSION
Immune checkpoint inhibitors have truly transformed the treatment of cancer. However, these new therapy modalities have brought with them significant adverse effects or immune-related adverse events with CIC involving the gastrointestinal tract.5,6 The true pathophysiology of CIC is not fully understood; however, it is believed to be due to a proinflammatory status consequence of infiltration of lymphocytes, hyperactivation, and proliferation of T cells, in addition to gut microbiome interaction.3,7
CIC can present with diarrhea and/or colitis. Diarrhea severity can be defined as grade 1, less than 4 stools per day above baseline; grade 2 is 4–6 stools per day; grade 3 is more than 7 stools per day; and grade 4 includes life-threatening complications such as hemodynamic instability.7 Similarly, colitis can be classified as grade 1, which is asymptomatic; grade 2 defined as abdominal pain, mucus, and blood in stools; and grade 3 colitis incorporates severe pain, fever, and peritoneal signs. Grade 4 exhibits life-threatening consequences such as bleeding, ischemia, perforation, necrosis, and toxic megacolon, and there is significant morbidity and mortality with grade 5 CIC.2,7
Underlying risk factors of CIC identified by a US-based population database include White race, age older than 65 years, obesity, female sex, and a history of alcohol abuse.8 Diagnosis is made using lower endoscopy with biopsies after other causes such as infection have been excluded.3,7 In our case, the patient presented with grade 3 diarrhea and grade 2 colitis. Despite treatment with steroids, infliximab, vedolizumab, and even FMT, his follow-up colonoscopy and biopsies revealed severely active chronic colitis with prominent crypt epithelial apoptosis.9
The incidence of CIC has been reported to be lower with PD1 and PD1 ligand inhibitors when compared with CTLA4 inhibitors.3 Pembrolizumab has the lowest risk of CIC.3 According to previous studies, the incidence of colitis varies between 0.7% and 31.6%.3,10,11
Ustekinumab, a p40 subunit antagonist of IL-12 and IL-23, is known to be effective in inducing and maintaining remission in patients with IBD.12,13 With this difficult case, we report improved clinical, endoscopic, and histopathologic response with ustekinumab as a treatment option for refractory CIC and propose further investigation to support this hypothesis.
DISCLOSURES
Author contributions: G. Perez Del Nogal and N. Patel wrote and edited the manuscript. N. Patel reviewed and edited the final manuscript. G. Perez Del Nogal is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Kwok G, Yau TC, Chiu JW, et al. Pembrolizumab (keytruda). Hum Vaccin Immunother. 2016;12(11):2777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. 2017. (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf). Accessed October 5, 2022. [Google Scholar]
- 3.Tang L, Wang J, Lin N, et al. Immune checkpoint inhibitor-associated colitis: From mechanism to management. Front Immunol. 2021;12:800879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis [published correction appears in Nat Med. 2018]. Nat Med. 2018;24(12):1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajwa R, Cheema A, Khan T, et al. Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): Results of a retrospective study. J Clin Med Res. 2019;11(4):225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Takahashi K, Terai S. A case of enteritis induced by nivolumab. Clin Gastroenterol Hepatol. 2019;17(6):A31–A32. [DOI] [PubMed] [Google Scholar]
- 7.Som A, Mandaliya R, Alsaadi D, et al. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Clin Cases. 2019;7(4):405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farha N, Alkhayyat M, Lindsey A, et al. Immune checkpoint inhibitor induced colitis: A nationwide population-based study. Clin Res Hepatol Gastroenterol. 2022;46(1):101778. [DOI] [PubMed] [Google Scholar]
- 9.Coutzac C, Adam J, Soularue E, et al. Colon immune-related adverse events: Anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entities. J Crohns Colitis. 2017;11(10):1238–46. [DOI] [PubMed] [Google Scholar]
- 10.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6(10):e1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Wu B, Zhang C, Xu T. Immune-related adverse events associated with immune checkpoint inhibitors: An updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int Immunopharmacol. 2021;95:107498. [DOI] [PubMed] [Google Scholar]
- 12.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. [DOI] [PubMed] [Google Scholar]
- 13.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375(20):1946–60. Images. [DOI] [PubMed] [Google Scholar]


