Background:
To construct a prognostic risk model of bladder cancer (BC) from the perspective of long non-coding RNAs (lncRNAs) and ferroptosis, in order to guide clinical prognosis and identify potential therapeutic targets.
Methods:
In-hours BC samples were collected from 4 patients diagnosed with BC, who underwent radical cystectomy. Single cell transcriptome sequencing was performed and Seurat package were used for quality control and secondary analysis. LncRNAs expression profiles of BC samples were extracted from The Cancer Genome Atlas database. And sex, age, tumor, node, metastasis stage and other clinical data was downloaded at the same time. Ferroptosis-related lncRNAs were identified by co-expression analysis. We constructed a risk model by Cox regression and least absolute shrinkage and selection operator regression analyses. The predictive strength of the risk model for overall survival (OS) of patients with BC was evaluated by the log-rank test and Kaplan–Meier method. Finally, the enrichment analysis was performed and visualized.
Results:
We identified and included 15 prognostic ferroptosis-related lncRNAs (AL356740.1, FOXC2AS1, ZNF528AS1, LINC02535, PSMB8AS1, AL590428.1, AP000347.2, OCIAD1-AS1, AP001347.1, AC104986.2, AC018926.2, LINC00867, AC099518.4, USP30-AS1, and ARHGAP5-AS1), to build our ferroptosis-related lncRNAs risk model. Using this risk model, BC patients were divided into high and low-risk groups, and their respective survival lengths were calculated. The results showed that the OS of the low-risk group was significantly longer than that of the high-risk group. A nomogram was utilized to predict the survival rate of BC patients. As indicated in the nomogram, risk score was the most important indicator of OS in patients with BC. The ferroptosis-related lncRNAs risk model is an independent tool for prognostic risk assessment in patients with BC. Single cell transcriptome sequencing suggests that ferroptosis-related lncRNAs express specifically in BC tumor microenvironment. AL356740.1, LINC02535 and LINC00867 were mainly expressed in tumor cells.
Conclusion:
The risk model based on the ferroptosis-related lncRNAs and the genomic clinico-pathological nomogram could be used to accurately predict the prognosis of patients with BC. The lncRNAs used to build this model might become potential therapeutic targets in the future.
Keywords: bladder cancer, ferroptosis, gene signature, risk score, TCGA
1. Introduction
The global incidence of bladder cancer (BC) remains quite high. Of the 549,393 new cases reported in 2018, nearly 77% were males and the mortality rate was as high as 47%.[1] Although the success of immunotherapy and attention to tumor microenvironment (TME) have improved the prognosis of patients to some extent, the diagnostic accuracy of BC still lags behind the current rapid progress in biomedical technology. Many BC patients miss the optimal window for treatment, resulting in the need for more complex or expensive treatment, which in turn seriously affects the quality of life and increases the financial burden.[2–5] Therefore, early diagnosis and prognostic risk assessment of BC have become urgent research topics.
Ferroptosis is a form of regulatory cell death. It differs from apoptosis or necrosis, in that it results from iron dependent lipid peroxidation, which is controlled by oxidation and antioxidant systems in the body. Earlier studies have confirmed that ferroptosis is different from regulatory cell death with respect to morphological changes, biochemical pathways and genetic levels. It has now been shown that several proteins such as glutathione peroxidase, NADPH oxidase, SLC7A11 molecules and heat shock protein β1 regulate ferroptosis by acting on iron metabolism and lipid peroxidation. Mounting evidence indicates that ferroptosis plays an important role in the occurrence and development of tumors and can be used for treatment of cancer.[6] However, the specific relationship between ferroptosis related genes and BC is unclear.
Long non-coding RNAs (lncRNAs) are RNAs that cannot be translated into proteins, are typically more than 200 bp in length, and include multiple transcripts that can monitor and regulate gene expression in specific ways depending on cell type, developmental stage and function.[7] It is becoming increasingly clear that abnormal expression of lncRNAs leads to abnormal regulation of cell growth, cell cycle, cell function, development, and apoptosis in many mammals, which finally leads to diseases such as leukemia, cerebral ischemic stroke, and tumors.[8–11]
In spite of the mounting evidence for the involvement of lncRNAs in the etiology and progression of several different cancers, no ferroptosis related lncRNA signature that has prognostic value in BC has been proposed to date. Therefore, we tried to build a new prognostic risk model of BC from a unique perspective.
2. Materials and Methods
2.1. Sample collection and single-cell isolation
Four BC samples were collected from 4 patients undergoing radical cystectomy. The patients were not receiving any anti-tumor treatment therapy prior to sampling, including chemotherapy, radiotherapy, immunotherapy and Chinese medicine. The preparation of the single-cell suspension was as described in a previous study.[12] Fresh BC samples were taken from the operating room to the laboratory in cold Hank’s Balanced Salt Solution (Gibco, State of California; C11875500BT) and transported throughout within 30 min. The samples were washed in Dulbecco’s phosphate-buffered saline (DPBS; WISENT, 311-425-CL) at 4°C and cut into 2 to 4 mm pieces using sterile scissors. The tissue pieces were resuspended in pre-chilled DPBS and washed twice. The tissues were digested for 30 minutes in an Hank’s Balanced Salt Solution digestion solution with gentle agitation. Subsequently, the suspended cells and tissue fragments were then filtered through a 70 µm cell filter (Falcon) with 5 mL of red blood cell lysis buffer (10X diluted to 1X; BioLegend, San Diego, 420301) on ice for 5 minutes to remove red blood cells, followed by filtering the cells through a 40 µm cell filter. The cells were then centrifuged at 300 g for 5 minutes and washed twice with DPBS. Finally, the cells were resuspended in DPBS containing 1% fetal bovine serum. Single-cell suspensions were obtained and trypan blue staining (Gibco; 15250-061) was used to calculate cell viability. If cell viability was >80%, 10x genomics samples were processed.[13] This study was approved by the ethics and human subjects committee of the First Affiliated Hospital of Guangxi Medical University. Written informed consent forms have been obtained from patients.
2.2. ScRNA-seq processing and data analysis
The Hiseq X10 (Illumina, San Diego, CA) was used to sequence all the samples using standard parameters. CellRanger (version 3.0.2, 10x Genomics, San Francisco, CA) was used to convert preliminary sequencing files (.bcl) to FASTQ files. The R Foundation and the (R Core Group, New Zealand) (version 3.5.2) and Seurat R package (version 3.1.1, New York University, NY) were used for quality control and secondary analysis.
2.3. Data acquisition and preliminary processing
The protocol of this study was approved by the Ethics and Human Subject Committee and all experiments and methods were in compliance with The Cancer Genome Atlas (TCGA’s) informed consent policy, data access policy, and HIPAA Privacy Rule policy. We obtained the main data from TCGA database (https://portal.gdc.cancer.gov/) including RNA sequencing (RNA-seq) files and clinical information data about BC. The initial dataset included 409 BC tumor samples. Samples with a follow-up period shorter than 1 month and samples from patients with incomplete clinical information were excluded from the study. The final dataset comprised 397 samples. We also extracted 60 ferroptosis related genes from published literature (Table S1, Supplemental Digital Content, http://links.lww.com/MD/I256, expression matrix of ferroptosis-related genes in BC).[14–17]
2.4. Screening of target lncRNAs and ferroptosis related genes
We separated and extracted lncRNA expression profiles from all RNA-seq data. LncRNA expression level read counts were normalized using The Fragments per Kilobase of transcript per Million mapped reads (FPKM) as previously described.[18] We then performed log2 transformation by (FPKM + 1 value) of lncRNA. The extraction of ferroptosis-related lncRNAs was mainly based on Pearson correlation algorithm of Limma R package. When the correlation coefficient satisfied the condition of | R2| > 0.3 and P < .001, it was considered ferroptosis-related lncRNA. Cytoscape (3.7.2) was used to construct the co-expression network.
2.5. Extraction of prognosis associated lncRNAs and construction of model
Univariate Cox regression analysis was used to identify prognosis associated lncRNAs of BC (P < .05). These lncRNAs with prognostic value were subjected to least absolute shrinkage and selection operator (Lasso) regression. Patient survival status and survival time were used as dependent variables and the lncRNA expression values were used as independent variables. Cross-validation was used to confirm the appropriate adjustment parameter (λ) for the LASSO regression. LncRNAs with non-zero coefficients at a defined (λ) value were selected for the multivariate COX model. We used the “Forward” model for the COX regression model construction. The final lncRNAs comprised in the model constituted the final risk model. The prognostic-related risk score of each patient was calculated using the following formula: Risk score = coefficient (β) × expression level of lncRNA. The median-risk score was calculated, and the cases were divided into high and low-risk groups according to the median value. The survival differences between high and low-risk groups were compared by log-rank test.
2.6. Development and validation of the model
The construction of an independent prognosis model is mainly based on Cox regression algorithm, while the survival time of patients is predicted using the nomogram. We tested the accuracy of the model using calibration curves, index of concordance (C-index) and area under the curve (AUC). AUC is a value between 0.5 and 1, with a higher value representing a stronger predictive power of the model. Multivariate Cox regression analysis was used to assess whether the risk score had independent prognostic value for BC patients.
2.7. Functional analysis
In order to gain a better understanding of the molecular mechanisms, biological processes, and related pathways involved in BC, we carried out gene set enrichment analysis and visualized the results of top 5 gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) pathways with ferroptosis-related lncRNAs.
2.8. Statistical analysis
Data processing and statistical analysis were carried out in R language (Institute for Systems Biology, Seattle, Washington 98103, The R Foundation and the R Core Group, New Zealand version 3.5.2). The statistical test was bilateral, and P ≤ .05 was considered statistically significant. We constructed the ferroptosis-related lncRNA co-expression network by using Cytoscape software (version 3.7.2). The survival curve was drawn based on Kaplan–Meier method, and log-rank test was used for comparison. The effectiveness of the ferroptosis-related lncRNA risk model in combination with clinico-pathological data for prognosis was evaluated using Cox regression and Lasso regression analysis.
3. Results
3.1. Ferroptosis-related lncRNAs co-expression network construction
We identified 14,132 lncRNAs from the TCGA-BLAC transcriptome dataset. We also extracted 60 genes related to ferroptosis from published literature. Constructing a ferroptosis-related gene lncRNA co-expression network yielded 4742 ferroptosis-related lncRNAs.
3.2. Identification of 15 ferroptosis-related prognostic lncRNAs
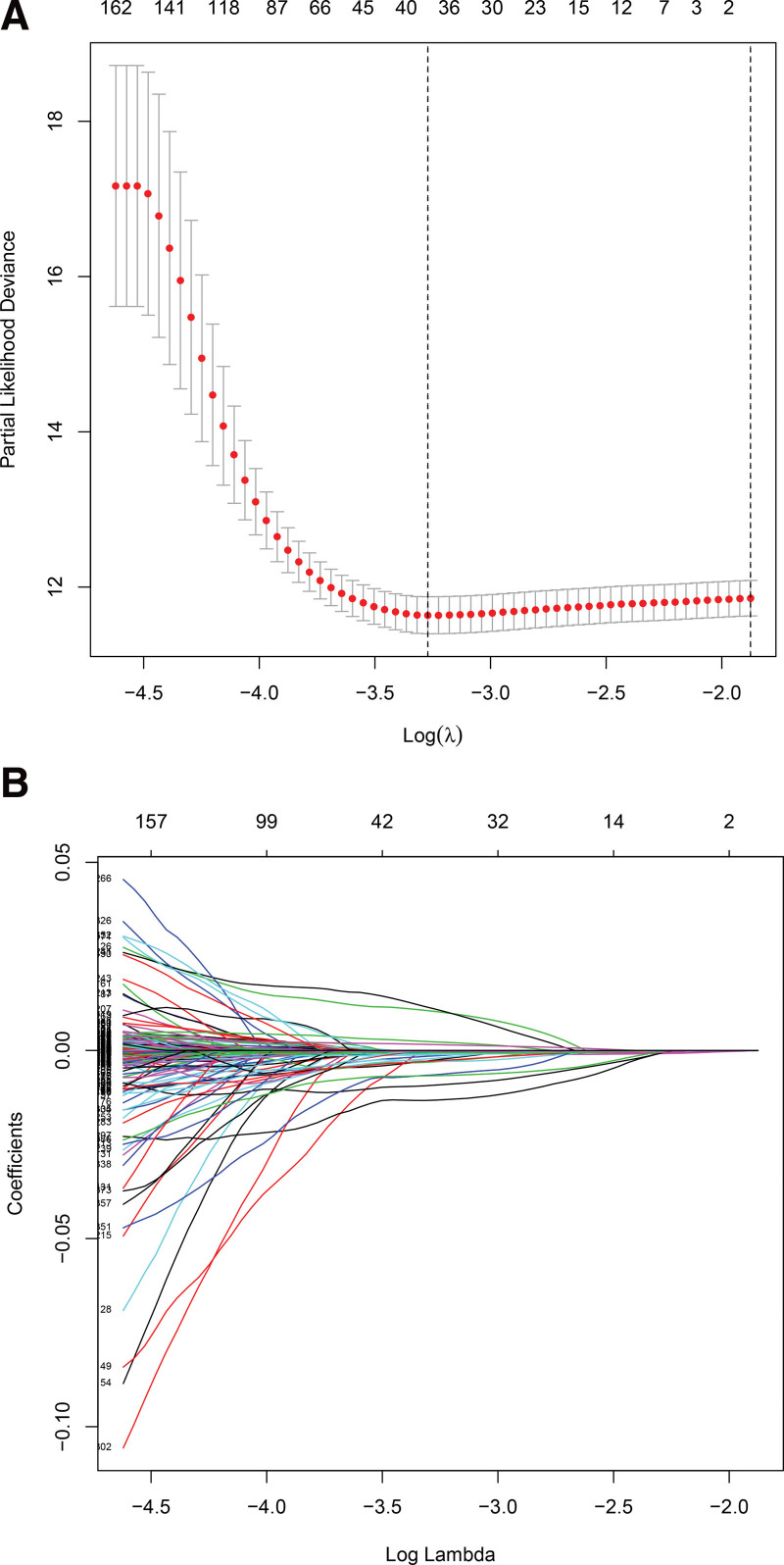
A total of 501 ferroptosis-related lncRNAs with prognostic value for BC were identified by univariate COX analysis (P < .05, Table S2, Supplemental Digital Content, http://links.lww.com/MD/I257, univariate Cox analysis results of ferroptosis-related lncRNAs based on survival length). Subsequent Lasso regression analysis brought this number down to 38 lncRNAs associated with ferroptosis (Fig. 1). Finally, multivariate COX regression analysis identified fifteen independent prognostic lncRNAs. (Fig. 2, Table 1). Four of them (AL590428.1, FOXC2-AS1, LINC00867, and ARHGAP5-S1) were harmful factors and the rest were protective factors (Fig. 3). Based on these fifteen ferroptosis-related LncRNAs, the risk model was successfully constructed. The risk score of each patient was calculated as follows:
Figure 1.
Screening for ferroptosis-related lncRNA using the Lasso regression. (A) Lasso coefficient values of 38 ferroptosis-related lncRNAs in BC. (B) Correlation of Lasso coefficients with λ values. BC = bladder cancer, Lasso = least absolute shrinkage and selection operator.
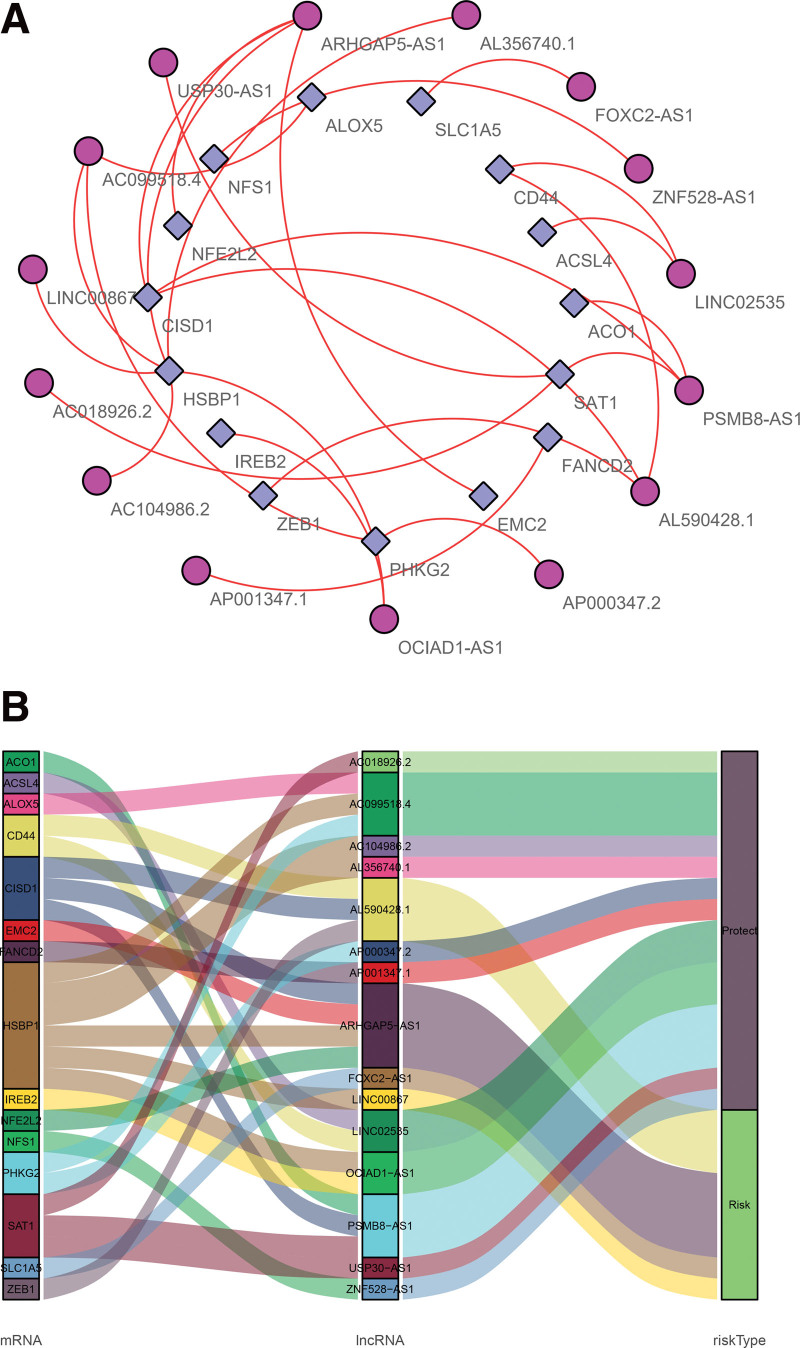
Figure 2.
Co-expression networks and Sankey plots of prognosis-associated ferroptosis-related lncRNAs. (A) Co-expression network of prognosis-associated lncRNAs and ferroptosis-related genes in BC. The purple circles represent prognosis-associated lncRNAs, and ferroptosis-related genes are represented by blue squares. (B) Sankey plots showing the association between prognostic ferroptosis-related lncRNAs, ferroptosis-related genes and patient risk type. BC = bladder cancer.
Table 1.
Results of multivariate Cox regression analysis of lncRNAs based on TCGA-BLCA data.
| LncRNA | Coef | HR | 95% CI of HR | P value |
|---|---|---|---|---|
| AL356740.1 | −0.004 | 0.996 | 0.992–1.001 | .099 |
| FOXC2-AS1 | 0.016 | 1.016 | 1.007–1.025 | .000 |
| ZNF528-AS1 | −0.000 | 1.000 | 0.999–1.000 | .135 |
| LINC02535 | −0.013 | 0.987 | 0.976–0.999 | .034 |
| PSMB8-AS1 | −0.001 | 0.999 | 0.999–1.000 | .094 |
| AL590428.1 | 0.028 | 1.028 | 1.010–1.046 | .002 |
| AP000347.2 | −0.008 | 0.992 | 0.984–1.000 | .049 |
| OCIAD1-AS1 | −0.026 | 0.974 | 0.947–1.002 | .068 |
| AP001347.1 | −0.005 | 0.995 | 0.989–1.001 | .093 |
| AC104986.2 | −0.003 | 0.997 | 0.992–1.001 | .140 |
| AC018926.2 | −0.020 | 0.980 | 0.963–0.997 | .021 |
| LINC00867 | 0.007 | 1.007 | 1.002–1.013 | .007 |
| AC099518.4 | −0.014 | 0.986 | 0.966–1.007 | .192 |
| USP30-AS1 | −0.003 | 0.997 | 0.992–1.001 | .131 |
| ARHGAP5-AS1 | 0.002 | 1.002 | 1.001–1.003 | .000 |
BLCA = bladder cancer, CI = confidential interval, lncRNAs = long non-coding RNAs, TCGA = The Cancer Genome Atlas, HR = hazard ratio.
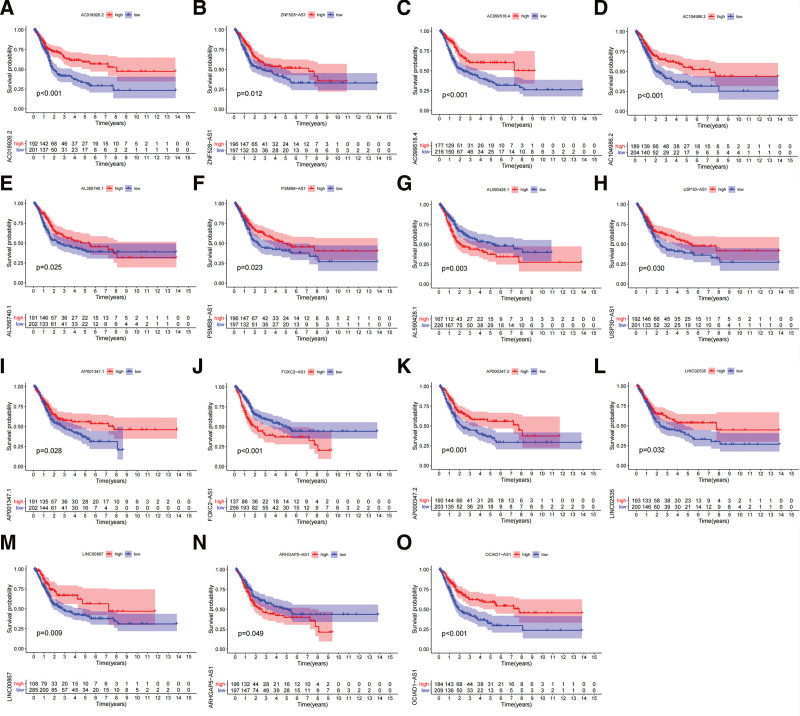
Figure 3.
KM survival curves of 15 prognostic ferroptosis-related lncRNAs. (A–O) KM survival curves of different lncRNAs, KM = kaplan meier.
Risk score = (0.02779 × L590428.1) + (0.01588 × FOXC2AS1) + (0.00723 × LINC00867) + (0.00223 × ARHGAP5-AS1) − (0.00040 × ZNF528-AS1) − (0.00063 × PSMB8-AS1) − (0.00324 × AC104986.2) − (0.00330 × USP30-AS1) − (0.00368 × AL356740.1) − (0.00498 × AP001347.1) − (0.00788 × AP000347.2) − (0.01286 × LINC02535) − (0.01380 × AC099518.4) − (0.02045 × AC018926.2) − (0.02614 × OCIAD1-AS1).
3.3. Prognostic value of the risk model
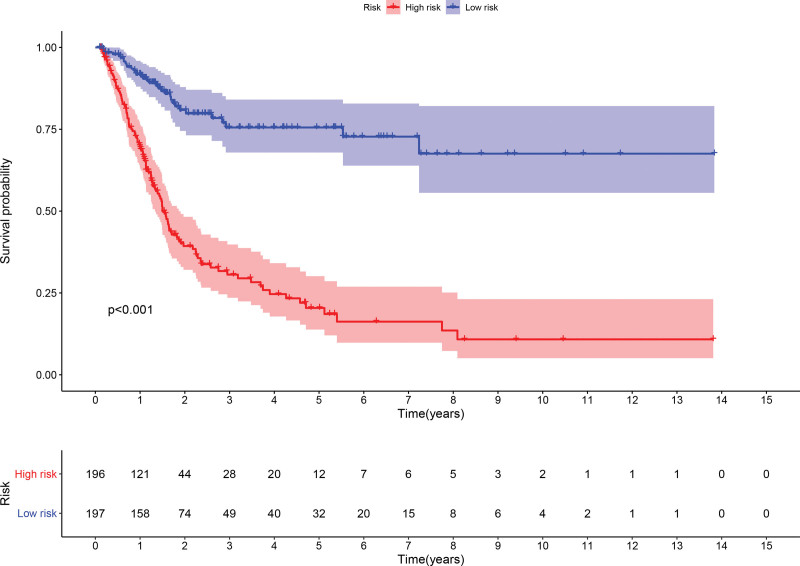
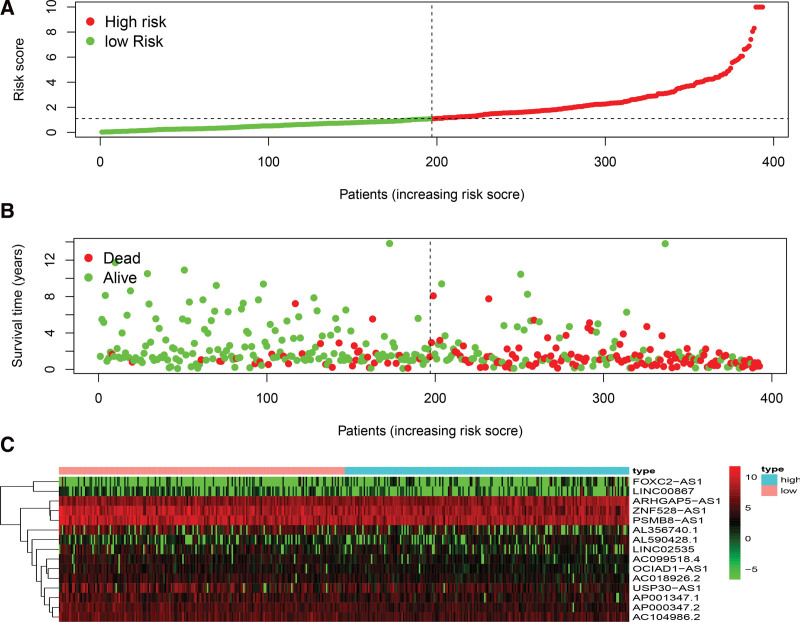
The high and low-risk groups were compared with respect to survival rates and durations. There was a significant difference in survival probability between the 2 groups. The probability of survival was higher in the low-risk group (P < .001, log-rank test) (Fig. 4). Cox regression also showed that the risk score was an independent indicator that predicted survival in BC patients with significant accuracy (Fig. 5).
Figure 4.
KM survival curves based on risk scores constructed from 15 ferroptosis-related lncRNAs, KM = kaplan meier.
Figure 5.
Correlation of ferroptosis-related lncRNAs expression with risk scores and clinical information in patients with BC. (A) The difference in risk scores between high and low-risk groups. (B) The survival time of the patients. (C) Heat map of the expression of the 15 ferroptosis-related lncRNAs. Green represents low expression. Red represents high expression. BC = bladder cancer.
3.4. Clinical value of the ferroptosis-related lncRNA risk model
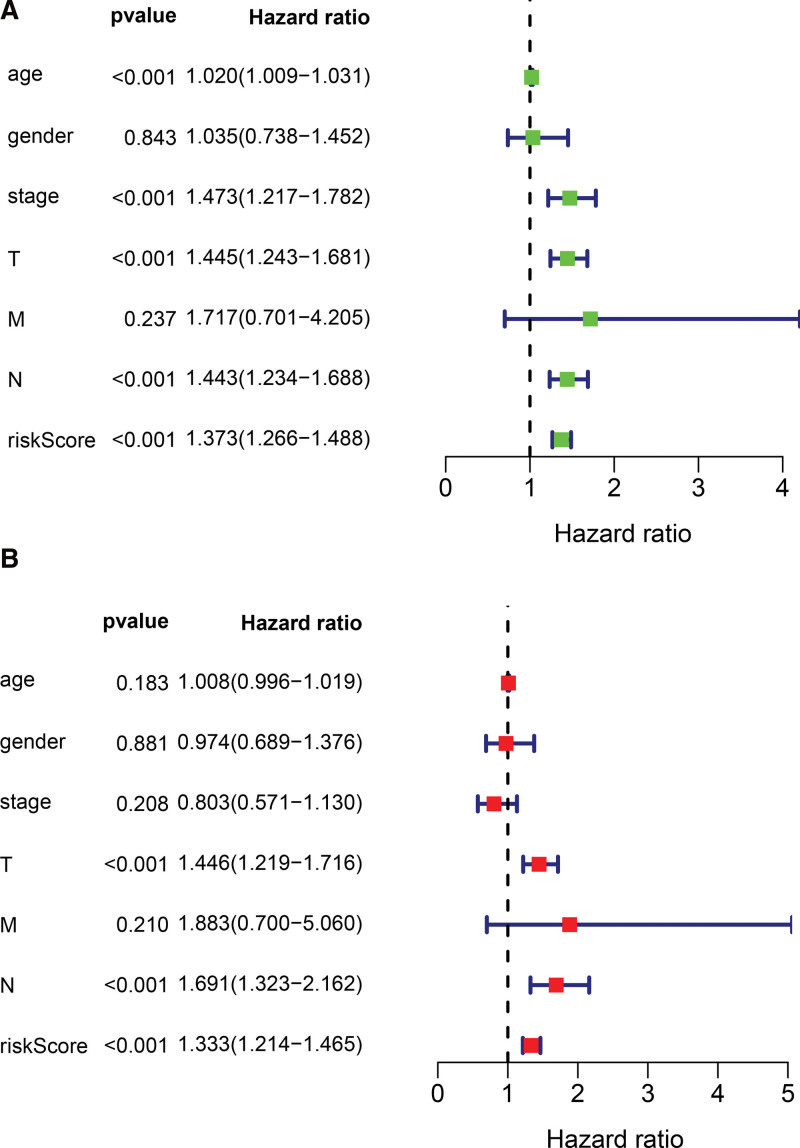
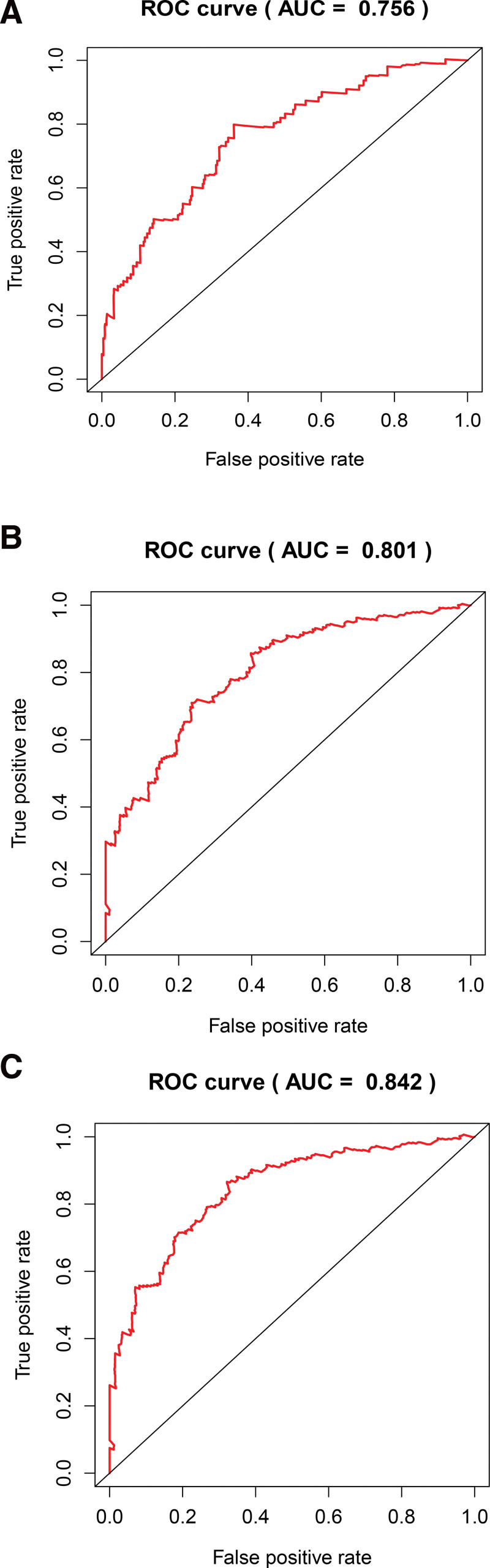
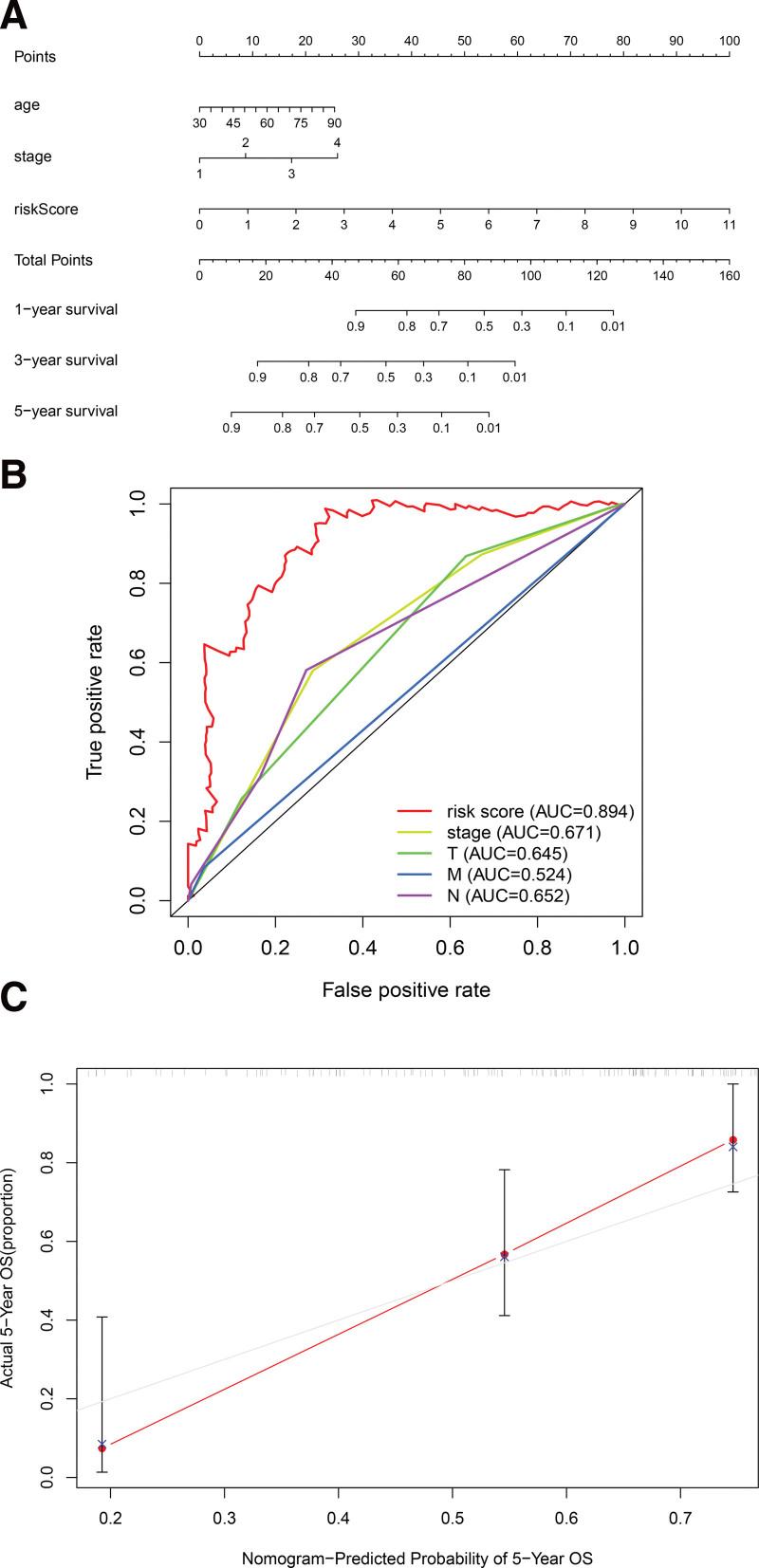
The results of univariate Cox regression analysis showed that indicators with independent predictive ability were age, risk score, and tumor, node, metastasis (TNM)-stage. The hazard ratio of risk score was 1.373 [95% Confidential interval = 1.266–1.488, P < .001, Fig. 6A, Table S3, Supplemental Digital Content, http://links.lww.com/MD/I258, results of univariate Cox analysis based on clinical characteristics and risk score of samples]. In forest plots of multivariate COX regression, the risk score continued to show strong predictive potential [Hazard ratio = 1.111, 95% confidential interval = 1.077–1.146, P < .001, Table 2, Fig. 6B]. The AUC values of the receiver operating characteristic (ROC) curves were 0.756, 0.801, and 0.842, at 1-year, 3-year, and 5- year survival, respectively (Fig. 7). From the nomogram composed of age, risk score, TNM stage, it was clear that the risk score was the main contributor to OS in patients with BC (Fig. 8A). The C-index of the prognostic model was 0.767. In the ROC curve of 5-year survival rate, the AUC value of risk score was 0.894, suggesting that it had a strong ability to predict the prognosis of patients with BC (Fig. 8B). The trend of the risk score curve was consistent with that of the staging curve, indicating that the increase in risk score might be indicative of the progression of the disease (Fig. 8C, Table 3).
Figure 6.
Univariate and multivariate analysis of ferroptosis-associated lncRNAs in BC (A) forest plot showing the results of univariate analysis of ferroptosis-associated lncRNAs; (B) forest plot showing the results of multivariate analysis of ferroptosis-associated lncRNAs. BC = bladder cancer.
Table 2.
Clinical characteristics and risk scores of BC using multivariate Cox regression.
| Variable | B | SE | Z | HR | HR.95L | HR.95H | P value |
|---|---|---|---|---|---|---|---|
| Age | 0.008 | 0.006 | 1.330 | 1.008 | 0.996 | 1.019 | .183 |
| Gender | −0.026 | 0.176 | −0.149 | 0.974 | 0.689 | 1.376 | .881 |
| Stage | −0.220 | 0.174 | −1.260 | 0.803 | 0.571 | 1.130 | .208 |
| T | 0.369 | 0.087 | 4.227 | 1.446 | 1.219 | 1.716 | .016 |
| M | 0.633 | 0.504 | 1.254 | 1.883 | 0.700 | 5.060 | .210 |
| N | 0.525 | 0.125 | 4.194 | 1.691 | 1.323 | 2.162 | .019 |
| Risk score | 0.288 | 0.048 | 5.995 | 1.333 | 1.214 | 1.465 | <.001 |
BC = bladder cancer, HR = hazard ratio.
Figure 7.
The areas under the ROC curves of 1 (A), 3 (B), and 5 (C) years, ROC = receiver operating characteristic.
Figure 8.
Prognostic model based on 15 ferroptosis-associated lncRNAs and clinical information. (A) Nomograms of predicted 1-, 3-, and 5-year OS; (B) ROC curves of risk scores and pathological staging; (C) calibration plots to assess the fit of the predicted and actual values of the prognostic model, ROC = receiver operating characteristic.
Table 3.
Clinical significance of risk score signature for TCGA-BLCA data.
| Clinical | N | Risk score | T | P | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Age | |||||
| >65 | 94 | 1.844 | 1.861 | 1.773 | .078 |
| ≤65 | 67 | 1.396 | 1.339 | ||
| Gender | |||||
| Female | 35 | 2.197 | 2.034 | 1.863 | .069 |
| Male | 126 | 1.508 | 1.535 | ||
| Stage | |||||
| Ⅰ–Ⅱ | 48 | 1.047 | 0.831 | −4.090 | <.001 |
| Ⅲ–Ⅳ | 113 | 1.917 | 1.867 | ||
| T | |||||
| T0–2 | 53 | 1.088 | 0.859 | −3.907 | <.001 |
| T3–4 | 108 | 1.937 | 1.895 | ||
| M | |||||
| M0 | 154 | 1.595 | 1.652 | −2.192 | .067 |
| M1 | 7 | 3.028 | 1.693 | ||
| N | |||||
| N0 | 110 | 1.388 | 1.482 | −2.801 | .006 |
| N1–2 | 51 | 2.238 | 1.917 | ||
BLCA = bladder cancer, TCGA = the cancer genome atlas.
3.5. Gene set enrichment analysis
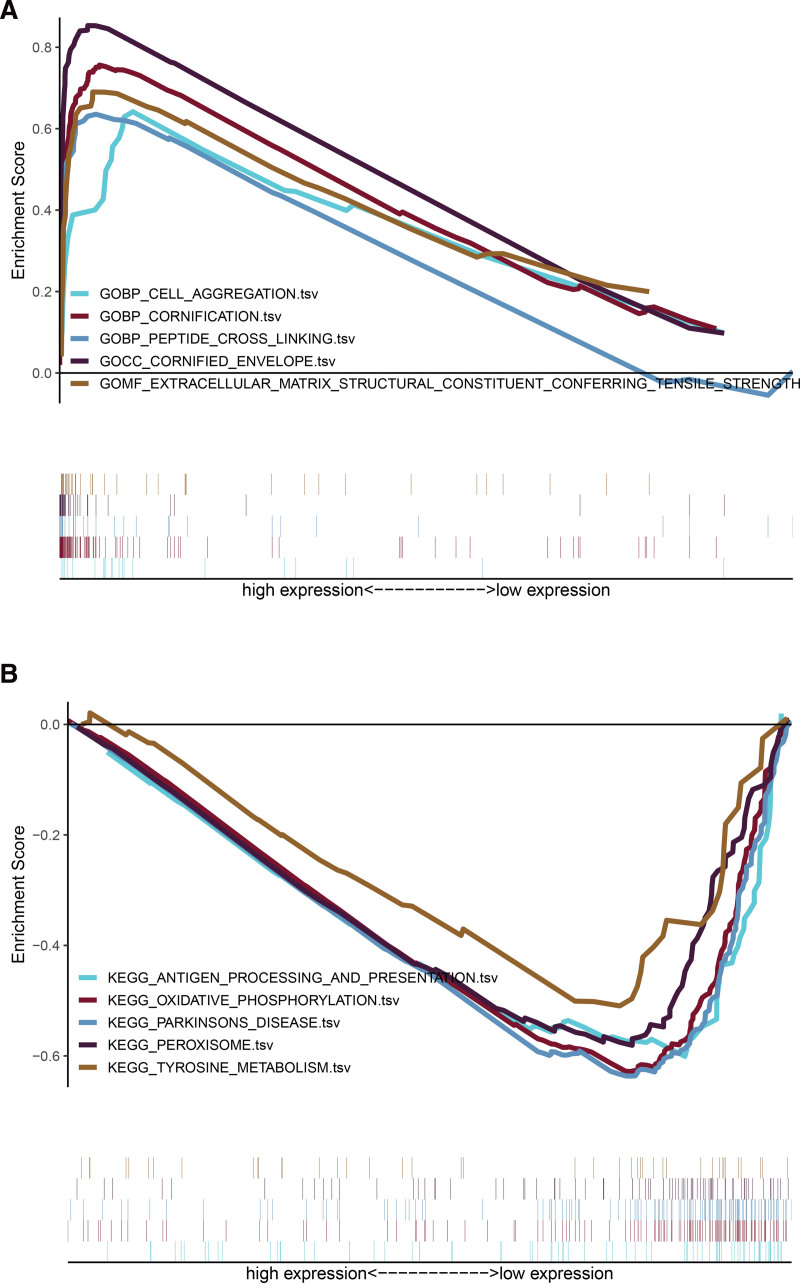
We further analyzed the functional enrichment of lncRNA by gene set enrichment analysis software. GO enrichment showed that the enrichment of ferroptosis-related lncRNAs was observed in cell aggregation, peptide cross linking, extracellular matrix structural constituent conferring tensile strength etc. (Fig. 9A). The KEGG pathway showed that the lncRNAs were mainly involved in the pathway of oxidative phosphorylation, peroxisome, tyrosine-metabolism etc. (Fig. 9B).
Figure 9.
Results of functional analysis (KEGG and GO) based on ferroptosis-related lncRNAs. Enrichment map of (A) GO and (B) KEGG enrichment analysis, GO = gene ontology, KEGG = kyoto encyclopedia of genes and genomes.
3.6. Single-cell transcriptional analysis of ferroptosis-related lncRNAs in the BC TME
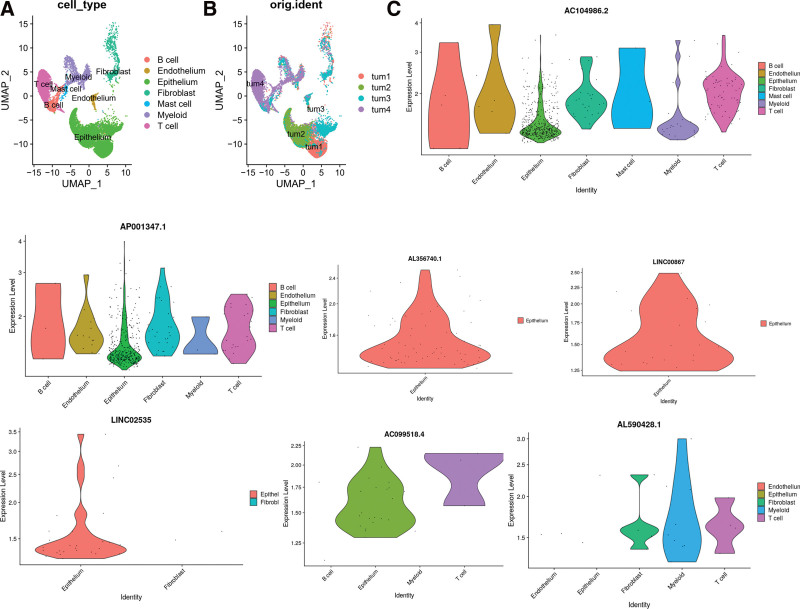
ScRNA-seq was performed on 4 in-house BC samples. After quality control using Seurat, 24,400 high-quality single-cell transcriptome information was used for subsequent analysis. Cell clustering analysis based on the uniform manifold approximation and projection algorithm showed that the above cells could be classified into 7 clusters, namely B cell, mast cells, epithelial cells, endothelial cells, fibroblast, T cells, and myeloid cells (Fig. 10A). Different cell clusters had significantly different expression levels of marker genes (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/I261, which demonstrates the expression of cell markers in different cell clusters). Tumor samples from different patients all contain the 7 cellular components but in different proportions (Fig. 10B). We compared the expression of ferroptosis-related lncRNAs in the BC TME. AC104986.2 and AP001347.1 were expressed in all 7 cells, especially in T cells, suggesting that the above 2 LncRNAs may play a role in the T cells of BC (Fig. 10C). AL356740.1, LINC02535 and LINC00867 were mainly expressed in epithelial cells (tumor cells), suggesting a possible involvement in tumor development. These results suggest that targeting ferroptosis-related lncRNAs could be a breakthrough in regulating the TME.
Figure 10.
Single-cell transcriptomic atlas of BC. (A) A UMAP plot of BC samples showing 7 distinct cell types. (B) A UMAP plot of BC from 4 different samples. (C) Expression of ferroptosis-related lncRNAs in different BC tumor microenvironment cells. BC = bladder cancer, UMAP = uniform manifold approximation and projection.
4. Discussion
The incidence of BC remains high, and the efficacy of current therapeutic options are not satisfactory. Radical cystectomy with bilateral pelvic lymph node dissection is the current gold standard for the treatment of muscle-infiltrating BC.[19] Unfortunately, 40% of patients with limited stage BC who undergo cystectomy will experience recurrence. It is now clear that TNM stage, grade and urinary tract obstruction are the key factors affecting prognosis.[20–22] However, these prognostic factors have limited value because of individual differences at molecular level. Considering the complex molecular types and regulatory mechanisms of BC, we took a unique approach to prognostic analysis from the perspective of ferroptosis-related lncRNAs. We aim to provide new insights into the prognostic assessment and individualized treatment of BC, and to lay the foundation for subsequent studies on molecular mechanisms.
Earlier studies showed that many lncRNAs are closely related to the occurrence and progression of tumors. We, therefore, looked for lncRNAs that might be involved in BC and identified fifteen ferroptosis-related lncRNAs (AL356740.1, FOXC2-AS1, ZNF528-AS1, LINC02535, PSMB8-AS1, AL590428.1, AP000347.2, OCIAD1-AS1, AP001347.1, AC104986.2, AC018926.2, LINC00867, AC099518.4, and USP30-AS1ARHGAP5-AS1) using cox regression analysis and lasso regression analysis. These lncRNAs were used to construct the co-expression network of lncRNA and ferroptosis-related genes.
Research showed that the expression of LINC02535 was significantly increased in cervical cancer. LINC02535 regulates cervical cancer cell proliferation, migration, DNA damage repair, and tumor progression by binding to poly-binding protein 2.[23]
In studies related to PSMB8-AS1, it has been found that PSMB8-AS1 can promote pancreatic cancer progression by regulating miR-382-3p/STAT1/PD-L1 axis and also promote glioma cell proliferation by regulating miR-574-5p/RAB1.[24,25] LncRNA OCIAD1-AS1 has been reported before, which is related to the prognosis of patients with BC.[26,27] However, no detailed study on the specific mechanism of lncRNA has been conducted to date. LncRNA FOXC2-AS1 is mainly located in the cytoplasm and is significantly up-regulated in osteosarcoma and can promote drug resistance of osteosarcoma cells leading to tumor progression and poor prognosis.[28] In addition, FOXC2-AS1 can also promote the proliferation and progression of prostate cancer by influencing the regulation of miR-1253/EZH2,[29] and enhance the stability of FOXC2 mRNA by activating Ca-FAK signaling pathway to promote the progression of colorectal cancer.[30]
It is reported that USP30-AS1 is associated with the prognosis of BC,[31,32] cervical cancer,[33] ovarian cancer, and glioblastoma.[34,35] However, there is no further research into these findings. Some studies have shown that the up-regulation of lncRNA ARHGAP5-AS1 promotes chemotherapy resistance of gastric cancer. It’s up-regulation is also related to the poor prognosis of gastric cancer.[36] However, how the rest of the lncRNAs play a regulatory role in the occurrence and development of tumors and affect the prognosis of patients is not clear. Therefore, more research is needed to unravel their mysteries.
Using risk scoring method and classification into high and low-risk groups, we confirmed that risk score could be used as an independent indicator to predict the prognosis of BC patients. The accuracy of nomogram prediction is further verified by discrimination and calibration plots. We also constructed multivariate ROC curve and calculated the AUC value (0.894), showing high predictive strength of the method. This can not only provide an individualized assessment of survival rates for BC patients, but also help make individualized disease management decisions.
The results of GO analysis in high-risk group showed that differentially expressed lncRNAs were mainly enriched in the cell aggregation, peptide cross linking, extracellular matrix structural constituent conferring tensile strength etc. (Table S4, Supplemental Digital Content, http://links.lww.com/MD/I259, the top 20 results of low-risk group analyzed by GO). Through the KEGG pathway analysis, we found that lncRNAs in low-risk group are mainly enriched in oxidative phosphorylation, peroxisome, tyrosine metabolism, involved in ferroptosis regulatory pathways (Table S5, Supplemental Digital Content, http://links.lww.com/MD/I260, the top 20 results of high-risk group analyzed by KEGG).
In this study we constructed a risk model based on 15 ferroptosis-related lncRNAs, which has good prognostic value for patients with BC. However, our research does have some limitations. Most of the data used in this study comes from a single database, TCGA database and the quantity of data included is relatively small, which may have introduced some bias in the results. Although the stability of lncRNA signals related to ferroptosis has been successfully verified, the exact molecular regulatory mechanism of these lncRNAs has not been effectively studied, and the significance of their clinical effect is unclear. Therefore, further experimental studies and clinical observations with a larger sample size are needed to verify these results.
5. Conclusion
In summary, the risk score model has an independent prognostic value. Genomic clinico-pathological nomogram could be used to accurately predict the prognosis of patients with BC. The fifteen ferroptosis-related lncRNAs might also be investigated as potential therapeutic targets.
Acknowledgments
We thank the staff in the Centre for Genomic and Personalized medicine Guangxi Medical University for supporting the research.
Author contributions
Conception: Xuebao Xiang, Yi Guo, Zhongyuan Chen, Fangxin Zhang, Jiefu Huang, Yan Qin.
Interpretation or analysis of data: Xuebao Xiang, Yi Guo, Zhongyuan Chen.
Preparation of manuscript: Xuebao Xiang, Fangxin Zhang, Jiefu Huang, Yan Qin.
Revision for important intellectual content: Xuebao Xiang, Yi Guo, Fangxin Zhang, Yan Qin.
Supervision: Xuebao Xiang, Yi Guo, Zhongyuan Chen, Fangxin Zhang, Yan Qin, Jiefu Huang.
Supplementary Material
Abbreviations:
- AUC =
- area under the curve
- BC =
- bladder cancer
- DPBS =
- Dulbecco’s phosphate-buffered saline
- GO =
- gene ontology
- KEGG =
- kyoto encyclopedia of genes and genomes
- Lasso =
- least absolute shrinkage and selection operator
- lncRNAs =
- long non-coding RNAs
- OS =
- overall survival
- RNA-seq =
- RNA sequencing
- ROC =
- receiver operating characteristic
- TCGA =
- The Cancer Genome Atlas
- TME =
- tumor microenvironment
- TNM =
- tumor, node, metastasis
This study was supported by Guangxi Zhuang Autonomous Region Health and Health Commission Self-Financed Research Projects (Z20200351); Guangxi Zhuang Autonomous Region People’s Hospital Youth Fund (QN2021-11); and Guangxi Natural Science Foundation (2018JJA140430).
TCGA database has obtained informed consent from patients.
This study was approved by the ethics and human subjects committee of the First Affiliated Hospital of Guangxi Medical University. Written informed consent forms have been obtained from patients.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Xiang X, Guo Y, Chen Z, Zhang F, Huang J, Qin Y. A prognostic risk prediction model based on ferroptosis-related long non-coding RNAs in bladder cancer: A bulk RNA-seq research and scRNA-seq validation. Medicine 2022;101:51(e32558).
Contributor Information
Xuebao Xiang, Email: xiangxuebao@outlook.com.
Yi Guo, Email: omr00373@163.com.
Zhongyuan Chen, Email: xjq77849@163.com.
Fangxin Zhang, Email: tbjb6211@163.com.
Jiefu Huang, Email: oqta4528@21cn.com.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Davis A, Patel V. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Segovia C, San José-Enériz E, Munera-Maravilla E, et al. Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat Med. 2019;25:1073–81. [DOI] [PubMed] [Google Scholar]
- [4].Morales-Barrera R, Suárez C, González M, et al. The future of bladder cancer therapy: optimizing the inhibition of the fibroblast growth factor receptor. Cancer Treat Rev. 2020;86:102000. [DOI] [PubMed] [Google Scholar]
- [5].Casadei C, Dizman N, Schepisi G, et al. Targeted therapies for advanced bladder cancer: new strategies with FGFR inhibitors. Ther Adv Med Oncol. 2019;11:1758835919890285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Louandre C, Marcq I, Bouhlal H, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356:971–7. [DOI] [PubMed] [Google Scholar]
- [7].Wang K, Chang H. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jin K, Lu Z, Lv J, et al. The role of long non-coding RNAs in mediating chemoresistance by modulating autophagy in cancer. RNA Biol. 2020;17:1727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang Y, Tao Y, Liao Q. Long noncoding RNA: a crosslink in biological regulatory network. Brief Bioinform. 2018;19:930–45. [DOI] [PubMed] [Google Scholar]
- [10].Wang Z, Chen X, Liu N, et al. A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2021;29:263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu J, Xu F, Lu H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 2020;260:118305. [DOI] [PubMed] [Google Scholar]
- [12].Su C, Lv Y, Lu W, et al. Single-Cell RNA sequencing in multiple pathologic types of renal cell carcinoma revealed novel potential tumor-specific markers. Front Oncol. 2021;11:719564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu P, Wang Z, Ou X, et al. The FUS/circEZH2/KLF5/ feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol Cancer. 2022;21:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Y, Tai W, Lu N, et al. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging. 2020;12:9085–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–49. [DOI] [PubMed] [Google Scholar]
- [16].Bersuker K, Hendricks J, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doll S, Freitas F, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. [DOI] [PubMed] [Google Scholar]
- [18].Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2018;174:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bruins HM, Arends TJ, Pelkman M, et al. Radical cystectomy in a Dutch University hospital: long-term outcomes and prognostic factors in a homogeneous surgery-only series. Clin Genitourin Cancer. 2014;12:190–5. [DOI] [PubMed] [Google Scholar]
- [20].Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- [21].Alfred Witjes J, Lebret T, Compérat E, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–75. [DOI] [PubMed] [Google Scholar]
- [22].Wan J, Guo C, Fang H, et al. Autophagy-related long non-coding RNA is a prognostic indicator for bladder cancer. Front Oncol. 2021;11:647236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wen D, Huang Z, Li Z, et al. LINC02535 co-functions with PCBP2 to regulate DNA damage repair in cervical cancer by stabilizing RRM1 mRNA. J Cell Physiol. 2020;235:7592–603. [DOI] [PubMed] [Google Scholar]
- [24].Zhang H, Zhu C, He Z, et al. LncRNA PSMB8-AS1 contributes to pancreatic cancer progression via modulating miR-382-3p/STAT1/PD-L1 axis. J Exp Clin Cancer Res. 2020;39:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].More S, Zhu Z, Lin K, et al. Long non-coding RNA PSMB8-AS1 regulates influenza virus replication. RNA Biol. 2019;16:340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang J, Shen C, Dong D, et al. Identification and verification of an immune-related lncRNA signature for predicting the prognosis of patients with bladder cancer. Int Immunopharmacol. 2021;90:107146. [DOI] [PubMed] [Google Scholar]
- [27].Li W, Zhang Y. Novel long non-coding RNA markers for prognostic prediction of patients with bladder cancer. Chin Med Sci J. 2020;35:239–47. [DOI] [PubMed] [Google Scholar]
- [28].Zhang C, Zhu K, Ma X. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66–75. [DOI] [PubMed] [Google Scholar]
- [29].Chen Y, Gu M, Liu C, et al. Long noncoding RNA FOXC2-AS1 facilitates the proliferation and progression of prostate cancer via targeting miR-1253/EZH2. Gene. 2019;686:37–42. [DOI] [PubMed] [Google Scholar]
- [30].Pan K, Xie Y. LncRNA FOXC2-AS1 enhances FOXC2 mRNA stability to promote colorectal cancer progression via activation of Ca-FAK signal pathway. Cell Death Dis. 2020;11:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tong H, Li T, Gao S, et al. An epithelial-mesenchymal transition-related long noncoding RNA signature correlates with the prognosis and progression in patients with bladder cancer. Biosci Rep. 2021;41:BSR20203944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun Z, Jing C, Xiao C, et al. An autophagy-related long non-coding RNA prognostic signature accurately predicts survival outcomes in bladder urothelial carcinoma patients. Aging. 2020;12:15624–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen P, Gao Y, Ouyang S, et al. A prognostic model based on immune-related long non-coding RNAs for patients with cervical cancer. Front Pharmacol. 2020;11:585255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gao M, Wang X, Han D, et al. A six-lncRNA signature for immunophenotype prediction of glioblastoma multiforme. Front Genet. 2020;11:604655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meng C, Zhou J, Liao Y. Autophagy-related long non-coding RNA signature for ovarian cancer. J Int Med Res. 2020;48:300060520970761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu L, Zhu Y, Han S, et al. Impaired autophagic degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in gastric cancer. Cell Death Dis. 2019;10:383. [DOI] [PMC free article] [PubMed] [Google Scholar]