Abstract
The atlC gene (1,485 bp), encoding an autolysin which binds fibronectin, and the ica operon, involved in biofilm formation, were isolated from the chromosome of an infectious isolate of Staphylococcus caprae and sequenced. AtlC (155 kDa) is similar to the staphylococcal autolysins Atl, AtlE, Aas (48 to 72% amino acid identity) and contains a putative signal peptide of 29 amino acids and two enzymatic centers (N-acetylmuramoyl-l-alanine amidase and endo-β-N-acetylglucosaminidase) interconnected by three imperfect fibronectin-binding repeats. The glycine-tryptophan (GW) motif found in the central and end part of each repeat may serve for cell surface anchoring of AtlC as they do in Listeria monocytogenes. The S. caprae ica operon contains four genes closely related to S. epidermidis and S. aureus icaA, icaB, icaC, and icaD genes (≥ 68% similarity) and is preceded by a gene similar to icaR (≥70% similarity). The polypeptides deduced from the S. caprae ica genes exhibit 67 to 88% amino acid identity to those of S. epidermidis and S. aureus ica genes. The ica operon and icaR gene were analyzed in 14 S. caprae strains from human specimens or goats' milk. Some of the strains produced biofilm, and others did not. All strains carry the ica operon and icaR of the same sizes and in the same relative positions, suggesting that the absence of biofilm formation is not related to the insertion of a mobile element such as an insertion sequence or a transposon.
Staphylococcus caprae (13) is the predominent species among the staphylococci recovered from mastitis-free goats' milk (5). It is also increasingly recognized as a human pathogen infecting implanted foreign bodies (1, 6, 14, 44, 46, 52). Despite the amended description of this species (25), its phenotypic identification remains difficult. Therefore, molecular identification methods such as the analysis of ribotypes (1, 5, 12, 52), DNA-DNA hybridization (25), sequencing of the 16S rRNA gene (46), or analysis of the banding patterns on gels of penicillin-binding proteins (24) have been used for ecological studies and investigation of the involvement of S. caprae in infections. Some S. caprae strains from human specimens and goats' milk form biofilms (1, 4). Other strains do not, although the genomes of all strains tested carry nucleotide sequences hybridizing, at low stringency, with the S. epidermidis genes involved in initial adherence (atlE) and biofilm accumulation (the ica operon) (1). S. caprae adherence to fibronectin- and gelatin-coated coverslips is very weak. Nevertheless, surface proteins binding fibronectin have been detected on all S. caprae strains tested (1). The N-terminal part of the 175-kDa fibronectin-binding protein released from the surface of S. caprae clinical isolate 96007 has more than 50% amino acid identity (1) to the N-terminal part of the staphylococcal autolysins Atl (38), AtlE (18), and Aas (20). The aim of this study was to isolate the atlC autolysin gene of isolate 96007 to check whether the purified protein encoded by this gene binds fibronectin. We also characterized the ica operon to determine whether the absence of biofilm production in some strains is due to the integration of a mobile element as reported for the S. epidermidis ica operon (56).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The relevant characteristics of the 14 S. caprae strains isolated from specimens from four infected patients and from milk samples have been described in a previous paper (1). S. aureus strain DU5883(pFNBA4) (17) was used as a positive control in fibronectin-binding experiments. Micrococcus luteus strain ATCC 9341 was used for the detection of bacteriolytic activity. Escherichia coli strain M15 harboring pREP4, which constitutively expresses the Lac repressor protein encoded by the lacI gene (QiaExpress System; Qiagen, Hilden, Germany), was used as a recipient.
Plasmid pQE31 (Qiagen) was used as a vector to produce a fusion protein with the His6 tag at the N terminus of the protein. Plasmid pIP1818 (this study) was constructed by cloning into pQE31 a 1,884-bp fragment amplified from within atlC with primers Atl5 and Atl6 (Table 1). The recombinant plasmids pIP1781, pIP1789, pIP1807, pIP1808, and pIP1823 used for sequencing atlC and ica genes (this study) are pUC18 carrying chromosomal restriction fragments from S. caprae strain 96007. Staphylococcal and M. luteus strains were grown on brain heart infusion (Difco, Detroit, Mich.) or Trypticase soy broth (Difco). E. coli strains were cultured in Luria-Bertani medium supplemented with 100 μg of ampicillin per ml and, as required, 25 μg of kanamycin per ml.
TABLE 1.
Oligonucleotides used for PCR experiments
| Primer | Sequence (5′-3′) | Gene | Accession no. | Position |
|---|---|---|---|---|
| Atl5 | CTGGTCAAGTGGATCCTTGG | atlC | AF244123 | 1883–1902 |
| BamH1 | ||||
| Atl6 | GTATCTCTGCAGCATGAGC | atlC | AF244123 | 3784–3766 |
| PstI | ||||
| RF | CAATTCTTATTTTCTTCAATAACC | icaR | AF246926 | 442–466 |
| AR | AACTCTTGTTGCGAATACGTGGG | icaA | AF246926 | 1604–1627 |
| BF | CAATTATCAGAAATGTATCGTACAGG | icaB | AF246926 | 3112–3138 |
| LR | CAAGACAGTTCAGATACAGTACGC | gehC | AF246926 | 4832–4855 |
| CF | CATTAAGTGAAAAAGCTGTCACTCC | icaC | AF246926 | 3368–3393 |
DNA isolation and analysis.
Total cellular DNA was isolated from staphylococcal strains and was purified using the QIAamp tissue kit from Qiagen. Plasmid DNA was extracted and purified from E. coli using the QIA-prep Spin plasmid kit from Qiagen.
Restriction endonucleases were obtained from Amersham-Pharmacia Biotech, Inc. (Piscataway, N.J.), and were used as specified by the manufacturer. Native or digested DNA was analyzed by electrophoresis in a 0.7% (wt/vol) agarose gel. DNA fragments of less than 1 kb amplified by PCR were separated by electrophoresis in 4% (wt/vol) Nusieve agarose gels (FMC Products, Rockland, Maine).
Blotting and hybridization.
DNA was transferred to Hybond-N+ membranes (Amersham) and hybridized under stringent conditions (65°C) as previously described (9) or at lower stringency, i.e., 42°C.
PCR.
PCR experiments were performed at high stringency (initial cycle of 5 min at 95°C followed by 30 cycles of 1 min at 60°C, 1 min 30 at 72°C, and 45 s at 95°C and a final extension step of 10 min at 72°C). The primers used are listed in Table 1 and were prepared by the phosphoramidite method with an Applied Biosystems (Foster City, Calif.) model 380B DNA synthesizer.
Sequencing.
An Applied Biosystems automated 373A DNA sequencer and the protocol described by the manufacturer were used for sequencing. The amino acid sequences deduced from the nucleotide sequences were analyzed with the GCG, Inc., package and compared with those deduced from nucleotide sequences in the GenBank/EMBL Database.
Detection of bacteriolytic enzyme activity.
Bacteriolytic activity was detected using renaturing gels as described by Sugai et al. (47). Dried cells of M. luteus strain ATCC 9341 or S. caprae 96007 (1) were incorporated into sodium dodecyl sulfate (SDS)-polyacrylamide gels (1 mg · ml−1). After electrophoresis, the gels were washed in distilled water to remove the SDS and incubated in renaturating buffer (100 mM phosphate buffer [pH 7]) at 37°C for 1 h to overnight with gentle shaking. When lytic bands appeared, photographs were taken under oblique translucent light from the back against a black background. Thus, lytic bands appear as dark zones on the photographs.
Protein production.
For generation of recombinant protein, plasmid pQE31 was used. Production and purification steps were performed as specified by the manufacturer (QIAexpress system; Qiagen).
Extraction of cell surface-associated proteins, SDS-PAGE, and Western affinity blotting.
LiCl extractions were carried out as described by Komatsuzawa et al. (27). Briefly, the pellet of a 40-ml cell culture (6 h at 37°C) was suspended in 200 μl of 3 M LiCl (Sigma, Aldrich Chemie, Deisenhofen, Germany) in an ice bath for 15 min. After centrifugation (10,000 × g for 15 min), the supernatant was extensively dialyzed against distilled water (4°C). SDS-polyacrylamide gel electrophoresis (PAGE) of the proteins, their transfer onto polyvinylidene difluoride membranes (Hybond-P; Amersham), and incubation of the membranes with 3 μg of fibronectin (Chemicon, Temecula, Calif.) per ml were carried out as described previously (1). Binding proteins were detected with mouse anti-human fibronectin N-terminal monoclonal antibody (Chemicon). The ECL kit (Amersham) was used for antibody detection by chemiluminescence.
Nucleotide sequence accession numbers.
The nucleotide sequences of the atlC gene and the ica operon from S. caprae strain 96007 have been submitted to GenBank under accession numbers AF244123 and AF246926, respectively. The nucleotide sequence of the icaC gene from S. caprae strain 89318 is registered under accession number AF246927.
RESULTS
Characterization of the atlC autolysin gene from the S. caprae isolate 96007.
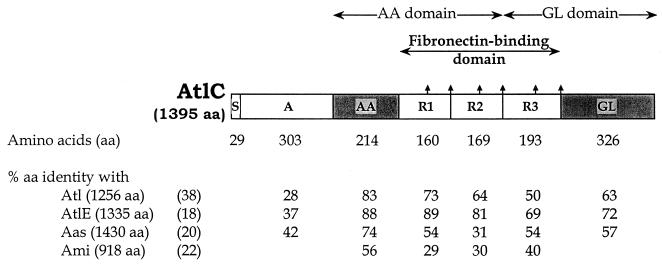
In S. caprae clinical isolate 96007 (1), the nucleotide sequences hybridizing at low stringency with the S. epidermidis atlE probe were found on 8.9-kb HindIII and 4.3-kb HincII fragments having a 1.6-kb overlapping region (results not shown). Each of these two restriction fragments was inserted into pUC18. The resulting recombinant plasmids, pIP1781 and pIP1808, respectively, were used to determine the sequence of the regions exhibiting similarity to atlE (18). The sequence (accession number AF244123), contains a 4,185-bp putative gene, atlC, delimited by the ATG start codon at nucleotides (nt) 271 to 273 and the TAA stop codon at nt 4456 to 4458. This gene exhibits at least 55% similarity to the three sequenced staphylococcal autolysin genes, atl, atlE, and aas. The ATG start codon of atlC is preceeded, 10 nt upstream, by a 6-nt putative ribosome-binding site. The ΔG of the interaction of the most stable structure between this putative ribosome-binding site and the 3′ terminus of the 16S rRNA, calculated by the method of Tinocco et al. (51), is −60.2 kJ · mol−1. The DNA sequence upstream from the start codon contains a putative promoter sequence, AATACAN17TATAAT (nt 206 to 234). This sequence has 9 of 12 possible matches with the consensus sequence of the Bacillus subtilis vegetative T-factor promoter, ςA (TTGACAN18TATAAT) (36) and the putative promoter of S. aureus atl (CGCACAN18TATAAT) (39) There is a putative transcriptional terminator consisting of inverted repeats (nt 4503 to 4538) followed by an AT-rich sequence downstream from the stop codon. The G+C content of atlC is 33.5%. This is similar to the value for the staphylococcal genome (32 to 36%) (26). The gene encodes a 1,395-amino-acid putative protein of 155 kDa.
Analysis of the AtlC sequence.
The N-terminal part of the deduced amino acid sequence is a putative signal peptide of 29 amino acids as assessed by the method of von Heijne (53). The overall sequence exhibits significant similarities to the staphylococcal Atl-related autolysins: Atl (38), AtlE (18), and Aas (20) (61, 72, and 48% amino acid identity, respectively). Three imperfect repeats are present in the central region of each of these autolysins (Fig. 1). A glycine-tryptophan (GW) dipeptide motif is present in the central part and at the end of each repeat.
FIG. 1.
Organization of the S. caprae autolysin AtlC
and percent amino acid identity to the corresponding domains of
S. aureus Atl (38), S. epidermidis
AtlE (18), and S. saprophyticus Aas
(20).
, Glycine-tryptophan (GW) dipeptide;
 , active
enzymatic center. The enzymatic domains (AA and GL) were deduced from
the similarities to those of the staphylococcal Atl-related autolysins.
Abbreviations: AA, N-acetylmuramoyl-l-alanine
amidase; GL, N-acetylglucosaminidase; R, repeats.
, active
enzymatic center. The enzymatic domains (AA and GL) were deduced from
the similarities to those of the staphylococcal Atl-related autolysins.
Abbreviations: AA, N-acetylmuramoyl-l-alanine
amidase; GL, N-acetylglucosaminidase; R, repeats.
The two enzymatic domains of AtlC (Fig. 1), N-acetylmuramoyl-l-alanine amidase and endo-ß-N-acetylglucosaminidase, were deduced from the sequence similarities to the staphylococcal autolysins Atl (2, 27, 38, 39, 48), AtlE (18), and Aas (20). The repeats are required for the active enzymatic centers of these autolysins to be expressed on the bacterial surface.
Cell-associated bacteriolytic enzymes.
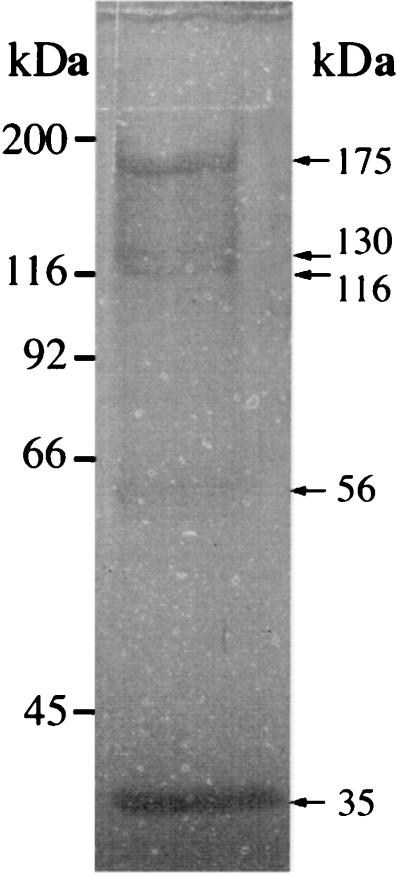
The proteins released from LiCl extracts of S. caprae clinical isolate 96007 were assayed for bacteriolytic activity by using renaturating gels containing either S. caprae strain 96007 (Fig. 2) or M. luteus strain ATCC 9341 (results not shown). The bacteriolytic banding profiles were similar on the two gels: there were major bands of ≈175, 130, 116, 56, and 35 kDa. Two of these bacteriolytic bands (175 and 116 kDa) gave a strong fibronectin-binding signal (1), whereas the signals observed for the three other bands were weak and not consistently reproducible. The N terminus of the larger bacteriolytic band binding fibronectin was sequenced (1). It appeared to be a secreted form of the unprocessed product of atlC (150 kDa, deduced from the sequence); hence the molecular mass of ≈175 kDa previously attributed to this protein (1) on the basis of mobility was overestimated. The 116-kDa band may correspond to the two enzymatic domains (57 and 59 kDa), and the 56-kDa bacteriolytic band may correspond to either or both these two enzymatic domains.
FIG. 2.
Bacteriolytic enzyme profile of S. caprae strain 96007 on an SDS-polyacrylamide gel containing dried S. caprae 96007 cells (1 μg · ml−1) as a substrate. Bands with lytic activity were observed as clear zones in the opaque gel and as dark bands after photography against a dark background. The sizes of marker proteins (in kilodaltons) are indicated on the left, and those of the bacteriolytically active proteins are shown on the right.
Characterization of the AtlC fibronectin-binding domain.
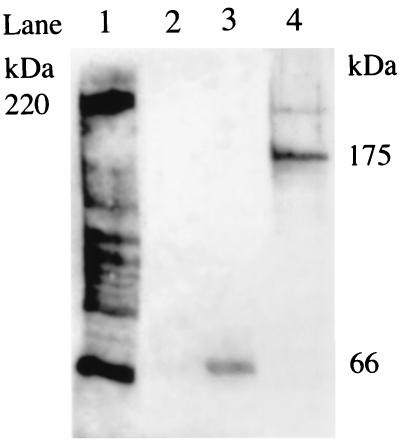
The repeats of the S. saprophyticus autolysin Aas are involved in fibronectin binding (20). Therefore, we checked whether the repeats of AtlC express fibronectin-binding activity. Primers Atl5 and Atl6 (Table 1) were used to amplify an 1,884-bp fragment from atlC by PCR. The amplified fragment was cleaved with BamHI and PstI and inserted into pQE31 (Qiagen) cut with the same restriction endonucleases. The resulting construct (pIP1818) encodes a fusion protein with His6 tag at the N terminus. pIP1818 was introduced into E. coli strain M15(pREP4). The transformant was grown in the absence or presence of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce the expression of the His-tagged protein. Aliquots were removed 1, 2, 3, and 4 h after induction and 4 h after growth without induction. The bacterial proteins were purified on Ni-nitrilotriacetic acid resin (Qiagen) under denaturating conditions. A single 66-kDa protein band was visualized by Coomassie staining in all the induced samples but was not detected in the uninduced culture (results not shown). The purified 66-kDa protein bound fibronectin (Fig. 3, lane 3) but not fibrinogen or collagen (results not shown). The protein binding fibronectin was detected in bacterial lysates of the IPTG-induced cultures (results not shown) but not in the noninduced cultures (lane 2).
FIG. 3.
Screening for fibronectin-binding proteins according to the Western affinity blotting technique described previously (1). Lanes: 1, surface proteins released from an LiCl extract of S. aureus strain DU5883 (pFNBA4) (17) used as positive control; 2, proteins released from uninduced culture of E. coli M15 harboring pREP4 and pIP1818; 3, 66-kDa protein resulting from purification on Ni-nitrilotriacetic acid resin (Qiagen) of the proteins released from an IPTG-induced culture of E. coli M15 harboring pREP4 and pIP1818; 4: proteins released from an LiCl extract of S. caprae isolate 96007.
Characterization of the ica locus in S. caprae strains.
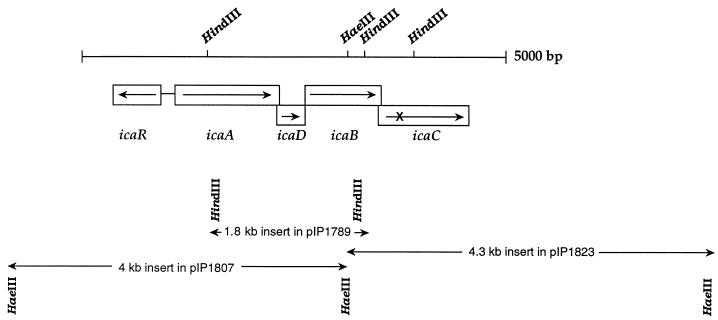
Nucleotide sequences hybridizing at low stringency with an S. epidermidis ica probe were detected in the genomes of all the S. caprae strains tested (1). In clinical isolate 96007, the hybridizing sequences were carried by contiguous HaeIII fragments of 4 and 4.3 kb overlapping a 1.8-kb HindIII fragment (Fig. 4). Each of the three fragments was inserted into pUC18 cleaved with SmaI or HindIII. The recombinant plasmids, pIP1807, pIP1823, and pIP1789 (Fig. 4), were used to sequence the regions similar to S. epidermidis and S. aureus ica operons (11, 19). The sequence of these regions in 96007 (accession number AF246926) contains three putative genes closely related (≥68% similarity) to icaA, icaB, and icaD of the S. epidermidis and S. aureus ica operons and a region similar to icaC (≥73% similarity) which is interrupted by a stop codon (nt 3743 to 3745) (Fig. 4). The organization of the locus is identical to those of S. epidermidis and S. aureus ica operons. A gene starting with a TTG codon and similar to icaR (≥70% similarity) was found upstream from the S. caprae ica operon and transcribed in the opposite direction from the four genes of the operon. The sequence of the incomplete open reading frame downstream from the S. caprae ica operon is similar to that of the end part of the S. epidermidis lipase gene gehC (accession number M95577), adjacent to the S. epidermidis and S. aureus ica operons. Isolate 96007, in which icaC is interrupted by a stop codon, does not produce biofilm. Therefore, primers CF and LR (Table 1) were designed to amplify icaC from an S. caprae isolate producing biofilm, 89318 (1). The amplicon of the expected size (1.5 kb) was sequenced (accession number AF246927): the icaC gene in strain 89318 was 99% identical to that in 96007 but was not interrupted by a stop codon. The sizes of the predicted polypeptides deduced from the S. caprae genes and the amino acid identities to the polypeptides encoded by the S. epidermidis and S. aureus ica genes are reported in Table 2.
FIG. 4.
Map of the ica locus and surrounding chromosomal region in S. caprae strain 96007 (accession number AF246926). The IcaC product is truncated. The fragments reported below the map were cloned into pUC18 for sequencing; their sizes and the designation of the recombinant plasmids are given.
TABLE 2.
Sizes of the polypeptides deduced from the sequenced S. caprae ica genes and the amino acid identity to those encoded by ica genes from S. epidermidis ATCC 35984 and S. aureus ATCC 35556 strains
| Polypeptide designation | Size (amino acids)a | %
Identity to polypeptides ofb:
|
|
|---|---|---|---|
| S. epidermidis | S. aureus | ||
| IcaR | 190 | 77 | 68 |
| IcaA | 412 | 88 | 82 |
| IcaD | 102 | 73 | 65 |
| IcaB | 289 | 72 | 66 |
| IcaC | 358 | 84 | 78 |
We tested whether the absence of ica expression in some strains was due to the integration of an insertion sequence as reported for the S. epidermidis ica operon (56). The 14 S. caprae isolates tested, including 96007, carry a HindIII fragment of 1.8 kb hybridizing with the S. epidermidis ica probe (1). Two pairs of primers, RF-AR and BF-LR (Table 1), were designed to test whether the regions flanking this central fragment in the 96007 ica operon are present in the other S. caprae isolates. Fragments of 1.2 and 1.7 kb were amplified from the genomes of all S. caprae strains tested including 96007 (results not shown). Thus, in all the strains, the ica operon and icaR have the same sizes, suggesting that no mobile elements are inserted in them.
DISCUSSION
We describe the S. caprae autolysin gene atlC and the ica operon. The inactivation of similar genes in other staphylococcal species is associated with the loss of biofilm production (11, 19) or a surface adhesin (18) and/or a decrease in virulence in animal infection models (31, 42, 43). It is therefore likely that these genes are involved in S. caprae virulence.
The S. caprae surface autolysin AtlC has the same overall organization as the three staphylococcal surface autolysins Atl (38), AtlE (18), and Aas (20), which are necessary for cluster dispersion during cell division. The presence in these autolysins of two enzymatic domains has probably resulted from the fusion of two genes. None of these autolysins contains the motifs typical of gram-positive surface proteins including the LPXTG motif (37) recognized by the sortase (32). However, the three imperfect repeats connecting the two enzymatic active centers are required for the exposure of proteins on the bacterial surface (2, 18, 20, 39). The L. monocytogenes InlB and Ami proteins also contain tandem repeats including GW dipeptides (7, 22). These repeats were shown to be responsible for displaying proteins on the cell surface by associating with lipoteichoic acids. Such GW repeats, also found in the staphylococcal autolysins LytA (54), Atl (39), AtlE (18), Aas (20), and AtlC (this study), may serve for cell surface anchoring in staphylococci as they do in L. monocytogenes. Synthetic 10- to 30-mer oligopeptides derived from repeat 1 of Atl were synthesized, and their effects on the autolysis of S. aureus cells were studied (50). All active peptides were located on the C-terminal side of repeat 1 and are suspected to affect the interaction between the autolytic enzymes and lipoteichoic acid.
In addition to the bacteriolytic activity, some staphylococcal autolysins have adhesive functions. The adhesion properties of the S. aureus autolysin Atl (38) have not been investigated. The S. epidermidis autolysin AtlE mediates the primary attachment to polystyrene and binds vitronectin, whereas its binding to fibronectin is very weak and not reproducible (19). The S. saprophyticus autolysin Aas binds fibronectin and sheep erythrocytes, and the binding domain has been mapped to the central region containing the repeats (20). Analysis of the ability of the Atl and AtlE regions containing the repeats to bind fibronectin may help to elucidate the correlation between the amino acid sequence of the repeats and binding. The repeats connecting the two enzymatic centers of the staphylococcal Atl-related autolysins are different from those involved in the fibronectin-binding activity of the described LPXTG surface proteins in staphylococcal and streptococcal strains (37, 40). Moreover, the three motifs GGXX(I/V)DF (33, 49), VETEDT (28), and HFDNXXP (21, 40), which are critical or important for the fibronectin-binding activity of the LPXTG proteins, were not found in the repeats of the staphylococcal Atl-related autolysins. The different fibronectin-binding proteins may recognize different parts of the fibronectin molecule. Moreover, the recombinant fusion proteins containing the repeats of the S. aureus FnBPA and FnBPB proteins (23, 45), which have been proposed as vaccines (8, 49), are not expected to inhibit the fibronectin-binding activity of the autolysins. Analysis of the contribution of S. caprae AtlC and in particular of its fibronectin-binding activity to infections associated with the implantation of foreign bodies will require the construction of isogenic S. caprae mutants in which atlC or part of this gene is deleted or modified. Such variants would also be useful to check whether an autolysin(s) or fibronectin-binding protein(s) other than AtlC is produced by S. caprae.
Some human and goat S. caprae strains produce biofilm, but production in vitro is variable (1, 4), despite the presence of the ica operon and icaR which are not interrupted by detectable mobile elements. In S. epidermidis, such variations have been attributed to the spontaneous insertion of IS256 in icaB (56) and to mutations triggered by insertion of Tn917 into the ica operon and three other loci contributing to the regulation of transcription of the genes involved in biosynthesis of the polysaccharide intercellular adhesin (PIA) (30). This regulation has not been elucitated and may also be involved in biofilm formation in S. caprae.
Biofilm may contribute to the virulence of S. caprae and protect the bacterium against antibiotics by preventing access, as it does in S. epidermidis (10, 41). Biofilm formation is thought to be a two-step process that requires the adhesion of bacteria to a substrate surface followed by cell-cell adhesion, forming the multiple layers of the biofilm. In S. epidermidis, atlE (18) is involved in initial adherence and the formation of multiple layers is due to the PIA (19, 29) also named PS/A (34). PIA is composed of linear ß-1,6-linked glucosaminylglycans. The genes icaA and icaD mediate the synthesis of the oligomers in vitro (16). The N-acetylglucosaminyltransferase activity, due to IcaA and IcaD, together with IcaC, is associated with the in vitro formation of a product that is recognized by an antibody raised against PIA. The ica locus of S. aureus (11, 35) was sequenced and is similar to S. epidermidis ica (19). It is present in all S. aureus strains tested and is required for biofilm formation (11). The biofilm polysaccharide, also named PNSG (poly-N-succinyl-ß1-6-glucosamine), is produced in vivo during human and animal infection by S. aureus and is a target for protective antibodies (35).
ica DNA probes from both S. epidermidis and S. aureus were used in hybridization experiments at low stringency to screen for related genes or operons in strains belonging to 21 staphylococcal taxa (1, 11). Cross-hybridizations with the probes have been detected in seven taxa including S. caprae. The organization of the S. caprae ica operon and flanking regions is identical to that in S. epidermidis and S. aureus, with close relatedness between the ica operon genes and the flanking genes, i.e., icaR and geh. The ica operon is significantly more prevalent among S. epidermidis isolates responsible for catheter-related and joint prosthesis infections than among those isolated from normal skin and mucosa of healthy controls (3, 15, 55). In contrast, ica was detected in all S. caprae isolates tested regardless of their source, whether from infected human specimens or mastitis-free goats' milk (1).
It is not known whether S. caprae strains produce PIA that reacts with antibodies directed against S. epidermidis PIA or S. aureus PNSG (35). If there is a cross-immunosensitivity with S. aureus PNSG, protection against S. aureus infection (35) may also be effective against S. caprae infection. In various other staphylococcal taxa, tests to detect PIA production by using the antibodies directed against S. epidermidis PIA were shown to be inconclusive since strains belonging to the same species (S. aureus or S. epidermidis) behave very differently (11). The immune system may recognize sugar moieties in the biofilm, and these moieties may be differently modified in different strains (deacetylated or succinylated). Therefore, an analysis of staphylococcal biofilm polysaccharides may help in the design of vaccines protecting against foreign body infections caused by S. aureus and coagulase-negative staphylococci.
ACKNOWLEDGMENT
We thank C. Tran for secretarial assistance.
REFERENCES
- 1.Allignet J, Galdbart J-O, Morvan A, Dyke K G H, Vaudaux P, Aubert S, Desplaces N, El Solh N. Tracking adhesion factors in Staphylococcus capraestrains responsible for human bone infections following implantation of orthopaedic material. Microbiology. 1999;145:2033–2042. doi: 10.1099/13500872-145-8-2033. [DOI] [PubMed] [Google Scholar]
- 2.Baba T, Schneewind O. Targeting of muralytic enzymes to the cell division site of Gram-positive bacteria: repeat domains direct autolysin to the equatorial surface ring of Staphylococcus aureus. EMBO J. 1998;17:4639–4646. doi: 10.1093/emboj/17.16.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbier-Frebourg N, Lefebvre S, Baert S, Lemeland J-F. PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidisstrains. J Clin Microbiol. 2000;38:877–880. doi: 10.1128/jcm.38.2.877-880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedidi-Madani N, Greenland T, Richard Y. Exoprotein and slime production by coagulase-negative staphylococci isolated from goats' milk. Vet Microbiol. 1998;59:139–145. doi: 10.1016/s0378-1135(97)00190-9. [DOI] [PubMed] [Google Scholar]
- 5.Bedidi-Madani N, Kodjo A, Villard L, Richard Y. Ribotyping of Staphylococcus capraeisolated from goat milk. Vet Res. 1998;29:149–158. [PubMed] [Google Scholar]
- 6.Blanc V, Picaud J, Legros E, Bes M, Etienne J, Moatti D, Raynaud M F. Infection sur prothèse totale de hanche àStaphylococcus caprae. Pathol Biol. 1999;47:409–413. [PubMed] [Google Scholar]
- 7.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InIB: an invasion protein of Listeria monocytogeneswith a novel type of surface association. Mol Microbiol. 1997;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 8.Brennan F R, Jones T D, Longstaff M, Chapman S, Bellaby T, Smith H, Xu F, Hamilton W D O, Flock J-I. Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureusexpressed on two different plant viruses. Vaccine. 1999;17:1846–1857. doi: 10.1016/s0264-410x(98)00485-x. [DOI] [PubMed] [Google Scholar]
- 9.Chesneau O, Allignet J, El Solh N. Thermonuclease gene as a target nucleotide sequence for specific recognition of Staphylococcus aureus. Mol Cell Probes. 1993;7:301–310. doi: 10.1006/mcpr.1993.1044. [DOI] [PubMed] [Google Scholar]
- 10.Christensen G D, Baldassarri L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. Washington, D.C.: American Society for Microbiology; 1994. pp. 45–78. [Google Scholar]
- 11.Cramton S E, Gerke C, Schnell N F, Nichols W W, Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureusand is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Buyser M L, Morvan A, Aubert S, Dilasser F, El Solh N. Evaluation of a ribosomal RNA gene probe for the identification of species and subspecies within the genus Staphylococcus. J Gen Microbiol. 1992;138:889–899. doi: 10.1099/00221287-138-5-889. [DOI] [PubMed] [Google Scholar]
- 13.Devriese L A, Poutrel B, Killper-Bälz R, Schleifer K H. Staphylococcus gallinarum and Staphylococcus caprae, two new species from animals. Int J Syst Bacteriol. 1983;33:480–486. [Google Scholar]
- 14.Elsner H-A, Dahmen G P, Laufs R, Mack D. Intra-articular empyema due to Staphylococcus capraefollowing arthroscopic cruciate ligament repair. J Infect. 1998;37:66–67. doi: 10.1016/s0163-4453(98)90733-2. [DOI] [PubMed] [Google Scholar]
- 15.Galdbart J-O, Allignet J, Tung H-S, Rydèn C, El Solh N. Screening for Staphylococcus epidermidismarkers discriminating between skin flora strains and those responsible for joint prosthesis infections. J Infect Dis. 2000;182:351–355. doi: 10.1086/315660. [DOI] [PubMed] [Google Scholar]
- 16.Gerke C, Kraft A, Süßmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidispolysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 17.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnbgenes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 18.Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidisto a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 20.Hell W, Meyer H-G W, Gatermann S G. Cloning of aas, a gene encoding a Staphylococcus saprophyticussurface protein with adhesive and autolytic properties. Mol Microbiol. 1998;29:871–881. doi: 10.1046/j.1365-2958.1998.00983.x. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe J, Natanson-Yaron S, Caparon M G, Hanski E. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two domains. Mol Microbiol. 1996;21:373–384. doi: 10.1046/j.1365-2958.1996.6331356.x. [DOI] [PubMed] [Google Scholar]
- 22.Jonquières R, Bierne H, Fiedler F, Gounon P, Cossart P. Interaction between the protein InlB of Listeria monocytogenesand lipoteichoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol Microbiol. 1999;34:902–914. doi: 10.1046/j.1365-2958.1999.01652.x. [DOI] [PubMed] [Google Scholar]
- 23.Jönsson K, Signäs C, Müller P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanda K, Suzuki E, Hiramatsu K, Oguri T, Miura H, Ezaki T, Yokota T. Identification of a methicillin-resistant strain of Staphylococcus capraefrom a human clinical specimen. Antimicrob Agents Chemother. 1991;35:174–176. doi: 10.1128/aac.35.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura Y, Hou X-G, Sultana F, Hirose K, Miyake M, Suh S-E, Ezaki T. Distribution of Staphylococcus species among human clinical specimens and emended description of Staphylococcus caprae. J Clin Microbiol. 1998;36:2038–2042. doi: 10.1128/jcm.36.7.2038-2042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloos W E, Lambe D W., Jr . Staphylococcus. In: Balows A, Hausler W J Jr, Hermann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; 1991. pp. 222–237. [Google Scholar]
- 27.Komatsuzawa H, Sugai M, Nakashima S, Yamada S, Matsumoto A, Oshida T, Suginaka H. Subcellular localization of the major autolysin, Atl, and its processed proteins in Staphylococcus aureus. Microbiol Immunol. 1997;41:469–479. doi: 10.1111/j.1348-0421.1997.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindmark H, Jacobsson K, Frykberg L, Guss B. Fibronectin-binding protein of Streptococcus equi subsp. zooepidemicus. Infect Immun. 1996;64:3993–3999. doi: 10.1128/iai.64.10.3993-3999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidisis a linear ß-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mack D, Rohde H, Dobinsky S, Riedewald J, Nedelmann M, Knobloch J K-M, Elsner H-A, Feucht H H. Identification of three essential regulatory gene loci governing expression of Staphylococcus epidermidispolysaccharide intercellular adhesin and biofilm formation. Infect Immun. 2000;68:3799–3807. doi: 10.1128/iai.68.7.3799-3807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mani N, Baddour L M, Offutt D Q, Vijaranakul U, Nadakavukaren M J, Jayaswal R K. Autolysis-defective mutant of Staphylococcus aureus: pathological considerations, genetic mapping, and electron microscopic studies. Infect Immun. 1994;62:1406–1409. doi: 10.1128/iai.62.4.1406-1409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazmanian S K, Liu G, Ton-That H, Schneewind O. Staphylococcus aureussortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 33.McGavin M J, Gurusiddappa S, Lindgren P-E, Lindberg M, Raucci G, Höök M. Fibronectin receptors from Streptococcus dysgalactiae and Staphylococcus aureus. Involvement of conserved residues in ligand binding. J Biol Chem. 1993;268:23946–23953. [PubMed] [Google Scholar]
- 34.McKenney D, Hübner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidisencodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenney D, Pouliot K L, Wang Y, Murthy V, Ulrich M, Döring G, Lee J C, Goldmann D A, Pier G B. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 36.Moran C P., Jr . RNA polymerase and transcription factors. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other Gram-positive bacteria. Washington, D.C.: American Society for Microbiology; 1993. pp. 653–667. [Google Scholar]
- 37.Navarre W W, Schneewind O. Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oshida T, Sugai M, Komatsuzawa H, Hong Y-M, Suginaka H, Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-ß-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci USA. 1995;92:285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshida T, Takano M, Sugai M, Suginaka H, Matsushita T. Expression analysis of the autolysin gene (atl) of Staphylococcus aureus. Microbiol Immunol. 1998;42:655–659. doi: 10.1111/j.1348-0421.1998.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 40.Rocha C L, Fischetti V A. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect Immun. 1999;67:2720–2728. doi: 10.1128/iai.67.6.2720-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rupp M E, Archer G L. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 42.Rupp M E, Ulphani J S, Fey P D, Bartscht K, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidisin the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect Immun. 1999;67:2627–2632. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rupp M E, Ulphani J S, Fey P D, Mack D. Characterization of Staphylococcus epidermidispolysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect Immun. 1999;67:2656–2659. doi: 10.1128/iai.67.5.2656-2659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuttleworth R, Behme R J, McNabb A, Colby W D. Human isolates of Staphylococcus caprae: association with bone and joint infections. J Clin Microbiol. 1997;35:2537–2541. doi: 10.1128/jcm.35.10.2537-2541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Signas C, Raucci G, Jonsson K, Lindgren P E, Anantharamaiah G M, Hook M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spellerberg B, Steidel K, Lütticken R, Haase G. Isolation of Staphylococcus capraefrom blood cultures of a neonate with congenital heart disease. Eur J Clin Microbiol Infect Dis. 1998;17:61–62. doi: 10.1007/BF01584369. [DOI] [PubMed] [Google Scholar]
- 47.Sugai M, Akiyama T, Komatsuzawa H, Miyake Y, Suginaka H. Characterization of sodium dodecyl sulfate-stable Staphylococcus aureusbacteriolytic enzymes by polyacrylamide gel electrophoresis. J Bacteriol. 1990;172:6494–6498. doi: 10.1128/jb.172.11.6494-6498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugai M, Komatsuzawa H, Akiyama T, Hong Y-M, Oshida T, Miyake Y, Yamaguchi T, Suginaka H. Identification of endo-ß-N-acetylglucosaminidase and N-acetylmuramyl-l-alanine amidase as cluster-dispersing enzymes in Staphylococcus aureus. J Bacteriol. 1995;177:1491–1496. doi: 10.1128/jb.177.6.1491-1496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Q, Smith G M, Zahradka C, McGavin M J. Identification of D motif epitopes in Staphylococcus aureusfibronectin-binding protein for the production of antibody inhibitors of fibronectin binding. Infect Immun. 1997;65:537–543. doi: 10.1128/iai.65.2.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takano M, Oshida T, Yasojima A, Yamada M, Okagaki C, Sugai M, Suginaka H, Matsushita T. Modification of autolysis by synthetic peptides derived from the presumptive binding domain of Staphylococcus aureusautolysin. Microbiol Immunol. 2000;44:463–472. doi: 10.1111/j.1348-0421.2000.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 51.Tinoco I, Jr, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acids. Nature New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 52.Vandenesch F, Eykyn S J, Bes M, Meugnier H, Fleurette J, Etienne J. Identification and ribotypes of Staphylococcus capraeisolates isolated as human pathogens and from goat milk. J Clin Microbiol. 1995;33:888–892. doi: 10.1128/jcm.33.4.888-892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Wilkinson B J, Jayaswal R K. Sequence analysis of a Staphylococcus aureusgene encoding a peptidoglyccan hydrolase activity. Gene. 1991;102:105–109. doi: 10.1016/0378-1119(91)90547-o. [DOI] [PubMed] [Google Scholar]
- 55.Ziebuhr W, Heilmann C, Götz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidisblood culture strains and mucosal isolates. Infect Immun. 1997;65:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziebuhr W, Krimmer V, Rachid S, Lößner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 1999;32:345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]