Abstract
The aim of the present study is to retrospectively analyze the surgical outcomes and complications of microscopic and endoscopic transsphenoidal surgery in the management of Rathke cleft cysts (RCCs) at a single institution. A total of 38 patients were enrolled in this study. All patients were diagnosed with RCCs, which were confirmed histopathologically. Endocrine function, radiological, and clinical outcomes were evaluated following surgery. This cohort study consisted of 16 male and 22 female patients. The median age was 48 years (range, 21–72). The median clinical follow-up duration was 57 months (range, 3–187). Twenty-five patients underwent microscopic transsphenoidal surgery and 13 patients underwent endoscopic surgery. The cysts were located within the intrasellar area in 18 (47.4%) patients, and extended to the suprasellar area in 20 (52.6%) patients. The radiological characteristics were similar in the microscope and endoscope groups, except mass volume (1.40 vs 0.65 mm3; P = .003) and peripheral rim enhancement (P = .036). After surgery, 30 (78.9%) of the 38 patients had no residual cysts. There was no significant difference in outcomes between both groups (76.0% vs 84.6%; P = .689). Four (10.5%) patients experienced cyst recurrence in only the microscope group. Twenty-four of the 25 patients who presented with headache showed improvement after surgery. Four patients with visual field defects recovered after surgery. Among the 8 patients with hormonal deficiencies, hormone levels normalized in 5 patients, did not change in 2 patients and worsened in 1 patient. New hormonal deficiencies occurred in 3 patients. Microscopic or endoscopic transsphenoidal surgery for RCCs is a safe and effective treatment option. Complete aspiration of the cyst contents with wide fenestration and wall biopsy, regardless of the surgical approach used, is usually a sufficient treatment for RCCs.
Keywords: outcome, pituitary, Rathke cleft cyst, recurrence, transsphenoidal approach
1. Introduction
Rathke cleft cysts (RCCs) are benign cysts that originate from the remnants of the embryological Rathke pouch.[1,2] According to autopsy studies, these cysts are found incidentally at a rate of 4% to 33%.[3–6] RCCs can cause neurological symptoms that are related to the mass effect on surrounding neurological structures such as the pituitary gland, optic chiasm, and/or hypothalamus.[2,7] Common symptoms include headache, visual disturbance, or endocrine dysfunction.[2,8,9] For symptomatic RCCs, a standard treatment is surgical decompression, usually through a transsphenoidal approach (TSA).[10–12] However, the treatment of small, asymptomatic RCCs is somewhat controversial.[13]
On the other hand, the traditional TSA has been used for the surgical resection of pituitary lesion, including RCCs.[11,14,15] More recently, the endoscopic transsphenoidal surgery has been advocated for the treatment of these lesions. To date, some authors reported surgical outcomes of endoscopic transsphenoidal surgery for RCCs.[16,17] In this situation, we aim to analyze the surgical outcomes of microscopic and endoscopic transsphenoidal surgery in the treatment of RCCs at a single institution.
2. Materials and methods
Our institutional review board approved this retrospective analysis. Between 2001 and 2021, a total of 48 patients underwent surgical treatment for RCCs. In our institution, we usually recommend regular follow-up for patients with asymptomatic, small RCCs. Surgical indications were as follows: symptomatic RCCs with or without an increase in cyst size. The diagnosis of RCC was determined based on magnetic resonance (MR) images and was confirmed by a histopathologic diagnosis. The initial examination included physical and neurological examinations, an ophthalmological evaluation, and an endocrine function evaluation. The radiological characteristics, such as volume and intensity of cystic fluid, were also evaluated based on preoperative MR images. The locations of the RCCs were classified, as described by Potts et al,[2] into 3 groups: intrasellar, intra- and suprasellar, and suprasellar. After the exclusion of 10 patients with less than 3 months of follow-up or insufficient medical records, a total of 38 patients were enrolled in this study.
2.1. Surgical treatment
At our institution, most patients with pituitary lesions underwent surgical resection through a microscopic TSA until 2010. Since endoscopic TSA equipment was introduced in early 2010, endoscopic TSA was also performed in patients with pituitary lesions. In this study, 25 of the 38 patients with RCCs underwent surgery through a microscopic TSA, and 13 of the 38 patients with RCCs underwent surgical treatment via an endoscopic TSA. The 38 patients with intrasellar or intrasellar and suprasellar RCCs underwent microscopic or endoscopic TSA for fenestration and aspiration of fluid in RCCs with excision of the RCC wall for biopsy. After removal of the RCC, we usually reconstructed the sellar defect using a multilayered reconstruction technique with fat tissue, a fibrin sealant patch and/or, autologous bone.[18,19] If any cerebrospinal fluid (CSF) leakage was suspected during surgery, we usually perform lumbar drainage. Lumbar drainage catheters were left in place for 2 to 3 days.
2.2. Follow-up
The initial volume of the cyst was defined as the volume of the cyst on preoperative MR images. On each MR image slice, the cyst area was measured by delineating a cyst margin. Then, the cyst volume was calculated as the summation of the cyst areas multiplied by the MR image slice thickness. The radiological outcome was classified as either “no cyst” (disappearance of the RCC on postoperative MR images) or “residual cyst” (any residual cyst of the RCC on postoperative MR images). Recurrence was defined as regrowth of the RCC after surgery or an increase in size in the residual cyst.
Medical record reviews and interviews with the patients were used to evaluate the outcome. The clinical outcome was defined as “new deficit” (newly developed symptoms and signs), “improvement” (disappearance or improvement in the initial symptoms and signs) or “no change” (other outcomes). During the follow-up period, an endocrine assessment was performed, following additional/adjuvant hormonal replacement by an endocrinologist. The measured pituitary function parameters included adrenocorticotropic hormone (ACTH), cortisol, thyroid-stimulating hormone (TSH), triiodothyronine, thyroxine, luteinizing hormone, follicle-stimulating hormone, growth hormone (GH), insulin-like growth factor 1, prolactin and testosterone or estrogen (for male or female patients, respectively). The hormonal outcome was classified as “normalized” or “deficiency” based on the final hormonal test. The outcome evaluation was conducted within 3 months after the initial diagnosis, then at 3 to 6-month intervals for 1 year, and finally at 1-year intervals.
2.3. Statistical analysis
The results were analyzed using IBM SPSS Statistics software (version 20.0; SPSS, Armonk, NY). The Mann-Whitney U-test, Pearson chi-squared test and Fisher exact test were used for comparisons between the microscopic and endoscopic TSA groups. A P-value of 0.05 or less was considered statistically significant.
3. Results
This cohort study consisted of 16 male patients and 22 female patients. The median patient age was 48 years (range, 21–72 years). The median clinical follow-up duration was 57 months (range, 3–187 months). The median radiological follow-up duration was 47 months (range, 3–185 months). The most common presenting symptom was headache, presenting in 25 (65.8%) of the 38 patients. Visual field defects were identified in 4 (10.5%) of the 38 patients. The other symptoms were dizziness (in 2 patients), amenorrhea (in 1 patient), asthenia (in 1 patient), Galactorrhea (in 1 patient) and hyponatremia (in 1 patient). Incidental findings were identified in 3 patients. The partial preoperative hormonal deficiencies were identified in 8 patients and hyperprolactinemia in 7 patients. The comparison of the clinical characteristics of the patients with RCCs is summarized in Table 1. There were no significant differences in clinical characteristics between the 2 groups.
Table 1.
Clinical characteristics of the 38 patients with Rathke cleft cysts.
| Factor | Microscopic TSA (n = 25) | Endoscopic TSA (n = 13) | Total | P value |
|---|---|---|---|---|
| Gender (male/female), No. | 10/15 | 6/7 | 16/22 | .715 |
| Age, median, years | 47 (28–64) | 49 (25–69) | 48 (21–72) | 1.000 |
| Clinical follow-up duration, months | 59 (3–187) | 42 (3–118) | 57 (3–187) | .286 |
| Radiological follow-up duration, months | 52 (3–185) | 42 (3–118) | 47 (3–185) | .761 |
| Clinical presentation, No. | ||||
| Headache | 17 | 8 | 25 (65.8) | .690 |
| Visual field defect | 3 | 1 | 4 (10.5) | 1.000 |
| Unilateral hemianopsia | 0 | 1 | 1 | |
| Bilateral hemianopsia | 3 | 0 | 3 | |
| Dizziness | 0 | 2 | 2 | |
| Amenorrhea | 1 | 0 | 1 | |
| Asthenia | 1 | 0 | 1 | |
| Galactorrhea | 1 | 0 | 1 | |
| Hyponatremia | 0 | 1 | 1 | |
| Incidental findings | 2 | 1 | 3 | |
| Partial preoperative hypopituitarism, No. | 7 | 1 | 8 (21.1) | .222 |
| Hyperprolactinemia, No. | 6 | 1 | 7 (18.4) | .385 |
Data are shown as the median (range) or number of participants (%).
TSA = transsphenoidal approach.
Based on the preoperative MR images, the median volume of RCCs was 1.28 cm3 (range, 0.25–5.72 cm3. According to the location of the RCCs, 38 patients were classified as follows: intrasellar type in 18 (47.4%) patients and intra- and suprasellar type in 20 (52.6%) patients. Moreover, 27 (71.1%) of the 38 RCCs were located on the dorsal side of the pituitary stalk. On MR images, peripheral rim enhancements were identified in 16 (42.1%) patients. The signal intensity of RCCs on MR images is described in detail in Table 2. Additionally, statistical analysis was performed to compare radiological characteristics between microscopic and endoscopic TSAs. There was no statistically significant difference in any parameter between the 2 groups except for cyst volume (P-value, 0.003) and peripheral rim enhancement (P-value, 0.036). The radiological characteristics are summarized in Table 2.
Table 2.
Radiological characteristics of the 38 patients with Rathke cleft cysts.
| Factor | Microscopic TSA (n = 25) | Endoscopic TSA (n = 13) | Total | P value |
|---|---|---|---|---|
| Volume, median (range), mm3 | 1.40 (0.32–5.72) | 0.65 (0.25–2.32) | 1.28 (0.25–5.72) | .003 |
| Location | .087 | |||
| Intrasellar, No. | 9 | 9 | 18 (47.4%) | |
| Intra- and suprasellar, No. | 16 | 4 | 20 (52.6%) | |
| Dorsal side location of the pituitary stalk, No. | 16 | 11 | 27 (71.1) | .268 |
| MR signal intensity (SI), No. | ND | |||
| Low T1WI/Low T2WI SI | 0 | 0 | 0 | |
| /Iso or mix T2WI SI | 0 | 0 | 0 | |
| /High T2WI SI | 2 | 1 | 3 | |
| Iso or mix T1WI/Low T2WI SI | 1 | 1 | 2 | |
| /Iso or mix T2WI SI | 4 | 3 | 7 (18.4) | |
| /High T2WI SI | 5 | 0 | 5 | |
| High T1WI/Low T2WI SI | 2 | 1 | 3 | |
| /Iso or mix T2WI SI | 4 | 4 | 8 (21.1) | |
| /High T2WI SI | 7 | 3 | 10 (26.3) | |
| Peripheral rim enhancement, No. | 14 | 2 | 16 (42.1%) | .036 |
Data are shown as the median (range) or number of participants (%).
Iso or mix = isointense or mixed intensity, MR = magnetic resonance, ND = not done, T1WI = T1 weighted imaging, T2WI = T2 weighted imaging, TSA = transsphenoidal approach.
3.1. Surgical outcome
All RCCs were removed through a microscopic or an endoscopic TSA. Among the 38 patients, 25 underwent surgery via a microscopic TSA and 13 underwent an endoscopic TSA. Surgical outcomes are summarized according to the surgical approach in Table 3. Additionally, statistical analysis for comparisons surgical outcomes between the microscopic and endoscopic TSA groups is described in Table 3. There was no statistically significant difference between the 2 groups. On postoperative MR images, 30 (78.9%) of the 38 patients had no residual cysts, whereas 8 (21.1%) patients had residual cysts. The cyst fluid status of RCCs is described in detail in Table 3. Complications after surgery for RCCs are detailed in Table 3. Transient diabetes insipidus (DI) occurred in 7 patients. No patient had permanent DI. Other complications included syndrome of inappropriate antidiuretic hormone secretion in 1 patient, postoperative meningitis in 2 patient, and epistaxis in 1 patient. Postoperative CSF leakage occurred in only 1 patient.
Table 3.
Surgical outcomes of the 38 patients with surgically-treated Rathke cleft cysts.
| Factor | Microscopic TSA | Endoscopic TSA | Total | P value |
|---|---|---|---|---|
| Number | 25 | 13 | 38 | |
| Outcome on postoperative MR | .689 | |||
| No cyst | 19 | 11 | 30 (78.9) | |
| Residual cyst (no shrinkage) | 6 | 2 | 8 (21.1) | |
| Fluid status | ND | |||
| No records | 5 | 0 | 5 | |
| Serous or xanthochromic | 1 | 3 | 4 | |
| Mucoid | 11 | 5 | 16 (42.1) | |
| Gelatinous | 5 | 4 | 9 (23.7) | |
| Motor oil-like | 1 | 1 | 2 | |
| Hemorrhagic | 2 | 0 | 2 | |
| Sellar floor reconstruction | .115 | |||
| Fat with or without bone graft | 23 | 10 | 33 (86.8) | |
| Fibrin sealant patch with or without flap | 1 | 3 | 4 | |
| Lumbar drainage* | 11 | 7 | 18 (47.4) | .564 |
| Complications | .391 | |||
| Diabetes insipidus, transient | 5 | 2 | 7 | |
| SIADH | 0 | 1 | 1 | |
| Postoperative meningitis | 1 | 1 | 2 | |
| Cerebrospinal fluid leakage | 1 | 0 | 1 | |
| Epistaxis | 0 | 1 | 1 | |
| Recurrence | 4 | 0 | 4(10.5) | .278 |
Data are shown as the number of participants (%).
MR = magnetic resonance, ND, not done, TSA = transsphenoidal approach, SIADH = syndrome of inappropriate antidiuretic hormone secretion.
Lumbar drainage was performed in patient with intraoperative cerebrospinal fluid leakage.
During the follow-up period, 4 (10.5%) of the 38 patients experienced cyst recurrence. Two of these 3 patients exhibited delayed recurrence (4 years and 13 years after surgery), with no residual cysts on postoperative MR images. The other 2 patient with an intra- and suprasellar type RCC experienced regrowth of a residual cyst 4 years and 5 years after surgery. None of the 4 patients had specific neurological deficits, and conservative treatment was recommended.
3.2. Clinical and endocrine function outcomes
Headache was observed in 25 (70.6%) of the 38 patients at the initial presentation. Twenty-four of the 25 patients experienced improvements, and only 1 patient experienced a partial improvement. Two patients with dizziness showed unchanged symptom. All 4 patients with visual field defects (3 with bitemporal hemianopsia and 1 with unilateral hemianopsia) also experienced improvements. There were no newly developed deficits following surgery.
At the initial assessment, 8 (21.1%) of the 38 patients had 1 or 2 hormonal deficiencies. The most common hormonal deficiency was GH deficiency (5 cases), followed by ACTH deficiency (4 cases), gonadal hormone deficiency (2 cases), and TSH deficiency (1 case). At the final assessment, 5 of the 8 patients recovered to a normal endocrinological status, and 2 patients had 1 or 2 hormonal deficiencies. The other patient (of the 8 patients) had panhypopituitarism. These patients required continuous hormonal replacement. Among the 30 patients with a normal hormonal status at the initial presentation, 3 developed a new hormonal deficiency, including TSH and ACTH deficiency. The clinical and endocrine outcomes of the patients with surgically treated RCCs are summarized in Table 4. There were no significant differences in clinical characteristics between the 2 groups.
Table 4.
Clinical and endocrine function outcomes of 38 patients with surgically treated Rathke cleft cysts.
| Factor | Microscopic TSA | Endoscopic TSA | Total | P value† |
|---|---|---|---|---|
| Headache, No. | 17 | 8 | 25 | 1.000 |
| Improved | 16 | 8 | 24 | |
| Unchanged | 1 | 0 | 1 | |
| Dizziness, No. | 0 | 2 | 2 | ND |
| Improved | 0 | 0 | 0 | |
| Unchanged | 0 | 2 | 2 | |
| Visual field defect, No. | 3 | 1 | 4 | ND |
| Improved | 3 | 1 | 4 | |
| Unchanged | 0 | 0 | 0 | |
| New deficit | 0 | 0 | 0 | |
| No preoperative hypopituitarism, No. | 18 | 12 | 30 | 1.000 |
| Unchanged | 16 | 11 | 27 | |
| New deficiency | 2 | 1 | 3 | |
| Partial preoperative hormonal dysfunction, No. | 7 | 1 | 8 | 1.000 |
| Improved | 4 | 1 | 5 | |
| Unchanged | 2 | 0 | 2 | |
| Worsening | 1 | 0 | 1 | |
| Hyperprolactinemia, No. | 6 | 1 | 7 | ND |
| Normalized | 6 | 1 | 7 | |
| Unchanged | 0 | 0 | 0 |
ND = not done, TSA = transsphsenoidal approach.
* The patient had intermittent headache similar to a previous surgery.
One patient had panhypopituitarism following surgery.
‡ Four of 23 patients with normal preoperative hormonal function had 1 or 2 newly developed hormonal deficiencies following surgery.
3.3. Illustrative cases after surgery for RCCs
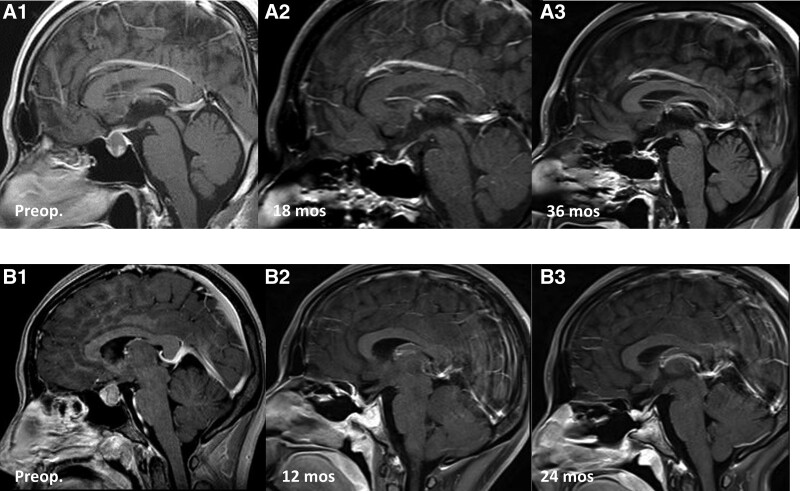
A 37-years-old male patient presented with headache. Preoperative MR images showed a cystic mass in the sellar region (Fig. 1. A1). The patient underwent a microscopic TSA to treat an RCC. No postoperative complications occurred. After surgery, his symptoms improved, and no residual cyst was observed on postoperative MR images (Fig. 1. A2 and A3). There was no recurrence at 36 months after surgery.
Figure 1.
Illustrative cases of surgically treated Rathke cleft cysts (RCCs). A1–3: A 37-year-old male patient presented with headache. Sagittal postcontrast T1-weighted MR image showed a cystic mass in the sellar region (A1). After surgery, his symptoms improved. Serial postoperative follow-up MR images at 18 months, and 36 months after surgery demonstrated no residual or recurrent cysts (A2–3). B1–3: A 21-years-old female patient presented with headache. Sagittal T1-weighted MR image demonstrated a cystic mass in the sellar region (B1). After surgery, postoperative MR image at 12 months after surgery showed no residual cyst (B2). Follow-up MR image at 24 months showed no recurrence (B3). MR = magnetic resonance, RCCs = Rathke cleft cysts.
Another patient underwent an endoscopic TSA. A 21-years-old female patient presented with headache. Preoperative MR images demonstrated a cystic mass in the sellar region (Fig 1. B1). After surgery, her symptoms improved, and no residual cyst identified on follow-up MR images 12 months and 24 months after surgery (Fig. 1. B2 and B3).
4. Discussion
RCCs are relatively common and benign intracranial lesions originating from remnants of Rathke pouch.[1,20] Many RCCs have been incidentally discovered while evaluating the cause of nonspecific or unrelated symptoms, such as headache or dizziness.[20] Asymptomatic and small RCCs generally do not require surgical treatment. However, the natural history of these asymptomatic RCCs is not clear.[20] To date, several studies have reported the natural history of RCCs. During the follow-up period, the growth rate of RCCs ranged from 3% to 28%; however, most patients remained asymptomatic.[11,13,21] The spontaneous regression of cysts has also been reported, with rates ranging from 10% to 15%.[13,22] However, symptomatic and large RCCs are generally indicated for surgical treatment.[13,20] Common presenting symptoms included headache, visual disturbance, and/or pituitary hormone dysfunction.[20] The TSA using a microscope and, more recently, an endoscope has been used for surgical treatment of RCCs.[8,10–12,17,23,24] Although very rare, craniotomy might be required for a giant or purely suprasellar RCC.[25]
4.1. Surgical outcomes of the RCCs
In this study, a total of 38 patients underwent surgical treatment for RCCs. The majority of the 38 patients RCCs were symptomatic (3 patients had incidental findings). On postoperative MR images, 30 (78.9%) of the 38 patients had no residual cysts. Additionally, 8 patients had small but residual cysts; among them, 6 patients (of 25) underwent microscopic TSA, and 2 patients (of 13) underwent endoscopic TSA. No difference was observed between microscopic and endoscopic TSA. Regarding the surgical technique, including decompression with cyst wall biopsy and aggressive resection of the cyst wall, various surgical outcomes have been reported. Aho et al reported one of the largest series of RCCs, consisting of 118 patients. They reported that complete cyst resection via a microscopic TSA was achieved in 97% of patients based on MR images obtained 3 months postoperatively.[11] Benvenistle et al reported that 90.3% of patients had complete cyst decompression via a mostly microscopic TSA.[10] More recently, an endoscopic TSA has widely been used to access sellar lesions, including RCCs. Madhok et al reported that complete removal of the cyst contents was achieved in 100% of patients.[12] They avoided removal of the cyst capsule to prevent injury to the surrounding microvasculature supplying the adjacent pituitary gland and stalk.[12] Solari et al reported outcomes of an endoscopic endonasal approach for RCCs and showed that gross total removal (with complete cyst wall removal) achieved a success rate of 55.1%.[16] However, there is no consensus on the most effective surgical method to relieve symptoms and prevent recurrence with minimal postoperative complications.
Postoperative complications, including CSF leakage, transient or permanent DI, hormonal deficiency, hyponatremia, infection and others, have been reported.[20] Postoperative complications noted in our series are provided in table III. Previous studies have shown that aggressive cyst resection (involving the cyst wall) is associated with new endocrine dysfunction following surgery.[10,11,23] DI has been reported to be the most common complication following RCC surgery.[11,20,26] According to the surgical strategy and technique, the rates of postoperative DI range from 0% to 23%.[10–12,16,17,23,26] In our series, 7 (18.4%) of the 38 patients experienced transient postoperative DI. No patient had permanent DI. On the other hand, postoperative CSF leakage occurred in only 1 (2.6%) patients in our series. The reported rate of postoperative CSF leakage is relatively low, ranging from 0 to 6.9% in both microscopic and endoscopic TSAs.[10–13,16]
4.2. Clinical and hormonal outcomes of surgically treated RCCs
Headache is the most common presenting symptom of RCCs.[20] Headaches associated with RCCs mostly tend to be frontal, episodic, nonpulsating, and bilateral or with deep retroorbital pain; however, they may also present in the occipital or temporal region or with whole head pain.[20,27] According to previous studies, the rate of headache improvement in patients with symptomatic RCCs is 71% to 100%.[13,20,27] In our study, most patients showed headache improvement (1 patient showed a partial improvement) following surgery.
Previous studies have reported that the improvement rate of visual dysfunction following surgery for RCCs ranges from 54% to 98%.[2,11,13,16,26,28] In our study, all 4 patients with visual field defects showed an improvement following surgery. For these patients, the volumes of each RCC were 4.26 cm3, 2.80 cm3, 1.84 cm3 and 1.54 cm3, while the median volume of RCCs in the 38 patients was 1.28 cm3.
Hyperprolactinemia resolved in most patients with surgically treated RCCs, while hormonal deficiencies, including GH deficiency, gonadotroph deficiency, TSH deficiency and ACTH deficiency, showed a recovery rate of 31% to 60% in patients with RCCs.[11,13,20,29] Our results are similar to those from previous reports. In this study, 7 (18.4%) of the 34 patients had hyperprolactinemia, which may be associated with the stalk effect, and all of these patients showed normalization of prolactin levels following surgery. Moreover, 8 (21.1%) of the 38 patients had 1 or 2 hormonal deficiencies at the initial assessment. At the final assessment, 5 (62.5%) of the 8 patients recovered to a normal endocrinological status.
4.3. Recurrence following RCC surgery
The reported recurrence rate after surgery ranges from 0% to 42%.[10,11,14,23,30] In one of the largest series to date, Aho et al reported a recurrence rate of 18%.[11] Solari et al used the endoscopic approach and reported a recurrence rate of 13.8%.[16] Several prognostic factors, such as a suprasellar location, inflammation, squamous metaplasia of the cyst capsule, or fat graft, are associated with the recurrence of RCCs.[2,10,11] Mendelson et al recently conducted a meta-analysis comparing the recurrence of RCCs after a TSA.[31] They reported insufficient evidence between more aggressive resection of the cyst capsule and low recurrence rates.[31] Moreover, the recurrence rate according to the approach used (microscopic and endoscopic) differed (14% vs 8%, respectively), although there were large differences in sample sizes between patients who received a microscopic TSA and those who received an endoscopic TSA.[31] More recently, Kino et al reported a novel technique for maintaining cyst drainage to prevent recurrence via the mucosa coupling method and showed no recurrence in patients treated with this method.[32] In this study, 4 (10.5%) of the 38 patients experienced cyst recurrence; 4 patients received a microscopic TSA, and 0 patients received an endoscopic TSA. This difference might be associated with the small sample size of patients who received an endoscopic TSA. Moreover, in the current study, the recurrence of RCCs occurred relatively late, at 4 years, 5 years and 13 years after surgery in 4 patients. To identify characteristics of recurrence, such as the true rate, related symptoms, and/or recurrence time, further large prospective studies should be performed.
4.4. Limitations
The limitations of the present study include those inherent to retrospective analyses. Moreover, compared with previously reported studies, this study included a relatively small number of patients. Additionally, a relatively short follow-up duration is a limitation of this study due to the possibility of delayed cyst recurrence. However, despite these limitations, our study, together with previously reported series, contributes to knowledge of the safety and effectiveness of surgical treatment for RCCs.
5. Conclusions
Surgical treatment is a reasonable treatment modality for patients with symptomatic RCCs regardless of the surgical approach used (whether microscopic or endoscopic). Complete aspiration of the cyst contents with wide fenestration and wall biopsy is usually sufficient for RCC treatment. However, the recurrence rate remains unclear, and a large study over a long-term follow-up period is needed.
Author contributions
Conceptualization: Youngbeom Seo.
Data curation: Yoon-Hee Choo.
Formal analysis: Yoon-Hee Choo, Youngbeom Seo.
Funding acquisition: Youngbeom Seo.
Investigation: Yoon-Hee Choo, Youngbeom Seo.
Methodology: Youngbeom Seo.
Resources: Youngbeom Seo, Oh Lyong Kim.
Supervision: Youngbeom Seo.
Writing – original draft: Yoon-Hee Choo.
Writing – review & editing: Youngbeom Seo, Oh Lyong Kim.
Abbreviation:
- ACTH =
- adrenocorticotropic hormone
- CSF =
- cerebrospinal fluid leakage
- DI =
- diabetes insipidus
- GH =
- growth hormone
- MR =
- magnetic resonance
- RCCs =
- Rathke cleft cysts
- TSA =
- transsphsenoidal approach
- TSH =
- thyroid stimulating hormone
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Grant no. 2021M3E5D1A02015269 to Y Seo).
The authors have no conflicts of interest to disclose.
How to cite this article: Choo Y-H, Seo Y, Kim OL. The surgical outcomes following transsphenoidal surgery for Rathke cleft cysts: Comparison of the surgical approaches at a single institution. Medicine 2022;101:51(e32421).
Contributor Information
Yoon-Hee Choo, Email: choo.ns.southkorea@gmail.com.
Oh Lyong Kim, Email: oryongkim@hira.or.kr.
References
- [1].Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst. 2005;21:622–7. [DOI] [PubMed] [Google Scholar]
- [2].Potts MB, Jahangiri A, Lamborn KR, et al. Suprasellar Rathke cleft cysts: clinical presentation and treatment outcomes. Neurosurgery. 2011;69:1058–68. discussion 1068-1057. [DOI] [PubMed] [Google Scholar]
- [3].Shanklin WM. On the presence of cysts in the human pituitary. Anat Rec. 1949;104:379–407. [DOI] [PubMed] [Google Scholar]
- [4].Fager CA, Carter H. Intrasellar epithelial cysts. J Neurosurg. 1966;24:77–81. [DOI] [PubMed] [Google Scholar]
- [5].McGrath P. Cysts of sellar and pharyngeal hypophyses. Pathology. 1971;3:123–31. [DOI] [PubMed] [Google Scholar]
- [6].Teramoto A, Hirakawa K, Sanno N, et al. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology. 1994;193:161–4. [DOI] [PubMed] [Google Scholar]
- [7].Jahangiri A, Molinaro AM, Tarapore PE, et al. Rathke cleft cysts in pediatric patients: presentation, surgical management, and postoperative outcomes. Neurosurg Focus. 2011;31:E3. [DOI] [PubMed] [Google Scholar]
- [8].Kim JE, Kim JH, Kim OL, et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 2004;100:33–40. [DOI] [PubMed] [Google Scholar]
- [9].Higgins DM, Van Gompel JJ, Nippoldt TB, et al. Symptomatic Rathke cleft cysts: extent of resection and surgical complications. Neurosurg Focus. 2011;31:E2. [DOI] [PubMed] [Google Scholar]
- [10].Benveniste RJ, King WA, Walsh J, et al. Surgery for Rathke cleft cysts: technical considerations and outcomes. J Neurosurg. 2004;101:577–84. [DOI] [PubMed] [Google Scholar]
- [11].Aho CJ, Liu C, Zelman V, et al. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005;102:189–93. [DOI] [PubMed] [Google Scholar]
- [12].Madhok R, Prevedello DM, Gardner P, et al. Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg. 2010;112:1333–9. [DOI] [PubMed] [Google Scholar]
- [13].Barkhoudarian G, Palejwala SK, Ansari S, et al. Rathke’s cleft cysts: a 6-year experience of surgery vs. observation with comparative volumetric analysis. Pituitary. 2019;22:362–71. [DOI] [PubMed] [Google Scholar]
- [14].el-Mahdy W, Powell M. Transsphenoidal management of 28 symptomatic Rathke’s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998;42:7–16. discussion 16-17. [DOI] [PubMed] [Google Scholar]
- [15].Liu JK, Das K, Weiss MH, et al. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083–96. [DOI] [PubMed] [Google Scholar]
- [16].Solari D, Cavallo LM, Somma T, et al. Endoscopic endonasal approach in the management of Rathke’s Cleft cysts. PLoS One. 2015;10:e0139609–e0139609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang Z, Yu M, Jiang Y, et al. Endoscopic endonasal resection of symptomatic Rathke cleft cysts: clinical outcomes and prognosis. Neurosurg Rev. 2019;42:699–704. [DOI] [PubMed] [Google Scholar]
- [18].Collins WF. Hypophysectomy: historical and personal perspective. Clin Neurosurg. 1974;21:68–78. [DOI] [PubMed] [Google Scholar]
- [19].Ziu M, Jimenez DF. The history of autologous fat graft use for prevention of cerebrospinal fluid rhinorrhea after transsphenoidal approaches. World . 2013;80:554–62. [DOI] [PubMed] [Google Scholar]
- [20].Trifanescu R, Ansorge O, Wass JA, et al. Rathke’s cleft cysts. Clin Endocrinol (Oxf). 2012;76:151–60. [DOI] [PubMed] [Google Scholar]
- [21].Sanno N, Oyama K, Tahara S, et al. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol. 2003;149:123–7. [DOI] [PubMed] [Google Scholar]
- [22].Culver SA, Grober Y, Ornan DA, et al. A case for conservative management: characterizing the natural history of radiographically diagnosed Rathke cleft cysts. J Clin Endocrinol Metab. 2015;100:3943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lillehei KO, Widdel L, Astete CA, et al. Transsphenoidal resection of 82 Rathke cleft cysts: limited value of alcohol cauterization in reducing recurrence rates. J Neurosurg. 2011;114:310–7. [DOI] [PubMed] [Google Scholar]
- [24].Zhong W, You C, Jiang S, et al. Symptomatic Rathke cleft cyst. J Clin Neurosci. 2012;19:501–8. [DOI] [PubMed] [Google Scholar]
- [25].Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31:E1. [DOI] [PubMed] [Google Scholar]
- [26].Han SJ, Rolston JD, Jahangiri A, et al. Rathke’s cleft cysts: review of natural history and surgical outcomes. J Neurooncol. 2014;117:197–203. [DOI] [PubMed] [Google Scholar]
- [27].Nishioka H, Haraoka J, Izawa H, et al. Headaches associated with Rathke’s cleft cyst. Headache. 2006;46:1580–6. [DOI] [PubMed] [Google Scholar]
- [28].Chotai S, Liu Y, Pan J, et al. Characteristics of Rathke’s cleft cyst based on cyst location with a primary focus on recurrence after resection. J Neurosurg. 2015;122:1380–9. [DOI] [PubMed] [Google Scholar]
- [29].Trifanescu R, Stavrinides V, Plaha P, et al. Outcome in surgically treated Rathke’s cleft cysts: long-term monitoring needed. Eur J Endocrinol. 2011;165:33–7. [DOI] [PubMed] [Google Scholar]
- [30].Raper DM, Besser M. Clinical features, management and recurrence of symptomatic Rathke’s cleft cyst. J Clin Neurosci. 2009;16:385–9. [DOI] [PubMed] [Google Scholar]
- [31].Mendelson ZS, Husain Q, Elmoursi S, et al. Rathke’s cleft cyst recurrence after transsphenoidal surgery: a meta-analysis of 1151 cases. J Clin Neurosci. 2014;21:378–85. [DOI] [PubMed] [Google Scholar]
- [32].Kino H, Akutsu H, Tanaka S, et al. Endoscopic endonasal cyst fenestration into the sphenoid sinus using the mucosa coupling method for symptomatic Rathke’s cleft cyst: a novel method for maintaining cyst drainage to prevent recurrence. J Neurosurg. 2019;133:1710–20. [DOI] [PubMed] [Google Scholar]