Abstract
Background
Adequate response to the SARS-CoV-2 vaccine represents an important treatment goal in caring for patients with multiple sclerosis (MS) during the ongoing COVID-19 pandemic. Previous data so far have demonstrated lower spike-specific IgG responses following two SARS-CoV-2 vaccinations in MS patients treated with sphingosine-1-phosphate (S1P) receptor modulators and anti-CD20 monoclonal antibodies (mAb) compared to other disease modifying therapies (DMTs). It is unknown whether subsequent vaccinations can augment antibody responses in these patients.
Objectives
The goal of this observational study was to determine the effects of a third SARS-CoV-2 vaccination on antibody and T cell responses in MS patients treated with anti-CD20 mAb or S1P receptor modulators.
Methods
Vaccine responses in patients treated with anti-CD20 antibodies (ocrelizumab and ofatumumab) or S1P receptor modulators (fingolimod and siponimod) were evaluated before and after third SARS-CoV-2 vaccination as part of an ongoing longitudinal study. Total spike protein and spike receptor binding domain (RBD)-specific IgG responses were measured by Luminex bead-based assay. Spike-specific CD4+ and CD8+ T cell responses were measured by activation-induced marker expression.
Results
MS patients and healthy controls were enrolled before and following SARS-CoV-2 vaccination. A total of 31 MS patients (n = 10 ofatumumab, n = 13 ocrelizumab, n = 8 S1P) and 10 healthy controls were evaluated through three SARS-CoV-2 vaccinations. Compared to healthy controls, total spike IgG was significantly lower in anti-CD20 mAb-treated patients and spike RBD IgG was significantly lower in anti-CD20 mAb and S1P-treated patients following a third vaccination. While seropositivity was 100% in healthy controls after a third vaccination, total spike IgG and spike RBD IgG seropositivity were lower in ofatumumab (60% and 60%, respectively), ocrelizumab (85% and 46%, respectively), and S1P-treated patients (100% and 75%, respectively). Longer treatment duration, including prior treatment history, appeared to negatively impact antibody responses. Spike-specific CD4+ and CD8+ T cell responses were well maintained across all groups following a third vaccination. Finally, immune responses were also compared in patients who were vaccinated prior to or following ofatumumab treatment. Antibody responses were significantly higher in those patients who received their primary SARS-CoV-2 vaccination prior to initiating ofatumumab treatment.
Conclusions
This study adds to the evolving understanding of SARS-CoV-2 vaccine responses in people with MS treated with disease-modifying therapies (DMTs) known to suppress humoral immunity. Our findings provide important information for optimizing vaccine immunity in at-risk MS patient populations.
Keywords: COVID-19, Vaccine, SARS-COV-2, Multiple sclerosis, Disease modifying therapy
Introduction
Effective vaccine-elicited immunity against COVID-19 requires engagement of robust humoral and cellular responses against SARS-CoV-2. Antibody reactivity against the receptor binding domain (RBD) of the spike protein is particularly important in preventing breakthrough COVID-19 infection (Cromer et al., 2022), with higher spike RBD antibody levels correlated with higher degrees of protection. We and others have previously demonstrated that two classes of certain MS disease modifying therapies (DMTs), in particular anti-CD20 monoclonal antibodies (mAb) and sphingosine-1-phosphate (S1P) receptor modulators, significantly reduce spike antibody responses following the first two doses of SARS-CoV-2 vaccination (Sormani et al., 2021; Sabatino et al., 2022). The impaired responses to initial vaccination appear to result in increased risk of breakthrough COVID-19 in vaccinated MS patients on ocrelizumab, rituximab, and fingolimod (Sormani et al., 2022; Jakimovski et al., 2022).
There is therefore significant need to understand how to optimize SARS-CoV-2 vaccination responses in MS patients on anti-CD20 mAb and S1P therapies. While several studies assessed antibody responses in MS patients following a third SARS-CoV-2 vaccination (König et al., 2022; Bajwa et al., 2022; Achtnichts et al., 2021; Sidler et al., 2022), more comprehensive data on antibody and T cell outcomes as well as comparisons of different types of anti-CD20 mAb therapies are still needed. Furthermore, an additional question that has emerged for vaccinated patients who plan to initiate anti-CD20 mAb treatment is how well vaccine-induced SARS-CoV-2 immunity is retained.
In this study, we assessed SARS-CoV-2 spike-specific antibody and T cell responses following a third vaccination in MS patients on anti-CD20 mAb (ofatumumab and ocrelizumab) and S1P treatments (fingolimod and siponimod) in comparison to healthy controls. In addition to measuring antibodies against the entire SARS-CoV-2 spike protein, we also assessed antibody reactivity specifically against the spike RBD, which has been previously demonstrated to be highly correlated with virus neutralization (Premkumar et al., 2020). We also explored the effects of treatment duration and prior treatment history on these immune outcomes. Finally, we compared the effects of ofatumumab on the generation of de novo SARS-CoV-2 immunity (i.e. vaccination after start of anti-CD20 treatment) with the retention of pre-existing immunity (i.e. primary vaccination prior to start of anti-CD20).
Material and methods
Sample collection and processing
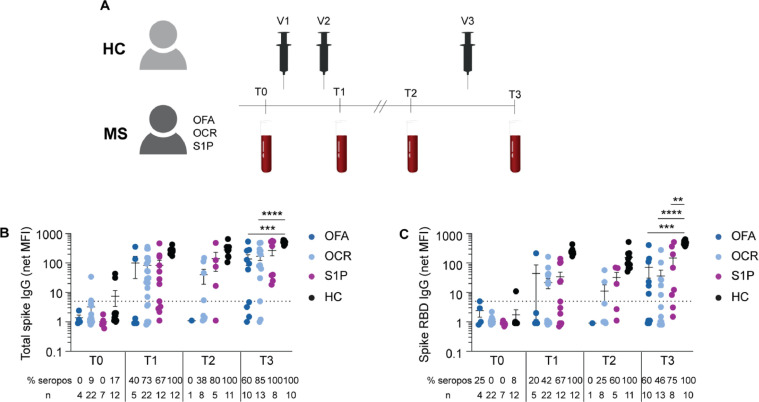
Consented participants included adults with diagnosed with MS based on 2017 McDonald criteria, as well as healthy adult controls (not immunocompromised or on immunosuppressive therapy) aged 18–75 years old. Only MS patients treated with S1P receptor modulators (fingolimod and siponimod) and anti-CD20 mAb (ocrelizumab and ofatumumab) were studied in this longitudinal cohort. Ofatumumab and ocrelizumab-treated patients were analyzed separately given the differences in their standard dosing routes and frequencies (subcutaneous monthly and intravenous every six months, respectively). All participants received only FDA-approved or authorized SARS-CoV-2 vaccines (Comiranty/BNT162b2 from Pfizer/BioNTech, Spikevax/mRNA-1273 from Moderna, or Ad26.COV2 from Johnson and Johnson) according to CDC guidelines. Participants with a known history of COVID-19 were excluded from the study. Samples were collected from the following time points as indicated in Fig. 1 A: prior to SARS-CoV-2 vaccination (T0), two weeks following two mRNA vaccinations or one adenoviral vaccination (T1), prior to third vaccination (T2), and following third vaccination (T3). Samples from patients who had previously received anti-SARS-CoV-2 monoclonal antibody treatment or prophylaxis were excluded. Blood samples were collected from the Neurosciences Clinical Research Unit at UCSF or the patient's residence through ExamOne (a Quest Diagnostics company). 90 mL of blood was collected in heparinized tubes and an additional 10 mL of blood was collected in serum separator tubes at each time point. All samples were processed within 24 h of collection as previously described (Sabatino et al., 2022). Plasma and serum were stored -80 °C until ready for use and PBMCs were stored in liquid nitrogen until the day of experimentation.
Fig. 1.
Antibody responses following serial SARS-CoV-2 vaccination.
Study overview including a cohort of healthy controls and MS patients on the indicated therapies who received three SARS-CoV-2 vaccinations (V1, V2, V3) with blood sample collections at the indicated time points (A): prior to SARS-CoV-2 vaccination (T0), two weeks following two mRNA vaccinations or one adenoviral vaccination (T1), prior to third vaccination (T2), and following third vaccination (T3). Mean net MFI ± SEM of total spike IgG (B) and spike RBD IgG (C) at the indicated time points for each cohort. The dotted line represents the cut-off for seropositivity (net MFI = 5). The percent seropositivity (% seropos) and number of subjects (n) is shown below each plot for all cohorts at each time point. Comparisons between all cohorts at each time point by ixed effects analysis with multiple comparisons (** p < 0.01, *** p < 0.001, **** p < 0.0001).
Semi-quantitative spike antibody analysis by Luminex assay
Spectrally distinct Luminex beads were conjugated with trimeric spike protein (residues 1-1213), spike RBD (residues 328-533) or bovine-specific albumin fraction V (BSA) as previously described (Zamecnik et al., 2020). Serum samples were mixed with pooled protein-coated beads at a final concentration of 1:500 in PBS + 0.05% Tween 20 (PBST) and 1% non-fat milk as in our prior study (Sabatino et al., 2022). Following secondary antibody staining with anti-human IgG Fc antibody PE (1:2000) and washing, beads were analyzed in a 96 well format on a Luminex LX 200 cytometer. The same lot of beads of was used for all sample analyses and all samples were run in duplicate. A normalized net median fluorescence intensity (MFI) was calculated by dividing the mean net MFI for total spike and spike RBD by BSA for each sample. A net MFI ≥ 5.0 as a threshold for seropositivity as before (Sabatino et al., 2022).
T cell analysis by activation-induced marker (AIM) expression
PBMCs were thawed, washed, and equilibrated in RPMI with 10% FBS for 2 h at 37 °C prior to use. CD19+ B cells and CD4+ and CD8+ T cell frequencies were assessed using the same antibody panel as previously described (Sabatino et al., 2022): CD3 (OKT3 clone), CD4 (A161A1 clone), CD8 (SK1 clone), CD19 (HIB19 clone), and live/dead viability stain. For AIM studies, PBMCs were resuspended in serum-free RPMI and plated at 1×106 cells per well in 96-well round-bottom plates. PBMCs were stimulated for 24 h in parallel with two spike peptide pools (S1 and S2 pools, JPT Peptide Technologies) at a final concentration of 1 µg/ml per peptide. 0.2% DMSO vehicle control was used for no stimulation in all assays. Cells were washed with FACS wash buffer and stained with antibodies to CD4 (OKT4 clone), CD8 (SK1 clone), CD14 (HCD14 clone), CD16 (B73.1 clone), CD19 (HIB19 clone), OX-40 (ACT35 clone), CD69 (FN-50 clone), and CD137 (4B4-1 clone) as well as live/dead viability dye. Cells were washed with FACS wash buffer, fixed with 2% paraformaldehyde (BD), and stored in FACS wash buffer in the dark at 4°C until ready for flow cytometry analysis. All samples were collected on an LSR Fortessa (BD). Flow cytometry analysis was completed using FlowJo (BD). AIM+ CD4+ and CD8+ T cells were identified using the same gating strategy as before by selecting live CD14/CD16/CD19-negative cells (Sabatino et al., 2022). The frequencies of spike-specific T cells were calculated by subtracting the no stimulation background from the corresponding S1 and S2 pool-stimulated samples, which were then summed together.
Statistical analysis
Kruskal–Wallis test with multiple comparisons was used to compare vaccine intervals and DMT treatment durations across different cohorts. Mixed effects analysis with multiple comparisons was used to analyze post-vaccination antibody and T cell responses across different groups and time points. Healthy controls were used as the comparison group for statistical significance unless stated otherwise. Simple linear regression was to analyze spike IgG and spike RBD IgG levels with treatment duration and Spearman rank was used for correlation analysis. The following p-values were used: not significant (ns) for p ≥ 0.05, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, **** for p < 0.0001.
Study approval
All enrolled participants provided written, informed consent for this study, which was approved by the UCSF Committee on Human Research (San Francisco, CA; IRB# 21-33240).
Results
Study overview
A cohort of healthy controls (HC, n = 12) and MS patients treated with anti-CD20 mAb (ofatumumab (OFA), n = 11; ocrelizumab (OCR), n = 25) or S1P receptor modulators (fingolimod, n = 11; siponimod n = 2) were longitudinally assessed for SARS-CoV-2 vaccine-induced antibody and T cell immunity (Table 1 ). Blood samples were collected before and following three SARS-CoV-2 vaccinations at the indicated time points (Fig. 1A); this analysis includes patients previously reported after two vaccinations (Sabatino et al., 2022). As not all participants had data at each time point, the number of participants at each sample collection point is shown in Supplemental Table 1. At the final time point, T3, there were n = 10 OFA, n = 13 OCR, n = 8 S1P, and n = 10 HC samples available for analysis. All participants received an mRNA vaccine as the third vaccination. The interval between second and third vaccinations tended to be shorter in MS patients compared to HC, and was significantly less in the case of OCR and S1P patients (Table 1).
Table 1.
Overview of study subjects.
| Group | HC | OFA | OCR | S1P |
|---|---|---|---|---|
| N | 12 | 11 | 25 | 13 |
| Age (years) | 35.9 | 48.1 | 44.2 | 48.8 |
| % Female | 67% | 64% | 64% | 69% |
| Days V2-V3 | 265 (204-306) | 189 (105-259) | 147*** (28-317) | 152** (106-197) |
| Days V3-T3 | 15 (12-20) | 42 (13-79) | 23 (0-53) | 33 (14-56) |
| Months treatment duration to V3 | N/A | 10.2 (1.8-42.7) | 37.8 (14-37.8) | 67.3 (4.7-123.7) |
| Months cumulative treatment duration to V3 | N/A | 53.5 (1.8-114.9) | 42.7 (21.8-96.9) | 77.9 (13.1-123.7) |
Values shown represent means unless otherwise indicated and values in parentheses reflect ranges. **p<0.01 and ***p<0.001 for the indicated groups compared to healthy controls (Kruskal–Wallis with multiple comparisons). Abbreviations: HC = healthy control, OFA = ofatumumab, OCR = ocrelizumab, S1P = sphingosine-1-phosphate receptor modulator.
The percentages of CD19+ B cells and CD4+ and CD8+ T cells were measured at each collection time point. As expected, the frequencies of CD19+ B cells were significantly lower in OFA, OCR, and S1P-treated MS patients compared to HC at all time points (Supplemental Fig. 1A). The frequencies of CD4+ and CD8+ T cells were significantly lower in S1P-treated patients only (Supplemental Fig. 1B and C), consistent with the mechanism of action of S1P therapies.
Longitudinal analysis of SARS-CoV-2 IgG responses following multiple vaccinations
Nearly all individuals were seronegative prior to vaccination, but low level seropositivity at T0 in a few individuals could reflect prior exposure to SARS-CoV-2 or cross-reactive coronaviruses (Fig. 1B and C). We focused our analysis on comparing the responses between the second and third vaccinations (i.e. between timepoints T1 and T3) given our prior report on responses following second vaccination (Sabatino et al., 2022). Between T1 and T3, total spike IgG titers increased by 1.4-fold in OFA, 2.1-fold in OCR, 3.2-fold in S1P, and 2.0-fold in HC; spike RBD IgG increased by 1.6-fold in OFA, 1.7-fold in OCR, 4.3-fold in S1P, and 2.0-fold in HC. Total spike IgG titers at T3 were significantly increased compared to T1 in HC and S1P-treated patients only (p < 0.0001 and p = 0.0494, respectively) (Fig. 1B). Only HC demonstrated a significant increase in spike RBD IgG levels at T3 compared to T1 (p < 0.0001) (Fig. 1C). At T3, total spike IgG was significantly lower in both OFA (p = 0.0002) and OCR (p < 0.0001) patients compared to HC, while spike RBD IgG was significantly lower in both OFA (p < 0.0001) and OCR (p < 0.0001) as well as S1P (p = 0.004) patients (Fig. 1B and C). Total spike IgG (p = 0.0215) and spike RBD IgG (p = 0.0215) seropositivity significantly increased in OCR patients between T1 and T3, but no significant changes were observed in OFA and S1P patients, possibly due to lower patient numbers in those groups (Fig. 1B and C). At T3, 100% of HC were total spike IgG and spike RBD IgG seropositive. In contrast, total spike IgG seropositivity was 60.0 ± 16.3% in OFA, 84.6 ± 10.4% in OCR, and 100% in S1P; spike RBD IgG seropositivity was 60.0 ± 16.3% in OFA, 46.2 ± 14.2% in OCR, and 75.0 ± 16.4% in S1P. The reduction in spike RBD IgG seropositivity was statistically significant in OCR patients compared to HC at T3 (p = 0.0054), but not in OFA patients (p = 0.1363).
While there were variable increases in total spike IgG and spike RBD IgG levels at T3, anti-CD20 mAb and S1P-treated patients had more limited serologic responses to a third vaccination resulting in significantly lower antibody levels compared to HC. To evaluate further, we investigated seropersistence between the second and third vaccinations (i.e. retention of seropositivity between T1 and T3). All subjects who were seropositive to total spike and spike RBD after two vaccinations remained seropositive following a third vaccination (Supplemental Table 2), acknowledging limited patient numbers in certain treatment groups, including only one OFA-treated patient. We also assessed seroconversion following a third vaccination in the limited number of patients who were seronegative at T1. Among the patients with longitudinal blood samples, anti-CD20 mAb-treated patients showed minimal seroconversion (2/7 for total spike IgG and 2/12 for spike RBD IgG), while 3/3 S1P patients seroconverted to total spike IgG and 2/4 seroconverted to spike RBD IgG (Supplemental Table 3).
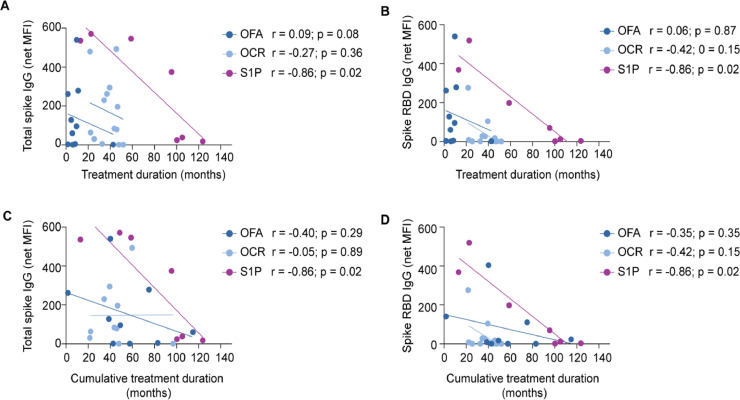
We then investigated factors previously demonstrated to influence SARS-CoV-2 vaccine-elicited antibody responses (Sormani et al., 2021; Sabatino et al., 2022), including age, CD19+ B cell levels and treatment duration. No significant difference in age was found between seropositive and seronegative anti-CD20 and S1P-treated patients (mean 48.2 and 37.3, respectively, for total spike IgG; mean 48.5 and 42.8, respectively, for spike RBD IgG). We did not observe a significant relationship between CD19+ B cells and total spike IgG or spike RBD IgG (Supplemental Fig. 2A and B). With respect to treatment duration, we evaluated both time on current therapy, as well as cumulative prior treatment with S1P receptor modulators and/or anti-CD20 mAbs. Only S1P-treated patients demonstrated a significant negative correlation between treatment duration and total spike IgG and spike RBD IgG levels (Fig. 2 A and B). We observed that treatment duration was longest in S1P-treated patients and shortest in ofatumumab-treated patients (Table 1), which is largely related to differences in FDA approvals for these DMTs. As expected, the mean cumulative treatment durations (i.e. total duration all consecutive anti-CD20 mAb and/or S1P treatments) of OFA-treated patients were increased the most with more modest increases in OCR and S1P patients (Table 1). Again, only S1P-treated patients showed a significant negative correlation between cumulative treatment duration and antibody responses (Fig. 2C and D). We further explored antibody levels at T3 in OFA patients based on pre-OFA treatment history. OFA patients with no prior DMT treatment trended toward higher total spike and spike RBD IgG levels compared to OFA patients who transitioned from other anti-CD20 mAb or S1P DMTs, but this did not reach statistical significance (Supplemental Fig. 3).
Fig. 2.
Correlation of MS treatment duration with SARS-CoV-2 antibody responses.
Total spike IgG (A and C) and spike RBD IgG (B and D) levels were correlated with duration of the indicated treatments. The duration of the current therapies is shown in panels A and B. The cumulative duration of the current therapies includes the current treatment duration plus prior anti-CD20 or S1P therapy is shown in panels C and D.
Longitudinal spike antigen-specific CD4+ and CD8+ T cell responses
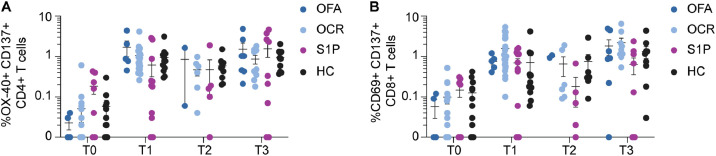
Frequencies of CD4+ and CD8+ T cells specific for spike antigens were investigated using pools of spike peptides in an activation-induced marker (AIM) expression assay. Spike-specific CD4+ and CD8+ T cells were highly stable between T1 and T3 (Fig. 3 A and B). Mean frequencies of spike-specific CD4+ and CD8+ T cells were similar across all MS treatment groups and HC at all timepoints, although several S1P patients had undetectable spike-specific T cells, consistent with the known sequestering effects of lymphocytes in lymphoid tissues of this DMT class.
Fig. 3.
Spike-specific CD4+ and CD8+ T cell responses following serial SARS CoV-2 vaccination.
The frequencies of spike-specific CD4+ T cells (A) and CD8+ T cells measured by AIM is demonstrated at each of the indicated time points.
Comparison of ofatumumab effects on de novo versus pre-existing SARS-CoV-2 vaccine-elicited immunity
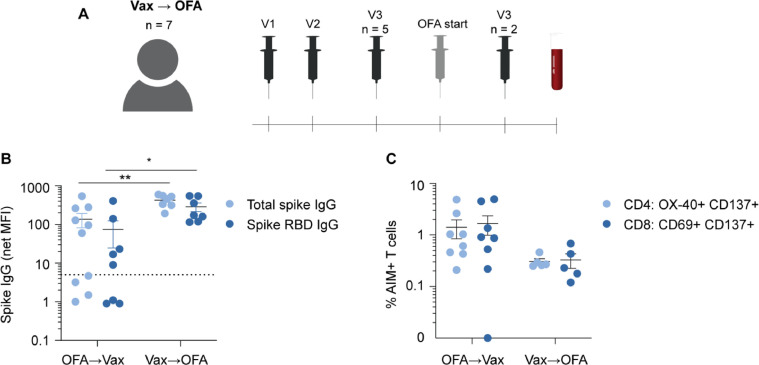
The above data indicate that anti-CD20 therapies blunt the generation of de novo spike-specific IgG responses following multiple SARS-CoV-2 vaccinations. However, it is not known at this time whether anti-CD20 mAb may attenuate pre-existing antibody responses to SARS-CoV-2 vaccinations. We therefore enrolled a separate cohort of MS patients who received 2-3 SARS-CoV-2 vaccinations prior to anti-CD20 therapy and then subsequently began treatment with OFA (Supplemental Table 4). All patients received full SARS-CoV-2 vaccinations (i.e. three mRNA or one adenoviral plus one mRNA vaccine) with five patients completing all vaccinations prior to OFA treatment and two patients receiving their final vaccination after OFA initiation. Blood samples were then collected and analyzed after OFA initiation (Fig. 4 A). We compared the immune outcomes in this cohort (termed ‘vax → OFA’) and compared to the post-third vaccination immune responses in patients who were on OFA throughout all three vaccinations (i.e. from T3 of Fig. 1A; referred to as ‘OFA → vax’ in Fig. 4). As shown in Fig. 4B, total spike IgG (p = 0.0031) and spike RBD IgG (p = 0.0392) were significantly higher in vax → OFA patients compared to OFA → vax patients. While T cell responses trended lower in OFA → vax patients, there was no significant difference in spike-specific CD4+ and CD8+ T cell responses in comparison to vax → OFA patients (Fig. 4C). These findings therefore suggest that humoral responses are largely retained when vaccination precedes initiation anti-CD20 therapy.
Fig. 4.
Impact of ofatumumab treatment on de novo versus pre-existing SARS-CoV-2 immunity.
Overview of second cohort of MS patients (n = 7) who completed at least two SARS-CoV-2 vaccinations prior to ofatumumab (OFA) initiation (termed ‘Vax → OFA’) (A). N = 5 completed three vaccinations prior to OFA and n = 2 completed a third vaccination after OFA start). In all patients, blood samples were collected following the third vaccination and OFA initiation. Comparison of total spike IgG and spike RBD IgG responses in patients who began OFA prior to SARS-CoV-2 vaccination (OFA → Vax) with Vax → OFA cohort (B). AIM+ CD4+ and CD8+ T cells were likewise compared between both cohorts (C). Note: two OFA → Vax and two Vax → OFA samples had insufficient cells to complete AIM analysis. * p < 0.05, ** p < 0.01.
Discussion
Anti-CD20 mAb and S1P therapies are highly effective and widely used treatments for MS. Significant disruption of the generation of de novo humoral immunity is an unfortunate consequence of their modes of action, however (Bar-Or et al., 2020). Previous studies have demonstrated that antibody responses in anti-CD20 mAb and S1P-treated patients are significantly blunted following two doses of SARS-CoV-2 vaccination (Sormani et al., 2021; Sabatino et al., 2022). Several recent prospective studies demonstrated a higher risk of breakthrough COVID-19 in vaccinated patients on these DMT classes, which was correlated with reduced spike-specific-antibody levels (Sormani et al., 2022; Jakimovski et al., 2022). It is therefore imperative to determine if additional vaccinations may improve immune responses. In this study, we assessed the effects of a third SARS-CoV-2 vaccination on antibody and T cell responses in patients on anti-CD20 mAb (ocrelizumab and ofatumumab) and S1P receptor modulators (fingolimod and siponimod).
We demonstrated that while the majority of anti-CD20 mAb and S1P patients mounted persistently detectable antibody responses, spike RBD IgG levels did not significantly increase following a third SARS-CoV-2 vaccination and remained significantly lower compared to healthy controls. Moreover, seroconversion between the second and third vaccinations was very limited amongst anti-CD20-treated patients, consistent with recent reports (König et al., 2022; Bajwa et al., 2022; Achtnichts et al., 2021; Sidler et al., 2022; Brill et al., 2022). In contrast, S1P patients showed a moderate degree of seroconversion following a third SARS-CoV-2 vaccination. These findings suggest that humoral immunity may not be disrupted to the same extent in S1P therapies as in anti-CD20 mAb and repeat vaccination may be more likely to augment antibody responses in S1P-treated patients. Thus, it may be reasonable to monitor post-vaccination antibody response and consider more frequent boosters (e.g. every four to six months) in S1P patients while the COVID-19 pandemic persists.
Although this study did not reveal a significant correlation between CD19+ B cell levels and spike-specific antibody responses, this relationship been demonstrated in other studies (Sabatino et al., 2022; Tolf et al., 2022). Our study provides further support that treatment duration may be an important factor in determining antibody responses to SARS-CoV-2 vaccination. S1P treatment duration showed a significant negative relationship with total spike and spike RBD IgG levels, consistent with another recent report (Meyer-Arndt et al., 2022). Moreover, our data suggest that cumulative treatment duration with consecutive of anti-CD20 mAb and/or S1P therapies is an important consideration when assessing the likelihood of patients on these therapies mounting effective vaccine-elicited antibody responses. In contrast to antibody responses, spike-specific CD4+ and CD8+ T cell responses were very stable between the second and third vaccinations for anti-CD20 and S1P-treated patients, consistent with findings reported in the general population. Increasing evidence supports an important role of T cell immunity in the resolution of SARS-CoV-2 infection (Sette and Crotty, 2021; Vardhana et al., 2022), which supports the favorable COVID-19 outcomes in most MS patients even in the absence of detectable antibodies (Sormani et al., 2022; Jakimovski et al., 2022; Cross et al., 2022). In the case of S1P therapies, it is likely that additional antigen-specific lymphocytes found outside of the blood help to account for the lack of an increased risk of serious COVID-19 infections.
This investigation provides increasing support that robust humoral immunity is difficult to achieve in patients who receive SARS-CoV-2 vaccinations while on anti-CD20 mAb treatment. We were further able to explore whether beginning anti-CD20 mAb attenuates SARS-CoV-2 immunity after primary vaccination off therapy. Our findings demonstrate that patients who received 2-3 doses of SARS-CoV-2 vaccinations prior to ofatumumab treatment retained significantly higher levels of spike-specific antibodies compared to patients who received all vaccinations while on ofatumumab therapy. While our study does not address the longevity of humoral immunity following initiation of anti-CD20 mAb treatment, these findings highlight the importance of optimizing vaccine-elicited antibody responses when circulating levels of B cells are high (Tolf et al., 2022; König et al., 2021).
There are limitations of the current study. Sample sizes for the different groups were smaller in some treatment groups. In addition, patient sample numbers varied across different collection timepoints, limiting the ability to compare groups as well as seropersistence and seroconversion analyses. This study also investigated only immune outcomes and was not intended to explore clinical outcomes. However, we have retrospectively investigated spike-specific antibody responses in our MS cohort who subsequently developed PCR-confirmed COVID-19 following SARS-CoV-2 vaccination. Three anti-CD20 mAb (OCR n = 2, OFA n = 1) and three S1P (fingolimod n = 2, siponimod n = 1) patients in the vaccinated cohort subsequently developed COVID-19. All six had high titer total spike IgG responses and five of the six were seropositive to spike RBD IgG. None of the patients were hospitalized and all recovered. While it is difficult to conclude a relationship between the vaccine-elicited antibody responses and clinical outcomes in this analysis, these data provide some reassurance that patients on these treatments can recover fully from breakthrough infection.
This study extends our understanding of the immunogenicity of repeated SARS-CoV-2 vaccination in MS patients on DMTs known to significantly blunt generation of de novo humoral immunity. While monitoring vaccination-induced antibody responses is feasible and can inform clinical decisions in anti-CD20 and S1P-treated patients (e.g. impacting infection risk assessment and risk mitigation recommendations), measuring cellular responses is much more challenging in clinical practice and has an unclear impact on decision making. Our findings and those of others indicate that repeat SARS-CoV-2 vaccination may lead to higher antibody responses in S1P-treated patients than in anti-CD20 mAb patients (Jakimovski et al., 2022; Bajwa et al., 2022; Tallantyre et al., 2022; Maglione et al., 2022). However, the presence of detectable B cells has been shown to be associated with seroconversion in CD20 mAb-treated patients (Tolf et al., 2022; König et al., 2021), although the clinical impact of extended dosing intervals is not entirely clear. The impaired antibody response, which is needed for virus neutralization, could potentially increase the risk of breakthrough COVID-19 in patients on these classes of DMTs. For additional protection, implementation of prophylactic monoclonal antibodies against the SARS-CoV-2 spike RBD to reduce the breakthrough infection risk in immunocompromised patients may be considered (Goulenok et al., 2022; Levin et al., 2022; Conte and Golzarri-Arroyo, 2022). On the other hand, T cell immunity appears to be generally well retained and increasing evidence supports an important role of T cell immunity in the resolution of SARS-CoV-2 infection (Sette and Crotty, 2021; Vardhana et al., 2022). This is consistent with recent reports that breakthrough infections with pre-omicron SARS-CoV-2 variants in vaccinated MS patients on anti-CD20 mAb and S1P therapies are generally mild (Sormani et al., 2022; Jakimovski et al., 2022; Cross et al., 2022; Januel et al., 2021). The findings from this study therefore provide important clinical guidance for optimizing COVID-19 immunity in MS patients on anti-CD20 mAb and S1P receptor modulators.
Funding
This study was funded by Novartis Pharmaceuticals.
CRediT authorship contribution statement
Joseph J. Sabatino: Supervision, Methodology, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Kristen Mittl: Formal analysis, Data curation, Writing – review & editing. William Rowles: Funding acquisition, Resources, Data curation, Formal analysis, Writing – review & editing. Colin R. Zamecnik: Formal analysis, Data curation, Writing – review & editing. Rita P. Loudermilk: Formal analysis, Data curation, Writing – review & editing. Chloe Gerungan: Formal analysis, Data curation, Writing – review & editing. Collin M. Spencer: Data curation, Writing – review & editing. Sharon A. Sagan: Data curation, Writing – review & editing. Jessa Alexander: Funding acquisition, Resources, Data curation, Writing – review & editing. Kira Mcpolin: Funding acquisition, Resources, Data curation, Writing – review & editing. PeiXi Chen: Formal analysis, Data curation, Writing – review & editing. Chinmay Deshpande: Supervision, Methodology, Data curation, Writing – review & editing. Kerri Wyse: Supervision, Methodology, Writing – original draft, Data curation, Writing – review & editing. Eric M. Maiese: Supervision, Methodology, Writing – original draft, Data curation, Writing – review & editing. Michael R. Wilson: Supervision, Methodology, Data curation, Writing – review & editing. Scott S. Zamvil: Supervision, Methodology, Data curation, Writing – review & editing. Riley Bove: Supervision, Methodology, Writing – original draft, Data curation, Writing – review & editing.
Disclosures
JJS has received research funding from Novartis and Roche/Genentech. MRW has received research grant funding from Roche/Genentech and speaking honoraria from Novartis, Takeda and Genentech. SSZ has received consulting honoraria from Alexion, Biogen-Idec, EMD-Serono, Genzyme, Novartis, Roche/Genentech, and Teva Pharmaceuticals, Inc and has served on Data Safety Monitoring Boards for Lilly, BioMS, Teva and Therapeutics. RB has received research grant funding from Novartis, Roche Genentech and Biogen, and consulting honoraria from Alexion, Biogen, EMD Serono, Genzyme Sanofi, Novartis, and Roche Genentech.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.104484.
Appendix. Supplementary materials
References
- Achtnichts L., et al. Humoral immune response after the third SARS-CoV-2 mRNA vaccination in CD20 depleted people with multiple sclerosis. Vaccines. 2021;9 doi: 10.3390/vaccines9121470. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa H.M., et al. Persistently reduced humoral and sustained cellular immune response from first to third SARS-CoV-2 mRNA vaccination in anti-CD20-treated multiple sclerosis patients. Mult. Scler. Relat. Disord. 2022;60 doi: 10.1016/j.msard.2022.103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., et al. Severe acute respiratory syndrome coronavirus 2 third vaccine immune response in multiple sclerosis patients treated with ocrelizumab. Ann. Neurol. 2022;91:796–800. doi: 10.1002/ana.26343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W.L., Golzarri-Arroyo L. Tixagevimab and Cilgavimab (Evusheld) boosts antibody levels to SARS-CoV-2 in patients with multiple sclerosis on b-cell depleters. Mult. Scler. Relat. Disord. 2022;63 doi: 10.1016/j.msard.2022.103905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A.H., et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol. Ther. 2022 doi: 10.1007/s40120-022-00341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulenok T., et al. Pre-exposure anti-SARS-CoV-2 monoclonal antibodies in severely immunocompromised patients with immune-mediated inflammatory diseases. Lancet Rheumatol. 2022 doi: 10.1016/S2665-9913(22)00099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakimovski D., et al. COVID-19 vaccination in multiple sclerosis and inflammatory diseases: effects from disease-modifying therapy, long-term seroprevalence and breakthrough infections. Vaccines. 2022;10 doi: 10.3390/vaccines10050695. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januel E., et al. Post-vaccine COVID-19 in patients with multiple sclerosis or neuromyelitis optica. Mult. Scler. J. 2021;13524585211049736 doi: 10.1177/13524585211049737. [DOI] [PubMed] [Google Scholar]
- König M., et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J. Neurol. Neurosurg. Psychiatry. 2021 doi: 10.1136/jnnp-2021-327612. jnnp-2021-327612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König M., et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. 2022;79:307–309. doi: 10.1001/jamaneurol.2021.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M.J., et al. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for prevention of Covid-19. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione A., et al. Humoral response after the booster dose of anti-SARS-CoV-2 vaccine in multiple sclerosis patients treated with high-efficacy therapies. Mult. Scler. Relat. Disord. 2022;61 doi: 10.1016/j.msard.2022.103776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Arndt L., et al. SARS-CoV-2 mRNA vaccinations fail to elicit humoral and cellular immune responses in patients with multiple sclerosis receiving fingolimod. J. Neurol. Neurosurg. Psychiatry. 2022;93:960–971. doi: 10.1136/jnnp-2022-329395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar L., et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5:eabc8413. doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino, J.J, Jr., et al., 2022. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine–induced antibody and T cell immunity and function. JCI Insight. 7(4) (2022):e156978. [DOI] [PMC free article] [PubMed]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler D., et al. Trajectories of humoral and cellular immunity and responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. eBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. eBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., et al. Response to COVID-19 booster vaccinations in seronegative people with multiple sclerosis. Mult. Scler. Relat. Disord. 2022;64 doi: 10.1016/j.msard.2022.103937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolf A., et al. Factors associated with serological response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with rituximab. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.11497. e2211497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhana S., Baldo L., Morice W.G., Wherry E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci. Immunol. 2022;7 doi: 10.1126/sciimmunol.abo1303. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik C.R., et al. ReScan, a multiplex diagnostic pipeline, pans human sera for SARS-CoV-2 antigens. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.