Abstract
Numerous studies have reported that asialo-GM1, gangliotetraosylceramide, or moieties serve as epithelial cell receptors for Pseudomonas aeruginosa. Usually this interaction is confirmed with antibodies to asialo-GM1. However, few, if any, of these reports have evaluated the binding of fresh clinical isolates of P. aeruginosa to asialo-GM1 or the specificity of the antibodies for the asialo-GM1 antigen. We confirmed that asialo-GM1 dissolved in dimethyl sulfoxide could be added to the apical membrane of Madin-Darby canine kidney cells growing as a polarized epithelium on Transwell membranes (J. C. Comolli, L. L. Waite, K. E. Mostov, and J. N. Engel, Infect. Immun. 67:3207–3214, 1999) and that such treatment enhanced the binding of P. aeruginosa strain PA103. However, no other P. aeruginosa strain, including eight different clinical isolates, exhibited enhanced binding to asialo-GM1-treated cells. Studies with commercially available antibodies to asialo-GM1 showed that these preparations had high titers of antibody to P. aeruginosa antigens, including whole cells, purified lipopolysaccharide (LPS), and pili. Inhibition studies showed that adsorption of an antiserum to asialo-GM1 with P. aeruginosa cells could remove the reactivity of antibodies to asialo-GM1, and adsorption of this serum with asialo-GM1 removed antibody binding to P. aeruginosa LPS. Antibodies in sera raised to asialo-GM1 were observed to bind to P. aeruginosa cells by immunoelectron microscopy. Antibodies to asialo-GM1 inhibited formation of a biofilm by P. aeruginosa in the absence of mammalian cells, indicating a direct inhibition of bacterial cell-cell interactions. These findings demonstrate that asialo-GM1 is not a major cellular receptor for clinical isolates of P. aeruginosa and that commercially available antibodies raised to this antigen contain high titers of antibody to multiple P. aeruginosa antigens, which do not interfere with the binding of P. aeruginosa to mammalian cells but possibly interfere with the binding of P. aeruginosa cells to each other.
Interactions of bacterial cells with host tissues initiates many processes, including the anchoring of microbes to host cells and extracellular matrices, the activation of innate host immune responses, and changes in gene expression in both the microbial and host cell (15, 25, 33, 48). A large array of adhesins for host mammalian receptors have been described for many bacterial species. Among the gram-negative bacteria, pili and flagella often play a prominent role in anchoring bacterial cells to host tissues (1, 45, 48). For Pseudomonas aeruginosa, numerous studies have implicated flagella and pili as bacterial adhesins that bind specifically to terminal or internal N-acetylgalactosamine (GalNAc) residues that are linked beta-1-4 to galactose (Gal) residues unsubstituted with sialyl residues (30). Such structures are found in the gangliotetraosylceramide (asialo-GM1) receptor on host cells (2, 7, 9, 10, 24). The number of asialo-GM1 receptors is reported to be increased on respiratory epithelial cells from patients with cystic fibrosis (CF) who are homozygous for the ΔF508 allele of the CF transmembrane conductance regulator (CFTR) (24, 39, 51). In addition, some investigators have reported that asialo-GM1 is a receptor for both pili and lipopolysaccharide (LPS) of P. aeruginosa present on murine and bovine corneal epithelial cells (16, 20, 47); others have disputed whether asialo-GM1 is expressed in the human cornea (52). Some of these studies confirmed that asialo-GM1 is a receptor for P. aeruginosa binding by using purified glycolipid to inhibit binding (24, 47) or commercially prepared antisera to this antigen (7, 9, 10, 20, 24). Finally, a role has been proposed for a possible neuraminidase in generating asialo-GM1 tetrasaccharide from the parental sialylated GM1 molecule (3, 9), although to date the only evidence for a gene that encodes a P. aeruginosa neuraminidase is the recent identification of a DNA sequence in P. aeruginosa PAO1 that has some homology to other bacterial neuraminidases (GenBank accession no. AAF60322).
Although the reports noted above suggest a strong case for the involvement of asialo-GM1 as a receptor for P. aeruginosa on mammalian cells, careful scrutiny of these studies indicates that their general applicability to this host-pathogen interaction may be limited. Few of the studies used clinical isolates of P. aeruginosa (30); most used well-characterized laboratory strains such as PAO1, PAK, ATCC 19660, and PA103 (7, 9, 10, 21, 24). Only two studies presented evidence that purified asialo-GM1 ganglioside, or the purified tetrasaccharide, could inhibit the adherence of P. aeruginosa to cells (24, 47). Furthermore, Imundo et al. (24) found a very high concentration of the asialo-GM1 ganglioside (25 mM) or tetrasaccharide (250 μM) was needed to inhibit binding to CF bronchial cells by only 57 to 75%, and Singh et al. (47) noted only a transient decrease in binding of P. aeruginosa to unwounded cornea after premixing the bacteria with asialo-GM1. In the Singh et al. study, monosialoganglioside (GM1), which is not considered a major receptor for P. aeruginosa, had efficacy comparable to that of asialo-GM1, whereas in the Imundo et al. study GM1 was not an effective inhibitor of P. aeruginosa binding to cells (24). Also, Davies et al. (9) could not inhibit binding of P. aeruginosa to CF epithelial cells with the asialo-GM1 tetrasaccharide.
Additional concerns center on the use of commercially prepared polyclonal antibodies to asialo-GM1 in these studies. Although numerous investigators have found these antibodies to be effective at inhibiting the binding of P. aeruginosa to cells (2, 7, 9, 10, 22), essentially none of the studies confirmed the specificity of the antibodies by blocking the biologic activity of the antibodies with appropriate adsorbing or inhibiting reagents to demonstrate the specificity of the antibodies to asialo-GM1. Of even greater concern is that these polyclonal antibodies are raised in rabbits to essentially a self-antigen purified from bovine tissues emulsified in methylated bovine serum albumin (BSA) and complete Freund's adjuvant. Such antisera would contain high levels of antibodies that would react with the BSA and possibly with contaminants from the bovine tissues used to purify the asialo-GM1. Since bovine antigens are present in cell culture media that include fetal calf serum (FCS), it is possible that antibodies raised under these conditions could bind to bovine antigens adsorbed onto the epithelial or bacterial cell surface. In addition, the presence of mycobacterial antigens in the adjuvant could elicit antibodies reactive with bacterial and mammalian cellular antigens, readily perturbing experimental outcomes.
Comolli et al. (7) showed that asialo-GM1 ganglioside dissolved in dimethyl sulfoxide (DMSO) could be transferred onto the surface of polarized epithelial monolayers of Madin-Darby canine kidney (MDCK) cells in culture, thereby increasing the level of asialo-GM1. These cells normally express little asialo-GM1 on their surface. Cells with increased asialo-GM1, but not those with increased GM1, bound more P. aeruginosa PA103 to their surface, were more susceptible to the ExoU-bacterial cytotoxic factor, and internalized a noncytotoxic mutant of strain PA103 better than those without the increased asialo-GM1. This all required intact type IV pili; a nonpiliated P. aeruginosa mutant had little interaction with the MDCK cells with asialo-GM1. However, as with the other studies, the effect was measured with only one laboratory strain and related isogenic mutants, and confirmation of the role of asialo-GM1 in this system that used commercially provided antibody to asialo-GM1 did not include studies that showed the specificity of the inhibiting antibodies to asialo-GM1. Nonetheless, using cells with increased levels of asialo-GM1 provides a robust system for comparing P. aeruginosa adherence to cells treated with asialo-GM1, other gangliosides, or with delivery vehicle only. In an attempt to determine the role of asialo-GM1 in the binding of clinical isolates of P. aeruginosa to mammalian cells and the value of the commercially available antibodies to asialo-GM1 for confirming this phenomenon, we analyzed the binding of typical laboratory strains of P. aeruginosa to asialo-GM1-treated MDCK cells growing in Transwells and minimally passaged clinical isolates from P. aeruginosa corneal and respiratory infections, including isolates obtained from patients with CF early in the course of infection that were thus representative of the initial, colonizing strain. We also characterized the binding activity of commercially available antisera to asialo-GM1 against asialo-GM1 and GM1 gangliosides and P. aeruginosa antigens. The overall findings indicated that one laboratory strain, but no clinical isolates, of P. aeruginosa use asialo-GM1 to bind to cells and that the cell-binding-inhibitory effects of antisera to asialo-GM1 are due not to antibodies to asialo-GM1 but rather to high titers of antibodies to multiple P. aeruginosa antigens, including LPS and pili, that are present in these antisera.
MATERIALS AND METHODS
Bacterial strains.
The strains of P. aeruginosa used in this study are listed in Table 1.The clinical isolates were obtained directly from the microbiology laboratory of the indicated hospital, stored frozen at −80°C, and used as stocks to prepare inocula for the assays. Escherichia coli DH5α was used as a control in serum adsorption experiments.
TABLE 1.
P. aeruginosa strains used in this study
| Isolate | Phenotype | Source or reference |
|---|---|---|
| Laboratory strains | ||
| PAO1-V | Chloramphenicol sensitive | M. Vasil, Denver, Colo. |
| PAO1-I | Chloramphenicol resistant | B. Iglewski, Rochester, N.Y. |
| PA103 | lasR mutant; exoU+; serogroup O11 | 14 |
| PAK | Serogroup O6 | S. Lory, Seattle, Wash. |
| PAC557 | Complete-core LPS; no O side chains | 29 |
| PAO1 algC::tet | algC mutant; incomplete-core LPS | 8 |
| Clinical isolates | ||
| 149 | CF patient | Children's Hospital, Boston, Mass. |
| 324 | CF patient | Children's Hospital, Boston, Mass. |
| 383 | CF patient | Children's Hospital, Boston, Mass. |
| 6294 | Ulcerative keratitis patient | Bascom-Palmer Eye Institute, Miami, Fla. |
| 6206 | Ulcerative keratitis patient | Bascom-Palmer Eye Institute, Miami, Fla. |
| 6487 | Ulcerative keratitis patient | Bascom-Palmer Eye Institute, Miami, Fla. |
| 2BI | Pneumonia patient | Beth Israel Hospital, Boston, Mass. |
| 10CH | Pneumonia patient | Children's Hospital, Boston, Mass. |
LPS and pilus antigens.
LPS was purified from strains PAC557 and PAO1 algC::tet as previously described (18). LPS purified from P. aeruginosa serogroup O6 was purchased from List Biochemicals (Campbell, Calif.). Purified pili from strain PAK was provided by Reuben Ramphal (Gainesville, Fla.).
Cell culture and bacterial adherence experiments.
MDCK type 1 cells (105) cultured in minimal essential medium (MEM) with Earle's balanced salt solution, 2 mM l-glutamine, 10% FCS, 50 Units of penicillin/ml, and 50 μg of streptomycin/ml were seeded onto 6.5-mm-diameter, 0.4-μm-pore-size polycarbonate Transwell filters (Corning Costar Corporation, Cambridge, Mass.) and grown to confluency. Polarized monolayers were tested for confluency by exclusion from the basal side of >98% of tritiated mannitol applied to the apical side of the cell cultures and by measurement of the transepithelial cellular resistance.
The addition of gangliosides and bacteria for the measurement of adherence was performed as previously described by Comolli et al. (7). Asialo-GM1 and GM1 were purchased from Matreya Inc. (Pleasant Gap, Pa.) and Wako Pure Chemicals (Richmond, Va.). The free carboxyl group on the sialic acid residue of GM1 was reduced by incubation in 0.1 M 2-(N-morpholino)ethanesulfonic acid (pH 7.0) at a concentration of 1 mg/ml and 10 mg of 1-ethyl-3-(3-dimethylamino- propyl)carbodiimide hydrochloride (Sigma-Aldrich, St. Louis, Mo.) per ml for 4 h at room temperature, followed by the addition of 20 mg of sodium borohydride/ml. This solution was dialyzed for 72 h against distilled water using a 1,000 molecular weight cutoff membrane and then lyophilized. The degree of reduction was verified by a thiobarbituric acid assay (28), which demonstrated reduction of the carboxyl group of the sialic acid in >50% of the GM1 molecules. GM1, reduced-GM1, and asialo-GM1 were suspended at 10 mg/ml in DMSO. DMSO alone served as an additional control. Then 14.8 μg of gangliosides, corresponding to the previously described concentration per surface area (7), was added to 100 μl of MEM–5% FCS–20 mM HEPES (pH 7.4) (MEM-lite). The 100 μl was added to the apical surface of the MDCK monolayer and incubated at 37°C for 1 h with gentle rocking and then washed twice with MEM-lite.
Laboratory and clinical P. aeruginosa strains were grown overnight in tryptic soy broth without shaking to enhance pilus expression (23), diluted in MEM-lite to a multiplicity of 10 bacteria to 1 MDCK cell, added in a volume of 100 μl/Transwell, and incubated for 2 h at 37°C. The monolayers were then washed eight times with 1 ml of MEM-lite; the number of washes was determined after preliminary studies showed that fewer washes left a significant number of nonadherent bacteria in the cell culture wells. With eight washes, <0.01% of the remaining bacterial cells were being removed in washes 6 through 8. The Transwell membranes were then cut from their support, incubated for 15 min at 22°C in MEM-lite with added 0.5% Triton X-100, and vortex mixed for 2 min. The solutions were then serially diluted and plated onto MacConkey agar plates for bacterial enumeration.
Immunofluorescence studies of asialo-GM1 binding to MDCK cells.
Immunofluorescence experiments with polarized MDCK cell monolayers treated with gangliosides in DMSO were performed as previously described (7), with the following modifications. A 1:500 dilution of rabbit antisera raised to asialo-GM1, GM1, or normal rabbit serum was made into phosphate-buffered saline (PBS) with 0.7% fish-skin gelatin, and 100 μl was added to the apical surface of the MDCK monolayers for 1 h at 37°C. After the monolayers were washed, a 1:1,000 dilution of goat-anti rabbit immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate (FITC) was added for 30 min at 37°C. After extensive washing of the monolayers, immunofluorescence images were obtained with a Nikon Diaphot fluorescent microscope with a ×40 objective and a Bio-Rad MRC-1024/2P multi-photon confocal laser array interfaced with a Zeiss Axiovert microscope using a ×100 C-Apochromat/1.2 NA water-immersion objective (Bio-Rad, Hercules, Calif.). Bound FITC was excited with the 488-nm line of a krypton-argon laser, and the emissions were collected with a DF bandpass filter of 522 ± 35 nm.
Antibodies.
Polyclonal antibodies to LPS purified from P. aeruginosa strains PAC557 and PAO1 algC::tet were generated in rabbits by subcutaneous immunization with the purified LPS emulsified in incomplete Freund's adjuvant for two doses administered 1 week apart, followed by a series of three intravenous immunizations of 10 μg in 0.5 ml of saline given 2 days apart. Normal serum was obtained from the corresponding rabbits before the immunization procedure, and immune serum was obtained multiple times after completion of the immunization schedule. Antibody to asialo-GM1 was purchased from Wako Pure Chemicals, and antibody to GM1 was purchased from Matreya. According to the manufacturer (Wako), the antibody to asialo-GM1 is a gamma globulin fraction derived from antisera raised in rabbits by “repeated immunization…with purified asialo-GM1 from bovine brain tissue, in conjunction with methylated bovine serum albumin and complete Freund's adjuvant.” For some experiments, antisera were adsorbed twice with lyophilized cells of P. aeruginosa strain PAC557 or PAO1 algC::tet bacteria (5 mg/ml of serum) or with asialo-GM1 (0.2 mg/ml) overnight at 4°C and then sterile filtered (0.45 μm, pore size).
ELISA.
Immulon II flat-bottom enzyme-linked immunosorbent assay (ELISA) plates were sensitized either with lyophilized whole bacteria (108 CFU/well) or purified LPS, BSA, asialo-GM1, GM1, or reduced GM1 at a concentration of 1 μg/well or with purified P. aeruginosa strain PAK pili at 50 ng/well. The coating buffer was 0.04 M phosphate (pH 7.0), and sensitization was done by overnight incubation at 4°C. The plates were washed three times in PBS with 0.05% Tween 20 (PBS-T). Unoccupied binding sites were blocked with 3% skim milk added in PBS and incubated for 2 h at 37°C. The different antisera were diluted 1:100 to 1:51, 200 in PBS-T with 1% skim milk and incubated for 1.5 h at 37°C. The plates were then washed again three times with PBS-T. Bound antibodies were detected with an alkaline phosphatase-conjugated goat anti-rabbit IgG antibody (whole molecule; Sigma), diluted 1:1,000 in PBS-T with 1% skim milk. The optical density at 405 nm (OD405) was measured after incubation for 1 h at room temperature in the dark. Titers and 95% confidence intervals (CI) were then determined as the serum dilution giving a reading of 0.2, as calculated from linear regression analysis of log-transformed serum dilutions and OD readings. All titers were calculated using regression analyses with a P > F value of ≤0.03 derived by analysis of variance (ANOVA). The smaller the P value the better the fit of the linear regression curve relating binding of the antibody over the range of dilutions used to the antigen, and thus it is a measure of the effectiveness of antibody binding.
Immunoelectron microscopy.
The electron microscopic visualization of antibody bound to bacteria was performed as previously described (23). In brief, bacteria were grown overnight at 37°C in tryptic soy broth. Next, 200-mesh Formvar-carbon-coated copper grids (Electron Microscopy Sciences, Fort Washington, Mass.) were put on top of 5-μl drops of bacterial growth solutions that had been placed on Parafilm paper. The grids were rinsed in PBS and blocked by placing them on top of 5 μl of a solution of 0.7% fish-skin gelatin for 10 min, washed again three times, and then placed on top of a 5-μl drop containing 1:50 dilutions of the antisera to PAC557 LPS or asialo-GM1 and incubated for 20 min at room temperature. Preimmune rabbit sera served as controls. After the grids were rinsed in PBS, gold-conjugated protein A (2-nm-diameter gold particles) was added. After 30 min of incubation at room temperature followed by washing, the preparations were visualized in the electron microscope (JEOL 1200EX). Photographs were taken at magnifications of ×10,000 to ×25,000.
Biofilm formation assay.
P. aeruginosa PAO1-V was grown in polyvinylchloride (PVC) microtiter dishes for biofilm formation, using a modification of a previously described assay (34). The antibodies to asialo-GM1 were then tested for their ability to inhibit biofilm formation. Bacteria were first grown overnight in 5 ml of M9 medium at 37°C. PVC microtiter plates were filled with 50 μl of a 1:50 dilution of this bacterial culture. The antibodies were added at 1:400 in an equal volume of M9 broth. The plates were incubated for 10 h at 37°C without shaking and then washed twice in distilled water before the addition of 125 μl of 1% crystal violet for 10 min at room temperature for staining the biofilm. The plates were then rinsed with water, and 100 μl of 95% ethanol was pipetted in the wells to release the crystal violet dye from the biofilm; the OD590 was measured. Additional evaluations were with PAO1-V grown without antibodies, with normal rabbit serum, with anti-asialo-GM1 adsorbed with 2 mg of asialo-GM1/ml, with anti-asialo-GM1 adsorbed with P. aeruginosa strain PAC557, or with anti-asialo-GM1 adsorbed with E. coli DH5α.
Statistical analysis.
Multigroup comparisons were made with ANOVA, and post hoc, pairwise comparisons between the groups were made by using the Fisher probable least-square differences (PLSD) method. Two-group comparisons were by unpaired t-tests. Simple regression was used in analysis of the results from the ELISA inhibition assays.
RESULTS
Effect of adding gangliosides to MDCK cells on P. aeruginosa binding.
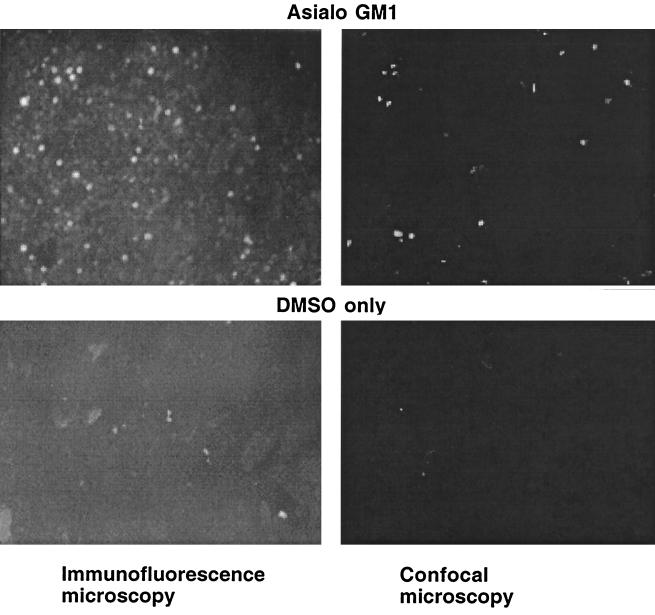
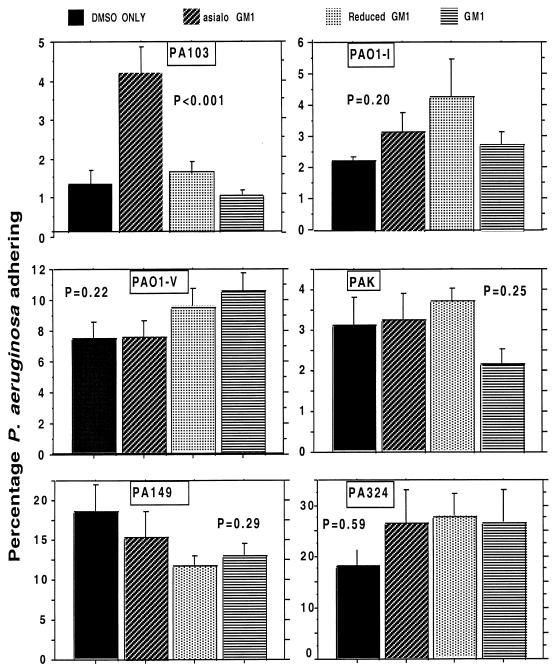
We initially examined the adherence of six strains of P. aeruginosa to MDCK type 1 cells treated with one of three gangliosides dissolved in DMSO—asialo-GM1, GM1, or reduced GM1—or with DMSO only. Figure 1 confirms the prior finding of Comolli et al. (7) that the addition of asialo-GM1 to MDCK cells in DMSO increases the level of this material on the cell surface, as documented by immunofluorescence. Although, as noted below, the antisera to asialo-GM1 contains high levels of antibodies to both asialo-GM1 and other antigens, the background level of fluorescence obtained with the control cells indicates that these other antibodies do not bind strongly to non-asialo-GM1 antigens in this system (Fig. 1). The patchy nature of the immunofluorescence was attributed to aggregation of the asialo-GM1 by the antibody used for visualization. Comparable findings were obtained with GM1 and reduced GM1 using antibody to GM1 (not shown). We also confirmed the finding of Comolli et al. (7) that the addition of asialo-GM1 to MDCK cells significantly (P < 0.001, ANOVA and Fisher PLSD) increased the binding of P. aeruginosa strain PA103 to these cells (Fig. 2), but that addition of either GM1 or reduced GM1 had little effect. With asialo-GM1, we achieved a fourfold increase in binding of strain PA103 to MDCK cells, which was not quite as high, but nevertheless in the same range, as the eightfold increase reported by Comolli et al. (7). However, when we examined the binding of six to nine replicates of five additional P. aeruginosa strains to MDCK cells treated with the different gangliosides, none showed statistically significant changes in binding to any of the gangliosides (Fig. 2), including strains 149 and 324 obtained from two patients with CF early in the course of infection. Strain PAO1-I, a chloramphenicol-resistant derivative of PAO1 (grows in 50 μg of chloramphenicol/ml, whereas PAO1-V and other PAO1 isolates we tested only grow at chloramphenicol concentrations of ≤3 μg/ml) showed about a 50% increase in binding to asialo-GM1 (from 2.2% of the inoculum adhering to 3.3%), but this difference did not reach statistical significance. However, this difference in binding is comparable to that reported by Prince and colleagues for adherence of P. aeruginosa PAO1 to CF cells compared with non-CF cells (24, 51) and may have been the strain of PAO1 used in these studies. Also, strain PAO1-I was even more adherent (about twofold) to reduced GM1, a ganglioside that was never examined in the other studies.
FIG. 1.
Demonstration by immunofluorescence and confocal microscopy of the presence of asialo-GM1 on the surface of MDCK cells growing in Transwells. After the addition of asialo-GM1 in DMSO to the cells, followed by incubation, washing, and staining with antibody to asialo-GM1, ganglioside was seen in cells treated with asialo-GM1 in DMSO but not in cells treated with DMSO only. Magnification, ×400.
FIG. 2.
Binding of strains of P. aeruginosa to MDCK cells treated with gangliosides (identified in the legend at the top of figure). Strains PA103, PAO1-I, PAO1-V, and PAK are laboratory strains. Strains PA149 and PA324 are clinical isolates obtained early in the course of infection from patients with CF. Only PA103 showed significantly increased binding to cells treated with asialo-GM1 by ANOVA and Fisher PLSD (P < 0.01). Bars represent the means of six to nine replicates; error bars represent the standard deviations.
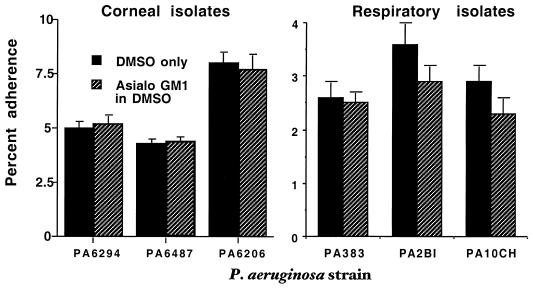
Further studies were conducted with six additional minimally passaged clinical isolates of P. aeruginosa to determine if binding to MDCK cells increased after the augmentation of asialo-GM1 levels on the cell surface. The P. aeruginosa strains were three isolates from human corneal infections and three from the respiratory tract (one additional strain from a patient with CF early in the course of infection, two strains from patients with acute pneumonia; Fig. 3.) As with the other studies, there was no increased binding of P. aeruginosa to MDCK cells with elevated levels of asialo-GM1 compared to the cells treated with DMSO alone. All P values for differences by t tests were 0.2 to 0.9.
FIG. 3.
Binding of six clinical isolates of P. aeruginosa to MDCK cells treated with the asialo-GM1 ganglioside. None of the strains showed a significantly increased binding to cells treated with asialo-GM1 by (P = 0.2 to 0.9, unpaired t tests). Bars represent the means of six to nine replicates; error bars represent the standard deviations.
Titer and specificity of antibodies in antisera raised to asialo-GM1.
A commercially available antibody preparation (Wako) raised to asialo-GM1 was examined by ELISA for the titer of antibodies to the immunizing antigen, BSA (included in the immunizing mixture), and P. aeruginosa LPS antigens. Because antibodies to asialo-GM1 are often added to P. aeruginosa adherence experiments to block bacterial binding to asialo-GM1 and thus confirm the specificity of the interaction, it is critical to know if the antisera contain antibodies to either P. aeruginosa antigens or other antigens such as BSA that might be present in the cell culture medium. As shown in Table 2, antibodies raised to asialo-GM1 had high titers to itself, to BSA, to LPS from P. aeruginosa serogroup O6, and to the complete-core LPS from strain PAC557. There were modest titers of antibody to GM1 and reduced GM1. The relative width of the 95% CI is also an indicator of the antibody affinity—the narrower the relative width, the steeper the binding curve and the higher the affinity of the antibody (41). The relatively narrow CI for the binding of antibody to asialo-GM1 to itself, to BSA, and to the two P. aeruginosa LPS indicates the presence of a population of mostly high-affinity antibodies in the preparation.
TABLE 2.
Calculated antibody titers in antisera raised to asialo-GM1, GM1, or the P. aeruginosa complete-core LPS from strain PAC 557
| Antigen | Titera (95%
CI) in antisera raised to:
|
|||
|---|---|---|---|---|
| Asialo-GM1 | GM1 | PA complete core LPS | Normal rabbit serum | |
| Asialo-GM1 | 91,833 (86,657–97,095) | 2,113 (1,432–4,442) | 719 (630–5,175) | <100 |
| GM1 | 4,550 (4,029–7,592) | 4,842 (4,029–5,123) | 3,908 (1,070–14,328) | <100 |
| Reduced GM1 | 853 (467–11,239) | 2,685 (1,763–3,607) | <100 | <100 |
| BSA | 38,815 (35,793–41,957) | 32,734 (30,535–35,108) | 455 (148–1,389) | <100 |
| PA serogroup O6 LPS | 21,627 (17,037–26,546) | 8,299 (3,822–18,030) | 136,773 (126,067–148,593) | <100 |
| PA complete-core LPS | 17,140 (10,607–27,708) | 16,218 (4,684–56,208) | 106,414 (106,316–153,108) | 454 (150–1,379) |
Titer and 95% CI values were calculated by linear regression. All titers are based on a significant dose-response effect, as determined by the F > t value of the associated ANOVA calculation with P values of 0.009 to 0.0001.
Antisera raised to GM1 had modest titers of antibody to itself, to asialo-GM1, and to reduced GM1 and had a high titer with a narrow CI to BSA (Table 2). The modest (1.8-fold) but significant (nonoverlapping 95% CI) decrease in titer of antibody to reduced GM1 indicates that the sialic acid residue on GM1 contributed to binding for about half of the antibodies in this antiserum. The serum raised to GM1 had an even higher titer to the P. aeruginosa LPS antigens from serogroup O6 and strain PAC557 than to itself, but the somewhat wider CIs suggested that these were not all high-affinity antibodies. As expected, antisera raised to the P. aeruginosa LPS core had a high titer and narrow CI to itself and cross-reactive antibodies to shared antigens in the serogroup O6 LPS but low antibody titers to both BSA and asialo-GM1 and modest titers to GM1. The wide relative CI (>8-fold) for the titers to the gangliosides is indicative of relatively low affinity, cross-reactive antibodies in the serum. Normal rabbit sera had titers of <100 to all of the antigens except the complete-core LPS from P. aeruginosa strain PAC557. Overall, the antibody titers indicate that some component of the asialo-GM1 immunogen (which included asialo-GM1 prepared from bovine brain, methylated BSA, and complete Freund's adjuvant) elicited high-affinity antibodies to epitopes in P. aeruginosa LPS but that antigenic determinants capable of eliciting antibody to asialo-GM1 were present at low levels or were poorly immunogenic in the P. aeruginosa complete-core LPS immunogen, eliciting only low-affinity antibodies.
Further investigations into the reactivity of the antibodies raised to asialo-GM1 showed extensive, high-titered binding to P. aeruginosa pili from strain PAK, incomplete-core LPS isolated from strain PAO1 algC::tet, and whole cells of P. aeruginosa strains PAO1 algC::tet and PAC557 (Table 3). Anti-asialo-GM1 bound strongly to pili, whereas antisera raised in rabbits to P. aeruginosa LPS from strains PAC557 and PAO1 algC::tet were only slightly more reactive than normal rabbit serum. Antibody to asialo-GM1 bound strongly to purified incomplete core LPS from P. aeruginosa strain PAO1 algC::tet, and higher titers were achieved with antisera to strain PAC557 LPS and homologous antibody to PAO1 algC::tet LPS. Antibodies to asialo-GM1 had high levels of binding to whole bacteria of P. aeruginosa strains PAC557 and PAO1 algC::tet, with essentially superimposable results, as evidenced by the nearly identical titers and identical CIs. The narrow CIs for the calculated titer of anti-asialo-GM1 antibody to the P. aeruginosa antigens indicate that these are high-affinity antibodies. As expected, antisera to purified LPS bound best to lyophilized bacteria expressing the immunizing LPS antigen, with good cross-reactivity between the antisera raised to the two different LPS and the heterologous P. aeruginosa strain.
TABLE 3.
Calculated antibody titers to P. aeruginosa pili, incomplete core LPS, and whole bacterial cells in antisera raised to asialo-GM1, the P. aeruginosa complete-core LPS from strain PAC 557, or the incomplete core from P. aeruginosa PAO1 algC::tet
| Antigen | Titera (95%
CI) in antisera raised to:
|
|||
|---|---|---|---|---|
| Asialo-GM1 | PA complete-core LPS | PA incomplete-core LPS | Normal rabbit serum | |
| P. aeruginosa pili | 10,116 (6,051–18,923) | 692 (391–1,225) | 521 (20–1,361) | <200 |
| Incomplete-core LPS | 26,363 (22,646–30,690) | 97,949 (93,541–102,329) | 117,490 (109,144–126,474) | <200 |
| PAC 557 whole cells | 19,320 (15,776–23,659) | 5,834,451 (4,174,457–8,143,292) | 36,644 (33,497–40,087) | <200 |
| PAO1 algC::tet whole cells | 19,275 (15,776–23,659) | 26,915 (22,699–31,989) | 66,096 (60,674–71,779) | <200 |
Titer and 95% CI values were calculated by linear regression. All titers were based on a significant dose-response effect, as determined by the F > t value of the associated ANOVA calculation with P values of 0.03 to 0.0001.
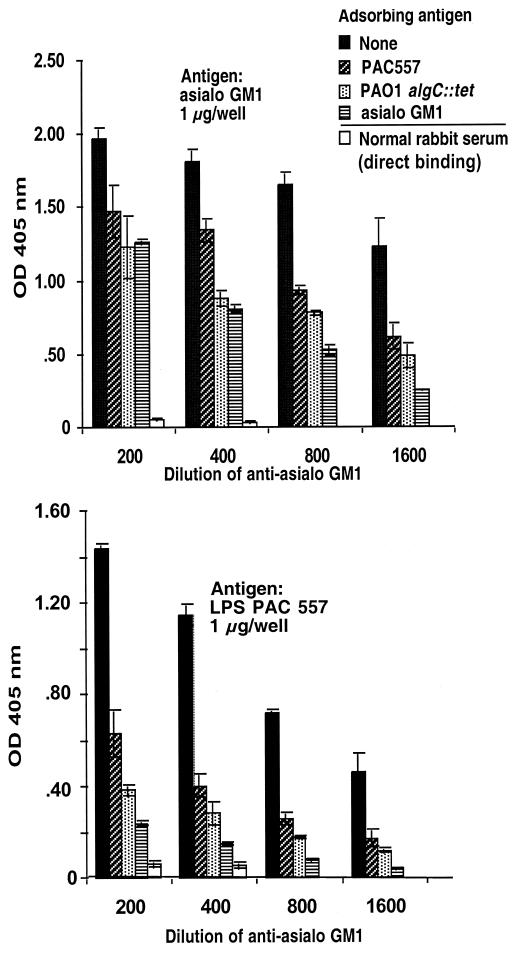
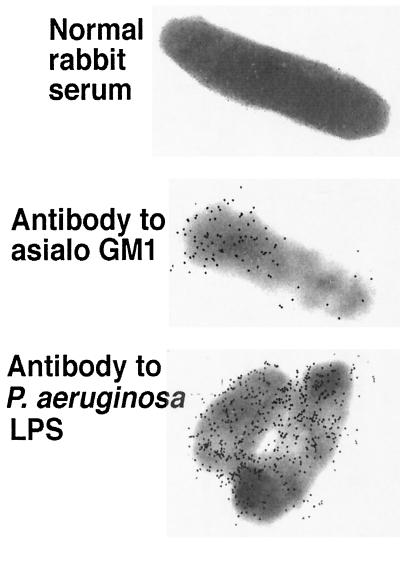
Adsorption studies confirmed that antisera raised to asialo-GM1 contained a heterogeneous mixture of antibodies to both the immunizing antigen and P. aeruginosa and that a significant portion were not only high-affinity, but also cross-reactive, antibodies. Twofold dilutions of anti-asialo-GM1 from 1:200 to 1:1,600 were adsorbed with the homologous immunogen or with whole cells of P. aeruginosa strains PAC557 or PAO1 algC::tet and then tested by ELISA for residual binding to asialo GM1 (Fig. 4). Although asialo-GM1 adsorbed out the reactivity to itself best at all of the serum dilutions tested, both P. aeruginosa strains also adsorbed out significant amounts of antibody specific to asialo-GM1. Comparisons of the goodness of fit (R2) values for the regression curves plotting the ratios of the OD405 of the adsorbed to unadsorbed serum versus the serum dilutions gave values of 0.84 for the asialo-GM1 inhibitor, 0.87 for P. aeruginosa strain PAC 557, and 0.77 for strain PAO1 algC::tet. Thus, the P. aeruginosa strains were comparable to asialo-GM1 in their ability to adsorb out specific antibodies to this antigen in its homologous antiserum. Similarly, when the same adsorbed sera were tested for binding to LPS from strain PAC557 LPS, there was a significant reduction in binding when the serum was absorbed with asialo-GM1 (R2 = 0.87). This analysis indicates the ability of asialo-GM1 antigen to elicit antibodies with a high degree of cross-reactivity between itself and P. aeruginosa LPS, although there are clearly non-cross-reactive antibodies specific to asialo-GM1 present as well (see titers in antisera to asialo-GM1 given in Table 2). Interestingly, there may be a better ability of asialo-GM1 to elicit antibodies to P. aeruginosa LPS than there is for the LPS to elicit antibodies to asialo-GM1, since there are no appreciable titers of antibody to asialo-GM1 (Tables 2 and 3) in antisera raised to the LPS. Alternately, the methylated BSA and components of complete Freund's adjuvant present in the asialo-GM1 immunizing mixture may be responsible for eliciting antibodies to P. aeruginosa antigens. Electron microscopic studies confirmed that antibodies raised to asialo-GM1 bound to P. aeruginosa cell surface antigens (Fig. 5).
FIG. 4.
Inhibition of binding of rabbit antibodies raised to asialo-GM1 to asialo-GM1 antigen or P. aeruginosa complete-core LPS from strain PAC557. The antigen (whole bacteria or purified asialo-GM1) used to adsorb antibody preparation is indicated in the legend to the figure. The bars represent the means; the error bars are the standard errors. Regardless of the target antigen, all adsorbing antigens reduced the antibody binding significantly (P < 0.001, ANOVA and Fisher PLSD). The binding of unabsorbed normal rabbit serum is shown for comparison.
FIG. 5.
Demonstration by immunoelectron microscopy of binding of antibodies in antisera raised to asialo-GM1 plus adjuvants to P. aeruginosa strain PAO1-I.
Effect of antibodies to asialo-GM1 on formation of P. aeruginosa biofilms.
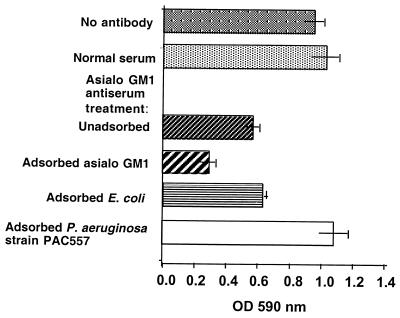
Binding of antibody to P. aeruginosa antigens by antibodies in the serum raised to asialo-GM1 suggested that inhibition of binding of P. aeruginosa to eukaryotic cells by such sera (9, 11, 24, 37, 39) might be confounded by a factor such as the inhibition of bacterial cell-cell interactions rather than to the inhibition of bacterial cell interactions with mammalian cells. Using the method of O'Toole and Kolter (34), we evaluated this possibility by adding the antibody to asialo-GM1 to cultures of P. aeruginosa PAO1-V forming a biofilm and measuring the results 10 h later. Antibody to asialo-GM1 readily reduced the formation of a P. aeruginosa biofilm, and this effect was not abrogated by adsorption with either asialo-GM1 antigen (P = 0.2, Fisher PLSD) or E. coli (P = 0.7, Fisher PLSD; Fig. 6.) In contrast, adsorption of this antiserum with the heterologous P. aeruginosa strain PAC557 eliminated the biofilm-reducing capacity of antibody to asialo-GM1 (P < 0.001, Fisher PLSD). This result indicates that the antisera raised to asialo-GM1 in methylated BSA and complete Freund's adjuvant reduces P. aeruginosa cell-cell interactions—a possible basis for the apparent inhibition of P. aeruginosa binding to mammalian cells in the presence of these antibodies.
FIG. 6.
Effect of antibodies in sera raised to asialo-GM1 plus adjuvants on formation of a biofilm by P. aeruginosa PAO1-V. The addition of antibody to asialo-GM1 significantly (P = 0.003, ANOVA and Fisher PLSD) reduced the formation of the biofilm compared with the formation in the absence of antibody or in the presence of normal serum. Adsorption of antibody with either asialo-GM1 antigen or E. coli DH5α, but not adsorption with P. aeruginosa strain PAC557, abrogated the inhibition of biofilm formation (P < 0.001, Fisher PLSD compared to unabsorbed serum). Bars represent the means; error bars are the standard errors.
DISCUSSION
Although numerous studies have implicated asialo-GM1 as a cellular adhesin for P. aeruginosa (7, 10, 16, 20, 22, 24, 37, 39, 42, 44, 47, 52), involving flagella (13), pili (38, 42, 50), and possibly LPS (16), almost all of these studies have been conducted with laboratory strains PA103, PAO1, and PAK. Some studies have also used two other laboratory strains, ATCC 19660 and PA1244, but the latter strain was observed to be hyperpiliated (21), a phenotype that decreases the cellular adherence of P. aeruginosa. Few, if any, studies have been carried out with minimally passaged clinical isolates. Using the recently described technique of Comolli et al. (7), we confirmed that asialo-GM1 can be added to the surface of MDCK cells growing in Transwells and that this treatment significantly enhanced binding of strain PA103 to the cells. However, no such enhancement was obtained with any other strain, particularly fresh clinical isolates. While it is possible than endogenous expression of asialo-GM1 residues on cells is different from what we achieved by membrane incorporation, it is difficult to see how this difference would manifest itself in the outcomes from simple adherence assays. Even if true, studies testing P. aeruginosa binding to endogenous asialo-GM1 on cells have, for the most part, not used clinical isolates. We therefore conclude that asialo-GM1 serves as an epithelial cell receptor principally for some laboratory passed strains of P. aeruginosa but not for clinical isolates.
Several studies have identified asialo-GM1 and, to a lesser extent, GM1 as receptors for P. aeruginosa pili (12, 19, 32, 38, 39, 44, 49), and Comolli et al. (7), whose system we used in the present study, previously found that P. aeruginosa adherence, cytotoxicity, and invasion of MDCK cells treated with asialo-GM1 were dependent on expression of type IV pili. However, the recent reporting of the crystal structure of the pilin of strain PAK (19), which is highly homologous to that of strain PAO1 (31, 44), and other P. aeruginosa strains (5), including PA103 (26), reveals that the previously identified disulfide-folded C-terminal tip of the pilin that binds to the GalNAc-Gal disaccharide in asialo-GM1 is either not surface exposed at all or not surface exposed as a multivalent polymer needed for high-affinity binding to the GalNac-Gal residues. While these authors are careful to point out that there are potential conformational or other changes that could occur in the pilin subunit to bring multivalent GalNac-Gal binding sites to the surface, Hazes et al. state that “given the current data, we cannot conceive of a pilus model that would provide a multivalent membrane-proximal binding surface” (19) to interact with GalNAc-Gal residues.
Others also have found pili to be important in the overall adherence of P. aeruginosa to mammalian cells (6, 17, 27, 46), and our results do not contradict this finding, except in the context of the pilus–asialo-GM1 interaction, which does not appear to be a primary mediator of adherence of clinical isolates of P. aeruginosa to epithelial cells. Two reports of the potential protective efficacy of antibodies to P. aeruginosa pili in murine models of infection have indicated that antibodies directed to epitopes that interrupt bacterial adherence in vitro are also protective (4, 43). However, there are no indications that these antibodies work by interrupting bacterial adherence in vivo. An analogous receptor-ligand interaction found in Staphylococcus aureus, that of fibronectin-binding protein and fibronectin, has also shown protective efficacy against S. aureus infection in mice (40), but these antibodies were shown to function as opsonins for phagocytosis. Thus, protection by antibody to P. aeruginosa pili does not necessarily indicate that the antibodies function by interrupting bacterial cell-epithelial cell interactions. Also, we found that antibodies to LPS, but not to pili, prevent colonization by P. aeruginosa of the murine gastrointestinal tract following antibiotic treatment to deplete resident aerobic microbes (35).
A prominent hypothesis to explain the hypersusceptibility of CF patients to infection has proposed that asialo-GM1 receptors are increased on the surface of respiratory epithelial cells of CF patients, allowing for increased binding of P. aeruginosa to these cells (2, 24, 37, 39). The studies reporting increased binding of P. aeruginosa to CF cells have not evaluated clinical isolates of P. aeruginosa obtained from patients with CF early in the course of infection. Almost all of the studies have been carried out with strain PAO1, and we found one variant of this strain, PAO1-I, that showed about a 50% increase in binding to MDCK cells treated with asialo-GM1, an increase in the range reported by Prince and co-workers in their studies, (2, 24, 39). Thus, the lack of statistical significance to our results could reasonably be attributed to greater variation in our outcomes compared with those of other investigators. However, we also showed that reduced GM1, lacking the carboxylic acid moiety of the sialic acid, promoted even greater adherence of PAO1-I to MDCK cells, thus causing us to question the specificity of this interaction for asialo-GM1. de Bentzmann et al. (10) have reported that expression of asialo-GM1 on intact CF respiratory epithelium is not increased compared with that on non-CF tissues but that expression of asialo-GM1 is increased on injured, regenerating CF epithelium compared with that on non-CF epithelium. They proposed that damage to respiratory tissues in CF sets the stage for P. aeruginosa colonization, but there are no data to support this idea. In addition, they carried out these studies using only strain PAO1 to document the role of asialo-GM1 receptors in the binding of P. aeruginosa to respiratory epithelium. Of additional interest is the finding by this same group that a clinical isolate of P. aeruginosa uses integrins and fibronectin to adhere to the dedifferentiated epithelial cells undergoing migration for repair after injury and during epithelial surface regeneration (36).
Our results clearly show the need to evaluate clinical isolates of P. aeruginosa in experiments designed to determine a role for adhesins in pathogenesis. Prior to this report, there were two major reasons to question the role of asialo-GM1 receptors on CF cells as a contributor to increased binding and susceptibility of patients with CF to infection. One was the rather modest differences in binding of P. aeruginosa to CF and non-CF cells that occurred only when the ratio of bacteria to mammalian cells was very high. For example, Bryan et al. (2) reported no difference in the binding of 106 CFU of P. aeruginosa to a wild-type respiratory cell line and one with a “CF phenotype” caused by overproduction of the regulatory domain of CFTR. Significant differences were noted only at inocula of 107 or 108 CFU, and only two- to threefold more CF epithelial cells than wild-type cells bound P. aeruginosa. Similar small differences of two- to threefold-increased binding of P. aeruginosa PAO1 to CF compared with non-CF cells were found when using determinations of the number of bound P. aeruginosa per cell; again, this was evident only with a high inoculum (107 CFU) (24). Also, a difference in binding of P. aeruginosa PAO1 to cultured nasal epithelial cells for CF patients homozygous for the ΔF508 CFTR allele was barely twofold greater than binding to cells from heterozygous carriers or normal subjects, and an inoculum of 5 × 108 CFU/ml was necessary for this difference to be observed. It seems highly unlikely that humans are ever naturally exposed to the doses of P. aeruginosa required to demonstrate differences in binding between CF and non-CF cells, meaning that at lower, more realistic, inocula, the binding of P. aeruginosa to CF and non-CF epithelial cells would be comparable, thus eliminating any colonization advantage for the bacterium in the CF lung due to increased levels of asialo-GM1. A second major concern is that Zar et al. (51) clearly showed no difference in the binding of P. aeruginosa PAO1 to cultured epithelial cells from CF patients who were not homozygous for the ΔF508 CFTR allele but had other mutations. Nonetheless, P. aeruginosa disease in these CF patients was comparable to that in CF patients homozygous for this allele (51). It is difficult to accept a role of increased binding of P. aeruginosa to asialo-GM1: in the pathogenesis of CF lung disease if this difference is manifest in only one subset of CF patients: the 49% who are homozygous for theΔF508 CFTR allele.
The other major experimental protocol used to document a role for binding of P. aeruginosa to asialo-GM1 involves the inhibition of binding to target cells with antibodies to asialo-GM1. These antibodies are available from commercial suppliers but were never reported to have been tested for specificity or titer. We found that antibodies from Wako Pure Chemicals, used in numerous other studies (2, 10, 24, 39), had high levels of antibody to multiple P. aeruginosa antigens and to BSA. None of the previous studies (7, 10, 16, 20, 22, 24, 37, 39, 42, 44, 47, 52) that used antibodies to asialo-GM1 to confirm the involvement of this receptor in P. aeruginosa binding conducted specificity studies whereby the antisera were adsorbed or inhibited with purified asialo-GM1, and none measured antibodies to P. aeruginosa antigens in the antiserum to asialo-GM1. It is abundantly clear how the antisera raised to multiple doses of asialo-GM1 purified from bovine brain emulsified in complete Freund's adjuvant and methylated BSA could contain high titers of antibodies cross-reactive to bacterial antigens or to BSA or other bovine antigens present in FCS and adsorbed onto bacterial surfaces. In both cases, the antibodies to P. aeruginosa antigens or to bovine antigens in the antisera could easily agglutinate the P. aeruginosa organisms, and if the agglutinated organisms are not fully dispersed prior to diluting and plating them for enumeration, there will be an apparent reduction in bacterial binding to cells.
Another possible way in which antisera raised to asialo-GM1 and containing high titers of antibody to P. aeruginosa could function to give an apparent reduction in P. aeruginosa adherence in epithelial cell binding assays is to interrupt bacterial cell-cell interactions. We thus used a biofilm assay to measure the effects of antibodies in sera raised to asialo-GM1 on cell-cell interactions. We showed that antibody to asialo-GM1 inhibited biofilm formation by P. aeruginosa and that the inhibitory antibodies were not neutralized when free asialo-GM1 was added to the serum but were removed by adsorption of the antiserum with P. aeruginosa cells.
Some assays showing inhibition of P. aeruginosa binding to epithelial cell surface asialo-GM1 add the antibodies to the cells to block the receptor that are then washed away prior to the addition of bacteria. In spite of this methodologic approach, it is possible that antibodies in asialo-GM1 antisera to other antigens could perturb the bacterial-mammalian cell interaction in ways other then by blocking P. aeruginosa access to asialo-GM1, e.g., by binding to a different receptor on the epithelial cell surface. Interestingly, we did not observe any binding of antibodies to the surface of MDCK cells by immunofluorescence in sera raised to asialo-GM1. However, immunofluorescence may not be a sensitive enough technique to detect antibodies to mammalian epithelial cell antigens or low levels of BSA bound to the cell surfaces. Along these lines, recent reports indicating that antibodies to asialo-GM1 could mimic the epithelial responses achieved when P. aeruginosa is added to the cells also lacked any specificity controls (11), and the possibility that the antisera reacted with cellular antigens or with BSA bound to the cultured cells was not adequately excluded. Thus, the contention that differences in P. aeruginosa binding and activation of CF cell compared to non-CF cells (2, 24, 37, 39) involve asialo-GM1 are not supported by studies that show that the differences were due to antibodies specific to asialo-GM1. Clearly, the antiserum raised to asialo-GM1 by commercial vendors needs to be checked for antigenic specificity in any assay in which it is used.
Overall, our results indicate that asialo-GM1 is a receptor for one laboratory strain of P. aeruginosa but not for fresh clinical isolates. We confirmed that the method of Comolli et al. (7) for adding asialo-GM1 to the surface of MDCK cells was effective, as well as their report that this treatment increased the binding of strain PA103 to these cells. The addition of the asialo-GM1 to the cell surface should readily increase binding of other P. aeruginosa strains to the cell, yet this was not found. Furthermore, we documented the presence of high titers of antibody to P. aeruginosa and BSA in commercially obtained antisera to asialo-GM1, showing the requirement for specificity studies when using these reagents. Few, if any, other studies evaluating P. aeruginosa adherence to epithelial cells and asialo-GM1 used strains other than laboratory ones. The need to be sure that any experimental results obtained with laboratory strains can also be obtained with fresh clinical isolates is clear. In the absence of bacterial adherence studies using fresh clinical isolates of P. aeruginosa and well-characterized antibodies, conclusions regarding a role for asialo-GM1 as a receptor for P. aeruginosa binding cannot be supported.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI22806 and HL58398 to G.B.P. and by a grant to T.H.S. from the Walter-Marget Foundation in Germany.
REFERENCES
- 1.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and characterization of Pseudomonas aeruginosa fliF, necessary for flagellar assembly and bacterial adherence to mucin. Infect Immun. 1996;64:2130–2136. doi: 10.1128/iai.64.6.2130-2136.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan R, Kube D, Perez A, Davis P, Prince A. Overproduction of the CFTR R domain leads to increased levels of asialoGM1 and increased Pseudomonas aeruginosabinding by epithelial cells. Am J Respir Cell Mol Biol. 1998;19:269–277. doi: 10.1165/ajrcmb.19.2.2889. [DOI] [PubMed] [Google Scholar]
- 3.Cacalano G, Kays M, Saiman L, Prince A. Production of the Pseudomonas aeruginosaneuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J Clin Investig. 1992;89:1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cachia P J, Glasier L M, Hodgins R R, Wong W Y, Irvin R T, Hodges R S. The use of synthetic peptides in the design of a consensus sequence vaccine for Pseudomonas aeruginosa. J Peptide Res. 1998;52:289–299. doi: 10.1111/j.1399-3011.1998.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 5.Castric P A, Deal C D. Differentiation of Pseudomonas aeruginosapili based on sequence and B-cell epitope analyses. Infect Immun. 1994;62:371–376. doi: 10.1128/iai.62.2.371-376.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervin M A, Simpson D A, Smith A L, Lory S. Differences in eukaryotic cell binding of Pseudomonas. Microb Pathog. 1994;17:291–299. doi: 10.1006/mpat.1994.1075. [DOI] [PubMed] [Google Scholar]
- 7.Comolli J C, Waite L L, Mostov K E, Engel J N. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect Immun. 1999;67:3207–3214. doi: 10.1128/iai.67.7.3207-3214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyne M J, Russell K S, Coyle C L, Goldberg J B. The Pseudomonas aeruginosa algCgene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J Bacteriol. 1994;176:3500–3507. doi: 10.1128/jb.176.12.3500-3507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J, Dewar A, Bush A, Pitt T, Gruenert D, Geddes D M, Alton E W. Reduction in the adherence of Pseudomonas aeruginosa to native cystic fibrosis epithelium with anti-asialoGM1antibody and neuraminidase inhibition. Eur Respir J. 1999;13:565–570. doi: 10.1183/09031936.99.13356599. [DOI] [PubMed] [Google Scholar]
- 10.de Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski M C, Puchelle E. Asialo-GM1 is a receptor for Pseudomonas aeruginosaadherence to regenerating respiratory epithelium. Infect Immun. 1996;64:1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMango E, Ratner A J, Bryan R, Tabibi S, Prince A. Activation of NF-kappa B by adherent Pseudomonas aeruginosain normal and cystic fibrosis respiratory epithelial cells. J Clin Investig. 1998;101:2598–2605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farinha M A, Conway B D, Glasier L M G, Ellert N W, Irvin R T, Sherburne R, Paranchych W. Alteration of the pilin adhesin of Pseudomonas aeruginosaPAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect Immun. 1994;62:4118–4123. doi: 10.1128/iai.62.10.4118-4123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosapulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosacorrelates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 15.Goosney D L, Knoechel D G, Finlay B B. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis. 1999;5:216–223. doi: 10.3201/eid0502.990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S K, Berk R S, Masinick S, Hazlett L D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo-GM1. Infect Immun. 1994;62:4572–4579. doi: 10.1128/iai.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn H P. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene. 1997;192:99–108. doi: 10.1016/s0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 18.Hatano K, Goldberg J B, Pier G B. Pseudomonas aeruginosalipopolysaccharide-evidence that the O-side chains and common antigens are on the same molecule. J Bacteriol. 1993;175:5117–5128. doi: 10.1128/jb.175.16.5117-5128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazes B, Sastry P A, Hayakawa K, Read R J, Irvin R T. Crystal structure of Pseudomonas aeruginosaPAK pilin suggests a main-chain-dominated mode of receptor binding. J Mol Biol. 2000;299:1005–1017. doi: 10.1006/jmbi.2000.3801. [DOI] [PubMed] [Google Scholar]
- 20.Hazlett L D, Masinick S, Barrett R, Rosol K. Evidence for asialo-GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosaadhesion. Infect Immun. 1993;61:5164–5173. doi: 10.1128/iai.61.12.5164-5173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazlett L D, Moon M M, Singh A, Berk R S, Rudner X L. Analysis of adhesion, piliation, protease production and ocular infectivity of several P. aeruginosastrains. Curr Eye Res. 1991;10:351–362. doi: 10.3109/02713689108996341. [DOI] [PubMed] [Google Scholar]
- 22.Hobden J A, Gupta S K, Masinick S A, Wu X, Kernacki K A, Berk R S, Hazlett L D. Anti-receptor antibodies inhibit Pseudomonas aeruginosabinding to the cornea and prevent corneal perforation. Immunol Cell Biol. 1996;74:258–264. doi: 10.1038/icb.1996.46. [DOI] [PubMed] [Google Scholar]
- 23.Huebner J, Wang Y, Krueger W A, Madoff L C, Martirosian G, Boisot S, Goldmann D A, Kasper D L, Tzianabos A O, Pier G B. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect Immun. 1999;67:1213–1219. doi: 10.1128/iai.67.3.1213-1219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci USA. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joh D, Wann E R, Kreikemeyer B, Speziale P, Hook M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 26.Johnson K, Parker M L, Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosapilin genes. J Biol Chem. 1986;261:15703–15708. [PubMed] [Google Scholar]
- 27.Kang P J, Hauser A R, Apodaca G, Fleiszig S M J, WienerKronish J, Mostov K, Engel J N. Identification of Pseudomonas aeruginosagenes required for epithelial cell injury. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 28.Keleti G, Lederer W H. Handbook of micromethods for the biological sciences. New York, N.Y: Van Nostrand Reinhold Co.; 1974. [Google Scholar]
- 29.Koval S F, Meadow P M. The isolation and characterization of lipopolysaccharide-defective mutants of Pseudomonas aeruginosaPAC1. J Gen Microbiol. 1977;98:387–398. doi: 10.1099/00221287-98-2-387. [DOI] [PubMed] [Google Scholar]
- 30.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4 Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K K, Sheth H B, Wong W Y, Sherburne R, Paranchych W, Hodges R S, Lingwood C A, Krivan H, Irvin R T. The binding of Pseudomonas aeruginosapili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee K K, Yu L, Macdonald D L, Paranchych W, Hodges R S, Irvin R T. Anti-adhesin antibodies that recognize a receptor-binding motif (adhesintope) inhibit pilus/fimbrial-mediated adherence of Pseudomonas aeruginosa and Candida albicans to asialo-GM1receptors and human buccal epithelial cell surface receptors. Can J Microbiol. 1996;42:479–486. doi: 10.1139/m96-065. [DOI] [PubMed] [Google Scholar]
- 33.Novak R, Tuomanen E. Pathogenesis of pneumococcal pneumonia. Semin Respir Infect. 1999;14:209–217. [PubMed] [Google Scholar]
- 34.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosabiofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 35.Pier G B, Meluleni G, Goldberg J B. Clearance of Pseudomonas aeruginosafrom the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun. 1995;63:2818–2825. doi: 10.1128/iai.63.8.2818-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roger P, Puchelle E, Bajolet-Laudinat O, Tournier J M, Debordeaux C, Plotkowski M C, Cohen J H, Sheppard D, de Bentzmann S. Fibronectin and α5β1 integrin mediate binding of Pseudomonas aeruginosato repairing airway epithelium. Eur Respir J. 1999;13:1301–1309. [PubMed] [Google Scholar]
- 37.Saiman L, Cacalano G, Gruenert D, Prince A. Comparison of adherence of Pseudomonas aeruginosato respiratory epithelial cells from cystic fibrosis patients and healthy subjects. Infect Immun. 1992;60:2808–2814. doi: 10.1128/iai.60.7.2808-2814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saiman L, Ishimoto K, Lory S, Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosato respiratory epithelial monolayers. J Infect Dis. 1990;161:541–548. doi: 10.1093/infdis/161.3.541. [DOI] [PubMed] [Google Scholar]
- 39.Saiman L, Prince A. Pseudomonas aeruginosapili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Investig. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schennings T, Heimdahl A, Coster K, Flock J I. Immunization with fibronectin binding protein from Staphylococcus aureusprotects against experimental endocarditis in rats. Microb Pathog. 1993;15:227–236. doi: 10.1006/mpat.1993.1073. [DOI] [PubMed] [Google Scholar]
- 41.Schots A, Van der Leede B J, De Jongh E, Egberts E. A method for the determination of antibody affinity using a direct ELISA. J Immunol Methods. 1988;109:225–233. doi: 10.1016/0022-1759(88)90247-5. [DOI] [PubMed] [Google Scholar]
- 42.Schweizer F, Jiao H, Hindsgaul O, Wong W Y, Irvin R T. Interaction between the pili of Pseudomonas aeruginosa PAK and its carbohydrate receptor beta-d-GalNAc(1→4)beta-d-Gal analogs. Can J Microbiol. 1998;44:307–311. [PubMed] [Google Scholar]
- 43.Sheth H B, Glasier L M, Ellert N W, Cachia P, Kohn W, Lee K K, Paranchych W, Hodges R S, Irvin R T. Development of an anti-adhesive vaccine for Pseudomonas aeruginosatargeting the C-terminal region of the pilin structural protein. Biomed Peptides Prot Nucleic Acids. 1995;1:141–148. [PubMed] [Google Scholar]
- 44.Sheth H B, Lee K K, Wong W Y, Srivastava G, Hindsgaul O, Hodges R S, Paranchych W, Irvin R T. The pili of Pseudomonas aeruginosastrains PAK and PAO bind specifically to the carbohydrate sequence beta GalNAc(1-4)beta Gal found in glycosphingolipids asialo-GM1 and asialo-GM2. Mol Microbiol. 1994;11:715–723. doi: 10.1111/j.1365-2958.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 45.Simpson D A, Ramphal R, Lory S. Characterization of Pseudomonas aeruginosa fliO, a gene involved in flagellar biosynthesis and adherence. Infect Immun. 1995;63:2950–2957. doi: 10.1128/iai.63.8.2950-2957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson D A, Ramphal R, Lory S. Genetic analysis of Pseudomonas aeruginosaadherence—Distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992;60:3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh A, Hazlett L, Berk R S. Characterization of pseudomonal adherence to unwounded cornea. Investig Ophthalmol Vis Sci. 1991;32:2096–2104. [PubMed] [Google Scholar]
- 48.Soto G E, Hultgren S J. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999;181:1059–1071. doi: 10.1128/jb.181.4.1059-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong W Y, Campbell A P, McInnes C, Sykes B D, Paranchych W, Irvin R T, Hodges R S. Structure-function analysis of the adherence-binding domain on the pilin of Pseudomonas aeruginosastrains PAK and KB7. Biochemistry. 1995;34:12963–12972. doi: 10.1021/bi00040a006. [DOI] [PubMed] [Google Scholar]
- 50.Yu L, Lee K K, Hodges R S, Paranchych W, Irvin R T. Adherence of Pseudomonas aeruginosa and Candida albicansto glycosphingolipid (asialo-GM1) receptors is achieved by a conserved receptor-binding domain present on their adhesins. Infect Immun. 1994;62:5213–5219. doi: 10.1128/iai.62.12.5213-5219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zar H, Saiman L, Quittell L, Prince A. Binding of Pseudomonas aeruginosato respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995;126:230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z, Panjwani N. Pseudomonas aeruginosa infection of the cornea and asialo GM1. Infect Immun. 1995;63:353–355. doi: 10.1128/iai.63.1.353-355.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]