Abstract
During the COVID-19 pandemic, some countries, such as Australia, China, Iceland, New Zealand, Thailand, and Vietnam successfully implemented an elimination strategy, enacting strict border control and periods of lockdowns to end community transmission. Atlantic Canada and Canada’s territories implemented similar policies, and reported long periods with no community cases. In Newfoundland and Labrador (NL), Nova Scotia, and Prince Edward Island a median of 80% or more of daily reported cases were travel-related from July 1, 2020 to May 31, 2021. With increasing vaccination coverage, it may be appropriate to exit an elimination strategy, but most existing epidemiological frameworks are applicable only to situations where most cases occur in the community, and are not appropriate for regions that have implemented an elimination strategy. To inform the pandemic response in regions that are implementing an elimination strategy, we extend importation modelling to consider post-arrival travel restrictions, and pharmaceutical and non-pharmaceutical interventions in the local community. We find that shortly after the Omicron variant had begun spreading in Canada, the expected daily number of spillovers, infections spread to NL community members from travellers and their close contacts, was higher than any time previously in the pandemic. By December 24, 2021, the expected number of spillovers was 44% higher than the previous high, which occurred in late July 2021 shortly after travel restrictions were first relaxed. We develop a method to assess the characteristics of potential future community outbreaks in regions that are implementing an elimination strategy. We apply this method to predict the effect of variant and vaccination coverage on the size of hypothetical community outbreaks in Mount Pearl, a suburb of the St. John’s metropolitan area in NL. Our methodology can be used to evaluate alternative plans to relax public health restrictions when vaccine coverage is high in regions that have implemented an elimination strategy. This manuscript was submitted as part of a theme issue on “Modelling COVID-19 and Preparedness for Future Pandemics”.
Keywords: Importations, COVID-zero, Elimination, SARS-CoV-2, Atlantic Canada, COVID-19, Pandemic preparedness, Travel-related, Reopening
1. Introduction
To manage SARS-CoV-2 infections, countries including Australia, China, Iceland, New Zealand, Thailand, and Vietnam used an elimination approach (also known as a zero-COVID policy). This approach combines strong border control to diminish travel-related cases with pharmaceutical (PIs) and non-pharmaceutical interventions (NPIs) that reduce or completely end community transmissions if border measures fail (Baker et al., 2020b, Heywood and Macintyre, 2020). Elimination differs from eradication in that its intended region of influence is localized, typically to the jurisdiction pursuing the goal. This policy was also used in infranational jurisdictions such as Atlantic Canada and Canada’s territories (Bignami, 2021, Contandriopoulos, 2021, Department of Health and Community Services, N.L., 2022).
Until the end of 2021, countries that used an elimination strategy had less SARS-CoV-2 mortality (Baker et al., 2020a, Nam et al., 2020) and less stringent local restrictions when there were no community cases, which resulted in less psychological distress (Aknin et al., 2022). Regions that implemented elimination strategies may have also had stronger economies (König and Winkler, 2021). Newfoundland and Labrador (NL), which implemented a containment approach (Department of Health and Community Services, N.L., 2022), achieved prolonged periods with no community cases and low SARS-CoV-2 mortality: 3.6 SARS-CoV-2 deaths per 100,000 people in NL, compared to 78.1 SARS-CoV-2 deaths per 100,000 people in Canada from the beginning of the pandemic until December 31, 2021 (NL: 19 deaths (Government of Newfoundland and Labrador, 2021a) for a provincial population of 521,854 people (Statistics Canada, 2021); Canada: 30,024 SARS CoV-2 deaths (Public Health Agency of Canada, 2022) for a national population of 38,426,473 (Statistics Canada, 2021). Success similar to that of NL occurred throughout Atlantic Canada and in Canada’s territories.
The feasibility of an elimination strategy depends on vaccine availability and uptake. Hong Kong had low numbers of SARS-CoV-2 cases through strict border control, quarantine and NPIs, but did not vaccinate abundantly, which exposed the population to severe disease outcomes when the Omicron variant emerged (Ma and Parry, 2022). The feasibility of an elimination strategy may depend on variant characteristics and jurisdictional geographic and social characteristics (Silver, 2022, Martignoni and Hurford, 2022, Department of Health and Community Services, N.L., 2022). In early 2022, following the establishment of the Omicron variant, NL shifted from a containment to a mitigation approach (Department of Health and Community Services, N.L., 2022), with many of the jurisdictions that had implemented an elimination strategy responding similarly (a notable exception is China who continued to pursue an elimination strategy). The elimination strategy is most likely appropriate in specific locations and for specific periods of time as the costs and benefits of the strategy likely depend on complex interactions between regional characteristics, public health policy, community behavioural responses, and variant epidemiological characteristics.
While implementing an elimination strategy, it is important to develop tools to assess the risk of community outbreaks, to evaluate whether border controls should be upscaled or released. In the following, we define an importation as an individual who arrives in the local jurisdiction from another jurisdiction while infected with SARS-CoV-2. An importation occurs when a traveller is infected at the point of origin, or during travel to their destination. A travel-related infection refers to both an importation and close contacts who become infected by the traveller. A spillover is an infection from an individual with a travel-related infection to a community member that is not a close contact of the traveller, and a community infection is when a community member is infected either as a spillover, or from another community member. We note that this terminology differs from that used in NL Public Service Advisories, which reported cases as ‘related to international (or domestic) travel’ or ‘close contacts of a known case’. Infections and cases differ in that cases are the infections that are reported.
Travel-related and community infections arise through different processes, and therefore carry different risks and occur at different rates. The rate of arriving importations is dependent on the prevalence of infection at the travellers’ points of origin, the risk of infection during travel, and the rates of inbound travel to the local community (Russell et al., 2021). The rate that travel-related infections generate subsequent infections depends on contact rates with community members and can be reduced through post-arrival travel restrictions (Arino et al., 2020, Chen et al., 2021, Dickens et al., 2020). When travellers are to self-isolate post-arrival, infections can be spread to household members. In regions with few community cases of SARS-CoV-2, it is necessary to distinguish between importations, close contacts who were infected by an imported infection, and community infections (see Price et al., 2020 for related comments).
Here, we develop an approach to estimate the potential future impact of SARS-CoV-2 in communities that have experienced long periods with a high percentage of cases that are travel-related. Many models focus on community spread, without distinguishing between travel-related and community cases, and are not suitable for this purpose. During the pandemic, new SARS-CoV-2 variants emerged (Otto et al., 2021), and our framework considers this evolving risk. Our approach uses two models in a pipeline, expected spillovers and community spread, rather than only a single model that couples both. Our first model predicts the expected number of community members that are infected by travellers (i.e., spillovers) and considers three categories of public health measures: post-arrival travel restrictions, NPIs in the local community, and vaccination. Our second model describes a hypothetical future community outbreak and considers different variants and levels of vaccine coverage. The first model, describing the expected number of spillovers, is not coupled to the second model, describing a community outbreak, because community outbreaks might hypothetically begin on any given day, and averages taken across hypothetical outbreak start dates obscure key information (Juul et al., 2021). Considering a pipelined uncoupled framework is useful because some decisions that public health officials make are conditional on whether a community outbreak has been detected (notably the implementation of NPIs as part of an elimination strategy), while other decisions are better informed by the average across community outbreaks with all possible hypothetical start dates (i.e., generally applicable measures, such as provincial mask mandates when surveillance is low and importations are frequent).
In July 2021, most Canadians had received at least one dose of a SARS-CoV-2 vaccine, and there was a need to transition to a sustainable approach for SARS-CoV-2 management should high immunity levels be maintained. After vaccination, continued isolation of regions that implemented elimination strategies might be unrealistic, particularly given the economic and social impacts of these strategies (Committee for the Coordination of Statistical Activities, 2021). At this time, there was a need to develop guidelines to advise regions with zero or low SARS-CoV-2 prevalence in exiting elimination strategies (Lokuge et al., 2021, Open Society Common Purpose Taskforce, 2021). This remains an important topic even as most Canadian provincial governments have relaxed COVID-19 control measures. Indeed, vaccine coverage still lags in a large proportion of the world, and the emergence risk of novel variants remains high (Otto et al., 2021).
2. Materials and methods
2.1. Data
Our analysis combines data from multiple sources (summarized in Table 1) including the Public Health Agency of Canada (PHAC), and the Newfoundland and Labrador Centre for Health Information (NLCHI). A data source for travel-related cases was the COVID-19 Canada Open Data Working Group (CCODWG) (Berry et al., 2020, Berry et al., 2021), a group of volunteers who curated data from government and non-government sources. We validated the CCODWG data with travel-related cases as reported by the NL and NB provincial governments and found that the CCODWG data accurately describes the number of travel-related cases in NL and NB (Figure S1). Another data source was the Bank of Canada NPI stringency index (Cheung et al., 2021), which was used to measure the severity of NPIs implemented in NL. All modelling was performed in R (R Core Team, 2022). All data and code are archived at https://github.com/ahurford/pandemic-COVID-zero. Parameter estimates are summarized in Table 2, Table 3.
Table 1.
Data sources.
| Data source | Variables | Timeframe | Jurisdictions | Figures |
|---|---|---|---|---|
| COVID Canada Open Data Working Group | Travel-related cases (daily) Close contact cases (daily) |
July 1, 2020–May 31, 2021 | NB, PEI, NS, NL, YT, NWT | Figs. 1; 2; and S1 |
| Newfoundland and Labrador Centre for Health Information | Travel-related cases (daily) | July 1, 2020–December 24, 2021 | NL | Figs. 2; 3A, E; and S1 |
| Government of New Brunswick public releases | Travel-related cases (daily) | January 1, 2021–May 31, 2021 | NB | Fig. S1 |
| Public Health Agency of Canada public data | New cases (daily) | March 15, 2020–December 24, 2021 | All Canadian provinces | Explanatory variable for model fit in Fig. 2 |
| Public Health Agency of Canada public data | Variant frequency (weekly) Vaccination levels (weekly) |
March 14, 2020–December 24, 2021 | Canada Canada and NL |
Fig. 3A, E; and S2A Fig. 3C, E; and S2B |
| Newfoundland and Labrador Centre for Health Information | Community cases (daily) Close contacts infected per travel-related case Number of cases and symptom onset date for the Mt. Pearl outbreak |
March 14, 2020–December 24, 2021 February 8–24, 2021 |
NL |
Fig. 3E Fig. 3E Fig. 4 |
| Bank of Canada | NPI stringency (daily) | July 1, 2020–December 24, 2021 | NL | Fig. 3D |
The line list for CCODWG (Berry et al., 2020) was discontinued on May 31, 2021, and as such, no data on travel-related cases or close contacts of travellers are available from CCODWG after this date.
Table 2.
Parameter estimates.
| Description | Value | Details |
|---|---|---|
| The number of close contacts infected per imported infection of the Original variant | c=0.149 | Estimated from NLCHI data as: (the number of close contacts)/(the number of importations) on any day. Estimate is the average, weighted by the Original variant prevalence in Canada, when this prevalence was greater than 1%. |
| The number of close contacts infected per imported infection of the Alpha variant | c=0.114 | Same estimation method and data as c |
| The number of close contacts infected per imported infection of the Delta variant | c=0.266 | Same estimation method and data as c |
| The number of close contacts infected per imported infection of the Omicron variant | c=0.756 | Same estimation method and data as c |
| Probability of no infection given exposure for unvaccinated individuals | z = 0 | Assumed. Constraint is 0 z1. |
| Probability of no infection given exposure for individuals with 1 dose of vaccine (original, Alpha and Delta variant) |
z=z =z =0.49 |
Based on Pfizer vaccine efficacy against symptomatic infection (Bernal et al., 2021). Original variant same as Alpha variant (Khateeb et al., 2021). Constraint is 0 z1. |
| Probability of no infection given exposure for individuals with 1 dose of vaccine (Omicron variant) | z = 0 | Assumed. Constraint is 0 z1. |
| Probability of no infection given exposure for individuals with 2 or 3 doses of vaccine (original and Alpha variant) |
z = z = z = z = 0.93 |
Based on Pfizer vaccine efficacy against symptomatic infection (Bernal et al., 2021). Original variant same as Alpha variant (Khateeb et al., 2021). Constraint is 0 z1. |
| Probability of no infection given exposure for individuals with 2 or 3 doses of vaccine (Delta variant) |
z=z =0.88 |
Based on Pfizer vaccine efficacy against symptomatic infection (Bernal et al., 2021). Constraint is 0 z1. |
| Probability of no infection given exposure for individuals with 2 doses of vaccine (Omicron variant) | z=0.09 | Andrews et al. (2022). Based on Pfizer vaccine efficacy against symptomatic infection after 25 weeks. Constraint is 0 z1. |
| Probability of no infection given exposure for individuals with 3 doses of vaccine (Omicron variant) | z=0.67 | Andrews et al. (2022). Based on Pfizer vaccine efficacy against symptomatic infection. Constraint is 0 z1. |
| Transmission rate | = 0.287 | Calibrated |
| Multiplicative change in transmission for Alpha variant relative to original variant | b=1.77 | Model 1a estimate from Table 1 in Davies et al. (2021) |
| Multiplicative change in transmission for Delta variant relative to original variant | b=1.97 | Campbell et al. (2021) |
| Multiplicative change in transmission for Omicron variant relative to original variant | b=2.97 | Relative risk for unvaccinated primary cases (1.51) from Table 1 in Jalai et al. (2022) |
| Proportion of travellers that comply with self-isolation requirements | =0.7 | Assumed. Constraint is 0 1. |
| Probability the traveller is infectious after completing x days of self-isolation | Figure S3E | See Supplementary Material for details |
| Probability a PCR test a days after arrival is a false negative | Figure S3F | See Supplementary Material for details |
| Probability 5 Rapid Antigen Tests are all false negatives | =0.1 | Assumed. Constraint is 0 1. |
All parameters are unitless.
Table 3.
Post-arrival travel restrictions in NL.
| Reopening step | Dates, t | New measures | Value |
|---|---|---|---|
| SMO (Travel Exemption Order), May 5 2020 | t = May 4, 2020–June 30, 2021 | All travellersself-isolate for 14-days |
m(t) = f(14) = 0.153 m(t) = 0.153 m(t) = 0.153 |
| SMO Reopening – Travel – Step 1, July 1, 2021 |
t = July 1, 2021–July 31, 2021 |
Partially vaccinatedtravellers must have a negative PCR test result at entry Fully vaccinated travellers have no restrictions |
m(t) = 0.153 m(t) = f(0,0) = 0.384 m(t) = f(0) = 0.509 |
| SMO Reopening – Travel – Step 2 - August 1, 2021 | t = Aug 1, 2021–Sept 29, 2021 |
Unvaccinatedtravellers complete a Polymerase Chain Reaction (PCR) test on day 7–9 of self-isolation, and can exit self-isolation if negative. Partially vaccinated travellers have no restrictions |
m(t) = 0.156 m(t) = f(8,8) = 0.509 m(t) = 0.509 |
| SMO Reopening – Travel – Step 2 – UPDATED, Sept 30, 2021 | t= Sept 30–Dec 20, 2021 | Partially vaccinatedtravellers complete a PCR test on day 7–9 of self-isolation, and can exit self-isolation if negative. |
m(t) = 0.156 m(t) = 0.156 m(t) = 0.509 |
| SMO Reopening – Travel – Step 2 – December 21, 2021 Update | t= Dec 21–24, 2021 | All travellersself-isolate for 5-days and complete a rapid antigen test each day |
m(t) = 0.1f(5) = 0.022 m(t) = 0.022 m(t) = 0.022 |
The restrictions for travellers with 3 doses of vaccine are the same as for 2 doses of vaccine. For the calculations, 1 dose of vaccine and partially vaccinated were considered equivalent. When a vaccination status is not listed under a Special Measures Order (SMO), the new SMO does not change the measures that apply to that vaccination status. The functions and are defined in the Supplementary Material.
2.2. Modelling framework
Central to our approach where we develop a method to quantify the expected number of spillovers are two quantities: , the number of travel-related infections, and , the probability that a traveller or their close contacts infects a community member, where both quantities depend on the date, . Travellers and their close contacts are indexed by their vaccination status, i.e., the number of vaccination doses completed, which can be , or , and the infecting variant, , referring to the Original (), Alpha (), Delta (), or Omicron (; BA.1 subvariant) variants. Post-arrival travel restrictions, NPIs and vaccine coverage in the local community, and variant transmissibility are all considered to calculate .
2.3. Statistical model of imported cases to NL
To model the daily number of imported cases arriving to NL, we used a Poisson regression. Explanatory variables were time series of the mean new cases per 10,000 population over the last 14 days for (from east to west) Nova Scotia, Quebec, Ontario, Manitoba, Saskatchewan, Alberta and British Columbia. Provincial population sizes were based on Statistics Canada estimates for the first quarter of 2021. Fitted coefficients were constrained to be non-negative because we hypothesized that high infection prevalence in other provinces should have a positive relationship with the number of imported cases arriving in NL from that province.
2.4. Model 1: Expected number of spillovers
2.4.1. Characteristics of travel-related cases
To estimate the expected number of travellers or their close contacts that infected NL community members (referred to as ‘spillovers’), we first obtain the number of travellers and their close contacts with vaccination status and infected with variant as
| (1) |
where is the number of close contacts infected per imported infection of the variant, , and is the number of imported infections reported on date . We assume all infected travellers are identified and reported. Eq. (1) assumes a similar frequency of vaccination statuses for travellers and their close contacts. Given a travel-related infection, is the probability that the traveller has vaccination status and is infected with variant , where
| (2) |
Here, is the fraction of travellers with vaccination status at time , is the frequency of the variant at the origin sites of travellers, and is the probability that a traveller with vaccination status is infected with the variant , where we assume no changes in over time, i.e., as might occur due to waning of the vaccination.
As data on variant frequencies is not reliably available for jurisdictions within Canada, we parameterize as the variant frequency in Canada. To parameterize , we equate reported vaccine efficacies against symptomatic infection with the probability of infection (see Table 2) . Realistically, vaccines prevent less against infection and transmission than symptomatic infection, however, data for vaccine efficacies against infection and transmission are less available.
2.4.2. Post-arrival travel restrictions
Post-arrival travel restrictions may include self-isolation for a specified number of days after arrival, and Polymerase Chain Reaction (PCR) or Rapid Antigen Tests (RATs). In NL, different post-arrival travel restrictions were implemented through Special Measures Orders at different times during the SARS-CoV-2 public health emergency and depended on the vaccination status of the travellers (Table 3). We let describe the efficacy of travel restrictions for a traveller with the vaccination status , given the post-arrival travel restrictions on a given date .
To estimate , we assumed that the efficacy of self-isolation for a given number of days could be calculated from the generation interval of SARS-CoV-2 (Ferretti et al., 2020), which was estimated for the Original variant. We assumed the generation interval was the same for all variants, although data suggests shorter generation times for the Delta variant (Hart et al., 2022). We felt this assumption was reasonable as our conclusions are likely more sensitive to other parameter estimates (as described in the Discussion). We estimated the probability of a false negative PCR test by considering (Hellewell et al., 2021). The complete details of how we parameterized the effect of post-arrival travel restrictions are provided in the Supplementary Material. We had no information on compliance with self-isolation requirements, or when travellers are usually infected prior to arrival, and so we assumed 70% compliance with self-isolation, and that infected travellers were exposed between zero and ten days prior to arrival, with exposure times following a uniform distribution.
We assumed that the travel restrictions that applied to travellers also applied to their close contacts (i.e., household members). Our assumption is an over-simplification because in NL sometimes household members of travellers were subject to restrictions and other times they were not. If close contacts were infected from a traveller the timing of the close contact’s infectious period would be later than that of the traveller, and potentially after even a long period of self-isolation that began when the traveller arrived. This suggests that our assumption that the same restrictions apply to the traveller and their close contacts could under-estimate the spillover risk. However, in NL during the period of this study, if the traveller tested positive or if the close contacts developed symptoms, the close contacts were required to complete a PCR test. If the PCR test was positive, the close contacts were required to self-isolate, and in this respect, our assumptions regarding the probability that a close contact of a traveller infects a community member are an under-estimate.
2.4.3. NPIs and vaccination in the local community
We let the susceptibility of the local community to infection be determined by NPIs and vaccination (PIs). We let be the stringency of NPIs in the local community on a given date . We used the Bank of Canada COVID-19 stringency index estimated for NL. The Bank of Canada COVID-19 stringency index is calculated from 12 sub-indices which include policy related to school and workplace closures, restrictions on public and private gathering, travel restrictions, enforcement mechanisms, and public information campaigns (Cheung et al., 2021). We let be a transmission rate parameter, and we let be a multiplier reflecting the relative transmission rates for different variants.
The susceptibility of the local community to infection when considering vaccination is,
| (3) |
where the fraction of the community with different vaccination statuses is and is the probability of infection given vaccination status and the infecting variant as previously defined. We assume four factors act independently to determine the probability that a traveller infects a local community member: (i) the efficacy of travel restrictions, ; (ii) the stringency of NPIs, ; (iii) the transmissibility of different variants; and (iv) the susceptibility of the local community after considering vaccination, . As such,
| (4) |
We assume that spillovers occur following a Binomial distribution with probability and trials. Then, on each date , the expected number of community members infected by a traveller or their close contact is
| (5) |
which is the expectation of a Binomial distribution summed across all vaccination and variant types.
This quantity, , describes the daily expected number of community members infected by travellers and their close contacts (spillovers). Quantifying SARS-CoV-2 risks in regions that do not have SARS-CoV-2 community cases was an area of need during the first 18 months of the SARS-CoV-2 pandemic, and Eq. (5) addresses this need.
2.5. Model 2: Modelling outbreaks in regions implementing an elimination strategy
The second model for quantifying SARS-CoV-2 risk in regions that do not have community cases is to answer the question ‘if a community outbreak is established, how will the number of cases change over time, and how many cases will occur in the outbreak?’ To illustrate this modelling for a region that had few community cases of SARS-CoV-2, we consider Mount Pearl, NL.
Prior to December 15, 2021 in NL, the largest community outbreak of SARS-CoV-2 occurred due to the Alpha variant, with symptom onset dates from February 1 to 27, 2021, and with spread predominately in the Mount Pearl region. Mount Pearl is a suburb of St. John’s, and is part of the St. John’s metropolitan area which in 2016 had a population size of 205,955 (Statistics Canada, 2017). In response to the outbreak, on February 11, a Special Measures Order enacted the strictest level of NPIs (Alert level 5) in the St. John’s region. Contacts of cases were traced and tested, and many cases were associated with Mount Pearl Senior High School (Government of Newfoundland and Labrador, 2021b). No new cases associated with the outbreak were reported with symptom onset dates after February 28, 2021.
We calibrated a stochastic Susceptible–Infected–Recovered (SIR) model to data describing daily new reported cases and their symptom onset dates for cases belonging to the Mount Pearl outbreak (see Supplementary Material for details). This parameterized model is then the basis to explore the dynamics of hypothetical future outbreaks in Mount Pearl, NL.
For comparison, hypothetical scenarios retain the pattern of NPI implementation that occurred in the actual Mount Pearl outbreak, i.e., implementation of strict NPIs 10 days after the start of the outbreak, although it is possible to explore scenarios without this assumption. We consider future scenarios where vaccination coverage may have changed, and where a different variant may have caused the outbreak. For simplicity in interpreting the results of vaccination scenarios, we assume that all individuals in the community are either unvaccinated or have had two doses of vaccine.
3. Results
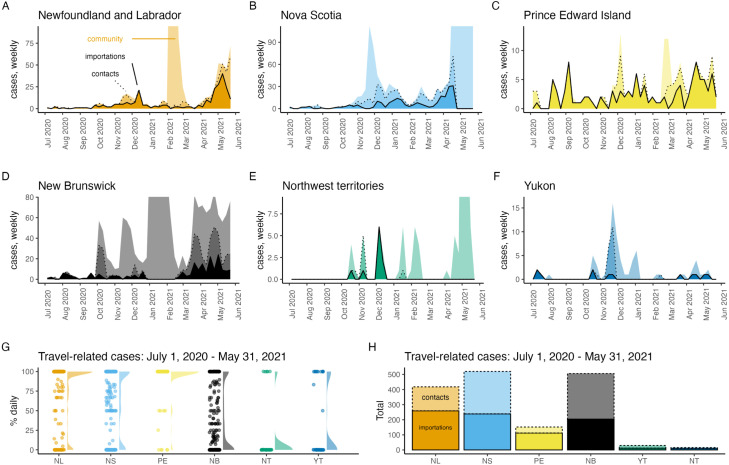
Most of the SARS-CoV-2 cases reported in Atlantic Canada and Canada’s territories were travel-related from July 1, 2020 to May 31, 2021 (Fig. 1). The period prior to July 1, 2020 was not considered because very few cases of any type were reported during this time. Notable differences that occur between these jurisdictions are that a much lower percentage of travel-related cases was reported each day in NB (mean = 36.7%, median = 13.4%), NT (mean = 12.8%, median = 0%) and YT (mean = 30.1%, median = 0%), as compared to NL (mean = 76.6%, median = 100%), NS (mean = 61.2%, median = 80%), and PE (mean = 91.3%, median = 100%) (Fig. 1G). The values reported for NB are likely still much higher than the provinces west of NB, which had community spread and likely near 0% of reported cases were travel-related on most days.
Fig. 1.
In Atlantic Canada and Canada’s territories most SARS-CoV-2 cases were importations and close contacts of these travellers from July 1, 2020 to May 31, 2021. Panels A–F show imported cases (dark shading, solid line), their close contacts (medium shading, dashed line), and community cases (light shading, no line) with the vertical axis limit as 20% more than the maximum number of reported weekly travel-related cases so that brief periods of large community outbreaks do not dominate the graphs. From July 1, 2020–May 31, 2021, panels G–H show the percentage of reported daily cases that were travel-related (dots; also shown as a shaded density plot, G), and the total number of imported cases and their close contacts (H).
During the same period, the total number of travel-related cases also differed between jurisdictions with NL (importations = 259, close contacts of travellers = 159), NS (importations = 239, close contacts = 281), and NB (importations 204, close contacts = 302) having reported at least 2.75 times more travel-related cases than PE (importations = 112, close contacts = 40), and with YT (importations = 12, close contacts = 18) and NT (importations = 10, close contacts = 6) having reported very few travel-related cases at all (Fig. 1H). Other Canadian provinces and Nunavut (NU) were not considered because travel-related case data was not reliably reported for these jurisdictions.
We found that the daily number of importations to NL was predicted as 1.12 times the mean number of new cases per 10,000 population in NS, where the mean is taken over the last 14 days (Fig. 2). Estimated coefficients for the contribution of other provinces to the prediction of daily imported cases to NL were not different than zero, and the estimated intercept was zero. This statistical relationship is reasonable since a pre-pandemic survey reported 26% of travel into NL was from the Maritimes, second only to Ontario (Government of Newfoundland Labrador, 2018). The island of Newfoundland was the destination for 93% of travellers into NL (Government of Newfoundland Labrador, 2018), the ferry to Newfoundland departs from NS, and many flights to Newfoundland are routed with layovers in NS.
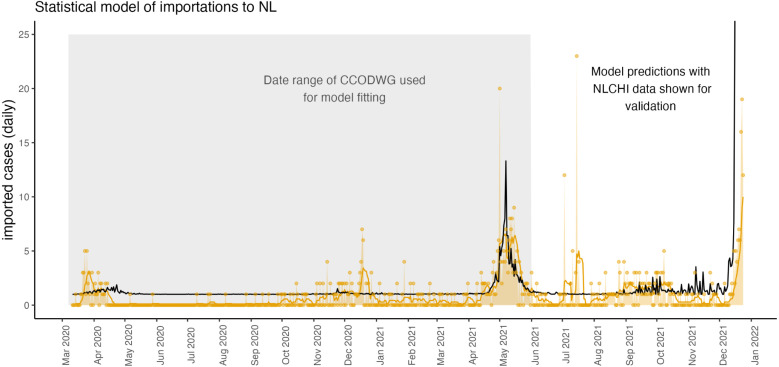
Fig. 2.
From March 15, 2020–December 24, 2021, the daily number of imported cases to NL is reliably predicted as 1.12 times the mean number of new cases per 10,000 population in NS, where the mean is taken over the last 14 days. This relationship was fit using the publicly available CCODWG data, where curation of these data ended on May 31, 2021 before the end of the study period. The model-predicted daily number of imported cases to NL (black line) extends beyond the time period of model fitting (grey shaded region) because data describing new cases in NS was available through to the end of the study period. To validate the predictions of the statistical model, we show the number of daily imported cases reported by NLCHI (yellow dots and yellow shading), and the 7-day rolling mean of daily imported cases (yellow line) where these data span the full study period.
The agreement of the model (Fig. 2, black line) with the data (yellow line) is good since the model was only parameterized with data to May 31, 2021 (grey shaded region), but the model predictions still agree with the validation data from June 1 to December 24, 2021 and the model predicts the rise in importations that occurred in early December 2021. Few importations were reported and so chance events disrupt the agreement between the model predictions and the data. For example, in July 2021, a Portuguese fishing boat anchored in Conception Bay, NL and 31 crew members tested positive for SARS-CoV-2 (Smelie, 2021). This event may explain the 23 imported cases reported on July 15, 2021. The arrival of such boats with SARS-CoV-2 positive crew members is a chance event rather than a regularly occurring event that can be predicted by a model.
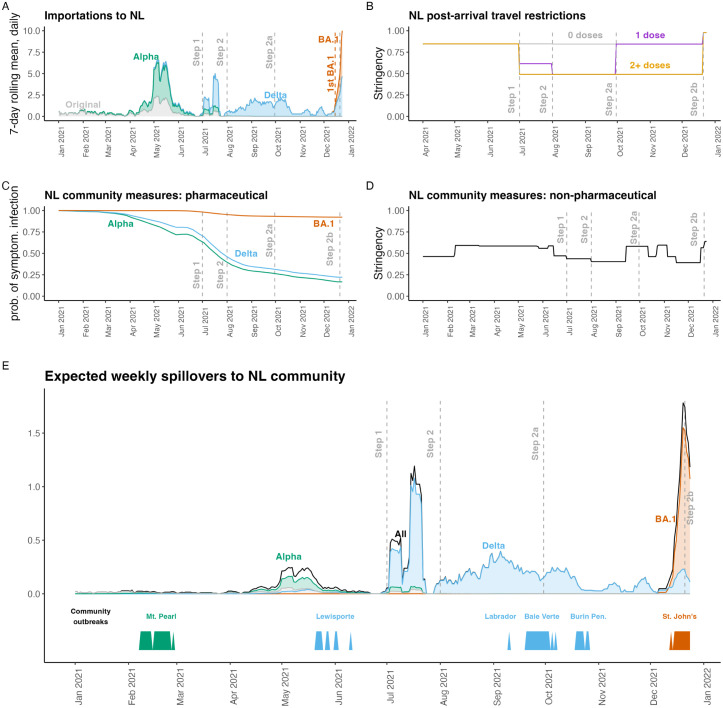
By December 24, 2021 (Fig. 3E), the expected number of spillovers, infections spread from travellers and their close contacts to NL community members, was as high as it had ever been (as calculated by Eq. (5)). At this time, a community outbreak involving the Omicron variant was already occurring, with the first Omicron variant case in NL reported in St. John’s on December 15, 2021. The expected number of spillovers in mid-December was 44% higher than the previous highest value, and due to both the high number of imported cases (Fig. 3A) and the reduced efficacy of two vaccine doses in protecting the NL community from infection with the Omicron variant (Fig. 3C). In late July 2021, the expected number of spillovers was also high (Fig. 3E). This was after NL relaxed entry requirements for Canadian travellers on July 1, 2021 (Fig. 3B; Table 3), but before most Newfoundlanders and Labradorians were fully vaccinated (Figure S2C). The peak in the expected number of spillovers due to the Alpha variant (early May 2021; Fig. 3E) was due to an increased number of importations occurring at that time (Fig. 3A). The expected number of spillovers occurring due to the Delta variant was higher than that of the Alpha variant for two reasons: (1) after July 1, 2021 travel restrictions into NL for Canadians were relaxed (Fig. 3B; Table 3), and (2) the Delta variant is more transmissible than the Alpha variant (Table 2).
Fig. 3.
In mid-December 2021, the expected number of spillovers to NL community members was the highest it had ever been. High spillover risk in mid-December 2021 was due to the establishment of the Omicron BA.1 variant in Canada and high numbers of imported cases (A), and low vaccine efficacy for NL community members with two doses of vaccine exposed to the Omicron variant (C). (A) Imported cases, . (B) The stringency of post-arrival travel restrictions, . (C) The probability of a symptomatic infection given exposure when considering vaccination of NL community members, . (D) The stringency of NPIs implemented in the NL community, . (E) The expected number spillovers, NL community members infected by travellers and their close contacts, (black - Eq. (5); with variant-specific numbers shown with colours). The timing of actual community outbreaks with more than 5 cases are shown along the bottom bar. Grey dashed vertical lines show post-arrival travel restrictions due to different Special Measures Orders (see Table 3).
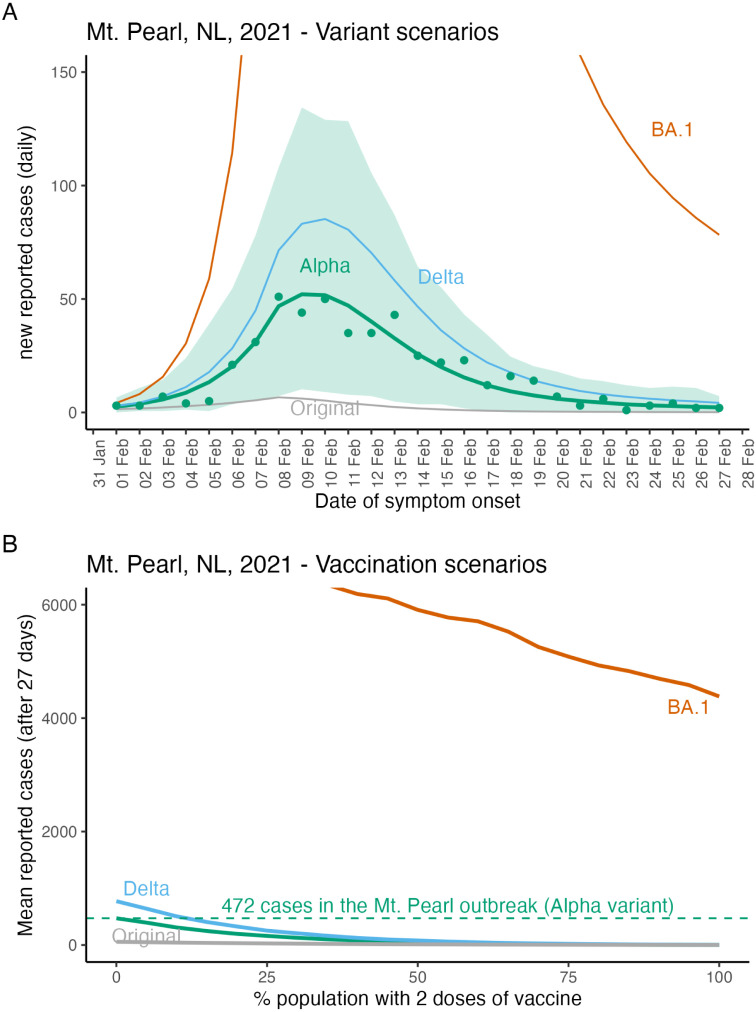
The stochastic SIR model (Fig. 4A, green lines and shading) shows close agreement with the data from the Mount Pearl outbreak in February, 2021 (Fig. 4A, green dots). When the Mount Pearl outbreak occurred few NL community members were vaccinated or had been infected, such that all the scenarios shown in Fig. 4A assume a fully susceptible community. The Omicron variant (BA.1 subvariant) is much more transmissible than the other variants, and a hypothetical BA.1 variant outbreak in a fully susceptible Mount Pearl, NL community (Fig. 4A, red line) cannot be completely shown given the vertical axis limits that were set to emphasize the actual Mount Pearl Alpha variant outbreak. Fig. 4A does not consider the arrival of imported cases. This is because the Mount Pearl data was strictly for cases known to belong to this outbreak.
Fig. 4.
Epidemiological model fit and hypothetical future variant and vaccination scenarios for Mount Pearl, NL. The Mount Pearl outbreak was due to the Alpha variant and the vertical axis limits of panel (A) were selected to show the Alpha variant (green line), and the Mount Pearl data (green dots) which meant that large values for the BA.1 variant are not shown. Lines show the mean and the shaded region shows the minimum and maximum values for 1000 simulations. The peak number of reported daily new cases for the BA.1 variant is 806 (not shown). In panel (B) vaccination scenarios assume community members are either unvaccinated or vaccinated with 2 doses. After 27 days of a BA.1 outbreak in a fully unvaccinated community, we estimate 7852 reported cases (not shown due to truncation). For more details describing parameter estimates see Table 2, and for model details see the Supplementary Material.
The number of cases reported in the Mount Pearl outbreak was 472 (Fig. 4B, green dashed line). For the simulations, the mean total number of reported cases after 27 days (the duration of the Mount Pearl outbreak) when the community is fully susceptible are Original variant, 56, Alpha variant, 472, Delta, 773, and Omicron variant, 7852. We assumed that community members could be either unvaccinated or have two doses of vaccine. The effect of vaccination is to substantially reduce the number of reported cases in the outbreak after 27 days for all variants (Fig. 4B).
4. Discussion
In regions that have extended periods with few community cases of SARS-CoV-2, for example, regions that effectively implemented an elimination strategy, travel-related cases are a high percentage of reported cases (Arino et al., 2021, Godin et al., 2021), and modelling importations is particularly important (Zhang et al., 2022). Here, we extend such importation modelling to incorporate post-arrival travel restrictions, community vaccination coverage, and NPIs into the risk assessment frameworks for regions with few community SARS-CoV-2 infections.
Atlantic Canada and Canada’s territories experienced few SARS-CoV-2 cases prior to June 2021, however, there were some differences between these jurisdictions. NT and YT reported few travel-related cases, while NL, NS, and NB reported similar numbers of travel-related cases, but with NB reporting a much lower percentage of daily cases that were travel-related (Fig. 1). Finally, while NL and NS had similar epidemiology until May 31, 2021, NL had enacted strict travel restrictions (Hurford et al., 2021), while NS enacted an extensive community testing program (Johnson-León et al., 2021). The YT implemented strict travel restrictions, but experienced an outbreak of the Gamma variant that overwhelmed hospital capacity (McPhee-Knowles et al., 2022).
We considered a statistical model describing the daily number of reported importations arriving in NL. During the pandemic response it was helpful to use this approach to forecast importations so that future risk could be assessed using Eq. (5). That was not done in this manuscript because such an exercise would never be current, but this could be valuable to assess border measures, the threat of a new variant, or the impacts of waning immunity. We found that importations to NL could be predicted from the mean new cases per 10,000 people in NS over the last 14 days (Fig. 2). These data were publicly available and regularly updated, but more generally better access to data describing travel volumes, travellers’ points of origin, reasons for travel, and granting of travel exemptions would aid real time importation modelling and risk quantification.
We applied our framework (Eq. (5)) to inform the potential for community outbreaks in NL. The estimated risk is somewhat consistent with the actual community outbreaks that occurred in NL (Fig. 3). Generally, it seems difficult to predict when community outbreaks might occur in regions without community cases even given the vast amounts of data that were available during the SARS-CoV-2 pandemic.
Our analysis considers only known travel-related infections, such that estimates per infected traveller equate to per known infected traveller. In NL, for the pandemic until July 1, 2021, testing of arriving travellers was intensive (owing to few ports of entry, reduced travel volumes (Hurford et al., 2021), testing requirements for rotational workers (Department of Health and Community Services, N.L., 2022), and requests for travellers potentially exposed during inbound flights to report for asymptomatic testing). This intensive testing, combined with few occurrences of community cases, suggests that a high proportion of imported cases were detected in NL during this time.
The main limitation of our analysis is parameter estimation and uncertainty. It is difficult to estimate the change in relative transmissibility due to a new variant because these data are estimated in different regions (or pooled across regions), and as the susceptible population changes owing to vaccination, infection, and waning of immunity during the time period that the estimation is made. We used 77% as the estimate of increased transmissibility of the Alpha variant relative to the Original variant (Table 2), however, the source of this estimate (Davies et al., 2021) gives a range of values from 43% to 90% depending on the population and assumptions of the estimation procedure. Vaccine efficacies are estimated in specific populations, and application to other regions assumes no differences in population structure with regard to age and immunity, and does not estimate protection against infection and onward transmission, which is a critical parameter for epidemiological models. Finally, the impact of NPIs on transmission is difficult to assess, and the impact of new variant characteristics on the effectiveness of NPIs is unknown. In some instances data were not available to estimate parameters, for example, we assumed 70% compliance with self-isolation requirements, and the transmission rate parameter was calibrated (Table 2).
Our work was motivated by a need for regions that successfully implemented an elimination strategy during the first 18 months of the COVID-19 pandemic to quantify the risk of SARS-CoV-2 spread in their communities, and a need for guidelines to exit an elimination strategy when high vaccination coverage has been achieved. While guidelines for reopening have been developed by many jurisdictions, those using criteria expressed as the number of observed community cases (Anderson et al., 2021, Nali et al., 2021) are not helpful for regions that are reopening when there are few community cases.
Existing theory applicable to developing such guidelines is importation modelling (i.e., considering infection prevalence at travellers’ origins and travel volumes into a destination, e.g. Russell et al., 2021) and branching process modelling that calculates the probability of a major outbreak (i.e., Allen, 2008). Extensions of classic branching process models consider the probability of an outbreak in age-structured populations with NPIs (Lovell-Read et al., 2022), and when importations occur (Ball et al., 2017). A related concept is the ‘event reproduction number’, a quantity that describes the number of secondary infections arising from one infected person attending an event (Tupper et al., 2020), since this quantity measures outbreak risk rather than simulating the entire outbreak. Some modelling studies have considered the efficacy of pre- and post-arrival travel restrictions (Steyn et al., 2021, Wells et al., 2021), but without linking to importation modelling as we have done. Future work to inform guidelines to exit an elimination strategy should further bridge these different research areas.
There is a need to communicate reasonable expectations to the public in regions where elimination has been implemented as relaxation of measures might have little or no impact on reported case numbers when infection prevalence is already high (Russell et al., 2021, Chen et al., 2020), but might bring risk in populations with zero or low SARS-CoV-2 prevalence (Russell et al., 2021, Chen et al., 2020, Arino et al., 2021). In regions that have implemented an elimination strategy, an increase in reported case numbers may occur even when measures are carefully and reasonably relaxed, and particularly if the prevalence of variants of concern is higher outside the jurisdiction than in (Wells et al., 2020, Grépin et al., 2021).
Prior to May 31, 2021, Atlantic Canada and Canada’s territories had experienced prolonged periods with few community SARS-CoV-2 cases. In this manuscript, we characterize differences within these jurisdictions, and distinguish between travel-related and community cases (Fig. 1). We illustrate a type of epidemic modelling that is useful in these regions. This framework extends importation modelling such that border restrictions, variants, NPIs and vaccination in the local community are considered. Additionally, hypothetical future outbreaks are considered by simulating variant and vaccination scenarios. Our framework can be used to inform the risk associated with different candidate reopening plans when vaccination coverage is high in regions that have experienced prolonged periods with few SARS-CoV-2 cases, and help inform plans to exit an elimination strategy.
CRediT authorship contribution statement
Amy Hurford: Developed the modelling framework, Wrote the code, Made the figures, Wrote the manuscript. Maria M. Martignoni: Wrote the manuscript. J. Concepción Loredo-Osti: Wrote the code, Comments on manuscript drafts. Francis Anokye: Wrote the code, Comments on manuscript drafts. Julien Arino: Wrote the manuscript, Comments on manuscript drafts. Bilal Saleh Husain: Collected data, Comments on manuscript drafts. Brian Gaas: Comments on manuscript drafts. James Watmough: Comments on manuscript drafts.
Acknowledgements
We acknowlege funding from the Natural Sciences and Engineering Research Council of CanadaEmerging Infectious Disease Consortium (the Canadian Network for Infectious Disease Modelling, Mathematics for Public Health, and the One Health Modelling Network for Emerging Infectious Diseases) and Discovery Grant program (RGPIN 2014-05413) and funding from the Newfoundland and Labrador Centre for Health Information, Canada . We acknowledge conversations with Proton Rahman, Sanjeev Sahara, and responding to modelling requests from the NL Department of the Health and Community Services that helped in the development of the modelling framework presented. NL COVID-19 data was provided by the NLCHI (Health Research Ethics Board reference number 2021.013).
Footnotes
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.jtbi.2022.111378.
Appendix A. Supplementary data
The following is the Supplementary material related to this article.
Additional details describing the data, model parameterization, and model formulation.
References
- Aknin Lara B., Andretti Bernardo, Goldszmidt Rafael, Helliwell John F., Petherick Anna, De Neve Jan-Emmanuel, Dunn Elizabeth W., Fancourt Daisy, Goldberg Elkhonon, Jones Sarah P., Karadag Ozge, Karam Elie, Layard Richard, Saxena Shekhar, Thornton Emily, Whillans Ashley, Zaki Jamil. Policy stringency and mental health during the COVID-19 pandemic: a longitudinal analysis of data from 15 countries. Lancet. Public Health. 2022;7:e417–e426. doi: 10.1016/S2468-2667(22)00060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Linda J.S. In: Mathematical Epidemiology. Brauer F., van den Driessche P., Wu J., editors. Springer; 2008. An introduction to stochastic epidemic models; pp. 81–130. [Google Scholar]
- Anderson Sean C., Mulberry Nicola, Edwards Andrew M., Stockdale Jessica E., Iyaniwura Sarafa A., Falcao Rebeca C., Otterstatter Michael C., Janjua Naveed Z., Coombs Daniel, Colijn Caroline. How much leeway is there to relax COVID-19 control measures? Epidemics. 2021;35 doi: 10.1016/j.epidem.2021.100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews Nick, Stowe Julia, Kirsebom Freja, Toffa Samuel, Rickeard Tim, Gallagher Eileen, Gower Charlotte, Kall Meaghan, Groves Natalie, O’Connell Anne-Marie, Simons David, Blomquist Paula B., Zaidi Asad, Nash Sophie, Iwani Binti Abdul Aziz Nurin, Thelwall Simon, Dabrera Gavin, Myers Richard, Amirthalingam Gayatri, Gharbia Saheer, Barrett Jeffrey C., Elson Richard, Ladhani Shamez N., Ferguson Neil, Zambon Maria, Campbell Colin N.J., Brown Kevin, Hopkins Susan, Chand Meera, Ramsay Mary, Lopez Bernal Jamie. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino Julien, Bajeux Nicolas, Portet Stephanie, Watmough James. Quarantine and the risk of COVID-19 importation. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino Julien, Boëlle Pierre-Yves, Milliken Evan M., Portet Stephanie. Risk of COVID-19 variant importation – how useful are travel control measures? Infect. Dis. Model. 2021;6:875–897. doi: 10.1016/j.idm.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker Michael G., Wilson Nick, Anglemyer Andrew. Successful elimination of COVID-19 transmission in New Zealand. N. Engl. J. Med. 2020;383 doi: 10.1056/NEJMc2025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker Michael G., Wilson Nick, Blakely Tony. Elimination could be the optimal response strategy for COVID-19 and other emerging pandemic diseases. BMJ. 2020;371 doi: 10.1136/bmj.m4907. [DOI] [PubMed] [Google Scholar]
- Ball Frank, Britton Tom, Trapman Pieter. An epidemic in a dynamic population with importation of infectives. Ann. Appl. Probab. 2017;27:242–274. [Google Scholar]
- Bernal Jamie Lopez, Andrews Nick, Gower Charlotte, Gallagher Eileen, Simmons Ruth, Thelwall Simon, Tessier Elise, Groves Natalie, Dabrera Gavin, Myers Richard, et al. 2021. Effectiveness of COVID-19 vaccines against the B. 1.617. 2 variant. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry Isha, O’Neill Meghan, Sturrock Shelby L., et al. A sub-national real-time epidemiological and vaccination database for the COVID-19 pandemic in Canada. Sci. Data. 2021 doi: 10.1038/s41597-021-00955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry Isha, Soucy Jean-Paul R., Tuite Ashleigh, Fisman David. Open access epidemiologic data and an interactive dashboard to monitor the COVID-19 outbreak in Canada. Canadian Med. Assoc. J. 2020;192:E420. doi: 10.1503/cmaj.75262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami Simona. The burden of COVID-19 in Canada. Canadian Stud. Popul. 2021;48(2):123–129. doi: 10.1007/s42650-021-00056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26(24):17. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Tiange, Huang Siwan, Li Guanqiao, Zhang Yuan, Li Ye, Zhu Jinyi, Shi Xuanling, Li Xiang, Xie Guotong, Zhang Linqi. An integrated framework for modelling quantitative effects of entry restrictions and travel quarantine on importation risk of COVID-19. J. Biomed. Inform. 2021;118 doi: 10.1016/j.jbi.2021.103800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Shi, Li Qin, Gao Song, Kang Yuhao, Shi Xun. State-specific projection of COVID-19 infection in the United States and evaluation of three major control measures. Sci. Rep. 2020;10(1):1–9. doi: 10.1101/2020.04.03.20052720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Calista, Lyons Jerome, Madsen Bethany, Miller Sarah, Sheikh Saarah. 2021. The Bank of Canada COVID-19 stringency index: measuring policy response across provinces. URL https://www.bankofcanada.ca/2021/02/staff-analytical-note-2021-1/ [Google Scholar]
- Committee for the Coordination of Statistical Activities . United Nations; 2021. How COVID-19 is changing the world: a statistical perspective. Volume III. New York, 2021. https://unstats.un.org/unsd/ccsa/documents/ [Google Scholar]
- Contandriopoulos Damien. The year public health lost its soul: a critical view of the COVID-19 response. Canadian J. Public Health. 2021;112(6):970–972. doi: 10.17269/s41997-021-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies Nicholas G., Abbott Sam, Barnard Rosanna C., Jarvis Christopher I., Kucharski Adam, Munday James D., Pearson Carl B. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Community Services, N.L. 2022. Report to the House of Assembly on the COVID-19 public health emergency. September 14, URL https://www.assembly.nl.ca/business/electronicdocuments/ReporttoHOACOVID-19PublicHealthEmergency2022.pdf/ [Google Scholar]
- Dickens Borame L., Koo Joel R., Lim Jue Tao, Sun Haoyang, Clapham Hannah E., Wilder-Smith Annelies, Cook Alex R. Strategies at points of entry to reduce importation risk of COVID-19 cases and reopen travel. J. Travel Med. 2020;27 doi: 10.1093/jtm/taaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti Luca, Wymant Chris, Kendall Michelle, Zhao Lele, Nurtay Anel, Abeler-Dörner Lucie, Parker Michael, Bonsall David, Fraser Christophe. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(6491) doi: 10.1101/2020.03.08.20032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin Arnaud, Xia Yiqing, Buckeridge David L., Mishra Sharmistha, Douwes-Schultz Dirk, Shen Yannan, Lavigne Maxime, Drolet Mélanie, Schmidt Alexandra M., Brisson Marc, Maheu-Giroux Mathieu. The role of case importation in explaining differences in early SARS-CoV-2 transmission dynamics in Canada - a mathematical modeling study of surveillance data. Int. J. Infect. Dis. 2021;102:254–259. doi: 10.1016/j.ijid.2020.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Newfoundland and Labrador . Department of Health and Community Services; 2021. News Releases. Public Advisory: Update on COVID-19 in Newfoundland and Labrador, December 31. URL https://www.gov.nl.ca/releases/2021/health/1231n06/ [Google Scholar]
- Government of Newfoundland and Labrador . 2021. Public advisory: 100 new cases of COVID-19 in newfoundland and labrador. February, 2021, URL https://www.gov.nl.ca/releases/2021/health/0211n05/ [Google Scholar]
- Government of Newfoundland Labrador . 2018. 2016 Exit survery - research highlights. URL https://www.gov.nl.ca/tcar/files/2016_Exit_Survey_Highlights_Report_FINAL_REVISED_June_2018.pdf. [Google Scholar]
- Grépin Karen Ann, Ho Tsi-Lok, Liu Zhihan, Marion Summer, Piper Julianne, Worsnop Catherine Z, Lee Kelley. Evidence of the effectiveness of travel-related measures during the early phase of the COVID-19 pandemic: a rapid systematic review. BMJ Glob. Health. 2021;6(3) doi: 10.1136/bmjgh-2020-004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart William S., Miller Elizabeth, Andrews Nick J, Waight Pauline, Maini Philip K., Funk Sebastian, Thompson Robin N. Generation time of the alpha and delta SARS-CoV-2 variants: an epidemiological analysis. Lancet Infect. Dis. 2022;22:603–610. doi: 10.1016/S1473-3099(22)00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021;19 doi: 10.1186/s12916-021-01982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood Anita E., Macintyre C. Raina. Elimination of COVID-19: what would it look like and is it possible? Lancet Infect. Dis. 2020;20(9):1005. doi: 10.1016/s1473-3099(20)30633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurford Amy, Rahman Proton, Loredo-Osti J. Concepción. Modelling the impact of travel restrictions on COVID-19 cases in newfoundland and labrador. R. Soc. Open Sci. 2021;8 doi: 10.1098/rsos.202266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalai N., Brustad Hilde K., Frigessi Arnoldo, MacDonald Emily A., Meijerink Hinta, Feruglio Siri L., Nygard Karin M. Increased household transmission and immune escape of the SARS-CoV-2 omicron compared to delta variants. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-33233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-León Maureen, Caplan Arthur L., Kenny Louise, Buchan Iain, Fesi Leah, Olhava Phoebe, Alugnoa Desmond Nsobila, Aspinall Mara G., Costanza Emily, Desharnais Brianna, et al. Executive summary: It’s wrong not to test: The case for universal, frequent rapid COVID-19 testing. eClinicalMedicine. 2021;33 doi: 10.1016/j.eclinm.2021.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul J.L., Græsbøll K., Christiansen L.E. Fixed-time descriptive statistics underestimate extremes of epidemic curve ensembles. Nat. Phys. 2021;17:5–8. doi: 10.1038/s41567-020-01121-y. [DOI] [Google Scholar]
- Khateeb Jasmin, Li Yuchong, Zhang Haibo. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Critical Care. 2021;25(1):1–8. doi: 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König Michael, Winkler Adalbert. The impact of government responses to the COVID-19 pandemic on gdp growth: Does strategy matter? PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0259362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuge Kamalini, Banks Emily, Davis Stephanie, Roberts Leslee, Street Tatum, O’Donovan Declan, Caleo Grazia, Glass Kathryn. Exit strategies: optimising feasible surveillance for detection, elimination, and ongoing prevention of COVID-19 community transmission. BMC Med. 2021;19(1):1–14. doi: 10.1186/s12916-021-01934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell-Read Francesca A., Shen Silvia, Thompson Robin N. Estimating local outbreak risks and the effects of non-pharmaceutical interventions in age-structured populations: SARS-CoV-2 as a case study. J. Theoret. Biol. 2022;535 doi: 10.1016/j.jtbi.2021.110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Arisina, Parry Jane. When Hong Kong’s “dynamic zero” COVID-19 strategy met omicron, low vaccination rates sent deaths soaring. BMJ (Clinical Research Ed.) 2022;377:o980. doi: 10.1136/bmj.o980. [DOI] [PubMed] [Google Scholar]
- Martignoni Maria M., Hurford Amy. Canadian Broadcasting Corportation, Newfoundland and Labrador; 2022. It’s not realistic to eliminate COVID-19 in Newfoundland and Labrador. Here’s why. URL https://www.cbc.ca/news/canada/newfoundland-labrador/covid-19-here-to-stay-1.6384033. [Google Scholar]
- McPhee-Knowles S., Hoffman B., Kanary L. The Yukon’s experience with COVID-19: Travel restrictions, variants and spread among the unvaccinated. Can. Commun. Dis. Rep. 2022;48:17–21. doi: 10.14745/ccdr.v48i01a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nali Luiz Henrique da Silva, Salvador Felipe Scassi, Bonani Graciela dos Santos Soares, Andrade Heitor Franco de, Luna Expedito José de Albuquerque, Fujita Dennis Minoru. 2021. Reopening borders: protocols for resuming travel during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Nguyen Hai, Quy Pham Nguyen, Pham Truong-Minh, Branch Joel. No new community COVID-19 infection in four consecutive weeks: what lesson can be learned from Vietnam. J. Infect. Dev. Countries. 2020;14(10):1125–1127. doi: 10.3855/jidc.13080. [DOI] [PubMed] [Google Scholar]
- Open Society Common Purpose Taskforce . University of Sydney; 2021. A roadmap to reopening. [Google Scholar]
- Otto Sarah P., Day Troy, Arino Julien, Colijn Caroline, Dushoff Jonathan, Li Michael, Mechai Samir, Van Domselaar Gary, Wu Jianhong, Earn David J.D., Ogden Nicholas H. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021;31:R918–R929. doi: 10.1016/j.cub.2021.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price David J., Shearer Freya M., Meehan Michael T. Early analysis of the Australian COVID-19 epidemic. eLife. 2020;9:e58785. doi: 10.7554/eLife.58785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada . 2022. COVID-19 epidemiology update. URL https://health-infobase.canada.ca/covid-19/ [Google Scholar]
- R Core Team . R Foundation for Statistical Computing, Vienna, Austria; 2022. R: A Language and Environment for Statistical Computing. URL https://www.R-project.org/ [Google Scholar]
- Russell Timothy W., Wu Joseph T., Clifford Sam, Edmunds W. John, Kucharski Adam J., Jit Mark, et al. Effect of internationally imported cases on internal spread of COVID-19: a mathematical modelling study. Lancet Public Health. 2021;6(1):e12–e20. doi: 10.1016/s2468-2667(20)30263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver Andrew. Covid-19: What went wrong after initial success in Laos? Br. Med. J. 2022;377 doi: 10.1136/bmj.o994. [DOI] [PubMed] [Google Scholar]
- Smelie Sarah. Third ship anchored off Newfoundland coast with COVID-19 cases among crew. The Globe and Mail. 2021:July 19. [Google Scholar]
- Statistics Canada . 2017. Census profile, 2016 census. URL https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. [Google Scholar]
- Statistics Canada . Government of Canada; 2021. Population Estimates, Quarterly. URL https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901. [Google Scholar]
- Steyn Nicholas, Plank Michael J., James Alex, Binny Rachelle N., Hendy Shaun C., Lustig Audrey. Managing the risk of a COVID-19 outbreak from border arrivals. J. R. Soc. Interface. 2021;18 doi: 10.1098/rsif.2021.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper Paul, Boury Himani, Yerlanov Madi, Colijn Caroline. Event-specific interventions to minimize COVID-19 transmission. Proc. Nat. Acad. Sci. 2020;17:32038–32045. doi: 10.1073/pnas.2019324117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells Chad R., Sah Pratha, Moghadas Seyed M., Pandey Abhishek, Shoukat Affan, Wang Yaning, Wang Zheng, Meyers Lauren A., Singer Burton H., Galvani Alison P. Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc. Natl. Acad. Sci. USA. 2020;117:7504–7509. doi: 10.1073/pnas.2002616117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells Chad R., Townsend Jeffrey P., Pandey Abhishek, Moghadas Seyed M., Krieger Gary, Singer Burton, McDonald Robert H., Fitzpatrick Meagan C., Galvani Alison P. Optimal COVID-19 quarantine and testing strategies. Nat. Commun. 2021;12:356. doi: 10.1038/s41467-020-20742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Xianghong, Song Yunna, Tang Sanyi, Xue Haifeng, Chen Wanchun, Qin Lingling, Jia Shoushi, Shen Ying, Zhao Shusen, Zhu Huaiping. Models to assess imported cases on the rebound of COVID-19 and design a long-term border control strategy in heilongjiang province, China. Math. Biosci. Eng. 2022;19:1–33. doi: 10.3934/mbe.2022001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional details describing the data, model parameterization, and model formulation.