Case Report
Dr. Ambrogio: We describe the case of a 39-year-old woman suffering from Hashimoto’s thyroiditis, without personal or family history of psoriasis, presenting with widespread erythematous and edematous plaques topped by non-follicular pustules.
Dr. Poli: In October 2021, the patient started treatment with metformin 500 mg/day, acetylsalicylic acid 100 mg/day, vaginal progesterone 200 mg/day and hydroxychloroquine (HCQ) 200 mg/day. These medications were prescribed by her gynecologist before in vitro fertilization (IVF) and embryo transfer. Metformin, acetylsalicylic acid and HCQ are sometimes used as adjuvant treatments in patients undergoing IVF [1–3], although the current evidence supporting their use appears to be limited. Another IVF attempt was previously made with a positive result, but the patient had a miscarriage after 7 weeks. She was not taking HCQ at that time.
Prof. Romita: No other drugs were taken during the last month. Eighteen days after introducing these drugs and 10 days after the concomitant injection of the booster dose of Pfizer-BioNTech COVID-19 vaccine (BNT162b2) and influenza vaccine, she developed erythematous itchy papules and plaques on the trunk, that spread over the entire body in a few days with the appearance of non-follicular pustules on targetoid plaques (Figs. 1 and 2).
Fig. 1.
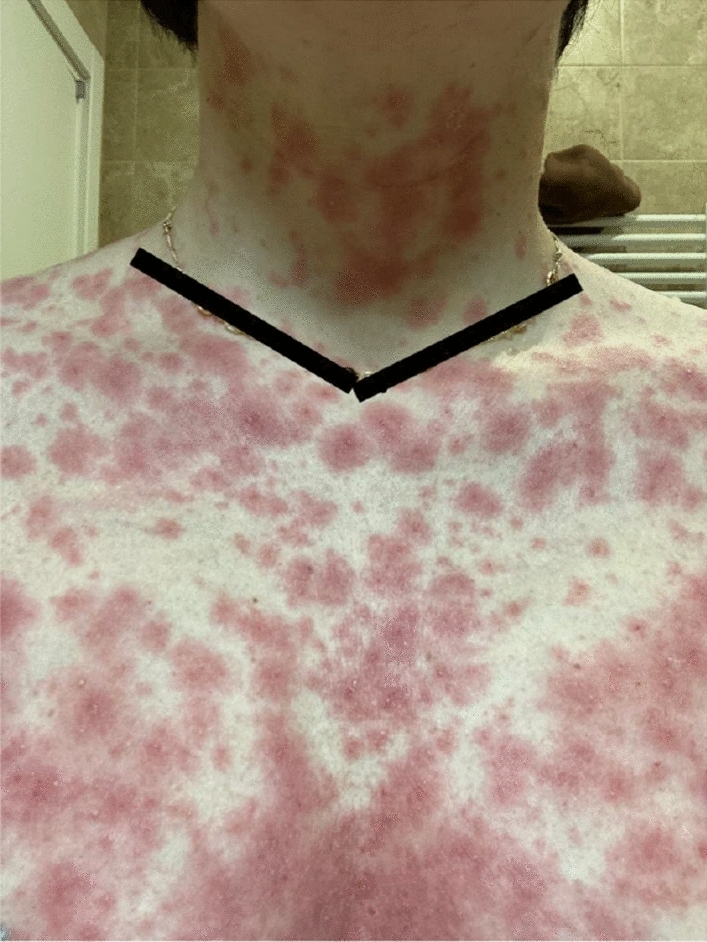
Targetoid lesions topped by pustules
Fig. 2.
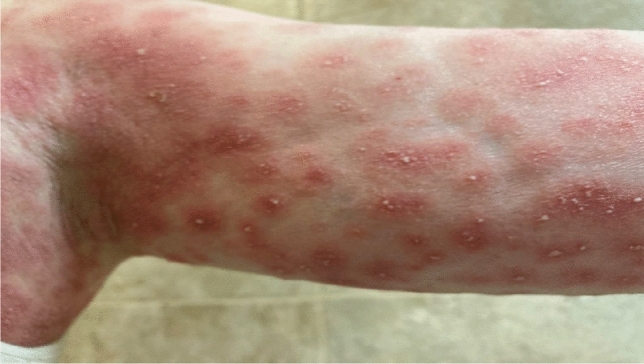
Millimetric non-follicular pustules on erythematous and edematous skin
Dr. De Marco: Skin folds, scalp, palms and soles were also involved. The mucous membranes were spared, and fever was not present. Nikolsky sign was negative and lymphadenopathy was absent.
Dr. Messina: Laboratory examinations showed neutrophilic leukocytosis (white blood cells > 20.000/μL; neutrophils 91.2%) without peripheral eosinophilia, an increase of erythrosedimentation rate (54 mm/h) and C-reactive protein (96.6 mg/L). Liver enzymes and serum creatinine were within the normal range. Bacterial cultures from pustules were negative. Viral serology and polymerase chain reaction for detection of DNA of Parvovirus B19, human herpesviruses 1, 2, 6, and 7, Epstein-Barr virus, varicella zoster virus and cytomegalovirus in blood and skin samples did not show an active infection.
Dr. Cazzato: Histopathological examination of a 5-mm punch biopsy specimen revealed spongiform subcorneal pustules with numerous neutrophils and some eosinophils. The superficial dermis showed edema and a diffuse perivascular infiltrate with a small amount of eosinophils and neutrophils. Keratinocyte necrosis and histological signs of psoriasis, interface dermatitis and vasculitis were absent.
Prof. Filotico: History, clinical examination and histopathological and laboratory findings suggested a diagnosis of acute generalized exanthematous pustulosis (AGEP) with atypical features, also possibly consistent with generalized pustular figurate erythema (GPFE) [4, 5].
Prof. Bonamonte: Following consultation with her gynecologist, treatment with all drugs was stopped. While waiting for the histopathological diagnosis, corticotherapy was started after receiving laboratory test results. The patient was treated with oral prednisone at the daily dose of 1 mg/kg for 21 days with subsequent gradual tapering, in association with levocetirizine that was administered soon after the onset of cutaneous lesions. The skin rash slowly improved with desquamation (Fig. 3) and resolved after approximately 10 weeks.
Fig. 3.
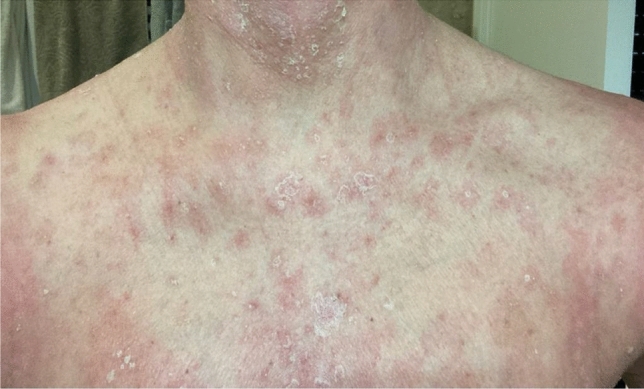
Improvement of the skin rash after 8 weeks with desquamation and reduction of erythema
Prof. Foti: One month after complete recovery, patch testing with all the above-mentioned drugs diluted at 20% in petrolatum was performed, and negative results were obtained.
Some months later, the patient was re-treated with metformin, acetylsalicylic acid and vaginal progesterone without any adverse event.
Discussion
Prof. Foti: Severe cutaneous drug reactions are well-known and include AGEP, Steven-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS). They can be caused by several different drugs, like antibiotics, anticonvulsants, allopurinol, HCQ and many others [6, 7].
Dr. De Marco: HCQ is an immunomodulatory drug that has been used to prevent and treat malaria and to treat rheumatoid arthritis and lupus erythematosus for several decades. HCQ use has exponentially increased during the initial phases of COVID-19 pandemic.
Dr. Ambrogio: Our patient received HCQ in combination with other drugs before IVF and embryo transfer. It has been suggested that immunomodulatory treatment may improve the reproductive outcomes of this procedure in patients with dysregulated immunity [8, 9]. HCQ can shift the immune response toward a Th2 pattern and has been considered a possible adjuvant tool for augmenting IVF success rates in women with repeated implantation failures and an elevated Th1/Th2 ratio [10].
Dr. Poli: It is well known that HCQ can induce cutaneous adverse reactions, including AGEP, DRESS, and SJS/TEN [11]. The onset of the skin eruption in our patient occurred 18 days after the start of treatment with metformin, acetylsalicylic acid, vaginal progesterone and HCQ. The same drugs without HCQ were administered again a few months after clinical resolution without any adverse effects, thus incriminating HCQ as the culprit agent.
Dr. Cassano: Moreover, our patient had received the COVID-19 and influenza vaccinations 10 days before the development of the cutaneous reaction. We cannot exclude the role of such vaccinations as an immunological trigger in the exacerbation of the HCQ-induced skin rash. The literature contains anecdotal reports of severe cutaneous reactions, including DRESS and AGEP, also showing overlapping features of DRESS, related to vaccines for SARS-CoV-2 [12–15]. Severe cutaneous reactions, such as DRESS, AGEP and SJS, have rarely been associated with influenza vaccination [16, 17], and in a few cases the dual synergistic role of the vaccination and a specific medication was suspected [17–19].
Dr. Messina: In our case, the diagnosis of pustular psoriasis was initially considered but it was excluded by the histological examination.
Prof. Romita: DRESS was ruled out as the typical peripheral eosinophilia and the visceral involvement were absent, as well as fever, atypical lymphocytosis, and lymphadenopathy. Viral reactivation was excluded.
Prof. Filotico: AGEP is a severe cutaneous adverse reaction characterized by the rapid development of sterile non-follicular pustules on an erythematous base [4, 20]. AGEP has been linked occasionally to infections and most commonly to medications such as pristinamycin, beta-lactams, quinolones, sulfonamides, terbinafine, diltiazem, ketoconazole, fluconazole, chloroquine and HCQ [4, 7, 20].
Prof. Vena: GPFE is a cutaneous drug reaction with widespread erythematous and edematous plaques, sometimes with targetoid erythema multiforme-like appearance or figurate pattern, scattered over the entire body, prominent on trunk and extremities, and topped by non-follicular pustules. This entity has recently been delineated and its association with HCQ has been emphasized [5], with approximately 30 cases present in the literature [5, 21]. Two cases of GPFE in SARS-CoV-2 positive patients treated with HCQ have also been reported [22]. In previous reports, alternative definitions have been used to describe similar HCQ-related cases of GPFE, such as atypical or recalcitrant AGEP, AGEP/SJS overlap, AGEP/TEN overlap, pustular DRESS syndrome, and others [5].
Prof. Bonamonte: Unlike AGEP, which has an onset typically within 1–2 days after drug administration, GPFE has a latency period of 2–3 weeks. Moreover, GPFE has a longer duration and is more recalcitrant to therapy, responding slowly to corticotherapy [21], while AGEP has usually a spontaneous resolution within 15 days from the discontinuation of the causative medication. Nevertheless, in previous reports of HCQ-induced AGEP, some authors noted more persistent and recalcitrant manifestations as compared to the usual course of AGEP, and this was attributed to the prolonged half-life of HCQ (approximately 40–50 days) [23].
Dr. Poli: In our case, the appearance of lesions during treatment with HCQ, their resolution after the discontinuation of this drug, and the description of similar cases in the literature might suggest a possible correlation.
Prof. Foti: In conclusion, when we observe a patient who develops a non-follicular pustular erythematous eruption after the administration of HCQ, it is important to consider the possible diagnosis of AGEP or GPFE, even though this appears to be a rare adverse event. The notification of new cases of GPFE can improve the recognition of clinical presentations, diagnostic criteria, and strategies for disease management.
Funding
None reported.
Declarations
Conflict of interest
None declared.
Consent to participate' and/or 'Consent to publish'
The patient gave her consent to the submission of the case report to the journal.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
The original online version of this article was revised: In this article Author name first and sur names are wrong, should be changed First name “Gino Antonio” and the Sur name “Vena”.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/22/2023
A Correction to this paper has been published: 10.1007/s11739-023-03234-7
References
- 1.Christianson MS. Metformin use in patients undergoing in vitro fertilization treatment: results of a worldwide web-based survey. J Assist Reprod Genet. 2015;32(3):401–406. doi: 10.1007/s10815-014-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mourad A, Antaki R, Jamal W, Albaini O. Aspirin for endometrial preparation in patients undergoing IVF: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2021;43:984–992.e2. doi: 10.1016/j.jogc.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghpour S. Effects of treatment with hydroxychloroquine on the modulation of Th17/Treg ratio and pregnancy outcomes in women with recurrent implantation failure: clinical trial. Immunopharmacol Immunotoxicol. 2020;42:632–642. doi: 10.1080/08923973.2020.1835951. [DOI] [PubMed] [Google Scholar]
- 4.De Groot AC. Results of patch testing in acute generalized exanthematous pustulosis (AGEP): a literature review. Contact Dermatitis. 2022;87:119–141. doi: 10.1111/cod.14075. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RA, Janniger CK. Generalized pustular figurate erythema: a newly delineated severe cutaneous drug reaction linked with hydroxychloroquine. Dermatol Ther. 2020;33:e13380. doi: 10.1111/dth.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Allergol Select. 2017;1:96–108. doi: 10.5414/ALX01508E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaabouni R, Bahloul E, Ennouri M, Atheymen R, Sellami K, Marrakchi S, et al. Hydroxychloroquine-induced acute generalized exanthematous pustulosis: a series of seven patients and review of the literature. Int J Dermatol. 2021;60(6):742–748. doi: 10.1111/ijd.15419. [DOI] [PubMed] [Google Scholar]
- 8.Sung N, Khan SA, Yiu ME, Jubiz G, Salazar MD, Skariah A, et al. Reproductive outcomes of women with recurrent pregnancy losses and repeated implantation failures are significantly improved with immunomodulatory treatment. J Reprod Immunol. 2021;148:103369. doi: 10.1016/j.jri.2021.103369. [DOI] [PubMed] [Google Scholar]
- 9.Meng S, Zhang T, Li C, Zhang X, Shen H. Immunoregulatory therapy improves reproductive outcomes in elevated Th1/Th2 women with embryo transfer failure. Biomed Res Int. 2022;2022:4990184. doi: 10.1155/2022/4990184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghasemnejad-Berenji H, Ghaffari Novin M, Hajshafiha M, Nazarian H, Hashemi SM, et al. Immunomodulatory effects of hydroxychloroquine on Th1/Th2 balance in women with repeated implantation failure. Biomed Pharmacother. 2018;107:1277–1285. doi: 10.1016/j.biopha.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Gisondi P, Piaserico S, Bordin C, Bellinato F, Tozzi F, Alaibac M, et al. The safety profile of hydroxychloroquine: major cutaneous and extracutaneous adverse events. Clin Exp Rheumatol. 2021;39:1099–1107. doi: 10.55563/clinexprheumatol/styx9u. [DOI] [PubMed] [Google Scholar]
- 12.Niebel D, Novak N, Wilhelmi J, Ziob J, Wilsmann-Theis D, Bieber T, et al. Cutaneous adverse reactions to COVID-19 vaccines: insights from an immuno-dermatological perspective. Vaccines (Basel) 2021;9:944. doi: 10.3390/vaccines9090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korekawa A, Nakajima K, Fukushi K, Nakano H, Sawamura D. Three cases of drug-induced hypersensitivity syndrome associated with mRNA-based coronavirus disease 2019 vaccines. J Dermatol. 2022;49:652–655. doi: 10.1111/1346-8138.16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96–97. doi: 10.1016/j.jdcr.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda T, Yokoyama K, Kawakami T. Overlapping acute generalized exanthematous pustulosis drug reaction with eosinophilia and systemic symptoms induced by a second dose of the Moderna COVID-19 vaccine. J Dermatol. 2022 doi: 10.1111/1346-8138.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone CA, Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt N, Levinson M, Stephenson G. Drug reaction with eosinophilia and systemic symptoms associated with H1N1 vaccination. Intern Med J. 2012;42:1365–1366. doi: 10.1111/imj.12012. [DOI] [PubMed] [Google Scholar]
- 18.Solak B, Dikicier BS, Kara RO, Erdem T (2016) DRESS syndrome potentially induced by allopurinol and triggered by influenza vaccine. BMJ Case Rep 2016:bcr2016214563 [DOI] [PMC free article] [PubMed]
- 19.Fleming JD, Fogo AJ, Creamer DJ. Stevens-Johnson syndrome triggered by seasonal influenza vaccination and flucloxacillin: a pathogenetic hypothesis. Eur J Dermatol. 2011;21:434–435. doi: 10.1684/ejd.2011.1314. [DOI] [PubMed] [Google Scholar]
- 20.Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843–848. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Bertello M, Rubegni G, Rubegni P, Tognetti L (2022) Authors' reply to: 'Generalized pustular figurate erythema first report in two COVID-19 patients on hydroxychloroquine'. J Eur Acad Dermatol Venereol in press. [DOI] [PMC free article] [PubMed]
- 22.Abadías-Granado I, Palma-Ruiz AM, Cerro PA, Morales-Callaghan AM, Gómez-Mateo MC, Gilaberte Y, et al. Generalized pustular figurate erythema first report in two COVID-19 patients on hydroxychloroquine. J Eur Acad Dermatol Venereol. 2021;35:e5–e7. doi: 10.1111/jdv.16903. [DOI] [PubMed] [Google Scholar]
- 23.Pearson KC, Morrell DS, Runge SR, Jolly P. Prolonged pustular eruption from hydroxychloroquine: an unusual case of acute generalized exanthematous pustulosis. Cutis. 2016;97:212–216. [PubMed] [Google Scholar]


