Abstract
Objective
Determine mid-term postoperative outcomes among COVID-19-positive patients compared to those who never tested positive before surgery.
Background
COVID-19 is thought to be associated with prohibitively high rates of postoperative complications. However, prior studies have only evaluated 30-day outcomes and most did not adjust for demographic, clinical, or procedural characteristics.
Methods
We analyzed data from surgeries performed at all Veterans Affairs hospitals between March 2020–2021. Kaplan-Meier curves compared trends in mortality and cox-proportional hazards models estimated rates of mortality and pulmonary, thrombotic, and septic postoperative complications between patients with a positive preoperative SARS-CoV-2 test (COVID (+)) and propensity score matched COVID(−) patients.
Results
Of 153,741 surgical patients, 4,778 COVID(+) were matched to 14,101 COVID(−). COVID(+) status was associated with higher postoperative mortality (p<0.0001) with a 6-month survival of 94.2% (95% CI 93.2–95.2 ) versus 96.0% (95.7.0–96.4 ) in COVID(−). The highest mortality was in the first 30 postoperative days. Hazards for mortality and postoperative complications in COVID(+) decreased with increasing time between testing COVID(+) and date of surgery. COVID(+) patients undergoing elective surgery had similar rates of mortality, thrombotic and septic complications, but higher rates of pulmonary complications than COVID(−) patients.
Conclusion
This is the first report of mid-term outcomes among COVID-19 patients undergoing surgery. COVID-19 is associated with decreased overall and complication-free survival primarily in the early postoperative period, delaying surgery by 5 weeks or more reduces risk of complications. Case urgency has a multiplicative effect on short- and long-term risk of postoperative mortality and complications.
Miniabstract
We present the first review of outcomes for COVID-19 positive patients up to one year after surgery. The greatest risk of complications is in the first month after surgery, risk diminishes with time from positive COVID-19 test, and emergency case designation produces a multiplicative increase in risk for complications.
Introduction
Over the last year, we have gained knowledge about the effect of performing surgery in patients who have active SARS-CoV-2 infection. This knowledge was gained during a period when elective surgery was largely suspended (approximately mid-March 2020-end of May 2020) and only emergency surgery was conducted. The bulk of these data have been analyzed for short-term outcome measures such as 30-day mortality and pulmonary complications. The results suggest a dramatically increased risk for 30-day postoperative complications when emergency surgery is performed in a patient with active or recent SARS-CoV-2 infection.1–3 One report from a large multicenter cohort showed a 30-day mortality rate of 24%.3 Our analysis of a large nationwide multicenter cohort including a mixture of emergency and elective surgeries collected both during the COVID-19 pandemic surge and subsequent recovery, demonstrated lower 30-day adverse events.4 Limited experience with elective surgery during the pandemic has precluded a reliable evaluation of differences in risks associated with performing elective versus emergency procedures in COVID-19 positive patients.
With the pandemic now extending beyond one year, the focus must shift from the short-term, 30-day postoperative complications and mortality to longer-term consequences of performing emergency and elective surgery in patients with active, recent or remote COVID-19.5–7 There is evidence that short-term pulmonary and cardiovascular complications are present when emergency and elective surgery are performed within 7 weeks of COVID-19 diagnosis by molecular testing.8 However, there are currently little data on how long the risks of morbidity and mortality among COVID-19 positive surgical patients extend past the 30-day postoperative period.
The Veterans Affairs (VA) Administration provides care to over 9 million individuals in the United States through 1,244 healthcare centers. Data from the electronic medical records on all patient encounters and surgical procedures are stored in a real-time central data repository.9 Given the lack of data on intermediate-term outcomes after surgery in COVID-19 positive patients, the prior inability to provide a detailed analysis of the interaction between case urgency and COVID-19 status, and the potential to provide more detailed and reliable estimates with larger sample size, a midterm analysis was undertaken. This report addresses 3 goals: 1) Provide the first report of mid-term postoperative outcomes in COVID(+) vs. COVID(−) patients, 2) Evaluate the impact of time from COVID(+) test to surgery on these outcomes, and 3) Evaluate the interplay between COVID(+) status and case urgency.
Methods
Study Design
This national multicenter, observational cohort study compared mid-term postoperative outcome measures in COVID-19 positive and negative patients undergoing identical surgical procedures with identical urgency classifications. The COVID-19 patients were further propensity score matched to patients who did not have COVID-19 prior to surgery. Study participants were obtained from all 1,244 VA health care centers across the US. The study was approved by the University of Maryland Institutional Review Board and VA Research and Development office. The study was determined to be minimal risk, and need for informed consent was waived.
Participants
The records for all patients who had a surgical procedure between March 1, 2020 and March 1, 2021 were analyzed. All Veterans with demographic, medical, and surgical data available in the VA electronic medical record and who had a COVID-19 status documented were considered for inclusion in the analytic cohort. If multiple procedures were performed during the study period in a COVID-19 positive patient, the procedure performed nearest to the date of the first positive test was defined as the index procedure. In the COVID-19 negative group, the first procedure was considered the index procedure.
Data collection
Data were obtained from the VA Informatics and Computing Infrastructure (VINCI) which compiles all data from the VA’s electronic medical record and updates it near real time as described previously.4 Our exposure was COVID-19 positive status (COVID (+)), defined as a positive result on SARS-CoV-2 RNA PCR test from a nasal swab or COVID-19 antigen test from a serum sample any time before surgery. Controls included patients who were COVID-19 negative (COVID (−)) before surgery. Data describing the patient’s age, sex, race (white, black, and other), ethnicity (Hispanic or Latino), body mass index (BMI), and the American Society of Anesthesiologists (ASA) physical status classification (grouped as I-II, III-IV, and V-VI)10 were obtained. Medical comorbidities were determined by reviewing International Classification of Disease system-10 (ICD-10) codes identified at inpatient and outpatient encounters in the two-years prior to the index surgical procedure and included congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), liver cirrhosis, human immunodeficiency virus (HIV), cancer, end stage renal disease (ESRD), chronic hepatitis, and diabetes. Smoking status was classified as current smoker, past smoker, or never smoker. The cumulative comorbidity burden was assessed using the Charlson Comorbidity Index (CCI), calculated from 17 different comorbidities.11 For each surgery, Current Procedural Terminology (CPT) codes were examined to determine procedural data from the full five-digit code) and organ system on which the procedure was performed (from the first two digits of the code). Anesthesia used during the procedure (general or other [composite of sedation, spinal, epidural and local]), and case urgency (elective, urgent, or emergency) were also recorded. The designation of an emergency case is reserved for immediate life- or organ-threatening conditions. Within the VA system, the protocol for designating urgent case status varies by facility. Per guidelines, an urgent procedure relates to conditions that do not require immediate surgical treatment but are deemed to warrant timely intervention to prevent deterioration of a clinical condition. Cases that do not fall under the emergency or urgent categories are designated as being elective. The final determination is made by the operating surgeon and stored under one of the above headings in the electronic medical record.
Outcomes
Our primary endpoint was all-cause mortality. Vital status and data of death, for those patients who died, is indicated in the VINCI data.12 Patients contributed person-time to the analysis from date of surgery until date of death or date of study closure (March 1, 2021). Secondary endpoints were composites of pulmonary complications (pneumonia, acute respiratory failure, or acute respiratory distress syndrome [ARDS]), thrombotic complications (venous thromboembolism [VTE], arterial thromboembolism, myocardial infarction [MI] or ischemic stroke) and septic complications (sepsis from unspecified source, gram-negative sepsis, post-procedural septic shock). The first appearance of an ICD-10 code associated with a composite outcome in the medical record counted as the date of the complication. If the earliest appearance of an ICD-10 code preceded the date of surgery, that code was excluded from the analysis to distinguish it as a pre-existing condition.
Statistical analysis
Demographic, clinical, and procedural characteristics of the two groups were compared using frequencies and percentages, means and standard deviations, or medians and interquartile ranges (IQR) as appropriate. Comparisons of categorical data were performed using Pearson’s test or Fisher’s Exact test. Student’s t-test or the Mann-Whitney-U tests were used for continuous data, as appropriate.
Propensity score matching was used to achieve balance in the distribution of covariates between the COVID (+) and COVID (−) group in a 1:3 ratio. Propensity scores were defined as the probability of testing positive for COVID-19 conditional on age, sex, race, ethnicity, BMI, smoking status, CPT code, anesthesia type, ASA class, case urgency, CCI, and cancer history. All covariates were selected a priori based on prior work4,13 and on known risk factors for COVID-19 infection and postoperative complications. The propensity-matching algorithm used an exact match on CPT code and case urgency, followed by a greedy nearest neighbor match without replacement for all other covariates. The balance was evaluated by comparing the standardized mean difference for each covariate between groups before and after propensity score matching.
For our primary and secondary outcomes, overall survival for each group was estimated using Kaplan Meier survival curves. Cox proportional hazards (CPH) models were used to calculate hazard ratios (HR) and 95% confidence intervals (95% CI) for each outcome. Survival was compared between all COVID (+) and COVID (−) patients in the matched cohort and CPH models only adjusted for preoperative COVID-19 status, as all other covariates were balanced by propensity score matching.
Second, the COVID (+) group was stratified into 2 groups according to the length of time between the positive COVID-19 test to the date of surgery: 0–4 and ≥5 weeks before surgery. These time intervals were chosen based on previous literature and available events in our cohort.8 Each level of the stratified COVID(+) group was compared to the COVID(−) group in CPH models that adjusted for age, sex, race, ethnicity, BMI, smoking status, anesthesia type, ASA class, case urgency, CCI, and cancer history to account for potential imbalances introduced by stratification.
Finally, overall and complication-free survival among the COVID (+) and COVID (−) groups was stratified by case urgency (elective, urgent, emergency). Log-rank tests were used to identify differences in survival curves, with Hochberg adjusted p-values when making multiple pairwise comparisons. Interaction between case urgency and preoperative COVID-19 status and confounding due to covariate imbalance were adjusted for in CPH models. The model output was presented as hazard ratios for mortality, pulmonary, thrombotic, or septic complications relative to patients in the COVID (−) group undergoing elective surgery. A sensitivity analysis adjusting for clustering by facility was performed but did not change our results.
Violations of the proportional hazards assumption and linear covariate relationships were assessed graphically using scaled Schoenfeld residual plots. If the proportional hazards assumption was violated, a stratified analysis was run using a cut-point determined by a visual analysis of the plot of the Schoenfeld residual. To simplify presentation of results, we present the non-stratified results in the manuscript, when no substantial differences existed. A two-sided α ≤0.05 was considered statistically significant. Statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
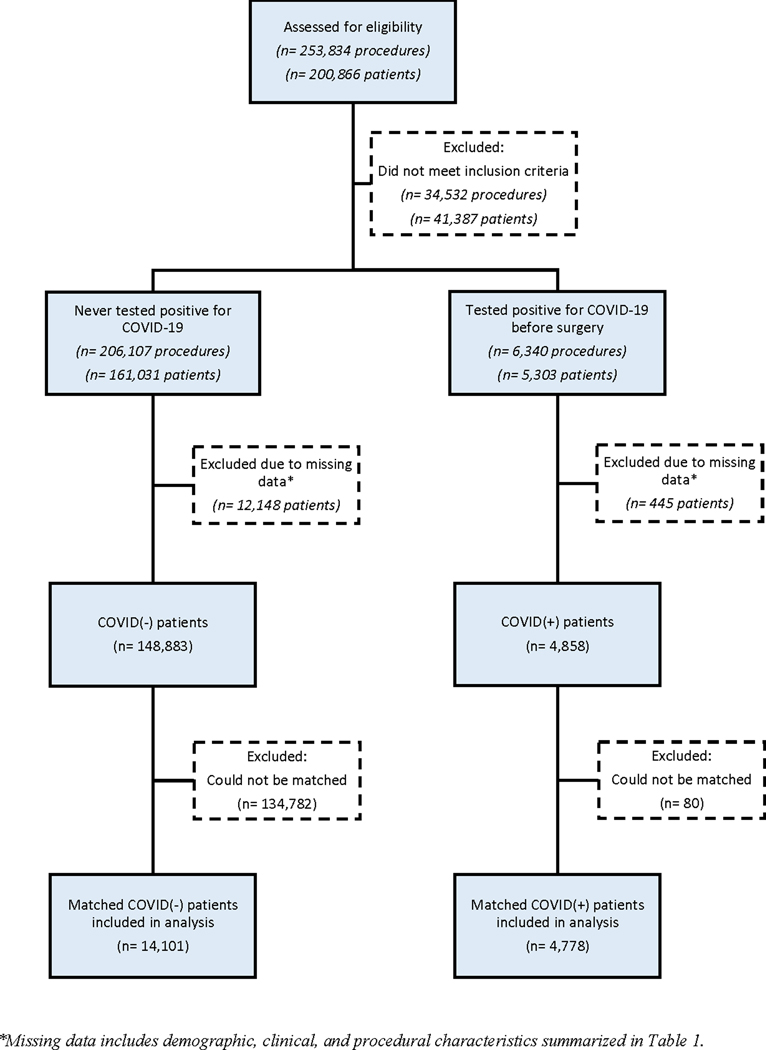
There were 200,866 patients who underwent 253,834 surgical procedures between March 1, 2020 and March 1, 2021 (Figure 1). After excluding those without data on COVID-19 status prior to surgery, or missing demographic, clinical, or procedural characteristics, 153,741 patients met criteria for inclusion in the analytic cohort. Propensity score matching yielded 4,778 COVID(+) and 14,101 COVID(−) patients. The two groups were matched in all covariates except for age and race, which demonstrated a statistically significant but biologically unimportant difference in distributions (Tables 1 and 2). Furthermore, the standardized mean differences of covariates confirmed that the two groups were well-balanced in the distribution of covariates between the COVID(+) and COVID(−) patients (data not shown). When evaluating time from COVID(+) test to surgery, patients undergoing emergency surgery were more likely to have an infection within 4 weeks (55%) compared to those undergoing elective surgery (17%; p<0.01).
Figure 1.
Flowchart for cohort creation
Table 1.
Demographic and clinical characteristics of patients undergoing surgery by COVID-19 status.
| COVID(−) N=14,101 |
COVID(+) N=4,778 |
p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age in years (median [IQR]) | 66 [56, 73] | 66 [55, 73] | 0.03 |
| Sex (% Male) | 12,776 (90.6) | 4,316 (90.3) | 0.60 |
| Race a | 0.004 | ||
| White | 9,698 (68.8) | 3,177 (66.5) | |
| Black | 3,499 (24.8) | 1,300 (27.2) | |
| Other | 904 (6.4) | 301 (6.3) | |
| Ethnicity (% Hispanic or Latino) | 1,030 (7.3) | 352 (7.4) | 0.91 |
| Body mass index (median, [IQR]) | 29.9 [26.0, 34.2] | 29.9 [26.2, 34.0] | 0.42 |
| Clinical Characteristics | |||
| Smoking | 0.74 | ||
| Never | 2,013 (14.3) | 666 (13.9) | |
| Former | 6,737 (47.8) | 2,273 (47.6) | |
| Current | 5,351 (37.9) | 1,839 (38.5) | |
| Charlson Comorbidity Index (median, [IQR]) | 2 [0, 4] | 2 [0, 4] | 0.75 |
| Congestive heart failure | 1,558 (11.0) | 566 (11.8) | 0.14 |
| Chronic obstructive pulmonary disease | 2,983 (21.2) | 986 (20.6) | 0.46 |
| Liver cirrhosis | 442 (3.1) | 146 (3.1) | 0.82 |
| Human immunodeficiency virus | 179 (1.3) | 54 (1.1) | 0.50 |
| Cancer | 4,642 (32.9) | 1,575 (33.0) | 1.00 |
| End stage renal disease | 580 (4.1) | 222 (4.6) | 0.12 |
| Chronic hepatitis | 50 (0.4) | 24 (0.5) | 0.20 |
| Diabetes | 5,748 (40.8) | 2,009 (42.0) | 0.12 |
Abbreviations: IQR, Interquartile range
Other includes Asian, American Indian or Alaskan Native, Native Hawaiian, or Other Pacific Islander.
Table 2.
Procedural characteristics of patients undergoing surgery by COVID-19 status.
| COVID(−) N=14,101 |
COVID(+) N=4,778 |
p-value | |
|---|---|---|---|
| Procedural Characteristics | |||
| ASA class | 0.11 | ||
| I or II | 2,925 (20.7) | 1030 (21.6) | |
| III or IV | 11,163 (79.2) | 3739 (78.3) | |
| V or VI | 13 (0.1) | 9 (0.2) | |
| Case Urgency | 0.40 | ||
| Elective | 10180 (72.2) | 3420 (71.6) | |
| Urgent | 3361 (23.8) | 1148 (24.0) | |
| Emergency | 560 (4.0) | 210 (4.4) | |
| Anesthesia (% General) | 7314 (51.9) | 2525 (52.8) | 0.25 |
| Organ system undergoing surgery a | |||
| Musculoskeletal | 2873 (20.4) | 979 (20.5) | 0.88 |
| Gastrointestinal | 2557 (18.1) | 860 (18.0) | 0.85 |
| Ophthalmological | 2226 (15.8) | 749 (15.7) | 0.88 |
| Urological | 2067 (14.7) | 693 (14.5) | 0.81 |
| Cardiovascular | 1147 (8.1) | 396 (8.3) | 0.76 |
| Integumentary | 1043 (7.4) | 353 (7.4) | 1.00 |
| Neurological | 895 (6.3) | 303 (6.3) | 1.00 |
| Respiratory | 800 (5.7) | 275 (5.8) | 0.86 |
| Other | 493 (3.5) | 170 (3.6) | 0.88 |
Abbreviations: ASA, American Society of Anesthesiologists; CPT, Current procedural terminology
Definition of the organ system is based on the first two digits of the CPT code. The following procedure types were grouped as “other” due to low proportions: miscellaneous, mediastinum/diaphragm, operating microscope, lymphatic, gynecological, maternity care, fine needle aspiration procedures, endocrine, auditory.
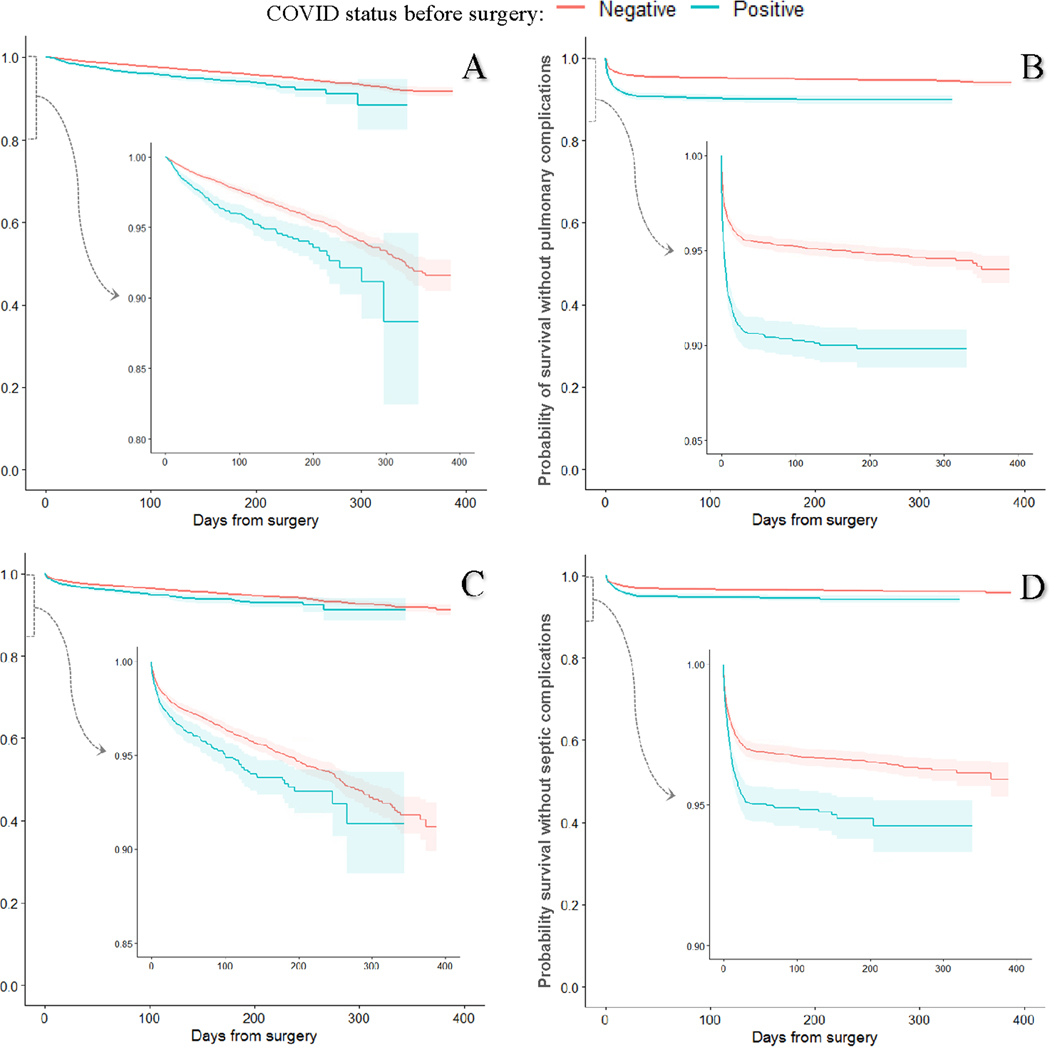
Over the study period, there were 698 deaths, and the median follow-up time was 156 (IQR 84–236) days for the entire matched cohort. There were 1,147 postoperative pulmonary complications, 859 thrombotic complications, and 709 septic complications over the study period in the entire cohort. Kaplan Meier-estimated 6-month overall survival was lower in COVID(+) compared to COVID(−) patients (94.2% vs. 96.0%, p<0.001) (Figure 2A). Pulmonary complication free and sepsis free survival fell rapidly for both groups over the first 30 days of follow-up and showed a slower decline thereafter (Figures 2B, 2D). The early rapid decrease in survival was less impressive for thrombosis-free survival (Figures 2C). Cox proportional hazards models estimated a cumulative hazard ratio of 1.60 (95% CI 1.40–1.1.00) for all-cause mortality in the COVID(+) group compared to the COVID(−) group (Table 3). The risk of pulmonary, thrombotic, and septic complications was also significantly higher in the COVID(+) group compared to the COVID(−) group (Table 3). The results of the CPH models when mortality was stratified by time to event are shown in Supplemental Table 1.
Figure 2.
Kaplan-Meier plots with 95%-confidence bands for survival among patients who tested positive for COVID-19 any time before surgery vs matched COVID-19 negative patients. Each COVID(+) patient was matched to up to 3 COVID(−) patients based on age, sex, race, ethnicity, BMI, smoking status, CPT code, anesthesia type, ASA class, case urgency, CCI, and cancer history. Outcomes were observed 3/1/20–3/26/21. (A) Survival from all-cause mortality; (B) pulmonary complications are a composite of pneumonia, acute respiratory failure, and acute respiratory distress syndrome; (C) thrombotic complications are a composite of ischemic stroke, myocardial infarction, venous thromboembolism, and arterial thromboembolism; (D) septic complications are a composite of sepsis from an unspecified source, gram-negative sepsis, and post-procedural septic shock.
Table 3.
Event rates and hazard ratios from Cox proportional hazards model for primary and secondary endpoints by COVID-19 status in the matcheda cohort
| Outcomeb | COVID(−) N (%) |
COVID(+) N (%) |
Hazard ratio (95% CI) |
|---|---|---|---|
| All-cause Mortality | 522 (0.04) | 176 (0.04) | 1.6 (1.4, 2.0) |
| Any pulmonary complication | 697 (0.05) | 450 (0.09) | 2.1 (1.9, 2.4) |
| Any thrombotic complication | 645 (0.05) | 214 (0.04) | 1.4 (1.2, 1.6) |
| Any septic complication | 472 (0.03) | 237 (0.05) | 1.6 (1.4, 1.9) |
Abbreviations: CI Confidence Interval; N/A Not applicable
Each COVID(+) patient was matched to up to 3 COVID(−) patients based on age, sex, race, ethnicity, BMI, smoking status, CPT code, anesthesia type, ASA class, case urgency, CCI, and cancer history.
Outcomes were observed 3/1/20–3/26/21. Pulmonary complications are a composite of pneumonia, acute respiratory failure, and acute respiratory distress syndrome. Thrombotic complications are a composite of ischemic stroke, myocardial infarction, venous thromboembolism, and arterial thromboembolism. Septic complications are a composite of sepsis from an unspecified source, gram-negative sepsis, and post-procedural septic shock.
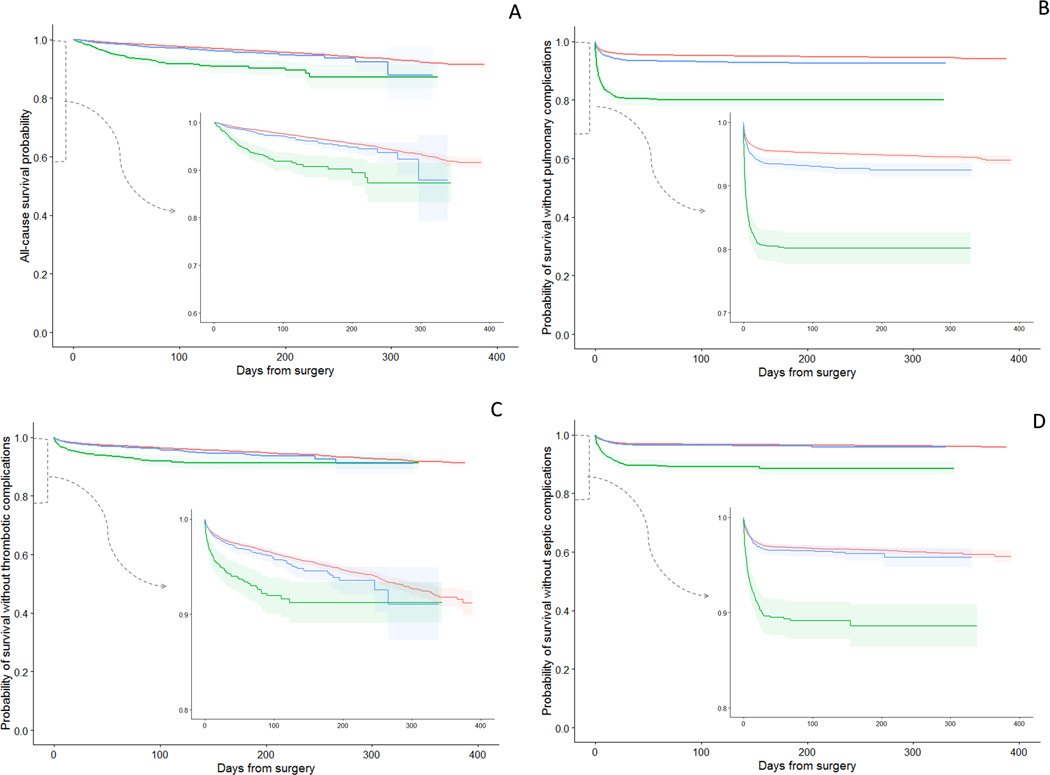
A Kaplan Meier analysis stratified by time intervals between positive preoperative COVID-19 test and the date of surgery (0–4 and ≥5 weeks) comparing matched COVID (+) and COVID (−) patients showed substantial differences in all 4 complications when the surgery was performed between 0 and 4 weeks from a COVID (+) test. When surgery was performed after a delay of 5 weeks from a positive COVID-19 test, these differences were minimal in overall survival (Figure 3A), thrombotic complications (Figure 3C), and septic complications (Figure 3D). The curves for pulmonary complications (Figure 3B) showed an improvement in complication-free-survival for those who waited 5 weeks compared to those who only waited 0–4 weeks, but at >5 weeks from a COVID-19 (+) test, pulmonary complications were still higher than those without a prior COVID-19 infection.
Figure 3.
Kaplan-Meier plots with 95% confidence bands for survival among patients who tested positive for COVID-19 at 0–4 and ≥5 weeks before surgery vs matched COVID-19 negative patients. Each COVID(+) patient was matched to up to 3 COVID(−) patients based on age, sex, race, ethnicity, BMI, smoking status, CPT code, anesthesia type, ASA class, case urgency, CCI, and cancer history. Outcomes were observed 3/1/20–3/26/21. (A) Survival from all-cause mortality; (B) pulmonary complications are a composite of pneumonia, acute respiratory failure, and acute respiratory distress syndrome; (C) thrombotic complications are a composite of ischemic stroke, myocardial infarction, venous thromboembolism, and arterial thromboembolism; (D) septic complications are a composite of sepsis from an unspecified source, gram-negative sepsis, and post-procedural septic shock.
In a Cox proportional hazards model, adjusting for all covariates originally included in the match (to account for imbalance from stratification), the cumulative hazard ratio for mortality was highest in the first month after a positive test (Table 4). In the ≥5 week group, the hazard ratio for mortality among COVID (+) patients remained significantly higher than in COVID (−) patients. The risk for all postoperative complications was highest among those who tested positive within 4 weeks before surgery (Table 4). Models for mortality, pulmonary and thrombotic complications were stratified (Supplemental table 2).
Table 4.
Event rates and hazard ratios from Cox proportional hazards model by COVID-19 status 0–4 and ≥5 weeks before surgery in the matched cohort
| Outcomea | Weeks from COVID(+) test to Surgeryb | Total events N (%) |
Hazard ratioc (95% CI) |
|
|---|---|---|---|---|
| All-cause Mortality | COVID-19(−) | 522 (0.40) | REF 1.0 | |
| 0–4 | 74 (0.07) | 2.1 (1.6, 2.7) | ||
| ≥5 | 102 (0.03) | 1.4 (1.1, 1.7) | ||
|
| ||||
| Any Pulmonary Complication | COVID-19(−) | 697 (0.05) | REF 1.0 | |
| 0–4 | 201 (0.19) | 3.4 (2.9, 4.0) | ||
| ≥5 | 249 (0.07) | 1.6 (1.4, 1.9) | ||
|
| ||||
| Any Thrombotic Complication | COVID-19(−) | 645 (0.05) | REF 1.0 | |
| 0–4 | 72 (0.07) | 1.7 (1.3, 2.1) | ||
| ≥5 | 142 (0.04) | 1.2 (1.0, 1.4) | ||
|
| ||||
| Any Septic Complication | COVID-19(−) | 472 (0.03) | REF 1.0 | |
| 0–4 | 108 (0.10) | 2.0 (1.6, 2.5) | ||
| ≥5 | 129 (0.03) | 1.2 (1.0, 1.5) | ||
Abbreviations: CI Confidence Interval; REF The reference group used to calculate all hazard ratios is the COVID(−) group, BMI body mass index; CCI Charlson comorbidity index
Outcomes were observed 3/1/20–3/26/21.Pulmonary complications are a composite of pneumonia, acute respiratory failure, and acute respiratory distress syndrome. Thrombotic complications are a composite of ischemic stroke, myocardial infarction, venous thromboembolism, and arterial thromboembolism. Septic complications are a composite of sepsis from an unspecified source, gram-negative sepsis, and post-procedural septic shock.
Time from COVID-19(+) test to surgery in weeks
Hazard-ratios from Cox-proportional hazards models adjusting for Age, race, sex, anesthesia type, ASA Class, Case urgency, Ethnicity, BMI, CCI
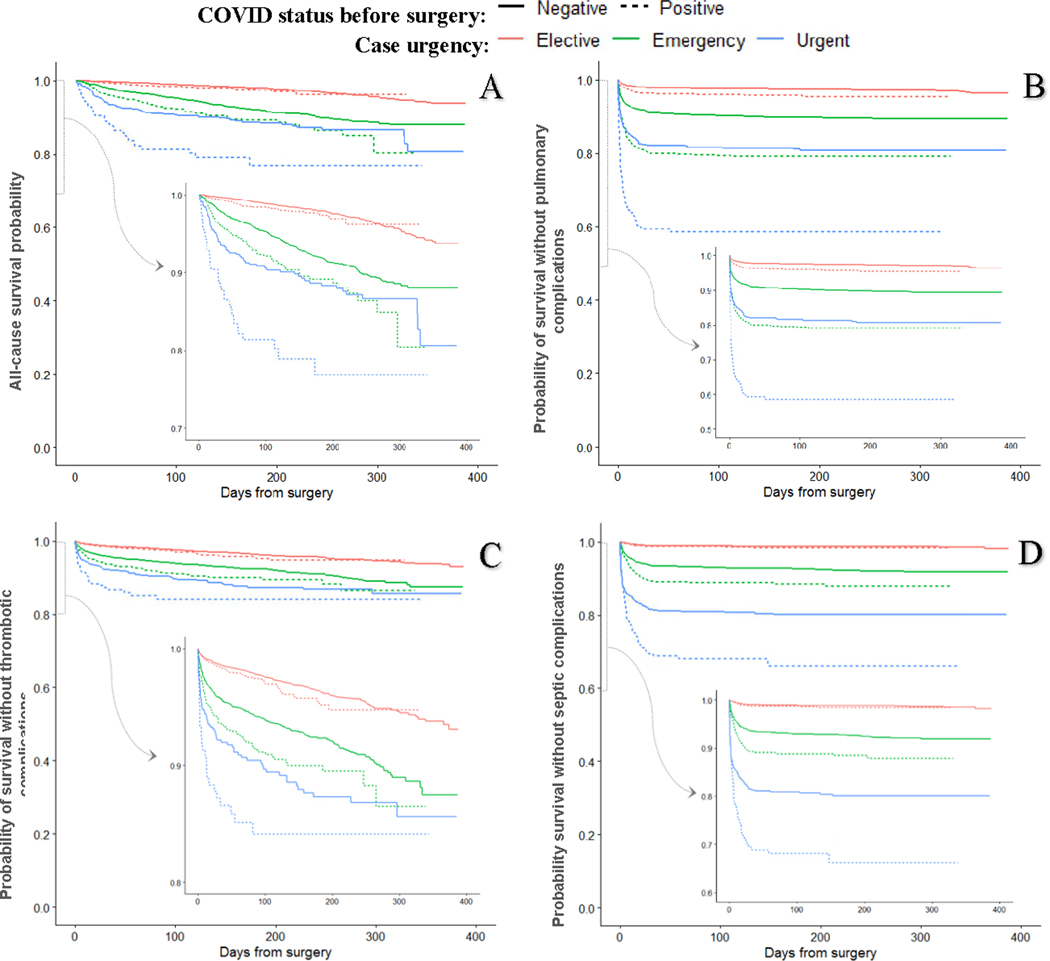
A Kaplan Meier analysis of COVID(+) versus COVID(−) patients from the matched cohort, stratified by case urgency (elective, urgent and emergency) showed no difference in 6-month overall survival (97.7 % vs. 97.9%, p= 0.06), thrombotic complication-free-survival (95.5% vs. 96.4%, p= 0.19) or septic complication-free-survival (98.4% vs. 98.8%, p=0.18) for patients undergoing elective surgery (Figure 4A, 4C, 4D). However, pulmonary-complication-free survival was lower (95.7% vs. 97.4%, p<0.001; Figure 4B). Among COVID(+) patients undergoing non-elective procedures, 6-month overall survival was lower in urgent (89.1 vs. 92.0, p=0.003) and emergency cases (76.8% vs. 88.7%, p=0.002) (Figure 4). CPH models adjusting for interaction between COVID status and case urgency, and covariates that may have become imbalanced due to stratification, showed no difference in hazard of mortality, thrombotic or septic complications comparing COVID(+) and COVID(−) undergoing elective surgery, but increased hazard for pulmonary complications (HR 1.71; 95% CI 1.39–2.11; Supplemental table 3).
Figure 4.
Kaplan-Meier plots for survival among patients who tested positive for COVID-19 any time before surgery vs matched COVID-19 negative patients, stratified by case urgency. Each COVID(+) patient was matched to up to 3 COVID(−) patients based on age, sex, race, ethnicity, BMI, smoking status, CPT code, anesthesia type, ASA class, case urgency, CCI, and cancer history. Outcomes were observed 3/1/20–3/26/21. (A) Survival from all-cause mortality; (B) pulmonary complications are a composite of pneumonia, acute respiratory failure, and acute respiratory distress syndrome; (C) thrombotic complications are a composite of ischemic stroke, myocardial infarction, venous thromboembolism, and arterial thromboembolism; (D) septic complications are a composite of sepsis from an unspecified source, gram-negative sepsis, and post-procedural septic shock.
Discussion
We present the impact of a history of SARS-CoV-2 viral infection on postoperative outcomes for a median of 156 days after surgery in a national cohort. COVID(+) patients undergoing an operation had higher mortality and pulmonary, thrombotic, and septic complications than propensity score matched COVID(−) patients. The risk for all complications was most pronounced during the early postoperative period. Postoperative mortality in COVID(+) patients was highest when surgery was performed within 4 weeks of testing positive, but some increased risk persisted even after 5 weeks from a COVID(+) test. Thrombotic and septic complications were highest within 4 weeks of a positive test but returned to near baseline after 5 weeks. The hazard for pulmonary complications persisted for greater than 5 weeks. Lastly, the increase in hazard for mortality and postoperative complications associated with COVID(+) relative to COVID(−) increased in a gradient effect with greater case urgency (Supplemental table 3).
Our data suggest that the risk of mortality and complications in COVID(+) patients is primarily elevated in the early postoperative period (Figure 2). In a recent report on the 6-month outcomes of non-surgical patients after a COVID-19 infection, a group in China found that 1.3% (33/2469) died after hospital discharge due to complications from pulmonary, cardiac, and renal dysfunction.14 The French COMBAC (Consultation Multi-Expertise de Bicêtre Après COVID-19) study performed chest computed tomography scans on COVID-19 patients 4 months after initial infection and found that 63% had persistent lung abnormalities such as subtle ground glass opacities, and 19% had overt fibrotic lesions.6 This may explain our findings that overall hazard of postoperative pulmonary complications remain elevated in patients even after delaying surgery for 5 or more weeks after a positive COVID-19 test.
Determining the impact of COVID-19 on postoperative outcomes is challenging and requires a large cohort, representing different regions and a breadth of procedures. Reports in the literature have typically presented heterogeneous single-center cohorts sampled during the early stages of the pandemic and followed only for 30 days after surgery. Inconsistent preoperative screening for SARS-CoV-2, variability in how COVID-19 was diagnosed, oversampling of emergency procedures, and pooling of data from different healthcare systems further contribute to confounded results.1,2,15–17 Our approach sampled all surgical procedures performed in a single healthcare system throughout the US, during the first full year of the pandemic that included the initial surge, plateau period, and the subsequent recurrences and recovery period. Our results offer reassurance that mortality and postoperative complications associated with preoperative SARS-CoV-2 infection are primarily elevated in the first 45 days after surgery with no significant subsequent difference in hazard relative to patients who never tested positive in the study period. While this does not argue against continued clinical surveillance, it does indicate that resources may be better allocated to monitoring other high-risk patient groups and conditions beyond the immediate postoperative period.
Our previous analysis of patients in the US based on 30-day follow-up showed similar findings of elevated postoperative risk for complications in those that tested COVID-19 positive up to 30 days before surgery.4 Our present analysis supports the findings from another multicenter study, which found elevated complications in patients testing positive up to 7 weeks prior to surgery.8 The current study also clarifies that the risk of surgery after a COVID-19 infection primarily impacts outcomes after urgent and emergency surgeries, although this may be partially due to the ability for elective cases to wait >5 weeks to reschedule their surgery. A course of two-dose vaccination (e.g. Pfizer or Moderna) against COVID-19 would require approximately 5 weeks to achieve full protection (three weeks between doses plus two weeks to develop immunity).18 The CDC recommends vaccination for all individuals, including those who have had prior COVID-19. Therefore, if a decision is made to wait at least 6 weeks before operating on patients that test positive for COVID-19, it may then be prudent to consider vaccination during the interim waiting period.
Determining the impact of COVID-19 alone on surgical outcomes is challenging as the factors that increase risk for COVID-19 also increase hazard for postoperative complications.13 The prohibitively high rates of complications for procedures performed in the early phase of the COVID-19 pandemic may have been due to interaction between SARS-CoV-2 infection, patient comorbidity burden, procedural complexity, and case urgency.1,2,15–17 We achieved balance in all of these factors between the COVID(+) and COVID(−) patients in this analysis. To assess the relationship between case urgency and COVID status, we performed a stratified survival analysis and adjusted for interaction using a Cox proportional-hazards model. This showed that the overall cumulative survival and complication-free survival for COVID(+) patients undergoing elective surgery was not significantly different than COVID(−) patients. Conversely, for patients undergoing non-elective surgery, COVID(+) status had a gradient effect on hazard of mortality and complications, with disproportionately higher mortality and complication rates in urgent and emergency procedures (Supplemental table 3). Given that it is not possible to anticipate the need for urgent or emergency procedures, this further supports the provision of COVID-19 vaccine for all individuals in the population who are at risk of infection.
Our study has several limitations inherent to working with administrative data, including reliance on ICD-10 codes for diagnosis of complications, and the inability to determine cause of death. The study sample consisted of primarily older male patients with a high comorbidity burden, limiting generalizability to other groups. As more knowledge was obtained about the diagnosis and management of COVID-19 over the course of the pandemic, this may have improved survival and reduced complications for COVID (+) patients undergoing surgery later in the study period. Despite our robust sample size, we had limited events, particularly among patients with more remote COVID-19 infections; therefore, we were not able to identify a waiting period where postoperative risks returned to baseline levels. We could not identify cases performed as a sequela of COVID-19 (i.e., tracheostomy); however, adjustment for exact CPT code and case urgency minimized differences in procedure types performed. However, our study does have several strengths. It is using data from a large national sample with broad inclusion criteria, which leads to a large sample size that increases power. The structure of the VA health system allows for close monitoring of all VA patients over time, with near-real-time availability of information about new diagnoses, hospital admissions, surgical procedures, and vital status, which decreases the likelihood of underestimation of adverse events. Overall, these data provide an estimate of the impact of SARS-CoV-2 on postoperative outcomes in a vulnerable population with high comorbidity burden, tracked since the beginning of the COVID-19 pandemic.
Conclusion
We present the first analysis of mid-term outcomes in COVID-19 positive patients undergoing surgery. The hazard for mortality and postoperative complications was higher among COVID-19 positive patients, particularly during the first 4 weeks after infection. For patients undergoing elective surgery, COVID-19 infection was associated with increased pulmonary complications but not mortality, thrombotic or septic complications. However, preoperative COVID-19 infection had a multiplicative effect on mortality and complication rates in urgent and emergency procedures.
Supplementary Material
Acknowledgments
Veterans Affairs awards HSRD C19-20-407, RRD RX000995 and CSRD CX001621, and NIH awards NS080168, NS097876 and AG000513 (BKL); National Institutes of Health awards AG028747, DK072488, and Baltimore VA Medical Centre GRECC (JDS); National Institutes of Health T32 AG00262 (NKP)
Footnotes
Conflicts of Interest and Source of Funding:
No conflicts of interest
References
- 1.Doglietto F, Vezzoli M, Gheza F, et al. Factors Associated with Surgical Mortality and Complications among Patients with and without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg. 2020. doi: 10.1001/jamasurg.2020.2713 [DOI] [PMC free article] [PubMed]
- 2.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal BK, Prasad NK, Englum BR, et al. Periprocedural complications in patients with SARS-CoV-2 infection compared to those without infection: a nationwide propensity-matched analysis. Am J Surg. 2020;In Press. [DOI] [PMC free article] [PubMed]
- 5.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin L, Savale L, Pham T, et al. Four-Month Clinical Status of a Cohort of Patients after Hospitalization for COVID-19. JAMA - J Am Med Assoc. 2021;325(15). doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Madhavan MV., Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborative C, Collaborative G. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. March 2021:anae.15458. doi: 10.1111/anae.15458 [DOI] [PMC free article] [PubMed]
- 9.U.S. Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI) homepage. 2018. https://www.hsrd.research.va.gov/for_researchers/vinci/. Accessed September 28, 2020.
- 10.Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status – historical perspectives and modern developments. Anaesthesia. 2019;74(3):373–379. doi: 10.1111/anae.14569 [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 12.Maynard C. Database & Methods Cyberseminar Series Ascertaining Veterans’ Vital Status: VA Data Sources for Mortality Ascertainment and Cause of Death. www.virec.research.va.gov. Accessed March 16, 2021.
- 13.Prasad NK, Lake R, Englum BR, et al. Increased complications in patients who test COVID-19 positive after elective surgery and implications for pre and postoperative screening. Am J Surg. 2021;0(0). doi: 10.1016/j.amjsurg.2021.04.005 [DOI] [PMC free article] [PubMed]
- 14.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayani B, Onochie E, Patil V, et al. The effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures: a multicentre cohort study. Bone Joint J. 2020;102(9):1–10. [DOI] [PubMed] [Google Scholar]
- 16.Egol KA, Konda SR, Bird ML, et al. Increased Mortality and Major Complications in Hip Fracture Care During the COVID-19 Pandemic: A New York City Perspective. J Orthop Trauma. 2020;34(8):395–402. doi: 10.1097/BOT.0000000000001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.When You’ve Been Fully Vaccinated | CDC. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html. Accessed March 23, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.