Abstract
Objective:
This article aimed to evaluate whether a substance-related diagnosis (SRD; i.e., alcohol, opioids, cannabis, stimulants, nicotine) predicts the likelihood and co-occurrence of preterm (20–37 weeks’ gestation) and cesarean delivery.
Methods:
This study reviewed electronic health record data on women (aged 18–44 years) who delivered a single live or stillbirth at ≥ 20 weeks of gestation from 2012 to 2019. Women with and without an SRD were matched on key demographic characteristics at a 1:1 ratio. Adjusting for covariates, odds ratios and 95% confidence intervals were calculated.
Results:
Of the 19,346 deliveries, a matched cohort of 2,158 deliveries was identified. Of these, 1,079 (50%) had an SRD, 280 (13%) had a preterm delivery, 833 (39%) had a cesarean delivery, and 166 (8%) had a co-occurring preterm and cesarean delivery. An SRD was significantly associated with preterm and cesarean delivery (AOR = 1.84 [95% CI, 1.41–2.39], p-value= <0.0001; AOR = 1.51 [95% CI, 1.23–1.85], p-value= <0.0001). An alcohol-related diagnosis (AOR = 1.82 [95% CI, 1.01–3.28], p-value= 0.0471), opioid-related diagnosis (AOR = 1.94 [95% CI, 1.26–2.98], p-value= 0.0027), stimulant-related diagnosis (AOR = 1.65 [95% CI, 1.11–2.45], p-value= 0.0142), and nicotine-related diagnosis (AOR = 1.54 [95% CI, 1.05–2.26], p-value= 0.0278) were associated with co-occurring preterm and cesarean delivery.
Conclusions:
Pregnant women with an SRD experienced disproportionally higher odds of preterm and cesarean delivery compared to pregnant women without an SRD. Substance-type predicts the type of delivery outcome. An SRD in pregnant women should be identified early to reduce potential harm through intervention and treatment.
Keywords: Substance use, pregnancy, preterm delivery, cesarean delivery, electronic health record data
Introduction
Women with a substance-related diagnosis (SRD; i.e., use, misuse, or dependence on substances) during pregnancy may be experiencing disproportionately higher odds of preterm (20–37 weeks’ gestation) and cesarean delivery compared to women without an SRD during pregnancy.1 Studies have shown that increases in preterm and cesarean delivery in the United States may be due to the changes in the population of women giving birth. Increases in maternal age,2 preexisting chronic health conditions (e.g., hypertension),3,4 and obesity5 have all been identified as common predictors of preterm and cesarean delivery. Studies that have examined the relationship between SRDs and preterm and cesarean delivery found mixed results and may have been limited to small sample sizes and failure to adequately control for confounding variables such as older age and preexisting chronic health conditions.6,7 Previous research has observed a significant relationship between substance use and preterm delivery.8–10 However, the type of substance and its association with preterm delivery vary. Much less is known about the relationships between prenatal substance use, cesarean delivery, and co-occurring preterm and cesarean delivery. Increasing rates of maternal substance use,11 the ongoing opioid epidemic, and the relatively recent legalization of cannabis in California12 may also impact rates of preterm and cesarean delivery in this vulnerable population. As such, updated prevalence and correlates of preterm and cesarean delivery in pregnant women with an SRD is needed.
To address the current gap in the literature, a retrospective cross-sectional study of pregnant women was conducted to evaluate whether an SRD predicts the likelihood and co-occurrence of preterm and cesarean delivery in a healthcare system that provides tertiary care and is a referral system for other providers in the community in Southern California. It was hypothesized that pregnant women with an SRD would have higher prevalence of preterm and cesarean delivery compared to pregnant women without an SRD.
Methods
Study participants and procedures
De-identified electronic health record (EHR) data on any woman (aged 18–44 years) who delivered a single live or stillbirth at ≥ 20 weeks of gestation were collected from a large health system in Southern California from January 1, 2012 through August 31, 2019 (7.8 years of available data). This healthcare system averages about 3000 deliveries per year. To protect adolescents under the age of 18 who are considered an especially vulnerable population, the sample was restricted to maternal aged ≥ 18 so those < 18 could not be identified due to small sample sizes.
Women with an International Classification of Diseases, 10th edition (ICD-10) code for a single live or stillbirth at ≥ 20 weeks of gestation were used for analysis (Table A in the supplemental material).13 Deliveries of multiple gestation were omitted due to potential differences in delivery outcomes related to more than one gestation. Data were collected from the antepartum (conception to ≤ 42 weeks) and intrapartum (labor and delivery) periods. When an individual record had more than one delivery carried to a gestational age of ≥ 20 weeks, each patient identification number (ID) and its unique delivery date represented one subject. The number of previous pregnancies for each delivery by ID number was identified by delivery codes that appeared before the most recent delivery in the dataset.
ICD-10 codes for SRDs and other mental illness diagnoses correspond with the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), which provides a more detailed description of each diagnosis (Table A in the supplemental material).13,14 An SRD, other mental illness (e.g., depression), or other preexisting health condition (e.g., cardiovascular disease) may be included in a patient chart during any outpatient visit (e.g., prenatal visit with their obstetrician, psychiatric visit), inpatient visit (e.g., hospitalization), emergency department visit (e.g., delivery), or during one of the many other types of healthcare-related visits. Each of these health-related variables is defined in detail below.
The Institutional Review Board approved the study protocol (#191588; Date of approval: October 1, 2019).15 Data were collected from the health center’s biomedical informatics team through their standardized data request process. Data were provided by staff in a secured Health Insurance Portability and Accountability Act (HIPAA)-approved Virtual Research Desktop (VRD). The VRD interface is protected by multi-factor authentication and is managed and monitored by the biomedical informatics team.
Measures
The primary outcomes of this study were preterm delivery (20–36-weeks’ gestation; yes/no), cesarean delivery (yes/no), and co-occurring preterm and cesarean delivery (yes/no). Because the type of delivery includes vaginal, cesarean, and spontaneous or therapeutic abortion, the sample used to assess cesarean delivery only included those with a cesarean or vaginal delivery. As such, those with a spontaneous or therapeutic abortion (n = 43) were omitted from the analysis for the cesarean delivery cohort but not for the preterm delivery or co-occurring preterm and cesarean delivery cohort.
The primary predictor variable was preexisting and/or new SRD (yes/no) during the antepartum and intrapartum period. Any SRD ICD-10 code for alcohol, opioids, cannabis, stimulants (i.e., cocaine and methamphetamines), nicotine, and nonspecific SRDs or other (i.e., sedatives, hallucinogens, and inhalants) were included in the SRD variable (Table A in the supplemental material). SRDs that were only identified after the intrapartum period were not included in the analysis. In addition, the relationship between an alcohol-, opioid-, cannabis-, stimulant-, or nicotine-related diagnosis and the delivery outcomes were assessed individually.
Covariates included age (18–44) and race/ethnicity (Hispanic/Latina, non-Hispanic/Latina Black, non-Hispanic/Latina White, and other race/ethnicity [American Indian/Alaskan Native, Asian/Pacific Islander, and other race or mixed)]) at delivery. Patients are asked to include their race (e.g., Black, White) and ethnicity (e.g., Hispanic/Latina, African American, and Caucasian) as separate categories on intake. To manage small cell sizes, those who did not select Hispanic/Latina, non-Hispanic/Latina Black or non-Hispanic/Latina White were grouped into the “other” category. Other variables include marital status (single, divorced/separated/widowed, or married), and Body Mass Index (BMI; calculated as weight in kilograms divided by height in meters squared) at delivery. To assess and control for the impact of previous pregnancies, one or more previous pregnancy at ≥ 20 weeks and ending in a livebirth or stillbirth (yes/no) was identified. Health insurance was defined as private (e.g., commercial), public (e.g., Medicaid) and no insurance. Those in the private insurance category could also have public insurance. Those grouped in the public insurance category did not have private insurance.
Table A in the supplemental material includes a full list of the ICD-10 codes used to identify serious mental illness (SMI; e.g., schizophrenia) and non-SMI (e.g., anxiety). A summary variable for preexisting health condition included cardiovascular disease, diabetes (non-gestational), anemia, kidney failure, hypertension, lupus erythematosus, epilepsy, pulmonary disease, cancer, human immunodeficiency virus, acquired immunodeficiency syndrome, hepatitis C virus, and tuberculosis (codes supplied on request).
Statistical analysis
Descriptive statistics were used to identify the number and type of SRD during the antepartum and intrapartum periods. Using propensity score matching, pregnant women with and without an SRD were matched 1:1 (50% with an SRD and 50% without an SRD) on known predictors of preterm and/or cesarean delivery (i.e., age at delivery, BMI at delivery, ≥ 1 previous pregnancy at ≥ 20 weeks and ending in a livebirth/stillbirth [yes/no], preexisting health condition [yes/no], and delivery year [2012–2019]17,18).16 For example, for every woman with a preexisting health condition and an SRD there is also a woman with a preexisting health condition and no SRD. Due to the reliance on ICD-10 codes, these data may represent an inconsistent distribution of ≥ 1 previous pregnancy compared to the rate in other studies (e.g., 92% vs. 73%).19 The analysis was repeated without matching on this variable, and again for primigravida women. Standardized mean differences were used to examine the balance of covariate distribution between the groups.
Unadjusted and adjusted analyses were conducted in the unmatched and matched cohorts using analysis of variance (ANOVA) for continuous data and Chi-square (χ2) tests for categorical data. To determine the effect/magnitude of the associations, unadjusted odds ratios (ORs) were calculated and reported. Two-sided tests with 95% confidence intervals (CIs) that cross one, indicating that there was no significant difference, and p-values ≥ 0.05 were used to determine whether a covariate would be included in the final adjusted regression models.
Regression analyses were performed and reported separately for (1) preterm delivery, (2) cesarean delivery, and (3) co-occurring preterm and cesarean delivery. The removal of the cases that resulted in spontaneous or elective abortion (n = 43) from the cesarean cohort created small differences in sample sizes for the preterm matched cohort (n = 2,158) and the cesarean matched cohort (n = 2,154). Due to this small difference in sample size, Figure 1 only represents the results for preterm and cesarean delivery in the preterm matched cohort.
Figure 1.
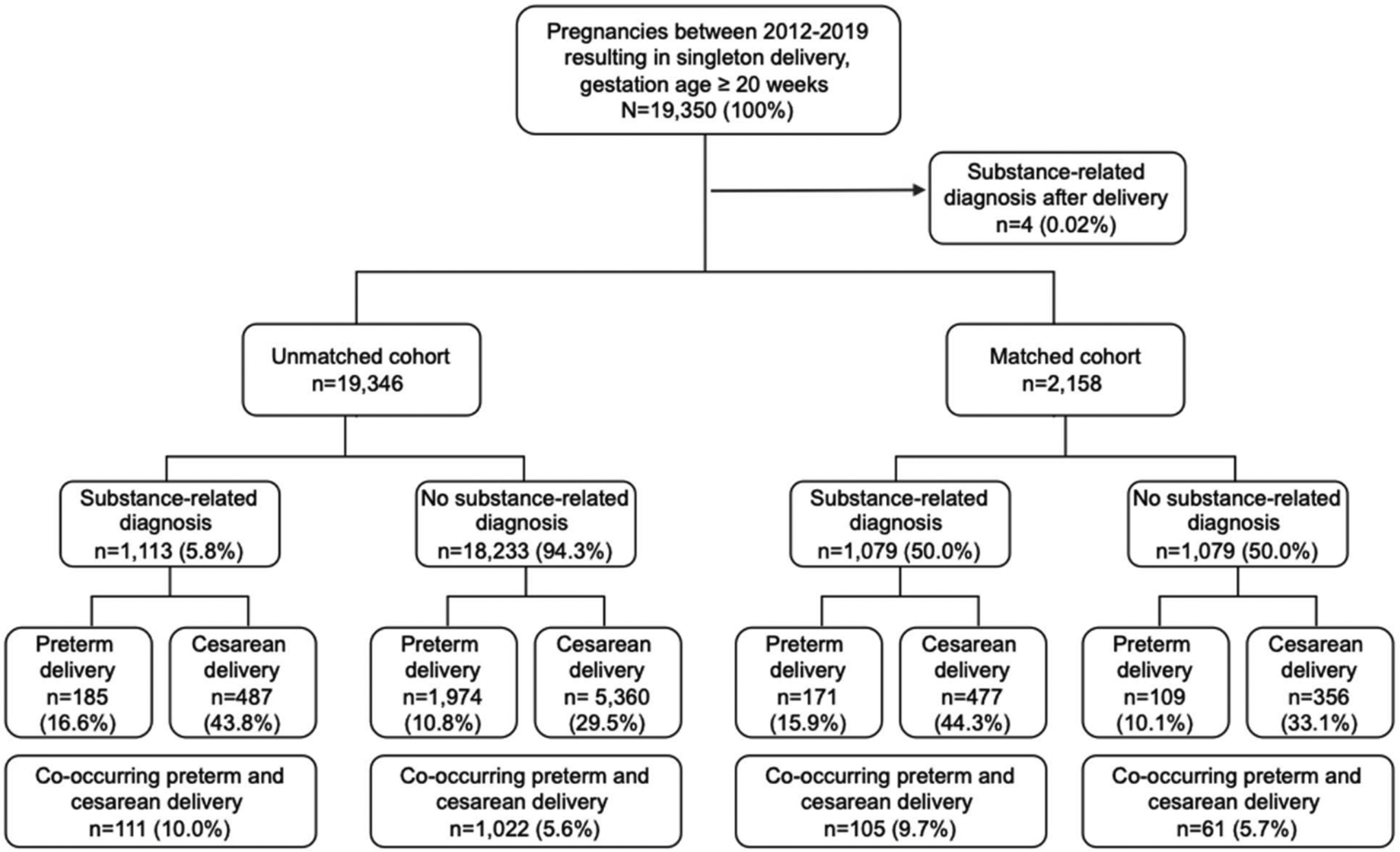
Flow chart of the study population for preterm and cesarean delivery in pregnant women in a large healthcare system from April 1st, 2011-September 30th, 2019.
Multivariable logistic regression models were conducted to determine the variables that were associated with having the three delivery outcomes compared to those without the three delivery outcomes. Standardized betas (B), standard errors (SE [B]), adjusted odds ratios (AOR), and the respective CIs and p-values were reported. Only variables significant in the unadjusted (bivariate) regression models (Tables 1 and 2) were included in the adjusted (multivariable) regression models (Table 3) for each outcome variable (preterm delivery, cesarean delivery, co-occurring preterm and cesarean delivery). As such, the variables included for the preterm delivery cohort (left side of this table) differ from the variables included in the cesarean delivery cohort (right side of this table).
Table 1.
Matched unadjusted demographic and health related characteristics and preterm delivery among women with a documented delivery from a large healthcare system’s electronic health record from April 1, 2012 to September 30, 2019 (n = 2158).
| Parameter | Total n (%)/Mean ± SD | Preterm Delivery n (%)/Mean ± SD | No preterm delivery n (%)/Mean ± SD | Odds ratio 95% (CI) | χ2 | P |
|---|---|---|---|---|---|---|
| All | 2158 (100.0) | 280 (13.0) | 1878 (87.0) | |||
| Age at delivery (ranges 18–44) | 29.9 ± 5.6 | 29.9 ± 6.1 | 29.9 ± 5.6 | 1.00 (1.00–1.02) | 0.01 | 0.9149 |
| Race/ethnicity | ||||||
| Hispanic/Latina | 201 (9.6) | 27 (9.8) | 174 (9.6) | 1.39 (0.88–2.21) | 0.04 | 0.8262 |
| Non-Hispanic/Latina Black | 214 (10.2) | 38 (13.8) | 176 (9.7) | 1.94 (1.28–2.93) | 3.92 | 0.0478 |
| Othera | 813 (38.8) | 123 (44.8) | 690 (37.9) | 1.61 (1.20–2.16) | 1.10 | 0.2948 |
| Non-Hispanic/Latina White | 869 (41.4) | 87 (31.6) | 782 (42.9) | – | ||
| Marital status | ||||||
| Single | 1017 (47.3) | 157 (56.3) | 860 (45.9) | 1.58 (1.22–2.06) | 2.06 | 0.1511 |
| Divorced/separated/widowed | 89 (4.1) | 13 (4.7) | 76 (4.1) | 1.49 (0.80–2.78) | 0.31 | 0.5804 |
| Married | 1046 (48.6) | 109 (39.1) | 937 (50.1) | – | ||
| Body Mass Index at delivery | 32.6 ± 7.6 | 31.8 ± 7.6 | 32.1 ± 7.6 | 0.98 (0.97–0.99) | 3.38 | 0.0540 |
| ≥1 previous pregnancyb | ||||||
| No | 1986 (92.0) | 258 (92.1) | 1728 (92.0) | 1.02 (0.64–1.63) | 0.01 | 0.9259 |
| Yes | 172 (8.0) | 22 (7.9) | 150 (8.0) | – | ||
| Health insurance | ||||||
| Public | 484 (22.4) | 71 (25.4) | 413 (22.0) | 1.18 (0.88–1.58) | 2.15 | 0.2528 |
| No insurance | 211 (9.8) | 22 (7.9) | 189 (10.1) | 0.83 (0.53–1.31) | 1..31 | 0.2528 |
| Private | 1463 (67.8) | 187 (66.8) | 1276 (67.9) | – | ||
| Substance-related diagnosis | ||||||
| Yes | 1079 (50.0) | 171 (61.1) | 908 (48.4) | 1.66 (1.28–2.14) | 15.0 | 0.0001 |
| No | 1079 (50.0) | 109 (38.9) | 970 (51.7) | – | ||
| Serious mental illness | ||||||
| Yes | 163 (7.6) | 29 (10.4) | 134 (7.1) | 1.50 (0.98–2.28) | 3.50 | 0.0614 |
| No | 1995 (92.5) | 251 (89.6) | 1744 (92.9) | – | ||
| Non-serious mental illness | ||||||
| Yes | 709 (32.9) | 100 (35.7) | 609 (32.4) | 0.97 (0.76–1.25) | 0.054 | 0.8165 |
| No | 1449 (67.1) | 180 (64.3) | 1269 (67.6) | – | ||
| Preexisting health condition | ||||||
| Yes | 1078 (19.9) | 175 (62.5) | 903 (48.1) | 1.78 (1.38–2.30) | 19.27 | <0.0001 |
| No | 1080 (50.1) | 105 (37.5) | 975 (51.9) | – |
Notes: Matched age at delivery by non-preterm delivery: n = 1878, median = 30, ranges = 18–44. Matched BMI at delivery by preterm delivery: n = 280, median = 31.4, ranges = 18.9–71.5. Matched BMI at delivery by non-preterm delivery: n = 1878, median = 32.7, ranges = 14.4–101.2.). Variable totals may not sum to column totals due to missing data.
Other race/ethnicity includes American Indian/Alaskan Native (n = 15), Asian/Pacific Islander (n = 139), and other race or mixed race/ethnicity (n = 659).
≥ 1 previous pregnancy at ≥20 weeks’ gestation ending in a livebirth or stillbirth. Matched age at delivery by preterm delivery: n = 280, median = 30, ranges = 18–42.
Table 2.
Matched unadjusted demographic and health-related characteristics by cesarean delivery among women with a documented delivery from a large healthcare system’s electronic health record from April 1, 2012 to September 30, 2019 (n = 2154).
| Parameter | Total n (%)/Mean ± SD | Cesarean delivery (%)/Mean ± SD | Vaginal delivery n (%)/Mean ± SD | Odds ratio 95% (CI) | χ2 | P |
|---|---|---|---|---|---|---|
| All | 2154 (100.0) | 833 (38.7) | 1321 (61.3) | |||
| Age at delivery (ranges 18–44) | 29.9 ± 5.6 | 30.8 ± 5.6 | 29.3 ± 5.5 | 1.05 (1.04–1.07) | 38.56 | <0.0001 |
| Race/ethnicity | ||||||
| Hispanic/Latina | 201 (9.6) | 80 (9.9) | 121 (9.4) | 1.13 (0.83–1.55) | 0.02 | 0.9133 |
| Non-Hispanic/Latina Black | 211 (10.1) | 95 (11.7) | 116 (9.1) | 1.40 (1.04–1.90) | 3.19 | 0.0739 |
| Othera | 813 (38.8) | 316 (39.0) | 497 (38.8) | 1.09 (0.90–1.33) | 0.45 | 0.5031 |
| Non-Hispanic/Latina White | 868 (41.5) | 320 (39.5) | 548 (42.8) | – | ||
| Marital status | ||||||
| Single | 1015 (47.3) | 418 (50.4) | 597 (45.3) | 1.32 (1.12–1.58) | 1.41 | 0.2352 |
| Divorced/separated/widowed | 88 (4.1) | 49 (5.9) | 40 (3.0) | 2.37 (1.53–3.70) | 10.89 | 0.0010 |
| Married | 1044 (48.6) | 362 (43.7) | 682 (51.7) | – | ||
| Body mass index at delivery | 32.6 ± 7.6 | 34.4 ± 8.3 | 31.4 ± 6.8 | 1.05 (1.04–1.07) | 73.08 | <0.0001 |
| ≥1 previous pregnancyb | ||||||
| No | 1982 (92.0) | 774 (92.9) | 1208 (91.4) | 1.23 (0.88–1.70) | 1.51 | 0.2205 |
| Yes | 172 (8.0) | 59 (7.1) | 113 (8.6) | – | ||
| Health insurance | ||||||
| Public | 482 (22.4) | 199 (23.9) | 283 (21.4) | 1.16 (0.94–1.43) | 1.37 | 0.2426 |
| No insurance | 211 (9.8) | 80 (9.6) | 131 (9.9) | 1.02 (0.76–1.37) | 0.15 | 0.6987 |
| Private | 1461 (67.8) | 554 (66.5) | 907 (62.1) | – | ||
| Substance-related diagnosis | ||||||
| Yes | 1077 (50.0) | 477 (57.3) | 600 (45.4) | 1.61 (1.35–1.92) | 28.50 | <0.0001 |
| No | 1077 (50.0) | 356 (42.7) | 721 (54.6) | – | ||
| Serious mental illness | ||||||
| Yes | 162 (7.5) | 82 (9.8) | 80 (6.1) | 1.69 (1.23–2.34) | 10.35 | 0.0013 |
| No | 1992 (92.5) | 751 (90.2) | 1241 (93.9) | – | ||
| Non-serious mental illness | ||||||
| Yes | 707 (32.8) | 287 (34.5) | 420 (31.8) | 1.18 (0.99–1.41) | 45.87 | 0.0725 |
| No | 1447 (67.2) | 546 (65.6) | 901 (68.2) | – | ||
| Preexisting health condition | ||||||
| Yes | 1076 (49.9) | 494 (59.3) | 582 (44.1) | 1.84 (1.54–2.19) | 45.87 | <0.0001 |
| No | 1078 (50.1) | 339 (40.7) | 739 (55.9) | – | ||
Notes: Matched age at delivery by cesarean delivery: n = 837, median = 31.0, Ranges = 18–44. Matched age at delivery by vaginal delivery: n = 1325, median = 29.0, ranges = 18–44. Matched BMI at delivery by cesarean delivery: n = 837, median = 32.8, ranges = 20.1–72.3. Matched BMI at delivery by non-preterm delivery: n = 1325, median = 30.2, ranges = 14.4–101.2.). Variable totals may not sum to column totals due to missing data.
Other race/ethnicity includes American Indian/Alaskan Native (n = 15), Asian/Pacific Islander (n = 139), and other race or mixed race/ethnicity (n = 659).
≥1 previous pregnancy at ≥ 20 weeks’ gestation ending in a livebirth or stillbirth.
Table 3.
Matched adjusted analysis of factors associated with a preterm and cesarean delivery among women with a documented delivery from a large healthcare system’s electronic health record from April 1, 2012 to September 30, 2019.
| Preterm delivery matched cohort (n = 2097) | Cesarean delivery matched cohort (n = 2148) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | B | SE (B) | Adjusted odds ratio (95% CI) | χ2 | B | SE (B) | Adjusted odds ratio (95% CI) | χ2 |
| Age at delivery (ranges 18–44) | 0.05 | 0.01 | 1.05 (1.03–1.07) | 33.47*** | ||||
| Race/ethnicity | ||||||||
| Hispanic/Latina | 0.01 | 0.17 | 1.48 (0.92–2.36) | 0.00 | ||||
| Non-Hispanic/Latina Black | 0.20 | 0.15 | 1.79 (1.17–2.73) | 1.67 | ||||
| Othera | 0.18 | 0.11 | 1.76 (1.30–2.38) | 2.83 | ||||
| Non-Hispanic/Latina White | – | |||||||
| Marital status | ||||||||
| Single | −0.04 | 0.09 | 1.17 (0.96–1.44) | 0.16 | ||||
| Divorced, separated, and widowed | 0.23 | 0.15 | 1.54 (0.96–2.45) | 2.29 | ||||
| Married | ||||||||
| Body Mass Index at delivery | −0.03 | 0.01 | 0.98 (0.96–0.99) | 1.18** | 0.05 | 0.01 | 1.05 (1.04–1.07) | 63.25*** |
| Substance-related diagnosis | ||||||||
| Yes | 0.27 | 0.07 | 1.84 (1.41–2.39) | 16.10*** | 0.20 | 0.05 | 1.51 (1.23–1.85) | 15.60*** |
| No | – | – | ||||||
| Serious mental illness | ||||||||
| Yes | 0.09 | 0.09 | 1.20 (0.85–1.69) | 1.03 | ||||
| No | – | |||||||
| Preexisting health condition | ||||||||
| Yes | 0.30 | 0.07 | 1.73 (1.32–2.26) | 20.29*** | 0.26 | 0.05 | 1.67 (1.39–2.01) | 30.21*** |
| No | – | – | ||||||
Notes: B, standardized betas, SE (B), standard errors; and CI, confidence interval.
Only variables significant in the unadjusted (bivariate) regression models (Tables 1 and 2) were included in the adjusted (multivariable) regression models (Table 3) for each outcome variable (preterm delivery, cesarean delivery). As such, the variables included for the preterm delivery cohort (left side of this table) differ from the variables included in the cesarean delivery cohort (right side of this table).
Other race/ethnicity includes American Indian/Alaskan Native (n = 15), Asian/Pacific Islander (n = 141), and other race or mixed race/ethnicity (n = 659).
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
The same analyses mentioned previously were then conducted by substance type. Only the results from the individual multivariable regressions by substance type, not the covariates (e.g., age, BMI), were reported. All analyses were conducted with SAS 9.4 (SAS Institute, Cary, North Carolina).
Results
Sample characteristics in the unmatched cohort
There were 19,350 deliveries with an ICD-10 code for a single delivery at ≥ 20 weeks’ gestation (Figure 1). Four individuals were diagnosed with an SRD after delivery and were removed from the analysis. Of the 19,346 deliveries in the unmatched cohort, preterm and cesarean deliveries were reported in 2159 (11%) and 5847 (30%) deliveries respectively (Table B in the supplemental material). Of these, 1133 (6%) had a co-occurring preterm cesarean delivery. An SRD was reported in 1113 (6%) in the preterm cohort and 1111 (6%) in the cesarean cohort. When grouped by SRD type, the most common SRD included nicotine (16%), cannabis (16%), stimulants (14%), opioids (10%), and alcohol (5%). In those with an SRD, 185 (17%) had a preterm delivery, 487 (44%) had a cesarean delivery, and 111 (10%) had a co-occurring preterm and cesarean delivery.
Sample characteristics in the matched cohort
In the preterm (n = 2158) and cesarean delivery (n = 2154) matched cohorts, 280 (13%) preterm deliveries and 833 (39%) cesarean deliveries were reported (Tables 1 and 2). Of these, 166 (8%) had a co-occurring preterm cesarean delivery. Due to matching on SRD, an SRD was reported in 1,079 (50%) in the preterm cohort and 1,077 (50%) in the cesarean cohort. Figure 2 represents the distribution of delivery outcomes by SRD type. In those with an SRD, 171 (16%) had a preterm delivery, 477 (44%) had a cesarean delivery, and 105 (10%) had a co-occurring preterm and cesarean delivery (Figures 1 and 2). Women with an SRD and co-occurring preterm and cesarean delivery accounted for 2 of the 4 spontaneous abortions.
Figure 2.
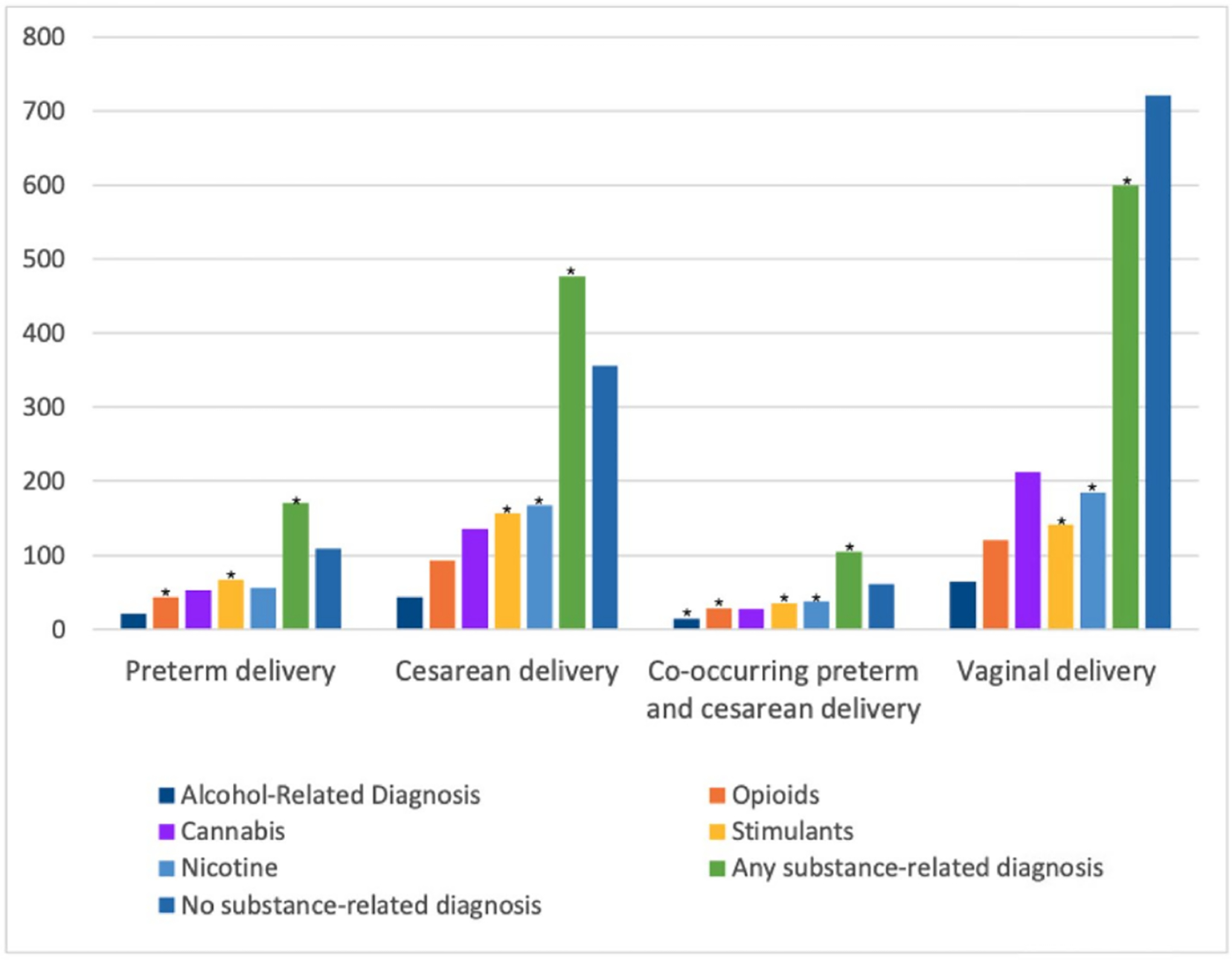
Distribution of delivery outcomes by substance-related type in matched cohort of pregnant women from January 1, 2012 to August 31st, 2019 (n = 2,158). *Results show that opioid-, stimulant-, and any substance-related diagnoses were significantly associated with preterm delivery. Stimulant-, nicotine-, and any substance-related diagnoses were significantly associated with cesarean delivery. Alcohol-, opioid-, stimulant-, nicotine-, and any substance-related diagnoses were significantly associated with co-occurring preterm and cesarean delivery. A cannabis-related diagnosis was not significantly associated with preterm or cesarean delivery. The sample size and percentage for each substance by those with and without preterm and cesarean delivery can be found in Table 4.
In the preterm and cesarean delivery matched cohorts, most were Non-Hispanic/Latina White (41%) or of other race/ethnicity (39%; i.e., American Indian/Alaskan Native [n = 15], Asian/Pacific Islander [n = 139], and other race or mixed race/ethnicity [n = 659]) with a mean age of 29.9 ([SD] = 5.6, ranges 18–44 years; Tables 1 and 2). Most were married (49%) or single (47%), had no previous pregnancies (92%), had private health insurance (68%), and a mean BMI at delivery of 32.6 (SD =7.6, ranges 14.4–101.2). SMI and non-SMIs were documented for 8% and 33%, respectively. Preexisting health conditions were documented for 50% due to matching.
Prevalence and correlates of preterm delivery
In the matched adjusted analysis, an SRD (AOR = 1.84 [95% CI, 1.41–2.39], p-value <0.0001), lower BMI at delivery (AOR = 0.98 [95% CI, 0.96–0.99], p-value = 0.0074), and preexisting health condition (AOR = 1.73 [95% CI, 1.32–2.26], p-value = <0.0001) were significantly associated with preterm delivery (Table 3).
In the matched adjusted analysis by substance type, opioid-related diagnosis (AOR = 2.00 [95% CI, 1.37–2.90], p-value = 0.0003), and stimulant-related diagnosis (AOR = 2.25 [95% CI, 1.64–3.09], p-value = <0.0001) were significantly associated with preterm delivery (Table 4).
Table 4.
Matched unadjusted and adjusted analysis of substance-related diagnosis types associated with preterm and cesarean delivery among women with a documented delivery from a large healthcare system’s electronic health record from April 1, 2012 to September 30, 2019.
| Parameter | Yes | No | Odds ratio 95% (CI) | χ 2 | B | SE (B) | Adjusted odds ratio (95% CI) | χ 2 |
|---|---|---|---|---|---|---|---|---|
| Preterm delivery unadjusted matched cohort (n = 2158) | Preterm delivery adjusted matched cohort (n = 2097) | |||||||
| Alcohol | ||||||||
| Yes | 21 (7.5) | 88 (4.7) | 1.65 (1.01–2.70) | 3.95* | 0.23 | 0.13 | 1.58 (0.95–2.64) | 3.07 |
| No | 260 (92.5) | 1789 (95.3) | – | – | ||||
| Opioids | ||||||||
| Yes | 44 (15.7) | 170 (9.1) | 1.87 (1.31–2.68) | 11.78*** | 0.34 | 0.10 | 2.00 (1.37–2.90) | 13.10*** |
| No | 237 (84.3) | 1707 (90.9) | – | – | ||||
| Cannabis | ||||||||
| Yes | 53 (18.9) | 296 (15.8) | 1.25 (0.90–1.73) | 1.79 | ||||
| No | 227 (81.1) | 1582 (84.3) | – | |||||
| Stimulants | ||||||||
| Yes | 67 (23.8) | 232 (12.4) | 2.23 (1.64–3.03) | 26.26*** | 0.40 | 0.08 | 2.25 (1.64–3.09) | 25.13*** |
| No | 214 (76.2) | 1645 (87.6) | – | |||||
| Nicotine | ||||||||
| Yes | 56 (19.9) | 298 (15.9) | 1.33 (0.97–1.82) | 3.03 | ||||
| No | 225 (80.1) | 1579 (84.1) | – | |||||
| Alcohol | ||||||||
| Yes | 44 (5.3) | 65 (4.9) | 1.08 (0.73–1.60) | 0.14 | ||||
| No | 789 (94.7) | 1256 (95.1) | – | |||||
| Opioids | ||||||||
| Yes | 93 (11.2) | 121 (9.2) | 1.25 (0.94–1.66) | 2.29 | ||||
| No | 740 (88.8) | 1200 (90.8) | – | |||||
| Cannabis | ||||||||
| Yes | 136 (16.3) | 212 (16.1) | 1.02 (0.81–1.29) | 0.03 | ||||
| No | 697 (83.7) | 1109 (84.0) | – | |||||
| Stimulants | ||||||||
| Yes | 157 (18.9) | 142 (10.8) | 1.93 (1.51–2.47) | 27.40*** | 0.22 | 0.69 | 1.54 (1.18–2.02) | 10.04*** |
| No | 676 (81.2) | 1179 (89.3) | – | – | ||||
| Nicotine | ||||||||
| Yes | 168 (20.2) | 184 (13.9) | 1.56 (1.24–1.97) | 14.41*** | 0.15 | 0.06 | 1.36 (1.06–1.74) | 5.94* |
| No | 665 (79.8) | 1137 (86.1) | – | |||||
| Alcohol | ||||||||
| Yes | 14 (8.4) | 95 (4.8) | 1.84 (1.03–3.30) | 4.17* | 0.30 | 0.15 | 1.82 (1.01–3.28) | 3.94* |
| No | 152 (91.6) | 1897 (95.2) | – | |||||
| Opioids | ||||||||
| Yes | 29 (17.5) | 185 (9.3) | 2.07 (1.35–3.17) | 11.05*** | 0.33 | 0.11 | 1.94 (1.26–2.98) | 9.00** |
| No | 137 (82.5) | 1807 (90.7) | – | |||||
| Cannabis | ||||||||
| Yes | 28 (16.9) | 321 (16.1) | 1.06 (0.70–1.61) | 0.06 | ||||
| No | 138 (83.1) | 1671 (83.9) | – | |||||
| Stimulants | ||||||||
| Yes | 35 (21.1) | 264 (13.3) | 1.75 (1.18–2.60) | 7.7** | 0.25 | 0.12 | 1.65 (1.12–2.45) | 6.08** |
| No | 131 (78.9) | 1728 (86.8) | – | – | ||||
| Nicotine | ||||||||
| Yes | 38 (22.9) | 316 (16.0) | 1.58 (1.08–2.31) | 5.44** | 0.22 | 0.10 | 1.54 (1.05–2.26) | 4.84* |
| No | 128 (77.1) | 1676 (84.1) | – | |||||
Notes: SD, standard deviation; CI, confidence interval; P-values based on Chi-square (χ2) tests of significance for categorical data and analysis of variance (ANOVA) for continuous data; B, standardized betas; SE (B), standard errors; CI, confidence interval.
Variable totals may not sum to column totals due to missing data. This table represents the results of 15 unadjusted and 15 adjusted regression models for the three outcomes: (1) preterm delivery, (2) cesarean delivery, (3) co-occurring preterm and cesarean delivery. As such, only the results from the individual multivariable regressions by substance type, not the covariates (e.g., age, BMI), are reported in this table.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
Prevalence and correlates of cesarean delivery
In the matched adjusted analysis, an SRD (AOR = 1.51 [95% CI, 1.23–1.85], p-value = <0.0001), age at delivery (AOR = 1.05 [95% CI, 1.03–1.07], p-value = <0.0001), BMI at delivery (AOR = 1.05 [95% CI, 1.04–1.07], p-value = <0.0001), and preexisting health condition (AOR = 1.67 [95% CI, 1.39–2.01], p-value = <0.0001) were associated with cesarean delivery (Table 3).
In the matched adjusted analysis for cesarean delivery by substance type, stimulant-related diagnosis (AOR = 1.54 [95% CI, 1.18–2.02], p-value = 0.0014), and nicotine-related diagnosis (AOR = 1.36 [95% CI, 1.06–1.74], p-value = 0.0155) were associated with cesarean delivery (Table 4).
Prevalence and correlates of co-occurring preterm and cesarean delivery
In the matched adjusted analysis, an SRD (AOR = 1.81 [95% CI, 1.30–2.52], p-value = 0.0004) and preexisting health condition (AOR = 2.42 [95% CI, 1.72–3.41], p-value = <0.0001) were associated with co-occurring preterm and cesarean delivery (data not shown in tables).
In the matched adjusted analysis for co-occurring preterm and cesarean delivery by substance type, alcohol-related diagnosis (AOR = 1.82 [95% CI, 1.01–3.28], p-value = 0.0471), opioid-related diagnosis (AOR = 1.94 [95% CI, 1.26–2.98], p-value = 0.0027), stimulant-related diagnosis (AOR = 1.65 [95% CI, 1.11–2.45], p-value = 0.0142), and nicotine-related diagnosis (AOR = 1.54 [95% CI, 1.05–2.26], p-value = 0.0278) were associated with co-occurring preterm and cesarean delivery (Figure 2 and Table 4).
Discussion
In a large matched pregnancy cohort at a tertiary care center from 2012 to 2019, women with a preterm delivery were more likely to have an SRD, an opioid- or stimulant-related diagnosis, lower BMI, and a preexisting health condition. Women with a cesarean delivery were more likely to have an SRD, a stimulant- or nicotine-related diagnosis, older age, a higher BMI, and preexisting health condition. Finally, women with a co-occurring preterm and cesarean delivery were more likely to have an SRD, an alcohol-, opioid, stimulant, or nicotine-related diagnosis, and a preexisting health condition. As such, only a cannabis-related diagnosis was not found to be significantly associated with preterm or cesarean delivery.
Findings from this study substantiate the previous findings of an increased risk for preterm delivery,8–10 but not cesarean delivery,10,20 in pregnant women who use opioids. This may be due to the regional variability associated with Southern California. Variance in these findings may also be related to differences in identifying pregnant women with an ICD-10 code for an opioid-related diagnosis (i.e., use, misuse, or dependence) compared to using a clinical assessment that requires a formal diagnosis per the DSM-5 to identify an opioid use disorder (OUD), which is a type of opioid-related diagnosis. Differences in these findings may also be related to the unmeasured and unknown impact of opioid agonist therapy (e.g., methadone) on delivery outcomes. Interestingly, an opioid-related diagnosis remained significantly associated with co-occurring preterm and cesarean delivery. This indicates that the strength of the relationship between opioid use and preterm delivery may be driving this observed association.
One study found that preterm delivery was more common in methadone-exposed deliveries (25%) compared to buprenorphine-exposed deliveries (14).21 This suggests that there may be maternal physiological changes related to different agonist therapies. Additional research on how untreated OUDs and treated OUDs with medications for opioid use disorder (e.g., methadone, buprenorphine) during pregnancy impacts delivery outcomes is needed to determine the safety and utility of these essential medications.
A similar association between stimulant use and preterm delivery has been observed in previous studies.22,23 A systematic review and meta-analysis of 31 studies found that cocaine use was associated with an increased risk for preterm delivery.24 Cocaine use in pregnant women has also been found to lead to severe hypertension, hyperreflexia, proteinuria, edema, and seizures, which are outcomes that may present as preeclampsia.25 In some older studies, an increased risk of placental abruption,26 uterine rupture,27 miscarriage,28 and stillbirth29 have been identified in women who use cocaine during pregnancy.23
Currently, there is limited research on the relationship between substance use and cesarean delivery. In this study, stimulant- and nicotine-related diagnoses were the strongest predictors of cesarean delivery. This finding is supported by a previous study which found that prenatal methamphetamine use was significantly associated with preterm delivery, cesarean delivery, and maternal intensive care unit admission.30 A significant relationship between nicotine use during the perinatal period and preterm or cesarean delivery has also been observed in the literature.31,32 In this current study, nicotine was significantly associated with cesarean delivery, but not preterm delivery. However, a nicotine-related diagnosis remained significantly associated with co-occurring preterm and cesarean delivery, indicating that the strength of the relationship between nicotine use and cesarean delivery may be driving this observed association.
An alcohol-related diagnosis was not found to be significantly associated with preterm or cesarean delivery individually However, a significant association with an alcohol-related diagnosis was observed in women with co-occurring preterm and cesarean delivery. Associations between alcohol use and preterm delivery has been observed in numerous studies,33,34 indicating that the strength of the relationship between alcohol use and preterm delivery may be driving this observed association. Further research on how alcohol use impacts delivery outcomes such as cesarean delivery is warranted.
Finally, a cannabis-related diagnosis was not found to be significantly associated with preterm delivery, cesarean delivery, or co-occurring preterm and cesarean delivery. This finding differs from other studies which have observed an association between cannabis use and preterm delivery.21 One study in France found that women who used cannabis demonstrated higher rates of preterm delivery and spontaneous preterm delivery.35 In addition, the association was observed in those who used cannabis once a month or more often, and especially in those who also used tobacco.
To our knowledge, no study has investigated the relationship between a cannabis-related diagnosis and cesarean delivery. As such, additional research on the relationship between a prenatal cannabis use and delivery outcomes is needed.
The findings in this study reinforce the need to identify SRDs in pregnant women early to minimize potential harm through intervention and treatment. Our data show high prevalence and risk of adverse delivery outcomes in those with an SRD, which supports a rational for robust SRD screening measures in all clinical settings. Because pregnant women with an SRD may be engaging with the health system in different capacities (prenatal visit vs. emergency department), questions regarding substance use should be posed often. Screening through questions related to substance use should be posed sensitively and should include questions specifically related to specific substances such as alcohol, opioids, cannabis, stimulants, and nicotine.36,37
Future studies should investigate the biological and environmental impact substance use, polysubstance use, and medication treatments (e.g., methadone) have on delivery outcomes, and differentiate between substance exposure, lifestyle factors, and the potential benefit of treatment on perinatal outcomes such as preterm and cesarean delivery. Screening and monitoring interventions should be implemented and tested in all types of clinical encounters including prenatal, primary care, and psychiatry visits to prevent substance use during pregnancy and subsequent adverse delivery outcomes. Investigating how stigma impacts these same delivery outcomes in these settings could also lead to increased recognition, appropriate diagnoses, and engagement in treatment.
Strengths and limitations
The research presented in this study used robust methodology in a large sample to evaluate previous potential negative outcomes and expand previous findings to address the relationships between SRDs and delivery outcomes. This study is strengthened by the large sample size over 7.5 years and the use of propensity score matching to control for confounding in the unstructured EHR data. By matching on key baseline characteristics, a greater portion of potential bias was eliminated when estimating the effects of an SRD on delivery outcomes.38
This study is limited by the reliance on ICD-10 codes for health-related diagnoses which can lead to misclassification bias, unmeasured confounding (e.g., SRD treatment), changes in eligibility over time, and missing data.39 These data represent an inconsistent distribution of ≥ 1 previous pregnancy compared to the rate in other studies.21 To address this concern, the analysis was repeated without matching on this variable, and again for primigravida women. The relationship between an SRD, the other covariates (e.g., age), and the outcome variables remained significant in both analyses, confirming that our decision to match on ≥ 1 previous pregnancy did not impact the final results. It may be difficult to generalize these results to other care settings due to the race/ethnicity distribution (e.g., low proportion of patients who report Black race or Hispanic/Latina ethnicity) in one healthcare system in Southern California.
Conclusion
The findings from this study reveal that pregnant women with an SRD are experiencing disproportionally higher odds of preterm and cesarean delivery compared to pregnant women without an SRD in a large matched pregnancy cohort. Substance type predicted the type of delivery outcome. Opioid- and stimulant-related diagnoses were significantly associated with preterm delivery while stimulant- and nicotine-related diagnoses were significantly associated with cesarean delivery. An alcohol-, opioid- stimulant-, and nicotine-related diagnosis were more likely to be observed in women with a co-occurring preterm cesarean delivery. A cannabis-related diagnosis was not significantly associated with preterm or cesarean delivery. These findings indicate that an SRD in pregnant women should be identified early to reduce potential harm through intervention and treatment.
Supplementary Material
Funding
This research is funded by Dr. Carla Marienfeld research funds through the Department of Psychiatry at the University of California, San Diego School of Medicine. Dr. Laramie R. Smith’s contributions to this work were facilitated by National Institute on Drug Abuse Career Development Award (grant no. K01 DA039767).
Footnotes
Supplemental data for this article can be accessed at http://doi:10.1080/10550887.2022.2082834.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Forray A, Foster D. Substance use in the perinatal period. Curr Psychiatry Rep. 2015;17(11):91. doi: 10.1007/s11920-015-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton BE, Osterman MJK, Driscoll AK, Rossen LM. 2018. Center for Disease Control and Prevention, births : provisional data for 2017 [Accessed May 9, 2019]. https://stacks.cdc.gov/view/cdc/55172.
- 3.Campbell KH, Savitz D, Werner EF, Pettker CM, Goffman D, Chazotte C, Lipkind HS. Maternal morbidity and risk of death at delivery hospitalization. Obstet Gynecol. 2013;122(3):627–33. doi: 10.1097/AOG.0b013e3182a06f4e. [DOI] [PubMed] [Google Scholar]
- 4.Small MJ, James AH, Kershaw T, Thames B, Gunatilake R, Brown H. Near-miss maternal mortality: cardiac dysfunction as the principal cause of obstetric intensive care unit admissions. Obstet Gynecol. 2012;119(2 Pt 1):250–5. doi: 10.1097/AOG.0b013e31824265c7. [DOI] [PubMed] [Google Scholar]
- 5.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med (Baltim). 2013;56(6):372–8. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson Julian N, Norwitz Errol R, Lockwood Charles J, Barss VA. Preterm birth: risk factors, interventions for risk reduction, and maternal prognosis; 2020. Accessed April 29, 2020. https://www.uptodate.com/contents/preterm-birth-risk-factors-interventions-for-risk-reduction-and-maternal-prognosis/print.
- 7.de Wit M, Goldberg A, Chelmow D. Alcohol use disorders and hospital-acquired infections in women undergoing Cesarean delivery. Obstet Gynecol. 2013; 122 (1) : 72–8. doi : 10.1097/AOG.0b013e318297be8d. [DOI] [PubMed] [Google Scholar]
- 8.Pinto SM, Dodd S, Walkinshaw SA, Siney C, Kakkar P, Mousa HA. Substance abuse during pregnancy: effect on pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):137–41. doi: 10.1016/J.EJOGRB.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Creanga AA, Sabel JC, Ko JY, Wasserman CR, Shapiro-Mendoza CK, Taylor P, Barfield W, Cawthon L, Paulozzi LJ. Maternal drug use and its effect on neonates. Obstet Gynecol. 2012; 119(5):924–33. doi: 10.1097/AOG.0b013e31824ea276. [DOI] [PubMed] [Google Scholar]
- 10.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–65. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 11.Chang G. Maternal substance use: consequences, identification, and interventions. Alcohol Res. 2020;40(2):06 doi: 10.35946/arcr.v40.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Conference of State Legislatures. State Medical Marijuana Laws. Accessed June 21, 2019. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- 13.World Health Organization. ICD-10 : International statistical classification of diseases and related health problems : tenth revision. 2nd ed. World Health Organization; 2004. https://apps.who.int/iris/handle/10665/42980 [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth Edition. Arlington, VA, American Psychiatric Association, 2013. [Google Scholar]
- 15.Institutional Review Board (IRB). Substance use diagnoses and maternal morbidity among women who presented for delivery in a large multi-site healthcare system in California. University of California San Diego IRB. [Google Scholar]
- 16.Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20(1): 1–24. doi: 10.1093/pan/mpr013. [DOI] [Google Scholar]
- 17.Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, Callaghan WM. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt)). 2014;23(1):3–9. doi: 10.1089/jwh.2013.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanc J, Resseguier N, Goffinet F, Lorthe E, Kayem G, Delorme P, Vayssière C, Auquier P, D’Ercole C. Association between gestational age and severe maternal morbidity and mortality of preterm cesarean delivery: a population-based cohort study. Am J Obstet Gynecol. 2019;220(4):399.e1–399-e9. doi: 10.1016/j.ajog.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Corsi DJ, Walsh L, Weiss D, Hsu H, El-Chaar D, Hawken S, Fell DB, Walker M. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322(2):145–52. doi: 10.1001/jama.2019.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nezvalová-Henriksen K, Spigset O, Nordeng H. Effects of codeine on pregnancy outcome: results from a large population-based cohort study. Eur J Clin Pharmacol. 2011;67(12): 1253–61. doi: 10.1007/s00228-011-1069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemon LS, Naimi A, Caritis SN, Platt RW, Venkataramanan R, Bodnar LM. The Role of Preterm Birth in the Association Between Opioid Maintenance Therapy and Neonatal Abstinence Syndrome. Paediatr Perinat Epidemiol. 2018;32(2):213–22. doi: 10.1111/ppe.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright TE, Schuetter R, Tellei J, Sauvage L. Methamphetamines and pregnancy outcomes. J Addict Med. 2015;9(2): 111–7. doi: 10.1097/ADM.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smid MC, Metz TD, Gordon AJ. Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin Obstet Gynecol. 2019;62 (1) : 168 – 84. doi : 10.1097/GRF.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouin K, Murphy K, Shah PS. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstet Gynecol. 2011;204(4):340.e1–e12. doi: 10.1016/J.AJOG.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Plessinger MA, Woods JR. Maternal, placental, and fetal pathophysiology of cocaine exposure during pregnancy. Clin Obstet Gynecol. 1993;36(2):267–78. doi: 10.1097/00003081-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Acker D, Sachs BP, Tracey KJ, Wise WE. Abruptio placentae associated with cocaine use. Am J Obstet Gynecol. 1983;146(2):220–1. doi: 10.1016/0002-9378(83)91060-8. [DOI] [PubMed] [Google Scholar]
- 27.Gonsoulin W, Borge D, Moise KJ. Rupture of unscarred uterus in primigravid woman in association with cocaine abuse. Am J Obstet Gynecol. 1990;163(2):526–7. doi: 10.1016/0002-9378(90)91189-J. [DOI] [PubMed] [Google Scholar]
- 28.Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, Kline J. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340(5):333–9. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]
- 29.Bingol N, Fuchs M, Diaz V, Stone RK, Gromisch DS. Teratogenicity of cocaine in humans. J Pediatr. 1987;110(1):93–6. doi: 10.1016/S0022-3476(87)80297-4. [DOI] [PubMed] [Google Scholar]
- 30.Good MM, Solt I, Acuna JG, Rotmensch S, Kim MJ. Methamphetamine use during pregnancy: maternal and neonatal implications. Obstet Gynecol. 2010;116(2 Pt 1):330–4. doi: 10.1097/AOG.0b013e3181e67094. [DOI] [PubMed] [Google Scholar]
- 31.The Surgeon General. 2014. Charpter 9: Reproductive outcomes in the health consequences of smoking: 50 years of progress. A report of the surgeon general. Accessed September 29, 2020. https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm.
- 32.Lurie S, Ribenzaft S, Boaz M, Golan A, Sadan O. The effect of cigarette smoking during pregnancy on mode of delivery in uncomplicated term singleton pregnancies. J Matern Neonatal Med. 2014;27(8):812–5. doi: 10.3109/14767058.2013.842551. [DOI] [PubMed] [Google Scholar]
- 33.Janisse JJ, Bailey BA, Ager J, Sokol RJ. Alcohol, tobacco, cocaine, and marijuana use: relative contributions to preterm delivery and fetal growth restriction. Subst Abus. 2014;35(1):60–7. doi: 10.1080/08897077.2013.804483. [DOI] [PubMed] [Google Scholar]
- 34.Ikehara S, Kimura T, Kakigano A, Sato T, Iso H. Association between maternal alcohol consumption during pregnancy and risk of preterm delivery: the Japan Environment and Children’s Study. BJOG. 2019;126(12): 1448–54. doi: 10.1111/1471-0528.15899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121(8):971–7. doi: 10.1111/1471-0528.12626. [DOI] [PubMed] [Google Scholar]
- 36.Courchesne NS, Meyers SA. Women and pregnancy. In: Absolute addiction psychiatry review. Springer International Publishing; 2020. p. 259–75. doi: 10.1007/978-3-030-33404-8_16. [DOI] [Google Scholar]
- 37.Meyers SA, Earnshaw VA, D’Ambrosio B, Courchesne N, Werb D, Smith LR. The intersection of gender and drug use-related stigma: a mixed methods systematic review and synthesis of the literature. Drug Alcohol Depend. 2021;223:108706 doi: 10.1016/j.drugalcdep.2021.108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171–84. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 39.Utter GH, Atolagbe OO, Cooke DT. The use of the international classification of diseases, tenth revision, clinical modification and procedure classification system in clinical and health services research: the devil is in the details. JAMA Surg. 2019;154(12):1089–90. doi: 10.1001/jamasurg.2019.2899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


