Structured Abstract
Objective:
To characterize incidence and outcomes for bariatric surgery patients who give birth.
Summary Background Data:
Patients of childbearing age comprise 65% of bariatric surgery patients in the United States, yet data on how often patients conceive and obstetric outcomes are limited.
Methods:
Using the IBM MarketScan database, we performed a retrospective cohort study of female patients ages 18–52 undergoing laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass from 2011–2017. We determined incidence of births in the first two years after bariatric surgery using Kaplan-Meier estimates. We then restricted the cohort to those with full two-year follow-up to examine obstetric outcomes and bariatric-related reinterventions. We reported event rates of adverse obstetric outcomes and delivery type. Adverse obstetric outcomes include pregnancy complications, severe maternal morbidity, and delivery complications. We performed multivariable logistic regression to examine associations between birth and risk of reinterventions.
Results:
Of 69,503 patients who underwent bariatric surgery, 1,464 gave birth. The incidence rate was 2.5 births per 100 patients in the 2 years after surgery. 85% of births occurred within 21 months after surgery. For 38,922 patients with full two-year follow-up, adverse obstetric event rates were 4.5% for gestational diabetes and 14.2% for hypertensive disorders. 48.5% were first-time Cesarean deliveries. Almost all reinterventions during pregnancy were biliary. Multivariable logistic regression analysis showed no association between post-bariatric birth and reintervention rate (OR: 0.93, 95%CI: 0.78–1.12).
Conclusions:
In this first national U.S. cohort, we find giving birth was common in the first 2 years after bariatric surgery and was not associated with increased risk of reinterventions. Clinicians should consider shifting the dialogue surrounding pregnancy after surgery to shared decisionmaking with maternal safety as one component.
Mini Abstract
Patients of childbearing age comprise 65% of bariatric surgery patients in the United States, yet rates of birth and adverse obstetric outcomes are unknown. Using the IBM MarketScan database, Kaplan-Meier estimates determined an incidence of 2.5 births per 100 female patients in the 2 years after surgery. Giving birth within this purported high-risk timeframe was also not associated with reinterventions.
Introduction:
Patients of childbearing age comprise 65% of bariatric surgery patients in the United States every year.1 Current consensus guidelines put forth by the American College of Obstetricians and Gynecologists and endorsed by the American Society for Metabolic and Bariatric Surgery recommend patients delay conception by 12–24 months after bariatric surgery due to possible risks to the mother and fetus.2 Despite these recommendations, we know little about the incidence of pregnancy and adverse birth outcomes for patients who become pregnant in this proposed higher-risk time frame.
To date, the largest studies of pregnancy after bariatric surgery have only addressed fetal outcomes. Maternal outcomes research after bariatric surgery is limited in a number of ways. Many studies are single center with small numbers, which may lead to biased results.3–5 Larger, population-based studies examined European6, 7 or Australian patients8 whose outcomes may not be generalizable to patients in the United States. Patients in the United States have higher pregnancy rates,9 greater racial and ethnic disparities in unintended pregnancies,10 and greater racial/ethnic diversity.11 Lastly, among studies examining maternal outcomes, sleeve gastrectomy, currently the most common bariatric surgery performed,12 is often missing.13
Healthcare providers lack crucial data to counsel reproductive-age patients about when it is safe to conceive after bariatric surgery, including potential obstetric risks for patients and their rates of bariatric-related surgical complications. To address these major gaps, we used a large, national claims database to identify the birth rate after laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass and to examine obstetric and bariatric surgical outcomes in the two years after their bariatric procedure.
Methods:
Data Source
We performed a retrospective cohort study using IBM MarketScan Commercial, a multi-payer administrative dataset with inpatient, outpatient, and physician claims.14 This database includes more than 40 million patients employed at large institutions from all 50 states. We examined claims from January 1, 2011 to two years after bariatric surgery or December 31, 2018.
Study Population
We included adult female patients of childbearing age who underwent laparoscopic sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass, the two most commonly performed bariatric procedures,12 from January 1, 2011 to December 31, 2017. Patients were included if they had at least 6 months of continuous follow-up after surgery. Childbearing age was defined as ages 18 to 55 years.15–17 While 55 is the upper range for childbearing age, we chose 52 years as the limit for our cohort to accommodate full-term pregnancy (40 weeks) and the two-year period after bariatric surgery. We identified bariatric patients using CPT codes 43775, 43644, 43645, 43844, 43846+43659, 43847+43659 for laparoscopic sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass when they also had ICD-9 and ICD-10 diagnosis codes for morbid obesity. Patients were excluded if they had a diagnosis code reflecting gastric or small bowel cancer associated with surgery to ensure our cohort only included patients undergoing bariatric surgery.
This cohort was used to examine the epidemiology of birth after bariatric surgery. Because we then used logistic regression to examine obstetric outcomes and reinterventions after birth, we further restricted the cohort to patients with full 2-year follow-up in order to account for differential follow-up lengths.
Outcomes
Births
Births were identified by ICD-9 diagnosis code (V27), ICD-10 diagnosis code (Z37), or diagnosis related group codes (765–768, 774, 775, 783–788, 796–798) for still births, live births, cesarean deliveries, and vaginal deliveries. Our primary outcomes were the incidence of births in the first 2 post-operative years and the proportion of these births occurring before 21 months. The mark of 21 months was chosen as this was thought to approximate conception at 12 months, the least conservative recommendation for conception after bariatric surgery, plus 9 months, the usual length of pregnancy. Births rather than pregnancy were used given the more accurate and complete capture of births in claims data. In secondary analyses we examined birth rate across subgroups defined by procedure type and patient age.
Obstetric Outcomes
We then examined obstetric outcomes by procedure type. We first identified obstetric outcomes for the first birth for each patient within the first two years after bariatric surgery. Obstetric outcomes were defined by CPT, ICD-9, or ICD-10 codes based on a review of the literature (Supplemental Digital Content, Table 1). Births were categorized as still births or live births.
Obstetric outcomes include pregnancy complications (gestational diabetes, gestational hypertension, mild/unspecified pre-eclampsia, severe pre-eclampsia, eclampsia, iron deficiency anemia, hyperemesis gravidarum), severe maternal morbidity (postpartum hemorrhage, peripartum hysterectomy, major puerperal infections, anesthesia complications) and delivery complications (PPROM/pre-term delivery, prolonged labor). Patients with more than one hypertensive disorder (gestational hypertension, mild pre-eclampsia, severe pre-eclampsia, and eclampsia) were classified as having only the most severe disorder to avoid counting patients multiple times. We additionally describe rates of delivery types: vaginal, operative, cesarean, vaginal after prior cesarean, and repeat cesarean.
Bariatric Surgery-Related Reinterventions
We examined bariatric reintervention rates in patients who gave birth compared to those who did not. Reintervention CPT codes were obtained from a review of the literature and CPT coding manual in line with the bariatric surgery comparative effectiveness literature.18–21 (Supplemental Digital Content, Table 2) Reinterventions were categorized as follows:
Revision: Operation modifying the index bariatric procedure including included conversion to another bariatric procedure (e.g. sleeve gastrectomy to gastric bypass), any gastrectomy, or anastomotic revision.
Enteral access: Surgical, endoscopic, or interventional.
Vascular Access: Procedure to reflect the need for parenteral nutrition.
Reoperation: Other abdominal operation potentially a sequela of the index bariatric procedure but not affecting bariatric physiology. We examine reoperations as a group as well as separately which include abdominal wall hernia repair, biliary procedure, internal hernia repair, paraesophageal hernia repair, and other abdominal procedure. We decided to examine reoperations separately also because of various physiologic changes of pregnancy which could affect the likelihood of each of these. For example, anatomic changes could increase intra-abdominal pressure and thus the risk of an abdominal wall hernia whereas the mechanism of pregnancy causing increased risk of biliary procedures would be through hormonal changes.
Other reintervention: Drainage, aspiration, diagnostic laparoscopy, etc.
Statistical Analysis
We first determined the incidence rate of births in the first two years after bariatric surgery using Kaplan-Meier estimates. We then examined the distribution of births over time and proportion of births occurring before 21 months. Next, we determined birth rates by procedure type (laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass) using Cox regression analysis, controlling for age, U.S. state,22 and Elixhauser comorbidities.23, 24 Age was treated as a categorical variable: 18–24, 25–29, 30–34, 35–39, 40–44, ≥45 years. U.S. state was the smallest geographic unit available for all patients. Finally, we conducted a Cox regression analysis of the association between age ranges and likelihood to give birth, adjusting for U.S. state and Elixhauser comorbidities. For all Cox regression analyses, we censored patients who did not have a qualifying event at the time of disenrollment from the plan or at 2 years after bariatric surgery.
To investigate obstetric outcomes for the first birth after bariatric surgery, we reported event rates for all patients who gave birth within the first two years after bariatric surgery. For bariatric reinterventions, we utilized the restricted cohort in which all patients had full 2-year follow-up time. Using multivariable logistic regression, we examined the association between giving birth and reinterventions over the entire the study period. In our analysis, we controlled for age, U.S. state, and Elixhauser comorbidities. For specific reinterventions with higher unadjusted event rates for women who gave birth compared to those who did not, we performed multivariable logistic regression adjusting for the same variables.
All statistical tests were two-sided and performed at the 5% significance level. All analyses were performed using Stata 16 (StataCorp LLP, College Station, TX). The study was exempt from Institutional Review Board review due to use of de-identified data.
Results:
Of 69,503 patients of childbearing age who underwent laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass from 2011–2017, 87% had full 1-year and 56% had full 2-year follow-up. Patient characteristics are included in Table 1. The median age of the entire cohort was 40 years (IQR: 34–46). Patients who gave birth were younger than patients who did not give birth. Patients who gave birth were less likely than patients who did not to have hypertension (29.0% vs 40.3%, P<.001), neurologic disorders (0.4% vs 1.1%, P=0.012), hypothyroidism (7.5% vs 9.4%, P=0.014), depression (11.0% vs 13.3%, P=0.010), and rheumatoid arthritis (0.3% vs 1.1%, P=0.006). There were no other observed differences in comorbidities, including diabetes with chronic complications (1.7% vs 1.6%, P=0.736). The cohort with full 2-year follow-up had 38,922 patients. This restricted cohort had a similar distribution of procedure type and comorbidities.
Table 1:
Baseline Characteristics of Patients of Childbearing Age Undergoing Bariatric Surgery 2011–2017
| Patients Who Did Not Give Birth | Patients Who Gave Birth |
P value | |
|---|---|---|---|
| n = 68,039 (97.9%) | n = 1,464 (2.1%) | ||
| Age at time of surgery, mean (SD) | 39.6 years (8.2) | 30.6 years (5.3) | <.001 |
| Age at time of surgery, No. (%) | |||
| 18–24 years | 3,730 (5.5%) | 207 (14.1%) | <.001 |
| 25–29 years | 4,995 (7.3%) | 396 (27.0%) | <.001 |
| 30–34 years | 9,487 (13.9%) | 520 (35.5%) | <.001 |
| 35–39 years | 13,163 (19.3%) | 291 (19.9%) | 0.61 |
| 40–45 years | 14,794 (21.7%) | 47 (3.2%) | <.001 |
| 45–52 years | 21,870 (32.1%) | 6 (0.4%) | <.001 |
| Year of operation, No. (%) | |||
| 2011 | 10,268 (15.1%) | 235 (16.0%) | 0.31 |
| 2012 | 10,725 (15.8%) | 245 (16.7%) | 0.31 |
| 2013 | 11,187 (16.4%) | 224 (15.3%) | 0.24 |
| 2014 | 10,173 (15.0%) | 233 (15.9%) | 0.31 |
| 2015 | 8,200 (12.1%) | 196 (13.4%) | 0.12 |
| 2016 | 8,478 (12.5%) | 215 (14.7%) | 0.01 |
| 2017 | 9,008 (13.2%) | 116 (7.9%) | <.001 |
| Comorbidities*, No. (%) | |||
| Hypertension | 27,428 (40.3%) | 424 (29.0%) | <.001 |
| Chronic pulmonary disease | 6,095 (9.0%) | 124 (8.5%) | 0.52 |
| Diabetes without chronic complications | 12,076 (17.8%) | 236 (16.1%) | 0.11 |
| Hypothyroidism | 6,406 (9.4%) | 110 (7.5%) | 0.01 |
| Liver disease | 7,815 (11.5%) | 163 (11.1%) | 0.68 |
| Depression | 9,057 (13.3%) | 161 (11.0%) | 0.01 |
Note: Percentages are reported by column.
Elixhauser comorbidities with a prevalence greater than 5% are listed.
Incidence of Births
Within the first 2 years after surgery, 1,464 (2.1%) patients gave birth. Five patients had two births within this time period. There were 17 still births and 1,452 live births. Examining time to first birth, the incidence rate was 0.0000347 births per person-day, or 0.025 births per 730 person-days. Thus, for every 100 patients of childbearing age, the incidence rate was 2.5 births in the first two years after bariatric surgery. The distribution of time to first birth by months after bariatric surgery is shown in Figure 1. Thirty-two births (2.2%) occurred prior to post-operative month 9, corresponding to conception during the first month after surgery. The majority of births (85%), occurred before 21 months after bariatric surgery, corresponding to conception during the first 12 months after surgery.
Figure 1:

Cumulative Distribution of Births Over Time after Bariatric Surgery
The number of births within every three months after bariatric surgery is shown. The total cumulative number of first births is 1,464 for women who underwent bariatric surgery from 2011–2017. The dashed line corresponds to births that would have had a conception date before 12 months after bariatric surgery.
Factors Associated with Birth
We examined the association between procedure type and age group with likelihood to give birth. Cox regression analysis showed no association between procedure type and likelihood to give birth (HR: 0.92, 95%CI: 0.82–1.03). The age group with the highest likelihood of birth was age 25–29 (HR: 1.46, 95%CI: 1.24–1.73, ref: age 18–24, Supplemental Digital Content, Table 3). The group least likely to give birth was age 45–52 (HR: 0.004, 95%CI: 0.0002–0.010).
Obstetric Outcomes
For the 1,079 patients with full two-year follow-up who gave birth, we examined rates of obstetric outcomes which are shown in Figure 2. Event rates for all obstetric outcomes are found in Table 2. Of the patients who gave birth there were 496 (46.0%) vaginal deliveries, 21 (2.0%) operative deliveries, 523 (48.5%) cesarean deliveries, 12 (1.1%) vaginal deliveries after prior cesarean delivery, and 27 (2.5%) repeat cesarean deliveries.
Figure 2:
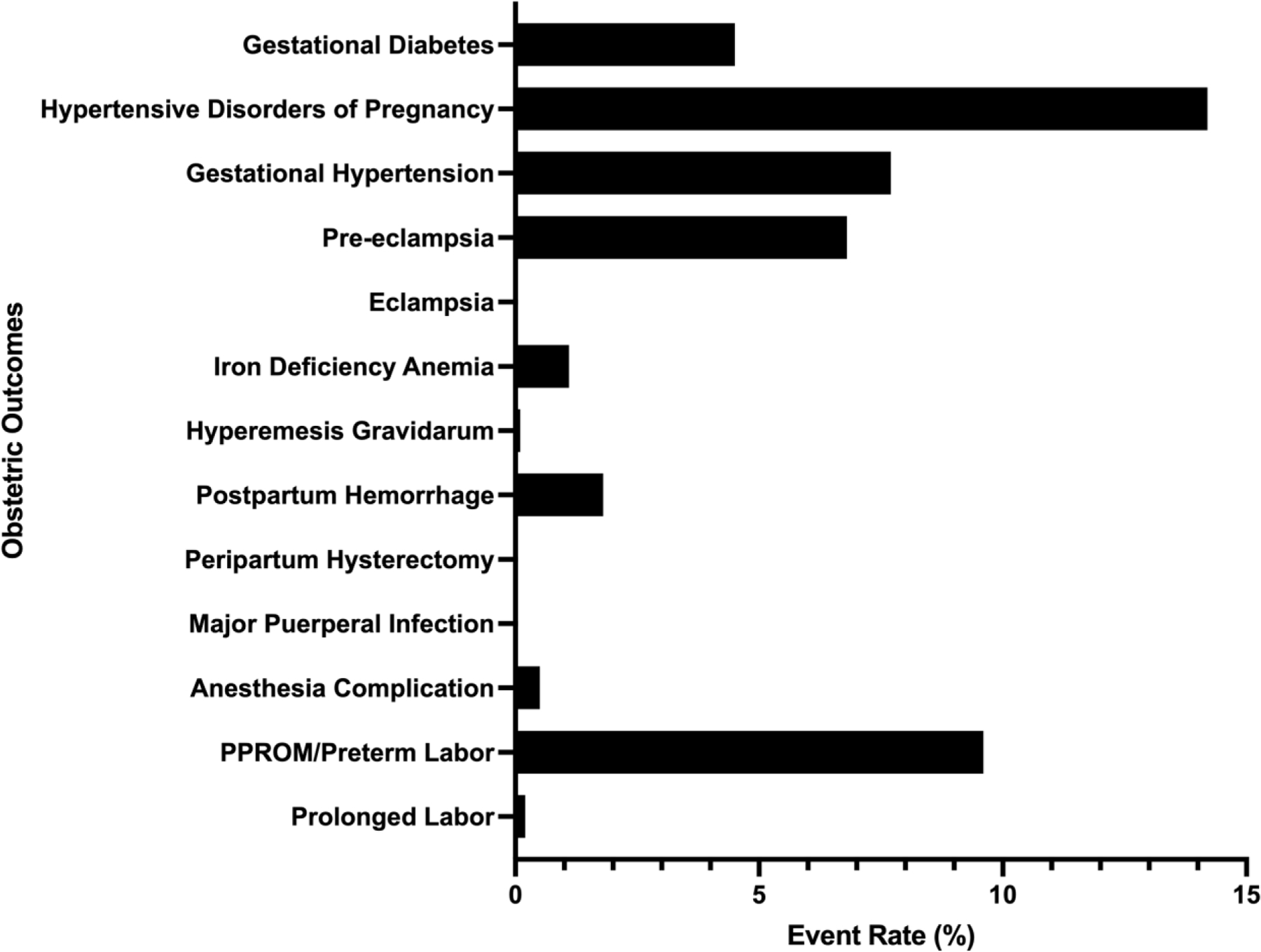
Obstetric Outcome Event Rates for Women Who Gave Birth Within 2 Years After Bariatric Surgery
Event rates for women who gave birth within the first two years after bariatric surgery. The event rates for eclampsia, prolonged labor, peripartum hysterectomy, and major puerperal infections were all 0%. The event rates for hyperemesis gravidarum was 0.1%, for prolonged labor was 0.2%, and for anesthesia complication was 0.5%.
Table 2:
Event Rates for Obstetric Outcomes in Patients Who Gave Birth 2 Years After Bariatric Surgery
| Patients Who Underwent Bariatric Surgery (n=1,079) |
|
|---|---|
| Outcome | % |
| Gestational Diabetes | 4.5% |
| Hypertensive Disorders of Pregnancy | 14.2% |
| Gestational Hypertension | 7.7% |
| Mild-unspecified Pre-eclampsia | 3.5% |
| Severe Pre-eclampsia | 3.3% |
| Eclampsia | 0% |
| Iron Deficiency Anemia | 1.1% |
| Hyperemesis Gravidarum | 0.1% |
| Postpartum Hemorrhage | 1.8% |
| Peripartum Hysterectomy | 0% |
| Major Puerperal Infections | 0% |
| Anesthesia Complications | 0.5% |
| PPROM/Pre-term Labor | 9.6% |
| Prolonged Labor | 0.2% |
Bariatric Reinterventions
There were 38,922 patients with full 2-year follow-up. Of these, 1,079 patients gave birth. Among patients who did not give birth, there were 4,575 (12.1%) patients who underwent a reintervention during the study period compared to 146 (13.5%, P=0.15) patients who gave birth. Of patients who gave birth and experienced a reintervention, 82 (56%) had a first reintervention before giving birth, 15 (10%) during pregnancy (9 months before birth), 3 (2%) the same day of birth (all abdominal wall hernia repairs), 4 (3%) within 6 months postpartum, and 42 (29%) patients had their first reintervention beyond 6 months postpartum. For reinterventions during pregnancy, there were 11 biliary operations, 1 vascular access procedure, 1 enteral access procedure, and 1 other operation. Table 3 shows unadjusted rates of each type of reintervention in patients who did not give birth and patients who gave birth. Multivariable logistic regression showed patients who gave birth were not more or less likely to experience a reintervention compared to patients who did not give birth (OR: 0.93, 95%CI: 0.78–1.12). Giving birth was also not associated with likelihood to undergo biliary procedures (OR: 1.15, 95%CI: 0.93–1.42).
Table 3:
Unadjusted Rates of Reinterventions in Patients After Bariatric Surgery with Full 2-Year Follow-up
| No Birth (n=37,843) |
Gave Birth (n=1,079) |
P value* | |
|---|---|---|---|
| Outcome | |||
| Revisions | 132 (0.3%) | 1 (0.1%) | 0.28 |
| Enteral Access | 62 (0.2%) | 2 (0.2%) | 0.86 |
| Vascular Access | 381 (1.0%) | 8 (0.7%) | 0.39 |
| Reoperation | |||
| Abdominal Wall Hernia Repair | 495 (1.3%) | 8 (0.7%) | 0.10 |
| Biliary Procedure | 2,203 (5.8%) | 102 (9.5%) | <.001 |
| Internal Hernia Repair | 102 (0.3%) | 1 (0.1%) | 0.54 |
| Paraesophageal Hernia Repair | 116 (0.3%) | 2 (0.1%) | 0.78 |
| Other Abdominal Procedure | 930 (2.5%) | 19 (1.8%) | 0.14 |
| Other Reintervention | 154 (0.4%) | 3 (0.3%) | 0.81 |
P values are shown for bivariate comparisons using Chi-square test if n > 5 or Fisher Exact test if n ≤ 5.
Discussion:
This is, to our knowledge, the first national cohort of commercially-insured patients of childbearing age in the United States. Despite consensus guidelines outlining the potential serious adverse effects of conception close to bariatric surgery on mothers,2 our findings show a relatively high incidence of births after bariatric surgery (2.5 births per 100 patients), at approximately half the birth rate of the general U.S. population (5.9 births per 100 patients).25 Overall, our data provide preliminary evidence that giving birth after bariatric surgery may be safe for mothers. Obstetric outcomes were generally comparable to U.S. population-level estimates, and there was no association between giving birth and having a bariatric reintervention.
Our study is the first to identify the birth rate of patients after bariatric surgery in the United States. At 2.5 births per 100 patients, this rate is relatively high considering current national recommendations to avoid conception during this purported high-risk period. Lack of concordance with guidelines brings up questions regarding efficacy of pre-conception counseling as well as alignment with patient preferences. Previous work in the Longitudinal Assessment of Bariatric Surgery-2 cohort showed pre-operatively rating pregnancy as important was associated with conception within 18 months.26 Researchers using the same cohort also found one third of patients ages 18–44 years who intended to become pregnant planned to do so within 2 years of bariatric surgery.27 4.3% of patients actively tried to conceive within the first year.26 Thus, pregnancy may be something patients value highly and may outweigh theoretical risks related to giving birth closely after bariatric surgery. Additionally, some patients may not be counseled that one of the effects of weight loss is increased fertility.
We only included full-term pregnancies in our study due to the limitations of identifying early pregnancy losses in claims data. Thus, the results of our study only explore complication rates in pregnancies resulting in birth and cannot be used to draw conclusions about overall fertility rates, pregnancy loss, or complications in early pregnancy losses. Data have shown mixed results for patients who undergo bariatric surgery regarding fertility, with some showing reduction in fertility28 and others showing increased fertility.29–31 Studies examining miscarriage rates also show mixed results, though data are from small studies.31, 32 Future work is needed to better understand this important period in pregnancy. Additionally, the method of conception (natural conception, medication-assisted conception, in vitro fertilization, etc) cannot be assessed in this dataset, potentially underestimating pregnancy complications such as preterm birth, placental disorders, and preeclampsia.
Our findings suggest that in patients with a history of bariatric surgery who give birth two years after their procedure, there is not an increased risk of surgical reinterventions, and obstetric outcomes are equivalent. We found patients who gave birth were not more likely than those who did not to undergo surgical reinterventions. Even when comparing surgical techniques with different risks, sleeve gastrectomy and gastric bypass, we found no elevated risk with procedures thought to cause more disruption to intra-abdominal anatomy. A recent study from Auger et al in a Canadian cohort also found no elevated obstetric risks in severe preeclampsia, sepsis, cardiac complications, and other morbidities for patients giving birth after bariatric surgery compared with patients without obesity.33
To place our obstetric findings into context, we turn to rates established in the literature. Gestational diabetes and pre-eclampsia rates are reported to be 11.2%34 and 9.1%35 for women with BMI ≥ 35kg/m2, respectively. Interestingly, the rate of iron deficiency anemia in the general U.S. population is estimated to be 18%36 regardless of BMI. Our findings of lower rates of iron deficiency anemia could indicate appropriate monitoring and treatment of pregnant patients after bariatric surgery or a limitation of claims data. Previous work has shown higher BMI is associated with more severe maternal morbidity and mortality.37 Thus, given more time to work, bariatric surgery may reduce obesity-related risks in pregnancy even further. Of note, a study using 113,818 U.S. patients with BMI ≥ 35 kg/m2 by Butwick et al found the cesarean delivery rate to be 45.5%.38
Fetal outcomes in births after bariatric surgery are also vital to consider. A study from the Swedish Medical Birth Register found pregnancies after bariatric surgery were associated with lower risk of large-for-gestational age infants but higher risk for small-for-gestational age infants and still birth compared to matched counterparts though this study included just 650 bariatric surgery patients.39 An Australian study examined infants born to the same patient before and after bariatric surgery as well as multiple births in the general population and found bariatric surgery was associated with fewer large-for-gestational age infants and fewer admissions to neonatal special or intensive care.8 A systematic review by Maggard et al found lower rates of macrosomia and neonatal complications after gastric bypass.3 The review’s findings also suggested biliopancreatic diversion was associated with lower adverse neonatal outcomes but higher miscarriage rates.3 More data are needed to ensure fetal safety and health.
Our preliminary data suggest it is time to rethink our counseling for pregnancy after bariatric surgery. Current recommendations are based on theoretical concerns for maternal and fetal risks: our data show births after bariatric surgery are not associated with increased rates of surgical intervention and obstetric outcomes are similar to other patients with obesity. Further, existing data demonstrate equivalent to improved fetal outcomes after bariatric surgery,.8 Given these findings, perhaps we need to readjust paradigms towards shared decisionmaking. Shared decisionmaking is a central component of the choice to pursue bariatric surgery; this should be mirrored in considerations of when to pursue pregnancy after bariatric surgery. Components that perhaps should weigh more heavily in the decisionmaking process are the risk of becoming pregnant too early (not achieving as much weight loss or comorbidity resolution), family planning desire (wanting a baby now), and the risk of delaying pregnancy (miscarriage and genetic disorders). Already established shared decisionmaking aids for reproductive goals40 and pregnancy clinical decisions can be adapted to explore these aspects of pregnancy and bariatric surgery. Many aids have already been effective in helping patients make other types of clinical decisions during pregnancy.41 Future work is needed to incorporate fertility and early pregnancy outcomes into the decisionmaking process.
One major component of this complex decisionmaking process we do not have is efficacy data surrounding weight loss after pregnancy. Studies show obesity is related to early or recurrent miscarriage,42, 43 gestational hypertension,44 pre-eclampsia,44, 45 gestational diabetes, cesarean delivery,46, 47 fetal growth disorders,48 and infants who develop childhood obesity.43 However, patients may be willing to accept less weight loss and not fully realize these benefits if they are able to have a pregnancy when younger or before reaching advanced maternal age. It is also possible patients may be seeking bariatric surgery in order to become pregnant3 due to obesity-related infertility.49, 50 If there is no hazard to the mother, this tradeoff between weight loss and other values and concerns, may be acceptable.
Our study is not without limitations. First, because we examine births rather than pregnancy, so it is possible that conception rates, and thus guideline discordance, are even higher than reported findings. Second, BMI prior to pregnancy, at conception, and at birth as well as clinical severity of comorbidities are not found in claims data, so we are unable to examine weight loss outcomes. Third, there may be important differences we were unable to account for between patients who did and did not give birth in this observational study, precluding causal inference. Fourth, race, ethnicity, and sociodemographic data were not available. Given the higher maternal mortality and morbidity among patients of color and low-income mothers, this is a vital part of future research. Additionally, these differences may represent sources of unmeasured confounding in our analysis of reinterventions. Fifth, prior cesarean delivery may have been undercounted since we relied on codes reflecting prior births. Sixth, time-to-event analysis which would account for censoring could not be used in our analysis because births were a rare event. Lastly, our study examines commercially-insured patients. Commercially-insured patients make up 73% of bariatric surgery patients,51 but findings may not be applicable to Medicare-, Medicaid-, and un-insured patients. Additionally, insured patients may have easier access to healthcare and be more likely to receive appropriate preventive care as well as timely diagnosis and management of pregnancy complications. They may also face fewer adverse social and structural determinants of health which are known to contribute to adverse maternity care and general health outcomes. Despite these limitations, this is the largest, nationwide cohort study examining births after bariatric surgery in the modern era of sleeve gastrectomy.
Conclusion
Our study provides important safety data to inform shared decisionmaking for pregnant patients and their healthcare providers. We found a high number of patients become pregnant after surgery. The decisionmaking process for childbirth after bariatric surgery is a complex one. We need to share these findings with patients to better inform them and perhaps revisit how we counsel patients.
Supplementary Material
Sources of Funding:
Dr. Chao received funding from the Veterans Affairs Center for Clinical Management Research, VA Ann Arbor Healthcare System; this work does not represent the views of the United States government nor the Department of Veterans Affairs. Dr. Yang, Dr. Peahl, and Ms. Thumma, have no conflicts of interest to disclose. Dr. Dimick receives grant funding from the NIH, AHRQ, and BlueCross BlueShield of Michigan Foundation. Dr. Dimick is a cofounder of ArborMetrix, Inc, a company that makes software for profiling hospital quality and efficiency, which had no role in the work herein. Dr. Arterburn receives grants from the National Institutes of Health and nonfinancial support from IFSO Latin America Chapter outside the submitted work. Dr. Telem receives funding from AHRQ K08 HS025778–01A1. Dr. Telem receives consulting fees from Medtronic, which had no role in the work herein.
Footnotes
Meeting Presentation: An earlier version of this study was presented as an oral presentation at the Academic Surgical Conference in February 2021.
Conflicts of Interest: The authors have no conflicts to report.
References:
- 1.The Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program 2019. Available at: https://www.facs.org/quality-programs/mbsaqip. Accessed April 13, 2020.
- 2.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 105: Bariatric Surgery and Pregnancy. Obstet Gynecol 2009; 113(6):1405–13. [DOI] [PubMed] [Google Scholar]
- 3.Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA 2008; 300(19):2286–96. [DOI] [PubMed] [Google Scholar]
- 4.Patel JA, Patel NA, Thomas RL, et al. Pregnancy outcomes after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 2008; 4(1):39–45. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub AY, Levy A, Levi I, et al. Effect of bariatric surgery on pregnancy outcome. Int J Gynaecol Obstet 2008; 103(3):246–51. [DOI] [PubMed] [Google Scholar]
- 6.Kjaer MM, Lauenborg J, Breum BM, et al. The risk of adverse pregnancy outcome after bariatric surgery: a nationwide register-based matched cohort study. Am J Obstet Gynecol 2013; 208(6):464 e1–5. [DOI] [PubMed] [Google Scholar]
- 7.Roos N, Neovius M, Cnattingius S, et al. Perinatal outcomes after bariatric surgery: nationwide population based matched cohort study. BMJ 2013; 347:f6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibiebele I, Gallimore F, Schnitzler M, et al. Perinatal outcomes following bariatric surgery between a first and second pregnancy: a population data linkage study. BJOG 2020; 127(3):345–354. [DOI] [PubMed] [Google Scholar]
- 9.Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann 2014; 45(3):301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med 2016; 374(9):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nargund G Declining birth rate in Developed Countries: A radical policy re-think is required. Facts Views Vis Obgyn 2009; 1(3):191–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Campos GM, Khoraki J, Browning MG, et al. Changes in Utilization of Bariatric Surgery in the United States From 1993 to 2016. Ann Surg 2020; 271(2):201–209. [DOI] [PubMed] [Google Scholar]
- 13.Rottenstreich A, Elchalal U, Kleinstern G, et al. Maternal and Perinatal Outcomes After Laparoscopic Sleeve Gastrectomy. Obstet Gynecol 2018; 131(3):451–456. [DOI] [PubMed] [Google Scholar]
- 14.Hansen L IBM MarketScan Research Databases for life sciences researchers. In IBM Watson Health, ed., 2018.
- 15.Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol 2016; 215(3):353 e1–353 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peahl AF, Dalton VK, Montgomery JR, et al. Rates of New Persistent Opioid Use After Vaginal or Cesarean Birth Among US Women. JAMA Netw Open 2019; 2(7):e197863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peahl AF, Morgan DM, Dalton VK, et al. New persistent opioid use after acute opioid prescribing in pregnancy: a nationwide analysis. Am J Obstet Gynecol 2020; 223(4):566 e1–566 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arterburn D, Wellman R, Emiliano A, et al. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study. Ann Intern Med 2018; 169(11):741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis KH, Arterburn DE, Callaway K, et al. Risk of Operative and Nonoperative Interventions Up to 4 Years After Roux-en-Y Gastric Bypass vs Vertical Sleeve Gastrectomy in a Nationwide US Commercial Insurance Claims Database. JAMA Netw Open 2019; 2(12):e1917603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao GF, Chhabra KR, Yang J, et al. Bariatric Surgery in Medicare Patients: Examining Safety and Healthcare Utilization in the Disabled and Elderly. Ann Surg 2020. [DOI] [PMC free article] [PubMed]
- 21.Chhabra KR, Telem DA, Chao GF, et al. Comparative Safety of Sleeve Gastrectomy and Gastric Bypass: An Instrumental Variables Approach. Ann Surg 2020. [DOI] [PMC free article] [PubMed]
- 22.Kaiser Family Foundation. Birth Rate per 1,000 Women Ages 15–44. State Health Facts 2017.
- 23.Moore BJ, White S, Washington R, et al. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data The AHRQ Elixhauser Comorbidity Index. Medical Care 2017; 55(7):698–705. [DOI] [PubMed] [Google Scholar]
- 24.Livingston EH. Development of bariatric surgery-specific risk assessment tool. Surg Obes Relat Dis 2007; 3(1):14–20; discussion 20. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. National Center for Health Statistics: Key Birth Statistics 2019 Available at: https://www.cdc.gov/nchs/nvss/births.htm. Accessed January 21, 2021.
- 26.Menke MN, King WC, White GE, et al. Contraception and Conception After Bariatric Surgery. Obstet Gynecol 2017; 130(5):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosman GG, King WC, Schrope B, et al. Reproductive health of women electing bariatric surgery. Fertil Steril 2010; 94(4):1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheiner E, Levy A, Silverberg D, et al. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. Am J Obstet Gynecol 2004; 190(5):1335–40. [DOI] [PubMed] [Google Scholar]
- 29.Bilenka B, Ben-Shlomo I, Cozacov C, et al. Fertility, miscarriage and pregnancy after vertical banded gastroplasty operation for morbid obesity. Acta Obstet Gynecol Scand 1995; 74(1):42–4. [DOI] [PubMed] [Google Scholar]
- 30.Deitel M, Stone E, Kassam HA, et al. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988; 7(2):147–53. [DOI] [PubMed] [Google Scholar]
- 31.Marceau P, Kaufman D, Biron S, et al. Outcome of pregnancies after biliopancreatic diversion. Obes Surg 2004; 14(3):318–24. [DOI] [PubMed] [Google Scholar]
- 32.Friedman D, Cuneo S, Valenzano M, et al. Pregnancies in an 18-Year Follow-up after Biliopancreatic Diversion. Obes Surg 1995; 5(3):308–313. [DOI] [PubMed] [Google Scholar]
- 33.Auger N, Ukah UV, Monnier M, et al. Risk of severe maternal morbidity after bariatric surgery: retrospective cohort study. Annals of Surgery 2021. [DOI] [PubMed]
- 34.Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes Among Women Who Had a Live Birth - United States, 2012–2016. MMWR Morb Mortal Wkly Rep 2018; 67(43):1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbah AK, Kornosky JL, Kristensen S, et al. Super-obesity and risk for early and late pre-eclampsia. BJOG 2010; 117(8):997–1004. [DOI] [PubMed] [Google Scholar]
- 36.Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr 2011; 93(6):1312–20. [DOI] [PubMed] [Google Scholar]
- 37.Lisonkova S, Muraca GM, Potts J, et al. Association Between Prepregnancy Body Mass Index and Severe Maternal Morbidity. JAMA 2017; 318(18):1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butwick AJ, Abreo A, Bateman BT, et al. Effect of Maternal Body Mass Index on Postpartum Hemorrhage. Anesthesiology 2018; 128(4):774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson K, Stephansson O, Neovius M. Outcomes of pregnancy after bariatric surgery. N Engl J Med 2015; 372(23):2267. [DOI] [PubMed] [Google Scholar]
- 40.Callegari LS, Nelson KM, Arterburn DE, et al. Development and Pilot Testing of a Patient-Centered Web-Based Reproductive Decision Support Tool for Primary Care. J Gen Intern Med 2021. [DOI] [PMC free article] [PubMed]
- 41.Say R, Robson S, Thomson R. Helping pregnant women make better decisions: a systematic review of the benefits of patient decision aids in obstetrics. BMJ Open 2011; 1(2):e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod 2004; 19(7):1644–6. [DOI] [PubMed] [Google Scholar]
- 43.Chandrasekaran S, Neal-Perry G. Long-term consequences of obesity on female fertility and the health of the offspring. Curr Opin Obstet Gynecol 2017; 29(3):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol 2018; 218(6):573–580. [DOI] [PubMed] [Google Scholar]
- 45.Anderson NH, McCowan LM, Fyfe EM, et al. The impact of maternal body mass index on the phenotype of pre-eclampsia: a prospective cohort study. BJOG 2012; 119(5):589–95. [DOI] [PubMed] [Google Scholar]
- 46.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. Am J Obstet Gynecol 2004; 190(4):1091–7. [DOI] [PubMed] [Google Scholar]
- 47.Poobalan AS, Aucott LS, Gurung T, et al. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women--systematic review and meta-analysis of cohort studies. Obes Rev 2009; 10(1):28–35. [DOI] [PubMed] [Google Scholar]
- 48.Liu P, Xu L, Wang Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev 2016; 17(11):1091–1102. [DOI] [PubMed] [Google Scholar]
- 49.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, et al. Subfecundity in overweight and obese couples. Hum Reprod 2007; 22(6):1634–7. [DOI] [PubMed] [Google Scholar]
- 50.van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod 2008; 23(2):324–8. [DOI] [PubMed] [Google Scholar]
- 51.Hennings DL, Baimas-George M, Al-Quarayshi Z, et al. The Inequity of Bariatric Surgery: Publicly Insured Patients Undergo Lower Rates of Bariatric Surgery with Worse Outcomes. Obes Surg 2018; 28(1):44–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


