Abstract
Oxygen is critical for neural metabolism, but under most physiological conditions oxygen levels in the brain are far more than are required. Oxygen levels can be dynamically increased by increases in respiration rate that are tied to the arousal state of the brain and cognition, and not necessarily linked to exertion by the body. Why these changes in respiration occur when oxygen is already adequate has been a long-standing puzzle. In humans, performance on cognitive tasks can be affected by very high or very low oxygen levels, but whether the physiological changes in blood oxygenation produced by respiration have an appreciable effect is an open question. Oxygen has direct effects on potassium channels, increases the degradation rate of nitric oxide, and is rate limiting for the synthesis of some neuromodulators. We discuss whether oxygenation changes due to respiration contribute to neural dynamics associated with attention and arousal.
Keywords: oxygen, respiration, neural excitability, cognition, nitric oxide
Introduction
Our state of mind is reflected in our breathing patterns. We breath rapidly when excited or scared, slowly when calm, and a surprise can make us gasp. Why we change our breathing patterns so drastically cannot be explained by metabolic concerns alone, and has been a longstanding mystery. There is a growing body of work showing that these changes in breathing can dynamically modulate blood oxygenation [22,81], and by consequence the oxygenation in the brain [125]. Given that the baseline supply of oxygen to the brain has a large safety margin that can easily accommodate the metabolic demands of increases in neural activity, the reason for these changes in respiration (as well as increases in the local flow of oxygenated blood due to changes in neural activity in the brain via neurovascular coupling) remains unexplained.
Here we review the speculative hypothesis that changes in local tissue oxygenation linked to normal respiratory fluctuations modulate neural activity in the brain. Experiments in humans have shown that un-physiologically high levels of blood oxygenation can improve cognitive performance [13] and low levels of oxygenation (like those that occur at high altitude) can impair performance [28,61], but whether the smaller changes in tissue oxygenation induced by changes in respiration (on a breath-by-breath basis and those caused by a change in respiratory rate) can have a meaningful effect is not known. In this hypothesis, oxygen functions like a neuromodulator, and respiration-driven changes in the level of oxygen [125] can affect the excitability of neurons, via direct actions on ion channels and by increasing the synthesis rate of many different neuromodulators. Like other canonical neuromodulators, oxygen levels are largely controlled by the activity of a small group of neurons that have reciprocal connections with other neuromodulatory nuclei [21,118,120,121]. The physiological processes that modulate brain oxygenation are modulated by sensory stimuli, arousal levels, and cognitive demands [94], just like other neuromodulatory signals. Un-physiologically large increases or decreases in oxygen can drive changes in cognition [61], as is seen when levels of neuromodulators are changed pharmacologically. We note that these effects of respiration on neural activity via oxygen could co-exist both with direct neural drive in respiratory and olfactory areas to other brain regions [106] [123] and local oxygenation changes driven by neurovascular coupling and functional hyperemia [19,65]. However, the effects we propose here would be distinct from directly mediated synaptic signaling (as inhalation cycle-linked oscillations [106]) and could be independent of changes in local blood flow (as in the hemo-neuro hypotheses [65]). The effects of oxygen on the brain that we discuss will depends on tissue oxygenation, which will be related to (but not the same as) hemoglobin saturation. The effects we discuss here could also act in parallel to (but distinct from) any changes in neural excitability caused by pH shifts secondary to changes in carbon dioxide levels which could impact neural excitability [50,5]. The amplitude and frequency of breath-related oxygen fluctuations will vary with species- and individual-specific breathing differences, and will also be impacted by the physiological state of the lung (dead space, etc.). Below we discuss the possibility that oxygen can function in a modulatory fashion in the brain.
Baseline oxygen levels can supply all the metabolic needs of neurons.
In many brain regions (but not all- [125,40,93]), sensory stimulation drives an increase in neural activity followed by local vasodilation (as known as functional hyperemia) mediated by many signaling mechanisms from neurons and other cells in the brain [91]. While this increase in flow is often attributed to the need to supply oxygen to active neurons, the increased oxygen is not necessary and usually greatly exceeds neuronal demands [63,54,59]. This clear oversupply of oxygen can be revealed with the application of vasoconstrictors like indomethacin [98] and caffeine [117], which can decrease cerebral blood flow by 30% without changing metabolism or any adverse cognitive consequences, consistent with a large safety margin in oxygen delivery. In anesthetized preparations, some brain areas even show inverted neurovascular coupling, with increases in activity driving vasoconstriction and oxygen decreases [88,97,96,16]. Anticipation of a stimulus can also drive increase in blood flow without increases in local neural activity [99], suggesting the existence of other preparatory mechanism in the brain that brings arterial blood to the cortex, other than to meet the metabolic demand generated by local neural activity. Vasodilation/blood flow increases can be elicited by the activation of a small set of neurons that express nitric oxide synthase without activation of other neurons in the cortical network [49,53,20], showing that the overall metabolic demands are disconnected from the neural control of the vascular system. The lack of tight coupling of the local regulation of blood flow to metabolism in so many instances suggests that the flow increases might serve other purposes than to supply a pressing metabolic need, an idea that has been noted previously [37,54]. It has been proposed that increase in flow with neurovascular coupling is not to service the bulk of the tissue, but regions where flow is limited [17,54]. However, simulations have suggested that increasing blood flow does not actually remove low flow regions, but rather relocates them, suggesting that functional hyperemia may not even remove these regions of lower oxygenation, but only shift them [82]. This unintuitive change of perfusion further suggests that increase in oxygenation is not specifically to meet metabolic demands.
Whether increasing oxygenation in the brain affects metabolic activity depends on whether the production of ATP is limited by concentration of oxygen. It is largely assumed that under physiological conditions, oxygen levels are far from rate limiting for neural metabolism, and increasing oxygen does not result in the increased production of ATP, as mitochondrial oxidative phosphorylation is saturated by oxygen concentrations well below 1 mmHg [55,110,29]. The oxygen dependence of mitochondrial oxidative phosphorylation depends on intracellular pH, and oxygen dependence becomes noticeable with alkaline pH [114]. However, the intracellular pH in the brain is near 7 [12,69]. In the physiological pH range, the oxygen becomes limiting below a few mmHg (~ 3 mmHg), still well below the state in most of the tissue. Supporting the idea that mitochondrial oxidative phosphorylation in the brain is not limited by oxygen levels, hyperoxia does not increase brain metabolic rate [116] (though these are bulk measures that may not detect elevations of metabolism in small, poorly-oxygenated regions). Systemic arterial oxygen levels of less than 20 mmHg (well below normal levels of ~90 mmHg [57]) are required to detectably decrease ATP levels in the brain [28]. Furthermore, measurements of mean cortical oxygen levels in the blood plasma (which will be higher than that in the tissue) in the cortex typically find oxygen concentrations in the range of 20-50 mmHg [52,125,11,57,87]. In the extracellular space of the cortex, tissue oxygen levels are in the range of 10 to 40 mmHg, though the levels can be much higher immediately adjacent to arteries [89,17], and veins passing near arteries can become oxygenated through diffusional shunting of oxygen [51]. While a small fraction of tissue may show oxygen levels below ~5 mmHg [125,57], this is still far above the levels at which oxygen is limiting for ATP production.
Oxygen levels impact neuronal excitability via effects on ion channels
Neuromodulators typically function to make distinct cell types more or less excitable, altering their responses to stimuli and changing network output [18,34]. While the oxygen sensitivity of neurons is often explored in the context of hypoxia, oxygen levels in neural tissue can fluctuate within the physiological range [57,125], often following changes in neural activity (functional hyperemia), during exercise, or in phase with breathing [125,124]. One tempting hypothesis is that the fluctuations in blood oxygenation due to alternating inspiration and expiration, as well as elevations and depression of respiration cycle-averaged blood oxygen over longer time scales, could modulate neuronal excitability and activity patterns. As most of the work we discuss here have been done in slices, one should bear in mind that the normal oxygen tension in slice experiments (where ionic mechanisms are probed) can be much higher than measured in vivo [43] [41]. With in vitro experiments, the oxygen gradient as a function of depth in the slice can be very large (~100mmHg per 100μm tissue) [33,8,43], leading to great heterogeneity of the measure due to effects of changing oxygen levels. As a result, in vitro conditions described as “normal or hypoxic” may range from hyperoxia to anoxia.
Several ion channels expressed widely in the brain are known to be sensitive to oxygen levels via a variety of mechanisms (Figure 1). TASK-1 and 3 channels (both hetero- and homodimers) are inhibited by hypoxia [107,9]. The channels are found in the carotid bodies and their activity contributes to (but is not necessary for) oxygen sensing [75]. A decrease in oxygen inhibits mitochondrial electron transport and subsequently leads to TASK channel inhibition. TASK-1 and 3 are also found in neurons in the basal forebrain [103,111], and single cell RNA sequencing has shown these channels are expressed by thalamic interneurons, cholinergic interneurons, and serotonergic neurons [122] (Figure 1). Kv3 channels are also sensitive to oxygen levels [76] and are expressed by cortical and hippocampal excitatory and inhibitory neurons [45] (Figure 1). A depression of oxygen will cause K+ channel inhibition. In addition to potassium channels, L-type calcium channels are also thought to contribute to oxygen sensitivity in peripheral tissue [113]. Cells in the vasculature (smooth muscle, endothelial cells and red blood cells), are also thought to be sensitive to oxygen, though the mechanisms are not clearly understood (see [42] for review). Changes in vessel tone require changes in the membrane potential [36], implying the activation of ion channels. While the molecular mechanisms linking oxygen levels to ion channel opening and closing are not well understood, several pathways have been implicated, including both NADPH oxidase dependent mitochondrial superoxide anion generation and changes in the ratio of reduced to oxidized glutathione[56]. Other possible mechanism includes changes in prostaglandin levels and nitric oxide (discussed below). The net effect of oxygen levels would depend on the relative distributions of oxygen-sensitive ion channels in excitatory and inhibitory neurons, as is the case with many neuromodulators.
Figure 1:
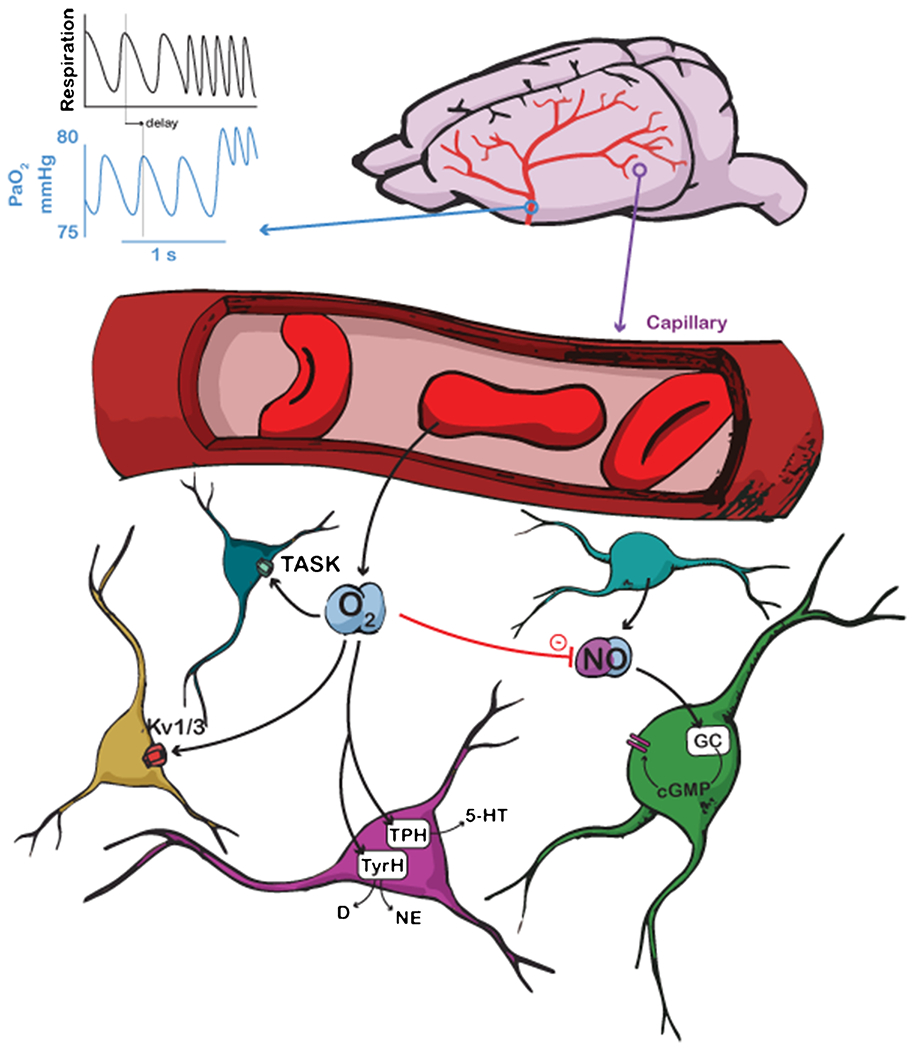
Schematic showing different pathways by which oxygen can modulate neural excitability. Top, oxygen levels in major supply arteries oscillate on a breath-by-breath basis, as well as showing an overall increase with respiration rate. Scale is for expected values in a mouse. Respiration shows idealized measurement from a thermocouple, with upswings representing exhalation. Bottom left, oxygen modulates K+ channels and TASK activity in neurons. Bottom middle, oxygen modulates tryptophan hydroxylase (TPH) synthesis of serotonin (5-HT) and tyrosine hydroxylase (TyrH) synthesis of dopamine (D) and norepinephrine (NE). Coloration of neurons is aesthetic. Bottom right, oxygen decreases nitric oxide (NO) concentrations which modulates neural activity.
At the cellular level, neurons and glial cells located in the medulla oblongata and hypothalamus, are able to sense oxygen levels and modulate respiratory rhythm accordingly [71,3]. Though the mechanism underlying these oxygen-induced changes are not completely understood, this demonstrates that some cells of the brain are able to change their activity in response to oxygen levels. Recent studies have shown that astrocytes (including astrocytes in the cortex which are not traditionally thought of as oxygen-sensing cells), respond to oxygen changes with altering calcium signals [108] [3], and modulating the respiratory network activity [3,31,4,30]. Given that astrocytes display regional diversity at the molecular and functional levels in the brain [7], astrocytes could vary in oxygen sensitivity according to their cellular function and metabolism.
Oxygen levels impact the level of the neuromodulator nitric oxide
In addition to any direct actions oxygen has on neuron excitability, oxygen has an antagonist relationship with nitric oxide (NO). While increases in NO will increase oxygenation (via its vasodilatory actions), increased tissue oxygen facilitates the removal of NO that is linearly dependent on the oxygen concentration [105] (Figure 1), creating a negative feedback loop important for equilibrium. Although the presence of central oxygen sensors in the CNS is a topic of debate, hypoxia will dilate and hyperoxia will constrict cerebral arterioles via modulation of NO levels [38,84,86,1]. These blood flow effects occur even under isocapnic conditions [109,68] and constant pH [85], which demonstrates that the traditional indirect sensing mechanisms of hypoxia via pH and CO2 are not required for hypoxia-induced vasodilation. The oxygen-dependent rate of NO removal in the tissue may be an additional mechanism of maintaining oxygenation under hypoxic conditions [35].
In addition to its cerebrovascular effects [39], NO can greatly affect neural excitability and has been shown to increase the activity of neurons in the cortex [46], cerebellum [101] and other brain areas [88,24]. Nitric oxide synthases, which produces NO, are found in the vascular endothelium (eNOS) and in neurons (nNOS). NO can also be produced in response to injury (iNOS). nNOS can exist in the cytoplasm of neurons or attached to the NMDA receptor where it can produce NO in response to glutamate and promote AMPA receptor trafficking to the synapse [83]. Regardless of the source of NO, NO-induced increases in neural excitability can be generated with exogenous application of NO donors or precursors [46] and occur through the GC/cGMP/PKG pathway [101]. In vivo electrophysiological recordings in the visual cortex show that L-arginine, an NO precursor, or DEA-NO, a NO donor, can increase neural excitability during visual stimulation, while inhibition of endogenous NO by a NOS inhibitor, L-MMA, decreases spiking [46]. In addition to modulating stimuli evoked firing rates, spontaneous firing of Purkinje neurons in cerebellar slices could also be increased by a NO donor via cGMP signaling [101]. Oxygen’s ability to directly control the rate of NO consumption also provides another avenue by which tissue oxygenation could potentially influence neural activity. Simulations of oxygen-NO diffusion/degradation dynamics in the brain have shown that increasing levels of oxygen in the blood stream led to decreases in NO levels in the tissue [35]. In these simulations, the decreases in NO were enough to drive vascular changes (vasoconstriction). These changes in blood oxygenation would drive changes in NO in the tissue that could impact neural excitability. However, oxygen’s interactions with NO degradation mean that cerebral blood flow will undergo compensatory changes that will tend to counter any changes in blood oxygenation on the time scale of seconds that it takes for a vascular response.
Oxygen levels impact the synthesis of many neuroactive substances
While mitochondrial respiration is not oxygen limited, the synthesis of many signaling molecules can be. For example, many of the enzymes involved in neuromodulator synthesis are rate limited by oxygen at physiological concentrations [110]. Acetylcholine synthesis is oxygen limited, and is impaired in low oxygen conditions [26]. Tyrosine hydroxylase (involved in the synthesis of dopamine and norepinephrine) has a Km for oxygen in the range of ~45 mmHg, and tryptophan hydroxylase (involved in the synthesis of serotonin) has a Km in the range of ~20 mmHg[110]. These Kms are high enough that physiological fluctuations in the level of oxygen in the brain [57][124,125] could affect local synthesis of these neuromodulators (Figure 1). Both chemically-induced and hypoxemic-induced hypoxia decrease the synthesis of acetylcholine and amino-acid based neurotransmitters [27]. Hypobaric hypoxia lowers the concentration of norepinephrine and dopamine in the brain [79], though sustained hypoxia can drive compensation in these systems [14]. Interestingly, there is evidence that altered serotonin metabolism may contribute to the increased incidence of mental illness in residents at increased altitude [47]. In contrast, increased respiration rate will increase tissue oxygenation [125] and could lead to greater availability of neuromodulators via increased synthesis.
Oxygenation as a link between the peripheral state and the ‘central governor’
Sustained physical exertion over the timescale of minutes drives many cardiovascular changes [44]: cardiac output and respiration rate increase, while systemic blood carbon dioxide and oxygen levels fall. When we physically exert ourselves voluntarily, we stop when we become fatigued. Traditionally, this fatigue has been attributed to the buildup of lactate in the muscles. However, there is some evidence that this fatigue is detected in the brain, and that the feeling of fatigue is centrally generated [74]. This idea is known as the ‘central governor’ hypothesis [73]. A variety of feedback signals, both via accumulating chemical signals and afferent neural feedback have been proposed. As oxygen levels fall in the blood during sustained exercise, it is a plausible candidate as a signal from the periphery to the brain, and experiments manipulating inhaled oxygen in exercising humans have shown effects on performance that cannot be entirely ascribed to peripheral effects [74]. Hyperoxymia during all-out exercise increases work output and increases brain (but not muscle) oxygenation, suggesting that cerebral oxygenation could act as a sensor of total cardiovascular state [72]. In humans, increasing or decreasing inhaled oxygen respectively increases or decreases motor output, but has no effect on peripheral fatigue (as measured with electrical stimulation of muscles), suggesting that oxygen’s role in preventing fatigue acts via its action on neurons in the brain [2].
Impact of oxygenation on cognitive performance and its relation to respiration
Anyone who has been at very high altitude knows how deleterious a reduction in oxygen can be for the performance of even the simplest of mental tasks. Hypoxia [28,61] and high altitude causes many cognitive impairments that increase with severity of oxygen deficit [119,70]. Many studies have shown that lower levels of brain oxygenation cause poorer performance in a wide range of cognitive tasks [61]. There are also numerous studies showing that respiratory entrainment of neural activity has important impacts on mood and cognition [58]. Oxygen levels in the blood decline with age [102], and these declines could contribute to the cognitive decline accompanying aging.
If oxygen serves a modulatory role in the brain, we would expect it to vary with behavioral state in a way that is not just due to increased exertion. In rodents, respiration locks with whisking [66]. In humans, respiration rate changes dynamically in response to stimuli and behavioral state [94]. Merely opening the eyes, reading, or listening to words causes a measurable increase in respiration that is hard to explain with metabolic factors alone[95]. Respiration locks to the onset of cognitive task, even ones that are not olfactory in nature and do not require a verbal answer [77]. Performance in the visuospatial task was significantly better during inhalation vs exhalation, potentially due to augmented brain oxygenation during inhalation [125]. As all these changes in respiration occur with minimal increases in exertion, their existence makes little sense unless oxygen serves some cognitive role.
For changes in respiration to modulate neural activity via changes in oxygen levels, changes in respiration rate and depth need to play an important role in setting arterial oxygenation (Figure 2). Arterial oxygenation varies over the inhalation-expiration cycle, though the amplitude of the variations is inversely related to the breathing rate of the animal [81,80,22,125]. Increases in respiration globally increase cerebral oxygenation, even in areas with no change or decrease in blood flow [125]. Although the average levels of oxygen and their respiration-linked changes are similar in both frontal and sensory cortices [125], there are wide variations in vascular density across the brain that could potentially make the oxygen fluctuations larger or smaller in different brain regions [115,48], analogous to greater levels of modulation in certain brain regions.
Figure 2.
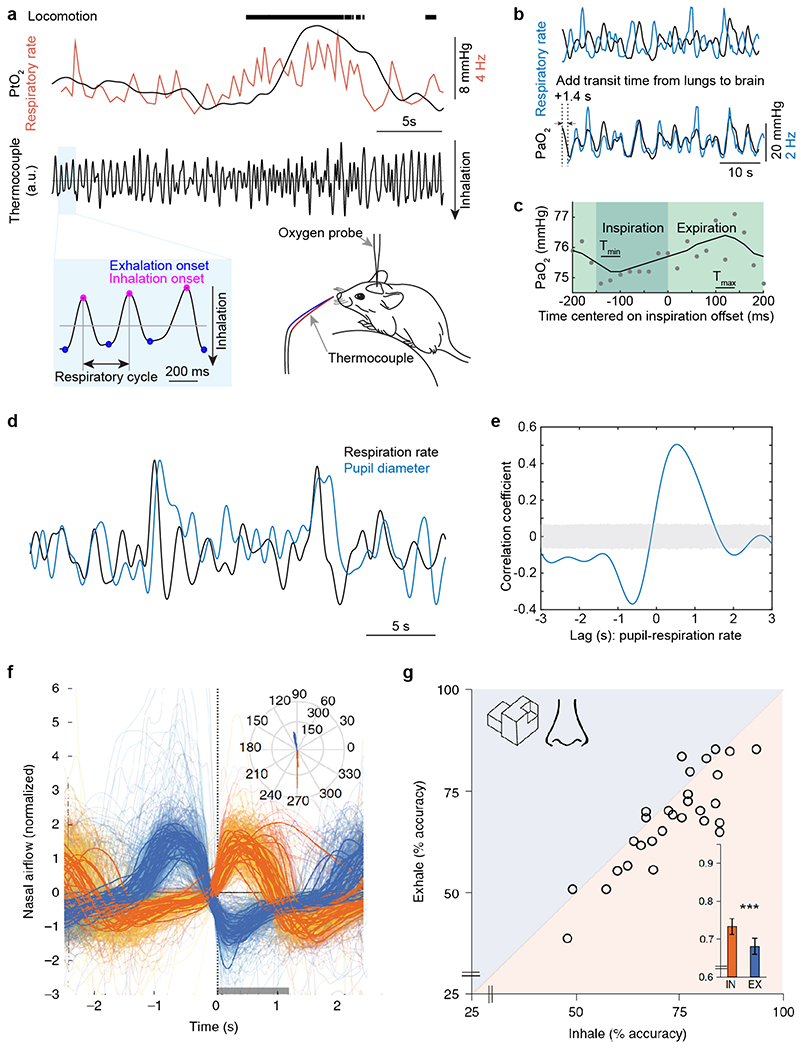
(a-b) Respiration drives changes in cerebral and blood oxygenation. (a) Measuring respiration using a thermocouple. Top, example data showing tissue oxygenation in the somatosensory cortex of an awake, headfixed mouse measured using an oxygen sensitive microelectrode (black trace) and respiratory rate (orange trace), during locomotion. Middle, signal from a thermocouple placed near the nostril of the mouse. The thermocouple voltage tracks inhalation and exhalation due to the higher temperature of exhaled air, which causes increase in the thermocouple signal. Bottom left, expanded thermocouple signal showing of the detection of the onset of inspiratory (magenta dot) and expiratory phase (blue dot). Bottom right, schematic showing respiration measurement using a thermocouple. (b) Example data showing the temporal relation between respiratory rate (black) and oxygen tension (PaO2, blue) in the center of one artery in somatosensory cortex of a mouse during periods of rest. The phase shift is caused by transit time from lungs to brain. (c) PaO2 fluctuates within the respiratory cycle. The PaO2 change in one artery of a headfixed, un-anesthetized mouse during the respiratory cycle at rest was measured using an intravascularly-injected phosphorescent oxygen dye using a two-photon microscope. This technique allows measurement of the concentration of oxygen in the blood plasma from a single location in the vasculature. PaO2 data (15 recordings with each of 50 seconds in duration) were aligned to the offset of inspiration. Each circle denotes averaged PaO2 over a short window (20 ms) and over the 15 recordings. The solid curve denotes filtering of data (first order binomial filter, 5 repetitions). Tmin denotes the time period (40 ms) PaO2 reaches minimum. Tmax denotes the time period (40 ms) PaO2 reaches maximum. (d) Example data showing the temporal relation between respiratory rate (black) and pupil diameter (blue, an indicator of noradrenergic activity) during periods of rest in an awake, headfixed mouse. (e) Cross-correlation between respiratory rate and pupil diameter during periods of rest. Gray shaded area indicates 95% confidence interval. (f-g) Nasal inhalation at visuospatial task onset is associated with improved performance in humans. (f) Mean event-related nasal respiratory signal used to trigger trial-onset time-locked to inhalation (orange) or exhalation (blue). Time 0 denotes task initiation. The grey rectangle along the x axis represents the stimulus (1,200 ms). Inset: a polar plot of the respiratory phase (in degrees) at trial onset is shown. The orange and blue bins are trials triggered by inhalation and exhalation, respectively (n = 28). (g) Scatter plot of performance in the EEG visuospatial task in inhalation and exhalation. Each point is a participant (n = 28). The diagonal line is the unit slope line (x = y). Thus, if points accumulate below the line, this means performance was better during inhalation. In the inlay, the mean group performance is shown. Error bars are SEM. a-e adapted from [125] , f-g adapted from [77].
Reciprocal interactions between respiratory related brain regions and modulatory regions
Neuromodulatory structures make brain wide projections and also form reciprocal connections with other neuromodulatory nuclei [112] [90]. If oxygen acts like a neuromodulator, we would expect there to be connections between the pre-Bötzinger complex and other respiratory-control nuclei and canonical neuromodulatory regions. Supporting this idea, a genetically-defined subset of neurons in the pre-Bötzinger complex has been found to send excitatory projections to the locus coeruleus (LC), and their activation affects LC activity enough to cause increases in arousal [118]. This coupling of oxygenation and modulation might take place on a breath-to-breath basis. As neurons from pre-Bötzinger complex neurons are linked with inspiratory phase of respiration [100], one would expect that there will be a direct relationship between the inspiratory phase of respiration and LC activity, if there is indeed a link between respiration and LC activity. Consistent with this idea, pupil diameter, an index of LC activity [104], rises in phase with the pre-inspiratory/inspiratory phase of respiration, and falls during the expiratory phase of respiration [62] (Figure 2), consistent with a direct role in activation of LC from pre-Bötzinger complex. Different pools of neurons in the pre-Bötzinger complex also project to many nuclei across the brain, including the dorsomedial hypothalamus and lateral preoptic area [120]. The dorsomedial hypothalamus sends orexinergic projections (which play a key role in maintaining wakefulness [78]) to many brain regions. The dorsomedial hypothalamus also sends projections back to the pre-Bötzinger complex [23]. The lateral preoptic area sends projections to the ventral tegmental area (VTA) [25], which sends dopaminergic projections to many brain regions. The retrotrapezoid nucleus (RTN) receives serotonergic input, which can then influence breathing rate independent of pH [67,15]. Thus the nuclei that control oxygen levels in the brain (via respiration) have connections with other modulatory nuclei.
Relationship to the hemo-neural hypotheses
Our hypothesis that oxygen modulates neural function partially overlaps with the hemo-neural hypothesis [10,65]. In the hemo-neural hypothesis, increases in blood flow accompanying functional hyperemia send mechanical and/or chemical signals to neurons to enhance information processing [64]. As both respiration (a global factor) and vasodilation (with accompanying increase in blood flow, a local factor) contribute to modulating brain oxygenation [125], we hypothesize that global cerebral oxygen changes, caused by changes in respiration and/or increased consumption by other organs modulate neural activity. Local oxygen changes due to functional hyperemia could potentially provide a more spatially restricted control of activity.
Issues with testing the modulatory oxygen hypothesis
Foremost, although extreme elevation and depression of blood oxygen (in the tens to hundreds of mmHg over minutes or longer) have been shown to have cognitive (and presumably neural) effects, it has not been determined if the much smaller and briefer changes that occur with respiration (in the range of few mmHg for a few seconds) can change neural activity. Testing this hypothesis is not an easy task. Experiments looking at the impact of respiration phase on cognitive tasks have the confound that in these experiments, other signals are present besides oxygen changes [77]. Oxygen’s effects on NO degradation will tend to function as a homeostatic regulator of oxygen levels in the brain, so any change in respiration will tend to drive a compensatory change in cerebral blood flow, reducing the size and duration of respiration-induced oxygenation changes. The flow of red blood cells is stochastic and results in a highly variable delivery of oxygen, the fluctuations in the oxygen levels that neurons experience [124] will tend to obscure any respiration-related changes. Finally, any experimental test will have to tease out any direct effects of oxygenation from changes mediated by other neural signals accompanying respiration [106].
There are also several non-neuronal factors that will contribute to the individual details of the oxygen fluctuations in the tissue and thus any potentially modulatory effects of oxygen. The oxygen-affinity curve for hemoglobin shift rightward with increasing animal size [92], and the tidal volume and respiration rate will vary with size as well [32], so the amplitude and frequency of a breath-by-breath oxygen changes in brain tissue will differ by species. The oxygen levels will also be affected by lung physiology and health. Thus, some of the effects may be more or less salient under different physiological or task conditions or in certain animal species.
Summary
The brain receives more oxygen than it needs to power the synthesis of ATP, yet oxygen levels are dynamically regulated by changes in local vessel dilation and by changes in respiration (both breath-by-breath cyclic changes and changes due to overall respiratory rate change). Cognitive tasks increase respiration [77], and large changes in oxygen levels bi-directionally affect mental tasks and reaction times. These puzzling facts suggest that in the brain, oxygen has some other dynamic function above and beyond its direct metabolic role. We discussed the possibility that oxygen serves a neuromodulator-like function in dynamically tuning the responsiveness of neurons. One direct evidence is that oxygen levels modulate neuron excitability, just like canonical neuromodulators. Low oxygen levels can inhibit some ion channels to increase the excitability of neurons. Besides the direct neural excitatory effects, oxygen levels affect the level of neuromodulator nitric oxide via a closed-loop feedback mechanism, i.e., the breakdown rate of nitric oxide will increase with increased oxygen concentration. This provides another pathway by which oxygen can impact neural excitability, as nitric oxide has many effects on neurons via second messengers. In addition, low oxygen levels will reduce the synthesis of many neuromodulators, such as acetylcholine and norepinephrine. The broad link between oxygenation and numerous modulatory pathways at different levels explains the relation between respiration and physical/cognitive performances. It explains how peripheral fatigue (due to sustained exercise) can provide brain-wide signals via changes in oxygen levels in the blood. It also suggests that the increase in oxygenation in the brain caused by increased respiration upon presentation of an unexpected stimulus may serve a similar purpose as the release of a burst of norepinephrine or acetylcholine, which may explain why cognition is locked with respiration and why we have better cognitive performance in high oxygen level environments. While testing this hypothesis will be challenging, it may give us insight into the purpose of our dynamic respiratory patterns.
Acknowledgments
QZ is supported by a Career Development Award from the American Heart Association (935961). PJD is supported by R01NS078168 and R01NS079737 from the National Institutes of Health. SC is supported by the FRM (EQU201903007811), the ANR (NR-16-RHUS-0004 [RHU TRT_cSVD]; IHU FOReSIGHT [ANR-18-IAHU-0001]) and the Fondation Leducq Transatlantic Networks of Excellence program (16CVD05).
Footnotes
Competing interests:
The authors have no competing interests to declare that are relevant to the content of this article.
Research involving Human Participants and/or Animals
This is a review of the published literature, and ethical approvals can be found within the cited references.
References
- 1.Agvald P, Adding LC, Artlich A, Persson MG, Gustafsson LE (2002) Mechanisms of nitric oxide generation from nitroglycerin and endogenous sources during hypoxia in vivo. Br J Pharmacol 135:373–382. doi: 10.1038/sj.bjp.0704489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA (2006) Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575:937–952. doi: 10.1113/jphysiol.2006.113936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak a, Zwicker J, Teschemacher aG, Ackland GL, Funk GD, Kasparov S, Abramov aY, Gourine aV (2015) Functional Oxygen Sensitivity of Astrocytes. Journal of Neuroscience 35:10460–10473. doi: 10.1523/JNEUROSCI.0045-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestrino M, Somjen GG (1988) Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol 396:247–266. doi: 10.1113/jphysiol.1988.sp016961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Band DM, Cameron IR, Semple SJ (1969) Oscillations in arterial pH with breathing in the cat. J Appl Physiol 26:261–267. doi: 10.1152/jappl.1969.26.3.261 [DOI] [PubMed] [Google Scholar]
- 7.Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18:31–41. doi: 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]
- 8.Bingmann D, Kolde G (1982) PO2-profiles in hippocampal slices of the guinea pig. Exp Brain Res 48:89–96. doi: 10.1007/BF00239575 [DOI] [PubMed] [Google Scholar]
- 9.Buckler KJ (2015) TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch 467:1013–1025. doi: 10.1007/s00424-015-1689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao R, Higashikubo BT, Cardin J, Knoblich U, Ramos R, Nelson MT, Moore CI, Brumberg JC (2009) Pinacidil induces vascular dilation and hyperemia in vivo and does not impact biophysical properties of neurons and astrocytes in vitro. Cleve Clin J Med 76 Suppl 2:S80–85. doi: 10.3949/ccjm.76.s2.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celaya-Alcala JT, Lee GV, Smith AF, Li B, Sakadzic S, Boas DA, Secomb TW (2021) Simulation of oxygen transport and estimation of tissue perfusion in extensive microvascular networks: Application to cerebral cortex. J Cereb Blood Flow Metab 41:656–669. doi: 10.1177/0271678X20927100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesler M, Kraig RP (1989) Intracellular pH transients of mammalian astrocytes. The Journal of Neuroscience 9:2011–2019. doi: 10.1523/jneurosci.09-06-02011.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damato EG, Flak TA, Mayes RS, Strohl KP, Ziganti AM, Abdollahifar A, Flask CA, LaManna JC, Decker MJ (2020) Neurovascular and cortical responses to hyperoxia: enhanced cognition and electroencephalographic activity despite reduced perfusion. J Physiol 598:3941–3956. doi: 10.1113/JP279453 [DOI] [PubMed] [Google Scholar]
- 14.Davis JN (1975) Adaptation of brain monoamine synthesis to hypoxia in the rat. J Appl Physiol 39:215–220. doi: 10.1152/jappl.1975.39.2.215 [DOI] [PubMed] [Google Scholar]
- 15.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG (2011) Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devor A, Hillman EMC, Tian P, Waeber C, Teng IC, Ruvinskaya L, Shalinsky MH, Zhu H, Haslinger RH, Narayanan SN, Ulbert I, Dunn AK, Lo EH, Rosen BR, Dale AM, Kleinfeld D, Boas DA (2008) Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. Journal of Neuroscience 28:14347–14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devor A, Sakadžić S, Saisan PA, Yaseen MA, Roussakis E, Srinivasan VJ, Vinogradov SA, Rosen BR, Buxton RB, Dale AM, Boas DA (2011) “Overshoot” of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. Journal of Neuroscience 31:13676–13681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Disney AA, Higley MJ (2020) Diverse Spatiotemporal Scales of Cholinergic Signaling in the Neocortex. J Neurosci 40:720–725. doi: 10.1523/JNEUROSCI.1306-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drew PJ (2019) Vascular and neural basis of the BOLD signal. Current Opinion in Neurobiology 58:61–69. doi: 10.1016/j.conb.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Echagarruga CT, Gheres KW, Norwood JN, Drew PJ (2020) nNOS-expressing interneurons control basal and behaviorally evoked arterial dilation in somatosensory cortex of mice. Elife 9. doi: 10.7554/eLife.60533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman JL, Del Negro CA, Gray PA (2013) Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75:423–452. doi: 10.1146/annurev-physiol-040510-130049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formenti F, Bommakanti N, Chen R, Cronin JN, McPeak H, Holopherne-Doran D, Hedenstierna G, Hahn CEW, Larsson A, Farmery AD (2017) Respiratory oscillations in alveolar oxygen tension measured in arterial blood. Sci Rep 7:7499. doi: 10.1038/s41598-017-06975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukushi I, Yokota S, Okada Y (2019) The role of the hypothalamus in modulation of respiration. Respir Physiol Neurobiol 265:172–179. doi: 10.1016/j.resp.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 24.Garthwaite G, Bartus K, Malcolm D, Goodwin D, Kollb-Sielecka M, Dooldeniya C, Garthwaite J (2006) Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci 26:7730–7740. doi: 10.1523/JNEUROSCI.1528-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geisler S, Zahm DS (2005) Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol 490:270–294. doi: 10.1002/cne.20668 [DOI] [PubMed] [Google Scholar]
- 26.Gibson GE, Duffy TE (1981) Impaired synthesis of acetylcholine by mild hypoxic hypoxia or nitrous oxide. J Neurochem 36:28–33. doi: 10.1111/j.1471-4159.1981.tb02373.x [DOI] [PubMed] [Google Scholar]
- 27.Gibson GE, Peterson C, Sansone J (1981) Decreases in amino acids and acetylcholine metabolism during hypoxia. J Neurochem 37:192–201. doi: 10.1111/j.1471-4159.1981.tb05308.x [DOI] [PubMed] [Google Scholar]
- 28.Gibson GE, Pulsinelli W, Blass JP, Duffy TE (1981) Brain dysfunction in mild to moderate hypoxia. The American Journal of Medicine 70:1247–1254. doi: 10.1016/0002-9343(81)90834-2 [DOI] [PubMed] [Google Scholar]
- 29.Gnaiger E (2003) Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. Adv Exp Med Biol 543:39–55. doi: 10.1007/978-1-4419-8997-0_4 [DOI] [PubMed] [Google Scholar]
- 30.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–575. doi: 10.1126/science.1190721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guyenet PG, Bayliss DA (2015) Neural Control of Breathing and CO2 Homeostasis. Neuron 87:946–961. doi: 10.1016/j.neuron.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyton AC (1947) Measurement of the respiratory volumes of laboratory animals. Am J Physiol 150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70 [DOI] [PubMed] [Google Scholar]
- 33.Hall CN, Klein-Flügge MC, Howarth C, Attwell D (2012) Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. Journal of Neuroscience 32:8940–8951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris-Warrick RM, Marder E (1991) Modulation of neural networks for behavior. Annual Review of Neuroscience [DOI] [PubMed] [Google Scholar]
- 35.Haselden WD, Kedarasetti RT, Drew PJ (2020) Spatial and temporal patterns of nitric oxide diffusion and degradation drive emergent cerebrovascular dynamics. PLoS Comput Biol 16:e1008069. doi: 10.1371/journal.pcbi.1008069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill CE (2012) Long Distance Conduction of Vasodilation: A Passive or Regenerative Process? Microcirculation 19:379–390. doi: 10.1111/j.1549-8719.2012.00169.x [DOI] [PubMed] [Google Scholar]
- 37.Hillman EMC (2014) Coupling Mechanism and Significance of the BOLD Signal: A Status Report. Annual Review of Neuroscience 37:161–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoiland RL, Bain AR, Tymko MM, Rieger MG, Howe CA, Willie CK, Hansen AB, Fluck D, Wildfong KW, Stembridge M, Subedi P, Anholm J, Ainslie PN (2017) Adenosine receptor-dependent signaling is not obligatory for normobaric and hypobaric hypoxia-induced cerebral vasodilation in humans. J Appl Physiol (1985) 122:795–808. doi: 10.1152/japplphysiol.00840.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosford PS, Gourine AV (2019) What is the key mediator of the neurovascular coupling response? Neurosci Biobehav Rev 96:174–181. doi: 10.1016/j.neubiorev.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huo BX, Smith JB, Drew PJ (2014) Neurovascular coupling and decoupling in the cortex during voluntary locomotion. J Neurosci 34:10975–10981. doi: 10.1523/JNEUROSCI.1369-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov A, Zilberter Y (2011) Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenergetics 3:9. doi: 10.3389/fnene.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson WF (2016) Arteriolar oxygen reactivity: where is the sensor and what is the mechanism of action? J Physiol 594:5055–5077. doi: 10.1113/JP270192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang C, Agulian S, Haddad GG (1991) O2 tension in adult and neonatal brain slices under several experimental conditions. Brain Research 568:159–164. doi: 10.1016/0006-8993(91)91392-e [DOI] [PubMed] [Google Scholar]
- 44.Joyner MJ, Casey DP (2015) Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95:549–601. doi: 10.1152/physrev.00035.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaczmarek LK, Zhang Y (2017) Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance. Physiol Rev 97:1431–1468. doi: 10.1152/physrev.00002.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kara P, Friedlander MJ (1999) Arginine analogs modify signal detection by neurons in the visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 19:5528–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kious BM, Kondo DG, Renshaw PF (2018) Living High and Feeling Low: Altitude, Suicide, and Depression. Harv Rev Psychiatry 26:43–56. doi: 10.1097/HRP.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 48.Kirst C, Skriabine S, Vieites-Prado A, Topilko T, Bertin P, Gerschenfeld G, Verny F, Topilko P, Michalski N, Tessier-Lavigne M, Renier N (2020) Mapping the Fine-Scale Organization and Plasticity of the Brain Vasculature. Cell 180:780–795 e725. doi: 10.1016/j.cell.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 49.Krawchuk MB, Ruff CF, Yang X, Ross SE, Vazquez AL (2020) Optogenetic assessment of VIP, PV, SOM and NOS inhibitory neuron activity and cerebral blood flow regulation in mouse somato-sensory cortex. J Cereb Blood Flow Metab 40:1427–1440. doi: 10.1177/0271678X19870105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krnjevic K, Randic M, Siesjoe BK (1965) Cortical Co2 Tension and Neuronal Excitability. J Physiol 176:105–122. doi: 10.1113/jphysiol.1965.sp007538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecoq J, Parpaleix A, Roussakis E, Ducros M, Goulam Houssen Y, Vinogradov SA, Charpak S (2011) Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med 17:893–898. doi: 10.1038/nm.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lecoq J, Parpaleix A, Roussakis E, Ducros M, Houssen YG, Vinogradov SA, Charpak S (2011) Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nature medicine 17:893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee L, Boorman L, Glendenning E, Christmas C, Sharp P, Redgrave P, Shabir O, Bracci E, Berwick J, Howarth C (2020) Key Aspects of Neurovascular Control Mediated by Specific Populations of Inhibitory Cortical Interneurons. Cereb Cortex 30:2452–2464. doi: 10.1093/cercor/bhz251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leithner C, Royl G (2014) The oxygen paradox of neurovascular coupling. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34:19–29. doi: 10.1038/jcbfm.2013.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Longmuir IS (1957) Respiration rate of rat-liver cells at low oxygen concentrations. Biochem J 65:378–382. doi: 10.1042/bj0650378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Barneo J, del Toro R, Levitsky KL, Chiara MD, Ortega-Saenz P (2004) Regulation of oxygen sensing by ion channels. J Appl Physiol (1985) 96:1187–1195; discussion 1170-1182. doi: 10.1152/japplphysiol.00929.2003 [DOI] [PubMed] [Google Scholar]
- 57.Lyons DG, Parpaleix A, Roche M, Charpak S (2016) Mapping oxygen concentration in the awake mouse brain. Elife 5. doi: 10.7554/eLife.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maric V, Ramanathan D, Mishra J (2020) Respiratory regulation & interactions with neurocognitive circuitry. Neurosci Biobehav Rev 112:95–106. doi: 10.1016/j.neubiorev.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masamoto K, Vazquez A, Wang P, Kim S-G (2009) Brain Tissue Oxygen Consumption And Supply Induced By Neural Activation. Advances in experimental medicine and biology 645:287–292 [DOI] [PubMed] [Google Scholar]
- 60.Matheson GO, McKenzie DC (1988) Breath holding during intense exercise: arterial blood gases, pH, and lactate. J Appl Physiol (1985) 64:1947–1952. doi: 10.1152/jappl.1988.64.5.1947 [DOI] [PubMed] [Google Scholar]
- 61.McMorris T, Hale BJ, Barwood M, Costello J, Corbett J (2017) Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci Biobehav Rev 74:225–232. doi: 10.1016/j.neubiorev.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 62.Melnychuk MC, Dockree PM, O’Connell RG, Murphy PR, Balsters JH, Robertson IH (2018) Coupling of respiration and attention via the locus coeruleus: Effects of meditation and pranayama. Psychophysiology 55:e13091. doi: 10.1111/psyp.13091 [DOI] [PubMed] [Google Scholar]
- 63.Mintun Ma, Lundstrom BN, Snyder aZ, Vlassenko aG, Shulman GL, Raichle ME (2001) Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proceedings of the National Academy of Sciences of the United States of America 98:6859–6864. doi: 10.1073/pnas.111164398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore CI, Cao R (2008) The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 99:2035–2047. doi: 10.1152/jn.01366.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore CI, Cao R (2008) The hemo-neural hypothesis: on the role of blood flow in information processing. Journal of Neurophysiology 99:2035–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore JD, Deschênes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D (2013) Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature 497:205–210. doi: 10.1038/nature12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG (2007) Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura T, Kajimura M, Morikawa T, Hattori K, Ishikawa M, Yukutake Y, Uchiyama SI, Suematsu M (2011) Acute CO2-independent vasodilatation of penetrating and pre-capillary arterioles in mouse cerebral parenchyma upon hypoxia revealed by a thinned-skull window method. Acta Physiologica 203:187–196. doi: 10.1111/j.1748-1716.2010.02212.x [DOI] [PubMed] [Google Scholar]
- 69.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA (1991) Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol 260:R581–588. doi: 10.1152/ajpregu.1991.260.3.R581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nesthus TE, Rush LL, Wreggit SS (1997) Effects of Mild Hypoxia on Pilot Performances at General Aviation Altitudes. [Google Scholar]
- 71.Neubauer JA, Sunderram J (2004) Oxygen-sensing neurons in the central nervous system. J Appl Physiol (1985) 96:367–374. doi: 10.1152/japplphysiol.00831.2003 [DOI] [PubMed] [Google Scholar]
- 72.Nielsen HB, Boushel R, Madsen P, Secher NH (1999) Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol 277:H1045–1052. doi: 10.1152/ajpheart.1999.277.3.H1045 [DOI] [PubMed] [Google Scholar]
- 73.Noakes TD (2011) Time to move beyond a brainless exercise physiology: the evidence for complex regulation of human exercise performance. Applied Physiology, Nutrition, and Metabolism 36:23–35 [DOI] [PubMed] [Google Scholar]
- 74.Noakes TD, Peltonen JE, Rusko HK (2001) Evidence that a central governor regulates exercise performance during acute hypoxia and hyperoxia. Journal of Experimental Biology 204:3225–3225 [DOI] [PubMed] [Google Scholar]
- 75.Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J (2010) Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol 135:379–392. doi: 10.1085/jgp.200910302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez-Garcia MT, Colinas O, Miguel-Velado E, Moreno-Dominguez A, Lopez-Lopez JR (2004) Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J Physiol 557:457–471. doi: 10.1113/jphysiol.2004.062281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perl O, Ravia A, Rubinson M, Eisen A, Soroka T, Mor N, Secundo L, Sobel N (2019) Human non-olfactory cognition phase-locked with inhalation. Nat Hum Behav 3:501–512. doi: 10.1038/s41562-019-0556-z [DOI] [PubMed] [Google Scholar]
- 78.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. The Journal of Neuroscience 18:9996–10015. doi: 10.1523/jneurosci.18-23-09996.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prioux-Guyonneau M, Cretet E, Jacquot C, Rapin JR, Cohen Y (1979) The effect of various stimulated altitudes on the turnover of norepinephrine and dopamine in the central nervous system of rats. Pflugers Arch 380:127–132. doi: 10.1007/BF00582147 [DOI] [PubMed] [Google Scholar]
- 80.Purves MJ (1966) The effect of a single breath of oxygen on respiration in the newborn lamb. Respiration Physiology 1:297–307. doi: 10.1016/0034-5687(66)90048-x [DOI] [PubMed] [Google Scholar]
- 81.Purves MJ (1966) Fluctuations of arterial oxygen tension which have the same period as respiration. Respiration Physiology 1:281–296. doi: 10.1016/0034-5687(66)90047-8 [DOI] [PubMed] [Google Scholar]
- 82.Qi Y, Roper M (2021) Control of low flow regions in the cortical vasculature determines optimal arterio-venous ratios. Proc Natl Acad Sci U S A 118. doi: 10.1073/pnas.2021840118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, Getzoff ED, Ziff EB (2007) Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci 27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ray CJ, Abbas MR, Coney AM, Marshall JM (2002) Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544:195–209. doi: 10.1113/jphysiol.2002.023440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reid JM, Davies AG, Ashcroft FM, Paterson DJ (1995) Effect of L-NMMA, cromakalim, and glibenclamide on cerebral blood flow in hypercapnia and hypoxia. Am J Physiol 269:H916–922. doi: 10.1152/ajpheart.1995.269.3.H916 [DOI] [PubMed] [Google Scholar]
- 86.Robinson JM, Lancaster JR Jr. (2005) Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide: mechanism(s) and physiologic versus pathophysiologic relevance. Am J Respir Cell Mol Biol 32:257–261. doi: 10.1165/rcmb.F292 [DOI] [PubMed] [Google Scholar]
- 87.Roche M, Chaigneau E, Rungta RL, Boido D, Weber B, Charpak S (2019) In vivo imaging with a water immersion objective affects brain temperature, blood flow and oxygenation. Elife 8. doi: 10.7554/eLife.47324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roy RK, Althammer F, Seymour AJ, Du W, Biancardi VC, Hamm JP, Filosa JA, Brown CH, Stern JE (2021) Inverse neurovascular coupling contributes to positive feedback excitation of vasopressin neurons during a systemic homeostatic challenge. Cell Rep 37:109925. doi: 10.1016/j.celrep.2021.109925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakadzic S, Mandeville ET, Gagnon L, Musacchia JJ, Yaseen MA, Yucel MA, Lefebvre J, Lesage F, Dale AM, Eikermann-Haerter K, Ayata C, Srinivasan VJ, Lo EH, Devor A, Boas DA (2014) Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat Commun 5:5734. doi: 10.1038/ncomms6734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saper CB, Fuller PM (2017) Wake-sleep circuitry: an overview. Curr Opin Neurobiol 44:186–192. doi: 10.1016/j.conb.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schaeffer S, Iadecola C (2021) Revisiting the neurovascular unit. Nat Neurosci 24:1198–1209. doi: 10.1038/s41593-021-00904-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt-Neilsen K, Larimer JL (1958) Oxygen dissociation curves of mammalian blood in relation to body size. Am J Physiol 195:424–428. doi: 10.1152/ajplegacy.1958.195.2.424 [DOI] [PubMed] [Google Scholar]
- 93.Shaw K, Bell L, Boyd K, Grijseels DM, Clarke D, Bonnar O, Crombag HS, Hall CN (2021) Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nature Communications 12. doi: 10.1038/s41467-021-23508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shea Sa (1996) Behavioural and arousal-related influences on breathing in humans. Experimental physiology 81:1–26. doi:VL - 81 [DOI] [PubMed] [Google Scholar]
- 95.Shea SA, Walter J, Pelley C, Murphy K, Guz A (1987) The effect of visual and auditory stimuli upon resting ventilation in man. Respiration Physiology 68:345–357. doi: 10.1016/s0034-5687(87)80019-1 [DOI] [PubMed] [Google Scholar]
- 96.Shih Y-YI, Wey H-Y, De La Garza BH, Duong TQ (2011) Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 31:832–841. doi: 10.1038/jcbfm.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shih YY, Chen CC, Shyu BC, Lin ZJ, Chiang YC, Jaw FS, Chen YY, Chang C (2009) A new scenario for negative functional magnetic resonance imaging signals: endogenous neurotransmission. J Neurosci 29:3036–3044. doi: 10.1523/JNEUROSCI.3447-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shoemaker LN, Wilson LC, Lucas SJE, Machado L, Walker RJ, Cotter JD (2020) Indomethacin markedly blunts cerebral perfusion and reactivity, with little cognitive consequence in healthy young and older adults. J Physiol. doi: 10.1113/JP280118 [DOI] [PubMed] [Google Scholar]
- 99.Sirotin YB, Das A (2009) Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457:475–479. doi: 10.1038/nature07664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254:726–729. doi: 10.1126/science.1683005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith SL, Otis TS (2003) Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade. J Neurosci 23:367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sorbini CA, Grassi V, Solinas E, Muiesan G (1968) Arterial oxygen tension in relation to age in healthy subjects. Respiration 25:3–13. doi: 10.1159/000192549 [DOI] [PubMed] [Google Scholar]
- 103.Talley EM, Sirois JE, Lei Q, Bayliss DA (2003) Two-pore-Domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist 9:46–56. doi: 10.1177/1073858402239590 [DOI] [PubMed] [Google Scholar]
- 104.Theis L, Berens P, Froudarakis E, Reimer J, Roman Roson M, Baden T, Euler T, Tolias AS, Bethge M (2016) Benchmarking Spike Rate Inference in Population Calcium Imaging. Neuron 90:471–482. doi: 10.1016/j.neuron.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr. (2001) The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A 98:355–360. doi: 10.1073/pnas.011379598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tort ABL, Brankack J, Draguhn A (2018) Respiration-Entrained Brain Rhythms Are Global but Often Overlooked. Trends Neurosci 41:186–197. doi: 10.1016/j.tins.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 107.Turner PJ, Buckler KJ (2013) Oxygen and mitochondrial inhibitors modulate both monomeric and heteromeric TASK-1 and TASK-3 channels in mouse carotid body type-1 cells. J Physiol 591:5977–5998. Doi 10.1113/jphysiol.2013.262022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uchiyama M, Nakao A, Kurita Y, Fukushi I, Takeda K, Numata T, Tran HN, Sawamura S, Ebert M, Kurokawa T, Sakaguchi R, Stokes AJ, Takahashi N, Okada Y, Mori Y (2020) O2-Dependent Protein Internalization Underlies Astrocytic Sensing of Acute Hypoxia by Restricting Multimodal TRPA1 Channel Responses. Curr Biol 30:3378–3396 e3377. doi: 10.1016/j.cub.2020.06.047 [DOI] [PubMed] [Google Scholar]
- 109.Van Mil AH, Spilt A, Van Buchem MA, Bollen EL, Teppema L, Westendorp RG, Blauw GJ (2002) Nitric oxide mediates hypoxia-induced cerebral vasodilation in humans. J Appl Physiol (1985) 92:962–966. doi: 10.1152/japplphysiol.00616.2001 [DOI] [PubMed] [Google Scholar]
- 110.Vanderkooi JM, Erecinska M, Silver IA (1991) Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol 260:C1131–1150. doi: 10.1152/ajpcell.1991.260.6.C1131 [DOI] [PubMed] [Google Scholar]
- 111.Vu MT, Du G, Bayliss DA, Horner RL (2015) TASK Channels on Basal Forebrain Cholinergic Neurons Modulate Electrocortical Signatures of Arousal by Histamine. J Neurosci 35:13555–13567. doi: 10.1523/JNEUROSCI.1445-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weber F, Dan Y (2016) Circuit-based interrogation of sleep control. Nature 538:51–59. doi: 10.1038/nature19773 [DOI] [PubMed] [Google Scholar]
- 113.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL (2005) Acute oxygen-sensing mechanisms. N Engl J Med 353:2042–2055. doi: 10.1056/NEJMra050002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson DF, Harrison DK, Vinogradov SA (2012) Oxygen, pH, and mitochondrial oxidative phosphorylation. J Appl Physiol (1985) 113:1838–1845. doi: 10.1152/japplphysiol.01160.2012 [DOI] [PubMed] [Google Scholar]
- 115.Wu Y-t, Bennett HC, Chon U, Vanselow DJ, Zhang Q, Muñoz-Castañeda R, Cheng KC, Osten P, Drew PJ, Kim Y (2021). doi: 10.1101/2021.05.19.444854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu F, Liu P, Pascual JM, Xiao G, Lu H (2012) Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab 32:1909–1918. doi: 10.1038/jcbfm.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu F, Liu P, Pekar JJ, Lu H (2015) Does acute caffeine ingestion alter brain metabolism in young adults? NeuroImage 110:39–47. doi: 10.1016/j.neuroimage.2015.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo LQ, Krasnow MA (2017) Breathing control center neurons that promote arousal in mice. Science 355:1411–1415. doi: 10.1126/science.aai7984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan X (2014) Cognitive impairments at high altitudes and adaptation. High Alt Med Biol 15:141–145. doi: 10.1089/ham.2014.1009 [DOI] [PubMed] [Google Scholar]
- 120.Yang CF, Feldman JL (2018) Efferent projections of excitatory and inhibitory preBotzinger Complex neurons. J Comp Neurol 526:1389–1402. doi: 10.1002/cne.24415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang CF, Kim EJ, Callaway EM, Feldman JL (2020) Monosynaptic Projections to Excitatory and Inhibitory preBotzinger Complex Neurons. Front Neuroanat 14:58. doi: 10.3389/fnana.2020.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular Architecture of the Mouse Nervous System. Cell 174:999–1014 e1022. doi: 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, Gottfried JA (2016) Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J Neurosci 36:12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q, Gheres KW, Drew PJ (2021) Origins of 1/f-like tissue oxygenation fluctuations in the murine cortex. PLoS Biol 19:e3001298. doi: 10.1371/journal.pbio.3001298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Q, Roche M, Gheres KW, Chaigneau E, Kedarasetti RT, Haselden WD, Charpak S, Drew PJ (2019) Cerebral oxygenation during locomotion is modulated by respiration. Nat Commun 10:5515. doi: 10.1038/s41467-019-13523-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


