Abstract
Periapical bone destruction occurs as a consequence of pulpal infection. In previous studies, we showed that interleukin-1 (IL-1) is the primary stimulator of bone destruction in this model. IL-6 is a pleiotropic cytokine that is induced in these infections and has both pro- and anti-inflammatory activities. In the present study, we determined the role of IL-6 in regulating IL-1 expression and bone resorption. The first molars of IL-6 knockouts (IL-6−/−) and wild-type mice were subjected to surgical pulp exposure and infection with a mixture of four common pulpal pathogens, including Prevotella intermedia, Fusobacterium nucleatum, Peptostreptococcus micros, and Streptococcus intermedius. Mice were killed after 21 days, and bone destruction and cytokine expression were determined. Surprisingly, bone destruction was significantly increased in IL-6−/− mice versus that in wild-type mice (by 30%; P < 0.001). In a second experiment, the effects of chronic (IL-6−/−) IL-6 deficiency and short-term IL-6 deficiency induced by in vivo antibody neutralization were determined. Both IL-6−/− (30%; P < 0.001) and anti-IL-6 antibody-treated mice (40%; P < 0.05) exhibited increased periapical bone resorption, compared to wild-type controls. The increased bone resorption in IL-6-deficient animals correlated with increases in osteoclast numbers, as well as with elevated expression of bone-resorptive cytokines IL-1α and IL-1β, in periapical lesions and with decreased expression of the anti-inflammatory cytokine IL-10. These data demonstrate that endogenous IL-6 expression has significant anti-inflammatory effects in modulating infection-stimulated bone destruction in vivo.
Bacterial infections of the dental pulp result in soft-tissue destruction and, ultimately, in periapical bone resorption (7). A proinflammatory cytokine cascade is induced in response to bacterial infection of the dental pulp. Some of these mediators stimulate bone resorption, in particular, interleukin-1α (IL-1α) and IL-1β, which have been shown to be key mediators of periapical bone destruction in vivo (21, 37, 38, 40, 46). IL-1 expression is induced by exposure of host cells to lipopolysaccharide (LPS) and other bacterial cell wall components (9, 12).
IL-6 is a pleiotropic cytokine that possesses activities that may enhance or suppress inflammatory bone destruction (44). IL-6 is produced locally in bone following stimulation by IL-1 and tumor necrosis factor (TNF) (14, 27). IL-6 stimulates the formation of osteoclast precursors from colony-forming unit–granulocyte-macrophage (25) and increases osteoclast numbers in vivo, leading to systemic increases in bone resorption (8, 20). However, emerging data suggest that IL-6 also has significant anti-inflammatory activities (3, 29, 33, 42). IL-6 fails to directly induce proteinase expression (3) and instead upregulates tissue inhibitor of metalloproteinases-1 (TIMP-1) (36). Many acute-phase proteins induced in the liver by IL-6 have anti-inflammatory properties (15, 18, 41). Finally, IL-6 has been reported to downregulate IL-1 (33) and upregulate IL-1 receptor antagonist (IL-1ra) expression (42).
The present study was undertaken to establish if the net effect of IL-6 is to increase or to decrease infection-stimulated infraosseus bone destruction in vivo. For this purpose, we employed animals genetically deficient in IL-6 (IL-6−/−), as well as wild-type animals treated acutely with neutralizing doses of anti-IL-6 antibody. Our results demonstrate that the predominant effects of IL-6 are anti-inflammatory and antiresorptive in this model.
MATERIALS AND METHODS
Animals.
Eight-week-old IL-6−/− male mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Eight-week-old C57BL/6 male mice were obtained from Charles River Breeding Laboratory (Wilmington, Mass.). All animals were maintained in a conventional environment in the Forsyth Institute Animal Facility, according to the guidelines of the Institutional Animal Care and Use Committee.
Periapical lesion induction.
For lesion induction, mice were mounted on a jaw retraction board and were anesthetized with ketamine HCl (62.5 mg/kg of body weight) and xylazine (12.5 mg/kg) in sterile phosphate-buffered saline (PBS) by intraperitoneal injection. All four first-molar pulps were exposed using a no. 1/4 round bur under a surgical microscope (model MC-M92; Seiler, St. Louis, Mo.) as described previously (46). The exposure size was approximately equivalent to the diameter of the bur. The pulp chamber was opened until the entrances of the canals could be visualized and probed with a no. 06 endodontic file. Animals without exposures served as controls.
Infection with pathogens.
Tryptic soy broth with yeast agar plates of four common endodontic pathogens, Prevotella intermedia ATCC 25611, Streptococcus intermedius ATCC 27335, Fusobacterium nucleatum ATCC 25586, and Peptostreptococcus micros ATCC 33270 were grown under anaerobic conditions (80% N2, 10% H2, and 10% CO2), harvested, and cultured in mycoplasma liquid media. The cells were centrifuged at 7,000 × g for 15 min and resuspended in prereduced anaerobically sterilized Ringer's solution under the influx of nitrogen. The final concentration of each organism was determined spectrophotometrically, and the four pathogens were mixed to yield a concentration of 1010 cells of each pathogen/ml in 10 μg of methylcellulose/ml. A total of 10 μl/tooth was introduced using a micropipette.
Antibody infusion.
Rat anti-mouse IL-6 monoclonal antibody (immunoglobulin G1 [IgG1]) was purchased from R&D Systems (Minneapolis, Minn.). Mice (n = 10) received 0.2 mg of antibody intramuscularly on days 0, 3, 6, 9, 12, 15, and 18 relative to pulp exposure and infection, for a total of 1.4 mg/mouse. Control mice received saline on the same schedule. On day 21 all mice were killed and samples were prepared as described below.
Sample preparation.
All animals were killed by CO2 asphyxiation on day 21 after pulp exposure. The left mandible was dissected free of soft tissue, fixed in 10% phosphate-buffered formalin, and subjected to microcomputed tomography (micro-CT). After micro-CT image acquisition, mandibles were demineralized in 14% EDTA, pH 7.2, at room temperature for 3 weeks. Samples were embedded in paraffin, and 7-μm-thick sections were prepared and were stained for tartrate-resistant acid phosphatase as a marker for osteoclasts as described previously (28). For the right mandibular and maxillary quadrants, periapical tissues surrounding root apices were carefully extracted together with surrounding bone in a block specimen under a surgical microscope. The gingiva, oral mucosa, tooth crown, and bone marrow were dissected free and discarded. Periapical tissues were rinsed in PBS, freed of clots, weighed, and immediately frozen in dry ice-ethanol for cytokine determinations.
Micro-CT.
Micro-CT was used to quantify the extent of bone destruction (4). Fixed mandibular samples were analyzed at the Orthopedic Biomechanics Laboratory, Beth Israel-Deaconess Medical Center, Harvard Medical School, using a compact fan beam-type tomograph (μCT 20; Scanco Medical AG, Bassersdorf, Switzerland). For each sample approximately 150 microtomographic slices with an increment of 17 μm, covering the entire mediolateral width of the mandible, were acquired. From the three-dimensional stack of images, a “pivot” section, which included the the crown and central portion of the distal root of the mandibular first molar, was selected. The cross-sectional area of the region of interest was analyzed by means of a semiautomatic histomorphometric system (Optimas Bioscan; Media Cybernetics, Bethell, Wash.). The area of evaluation included the area of periapical destruction (in units of millimeters squared) and/or the periodontal ligament space surrounding the distal root of the first molar, the coronal extension of which was standardized by using a predrawn template as described previously (4).
Cytokine assays.
For protein extract preparation, frozen periapical tissue samples were ground using a precooled sterile mortar and pestle, and the tissue fragments were dispersed in 650 to 800 μl of lysis buffer consisting of 100 μg of bovine serum albumin (fraction V; Sigma), 100 μg of Zwittergent-12 (Boehringer Mannheim, Indianapolis, Ind.), and 50 μg of gentamicin (Life Technologies, Gaithersburg, Md.)/ml, 10 mM HEPES buffer (Life Technologies), 1 μg of aprotinin (Sigma) and leupeptin (Sigma)/ml, and 0.1 μM EDTA (Fisher Scientific, Pittsburgh, Pa.) in RPMI 1640 (Mediatech, Herndon, Va.), as described previously (45). The incubation mixture was placed on ice and was subjected to a 20- to 30-s sonication. The supernatant was collected after centrifugation and stored frozen at −70°C until the assay.
Assays for cytokines in tissue extracts were carried out using commercially available enzyme-linked immunosorbent assay (ELISA) kits obtained from the following sources (sensitivities are in parentheses): IL-1α, Endogen, Cambridge, Mass. (6 pg/ml); IL-2 (<8 pg/ml), IL-4 (<5 pg/ml), IL-6 (<3 pg/ml), IL-12 (<2 pg/ml), gamma interferon (IFN-γ; <1 pg/ml), and TNF-γ (<3 pg/ml), BioSource International, Camarillo, Calif.; transforming growth factor β, R&D Systems (<5 pg/ml). All assays were conducted in accordance with the manufacturer's instructions. The concentration of each cytokine was calculated with reference to a standard curve that was constructed using recombinant cytokine provided with each kit. Results were expressed as picograms of cytokine per milligram of periapical tissue.
Antibacterial antibody responses.
Serum samples were obtained by cardiac puncture at sacrifice. Ninety-six-well plates were coated with formalin-killed microorganisms (P. intermedia, S. intermedius, F. nucleatum, and P. micros) in PBS (optical density at 580 nm [OD580] = 0.3) and incubated for 3 h at 37°C. After 2 days at 4°C, plates were washed three times with buffer II (0.9% NaCl, 0.05% Tween 20). For determination of the specific antibody against pathogens, the plates were incubated with diluted serum (1/1,000) in buffer III (PBS with 0.05% Tween 20 and 0.02% NaN3) for 2 h at room temperature on a shaker. The optimal serum dilution of 1/1,000 was determined after testing a range of serum dilutions. The plates were washed three times with buffer II, and the bound Ig was detected by a reaction with goat anti-mouse Ig coupled to alkaline phosphatase (Biosource), diluted 1/1,500 in buffer III overnight at room temperature. Conversion of the substrate (p-nitrophenylphosphate; 1 mg/ml) was determined at OD405 using an ELISA reader (BIO-TEK Instruments, Winooski, Vt.).
Statistical analysis.
Differences in bone destruction were analyzed by Student's t test. ELISA data were analyzed by the nonpaired Student t test with Bonferroni's correction for multiple comparisons.
RESULTS
Effect of genetic deletion of IL-6 on infection-stimulated bone destruction.
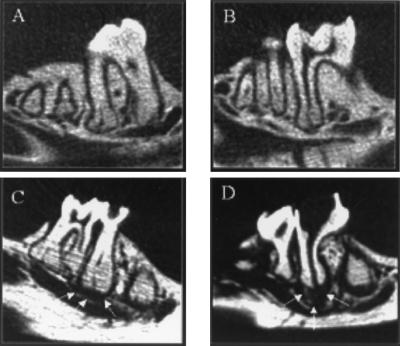
The role of IL-6 in regulating infraosseus bone destruction was assessed in IL-6 knockouts (IL-6−/−) and wild-type mice. The molar teeth of both groups (n = 10 each) were subjected to surgical pulp exposure and infection with a mixture of four bacterial pathogens. The teeth of controls of both genotypes remained unexposed and uninfected (n = 3 each). After 21 days, the extent of periapical bone destruction was quantified by micro-CT (Fig. 1). As shown, unexposed and uninfected animals have a narrow periodontal ligament space surrounding the roots of the first molar, whereas both wild-type and IL-6−/− mice with pulpal infections exhibit a clear radiolucent area, indicative of an inflammatory infiltrate and concomitant bone resorption.
FIG. 1.
Micro-CT images of periapical bone destruction in wild-type (A and C) and IL-6−/− (B and D) mice. (A and B) No pulp exposure, no infection; (C and D) pulp exposure, infection. Arrows, perimeter of the area of bone resorption.
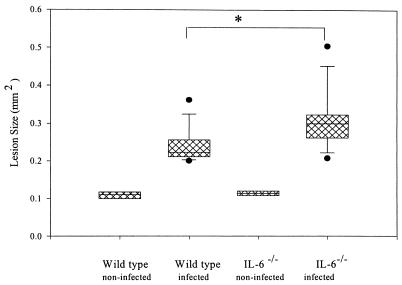
The extent of resorption is shown in Fig. 2. As indicated, both wild-type and IL-6−/− mice have significant periapical bone resorption compared to uninfected control mice (P < 0.002). Note that the indicated area of resorption in the controls represents the normal periodontal ligament space. Somewhat surprisingly, IL-6−/− mice exhibited increased periapical resorption compared to the wild-type controls (30%; P < 0.001).
FIG. 2.
Infection-stimulated bone resorption in IL-6−/− versus wild-type mice. Boxes, 25th to the 75th percentile; horizontal line, 50th percentile; vertical lines and cross bars, standard deviations; circles, outliers. Note that the area for noninfected teeth represents the normal periodontal ligament. ∗, P < 0.001.
Effect of short-term neutralization of IL-6.
Because knockout mice may have developmental alterations, the effect of short-term versus long-term IL-6 deficiency was also assessed by comparing bone destruction in IL-6−/− mice, wild-type mice, and wild-type mice treated with a neutralizing anti-IL-6 antibody. Assessment of the serum IL-6 concentrations for the three groups showed that, while significant levels of IL-6 were present in wild-type mice following pulp exposure and infection, as expected this cytokine was essentially undetectable in IL-6−/− mice (Table 1). Anti-IL-6 antibody-treated animals had levels of circulating IL-6 that were reduced but not completely absent, indicating that the efficiency of antibody neutralization was approximately 95%.
TABLE 1.
IL-6 concentration in serum
| Group | Mean IL-6 concn (pg/ml) ± SD in:
|
|
|---|---|---|
| Noninfected teeth | Infected teeth | |
| Wild type | 2.3 ± 0.5 | 21.0 ± 0.1 |
| IL-6−/− | NDa | 0.0 |
| Wild type + anti-IL-6 antibody | ND | 1.0 ± 0.1 |
ND, not done.
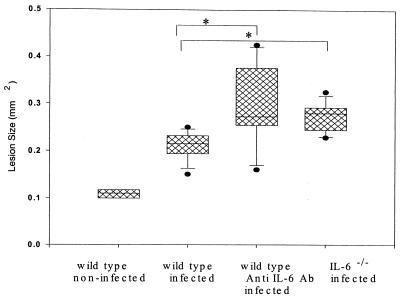
Bone destruction was again assessed by micro-CT. As shown in Fig. 3, IL-6−/− (32%; P < 0.003) and anti-IL-6 antibody-treated mice (38%; P < 0.05) exhibited similar increases in periapical bone resorption compared to wild-type mice confirming the result of the first experiment. Taken together, these data demonstrate that animals with either acute or chronic IL-6 deficiency have increased infection-stimulated infraosseus bone destruction.
FIG. 3.
Infection-stimulated bone resorption in IL-6−/−, anti-IL-6-treated, and wild-type mice. Boxes, 25th to the 75th percentile; horizontal line, 50th percentile; vertical lines and cross bars, standard deviations; circles, outliers. ∗, P < 0.001.
Osteoclast responses in IL-6-deficient mice.
To determine if osteoclast number correlated with the extent of resorption, the numbers of osteoclasts in the periapical region in wild-type and IL-6-deficient mice were quantified. As shown in Table 2, osteoclast counts increased after pulp exposure and infection. Consistent with the bone resorption results, there were more osteoclasts observed in lesions in the IL-6−/− and the anti-IL-6 antibody-treated mice than in wild-type mice, although these differences did not reach statistical significance.
TABLE 2.
Quantitation of osteoclasts in periapical lesions
| Group | Mean no. of osteoclasts/lesion ± SD in:
|
|
|---|---|---|
| Noninfected teeth | Infected teeth | |
| Wild type | 0.0 ± 0.0 | 2.8 ± 1.7 |
| IL-6−/− | NDa | 9.3 ± 4.6 |
| Wild type + anti-IL-6 antibody | ND | 6.3 ± 5.2 |
ND, not done.
Cytokine responses in infraosseus lesions.
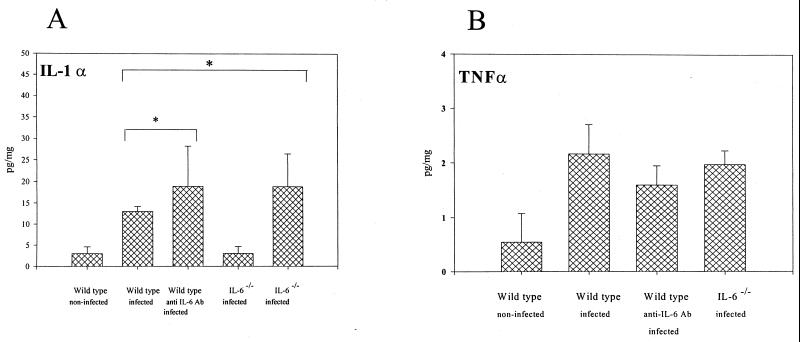
The cytokine responses in the local microenvironment of periapical lesions were assessed on day 21. As shown in Fig. 4 to 6, a significant elevation of most mediators occurred as a consequence of pulp exposure and infection in all of the experimental groups compared with the uninfected controls. For tissues from infected teeth, the levels of bone-resorptive mediators IL-1α and IL-1β were markedly increased in the IL-6−/− and anti-IL-6 antibody-treated mice versus those in wild-type mice (P < 0.07 and P < 0.05 for IL-1α, and P < 0.002 and P < 0.06 for IL-1β, respectively) (Fig. 4A). In contrast, levels of TNF-α, another bone-resorptive cytokine, were not significantly different among the experimental groups (Fig. 4B).
FIG. 4.
Expression of bone-resorptive cytokines in periapical inflammatory tissues. Vertical lines and cross bars, standard deviations; ∗, P < 0.01. Ab, antibody.
FIG. 6.
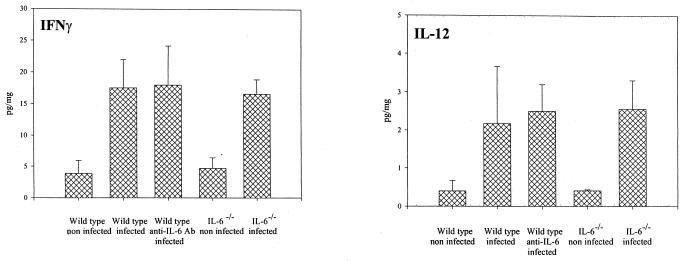
Proinflammatory Th1-type cytokines in periapical inflammatory tissues. Vertical lines and cross bars, standard deviations. There were no significant differences among the infected groups. Ab, antibody.
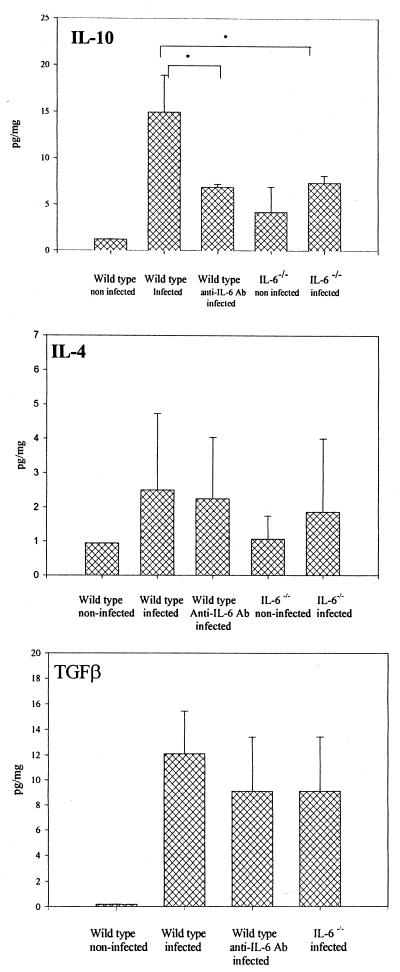
Levels of anti-inflammatory cytokine IL-10 in tissue were lower in the IL-6−/− and anti-IL-6 antibody-treated mice than in wild-type mice (P < 0.0008 and P < 0.0005, respectively; Fig. 5). On the other hand, two other anti-inflammatory cytokines, IL-4 and transforming growth factor β, did not show significant differences. Levels of proinflammatory Th1-type cytokines IFN-γ and IL-12 for the experimental groups also showed no significant differences (Fig. 6). Of note, many of the cytokines evaluated were also expressed in low levels in periapical tissues from unexposed teeth, which represent normal periodontal ligament and some surrounding bone. In particular, the level of IFN-γ was higher in the knockout strain than in the wild type.
FIG. 5.
Expression of anti-inflammatory cytokines in periapical inflammatory tissues. Vertical lines and cross bars, standard deviations; ∗, P < 0.01. Ab, antibody.
Systemic antibody responses to pathogens.
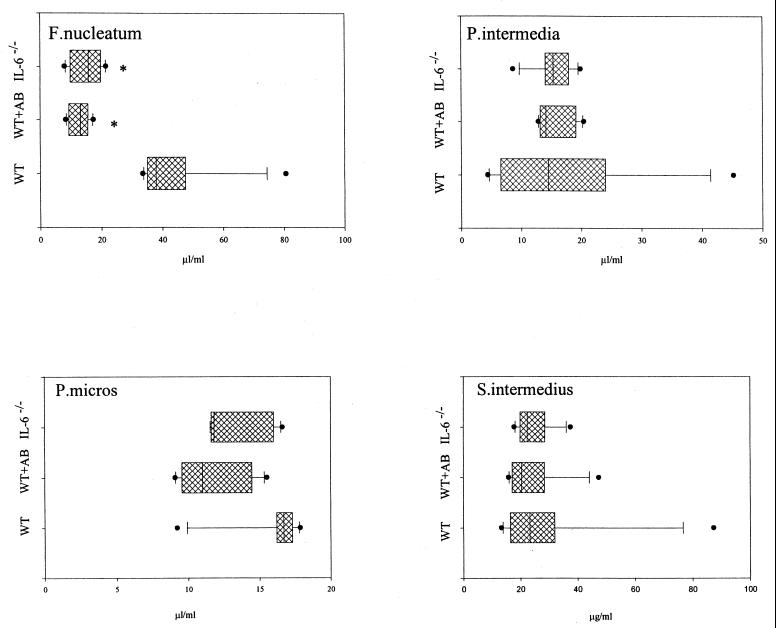
Antibodies are protective in reducing infection dissemination and bone destruction in this model (18). IL-6 promotes the terminal differentiation of B cells to plasma cells, and IL-6 deficiency could affect antipathogen antibody responses. The levels of specific antibodies against the four pathogens were therefore assessed in the three experimental groups. As seen in Fig. 7. wild-type mice had levels of antibodies against three of the pathogens similar to those of the IL-6-deficient mice. Only the response to F. nucleatum was significantly reduced, although considerable levels of antibody were still present in IL-6-deficient groups. These data indicate that modulation of antipathogen antibody responses in the IL-6-deficient groups did not play a significant role in the observed increase in bone destruction.
FIG. 7.
Antibody responses to infecting pathogens in IL-6-deficient and wild-type (WT) mice. Boxes, 25th to the 75th percentile; vertical lines, 50th percentile; horizontal lines and cross bars, standard deviations; circles, outliers. ∗, P < 0.05 versus the wild type. AB, antibody.
DISCUSSION
Proinflammatory cytokines, including IL-1α, enhance inflammation and promote bone resorption, whereas Th2-type mediators such as IL-4, IL-10, and IL-13 are known to exert anti-inflammatory effects. The results of the present investigation indicate that, with respect to regulating infection-stimulated bone resorption, IL-6 belongs to the latter category of anti-inflammatory cytokines. IL-6 is present in normal periodontal ligament and bone and is further induced after pulpal infection in mice (21). It has also been shown to be present in human periapical lesions and cysts (5, 39).
Our principal findings demonstrate that IL-6−/− mice have significantly increased bone resorption following pulpal infection with anaerobic bacteria, compared to wild-type controls. Similarly, the neutralization of endogenous IL-6 with anti-IL-6 antibody resulted in significantly increased bone destruction comparable to that seen in IL-6−/− mice. The bone destruction in both groups of IL-6-deficient animals correlated with increased expression of locally produced IL-1, previously shown to be the primary mediator of bone resorption in this model (11, 21, 37, 38, 40, 45, 46), and with increased numbers of osteoclasts. These results suggest that a deficiency of endogeneous IL-6 results in an exaggerated inflammatory response to infection with anaerobic bacteria, leading to increases in cytokine expression and bone destruction.
IL-6 has traditionally been considered to be a proinflammatory mediator, since it is induced by IL-1 and TNF-α early in the inflammatory cascade and because it stimulates expression of acute-phase proteins. However, recent data demonstrate that IL-6 lacks many typical proinflammatory properties and furthermore exerts a number of anti-inflammatory activities. For example, IL-6 does not directly stimulate the production of collagenase, matrix metalloproteinase, or stromelysin (3), although it does potentiate IL-1- and TNF-stimulated collagenase and prostaglandin E2 production by chondrocytes (43). IL-6 is a potent inducer of TIMP-1 (24, 32, 36). In a model of arthritis, IL-6 significantly enhanced synthesis of TIMP-1 in chondrocytes, inhibited superoxide production, and suppressed spontaneous and IL-1-mediated degradation of cartilage matrix (36).
Infusion of IL-6 in humans results in fever but does not cause shock or a capillary leakage syndrome as is observed with proinflammatory cytokines such as IL-1β and TNF (26). This is also the case in animal models (3). Although circulating-IL-6 levels correlate with the outcome of septic shock, the involvement of IL-6 in the pathogenesis of this syndrome is questionable, as demonstrated by the lack of effect of monoclonal antibodies against IL-6 or its receptor in various murine models (26).
IL-6 inhibits LPS-induced IL-1 and TNF production in monocytes (1, 25, 33), and LPS-treated IL-6-deficient mice produce threefold more TNF-α than do wild-type controls (13). IL-6 also fails to induce the expression of adhesion molecules on endothelial cells and suppresses the acute neutrophil exodus and TNF production stimulated by LPS, providing evidence that IL-6 may represent an endogenous negative-feedback mechanism to inhibit endotoxin-initiated cytokine-mediated acute inflammation (13). Of note, IL-6 induces IL-1ra in monocytes in vitro, as well as levels of circulating IL-1ra in immunotherapy patients (42). Furthermore, infection of mice with Yersinia enterocolitica stimulates expression of IL-1ra in Peyer's patches, an increase that is completely blocked by administration of anti-IL-6 antibody (20).
There has been accumulating evidence that the acute phase proteins regulated by IL-6 also have anti-inflammatory and immunosuppressive properties and act as antiproteinases and oxygen scavengers (6, 20, 41, 42). Following injections of croton oil, the presence of C-reactive protein (CRP) results in diminished polymorphonuclear leukocyte (PMN) infiltration and vascular permeability in the lung (15). Transgenic mice expressing rabbit CRP exhibit reduced chemotactic-factor-induced alveolitis with diminished infiltration of PMNs (2). α1-Antitrypsin ameliorates bleomycin-induced pulmonary fibrosis in hamsters by reducing the number of infiltrating neutrophils and lymphocytes (18). On the other hand, IL-6 has been shown to be the major inducer of phospholipase A2 (PLA2) (10), which plays an important role in producing potent lipid mediators, such as leukotrienes, prostaglandins, and platelet-activating factor. Levels of PLA2 activity in serum are elevated in septic shock and rheumatoid arthritis.
IL-6−/− mice develop normally with no induction of inflammatory or immunological disturbances (49). The redundancy of IL-6 function with IL-10 might also explain why IL-6-deficient animals do not suffer from severe inflammatory disorders, unlike IL-10-deficient mice (24, 41). Osteoclast development is normal in IL-6−/− mice (P. Stashenko and Y. Kwong, unpublished findings). Of interest, IL-1 and TNF-α but not IL-6 have been found to stimulate steady-state levels of osteoprotegerin ligand mRNA, which is a critical factor for inducing osteoclastogenesis by various human osteoblastic lineage cells (16, 30). Finally, TNF-α but not IL-6 plays a key role in estrogen-deficiency bone loss (22), again suggesting a nonessential role for IL-6 in this process.
Our data also showed a reduction of IL-10 in the IL-6-deficient animals, which points to the possibility of the participation of an indirect mechanism of IL-1 suppression by IL-6. IL-10 reduces steady-state levels of IL-1 mRNA (48), decreases mRNA stability (23), and inhibits IL-1 at the transcriptional level (47) by preventing activation of NF-κB. IL-10 also increases IL-1ra (34, 35). Recently, we have found that IL-10-deficient mice exhibit dramatically increased infection-stimulated resorption in this model (31).
Taken together, our data demonstrate that the anti-inflammatory properties of IL-6 predominate in inflammatory responses. Although the mechanisms of action still need to be defined, these may involve the direct suppression of IL-1 or the induction of endogenous antagonists or inhibitors of IL-1 such as IL-1ra and IL-10.
ACKNOWLEDGMENTS
We thank R. Kent for statistical analysis, T. Uchiyama and R. Muller of the Orthopedic Biomechanics Laboratory, Beth Israel-Deaconess Medical Center, for micro-CT analysis, Justine Dobeck for histology, and S. Yoganathan for expert animal care.
This work was supported by grants DE-09018 and DE-11664 (P.S.) from the N.I.D.C.R., N.I.H., and a grant from the American Association of Endodontists (K.B.).
REFERENCES
- 1.Aderka D, Le J, Wallach D. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989;143:3517–3523. [PubMed] [Google Scholar]
- 2.Ahmed N, Thorley R, Xia D, Samols D, Webster R O. Transgenic mice expressing rabbit C-reactive protein exhibit diminished chemotactic factor-induced alveolitis. Am J Respir Crit Care Med. 1996;153:1141–1147. doi: 10.1164/ajrccm.153.3.8630558. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 4.Balto K, Muller R, Carrington D, Dobeck J, Stashenko P. Quantification of periapical bone destruction in mice by micro-computed tomograhy. J Dent Res. 2000;79:35–40. doi: 10.1177/00220345000790010401. [DOI] [PubMed] [Google Scholar]
- 5.Barkhodar R, Hayashi C, Hussain M. Detection of interleukin-6 in human dental pulp and periapical lesions. Endod Dent Traumatol. 1999;15:26–27. doi: 10.1111/j.1600-9657.1999.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 6.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 7.Bergenholtz G. Pathogenic mechanisms in pulpal disease. J Endod. 1990;16:98–101. doi: 10.1016/S0099-2399(06)81571-2. [DOI] [PubMed] [Google Scholar]
- 8.Black K, Garrett I R, Mundy G R. Chinese hamster ovarian cells transfected with the murine interleukin-6 gene cause hypercalcemia as well as cachexia, leukocytosis and thrombocytosis in tumor bearing nude mice. Endocrinology. 1991;128:2657–2659. doi: 10.1210/endo-128-5-2657. [DOI] [PubMed] [Google Scholar]
- 9.Burysek L, Houstek J. Multifactorial induction of gene expression and nuclear localization of mouse interleukin 1 alpha. Cytokine. 1996;8:460–467. doi: 10.1006/cyto.1996.0062. [DOI] [PubMed] [Google Scholar]
- 10.Crowl R M, Stoller T J, Conroy R R, Stoner C R. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J Biol Chem. 1991;266:2647–2651. [PubMed] [Google Scholar]
- 11.Dinarello C A, Wolff S. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 12.Dunn A J, Swiergiel A H. The role of cytokines in infection-related behavior. Ann N Y Acad Sci. 1998;840:577–585. doi: 10.1111/j.1749-6632.1998.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 13.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feyen J H M, Elford P, Dipadova F E, Trechsel U. Interleukin-6 is produced by bone and modulated by parathyroid hormone. J Bone Miner Res. 1989;4:633–638. doi: 10.1002/jbmr.5650040422. [DOI] [PubMed] [Google Scholar]
- 15.Heuertz R M, Piquette C A, Webster R O. Rabbits with elevated serum C-reactive protein exhibit diminished neutrophil infiltration and vascular permeability in C5a-induced alveolitis. Am J Pathol. 1993;142:319–328. [PMC free article] [PubMed] [Google Scholar]
- 16.Hofbauer L C, Lacey D L, Dunstan C R, Spelsberg T C, Riggs B L, Khosla S. Interleukin-1β and tumor necrosis factor-α, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 17.Hou L, Sasaki H, Stashenko P. B-cell deficiency predisposes mice to disseminating anaerobic infections: protection by passive antibody transfer. Infect Immun. 2000;68:5645–5651. doi: 10.1128/iai.68.10.5645-5651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter N, Weston K M, Bowern N A. Suppression of experimental allergic encephalomyelitis by alpha 2-macroglobulin. Immunology. 1991;73:58–63. [PMC free article] [PubMed] [Google Scholar]
- 19.Ishimi Y, Miyaura C, Jin C H, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 20.Jordan M, Otterness I G, Ng R, Gessner A, Rollinghoff M, Beuscher H U. Neutralization of endogenous IL-6 suppresses induction of IL-1 receptor antagonist. J Immunol. 1995;154:4081–4090. [PubMed] [Google Scholar]
- 21.Kawashima N, Stashenko P. Expression of bone resorptive and regulatory cytokines in infraosseus inflammation. Arch Oral Biol. 1998;44:55–66. doi: 10.1016/s0003-9969(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 22.Kimble R, Bain S, Pacifici R. The functional block of TNF but not IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 23.Kishore R, Tebo J M, Kolosov M, Hamilton T A. Clustered AU-rich elements are the target of IL-10 mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162:2457–2461. [PubMed] [Google Scholar]
- 24.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute phase responses in interleukin-6 deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 25.Kurihara N, Bertolini D, Akiyam Y, Roodman G D. IL-6 stimulates osteoclast like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J Immunol. 1990;144:4226–4230. [PubMed] [Google Scholar]
- 26.Libert C, Vink A, Couli P. Limited involvement of interleukin-6 in the pathogenesis of lethal septic shock as revealed by the effect of monoclonal antibodies against interleukin-6 or its receptor in various murine models. Eur J Immunol. 1992;22:2625–2630. doi: 10.1002/eji.1830221023. [DOI] [PubMed] [Google Scholar]
- 27.Lowik C, van der Pluijm H, Bloys K, Hoekman K, Bijvoet O, Aarden L, Papapoulos S. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogenesis. Biochem Biophys Res Commun. 1989;162:1546–1552. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- 28.Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;3:285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 29.Ramsay A J, Husband A J, Ramshaw I A. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 30.Sakata M, Shiba H, Komatsuzawa H, Fujita T, Ohata K, Sugai M, Suginaka H, Kurihara H. Expression of osteoprotegerin (osteoclastogenesis inhibitory factor) in cultures of human dental mesenchymal cells and epithelial cells. J Bone Miner Res. 1999;14:1486–1492. doi: 10.1359/jbmr.1999.14.9.1486. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki H, Hou L, Wang C Y, Belani A, Uchiyama T, Muller R, Stashenko P. IL-10 but not IL-4 suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–3630. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, Ito A, Mori Y. Interleukin 6 enhances the production of tissue inhibitor of metalloproteinases (TIMP) but not that of matrix metalloproteinases by human fibroblasts. Biochem Biophys Res Commun. 1990;170:824–829. doi: 10.1016/0006-291x(90)92165-v. [DOI] [PubMed] [Google Scholar]
- 33.Schindler R, Mancilla J, Endres S, Ghorgani R, Clark S C, Dinarello C A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 34.Seitz M, Loetscher P, Dewald B, Towbin H, Gallati H, Baggiolini M. Interleukin-10 differentially regulates cytokine inhibitor and chemokine release from blood mononuclear cells and fibroblasts. Eur J Immunol. 1995;25:1129–1135. doi: 10.1002/eji.1830250443. [DOI] [PubMed] [Google Scholar]
- 35.Shimauchi H, Ogawa T, Oduda K, Kusumoto Y, Okada H. Autoregulatory effect of interleukin-10 on proinflammatory cytokine production by Porphyromonas gingivalis lipopolysaccharide-tolerant human monocytes. Infect Immun. 1999;67:2153–2159. doi: 10.1128/iai.67.5.2153-2159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shingu M, Miyauchi S, Nagai Y, Yasutake C, Horie K. The role of IL-4 and IL-6 in IL-1-dependent cartilage matrix degradation. Br J Rhetumatol. 1995;34:101–106. doi: 10.1093/rheumatology/34.2.101. [DOI] [PubMed] [Google Scholar]
- 37.Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9:498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 38.Stashenko P, Wang C, Tani-Ishii N, Yu S. Pathogenesis of induced rat periapical lesions. Oral Surg Oral Med Oral Pathol. 1994;78:494–502. doi: 10.1016/0030-4220(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 39.Takeichi O, Saito I, Tsurumachi T, Moro I, Saito T. Expression of inflammatory cytokine genes in vivo by human alveolar bone-derived polymorphonuclear leukocytes isolated from chronically inflamed sites of bone resorption. Calcif Tissue Int. 1996;58:244–248. doi: 10.1007/BF02508643. [DOI] [PubMed] [Google Scholar]
- 40.Tani-Ishii N, Wang C, Stashenko P. Immunolocalization of bone resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol Immunol. 1995;10:213–219. doi: 10.1111/j.1399-302x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 41.Tilg H, Dinarello C A, Mier J W. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–433. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 42.Tilg H, Trehu E, Atkins M B, Dinarello C A, Mier J W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1991;83:113–118. [PubMed] [Google Scholar]
- 43.van de Loo F A, Kuiper S, van Enckevort F H, Arntz O J, van den Berg W B. Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am J Pathol. 1997;151:177–191. [PMC free article] [PubMed] [Google Scholar]
- 44.van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 45.Wang C Y, Stashenko P. The role of interleukin-1 alpha in the pathogenesis of periapical bone destruction in a rat model system. Oral Microbiol Immunol. 1993;8:50–56. doi: 10.1111/j.1399-302x.1993.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang C Y, Stashenko P. Kinetics of bone resorbing activity in induced periapical lesions. J Dent Res. 1991;70:1362–1366. doi: 10.1177/00220345910700100901. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–818. [PubMed] [Google Scholar]
- 48.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. Interleukin-10 (IL-10) inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 49.Woodroofe C, Muller W, Ruther U. Long-term consequences of interleukin-6 overexpression in transgenic mice. J Cell Biol. 1992;11:587–592. doi: 10.1089/dna.1992.11.587. [DOI] [PubMed] [Google Scholar]