Cancer stem cells (CSCs), also known as tumor initiating cells, are a minority subpopulation of undifferentiated cancerous cells within the entire tumor that hold self-renewal capacity and differentiation potential. CSCs are critical for tumor progression, heterogeneity and metastasis and play a major role in chemo- and radiation-resistance and tumor recurrence [1]. Although the molecular mechanisms underlying the ability of CSCs to give rise to new tumors and promote resistance to many cancer therapies are not completely understood, increasing knowledge suggests potential roles for these cells in the evasion of immune surveillance [2, 3].
The immune system plays a main role in the surveillance against tumors, but tumor cells can develop different strategies to avoid immunogenic cell death (ICD), a form of regulated cell death that is sufficient to activate an adaptive immune response [4, 5]. Damaged or dying cancer cells release intracellular molecules with specific patterns that alert the immune system, thereby triggering antitumor-specific immune responses. These molecules, coined DAMPs (damage-associated molecular patterns), are recognized by specific pattern recognition receptors (PRRs) expressed by innate immune cells such as macrophages, dendritic cells, or NK cells, leading to the activation of T-lymphocytes directed against the tumor.
Although the mechanisms that link ICD with the antitumor immune response are complex and not fully understood, it is universally accepted that the rapid release of massive amounts of type I interferons (IFN-I) plays a pivotal role. In humans, IFN-Is are a family of proteins consisting of 13 isoforms of IFN-α, IFN-β, IFN-ω, and IFN-ε. All IFN-I subtypes exert their actions through the same receptor, the IFN-α/β receptor (IFNAR), which is expressed in all nucleated cells [6]. IFN-Is are essential for antiviral immunity, but they are also critical drivers of antitumor immunity. IFN-Is may act directly on tumor cells to activate a wealth of signaling pathways that impact cell proliferation, differentiation, survival, invasion, and metastasis. In addition, IFN-Is can also indirectly regulate tumor growth by affecting different biological processes involved in tumor progression [6, 7]. As a result, IFN-I-based therapies have received considerable clinical attention and, despite several rounds of optimism and discouragement, they are still considered a promising therapeutic approach to reduce cancer morbidity and mortality [8, 9].
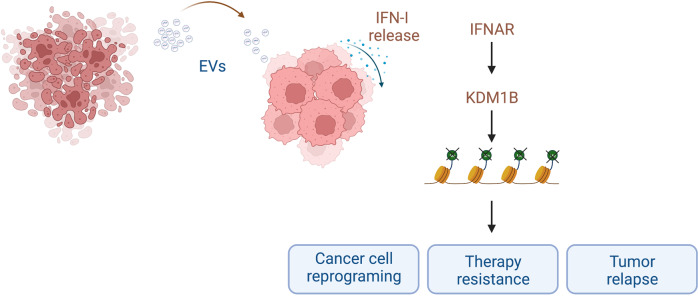
However, the role of IFN-Is in ICD is far more complex, and some untoward effects that, directly or indirectly, permit cancer cells to escape immune clearance have been described [6, 10]. In addition to immune cells, IFN-Is can be secreted by many other cell types in response to activation of PRR, including tumor cells and several types of tumor-associated cells [9], which collectively contribute to generate the tumor immune microenvironment (TIME). Although it has yet to be conclusively determined which cells act as the primary IFN-I producers, tumor cells are the most abundant components in the TIME, and accumulating evidence indicates that IFN-Is produced by malignant cells notably contribute to controlling the autocrine or paracrine circuits that underlie cancer immunosurveillance. In this regard, Musella and collaborators have recently described [3] how IFN-I is able to reprogram cancer cells toward a stem-like phenotype and immune escape, thus inducing a more aggressive tumor phenotype. These results are in keeping with previous findings [11–13], showing an unfavorable effect of exogenous administration of IFN-I and providing a conceivable mechanism of action that involves the expansion of CSCs through the upregulation of lysine-specific histone demethylase 1B (KDM1B, also known as LSD2). KDM1B is a well-known epigenetic regulator that removes repressive H3K4 methylation marks (H3K4me1 or H3K4me2), promoting gene expression. It is well established that KDM1B plays important roles in gene silencing, transcription factor activity, and cell cycle regulation, and alterations in KDM1B expression have been described in several tumor types, such as glioblastoma, breast, pancreas, gastric, colorectal, and prostate cancers [14]. The results from Musella et al. [3] further support the role of KDM1B in tumor progression by demonstrating its ability to promote INF-I-induced transcriptional rewiring of cancer cells toward stemness and immune escape. Interestingly, the authors also propose that CSC induction relies on the transfer of extracellular vesicles (EVs) containing nucleic acids from dying cancer cells to bystander viable cancer cells (Fig. 1). Once internalized by viable cells, these cargos act as DAMPs, inducing IFN-I production, which further activates the IFN-I → KDM1B pathway, leading to cell reprogramming and stemness.
Fig. 1.
Horizontal transfer of IFN-I-induced stemness in cancer. Dying tumor cells release extracellular vesicles (EVs) containing nucleic acids that are incorporated by neighboring viable cancer cells. EV cargos act as DAMPs in these cells, inducing further release of IFN-I that contributes to the stimulation of KDM1B transcriptional activity, which results in extensive chromatin reprogramming and subsequent induction of stemness. The final outcome of the process is the generation of novel populations of CSCs that will lead to boosted tumor resistance and increased relapse frequency. Created with BioRender.com
Based on these findings, it can be predicted that while massive IFN-I signaling may trigger a robust immune response, leading to the destruction of tumor cells, the presence of suboptimal IFN-I levels within the TIME would lead to a non-resolving immune response that may result in therapy resistance and tumor relapse. Given the prominent role of KDM1B in cancer cell reprogramming leading to CSC expansion, inhibition of this regulator offers an attractive therapeutic approach to increase the beneficial effects of antitumoral therapies.
Acknowledgements
This work received financial support from the Ministerio de Ciencia e Innovación (PID2020-113501RB-I00; JAC), the Axencia Galega de Innovación (Galician Agency of Innovation (2020-PG068; JAC), the Centro Singular de Investigación de Galicia accreditation 2016–2019, ED431G/05) and the European Regional Development Fund (ERDF).
Competing interests
The authors declare no competing interests.
Contributor Information
Jose A. Costoya, Email: josea.costoya@usc.es
Victor M. Arce, Email: victor.arce@usc.es
References
- 1.Bajaj J, Diaz E, Reya T. Stem cells in cancer initiation and progression. J Cell Biol. 2020;219:e201911053. doi: 10.1083/jcb.201911053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei MML, Lee TKW. Cancer stem cells: emerging key players in immune evasion of cancers. Front Cell Dev Biol. 2021;9:692940. doi: 10.3389/fcell.2021.692940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musella M, Guarracino A, Manduca N, Galassi C, Ruggiero E, Potenza A, et al. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat Immunol. 2022;23:1379–92. doi: 10.1038/s41590-022-01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14:2994–3006. doi: 10.1002/1878-0261.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukhaled GM, Harding S, Brooks DG. Opposing roles of type I interferons in cancer immunity. Annu Rev Pathol. 2021;16:167–98. doi: 10.1146/annurev-pathol-031920-093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Franco S, Turdo A, Todaro M, Stassi G. Role of type I and II interferons in colorectal cancer and melanoma. Front Immunol. 2017;8:878. doi: 10.3389/fimmu.2017.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aricò E, Castiello L, Capone I, Gabriele L, Belardelli F. Type I interferons and cancer: an evolving story demanding novel clinical applications. Cancers (Basel) 2019;11:1943. doi: 10.3390/cancers11121943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu R, Zhu B, Chen D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci. 2022;79:19. doi: 10.1007/s00018-022-04219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musella M, Manic G, De Maria R, Vitale I, Sistigu A. Type-I-interferons in infection and cancer: Unanticipated dynamics with therapeutic implications. Oncoimmunology. 2017;6:e1314424. doi: 10.1080/2162402X.2017.1314424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Karakhanova S, Huang X, Deng SP, Werner J, Bazhin AV. Influence of interferon-α on the expression of the cancer stem cell markers in pancreatic carcinoma cells. Exp Cell Res. 2014;324:146156. doi: 10.1016/j.yexcr.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Qadir AS, Ceppi P, Brockway S, Law C, Mu L, Khodarev NN, et al. CD95/Fas increases stemness in cancer cells by inducing a STAT1-dependent type I interferon response. Cell Rep. 2017;18:2373–86. doi: 10.1016/j.celrep.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Ruiz ME, Buqué A, Hensler M, Chen J, Bloy N, Petroni G, et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology. 2019;8:e1655964. doi: 10.1080/2162402X.2019.1655964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang D, He J, Dai Y, Geng X, Leng Q, Jiang H, et al. Targeting KDM1B-dependent miR-215-AR-AGR2-axis promotes sensitivity to enzalutamide-resistant prostate cancer. Cancer Gene Ther. 2022;29:543–57. doi: 10.1038/s41417-021-00332-6. [DOI] [PubMed] [Google Scholar]