Abstract
Lipoteichoic acids (LTA), cell wall components of gram-positive bacteria, have been reported to induce various inflammatory mediators and to play a key role in gram-positive-microbe-mediated septic shock. In a large number of these studies, investigators used commercially available LTA purified from a variety of gram-positive bacteria, including Staphylococcus aureus, Bacillus subtilis, and Streptococcus sanguis. We report here that, although these commercially available LTA could be readily shown to stimulate production of nitric oxide (NO) in RAW 264.7 mouse macrophages, the activity was dramatically inhibited by polymyxin B, a relatively specific inhibitor of endotoxin biological activity. One-step purification of the commercially available S. aureus LTA using hydrophobic interaction chromatography resulted in two well-separated peak fractions, one highly enriched for LTA and a second highly enriched for endotoxin. The LTA-enriched fractions did not induce production of NO in RAW 264.7 macrophages, although they caused a dose-dependent induction of NO in the presence of low concentrations of gamma interferon (IFN-γ) (which by itself induced little NO), regardless of the presence of polymyxin B. In contrast, the endotoxin-enriched fractions by themselves inhibited in high levels of NO in RAW 264.7 macrophages but activity was almost completely inhibited in the presence of polymyxin B. Consistent with these findings, our data also indicate that commercial LTA preparations from S. aureus, B. subtilis, and S. sanguis were not able to induce NO from lipopolysaccharide-hyporesponsive C3H/HeJ mouse peritoneal macrophages, but in the presence of IFN-γ, these LTA preparations were able to induce relatively high levels of NO from C3H/HeJ macrophages. These results indicate that commercially available LTA can contain contaminating and potentially significant levels of endotoxin that can be expected to contribute to the putative macrophage-stimulating effects of LTA as assessed by NO production. The fact that the purified LTA, by itself, was not able to induce significant levels of NO secretion in RAW 264.7 macrophages supports the conclusion that caution in attributing high-level biological activity to this microbial cell wall constituent should be exercised.
Lipoteichoic acids (LTA) are structural components in the outer walls and the cytoplasmic membranes of gram-positive bacteria. Recently, an increasing number of reports have indicated that LTA can function as immune system-stimulating agents and may play a role in the process of septic shock induced by gram-positive bacteria (reviewed in reference 18). In this respect, LTA from a variety of different species of gram-positive bacteria have been reported to stimulate both murine and human immune/inflammatory cells to produce cytokines, including interleukin-1 (IL-1) (3, 16, 22), tumor necrosis factor alpha (TNF-α) (3, 22), IL-6 (3), IL-8 (19), IL-12 (4), and macrophage inflammatory protein-1 alpha (5). In addition, LTA have also been shown to stimulate murine macrophages to produce nitric oxide (NO) (9, 11), which most likely occurs as the result of induced production of the inducible NO synthase gene (2, 9, 14). Induction of NO by LTA has been suggested to involve a molecular pathway similar to that used by lipopolysaccharide (LPS), which would be initiated by the binding of LTA to cell surface receptor CD14 (4, 9) and/or transmembrane receptor Toll-like receptor (Toll-2 in this case [17]) and subsequent activation of transcription factor NF-κB (13, 17). Furthermore, LTA have been reported to mediate delayed circulatory failure (7) and induce (through synergistic interaction with another bacterial cell wall component, peptidoglycan) multiple organ failure and lethal shock in experimental models of sepsis in rats (6).
In a number of the above reports, investigators used commercial preparations of LTA purified from various species of gram-positive bacteria to conduct their experimental studies. We have recently undertaken similar studies of NO induction in mouse macrophage-like cell line RAW 264.7 using similar commercial preparations of LTA purified from Staphylococcus aureus, Bacillus subtilis, and Streptococcus sanguis. Although we were able to reproduce the earlier-reported LTA-induced NO production, we also observed that the LTA-induced production of NO was dramatically inhibited by polymyxin B, a relatively specific inhibitor of endotoxin. While it is possible that, like LPS, LTA binds to polymyxin B, we also considered that the LTA might be contaminated with small amounts of biologically active endotoxin. To explore this possibility, purification of the commercially available S. aureus LTA was carried out using an octyl-Sepharose column. This procedure resulted in elution of two well-separated peaks, an LTA-enriched peak and an endotoxin-enriched peak. Contrary to previously reported studies, we found that the LTA peak fractions did not induce NO production in RAW 264.7 macrophages unless gamma interferon (IFN-γ) was present. However, the endotoxin peak fractions induced high levels of NO production, which was virtually totally inhibited by polymyxin B. In agreement with the above studies, our results also indicate that none of the commercial LTA preparations from S. aureus, B. subtilis, or S. sanguis was able to induce NO production from peritoneal macrophages isolated from LPS-hyporesponsive C3H/HeJ mice, even though, together with IFN-γ, these LTA preparations induced relatively high levels of NO from these cells. Collectively, our data indicate that commercially available LTA can contain contaminating detectable levels of endotoxin, which contributes to at least some of the observed biological activities of LTA. Therefore, purification of the commercially available LTA appears to be a necessary preparatory step before undertaking comprehensive experimental studies to assess the biological potential of such materials.
MATERIALS AND METHODS
Materials.
LTA, partially purified from S. aureus, B. subtilis, and S. sanguis, was purchased from Sigma (St. Louis, Mo.). Two different lots of the same preparations of LTA were used in these studies. Polymyxin B was purchased from Pfizer-Roerig (New York, N.Y.). Octyl-Sepharose was also purchased from Sigma.
Cell culture.
Female C3H/HeJ mice from the Jackson Laboratory (Bar Harbor, Maine) were used at 6 to 8 weeks. Mice were injected intraperitoneally with 1.5 ml of 4% Brewer thioglycolate (Difco, Detroit, Mich.), and peritoneal macrophages were harvested 5 days later by lavage with RPMI 1640 culture medium (Life Technologies, Grand Island, N.Y.). Both C3H/HeJ peritoneal macrophages and the murine macrophage-like cell line RAW 264.7 (American Type Culture Collection) were cultured in RPMI 1640 tissue culture medium supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% heat-inactivated fetal bovine serum (endotoxin content of less than 0.06 ng/ml; Sigma) at 37°C in a humidified, 5% CO2 environment. In experiments involving macrophage activation, macrophages were plated at 5 × 104 cells/well in 96-well plates and cultured for 24 h until they reached confluence. They were then incubated with culture medium containing LTA or other activators for 20 h, at which time aliquots of the culture supernatants were assayed for the presence of nitrite (as a measure of NO production).
LTA purification.
S. aureus LTA, purchased from Sigma, was further purified essentially as described by Kengatharan et al. (12). Briefly, LTA was dissolved in equilibration buffer (0.1 M sodium acetate [pH 4.7], 15% [vol/vol] propan-1-ol) and applied to an octyl-Sepharose column (CL-4B; 2.5 by 18 cm). The column was washed with at least three column volumes of equilibration buffer, and LTA was then eluted using a linear gradient of propan-1-ol (15 to 100% in equilibration buffer). Fractions of 2.0 ml were collected, and concentrations of propan-1-ol were estimated by measurement of refractive index. The fractions were dialyzed extensively against endotoxin-free distilled water, lyophilized, and resuspended in 1 ml of endotoxin-free distilled water.
LAL assay.
The relative endotoxin levels in column elute fractions were determined using Limulus amebocyte lysate (LAL) test kits purchased from Bio Whittaker (Walkersville, Md.). The manufacturer's protocol was followed exactly for the assay.
Phosphate assay.
The phosphate content of the eluted column fractions was determined using a protocol modified from that originally described by Ames (1). Briefly, to each Pyrex test tube (13 by 100 mm) containing an aliquot of 20 μl of each column elute sample, 20 μl of 10% Mg(NO3)2 · H2O (in ethanol) was added. The mixture was evaporated to dryness by heating the test tube over a flame. Subsequently, 0.45 ml of 1.0 N HCl was added to the tube and the samples were hydrolyzed at 100°C for 15 min to generate inorganic phosphate. Freshly prepared 10% ascorbic acid (1.0 ml)–0.42% ammonium molybdate (1:6 [vol/vol]) was then added, the mixture was incubated at 37°C for 60 min, and absorbance at 820 nm was then determined spectrophotometrically. Phosphate concentrations were calculated by comparison against a standard curve generated using sodium phosphate monohydrate (NaH2PO4 · H2O).
Nitrite assays.
NO in cell culture supernatants was measured as the concentration of nitrite, a stable reaction product of NO with molecular oxygen, using the Griess reagent, exactly as previously described (20). Absorbance (at 570 nm) was determined using a Dynatech MR5000 microtiter plate reader. Nitrite concentrations were calculated by comparison with a standard curve generated using sodium nitrite dissolved in culture medium. All data for nitrite represent the averages of triplicate samples. Each experiment was repeated at least two times.
RESULTS
Induction of NO by commercially available LTA.
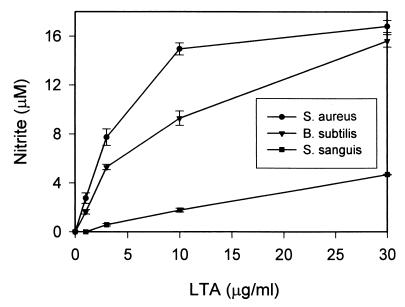
Since various types of LTA have been reported to stimulate NO production in in vitro cultures of mouse macrophages, rat macrophages, and vascular smooth muscle cells (2, 9, 11), we tested whether commercially available LTA extracted from S. aureus, B. subtilis, or S. sanguis would also be able to induce the mouse macrophage-like cell line RAW 264.7 to produce NO. These preliminary studies were carried out preparatory to more comprehensive studies designed to evaluate the consequences to macrophage activation in response to a variety of microbial stimuli. The results of these experiments essentially confirmed earlier published reports in that increasing doses of LTA also induced increasing amounts of NO production in macrophages. As indicated by the data in Fig. 1, although all three types of commercial LTA preparations were able to induce NO production from RAW 264.7 macrophages, S. sanguis LTA induced much lower levels of NO than S. aureus and B. subtilis LTA. At relatively low concentrations (1 to 3 μg/ml), S. aureus and B. subtilis LTA induced 2 to 8 μM NO from macrophages, while S. sanguis LTA induced less than 1 μM NO. At higher concentrations (10 to 30 μg/ml), S. aureus and B. subtilis LTA induced at least 10 to 18 μM NO from RAW 264.7 macrophages, while S. sanguis LTA induced less than 5 μM NO.
FIG. 1.
Induction of NO by commercial preparations of S. aureus, B. Subtilis, and S. sanguis LTA in RAW 264.7 macrophages. RAW 264.7 macrophages were stimulated with increasing concentrations of S. aureus, B. subtilis, and S. sanguis LTA for 20 h before supernatants were taken for nitrite assay as described in Materials and Methods. Results represent averages of triplicate determinations ± standard errors of the means from one representative experiment repeated three times under equivalent conditions.
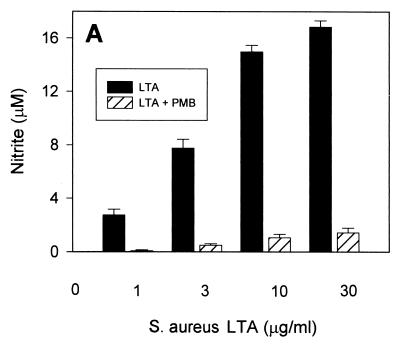
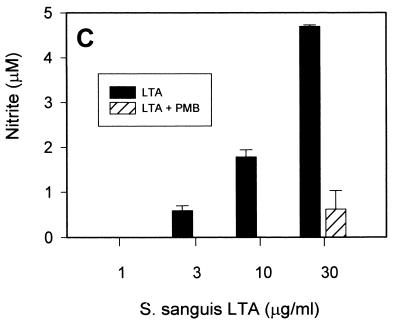
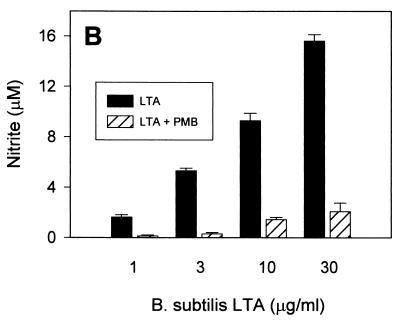
In order to assess whether such NO-inducing activity would be specific to LTA, we tested whether polymyxin B, a relatively specific endotoxin inhibitor, would inhibit the LTA-induced production of NO in RAW 264.7 macrophages. Somewhat unexpectedly, virtually all of the NO production induced by all three types of LTA was abrogated in the presence of 10 μg of polymyxin B/ml at low LTA concentrations (Fig. 2). At higher LTA concentrations (10 to 30 μg/ml), polymyxin B inhibited greater than 80% of NO production. These findings support the hypothesis that either the commercially available LTA contain relevant amounts of biologically active contaminating endotoxin or that LTA, like LPS, is able to bind polymyxin B.
FIG. 2.
Induction of NO by commercially available S. aureus, B. subtilis, and S. sanguis LTA in the presence of polymyxin B. RAW 264.7 macrophages were stimulated with increasing concentrations of S. aureus (A), B. subtilis (B), and S. sanguis (C) LTA in either the absence (solid columns) or the presence (hatched columns) of 10 μg of polymyxin B (PMB)/ml for 20 h before supernatants were taken for a nitrite assay as described in Materials and Methods. Cell viability was not affected by treatment with LTA either alone or in combination with polymyxin B, as judged by more than 95% cellular exclusion of trypan blue in parallel cultures. Data represent averages of triplicate samples ± standard errors of the means. Experiments were repeated three times under equivalent conditions.
Purification of commercially available S. aureus LTA.
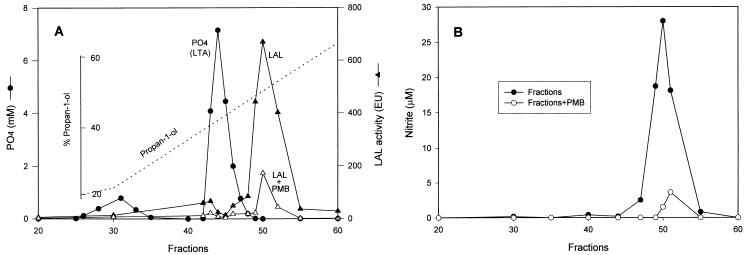
In light of the above results, we initiated experiments to further purify these preparations of LTA using an octyl-Sepharose column. For this purpose, S. aureus LTA was applied to an octyl-Sepharose affinity column and fractions were eluted and treated as described in Materials and Methods using a linear gradient of propan-1-ol. Each column fraction was then tested for phosphate content in order to monitor the elution of LTA, since LTA is known to contain a significant amount of phosphate (12). Each eluted fraction was also assayed for endotoxin content using the LAL test to monitor the presence of endotoxin. As shown by the results of these assays (Fig. 3A), a relatively minor peak of phosphate-containing material, which did not correspond to the anticipated abundance of LTA, was detected in fractions 25 to 35, whereas factions 41 to 47 contained a large amount of phosphate. Since this elution profile corresponds exactly with the previously reported elution concentration of propan-1-ol, these results strongly suggest that fractions 41 to 47 are the major peak of LTA (12). When all of the affinity column-eluted fractions were subjected to LAL assay, only a minimal amount of LAL activity was detected in fractions 41 to 47, which as pointed out above correspond to the major LTA peak. However, in fractions 48 to 54, a substantial amount of LAL activity (corresponding to approximately 680 endotoxin units/ml in fraction 50) was detected (Fig. 3A). As expected, the endotoxin levels in fractions 48 to 54 detected by LAL assay were dramatically reduced in the presence of polymyxin B (Fig. 3A). These results strongly support the conclusions that, (i) the commercially available S. aureus LTA contains a significant amount of LAL-reactive endotoxin and (ii) the LAL-reactive biologically active constituent is readily separable from high-phosphate-containing LTA using octyl-Sepharose affinity chromatography.
FIG. 3.
(A) Purification of commercially available S. aureus LTA. LTA was purified with an octyl-Sepharose column as described in Materials and Methods. The column was successfully eluted with a linear gradient of 15 to 80% propan-1-ol in equilibration buffer (dashed line). Fractions of 2 ml were collected, dialyzed, lyophilized, and resuspended in endotoxin-free water and tested for phosphate content as described in Materials and Methods. The same fractions were also subjected to an LAL assay to test endotoxin levels either in the absence (solid triangles) or the presence (open triangles) of 10 μg of polymyxin B (PMB)/ml. Data represent averages of duplicate samples. Experiments were repeated two times. (B) Induction of NO by eluted LTA fractions. Macrophages were stimulated with different LTA fractions (1:33 dilution in culture medium) for 20 h in either the absence (solid circles) or the presence (open circles) of 10 μg of polymyxin B/ml before supernatants were harvested for a nitrite assay as described in Materials and Methods. Data represent averages of triplicate samples. Experiments were repeated two times under equivalent conditions.
Induction of NO by eluted S. aureus LTA fractions.
In order to further explore which of the two constituents present in the original S. aureus LTA identified in the octyl-Sepharose elution profile is responsible for the observed secretion of NO from RAW 264.7 macrophages (Fig. 1), RAW 264.7 macrophages were cultured in vitro with aliquots of the eluted column fractions and subsequently assayed for NO production present in culture supernatants. As shown by the data in Fig. 3B, fractions 41 to 47, which correspond to the major LTA peak, did not induce any detectable NO production. In other experiments, LTA purified by affinity chromatography and used at concentrations as high as 100 μg/ml were also not able to induce any observable NO production in RAW 264.7 macrophages (data not shown). In contrast, fractions 48 to 54, which correspond to the LAL-reactive endotoxin peak, initiated significant induction of NO. Furthermore, NO secretion in response to the biologically active material present in fractions 48 to 54 (LAL reactive) was almost completely abrogated in the presence of endotoxin-specific inhibitor polymyxin B.
We also investigated the potential biological activity of a combination of LTA-enriched fractions and endotoxin-enriched fractions to stimulate NO production in cultures of macrophages. The LTA-enriched fractions neither increased nor inhibited NO production induced by the endotoxin-enriched fractions (data not shown), suggesting that the NO induction by the commercially available S. aureus LTA observed in Fig. 1 is not the result of the synergistic interaction of its endotoxin and LTA components. These data strongly suggest that it is the LAL-reactive endotoxin component, rather than the LTA component, in the commercially available S. aureus LTA that contributes to induction of NO production in the RAW 264.7 macrophage-like cell line.
Induction of NO by purified S. aureus LTA in the presence of IFN-γ.
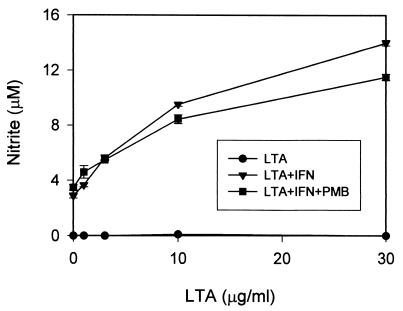
The data shown in Fig. 3 indicate that purified S. aureus LTA was not able to induce NO production in RAW 264.7 macrophages, strongly suggesting that it is the contaminating endotoxin component that contributes to the observed biological activity of commercial preparations of S. aureus LTA. While this conclusion is supported by the evidence presented above, these data do not completely exclude the possibility that the lack of NO induction by purified S. aureus LTA might be the result of a loss of biological activity during the purification process. In order to address this possibility, studies to evaluate whether or not the purified S. aureus LTA had any biological activity were carried out. Since it has been reported that IFN-γ can synergize with LTA to induce NO production from a variety of cell types (8, 10), we examined whether the column-purified LTA is able to induce NO from RAW 264.7 macrophages in the presence of IFN-γ. As shown by the data in Fig. 4, while the purified S. aureus LTA alone did not induce production of NO, it caused a significant induction of NO in the presence of 30 U of IFN-γ/ml (which by itself induced only low levels of NO production). Of particular interest is that the NO induced by purified LTA plus IFN-γ was not significantly inhibited by polymyxin B at a concentration of 10 μg/ml (Fig. 4).
FIG. 4.
Induction of NO by column-purified S. aureus LTA in the presence of IFN-γ. RAW 264.7 macrophages were incubated with increasing concentrations of purified S. aureus LTA either in the absence (circles) or presence (triangles) of 10 U of IFN-γ/ml for 20 h before culture supernatants were collected for a nitrite assay as described in Materials and Methods. To test whether the purified LTA–IFN-γ-induced NO can be inhibited by polymyxin B (PMB), 10 μg of polymyxin B/ml was included in the treating mixture (squares). Results represent averages of triplicate samples ± standard errors of the means. Experiments were repeated three times.
In combination with the data in Fig. 1, these results indicate that (i) the inability of purified S. aureus LTA to induce NO was not caused by a loss of biological activity during the purification process and (ii) inhibition of the unpurified commercial S. aureus LTA-induced NO by polymyxin B (Fig. 2A) was not due to a direct inhibitory effect of polymyxin B on the LTA component. Therefore, these findings further support the conclusion that it is the contaminating endotoxin component in commercial S. aureus LTA that contributes the NO-inducing activity in RAW 264.7 macrophages.
Induction of NO from C3H/HeJ peritoneal macrophages by unpurified commercial LTA with or without IFN-γ.
As an alternative approach to evaluate the intrinsic biological activity of the LTA component alone in the unpurified commercial preparations of LTA, we treated peritoneal macrophages isolated from the LPS-hyporesponsive C3H/HeJ mouse strain with commercial preparations of LTA from S. aureus, B. subtilis, or S. sanguis. Since it is well recognized that C3H/HeJ macrophages do not respond to LPS (21, 23), it was anticipated that the unpurified commercial preparations of LTA would be incapable of stimulating C3H/HeJ macrophages to produce NO even in the presence of a significant amount of contaminating endotoxin. As shown by the data in Fig. 5, neither S. aureus LTA, B. subtilis LTA, nor S. sanguis LTA alone was able to stimulate NO production from C3H/HeJ peritoneal macrophages even at the highest level tested, 30 μg/ml (data not shown). However, in the presence of IFN-γ, all three species of LTA were able to induce NO production from C3H/HeJ macrophages. Interestingly, while unpurified preparations of S. aureus LTA and B. subtilis LTA induced about equivalent NO production from RAW 264.7 macrophages (Fig. 1), the former (in the presence of IFN-γ) induced much lower levels of NO from C3H/HeJ macrophages (Fig. 5). The NO levels induced by S. sanguis LTA plus IFN-γ from C3H/HeJ macrophages are about equivalent to those induced by S. sanguis LTA in RAW 264.7 macrophages. These data indicate that the LTA component alone in commercial LTA preparations is not sufficient to induce NO production from mouse macrophages. However, in the presence of IFN-γ, LTA is able to induce NO production from LPS-hyporesponsive mouse macrophages. These findings further support the conclusion that it is the contaminating endotoxin that contributes to the NO-inducing activity of commercial LTA from LPS-responsive mouse RAW 264.7 macrophages.
FIG. 5.
Induction of NO by commercial LTA preparations from C3H/HeJ peritoneal macrophages. C3H/HeJ macrophages were treated with increasing concentrations of S. aureus, B. subtilis, and S. sanguis LTA in the presence of 30 U of IFN-γ/ml for 20 h. Culture supernatants were then collected for a nitrite assay as described in Materials and Methods. Data represent averages of triplicate samples ± standard errors of the mean. Experiments were repeated three times. None of the three types of commercial LTA alone induced detectable NO production (data not shown).
DISCUSSION
Collectively, the data presented in this paper indicate that commercially available LTA extracted from S. aureus, B. subtilis, and S. sanguis can be demonstrated to contain significant amounts of biologically active endotoxin contaminants that contribute to biological activity as assessed by in vitro macrophage activation studies. Purification of at least one of these commercial LTA preparations (S. aureus LTA) using an octyl-Sepharose column resulted in an LTA-enriched peak and an endotoxin-enriched peak. Although the fractions corresponding to the LAL-reactive endotoxin peak induced NO production from RAW 264.7 macrophages, fractions from the LTA peak were not able to induce NO from RAW 264.7 macrophages (Fig. 3B). This inability of purified LTA to induce NO production was not the result of loss of activity of LTA during the process of purification, since, in the presence of low concentrations of IFN-γ, purified S. aureus LTA was able to stimulate macrophages to produce high levels of NO (Fig. 4). Purified S. aureus LTA alone was also able to induce relatively high levels of TNF-α in RAW 264.7 macrophages (e.g., 10 μg of LTA/ml for about 13,600 pg of TNF-α/ml). Of additional importance is the fact that, in contrast to that of LPS, the biological activity of endotoxin-free S. aureus LTA in the presence of IFN-γ in RAW 264.7 macrophages was not significantly inhibited by polymyxin B (Fig. 4). This finding lends support to the conclusion that the inhibitory effect of polymyxin B on commercial preparations of S. aureus LTA is not due to its binding to and inhibition of the LTA component. In agreement with the above findings, none of the commercial LTA preparations from S. aureus, B. subtilis, and S. sanguis induced NO production from LPS-hyporesponsive C3H/HeJ peritoneal macrophages, although together with IFN-γ each of the three commercial LTA preparations was able to induce significant levels of NO production (Fig. 5). Taken together, these data allow the conclusion that commercially available LTA can be contaminated with a significant amount of biologically active endotoxin, which contributes to the observed NO induction from RAW 264.7 macrophages by such preparations. More importantly, our results also indicate that the endotoxin component in commercially available S. aureus LTA could be separated by hydrophobic interaction chromatography using an octyl-Sepharose column (Fig. 3A). This will provide investigators a necessary but relatively straightforward means for purification of commercially available LTA preparations in studies involving LTA.
Our findings reported here also raise the possibility that the induction of NO as well as other inflammatory mediators by commercially available LTA in various types of cells reported in some previously published studies may be, at least in part, due to endotoxin contamination. One of the primary pieces of supportive evidence is the fact that, in the present study, commercial preparations of S. aureus LTA subjected to additional purification steps were not able to induce NO production in RAW 264.7 macrophages (Fig. 3B) in the absence of the supplemental costimulator. Another important piece of evidence supporting this conclusion is results of studies showing that commercial preparations of LTA are biologically inert in the induction of NO secretion from LPS-hyporesponsive C3H/HeJ macrophages.
Further supportive evidence includes recent studies from two other groups. Keller et al. (11) reported that, although purified S. aureus LTA retains the ability to stimulate rat bone marrow-derived macrophages to produce modest levels of TNF-α, it failed to induce NO production. Moreover, Bhakdi et al. (3) showed that purified LTA from S. aureus failed to stimulate human monocytes to produce IL-1 and IL-6. However, the latter authors also reported that purified S. aureus LTA was unable to induce TNF-α production in human monocytes, a finding that conflicts with observations by Keller et al. (11) and our laboratory. Whether this discrepancy is due to distinct responses in different cell types is not known. Nevertheless, all of these studies support the notion that contaminating endotoxin in commercially available LTA preparations can and does contribute significantly to the observed biological activity of LTA, even though the role of pure LTA alone in inflammatory cell activation cannot be overlooked. Also worthy of note is a recent report by English et al. (8). In those studies, commercially available LTA from gram-positive bacteria S. sanguis and Streptococcus mutans were shown to induce TNF-α and NO production from RAW 264.7 macrophages. However, induction of TNF-α and NO by these two types of LTA was significantly reduced by treatment with polymyxin B. Together with our studies on S. aureus, B. subtilis, and S. sanguis LTA, these findings suggest once again that commercially available LTA extracts of gram-positive bacteria may contain endotoxin contaminants.
It would be of interest to speculate on the possible origin of the contaminating endotoxin, particularly given that fact that the microbial sources of the three commercial preparations of LTA used in these studies were all from gram-positive organisms. However, in the present study, LTA concentrations of about 10 μg/ml were usually required to generate significant levels of NO secretion in cultures of RAW 264.7 macrophages, and previously published reports from several laboratories have confirmed that endotoxin at the level of 100 pg/ml can manifest biological activity in these cells. Thus, levels of contamination of only 1 part in 105 would be sufficient to generate levels of endotoxin contamination adequate to explain the resulting macrophage responses.
In summary, the data presented in this report indicate that commercially available LTA contains significant amounts of endotoxin, which contributes to the observed biological activities of LTA such as NO induction. The contaminating endotoxin component is separable from LTA by hydrophobic interaction chromatography. Since endotoxin is reportedly not a structural component of gram-positive bacteria (15, 24), the source of the contamination is not known. However, regardless of the source of endotoxin contamination, purification of commercially available LTA before using it for both in vitro and in vivo studies is highly necessary.
ACKNOWLEDGMENTS
We thank Christopher Papasian, Alexander Shnyra, and Mei-Guey Lei for their constructive advice and Kathy Rode for her administrative assistance in the process of manuscript preparation.
This research was supported by National Institutes of Health grants AI-23447 and AI-44936, the Kansas Health Foundation, the Saint Luke's Hospital Foundation, and an unrestricted medical research grant from Merck & Co., West Point, Pa. David C. Morrison was supported in part by the Westport Anesthesia Services/State of Missouri Endowed Chair in Research.
REFERENCES
- 1.Ames B N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;3:115–118. [Google Scholar]
- 2.Auguet M, Lonchampt M O, Delaflotte S, Goulin-Schulz J, Chabrier P E, Braquet P. Induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in vascular smooth muscle cells. FEBS Lett. 1992;297:183–185. doi: 10.1016/0014-5793(92)80356-l. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danforth J M, Strieter R M, Kunkel S L, Arenberg D A, VanOtteren G M, Standiford T J. Macrophage inflammatory protein-1 alpha expression in vivo and in vitro: the role of lipoteichoic acid. Immunol Immunopathol. 1995;74:77–83. doi: 10.1006/clin.1995.1011. [DOI] [PubMed] [Google Scholar]
- 6.De-Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De-Kimpe S J, Hunter M L, Bryant C E, Thiemermann C, Vane J R. Delayed circulatory failure due to the induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in anaesthetized rats. Br J Pharmacol. 1995;114:1317–1323. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English B K, Patrick C C, Orlicek S L, McCordic R, Shenep J L. Lipoteichoic acid from viridans streptococci induces the production of tumor necrosis factor and nitric oxide by murine macrophages. J Infect Dis. 1996;174:1348–1351. doi: 10.1093/infdis/174.6.1348. [DOI] [PubMed] [Google Scholar]
- 9.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375–376. doi: 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 10.Hattori Y, Kasai K, Nakanishi N, Gross S S, Thiemermann C. Induction of nitric oxide and tetrahydrobiopterin synthesis by lipoteichoic acid from Staphylococcus aureus in vascular smooth muscle cells. J Vasc Res. 1998;35:104–108. doi: 10.1159/000025571. [DOI] [PubMed] [Google Scholar]
- 11.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kengatharan K M, De-Kimpe S, Robson C, Foster S J, Thiemermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kengatharan M, De-Kimpe S J, Thiemermann C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol. 1996;117:1163–1170. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lonchampt M O, Auguet M, Delaflotte S, Goulin-Schulz J, Chabrier P E, Braquet P. Lipoteichoic acid: a new inducer of nitric oxide synthase. J Cardiovasc Pharmacol. 1992;12(Suppl.):S145–S147. doi: 10.1097/00005344-199204002-00041. [DOI] [PubMed] [Google Scholar]
- 15.Natanson C, Danner R L, Elin R J, Hosseini J M, Peart K W, Banks S M, MacVittie T J, Walker R I, Parrillo J E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock J. Clin Investig. 1989;83:243–251. doi: 10.1172/JCI113866. . (Erratum, 83:1087.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riesenfeld-Om I, Wolpe S, Garcia-Bustos J F, Hoffmann M K, Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989;57:1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan-and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 18.Sriskandan S, Cohen J. Gram-positive sepsis. Mechanisms and differences from gram-negative sepsis. Infect Dis Clin N Am. 1999;13:397–412. doi: 10.1016/s0891-5520(05)70082-9. [DOI] [PubMed] [Google Scholar]
- 19.Standiford T J, Arenberg D A, Danforth J M, Kunkel S L, VanOtteren G M, Strieter R M. Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: a cellular and molecular analysis. Infect Immun. 1994;62:119–125. doi: 10.1128/iai.62.1.119-125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuehr D J, Nathan C F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultzer B M. Endotoxin-induced resistance to a staphylococcal infection: cellular and humoral responses compared in two mouse strains. J Infect Dis. 1968;118:340. doi: 10.1093/infdis/118.3.340. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui O, Kokeguchi S, Matsumura T, Kato K. Relationship of the chemical structure and immunobiological activities of lipoteichoic acid from Streptococcus faecalis (Enterococcus hirae) ATCC 9790. FEMS Microbiol Immunol. 1991;3:211–218. doi: 10.1111/j.1574-6968.1991.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 23.Vogel S N, Bhat N, Qureshi S T, Malo D. Genetic control of endotoxin responsiveness: the Lps gene revisited. In: Morrison D C, Brade H, Opal S M, Vogel S N, editors. Endotoxin in health and disease. New York, N.Y: Marcel Dekker; 1999. pp. 735–750. [Google Scholar]
- 24.Wakabayashi G, Gelfand J A, Jung W K, Connolly R J, Burke J F, Dinarello C A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Investig. 1991;87:1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]