Abstract
Artificial Intelligence (AI) plays an integral role in enhancing the quality of surgical simulation, which is increasingly becoming a popular tool for enriching the training experience of a surgeon. This spans the spectrum from facilitating preoperative planning, to intraoperative visualisation and guidance, ultimately with the aim of improving patient safety. Although arguably still in its early stages of widespread clinical application, AI technology enables personal evaluation and provides personalised feedback in surgical training simulations. Several forms of surgical visualisation technologies currently in use for anatomical education and presurgical assessment rely on different AI algorithms. However, while it is promising to see clinical examples and technological reports attesting to the efficacy of AI-supported surgical simulators, barriers to wide-spread commercialisation of such devices and software remain complex and multifactorial. High implementation and production costs, scarcity of reports evidencing the superiority of such technology, and intrinsic technological limitations remain at the forefront. As AI technology is key to driving the future of surgical simulation, this paper will review the literature delineating its current state, challenges, and prospects. In addition, a consolidated list of FDA/CE approved AI-powered medical devices for surgical simulation is presented, in order to shed light on the existing gap between academic achievements and the universal commercialisation of AI-enabled simulators. We call for further clinical assessment of AI-supported surgical simulators to support novel regulatory body approved devices and usher surgery into a new era of surgical education.
Keywords: artificial intelligence (AI), surgical training, surgical simulation, machine learning, deep learning, virtual reality, augemented reality, mixed reality
Introduction
In recent years, artificial intelligence (AI) technology has fuelled the advancement of surgical simulators, enhancing their accuracy and capabilities. Surgical simulators are increasingly used in modern medical practice to support preoperative planning and enhance the hands-on experience of training a surgeon. The application of modern AI technology can support simulators in providing personalised feedback to the user, while also automating an immersive surgical experience for visualisation of patient anatomy (1–5). In this review, we aim to discuss the current state of AI technology in surgical simulation. By reviewing the available literature and through our consolidated list of FDA/CE approved AI-enabled medical devices, we attempt to highlight recent advancements in the field as well as discuss some gaps between the research and industry communities. The key terms relevant to this article are summarised in Table 1.
Table 1.
Definitions.
| Terminology | Definition |
|---|---|
| Artificial intelligence (AI) | Mimicking of human intelligence through ability to predict, classify, predict, learn, plan, reason and/or perceive to make decisions |
| Machine learning (ML) | Sub-branch of AI technology that processes calculations and statistics to learn from data without human supervision |
| Deep learning (DL) | Subset of ML that uses neural networks to solve more complex challenges such as image, audio, and video classifications. |
| Artificial neural network (ANN) | A collection of simple interconnected algorithms that process information in response to external input |
| Convolutional neural network (CNN) | A deep learning neural network designed for processing structured arrays of data such as images. |
| Augmented reality (AR) | A class of ANN; deep learning neural network designed for processing structured arrays of data such as images. |
| Virtual reality (VR) | A computer-generated environment that replaces the real environment with a digital environment where users interact with virtual objects |
| Mixed reality (MR) | A hybrid environment that merges both real and virtual worlds with digital and physical objects that can interact in real-time |
| Extended reality (XR) | A complete immersive environment which integrates AR, VR, and MR technologies to provide an entire spectrum of reality to virtuality. |
| Head-mounted display (HMD) | A display device, worn on the head or as part of a helmet, that has a small display optic in front of one or each eye. |
The application of AI in surgical simulation assessment
AI can improve surgical training simulators by evaluating performance and providing personalised feedback to the end user (6). Several AI-based algorithms have been described in recent years. For instance, a neurosurgical group at McGill University, Montreal, developed a machine learning (ML) algorithm that classifies participants’ skill levels while performing a VR-based hemilaminectomy or brain tumour resection task (7, 8). More recently, the same group developed a “Virtual operative assistant (VOA)”—an open-sourced AI-based software that, in addition to determining the skill level, also provides personalised feedback in relation to expert proficiency performance benchmarks.
However, like any form of emerging technology, AI-driven performance evaluation and feedback generation remains imperfect with numerous limitations. Lam et al.'s systematic review explored several different ML techniques assessing surgical performance and noted the promising accuracy of ML-based assessment software, while delineating several major challenges and limitations of this technology (9). One limitation is that the surgical skill assessment of a simulated benchtop task might not accurately correlate with the trainee's performance in the operating room, as the real-life surgical environment is highly complex. Furthermore, there is a lack of validated assessment criteria against which participants’ performance should be assessed by the AI-algorithm. Another major barrier is the exorbitant volume of surgical data required to effectively train the ML-algorithms. Potential solutions to some of these challenges include developing a surgeon-led consensus statement outlining the core elements of a surgical technique to be evaluated by ML-algorithms and facilitating surgical data exchange through cross-institutional open-source initiatives (10).
Interestingly, we note that our review of the FDA/CE list did not identify any approved AI-powered software for appraising and providing feedback in surgical simulation. However, it is conceivable that such software may be commercially available but have not been evaluated through the regulatory process as they do not directly interact with patients (11).
The application of AI in surgical visualisation
3D visualisation has been a major development in surgical simulation across all surgical specialties ranging from neurosurgery and orthopaedics to maxillofacial, plastics, and general surgery (12, 13). Such universal demand for 3D presurgical planning and its steadfast advancement since the 1980s testifies to the substantial benefits in reducing operation duration, blood loss, and hospital stay (14–18) whilst improving patient survival. Our review of the FDA/CE list identified 11 AI-enabled visualisation devices (Table 2). It is important to note that this list does not include visualisation technology without obvious AI application.
Table 2.
List of FDA/CE approved AI-powered devices.
| Device name | Approval date | FDA submission number | Type of approval | Other approval | Approval date | Description | Company | Country | Software function | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| UNiD Spine Analyzer | 5/24/2017 | K170172 | 510 (k) premarket notification | Device that allows surgeons to view, measure images, and to perform generic spine related presurgical simulations for placement of surgical implants | Medicrea International (Device acquired by Medtronic) | USA | 2D simulation | 1,3 | ||
| D2P | 8/29/2019 | K183489 | 510 (k) premarket notification | CE | 10/2017 | Pre-operative image segmentation and DICOM visualisation software for surgical planning | 3D Systems, Inc. | USA | 3D printing, VR simulation | 1,2,3,4 |
| HealthJOINT | 12/04/2020 | K202487 | 510 (k) premarket notification | NA | NA | Software for 3D reconstruction from 2D images for preoperative planning of knee orthopaedic surgical procedures | Zebra Medical Vision Ltd (Acquired by Nanox.ai) | USA | 3D reconstruction | 1,3 |
| Innersight3D | NA | NA | NA | CE | 06/2019 | Modelling technology for surgery planning using models from MRI and CT scans | InnersightLabs Ltd. | UK | 3D reconstruction | 4 |
| MEDIP PRO | 11/07/2019 | K191026 | 510 (k) premarket notification | CE | 12/2019 | Software interface and image segmentation system for transfer of DICOM imaging information from medical scanner to output file; pre-operative software for treatment planning | Medical IP Co., Ltd. | South Korea | 3D reconstruction, 3D Printing, MR/XR Simulation | 1,3,4,5 |
| NemoScan | 12/11/2019 | K192571 | 510 (k) premarket notification | NA | NA | Diagnostic and treatment support for dental implantology | Software Nemotec S.L. | Spain | 3D reconstruction, 3D Printing | 1,4 |
| Profuse CAD | 11/21/2018 | K173744 | 510 (k) premarket notification | NA | NA | Standalone Computer Aided Detection (CAD) software used to visualise, analyse, plan and interpret CT, MRI, PET images | Eigen | USA | 3D reconstruction | 2 |
| SurgicalAR | 5/13/2019 | K190764 | 510 (k) premarket notification | NA | NA | Display of medical images and other healthcare data, including functions for image review, basic measurements and 3D visualization | Medivis, Inc. | USA | MR/XR Simulation | 1,4 |
| Synapse 3D, Synapse 3D Base Tools v6.1 | 02/09/2021 | K203103 | 510 (k) premarket notification | NA | NA | Software for advanced processing and analysis of imaging aiding in surgical decision support and preoperative simulation | Fujifilm Corporation | USA | 3D Reconstruction | 1 |
| Syngo Application Software | 06/09/2017 | K170747 | 510 (k) premarket notification | CE | 03/2017 | Provides real-time viewing, image manipulation, 3D-visualisation, communication, and storage of medical images | Siemens Medical Solutions, USA, Inc. | USA | 3D reconstruction | 1,2,4 |
| VSI Holomedicine | NA | NA | NA | CE | 12/2019 | Surgery support through mixed reality | apoQlar GmbH | Germany | VR simulation, MR/XR Simulation | 4 |
1. Artificial Intelligence and Machine Learning (AI/ML) Medical Devices [Internet]. U.S. Food and Drug Administration. 2021. Available from: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices#resources.
2. Wu E, Wu K, Daneshjou R, Ouyang D, Ho D, Zou J. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nature Medicine. 2021;27(4):582-584.
3. Dreyer K, Wald C, Allen Jr. B, Agarwal S, Bizzo B, Gichoya J et al. AI Central [Internet]. AI Central. 2022. Available from: https://aicentral.acrdsi.org/.
4. Muehlematter U, Daniore P, Vokinger K. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis. The Lancet Digital Health. 2021;3(3):e195-e203.
5. STAT Database: AI Tools [Internet]. Statnews.com. 2021. Available from: https://www.statnews.com/wp-content/uploads/2021/02/STAT_FDA_cleared-_AI_tools.xlsx.
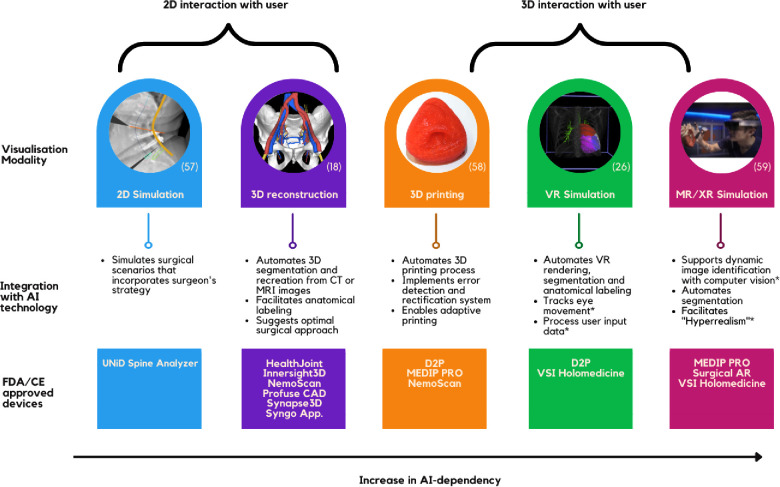
The most widely accepted technique for creating 3D simulations from 2D images involves convoluted neural network (CNN) algorithms. AI can complement this method by facilitating 3D segmentation and anatomical labeling (19–21). In addition, AI plays a crucial role in facilitating advanced visualisation modalities such as 3D printing, virtual reality (VR) simulation, and extended reality (XR) environment (Figure 1).
Figure 1.
Summary of AI function in visualisation technology.
3D-printed simulators have been shown to facilitate preoperative planning, improve surgical outcomes and decrease operation duration (22); however, several challenges have hindered its daily application (23). AI has removed the largest barrier of implementing 3D printing—the need for expert knowledge in the 3D-printing process and computer-based design. Manual processes have been automated through AI algorithms that can efficiently process large amounts of data needed to prefabricate a model, convert through slicer, detect, and rectify any errors, and print. Moreover, AI-driven fabrication that adopts real-time adaptation poses a solution to 3D-printing's challenge of reproducing in-vivo tissue (24). AI technology is essential to support a VR environment and automating the workflow. Software such as DICOM to Print (D2P) and MEDIP PRO produces instantaneous 3D Printing and VR rendering options. Moreover, AI is effectively used for segmentation and anatomical annotation of the VR environment and, more importantly, processing user input needed to facilitate user interaction (25, 26). Furthermore, AI is required to support the XR-environment—the most practical form and the holy-grail of surgical simulation. In conjunction with computer vision technology, deep learning (DL) based object detection allows dynamic image identification (27). Such complex feedback processing from DL models bridges the mismatch between virtual and the real world in 3D holographic projections (28).
The simulation techniques are also integral in surgical training. VSI Holomedicine, for example, provides a platform for both preoperative planning and anatomical education. Furthermore, hyperrealism is an AR concept used in training where artificial objects, such as a silicon replica, are visually enhanced by DL algorithms to simulate a realistic scene of surgery (29).
Discussion
Technological challenges
Current AI frameworks used in surgical simulation have some technological limitations. To begin with, AI-powered feedback platforms could address the challenges experienced with the VOA (3). For example, VOA is restricted to categorising two expertise groups, whilst machine learning techniques such as artificial neural networks can be utilised to classify multiple groups of expertise (9, 30). VOA also highlighted the downfalls of using linear machine algorithms; there were instances of misclassification due to high positive scoring metrics overcompensating for other negative scoring metrics (3). Moreover, recent clinical experience with AI models demonstrated difficulties in measuring subjective combinations of actions such as “instrument handling (31).” In addition to overcoming these fundamental obstacles, a leap to translation will require succeeding platforms to be able to recognise and capture different frames from an entire simulation, in order to account for users’ variety of approaches and techniques that can adequately fulfil a single metric (3).
As seen in Figure 1, segmentation and anatomical labelling seems to be the major application of AI in surgical simulation; however, generalisability of these algorithms remains a challenge (32). Although segmentation has proven its efficacy in small clinical studies, its accuracy is yet to reach perfection (33). Studies reported that their algorithms failed to identify certain structures such as complex vasculature or certain types of tumours and that their accuracy can be highly dependent on the fidelity of different imaging modalities (32–34). Furthermore, to train AI algorithms to an absolute precision without bias and to be generalisable, experts are needed to construct comprehensive datasets which require numerous annotations and segmentation of anatomical structures. However, this manual process is tedious and resource intensive, hence, this approach cannot be translated into clinical practice (35). Such technical impediments are closely related to the fact that AI segmentation and anatomical labelling technology has varying progress across different specialties, pathologies, and procedures. Technologies currently in practice such as Syngo Application Software allow automated segmentation across several different specialties. Nevertheless, there is a lack of an overarching AI framework that can learn, train, and segment or label any anatomical structures in a human body. Some intraoperative AI models have been applying deep learning architectures to predict objects that were previously unseen by the AI (36). Albeit its infancy, this seems to be the future in AI-powered segmentation and anatomical labelling.
Additional technical challenges in the visualisation field are attributed to the intrinsic limitations of technological devices, [i.e., VR-sickness, head-mounted-displays (HMD), computing power of graphic processors] which is beyond the scope of this review. However, few AI-based solutions are proposed to overcome these challenges. VR-sickness is commonly experienced due to the delay between sensory input and VR. To reduce such latency, CNN models are employed to improve gaze-tracking by pre-rendering subsequent scenes and predicting future frames (37, 38). Another ground breaking area of development is in producing 3D displays for human eyes. Current AR/VR displays display 2D images to each of users’ eyes instead of 3D which is how individuals perceive the real world. Choi et al. recently proposed an AI algorithm and calibration technique for producing 3D images, namely the “Neural holography,” that allows a more realistic fidelity of VR rendering, comparable to that of an LCD display (39).
Affordable cost
At the moment, the use of AI-based simulation in healthcare comes at a significant financial cost. As traditional practice for both surgical training and pre-operative planning rely on largely inexpensive methods such as near-peer teaching and group discussions, the introduction of AI-based simulators incurs additional costs. The price of such simulators is rarely publicly disclosed and varies greatly depending on quality, capabilities, and use cases. For instance, none of the companies we identified in our FDA/CE database search provided public information on the prices of their product. However, it is well known that the cost of simulator technology is steep. According to some estimates, the price to equip a simulation lab can range anywhere between $100,000 to several millions (40). The use of AI-algorithms adds an additional layer of costs, especially when considering that such algorithms need to be regularly updated to ensure optimal performance and reliability (41).
It is, therefore, important to understand if the clinical benefit from AI-driven surgical simulators would justify the significant costs associated with their purchase and maintenance. Interestingly, the majority of published research failed to yet report a significant superiority of surgical simulators as compared to traditional methods in the realm of surgical education (42). For instance, Madan et al. reported that substituting virtual reality trainers with an inanimate box does not decrease the rate and level of laparoscopic skill acquisitions. Similarly, a systematic review by Higgins et al. failed to identify any studies directly comparing the surgical simulation with traditional surgical skill development in the operating room (43). However, in the area of surgical visualisation for pre-operative planning, there is some, albeit still limited, evidence that supports the superiority of AI-driven simulators compared to traditional surgical techniques (14, 15, 18).
At the same time, it is important to explore if the cost of AI-driven surgical simulators can be reduced while maintaining the value they offer in patient care. The concept of “fidelity” refers to how well a surgical simulator represents reality (44). A range of studies found that higher fidelity simulators were rarely superior to their lower fidelity counterparts in the area of surgical education (45, 46). However, perceptual fidelity might prove to be important in simulators aiming to support surgical visualisation and preoperative planning process (47), although there is currently limited literature on the topic. Therefore, the choice to pursue low-cost, low-fidelity simulator variants needs to be carefully reviewed and balanced with the extent of benefits they can provide.
In addition to pursuing lower-fidelity simulators, it is reasonable to expect the price of simulators will be kept within a reasonable range with growing market competition and widespread adoption of this technology. Furthermore, national healthcare services and large healthcare institutions may have the negotiating power to further reduce the prices when supplying their hospitals with AI-driven surgical simulators and related equipment.
Regulatory challenges
Presently, a clear overview of all approved AI-based medical devices and algorithms does not exist (48). Few efforts are underway to curate an inclusive database of these devices, in which FDA is at the forefront (49–51). Additionally, multiple other regulatory bodies such as the UK's Medicines and Healthcare products Regulatory Agency (MHRA), are making headway in developing regulatory guidelines to ensure safety and effectiveness of AI-powered devices and softwares (52). As valuable as these national endeavours are, it is crucial for internationally governing bodies to bring forth a guideline for implementing AI-powered surgical simulators universally (41). International Medical Device Regulators Forum (IMDRF) is a prime example of a group that can strive towards this goal (53).
To our knowledge, there are only a handful of AI-powered surgical simulators that are regulatory approved. Nevertheless, we emphasise the need for strict scrutiny of these products to ensure patient safety, transparency, and cyber security (41, 54). Although feedback softwares do not directly interact with patients, quality of training indirectly impacts patient care. For AI-based surgical simulation assessments to become the norm, like flight simulators, products should be driven to adhere to rigid requirements that uplift the quality of surgical training. From our experience with reviewing approved preoperative planning simulators, we highlight the significance of regulatory approval for several reasons. Products are often exaggerated or falsely marketed to be AI-powered, to boost their sales (48). Additionally, even for approved devices, the extent of use or the function of AI in their devices are only vaguely communicated to the public and the users. Ultimately, as preoperative simulators hold and analyse important patient information, they need to strictly abide by established data security guidelines for medical devices. Some preoperative simulators aim to automate the decision-making process which makes regulation even more pivotal. Despite the lack of data protection guidelines for AI specifically, the General Data Protection Regulation (GDPR) for example, contains elements that are relevant to such AI-based surgical planning simulators, including use of personal data, profiling, and automation of decision making (55). FDA's most recent endeavours share similar notions (50, 51).
Moving forward, global organisations like IMDRF need to corroborate on a consensus on AI regulation since the aforementioned issues are ubiquitous around the globe. As we aim towards universalisation of AI-based surgical simulators, legal and ethical issues regarding accountability and performance transparency of AI-based surgical simulators are crucial components that should be overseen by such governing bodies (54, 56).
Conclusion
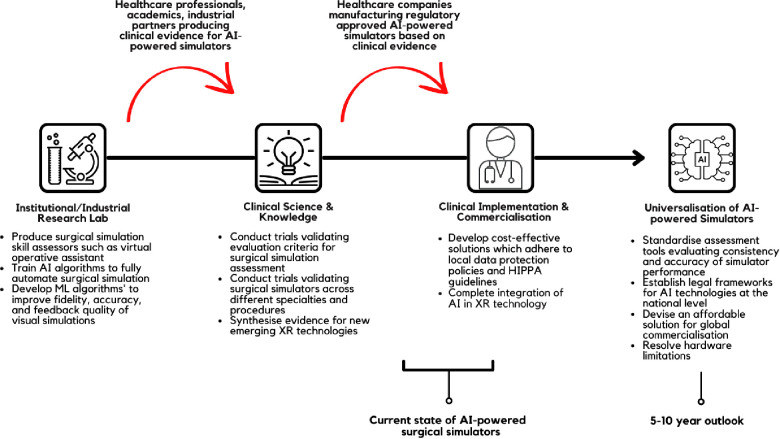
Application of AI has the potential to enhance the quality of surgical simulators and expand their capabilities. In this review article, we have discussed the elements of surgical simulation which are enhanced using AI-driven technologies and reviewed the available literature and FDA/CE-approved products. We summarised the current landscape of AI in surgical simulations and provided suggestions for further clinical implementation in Figure 2. It is promising to see clinical evidence and technological reports attesting to the efficacy of AI-supported surgical simulators. However, the barriers to wide-spread commercialisation of these devices and software are complex and multifactorial. High implementation and production cost, scarcity of reports evidencing the superiority of such technology, and intrinsic technological limitations remain at the forefront. With this in mind, we call for further clinical assessment of AI-supported surgical simulators to support novel regulatory body approved devices and usher surgery into a new era of surgical education.
Figure 2.
Recommendation and suggestions for AI-powered surgical simulators.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Bakshi SK, Lin SR, Ting DSW, Chiang MF, Chodosh J. The era of artificial intelligence and virtual reality: transforming surgical education in ophthalmology. Br J Ophthalmol. (2021) 105(10):1325–8. 10.1136/bjophthalmol-2020-316845 [DOI] [PubMed] [Google Scholar]
- 2.Bravo J, Wali AR, Hirshman BR, Gopesh T, Steinberg JA, Yan B, et al. Robotics and artificial intelligence in endovascular neurosurgery. Cureus. (2022) 14(3):e23662. 10.7759/cureus.23662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirchi N, Bissonnette V, Yilmaz R, Ledwos N, Winkler-Schwartz A, Del Maestro RF. The virtual operative assistant: an explainable artificial intelligence tool for simulation-based training in surgery and medicine. PLoS One. (2020) 15(2):e0229596. 10.1371/journal.pone.0229596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler-Schwartz A, Bissonnette V, Mirchi N, Ponnudurai N, Yilmaz R, Ledwos N, et al. Artificial intelligence in medical education: best practices using machine learning to assess surgical expertise in virtual reality simulation. J Surg Educ. (2019) 76(6):1681–90. 10.1016/j.jsurg.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Winkler-Schwartz A, Yilmaz R, Mirchi N, Bissonnette V, Ledwos N, Siyar S, et al. Machine learning identification of surgical and operative factors associated with surgical expertise in virtual reality simulation. JAMA Network Open. (2019) 2(8):e198363. 10.1001/jamanetworkopen.2019.8363 [DOI] [PubMed] [Google Scholar]
- 6.Sewell C, Morris D, Blevins NH, Dutta S, Agrawal S, Barbagli F, et al. Providing metrics and performance feedback in a surgical simulator. Comput Aided Surg. (2008) 13(2):63–81. 10.3109/10929080801957712 [DOI] [PubMed] [Google Scholar]
- 7.Siyar S, Azarnoush H, Rashidi S, Del Maestro RF. Tremor assessment during virtual reality brain tumor resection. J Surg Educ. (2020) 77(3):643–51. 10.1016/j.jsurg.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 8.Bissonnette V, Mirchi N, Ledwos N, Alsidieri G, Winkler-Schwartz A, Del Maestro RF. Artificial intelligence distinguishes surgical training levels in a virtual reality spinal task. J Bone Joint Surg Am. (2019) 101(23):e127. 10.2106/JBJS.18.01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam K, Chen J, Wang Z, Iqbal FM, Darzi A, Lo B, et al. Machine learning for technical skill assessment in surgery: a systematic review. NPJ Digit Med. (2022) 5(1):24. 10.1038/s41746-022-00566-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maier-Hein L, Eisenmann M, Sarikaya D, März K, Collins T, Malpani A, et al. Surgical data science - from concepts toward clinical translation. Med Image Anal. (2022) 76:102306. 10.1016/j.media.2021.102306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Administration USFD. FDA rules and regulations (2020). Available at: https://www.fda.gov/regulatory-information/fda-rules-and-regulations
- 12.Soon DSC, Chae MP, Pilgrim CHC, Rozen WM, Spychal RT, Hunter-Smith DJ. 3D Haptic modelling for preoperative planning of hepatic resection: a systematic review. Ann Med Surg. (2016) 10:1–7. 10.1016/j.amsu.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boaro A, Moscolo F, Feletti A, Polizzi GMV, Nunes S, Siddi F, et al. Visualization, navigation, augmentation. The ever-changing perspective of the neurosurgeon. Brain Spine. (2022) 2:100926. 10.1016/j.bas.2022.100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang CH, Tao HS, Yang J, Fang ZS, Cai W, Liu J, et al. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg. (2015) 220(1):28–37. 10.1016/j.jamcollsurg.2014.09.023 [DOI] [PubMed] [Google Scholar]
- 15.Fang CH, Liu J, Fan YF, Yang J, Xiang N, Zeng N. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. (2013) 217(2):280–8. 10.1016/j.jamcollsurg.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 16.Uchida M. Recent advances in 3D computed tomography techniques for simulation and navigation in hepatobiliary pancreatic surgery. J Hepatobiliary Pancreat Sci. (2014) 21(4):239–45. 10.1002/jhbp.82 [DOI] [PubMed] [Google Scholar]
- 17.Mutter D, Dallemagne B, Bailey C, Soler L, Marescaux J. 3D Virtual reality and selective vascular control for laparoscopic left hepatic lobectomy. Surg Endosc. (2009) 23(2):432–5. 10.1007/s00464-008-9931-y [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto R, Oshiro Y, Nakayama K, Kohno K, Hashimoto S, Fukunaga K, et al. Three-dimensional simulation of pancreatic surgery showing the size and location of the main pancreatic duct. Surg Today. (2017) 47(3):357–64. 10.1007/s00595-016-1377-6 [DOI] [PubMed] [Google Scholar]
- 19.Hamabe A, Ishii M, Kamoda R, Sasuga S, Okuya K, Okita K, et al. Artificial intelligence-based technology to make a three-dimensional pelvic model for preoperative simulation of rectal cancer surgery using MRI. Ann Gastroenterol Surg. (2022) 6(6):788–94. 10.1002/ags3.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Jung J, Kim J, Cho B, Kwak J, Jang JY, et al. Abdominal multi-organ auto-segmentation using 3D-patch-based deep convolutional neural network. Sci Rep. (2020) 10(1):6204. 10.1038/s41598-020-63285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neves CA, Tran ED, Kessler IM, Blevins NH. Fully automated preoperative segmentation of temporal bone structures from clinical CT scans. Sci Rep. (2021) 11(1):116. 10.1038/s41598-020-80619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. (2016) 15(1):115. 10.1186/s12938-016-0236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segaran N, Saini G, Mayer JL, Naidu S, Patel I, Alzubaidi S, et al. Application of 3D printing in preoperative planning. J Clin Med. (2021) 10(5):1–13. 10.3390/jcm10050917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Ng DWH, Park HS, McAlpine MC. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat Rev Mater. (2021) 6(1):27–47. 10.1038/s41578-020-00235-2 [DOI] [Google Scholar]
- 25.Gupta D, Hassanien AE, Khanna A. Advanced computational intelligence techniques for virtual reality in healthcare. 1 ed. Switzerland, AG: Springer Cham; (2020). [Google Scholar]
- 26.Sadeghi AH, Maat A, Taverne Y, Cornelissen R, Dingemans AC, Bogers A, et al. Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech. (2021) 7:309–21. 10.1016/j.xjtc.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devagiri JS, Paheding S, Niyaz Q, Yang X, Smith S. Augmented reality and artificial intelligence in industry: trends, tools, and future challenges. Expert Syst Appl. (2022) 207:118002. 10.1016/j.eswa.2022.118002 [DOI] [Google Scholar]
- 28.Morimoto T, Kobayashi T, Hirata H, Otani K, Sugimoto M, Tsukamoto M, et al. XR (extended reality: virtual reality, augmented reality, mixed reality) technology in spine medicine: status quo and quo vadis. J Clin Med. (2022) 11(2):470. 10.3390/jcm11020470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang DD, Qian Z, Vukicevic M, Engelhardt S, Kheradvar A, Zhang C, et al. 3D Printing, computational modeling, and artificial intelligence for structural heart disease. JACC Cardiovascular Imaging. (2021) 14(1):41–60. 10.1016/j.jcmg.2019.12.022 [DOI] [PubMed] [Google Scholar]
- 30.Reich A, Mirchi N, Yilmaz R, Ledwos N, Bissonnette V, Tran DH, et al. Artificial neural network approach to competency-based training using a virtual reality neurosurgical simulation. Oper Neurosurg. (2022) 23(1):31–9. 10.1227/ons.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 31.Fazlollahi AM, Bakhaidar M, Alsayegh A, Yilmaz R, Winkler-Schwartz A, Mirchi N, et al. Effect of artificial intelligence tutoring vs expert instruction on learning simulated surgical skills among medical students: a randomized clinical trial. JAMA Network Open. (2022) 5(2):e2149008. 10.1001/jamanetworkopen.2021.49008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamabe A, Ishii M, Kamoda R, Sasuga S, Okuya K, Okita K, et al. Artificial intelligence–based technology for semi-automated segmentation of rectal cancer using high-resolution MRI. PLoS One. (2022) 17(6):e0269931. 10.1371/journal.pone.0269931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding X, Zhang B, Li W, Huo J, Liu S, Wu T, et al. Value of preoperative three-dimensional planning software (AI-HIP) in primary total hip arthroplasty: a retrospective study. J Int Med Res. (2021) 49(11):1–21. 10.1177/03000605211058874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chidambaram S, Stifano V, Demetres M, Teyssandier M, Palumbo MC, Redaelli A, et al. Applications of augmented reality in the neurosurgical operating room: a systematic review of the literature. J Clin Neurosci. (2021) 91:43–61. 10.1016/j.jocn.2021.06.032 [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Zhang Y, Yan Z, Dong J, Cai W, Ma Y, et al. Artificial intelligence assisted display in thoracic surgery: development and possibilities. J Thorac Dis. (2021) 13(12):6994–7005. 10.21037/jtd-21-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanuma H, Endo Y, Fujinaga A, Kawamura M, Kawasaki T, Masuda T, et al. An intraoperative artificial intelligence system identifying anatomical landmarks for laparoscopic cholecystectomy: a prospective clinical feasibility trial (J-SUMMIT-C-01). Surg Endosc. (2022). 10.1007/s00464-022-09678-w [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Ma KT, Lim JH, Zhao Q, Feng J. Deep future gaze: gaze anticipation on egocentric videos using adversarial networks. 2017 IEEE conference on computer vision and pattern recognition (CVPR); 2017 July 21-26 (2017). [Google Scholar]
- 38.Xu Y, Dong Y, Wu J, Sun Z, Shi Z, Yu J, et al. Gaze prediction in dynamic 360° immersive videos. IEEE Conference on computer vision and pattern recognition (CVPR) (2018). [Google Scholar]
- 39.Choi S, Gopakumar M, Peng Y, Kim J, Wetzstein G. Neural 3D holography: learning accurate wave propagation models for 3D holographic virtual and augmented reality displays. ACM Trans Graph. (2021) 40(6):240. 10.1145/3478513.3480542 [DOI] [Google Scholar]
- 40.Bernier GV, Sanchez JE. Surgical simulation: the value of individualization. Surg Endosc. (2016) 30(8):3191–7. 10.1007/s00464-016-5021-8 [DOI] [PubMed] [Google Scholar]
- 41.He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. (2019) 25(1):30–6. 10.1038/s41591-018-0307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madan AK, Frantzides CT. Substituting virtual reality trainers for inanimate box trainers does not decrease laparoscopic skills acquisition. J Soc Laparoendosc Surg. (2007) 11(1):87–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. (2021) 19(4):e67–77. 10.1016/j.surge.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 44.Lewis R, Strachan A, Smith MM. Is high fidelity simulation the most effective method for the development of non-technical skills in nursing? A review of the current evidence. Open Nurs J. (2012) 6:82–9. 10.2174/1874434601206010082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman G, Dore K, Grierson L. The minimal relationship between simulation fidelity and transfer of learning. Med Educ. (2012) 46(7):636–47. 10.1111/j.1365-2923.2012.04243.x [DOI] [PubMed] [Google Scholar]
- 46.Grober ED, Hamstra SJ, Wanzel KR, Reznick RK, Matsumoto ED, Sidhu RS, et al. The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures. Ann Surg. (2004) 240(2):374–81. 10.1097/01.sla.0000133346.07434.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoyanov D, Mylonas GP, Lerotic M, Chung AJ, Yang GZ. Intra-operative visualizations: perceptual fidelity and human factors. J Disp Technol. (2008) 4(4):491–501. 10.1109/JDT.2008.926497 [DOI] [Google Scholar]
- 48.Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. (2020) 3:118. 10.1038/s41746-020-00324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S Food & Drug Administration. Artificial intelligence and machine learning (AI/ML)-enabled medical devices (2022). Available at: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices
- 50.U.S Food & Drug Administration. Artificial intelligence/machine learning (AI/ML)-based software as a medical device (SaMD) action Plan (2021).
- 51.U.S Food & Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)-based software as a medical device (SaMD). Department of Health and Human Services (United States) (2019).
- 52.Clarke O. UK digital health – the future of software as a medical device (2021).
- 53.International Medical Device Regulators Forum. Artificial intelligence medical devices (2022). Available at: https://www.imdrf.org/working-groups/artificial-intelligence-medical-devices.
- 54.Kiseleva A. AI As a medical device: is it enough to ensure performance transparency and accountability in healthcare? SSRN. (2019) 1(1):5–16. 10.21552/eplr/2020/1/4 [DOI] [Google Scholar]
- 55.Sartor G, Lagioia F. The impact of the general data protection regulation (GDPR) on artificial intelligence (2020).
- 56.Jamjoom AAB, Jamjoom AMA, Thomas JP, Palmisciano P, Kerr K, Collins JW, et al. Autonomous surgical robotic systems and the liability dilemma. Front Surg. (2022) 9:1015367. 10.3389/fsurg.2022.1015367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medtronic. UNID adaptive spine intelligence (ASI). Available at: https://www.medtronic.com/us-en/healthcare-professionals/products/spinal-orthopaedic/internal-fixation-systems/unid.html.
- 58.Qiu K, Zhao Z, Haghiashtiani G, Guo S-Z, He M, Su R, et al. 3D Printed organ models with physical properties of tissue and integrated sensors. Adv Mater Technol. (2018) 3(3):1700235. 10.1002/admt.201700235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shieber J. Medivis has launched its augemented reality platform for surgical planning: TechCrunch (2019). Available at: https://tcrn.ch/2NbGMG5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.