Abstract
Child lead poisoning damages central nervous system, immune, and renal function, and is the longest-standing public health epidemic in U.S. history. While primary prevention is the ultimate goal, secondary intervention is critical for curbing effects among children already exposed. Despite the lowering of child blood lead level (BLL) reference value in 2012 and again in 2021, few changes to secondary intervention approaches have been discussed. This study tested a novel interdisciplinary approach integrating ongoing child BLL-monitoring with education and home mitigation for families living in neighborhoods at high-risk of child lead exposure. In children ages 6 months to 16 years, most of whom had lowest range exposures, we predicted significantly reduced BLLs following intervention.
Methods
Twenty-one families with 49 children, were offered enrollment when at least 1 child in the family was found to have a BLL > 2.5 µg/dL. Child BLLs, determined by ICPMS, were monitored at 4- to 6-month intervals. Education was tailored to family needs, reinforced through repeated parent engagement, and was followed by home testing reports with detailed case-specific information and recommendations for no-cost/low-cost mitigation.
Results
Ninety percent of enrolled families complied with the mitigation program. In most cases, isolated, simple-to-mitigate lead hazard sources were found. Most prevalent were consumer products, found in 69% (11/16) of homes. Lead paint was identified in 56% (9/16) of homes. Generalized linear regression with Test Wave as a random effect showed that children’s BLLs decreased significantly following the intervention despite fluctuations.
Conclusion
Lower-level lead poisoning can be reduced through an interdisciplinary approach that combines ongoing child BLL monitoring; repeated, one-on-one parent prevention education; and identification and no-cost/low-cost mitigation of home lead hazards. Biannual child BLL monitoring is essential for detecting and responding to changes in child BLLs, particularly in neighborhoods deemed high-risk for child lead poisoning
Keywords: Child lead poisoning, Developmental lead exposure, Secondary prevention, Developmental neurotoxicity, Health disparities
Highlights
-
•
A novel team-based low-cost mitigation strategy for reducing dangerous lower-range child BLLs is presented.
-
•
Pre- and post-mitigation child BLL data from 21 families with 49 children were analyzed.
-
•
Consumer products and lead paint were the most frequent lead sources identified and were readily mitigated.
-
•
Following ongoing parent engagement, education, and mitigation, significant reduction in children’s BLLs was achieved.
-
•
Bi-annual child BLL testing is critical for monitoring possible fluctuations in child lead exposure over time.
1. Introduction
Childhood lead poisoning is the longest standing public health epidemic in U.S. history [1] and it has long been recognized that minority children living in lower-income neighborhoods with a high density of older unrenovated housing (“high-risk” neighborhoods) are at disproportionate risk [2], [3], [4], [5], [6], [7]. While child lead exposure constitutes a major unresolved child health disparity [8], it simultaneously contributes to a broader profile of child health inequities. Studies have shown that developmental lead exposure yielding BLLs as low as 2.5 µg/dL damages the central nervous [9], [10] and immune systems [11], disrupts kidney function [12], and promotes obesity [13], [14]. Following the child lead exposure crisis in Flint, Michigan, in which the numbers of children with BLLs exceeding 5 µg/dL doubled following changes in the city’s water supply, investigative reporting by Reuters [15] concluded that child lead exposure in over 3000 cities nationwide exceeded levels observed in Flint, with damaging effects for millions of children. Moreover, due to testing barriers and inconsistencies, the problem is likely to be larger than has been estimated. Modeling studies have suggested that at least 500,000 children per year, and as many as 2 million, are never tested [16], [17]. Moreover, children living in lower-income neighborhoods are significantly less likely to be tested and monitored [18], [19].
The clinical interpretation of child blood lead levels (BLLs) has changed dramatically over the past 50 years as indicated by changes in child BLL reference/threshold values, from 30 µg/dL in 1975, to 25 µg/dL in 1985, 10 µg/dL in 1991, 5 µg/dL in 2012, and 3.5 µg/dL in 2021. Our public health response strategies have not been able to keep pace with growing knowledge regarding the dangers of lower-range child BLLs. The increasing acceptance of “no safe level,” promoted since 1991 by the CDC, and reduction of the national child BLL “elevated” reference value to 3.5 µg/dL in October 2021, provides a mandate to reduce child lead exposure to non-detectable levels. The sheer numbers of children affected simply overwhelms current approaches [17].
One barrier to reducing lead exposure in U.S. children may be the continuing use of state-defined mitigation strategies originally conceived for response to individual cases of children with higher-range BLLs. As of April 2022, 66% of states (33/50) continued to rely on high-cost, labor-intensive home intervention approaches for secondary intervention which typically require contracting with specially trained contractors for whole-home lead abatement;, and these interventions are initiated only after the report of a BLL confirmed by a medical professional based on a venous sample blood draw. Moreover, a majority of state and local agencies initiate home mitigation only in response to child BLLs that are 3–7 times higher than the current federal “elevated” reference level of 3.5 µg/dL. In many states, limited resources result in time-lags of months to a year or more between the reporting of an elevated child BLL, as defined by each state, and the initiation of home mitigation efforts.
While removing lead from children’s environments before exposure occurs must remain the ultimate goal (“primary prevention”), mitigation approaches (“secondary prevention”) that are better suited to the magnitude of the current problem need to be tested; with new approaches built on past successes. For example, the Lead-Based Paint Hazard Control grant program started in 1993 by the U.S. Department of Housing and Urban Development (HUD) was a major step towards helping states and local agencies reduce household lead hazards in lower-income neighborhoods. The resulting studies examined the efficacy of a broad range of low- (cleaning, spot or complete paint stabilization, windowsill/trough caps, floor treatments); medium- (cleaning, complete paint encapsulation/stabilization, floor treatments, window treatments/replacement, selected abatement) and high-intensity (all lead-based paint enclosed, encapsulated, or removed) interventions. The short- and long-term efficacy of these have been summarized [20], [21], [22], [23], [24], [25], [26]. Conclusions from studies summarized in 2006 [26] suggest that both low-intensity (e.g., parent education and increased wet-dusting and wet-mopping) and high-intensity (e.g., major lead abatement activities) control measures were effective in significantly reducing floor and window sill dust lead loadings in urban and rural homes. Importantly, studies that tested efficacy differences between low- and high-intensity methods found no significant differences [27]. Similarly, short- and long-term tests of child blood lead burden for pre-mitigation BLLs > 10 µg/dL, showed reduction of nearly 40%, with continuing BLL decline for up to three years post-intervention [20]. More recent studies have demonstrated the efficacy of programs that integrate intensive community outreach with education, comprehensive screening and community-wide soil metal abatement efforts for lowering high-range child BLLs [28].
The work described here was part of an intended 3-year study that began in January 2019. Over the 12 years preceding this study, we witnessed many examples of how the current public health response to child lead exposure had become increasingly fragmented. The three main public health response components for child lead exposure – parent education, BLL testing, and home screening and mitigation – were segregated by trade, with little means for communication or data sharing. A comprehensive, interdisciplinary approach to reducing child BLLs was called for but had yet to be developed.
A team-based strategy that integrated ongoing parent and community education, child BLL testing and monitoring, and safe, simple, low-cost home screening and mitigation, was conceptualized, outlined, and implemented. Based on our experiences over the past 16 years working with families in the neighborhoods targeted in this study, we predicted a minimum 80% parent compliance with offered mitigation, and following mitigation, reduced child BLLs, and reduced home dust lead levels. The COVID pandemic ended data collection for this 36-month study at month 15; data reported here were collected through mid-March 2020.
2. Methods
2.1. Study preparation
The methods, procedures, forms, and materials for this study were reviewed and approved on an annual basis by the University of Texas at El Paso Institutional Review Board (#1309985, C. Sobin, PI), formally designated to provide ethical oversight and review of research protocols and all study materials for the protection of human participants. No major changes to the protocol were requested by the IRB. Detailed instructions and standard operating procedures for all methods, and tracking forms for recruitment, child BLL testing, home visits, home screening, and follow-up clearance testing, were included in a 61-page manual created for this study (available by request from the authors). All educational materials, forms, and questionnaires provided to parents were available in Spanish and/or English.
The study targeted downtown neighborhoods in El Paso, Texas with a high density of pre-1978 homes (> 85%). Since the study focused on detection and mitigation of interior and exterior lead paint, three core team members completed U.S. Environmental Protection Agency (U.S. EPA) training and certification as Lead Risk Assessors and Lead-Based Paint Inspectors; and 3 team members completed training and certification in Renovation, Repair, and Painting (RRP) for homes with lead paint. At least one certified Lead Risk Assessor/Lead-Based Paint Inspector and RRP certified team member were present for all home visits that involved environmental screening, testing, and/or mitigation.
2.2. Analytics
A Niton XLp 300 pXRF analyzer (Thermo Fisher Scientific, Tewksbury, MA), calibrated for Pb detection on interior and exterior surfaces, soil, and consumer products, was used for interior, exterior, and consumer product screening (see Supplementary Material, Table 3 for additional information). Four team members completed specific radiation safety training and safe handling practices for the pXRF device. All screening and sample testing followed current EPA and HUD guidelines; all ICPMS/ICPOES analyses were conducted at Kansas State University (laboratory of GH). (Validity studies of pXRF and ICPMS methods for this study were previously published [29].) Total lead concentration in dust wipe samples was measured using methods from ASTM E1644–17 (ASTM 2017) and followed by inductively-couple plasma mass spectrometry (ICPMS, Agilent 7500cx) analysis. For soil testing, total lead concentrations were determined using methods provided in EPA 3051 [30], followed by inductively-coupled plasma optical emission spectrometry (ICPOES) analysis. Bioaccessible lead was measured using a physiologically-based extraction procedure (PBET) modified gastric-phase method [31]. EPA Guidelines for home tap water sample collection were followed. Relevant research team members completed comprehensive training on clean, safe, multi-step procedures for the collection and handling of finger-stick blood samples; and requisite IRB training in human subjects research, laboratory safety, and blood borne pathogens.
All blood samples were analyzed for lead by inductively coupled plasma mass spectrometry [32] (Agilent ICP-MS 7500cx, Santa Clare, CA). Standards were prepared using stock solutions of Pb; Bi-209 was used as the internal standard element for Pb. Lyphochek Whole Blood Metals Control, Level 1 #527, Level 2 #528, and Level 3 # 529 (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.) were used as the reference (QA/QC) samples to confirm percent recovery. Recoveries were in the range of 95 – 105% for the elements of interest. The ICPMS limit of detection (LOD) for Pb was 0.04 µg/dL.
2.3. Mitigation
“Mitigation” was any intervention that could be safely and correctly carried out at relatively low-cost by a typical homeowner who had been provided with detailed training and guidance specifically relevant to the lead hazard source identified (see Supplementary Material, Table 3). Prior to sampling, the team prepared specific intervention plans for each type of anticipated lead hazard. Comprehensive educational materials for parents regarding the common home lead hazard sources, simple interventions for reducing risk of child lead exposure in the home, and guidelines for safe mitigation of lead paint surfaces in fair or deteriorating condition, were developed and provided to parents. The mitigation interventions used in this study were a combination of low- and medium-intensity methods previously shown to be effective in reducing high-range child BLLs (see Introduction above) including lead-encapsulating paint with required patch testing; wet-mop/wet-rag and HEPA vacuum cleaning for household dust removal; testing and removal and/or replacement of lead-contaminated consumer products; and, for soil, in situ phosphorous/other amendment stabilization and/or soil containment using fabric, net or mulch.
2.4. Family recruitment
This convenience sample of families was recruited through community outreach efforts including educational sessions at a local elementary school; child BLL screening events following Sunday church services; local health fairs; local “back-to-school” events; and a door-to-door educational campaign in downtown neighborhoods. Inclusion criteria included any family with children between the ages of 6 months and 16 years; exclusion criteria included residing outside of El Paso city limits and/or unwillingness of the child to participate in finger-stick blood sample collection. During recruitment, parents were invited to learn about child lead exposure through a brief presentation and provided with a colorful, illustrated 10-page educational booklet. Parents who received the educational presentation were invited to provide their contact information and indicate whether interested in having their child(ren) screened for lead exposure. Following each event, research staff contacted interested parents to confirm their participation, and scheduled child BLL screening.
2.5. Study overview
The study consisted of two phases. Phase I included baseline BLL testing for all children in the family, repeated at rolling 3- to 4-month intervals depending on the availability of the parents and child, for as long as the parents agreed and were available. Comprehensive education on prevention of child lead exposure in the home was reviewed with all parents enrolled for BLL monitoring. Child BLL results were made available to parents as soon as possible after testing, typically within 2–3 weeks, in the form of a written report. Families were considered for home screening and mitigation (Phase II) when a BLL of one or more children in the family exceeded 2.5 µg/dL, the lower level previously associated with central nervous system damage (see Introduction above). Within any given monitoring cycle, the families of children with the highest BLLs were offered participation first. Phase II included home screening; identification of likely child lead hazard sources; presentation and discussion of the Home Testing Report and recommendations; provision of materials as needed; and follow-up testing with additional updates and recommendations as needed.
2.6. Study phase I – home visit 1, baseline BLL testing
Parent consent and child assent were obtained, and parents were assisted with completing family demographics, home history, and child health history forms. Anthropometric data were collected for each child, after which hands were carefully cleaned following a documented 3-step cleaning method that included hand-washing, application of foam cleanser, and repeated wiping with industry-standard metal removing wipes (D-Wipes, Esca Tech, Inc., Milwaukee, Wisconsin). The fourth finger was lanced and a 50 µL blood sample was collected into sterile microvials, stored in a refrigerated container for transport back to the laboratory, and transferred to the analyzing laboratory. Results were presented to parents in confidential results brochures that also reinforced lead prevention education.
For children whose BLLs were < 2.5 µg/dL, results were delivered to parents via telephone, after which result reports were sent to parents in the mail and follow-up BLL monitoring in 3–4 months was offered. For children with blood lead levels ≥ 2.5 µg/dL, an in-person home appointment was scheduled for delivery of results and recruitment into Phase II. Discrepancies between the then-current “elevated” BLL recommended by CDC (5 µg/dL) and state guidelines were explained to parents, and parents were supported in seeking follow-up testing with their pediatrician for follow-up confirmatory BLL testing as they deemed necessary. Consistent with Texas state guidelines, a mandatory referral to a pediatrician for follow-up BLL testing was given for any child with a BLL > 10 µg/dL.
2.7. Study phase II – home visit 2
After obtaining informed consent for home screening and mitigation recommendations, additional child BLL testing was completed. A 20–30-minute interior and exterior visual inspection of the home was conducted with the parents at their convenience. Parents were asked to identify primary interior and exterior child play areas, and areas with deteriorating paint or structure, and an interior floorplan sketch within the outline of the home and property line, was created.
2.8. Study phase II – home visit 3
Prior to Home Visit 3, plans for dust wipe sample collection, pXRF interior and exterior paint testing, and pXRF exterior soil testing were made based on the size and configuration of yard. Throughout interior and exterior testing, each result was recorded on data tracking forms. Dust wipe samples and soil samples determined to be EPA designated Pb+ by pXRF were sent for confirmation by ICPMS or ICPOES (in the case of soil samples); criteria details provided in Supplementary Material, Table 3. Strategies for identifying and mitigating home lead hazard sources were guided by the environmental testing results. When the team agreed that the likely sources of child lead exposure had been identified, and a mitigation plan decided on, a Home Testing report was prepared.
2.9. Study phase II – home visit 4 and beyond
One or more team members discussed the Home Testing report and the recommendations for mitigation with the head of household. The team provided families with mitigation materials as needed (e.g., lead encapsulating paint, plastic sheeting, personal protective equipment [PPE]). Parents were informed that when mitigation was complete, their children would be re-tested, preferably within 3–4 months. Additional visits were scheduled based on the needs of the families enrolled in Phase II, for example, if one child’s test had to be rescheduled, and/or if parents required additional guidance with mitigation.
At the time of the COVID-19 city wide shut down in March of 2020, 19 families and 45 children were enrolled in Phase II of the home study. Collection of post-mitigation dust wipe “clearance” samples was interrupted by the timing of the COVID shutdowns for all but 4 of the mitigated homes.
2.10. Data management and analyses
All data were entered in Excel databases and checked for accuracy during and after entry. SPSS (IBM Version 27) was used for all descriptive and comparative analyses. The procedure for testing change in child BLLs followed recommendations for the analysis of unbalanced longitudinal data with irregular testing intervals [33]. Generalized linear models (“mixed” models) were used for all levels of model analyses. The first level model tested (only) the linear rate of change in BLL within individuals; the intercept was included as a fixed and random effect using an unstructured covariance matrix [34]. The second level model added Test Wave (1–5) and tested the rate of BLL change across children, and more specifically, whether children’s BLLs decreased significantly following education and mitigation. For additional modeling possibilities, the pattern of change over time was examined to determine whether a quadratic or cubic solution might provide better fit. While unlikely to return significant effects given the unbalanced nature of the data, third level models tested whether age group (< 5 years, 6–10 years, > 11 years) or age of house (pre-1950, 1951 – 1970, post-1970) were significantly associated with BLL change over time. In this sample, BLLs of males and females did not differ significantly (t = −0.44, df = 43, p = 0.66) and sex was not tested in regression models.
3. Results
A sample pool of 110 families with 223 children were recruited for Phase I of the study; 9 children did not provide assent at T1 BLL testing; 1 child was too young to participate (4.5 months); 2 children exceeded the enrollment age (> 16 years old); 3 children were not available during the scheduled test appointment; and 2 families could not be reached after their first visit (“lost to follow-up”). The final sample pool from which families were selected for Phase II enrollment based on current child BLL included 107 families and 206 children who completed Time 1 baseline BLL testing. The 19 homes with 45 children (19 males, 26 females) described below were identified for mitigation based on current child BLLs determined through ongoing child BLL monitoring of 206 children. A total of 21 families were offered Phase II home mitigation; enrollment compliance was 90%; 2/21 (10%) did not follow through with offered mitigation.
3.1. Child, family, and home characteristics
The child sample included 26 females and 19 males with a mean age of 7.81 years (males = 7.01 years, females = 8.72 years). As expected, baseline BLLs were positively skewed (skew=2.1, SE=0.4). Approximately 94% of the sample was of Hispanic descent. Mean (SD) child BLLs in µg/dL were 5.17 (7.5); males = 4.65 (8.5); females 5.54 (6.8) (additional clinical and demographic details provided in Supplementary Material Table 1).
The homes included 14 houses and 5 apartment units. None of the apartment dwellers (0/5) and 65% (9/14) house dwellers owned their homes. Regarding home structure age, 71% of houses (10/14) and 60% of apartments (3/5) exceeded the associated census tract home age median. The mean (SD) household size was 5.2 (1.2) for families living in houses and 4.5 (1.1) for families living in apartments. Of 19 enrolled families, 74% (14/19) provided annual income information including 71% (10/14) of house dwellers and 80% (4/5) of apartment dwellers. Following the U.S. Health and Human Services Poverty Guidelines for 2020 (updated periodically in the Federal Register by the U.S. Department of Health and Human Services under the authority of 42 U.S.C. 9902–2), those living at or below the current guideline included 29% (4/10) of house dwellers and 75% (3/4) of apartment dwellers (details provided in Supplement Table 2).
3.2. Lead hazard sources identified
Table 1 integrates child BLL data with an overview of lead hazard sources identified, mitigation provided, and changes in child BLLs. Homes are organized and numbered by home age (oldest to newest); houses are listed first followed by apartments (Table 1, column 1). Baseline child BLLs that triggered mitigation (Table 1, column 2) are shown in red; follow-up child BLLs (Table 1, column 5) that increased (rather than decreased) are shown in red.
Table 1.
Child BLLs triggering intervention, lead hazards identified, mitigation recommended, and BLL monitoring outcomes for children in mitigated homes (n = 14) and apartments (n = 5).
 | |||||||
 |
Child BLLs triggering mitigation ranged broadly from 3.1 to 27.3 µg/dL. In 4 cases, mitigation was triggered by a second (rather than first) BLL monitoring test, indicated with “*” in Table 1. (Supplementary Material Tables 3 and 4 provide quantitative and qualitative information regarding lead hazard source identification and mitigation.) At the time of the COVID shutdown (March 2020), interior and exterior home lead hazard screening had been completed for 89% of enrolled homes (16/19).
The total number of screening tests completed by pXRF included 1344 interior paint locations; 142 dust wipe samples; and 189 exterior paint locations. Positive soil and dust pXRF results were confirmed by ICPMS. Soil testing was indicated by pXRF for 14/19 homes from which a total of 124 soil samples were collected and tested. With regard to water testing, all enrolled homes were served by the city water utility which takes many precautions to ensure that home water supplies are free of lead contamination, including regular comprehensive testing and water treatment. Tap water was tested in our study only when possible lead hazard sources were ambiguous (H3, H7, H10). Tested water samples from these homes were negative for lead contamination.
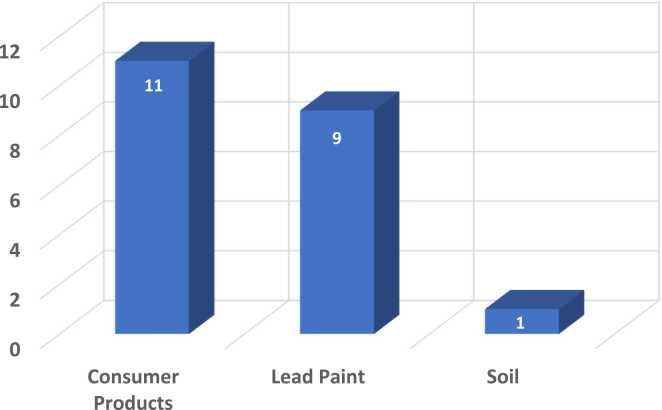
The potential sources of lead identified included interior and exterior lead paint, soil, water, and/or consumer products (details shown in Supplementary Material Table 3). Fig. 1 shows the numbers of different lead sources found in mitigated homes; Fig. 2 shows the numbers of different types of lead hazards found. In 44% of homes (7/16) child lead exposure appeared to be attributable to 1 lead hazard source. In 5 of these homes (H10, H11, H12, H13, A3) child lead exposure was likely attributable to 1 or more consumer products, including toys, pottery/cookware, mini-blinds, and/or consumption of Mexican candies. In the other 2 homes, lead paint was the single source identified (H4, A1). In 38% of homes (6/16), 2 lead hazard sources were identified including consumer products (Mexican dinnerware and deteriorating vintage door knobs) and soil with high bioaccessibility (H2); lead paint and sanded floor shellac (H3); lead paint and consumer products (mini-blinds) (H8); consumer products (toys) and parent occupation (A4); and lead paint and consumer products (toys) (H6, A2). In 13% of homes (2/16), 3 likely sources were identified including lead paint, soil with moderate bioaccessibility, and consumer products (Mexican candies) (H9); lead paint, consumer products (toys and Mexican candies), and parent occupation (car shop) (H7); in one home, 4 sources were identified including lead paint, sanded old shellac, soil with high bioaccessibility, and consumer products (mini-blinds) (H1).
Fig. 1.
Frequency of the number of lead hazard sources identified in mitigated homes (n = 16).
Fig. 2.
Frequency of specific lead hazard sources identified in mitigated homes (categories are not mutually exclusive).
3.2.1. Consumer products
Considering the individual sources of child lead exposure, consumer products were a likely source of child lead exposure in 69% (11/16) of homes, including 10/14 houses and 3/5 apartments. Children’s toys and child-specific products (mugs designed for children) were found in 26% (5/19) of homes (H6, H7, H13, A2, A4). Parents from 3 homes (H7, H9, A3) reported that children ate Mexican candies, a known source of lead exposure [35] due to leaching of lead paint from brightly colored candy wrappers, or the use of lead-contaminated chilis. Deteriorating plastic miniblinds testing as high as 3000 ppm were found and removed from 2 homes (H1 and H8). High Pb positive dishware (traditional pottery used as dinnerware and traditional Mexican beanpots) were found in 2 homes (H2, H11), and inexpensive cookware that had been removed from the home and could not be tested was a suspected child lead hazard source in 1 home (H12).
3.2.2. Lead paint
Deteriorating lead paint was the second most prevalent likely source of child lead exposure, found in 50% of homes (8/16) and all of these homes were built before 1970 (H1, H3, H4, H6, H7, H8, H9, A2). In each of these homes, dust wipe samples were positive for Pb. In 44% of the remaining homes found to be free of lead paint (7/16), dust wipe samples were uniformly negative for Pb. In one home (A1), bioaccessible soil lead appeared to be the sole source of Pb dust wipe samples and elevated child BLLs.
Deteriorating lead paint was prevalent throughout the home in only 1 case (H1, built in 1916 and undergoing a parent-initiated do-it-yourself renovation at the time the family contacted the study team). In all of the other homes, lead paint in fair/deteriorating condition was found on sporadic isolated surfaces, such as a child’s bedroom windowsill used as a toy shelf and adjoining the bed; chipping interior door frames; or a chipping exterior window sashes on windows that were opened in warm weather. With regard to exterior paint, 29% of homes (5/17) had sporadic isolated exterior locations positive for lead paint with fair or deteriorating surfaces, and all were built before 1955 (H1, H3, H4, H6, H7).
3.2.3. Lead concentration in soil
For the purposes of this study, > 400 ppm (mg/kg) was considered excessive Pb, and 100–399 ppm was considered “elevated” Pb. Soil sampling and testing were interrupted by COVID shutdowns in 4/19 homes, and in 1 additional home (A3) there were no soil areas to test. Soil sampling was indicated and completed for the remaining 14 homes. While only 1 home (H1) was found to have an excessive Pb soil lead concentration (591 ppm), elevated soil lead concentrations (between 100 and 336 ppm) were found in 36% of homes tested (5/14). The bioaccessibility of Pb in 4 of 5 homes with elevated levels was analyzed. Based on the bioaccessibility of these samples, soil lead appeared to be a plausible source of child lead exposure (H1, H2, H9, A1). In one home (A1) with Pb positive dust wipe samples, soil lead with bioaccessibility estimated to be from 49% to 60% was the only plausible source of child lead exposure identified. In the remaining 3 homes, bioaccessible soil lead co-occurred with deteriorating lead paint.
3.3. Mitigation
While the specific types of mitigation offered for each type of lead hazard source was consistent across families, the mitigation process was determined on a case-by-case basis (details in Supplementary Material Table 4). For example, in the case H1, educational intervention was started immediately during the first phone contact, to ensure that the parent understood the gravity of the situation and immediately halted and contained the DIY project; the team guided the parent in cleaning and containing the interior to the greatest extent possible using appropriate PPE. In another home, a single mother ended up requiring the assistance of the study team to complete the application of lead encapsulating paint (which delayed follow-up testing).
The timing of COVID shutdowns interrupted post-mitigation dust wipe sample collection and testing for 12 of 16 homes that completed interior and exterior screening. With regard to the 4 homes for which post-mitigation dust wipe sample testing was completed, in 2 homes with Pb positive screening dust wipe samples (H1, H8), post-mitigation dust wipe samples were negative for lead. In 2 homes with initial negative screening dust wipe samples (H7, H10) post-mitigation dust wipe samples remained Pb negative (Fig. 2).
3.4. Post-mitigation change in children’s BLLs
Of 19 mitigated homes, 47% (9/19) completed at least 3 BLL tests before pandemic interruption. In 89% of these homes (8/9), children’s BLLs decreased. In one home (H7), BLLs decreased gradually over 4 tests, and at an additional testing (T5), the child’s BLL increased to 7.5 µg/dL (it was suspected but not confirmed that the child may have resumed spending time in his father’s car shop). Among the remaining 10 homes for which follow-up testing was interrupted by the pandemic, 60% (6/10) completed 2 tests, and in those homes, child BLLs for 50% (3/6) decreased; in 2 of the 3 remaining homes, BLLs simultaneously increased and decreased across children. In 1 home, the child’s BLL increased from T1 to T2 testing. The diminishing completion across waves was due to the COVID shutdown and not study attrition. Fig. 3 shows the change in child BLLs over time. The observed increase at Test Wave 5 was attributable to one male below the age of 5 who may have returned to playing in his father’s car shop; and one female who may have resumed eating Mexican candies. (Supplementary Material Table 5 provides follow-up BLL testing details.) Overall, the change trend was a linear downward slope, and quadratic or cubic solutions were not tested. Generalized linear models (linear mixed models, SPSS) and a 3-level approach (see Methods) were used. The results for all model results are summarized in Table 2.
Fig. 3.
BLL means (µg/dL) by test wave and child age group (n).
Table 2.
Generalized linear (“mixed”) model results testing change in child BLLs over time.
| MODEL | Information Criteria (smaller is better) |
Type III Tests of Fixed Effects |
Estimates of Fixed Effects |
Estimates of Covariance Parameters |
|---|---|---|---|---|
| Baseline Intercept Only BLL Variability |
-2 Log Likelihood 753.30 Akaike’s Information Criterion (AIC) 759.30 |
Intercept F = 45.32 df= 1/56, p < 0.001 |
Intercept est. 3.52 SE = 0.52 95% CI (2.47/ 4.57) t = 6.73, df= 56, p < 0.001 |
|
| Linear Change Model (Unconditional) Test Wave |
-2 Log Likelihood 723.60 Akaike’s Information Criterion (AIC) 735.60 |
Intercept, F = 26.46 df= 1/44, p < 0.001 Test Wave, F= 9.76 df= 1/25, p = 0.005 |
Intercept est 5.84 SE= 1.14 95% CI (3.56/8.13) t = 5.14 df= 44, p < 0.001 Test Wave est − 1.09 SE= 0.35 95% CI (−1.81/−0.37) t = −3.12 df= 25, p = 0.005 |
Test Wave/Intercept Covariance Parameter est = −7.71 SE= 3.52 95% CI (−14.61/−0.80) Wald Z = −2.19, p = 0.029 |
| Linear Change Model (Conditional) Test Wave AgeGroup HomeAge |
-2 Log Likelihood 710.12 Akaike’s Information Criterion (AIC) 730.12 |
Intercept, F = 27.53 df= 1/82, p < 0.001 Test Wave, F = 7.93 df= 1/32, p = 0.008 AgeGroup, F = 2.30 df= 2/4, p = 0.219 HomeAge F= 4.69 df= 2/3, p = 0.130 |
3.4.1. “No predictor” model of within-subject change over time
The first model provided a baseline assessment of BLL variability for individuals without regard to time, that is, the differences between each child’s mean BLL and the estimated sample mean. The inter-correlation coefficient (ICC, [Intercept/Residual+Intercept], [2.94/24.14 +2.94] = 11% suggested an estimated 11% of the total variability in BLLs was attributable to differences between subjects. This relatively low level of variability was expected given the overall positive skew distribution of child BLLs.
3.4.2. BLL change over time (unconditional) model
The second model added only the variable “time” as a random effect. This model tested whether the rate of change over time was statistically significant. Consistent with the primary hypothesis of the study, the model suggested that children’s BLLs decreased after the initiation of mitigation and over the course of the study. The specific association between the intercept and Test Wave (Est = −7.71, column 5) suggested that children with higher initial BLLs had a slower decrease in BLL while children with lower initial BLL levels decreased more rapidly.
3.4.3. BLL change over time with additional factors (conditional) model
In the final model we examined the possible contribution to BLL change over time of two additional factors, age of home (3 categories) and age group (3 categories). As shown in Fig. 3, BLLs of children below the age of 6 years tended to be higher and stay higher over the course of the study, as compared to children over the age of 6 years. Neither of these factors contributed significantly to BLL change over time.
4. Discussion
The CDC revisions of “elevated” child BLL standards, from 10 to 5 µg/dL in January 2012, and from 5 to 3.5 µg/dL in October 2021, were major steps towards improving the health of particularly lower-income children nationwide. At the same time, these changes have substantially altered the magnitude of the challenge for mitigation. No interventions have yet been developed to protect children already exposed to lead, and new strategies are needed for identification and removal of home lead hazard sources, and that reduce children’s BLLs. Interventions must be safe, cost-effective, and accessible to the average parent. This study was undertaken to test an interdisciplinary team-based strategy that used ongoing child BLL monitoring to identify homes for screening and mitigation, with the goal of reducing lower-range child BLLs. Prior mitigation studies [26], [27], [28] have focused on reducing child BLLs that were higher than those observed in this study. This study focused primarily on children with lowest-range BLLs. The results are valuable for considering the most common sources contributing to dangerous lower-range child BLLs; and as a demonstration that even lowest-range child BLLs can be reduced; that sources can be readily identified in home environments; and very importantly, that safe, low-cost parent-deployed mitigation, is effective in lowering children’s BLLs.
A central feature of this study was the use of current child BLLs to guide home mitigation, which included ongoing child BLL monitoring for children and adolescents enrolled in Phase I (Methods, page 9). The study included an intentionally broad age range from 6 months to 16 years, because there is abundant clinical evidence (see Introduction above) showing that younger children, middle-school aged children, and adolescents are vulnerable to cognitive and/or motor function damage from lead exposure. Ongoing BLL monitoring is unquestionably necessary because lead absorption, storage, and redistribution via the lungs and/or gut, and thus the amount of detectable lead in a child’s blood at any given point in time, involves innumerable complex interacting physiological mechanisms that drive variability in children’s BLLs over time [36]. One or two BLL tests administered to only youngest children cannot be assumed to be sufficient for identifying lead poisoning, particularly for children living in high-risk neighborhoods. Importantly, this study used only ICPMS for determination of child BLLs to ensure accurate and precise BLL estimates in lowest BLL ranges. While the overall trend in BLLs was towards reduction, individual children’s BLLs fluctuated, demonstrating the need for ongoing monitoring throughout childhood, particularly for children living in high-risk neighborhoods.
4.1. Common sources of lead exposure for children with dangerous lower-range BLLs
4.1.1. Consumer products
The majority of homes in the targeted neighborhoods were built before 1978 when federal laws banning lead paint for homes were first enacted, and the team had prepared to target lead paint as a primary source of child lead exposure. While deteriorating lead paint was found in many homes, in an even larger proportion of homes, lead-contaminated consumer products were the most likely sources of child lead exposure. In fact, since at least 1991 [2] information regarding lead poisoning in U.S. children has emphasized the ubiquity of lead in our modern environments. In recent decades, lead production has continued to increase worldwide, dropping slightly only during the COVID pandemic in 2020, to 11.7 million metric tons [3]. Apparent lead consumption in the U.S. in 2020 was estimated to be 1,520,000 metric tons [5]. The lead contaminated products we identified as primary sources of child lead exposure ranged from children’s toys and mugs, to traditional pottery, decorative dinnerware, deteriorating plastic mini-blinds, and a tile-top table. The levels of lead in some of these products exceeded 3000 ppm.
The prevalence of lead in toys tested for this study was of particular concern. Consumer item recalls may warn the public, but apparently little to nothing can be done to ensure that lead contaminated products do not reappear in the marketplace. In two homes, we identified older “Thomas the Tank Engine” locomotives, recalled in 2007, as the only likely lead hazard sources (1500–3000 ppm, BLLs of 4 children, 4.1–12.3 µg/dL). A disturbing revelation was that new replacement toys of the same brand purchased from Walmart, screened for lead via pXRF and ICPMS, were found to have lead concentrations equivalent to or higher than the older recalled toys. In other homes, a variety of metal cars and trucks, including some Hot Wheels brand cars, had high lead content. Mexican made candies were also a significant source in our study, and despite repeated consumer warnings, some parents in our study were still unsure as to whether Mexican candies should be avoided.
There is no simpler nor more “cost-effective” solution for reducing children’s BLLs than removing lead-contaminated objects from a child’s environment. Knowing when to do so however requires ongoing child BLL monitoring. Until removal of lead from children’s environments accomplishes the ultimate goal of primary prevention, universal child BLL monitoring of children living in highest risk neighborhoods is currently the only approach that can ensure timely detection of lead poisoning in children requiring secondary prevention of effects from lead poisoning. A feasible approach for conducting wide-scale bi-annual universal monitoring has been described [37].
4.1.2. Lead paint
As expected, lead paint was found in many of the homes tested, but perhaps not in ways that were anticipated. Lead paint and lead contaminated dust were pervasive in only one home (the DIY renovation). In the rest of the homes, lead paint and Pb positive dust wipe samples were found in isolated locations, for example, a single wall, or on isolated sections of trim features such as door jambs, window casings, and/or one or more windowsills/troughs. In some cases, exterior deteriorating lead paint on window casings became a serious child lead hazard within the home. When deteriorating windows were opened and closed, deteriorating lead particles fell onto the aging, uneven surfaces of exterior windowsills and into window troughs, and could then be carried onto interior windowsills and into rooms via air currents. Finding and mitigating these isolated sources reduced dangerous lower-range child BLLs.
We noted that all homes with deteriorating lead paint also had dust wipe samples positive for lead and as expected, dust wipe samples were useful, valid “first-level” indicators of lead paint issues. Importantly, the pXRF device provides “depth” estimates for lead paint. This is a critical metric to share with parents. While lead paint “at depth” coated by non-leaded paint on surface layers, does not pose a child lead hazard risk, parents need to know where lead paint exists at depth, so surfaces can be maintained and not disrupted through DIY renovation or home improvement projects, for example.
4.1.3. Soil
Soil lead concentrations can be difficult to estimate and interpret, and safety guidelines and suggestions vary. Lead exists naturally in soil and there is broad agreement that levels below approximately 50 ppm are considered “normal” and not a source of health risks for children [38]. The current US EPA guidelines suggest that > 400 ppm soil lead constitutes “risk,” while lead concentrations in gardening soil to which children are exposed should fall below 100 ppm due to risk of contaminated soil ingestion [39]. The results of this study suggested that the possible dangers to children of lower-range soil lead hazards above “normal” (approximately 50 ppm) but below the 400 ppm “excessive” benchmark should not be discounted.
As one result in this study may suggest, a critical needed refinement is the estimation of not simply lead concentration, but assessment of the bioaccessibility of the lead source. Doing so may substantially increase our accuracy for estimating risk to child health from soil lead. For the purposes of this study focusing on dangerous lower-range child BLLs, we designated soil lead concentrations between 100 and 399 ppm as “elevated,” and tested these samples for bioaccessibility. In 5 homes, lead concentrations between 100 and 399 were found; in 4 of these homes, deteriorating lead paint was also found. In 1 home (A1), relatively low concentration soil lead (83–209 ppm) found to be 49–60% bioaccessible, was the only plausible source of elevated Pb in interior dust wipe samples and child lead exposure. Few homes in this study had soil lead concentrations of concern and many more studies are needed to clarify the relationships between soil lead bioaccessibility at different soil lead concentrations, and the impact of these on dangerous lower-range child BLLs. If these preliminary findings are supported, revisions to national guidelines that account for soil lead bioaccessibility could be needed.
4.1.4. Water
The city water utility in El Paso, Texas carries out many precautions to ensure that the residential city water supply is safe for human consumption. Monthly and annual tests are conducted and posted online, and comprehensive water treatment ensures that lead does not leach into the water supply from older pipe infrastructure. From the start, this study planned to test water only if all other possible sources of child lead exposure had been ruled out. Water was tested in 3 homes where lead hazard sources were ambiguous, and lead was not identified in any of the samples collected. As was demonstrated in Flint, Michigan however, poorly considered operational decisions can suddenly reverse favorable conditions, with massive impacts for thousands of children. Any program to reduce child lead exposure, particularly in high-risk neighborhoods, needs to begin by considering the water infrastructure, pipeline material, public reporting, and current preventative actions of the city water utility. Orthophosphate added to the water supply can limit lead leaching in old pipes, however debate continues regarding optimal water treatment methods [40], [41], [42]. Again, in order to identify possible changes in lead absorption among children, repeated BLL testing must be conducted, and should be considered the gold standard for prevention of lead related health threats. For example, instituting universal bi-annual child BLL monitoring would significantly improve our capacity for detection and surveillance.
4.2. Mitigation strategy
The mitigation approach that we tested in this study included three elements that perhaps distinguish it from previous efforts, with the goal of substantially improving the feasibility, cost-effectiveness and most importantly, short- and long-term efficacy of home lead hazard mitigation. These elements included: 1) a coordinated interdisciplinary team of experts in public health promotion and parent education; child lead exposure and testing; and environmental screening and home mitigation; 2) mitigation guided by ongoing monitoring of child BLLs measured by ICPMS to ensure accurate and precise lower-range BLL estimation; and 3) repeated and reinforced one-on-one parent education, detailed home testing reports, and training in home lead hazard sources coupled with home screening and safe, no-cost/low-cost home mitigation interventions.
4.3. Motivate to mitigate
All of the parents in this study were highly motivated to protect their children from harmful environmental exposures. At the same time, motivation to change must be cultivated through knowledge coupled with access to low-cost materials and methods, and guidance for their safe and effective use. Through all phases of this study, building parental knowledge served as the foundation for motivating parents to adopt primary and secondary prevention measures. Education began at recruitment, with the presentation of colorful, illustration-based educational materials presented in a one-on-one format, and that included pictures of potentially lead-contaminated consumer products that were unique to the culture of the region. The booklets showed the possible effects of lead exposure on children; possible sources of child lead exposure in the home; nutritional factors that could help to limit lead absorption; no-cost cleaning methods for reducing the accumulation of contaminated household dust; and safe, low-cost mitigation for lead paint and lead contaminated soil.
Education continued and was reinforced through discussion and conversation during the home walk-through screening; as the team completed diagnostic home questionnaires; and with the return of each child’s most recent BLL testing result. Education was fine-tuned and expanded as needed to address the mitigation needs of individual families. A second component that served to educate and motivate parents were the home testing reports provided to each family (anonymized sample available upon request). The reports gave an easy-to-interpret summary of the home findings, detailed results of all of the testing completed, detailed step-by-step recommendations for mitigation of whatever child lead hazard sources had been identified, and detailed “safety-first” instructions for carrying out each recommendation. The importance of providing parents with low-cost materials and the guidance to use them should not be under-estimated. When lead paint was an issue, the study provided lead encapsulating paint, appropriate PPE, and other materials as needed (e.g., plastic sheeting, masking tape). For the two homes in which soil was an issue, the study team provided phosphate soil amendment and/or landscaping materials to contain the topsoil.
Parents were highly receptive to this multi-phased, supportive approach. Compliance with the offered mitigation (90%) exceeded our predictions. Virtually no parent was oblivious to child lead exposure, many parents had seen “some brochure” on child lead exposure and potential lead hazard sources, but nearly all parents lacked critical details regarding the specific risks and prevention, and “real-life” examples. In some homes, dust accumulation was notable. For these homes, “no-cost” methods of wet-dusting and wet-mopping, leaving shoes at the door, frequent handwashing, brushing soil off of pets, and lead-safe precautionary cleaning actions for those in occupations that may expose parents to lead in the workplace (e.g., car repair shops, factories, outdoor work in highly polluted areas), were emphasized.
We noted that it can be difficult for parents to believe that everyday consumer objects can be main sources of ongoing child lead exposure. More specifically, parents had difficulty believing that seemingly “high-quality” children’s toys, bought at well-known common retail outlets may be exposing their children to dangerous amounts of lead; that a favorite candy was potentially toxic; that miniblinds present in a home for decades, were contaminating household dust with lead; or that lead contamination from a parent’s workplace could pose serious health dangers for their children.
Compliance in this study was high and likely attributable to our focus on parent engagement. Relationships with working parents living in high-risk neighborhoods must be built over time through repeated contact. Parents need support to process the implications of the information provided, to question the information provided, and eventually, to become motivated to take action, and importantly, maintain efforts needed in a particular home. Repeated home visits with ongoing child BLL monitoring ensured multiple opportunities for keeping parents engaged and moving forward with home lead hazard reduction. The costs and feasibility of this approach have been described in detail [37].
4.4. Limitations
This study was conducted in a largely white Hispanic sample and the relevance of the results for other cultural and/or ethnic subgroups cannot be assumed. Also, the relevance of the tested strategy and results for countries other than the U.S. in which higher child BLLs may be common, remains to be seen. As was true for most community-based research programs, data collection for these studies were stopped by the COVID-19 global pandemic. The shutdowns interrupted child BLL testing, reducing the numbers of children in the study who completed the full set of a minimum of 4 BLL tests, and reducing the total number of homes that could be completed. Using all available data, generalized linear model analyses demonstrated a statistically significant reduction in child BLLs. We interpret these findings with caution, and with the understanding that the results require replication with larger samples of subjects.
5. Conclusions
With regard to lead exposure that yields dangerous lower-range child BLLs, this study demonstrated that single and multiple sources in the home environment can be effectively identified and mitigated. Ongoing child BLL monitoring, one-on-one repeated and reinforced parent education and training coupled with targeted screening and mitigation of the home environment, significantly reduced child BLLs in children of all ages. In most cases, it appeared that dangerous lower-range child BLLs were attributable to multiple isolated low-level sources that, once identified, could be safely, quickly, inexpensively, and effectively mitigated.
It is important to note that incorporating ongoing child BLL testing as the basis for mitigation ensured feasibility by targeting resources according to children’s current lead exposure status. Just as importantly, using ICPMS (an alternative approach could be GFAAS with a lower detection limit of < 0.2 µg/dL), was essential for accurate and precise monitoring of decreases and increases in child BLLs. Also, elevated BLLs in children were somewhat dynamic over time; one or two BLL tests for only youngest children at discrete points in time is not sufficient for solving the problem of lead poisoning in U.S. children, nor is the use of analytic methods that do not provide accurate, precise estimates of dangerous lower-range BLLs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by U.S. Department of Housing and Urban Development, Grant No. TXLTS-10018-01, USA, and U.S. Environmental Protection Agency, Grant No. 01F67301, USA, and University of Texas System, J. Edward and Helen M. C. Stern Professorship, award to C. Sobin, (no Grant No.), USA.
Handling Editor: Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.12.004.
Appendix A. Supplementary material
Supplementary material.
.
Supplementary material.
.
Supplementary material.
.
Supplementary material.
.
Supplementary material.
.
Data availability
The data that has been used is confidential.
References
- 1.P.N. Breysse, Lead elimination for the 21st century, J. Public Health Manag. Pract., Lead Poisoning Prevention (Suppl 1. Lead Poisoning Prevention), 2019; 25(Suppl 1.), pp. S3–S4. [DOI] [PMC free article] [PubMed]
- 2.Centers for Disease Control and Prevention, Preventing Lead Poisoining in Young Children, U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 1991.
- 3.Garside M. Statista, Inc.; New York, NY: 2020. Mine Production of Lead in the Leading Countries Worldwide, 2010-2020. [Google Scholar]
- 4.Gottesfeld P. Time to ban lead in industrial paints and coatings. Front. Public Health. 2015;3:144. doi: 10.3389/fpubh.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.K. Klochko, Mineral Industry Surveys: Lead, U.S. Geological Survey, Reston, VA, 2021.
- 6.Jones R.L., Homa D.M., Meyer P.A., et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988-2004. Pediatrics. 2009;123(3):e376–e385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- 7.Levin R., Brown M.J., Kashtock M.E., et al. Lead exposures in U.S. Children, 2008: implications for prevention. Environ. Health Perspect. 2008;116(10):1285–1293. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClure L.F., Niles J.K., Kaufman H.W. Blood lead levels in young children: US, 2009-2015. J. Pediatr. 2016;175:173–181. doi: 10.1016/j.jpeds.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim K.-N., Kwon H.-J., Hong Y.-C. Low-level lead exposure and autistic behaviors in school-age children. NeuroToxicology. 2016;53(Suppl. C):S193–S200. doi: 10.1016/j.neuro.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Sobin C., Flores-Montoya M.G., Gutierrez M., Parisi N., Schaub T. δ-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD2) and peptide transporter 2*2 haplotype (hPEPT2*2) differently influence neurobehavior in low-level lead exposed children. Neurotoxicol. Teratol. 2015;47(0):137–145. doi: 10.1016/j.ntt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasten-Jolly J., Lawrence D.A. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol. Appl. Pharmacol. 2017;334:142–157. doi: 10.1016/j.taap.2017.09.009. Epub Sep 11. [DOI] [PubMed] [Google Scholar]
- 12.Basgen J.M., Sobin C. Early chronic low-level lead exposure produces glomerular hypertrophy in young C57BL/6J mice. Toxicol. Lett. 2013;225(1):48–56. doi: 10.1016/j.toxlet.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Wen X.W., Faulk C., et al. Perinatal lead exposure alters gut microbiota composition and results in sex-specific bodyweight increases in adult mice. Toxicol. Sci. 2016;151(2):324–333. doi: 10.1093/toxsci/kfw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G., DiBari J., Bind E., et al. Association between maternal exposure to lead, maternal folate status, and intergenerational risk of childhood overweight and obesity. JAMA Netw. Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.12343. e1912343-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M.B. Pell, J. Schneyer, The Thousands of U.S. Locales Where Lead Poisoning Is Worse than in Flint, 2016 (Accessed 19 December 2016). 〈https://www.reuters.com/investigates/special-report/usa-lead-testing/〉, (Accessed 30 November 2022).
- 16.Roberts E.M., Madrigal D., Valle J., King G., Kite L. Assessing child lead poisoning case ascertainment in the US, 1999–2010. Pediatrics. 2017 doi: 10.1542/peds.2016-4266. [DOI] [PubMed] [Google Scholar]
- 17.J. Schneyer, M.B. Pell, Millions of American Children Missing Early Lead Tests, Reuters Finds, Reuters Investigates: Reuters.com, 2016. 〈https://www.reuters.com/investigates/special-report/usa-lead-testing/〉, (Accessed 30 November 2022).
- 18.Kamai E.M., Daniels J.L., Delamater P.L., Lanphear B.P., MacDonald Gibson J., Richardson D.B. Patterns of children's blood lead screening and blood lead levels in North Carolina, 2011-2018-who is tested, who is missed? Environ. Health Perspect. 2022:67002. doi: 10.1289/EHP10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knighton A.J., Payne N.R., Speedie S. Lead testing in a pediatric population: underscreening and problematic repeated tests. J. Public Health Manag. Pract. JPHMP. 2016;22(4):331–337. doi: 10.1097/PHH.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 20.Clark S., Galke W., Succop P., et al. Effects of HUD-supported lead hazard control interventions in housing on children's blood lead. Environ. Res. 2011;111(2):301–311. doi: 10.1016/j.envres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Dixon S.L., Jacobs D.E., Wilson J.W., Akoto J.Y., Nevin R., Scott, Clark C. Window replacement and residential lead paint hazard control 12 years later. Environ. Res. 2012;113:14–20. doi: 10.1016/j.envres.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Dixon S.L., Wilson J.W., Scott Clark C., Galke W.A., Succop P.A., Chen M. Effectiveness of lead-hazard control interventions on dust lead loadings: findings from the evaluation of the HUD Lead-Based Paint Hazard Control Grant Program. Environ. Res. 2005;98(3):303–314. doi: 10.1016/j.envres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Dixon S.L., Wilson J.W., Succop P.A., et al. Residential dust lead loading immediately after intervention in the HUD lead hazard control grant program. J. Occup. Environ. Hyg. 2004;1(11):716–724. doi: 10.1080/15459620490520792. [DOI] [PubMed] [Google Scholar]
- 24.Galke W., Clark S., McLaine P., et al. National evaluation of the US Department of Housing and Urban Development Lead-Based Paint Hazard Control Grant Program: study methods. Environ. Res. 2005;98(3):315–328. doi: 10.1016/j.envres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Galke W., Clark S., Wilson J., et al. Evaluation of the HUD lead hazard control grant program: early overall findings. Environ. Res. 2001;86(2):149–156. doi: 10.1006/enrs.2001.4259. [DOI] [PubMed] [Google Scholar]
- 26.Wilson J., Pivetz T., Ashley P., et al. Evaluation of HUD-funded lead hazard control treatments at 6 years post-intervention. Environ. Res. 2006;102(2):237–248. doi: 10.1016/j.envres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Dixon S.L., Wilson J.W., Clark C.S., Galke W.A., Succop P.A., Chen M. The influence of common area lead hazards and lead hazard control on dust lead loadings in multiunit buildings. J. Occup. Environ. Hyg. 2005;2(12):659–666. doi: 10.1080/15459620500403737. [DOI] [PubMed] [Google Scholar]
- 28.Schoof R.A., Johnson D.L., Handziuk E.R., et al. Assessment of blood lead level declines in an area of historical mining with a holistic remediation and abatement program. Environ. Res. 2016;150 doi: 10.1016/j.envres.2015.12.028. 582-91. [DOI] [PubMed] [Google Scholar]
- 29.Obeng A.B., Del Rio M., Costa C., et al. Validity of a portable X-ray fluorescence device for analyzing field dust wipe samples for lead. Int. J. Environ. Sci. Technol. 2022 [Google Scholar]
- 30.US Environmental Protection Agency, Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, 2022.
- 31.Ruby M.V., Davis A., Schoof R., Eberle S., Sellstone C.M. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 1996;30(2):422–430. [Google Scholar]
- 32.Bazzi A., Nriagu J.O., Linder A.M. Determination of toxic and essential elements in children's blood with inductively coupled plasma-mass spectrometry. J. Environ. Monit. JEM. 2008;10(10):1226–1232. doi: 10.1039/b809465a. [DOI] [PubMed] [Google Scholar]
- 33.Shek D.T., Ma C.M. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. Sci. World J. 2011;11:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr D.J., Levy R., Scheepers C., Tily H.J. Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 2013;68:3. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamayo-Ortiz M., Sanders A.P., Rosa M.J., et al. Lead concentrations in mexican candy: a follow-up report. Ann. Glob. Health. 2020;86(1):20. doi: 10.5334/aogh.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Rio M., Sobin C., Hettiarachchi G.M. Biological factors that impact variability of lead absorption and BLL estimation in children: implications for child blood lead level testing practices. J. Environ. Health. 2022 (In press) [Google Scholar]
- 37.Sobin C., Gutierrez-Vega M., Flores-Montoya G., et al. Improving equitability and inclusion for testing and detection of lead poisoning in U.S. children. Milbank Q. 2022 doi: 10.1111/1468-0009.12596. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soil and Plant Nutrient Testing Laboratory UoM, Soil Lead: Testing, Interpretation, and Recommendations, University of Massachusetts, Amherst, Amherst, MA, 2020.
- 39.U.S. Environmental Protection Agency RI, Lead in Soil: U.S. Environmental Protection Agency, 2020.
- 40.Klučáková M., Pavlíková M. Lignitic humic acids as environmentally-friendly adsorbent for heavy metals. J. Chem. 2017;2017 [Google Scholar]
- 41.Pelley J. Treatment for lead in drinking water evolves. CEN Glob. Enterp. 2018;96(47):18–19. [Google Scholar]
- 42.Trueman B.F., Gregory B.S., McCormick N.E., et al. Manganese increases lead release to drinking water. Environ. Sci. Technol. 2019;53(9) doi: 10.1021/acs.est.9b00317. 4803-12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary material.
Supplementary material.
Supplementary material.
Supplementary material.
Data Availability Statement
The data that has been used is confidential.