Abstract
During Pneumocystis carinii pneumonia (PCP) in mice, the degree of pulmonary inflammation correlates directly with the severity of lung function deficits. Therefore, studies were undertaken to determine whether the host inflammatory response contributes to PCP-related respiratory impairment, at least in part, by disrupting the pulmonary surfactant system. Protein and phospholipid content and surfactant activity were measured in the lavage fluid of infected mice in either the absence or presence of an inflammatory response. At 9 weeks postinfection with P. carinii, nonreconstituted SCID mice exhibited no signs of pulmonary inflammation, respiratory impairment, or surfactant dysfunction. Lavage fluid obtained from these mice had protein/phospholipid (Pr/PL) ratios (64% ± 4.7%) and minimum surface tension values (4.0 ± 0.9 mN/m) similar to those of P. carinii-free control mice. However, when infected SCID mice were immunologically reconstituted, an intense inflammatory response ensued. Pr/PL ratios (218% ± 42%) and minimum surface tension values (27.2 ± 2.7 mN/m) of the lavage fluid were significantly elevated compared to those of the lavage fluid from infected, nonreconstituted mice (P < 0.05). To examine the specific role of CD8+ T-cell-mediated inflammation in surfactant dysfunction during PCP, mice with defined T-cell populations were studied. P. carinii-infected, CD4+-depleted mice had elevated lavage fluid Pr/PL ratios (126% ± 20%) and elevated minimum surface tension values (16.3 ± 1.0 mN/m) compared to normal mice (P < 0.05). However, when infected mice were additionally depleted of CD8+ cells, Pr/PL ratios were normal and surfactant activity was improved. These findings demonstrate that the surfactant pathology associated with PCP is related to the inflammatory process rather than being a direct effect of P. carinii. Moreover, CD8+ lymphocytes are involved in the mechanism leading to surfactant dysfunction.
Pneumocystis carinii pneumonia (PCP) is a life-threatening opportunistic infection of the immunocompromised host. The clinical hallmark of PCP at presentation is tachypnea and hypoxia. Few rales are present upon auscultation of the chest, and X ray shows a diffuse pattern of alveolar disease. The mechanism by which P. carinii produces this clinical picture is poorly understood. One possible contributor to the constellation of signs and symptoms of PCP is direct or indirect disruption of the pulmonary surfactant system. Surfactant is a macromolecular complex of various phospholipids and specific proteins that regulates surface tension at the air-liquid interface within the alveolus. Surfactant-mediated reduction and variation of surface tension stabilize alveolar inflation and deflation behavior and reduce the work of breathing. Loss of surfactant activity results in decreased lung compliance, atelectasis, and impaired gas exchange.
There have been several experimental observations that suggest that P. carinii has the potential to cause physiological disruption of the surfactant system. Sheehan et al. (43) initially described a decrease in surfactant phospholipids in rats with PCP, and alterations in surfactant composition have also been noted in humans with PCP (9, 17, 46). P. carinii and at least one specific cell wall component of P. carinii, gpA (major surface glycoprotein, gp120), have also been shown to bind to surfactant components. For example, surfactant protein (SP)-A has homology with mannose-binding proteins and has lectin-like activity (39). Since gpA is a mannosylated glycoprotein, an interaction between these two molecules would be expected and has been demonstrated in vitro (31, 59). SP-D, which may also play a role in surfactant homeostasis, also binds to P. carinii (33). In addition to interacting physically with surfactant proteins, P. carinii and P. carinii gpA have been shown to interfere with synthesis and secretion of various surfactant components by alveolar cells (4, 29, 36). Furthermore, there have been reports of functional impairment of surfactant activity in steroid-treated rats (52) and severe combined immunodeficient (SCID) mice (3) with PCP.
We have been using the SCID and CD4+ T-cell-depleted mouse models of PCP to examine the inflammatory processes occurring during disease (57). The advantage of these models is that they avoid the use of corticosteroids which may have confounding physiological and immunologic effects, including an increase in surfactant lipid production (58). Furthermore, the availability of well-characterized mouse-specific immunologic reagents allows for more-precise definition of humoral and cellular events taking place in response to infection by P. carinii. This report describes experiments analyzing surfactant composition and function in the lavage fluid of different groups of P. carinii-infected mice which vary in their ability to mount an inflammatory response against the organism. We chose to examine whole lavage fluids rather than fractionated surfactant preparations so that the contribution of inflammation-associated inhibitory components present in the lavage fluid could be assessed. Nonreconstituted SCID mice provided a model to study the direct effects of P. carinii on surfactant in the absence of inflammation, while reconstituted SCID mice were employed to examine the contribution of immune system-mediated inflammation to pulmonary surfactant dysfunction. In addition, by utilizing mice specifically depleted of CD4+ and CD8+ lymphocytes, the role and importance of these T-cell subpopulations in inflammation-induced surfactant dysfunction was also examined.
MATERIALS AND METHODS
SCID mouse model of PCP.
CB.17 scid/scid mice were obtained from the Trudeau Institute Animal Breeding Facility (Saranac Lake, N.Y.). The mice were maintained in microisolator cages and fed sterilized food and water. Beginning at 3 weeks of age, SCID mice were cohoused with P. carinii-infected SCID mice in order to induce PCP in the experimental animals. Seven weeks after commencing cohousing (postexposure), when SCID mice were known to have developed PCP, one group of mice was immunologically reconstituted with 5 × 107 spleen cells from normal unimmunized congenic CB.17 mice. At 12 days postreconstitution (DPR), during the peak inflammatory phase, these mice were sacrificed and surfactant composition and activity were measured. The remaining infected mice were not reconstituted and were sacrificed at 9, 12, and 15 weeks after exposure to P. carinii. P. carinii-free SCID mice were used as controls.
CD4+ T-cell-depleted mouse model of PCP.
Female C57BL/6 mice, 4 weeks of age, were obtained from the Trudeau Institute Animal Breeding Facility. Three days after arrival, mice were assigned to receive an anti-CD4 monoclonal antibody (MAb) (clone GK 1.5; American Type Culture Collection [ATCC]), both anti-CD4 and anti-CD8 MAbs (clone TIB210; ATCC), or no antibody as previously described (16). Mice treated with MAbs received intraperitoneal injections two times per week of 0.25 mg of the MAb in 0.5 ml of Hanks balanced salt solution. Injections of MAbs were continued for the entire duration of the experiments. Experimental groups of mice were inoculated with 107 P. carinii nuclei and then sacrificed 34 days after inoculation.
P. carinii inoculation.
Lungs from CB.17 SCID mice maintained in a P. carinii-infected colony were used as a source of P. carinii (16). Recipient mice were anesthetized with halothane gas and given intratracheal inoculations of 100 μl of lung homogenates containing 108 P. carinii nuclei/ml with a blunted 20-gauge needle inserted into the trachea through the oral pharynx as described previously (15).
BAL.
Each mouse was sacrificed by an overdose of sodium pentobarbital, and the trachea was surgically exposed and cannulated with a sterile 20-gauge Luer-lok stub adapter. The lungs were gently lavaged with eight 1-ml volumes of sterile 0.15 M NaCl (normal saline) administered through the tracheal catheter. Recovered bronchoalveolar lavage (BAL) fluid, averaging 6 to 7 ml per mouse for each experimental group, was immediately centrifuged at 150 × g for 5 min. This force was sufficient to pellet lavage cells while allowing surface-active surfactant aggregates to remain in solution. The cell-free supernatant was collected and stored at −80°C for subsequent surfactant composition measurements and surface tension studies.
For surface tension measurements of the large-aggregate (LA) surfactant fraction from normal C57BL/6 mice, the cell-free BAL fluids collected from two mice were pooled and centrifuged at 12,000 × g for 30 min. The supernatant was discarded, and the pellet was resuspended to a final concentration of 2.5 mg of phospholipid/ml in 0.9% sterile saline.
Surfactant composition measurements.
The total phospholipid concentration in BAL fluid was assessed by the colorimetric assay of Ames (1). Lavage fluid protein concentration was determined by the assay of Lowry et al. (30), with 15% sodium dodecyl sulfate added to allow measurement of protein in the presence of lipid.
Surface tension measurements.
Surface tension measurements in BAL fluid were made with a pulsating-bubble surfactometer (Electronetics, Amherst, N.Y.) based on the original design of Enhorning (8). Lavage fluid samples from control and experimental animals were evaporated under nitrogen and resuspended in 0.15 M NaCl–2 mM CaCl2 at a uniform phospholipid concentration of 2.5 mg/ml. Aliquots of standardized BAL fluid were then placed in the sample chamber of the bubble apparatus and assessed for surface activity. A small air bubble, communicating with ambient air, was formed and was pulsated between minimum and maximum radii of 0.4 and 0.55 mm by a precision pulsator moving liquid into and out of the sample chamber at 37°C. Bubble size was monitored through a microscope during continuous cycling at a rate of 20 cycles/min. The pressure drop across the air-liquid interface in the bubble (ΔP) was measured with a pressure transducer, and surface tension (γ) was calculated from the Laplace equation for a sphere: ΔP = 2γ/radius. The accuracy of this data analysis procedure has been verified for air bubbles of the small size studied in this apparatus despite nonspherical shape deformations that occur at low surface tension (12). Surface activity data are reported as surface tension at minimum bubble radius (minimum surface tension) as a function of time from the initiation of bubble pulsation.
RNA isolation and S1 nuclease protection assays.
Total lung RNA was isolated from mice using TRIzol reagent according to the manufacturer's instructions (Life Technologies Inc., Grand Island, N.Y.). Steady-state levels of SP-B mRNA were determined using an S1 nuclease protection assay as previously described (50, 51). Complementary DNA probes corresponding to murine SP-B and murine ribosomal protein L32 were end labeled with [γ32-P]ATP using T4 polynucleotide kinase (Life Technologies). Radiolabeled probes were hybridized to 5 μg of total RNA at 50°C for 16 h and then digested with S1 nuclease (Boehringer Mannheim, Indianapolis, Ind.). Protected DNA fragments were resolved by denaturing electrophoresis on an 8 M urea–6% acrylamide gel and quantitated using a PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). SP-B mRNA levels were normalized to the murine L32 levels to correct for sample loading.
Statistical analyses.
All values reported for each experimental group are means ± 1 standard error measurement. For each experiment, P values were determined by performing a one-way analysis of variance with the SigmaStat software package (Jandel Scientific, San Rafael, Calif.). The Student-Newman-Keuls method was used for pairwise multiple comparisons of experimental groups.
RESULTS
Effect of T-cell-mediated inflammation on surfactant function during PCP.
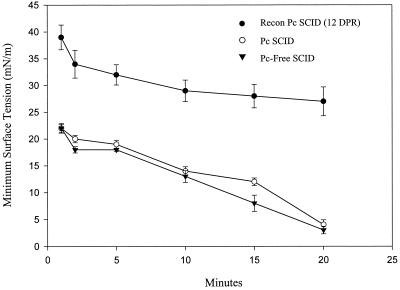
To determine whether P. carinii-induced pulmonary inflammation affects surfactant homeostasis, physiological measurements of surfactant function were performed on lavage fluid from nonreconstituted and immunologically reconstituted SCID mice. Seven weeks after initial exposure to P. carinii, one group of infected SCID mice was left nonreconstituted and one group was immunologically reconstituted. Both groups were sacrificed 12 days later, and surfactant composition and activity in the lavage fluid were measured. Nonreconstituted, P. carinii-infected SCID mice demonstrated normal pulmonary function and normal surfactant activity at 9 weeks after exposure to P. carinii. Lavage fluids from these mice had phospholipid and protein contents similar to those of P. carinii-free mice (Table 1, Fig. 1). In addition, lavage fluids from the nonreconstituted mice had normal minimum surface tensions that reached an average of 4.0 ± 0.9 mN/m after 20 min of pulsation at 20 cycles/min (Fig. 1). In contrast, immunologically reconstituted SCID mice (12 days postreconstitution) mounted an intense T-cell-mediated inflammatory response against P. carinii and exhibited severe surfactant abnormalities. Although BAL fluid phospholipid concentrations were normal in these mice, a fourfold increase in lavage fluid protein concentration was present relative to that for nonreconstituted SCID mice at the same stage of P. carinii infection (123 ± 19 versus 34 ± 6.0 μg/ml; P < 0.05) (Table 1). This increased protein concentration was associated with decreased surface activity of the lavage fluid. Lavage fluid from reconstituted SCID mice had minimum surface tension values that only reached an average of 27.2 ± 2.7 mN/m after 20 min of pulsation (P < 0.05 compared to values for nonreconstituted, infected mice) (Fig. 1).
TABLE 1.
Biochemical composition of BAL fluid from P. carinii-infected SCID mice
| Experimental group | n | Wk postexposure | Immune, Recon.b | DPR | Dynamic-compliancec (% control) | Concn of:
|
Protein/phospholipid ratio (%) | |
|---|---|---|---|---|---|---|---|---|
| Phospholipid (mg/ml) | Protein (μg/ml) | |||||||
| P. carinii free | 5 | 0 | No | 0 | 100 ± 17 | 0.063 ± 0.004 | 37 ± 4.0 | 58 ± 4.0 |
| P. carinii infected | 6 | 9 | No | 0 | 97 ± 11 | 0.053 ± 0.006 | 34 ± 6.0 | 64 ± 4.7 |
| P. carinii infected | 6 | 9 | Yes | 12 | 44 ± 8.4 | 0.058 ± 0.004 | 123 ± 19a | 218 ± 42a |
| P. carinii infected | 6 | 12 | No | 0 | 46 ± 18 | 0.060 ± 0.006 | 75 ± 10a | 123 ± 12a |
| P. carinii infected | 6 | 15 | No | 0 | 36 ± 10 | 0.063 ± 0.004 | 97 ± 7.0a | 160 ± 19a |
P < 0.05 compared to values for P. carinii-free SCID mice and for nonreconstituted, P. carinii-infected SCID mice at 9 weeks postexposure.
Recon., reconstitution.
Dynamic lung compliance data are representative values from an independent, controlled experiment previously reported by Wright et al. (57).
FIG. 1.
Effect of inflammation on surfactant activity in the lungs of P. carinii-infected SCID mice. At 9 weeks after initial exposure to P. carinii (Pc), minimum surface tensions of the BAL fluid from nonreconstituted (open circles) and immune system-reconstituted (recon) (solid circles) SCID mice were measured. The reconstituted mice were examined during the peak inflammatory response at 12 DPR. P. carinii-free SCID mice (solid inverted triangles) were used as controls for normal surfactant activity. Minimum surface tension measurements were made with a pulsating-bubble surfactometer at 37°C during continuous cycling at a rate of 20 cycles/min. Each value is the mean ± 1 standard error for six mice.
T-cell-independent surfactant dysfunction during severe PCP in SCID mice.
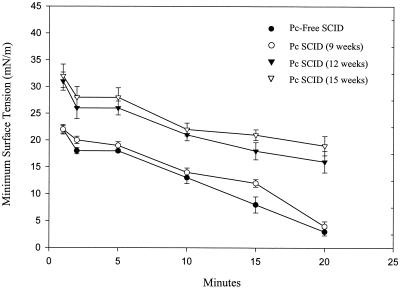
Surfactant composition and activity in nonreconstituted, P. carinii-infected SCID mice during the latter stages of infection were also examined. At 12 and 15 weeks after initial exposure to P. carinii, lavage fluid from SCID mice had normal phospholipid concentrations (0.06 ± 0.006 and 0.06 ± 0.004 mg/ml, respectively) but significantly elevated protein concentrations (75 ± 10 and 97 ± 7.0 μg/ml, respectively; P < 0.05 compared to that from P. carinii-free SCID mice) (Table 1). Again, elevated BAL fluid protein concentrations correlated with decreased surface activity. At 12 and 15 weeks postexposure, lavage fluid from P. carinii-infected SCID mice had average minimum surface tensions of 15.5 ± 2.0 and 18.7 ± 1.8 mN/m, respectively, after 20 min (P < 0.05 compared to those of P. carinii-free SCID mice; Fig. 2).
FIG. 2.
Measurement of surfactant activity during the course of P. carinii (Pc) infection in SCID mice. Minimum surface tensions of the BAL fluid from nonreconstituted SCID mice at 9, 12, and 15 weeks after initial exposure to P. carinii were measured. P. carinii-free SCID mice were used as controls for normal surfactant activity. Minimum surface tension measurements were made with a pulsating-bubble surfactometer at 37°C during continuous cycling at a rate of 20 cycles/min. Each value is the mean ± 1 standard error for six mice.
Effect of CD8+ T cells on surfactant function during PCP.
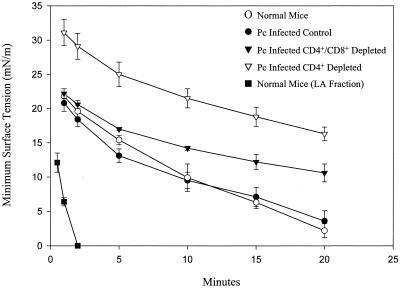
Uninfected C57BL/6 mice were used as baseline controls for normal surfactant composition and surface activity in whole-cell-free lavage fluid. The BAL fluid from normal C57BL/6 mice had an average phospholipid concentration of 0.078 ± 0.004 mg/ml, an average protein concentration of 54 ± 1.8 μg/ml, and a protein/phospholipid ratio of 70% ± 5.0% (Table 2). At a phospholipid concentration of 2.5 mg/ml, the lavage fluids from these mice reached an average minimum surface tension of 2.2 ± 1.0 mN/m after 20 min of cycling (Fig. 3). The LA surfactant fraction from normal C57BL/6 mice was also examined. As expected, the LA fraction lacked many of the inhibitory components present in whole lavage fluid and was much more active at the same phospholipid concentration. These samples reached minimum surface tensions of <1.0 mN/m after only 2 min of cycling (Fig. 3).
TABLE 2.
Biochemical composition of BAL fluid from lymphocyte-depleted P. carinii-infected mice
| Experimental group | n | Days post inoculation | Dynamic compliancea (% control) | Concn of:
|
Protein/phospholipid ratio (%) | |
|---|---|---|---|---|---|---|
| Phospholipid (mg/ml) | Proteins (μg/ml) | |||||
| C57BL/6 (uninfected) | 3 | 0 | NDb | 0.078 ± 0.004 | 54 ± 1.8 | 70 ± 5.0 |
| C57BL/6 | 5 | 34 | 100 ± 3.0 | 0.087 ± 0.015 | 54 ± 6.0 | 64 ± 4.5 |
| CD4+ and CD8+ depleted | 4 | 34 | 86 ± 6.0 | 0.070 ± 0.005 | 44 ± 8.0 | 63 ± 6.1 |
| CD4+ depleted | 5 | 34 | 49 ± 4.4 | 0.137 ± 0.026c | 167 ± 16c | 126 ± 20c |
Dynamic lung compliance data previously reported by Wright et al. (57).
ND, measurements not determined for the specific experimental group.
P < 0.05 compared to values for both uninfected and infected C57BL/6 control mice and for CD4+- and CD8+-depleted mice.
FIG. 3.
Effect of CD8+ T-cell-mediated inflammation on surfactant activity in the lungs of P. carinii-infected mice. Average minimum surface tensions of the lavage fluid from immunocompetent, CD4+-depleted, and CD4+- and CD8+-depleted mice at 34 days after inoculation with P. carinii were measured. Lavage fluid and the LA surfactant fraction from immunocompetent, uninoculated mice were used as controls for surfactant activity. Minimum surface tension measurements were made with a pulsating-bubble surfactometer at 37°C during continuous cycling at a rate of 20 cycles/min. Each value is the mean ± 1 standard error.
At 34 days after inoculation with P. carinii, BAL fluid from C57BL/6 mice had a composition similar to that from uninfected C57BL/6 mice, with an average phospholipid concentration of 0.087 ± 0.02 mg/ml, an average protein concentration of 54 ± 6.0 μg/ml, and a protein/phospholipid ratio of 64% ± 4.5% (Table 2). Minimum surface tension values in lavage fluid from these mice were also unchanged from those for control mice and reached a final value of 3.6 ± 1.5 mN/m after 20 min of cycling (Fig. 3). In contrast, lavage fluid from P. carinii-infected CD4+ T-cell-depleted mice had elevated phospholipid concentrations (0.137 ± 0.03 mg/ml), protein concentrations (167 ± 16 μg/ml), and protein/phospholipid ratios (126% ± 20%). Surface activity was also significantly decreased in lavage fluid from these CD4+-depleted mice and reached an average minimum surface tension of only 16.3 ± 1.0 mN/m after 20 min of cycling (P < 0.05 compared to values for normal controls). However, when CD4+-depleted mice were additionally depleted of CD8+ cells, the lavage fluid had phospholipid concentrations, protein concentrations, and protein/phospholipid ratios similar to those for normal mice (Table 2). In addition, the surface activity of the BAL fluid was improved in the absence of CD8+ cells, with an average minimum surface tension of 10.6 ± 1.3 mN/m after 20 min of cycling (Fig. 3).
Surfactant protein B gene expression during PCP.
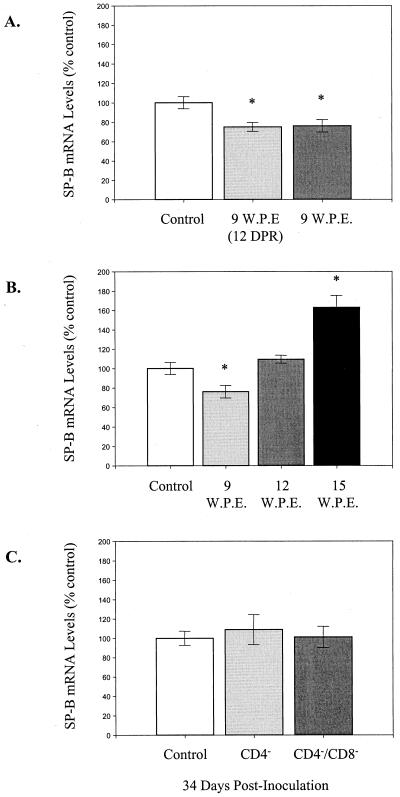
Beers et al. (4) have recently demonstrated a decrease in SP-B expression during P. carinii infection in mice. Therefore, to determine if decreased SP-B expression could explain the inflammation-associated surfactant dysfunction described here, SP-B levels in each experimental group of mice were evaluated. S1 nuclease protection assays were used to determine whether deficiencies in surfactant function correlated with altered lung mRNA levels for SP-B during PCP. At 9 weeks after the initial exposure to P. carinii, both reconstituted and nonreconstituted SCID mice demonstrated a 25% decrease in SP-B mRNA levels compared to P. carinii-free SCID controls (P < 0.05) (Fig. 4A). However, the changes were similar in both groups and did not explain the functional surfactant abnormalities observed in reconstituted mice. SP-B mRNA levels in nonreconstituted SCID mice through the course of P. carinii infection were also examined (Fig. 4B). SP-B mRNA levels were slightly decreased to 76% of those of controls at 9 weeks (P < 0.05), were elevated to 109% of those of controls at 12 weeks, and were elevated further to 163% of those of controls at 15 weeks after exposure to P. carinii (P < 0.05). Thus, there were no significant decreases in SP-B that could account for the observed surfactant dysfunction either in reconstituted SCID mice or in nonreconstituted SCID mice at 12 and 15 weeks postexposure.
FIG. 4.
SP-B mRNA expression in the lungs of P. carinii-infected mice. Steady-state levels of SP-B mRNA were measured in the lung by S1 nuclease protection assays. (A) mRNA levels in reconstituted (light gray) and nonreconstituted (dark gray) P. carinii-infected SCID mice at 9 weeks post exposure (W.P.E.). Reconstituted mice were examined at 12 DPR during the peak inflammatory phase. P. carinii-free SCID mice were used as normal controls. (B) SP-B levels during the progression of P. carinii infection in nonreconstituted SCID mice. Again, P. carinii-free SCID mice were used as normal controls. (C) SP-B levels in normal (control), CD4+-depleted, and CD4+- and CD8+-depleted mice at 34 days after inoculation with P. carinii. In all experiments SP-B mRNA levels were normalized to murine ribosomal protein L32 and then expressed as percentages of the control. Each value is the mean ± 1 standard error for four mice. ∗, experimental groups that are statistically different (P < 0.05) from the control group.
SP-B mRNA levels in control, CD4-depleted, and CD4- and CD8-depleted mice at 34 days after inoculation with P. carinii were also examined (Fig. 4C). Although CD4-depleted mice demonstrated surfactant dysfunction at this time point, they had normal SP-B mRNA levels in the lung. Therefore, under the conditions of our experiment, we were unable to document a decrease in SP-B mRNA levels that could have contributed to surfactant dysfunction in this model of PCP.
DISCUSSION
We have previously demonstrated that both the immune reconstitution of P. carinii-infected SCID mice and the action of CD8+ T cells in chronically infected CD4-depleted mice cause the accumulation of lymphocytes, macrophages, and neutrophils in the lung. This immune system-mediated pulmonary inflammation directly contributes to the impaired lung function observed during PCP (57). In the present study we expand upon these findings by demonstrating that pulmonary inflammation contributes to respiratory impairment during PCP, at least in part, by disrupting the pulmonary surfactant system. In order to study inflammation-related surfactant inhibition, the surface tension measurements reported here were taken on whole-cell-free lavage fluid. Although other groups have utilized the more active LA surfactant fraction for their studies, we did not want to separate out plasma proteins and related inhibitors and mask the inactivation phenomena that were the target of this investigation.
At 9 weeks postexposure, nonreconstituted P. carinii-infected SCID mice neither display evidence of pulmonary inflammation nor exhibit measurably impaired pulmonary function (57). In addition, surfactant composition and activity are normal. However, when these mice are immunologically reconstituted, they mount an intense T-lymphocyte-dependent inflammatory response against the infection. This inflammatory response causes severe respiratory impairment, increased lavage fluid protein concentrations, and a significant decrease in the in vitro surface activity of the lavage fluid (Table 1; Fig. 1). These findings indicate that in moderately infected mice surfactant is inactivated by P. carinii-induced pulmonary inflammation and not by direct effects of P. carinii. The effect of CD8+ T-cell-mediated inflammation on surfactant composition and activity during PCP was also examined. P. carinii-infected, CD4+-depleted mice display signs of pulmonary inflammation, exhibit severe respiratory impairment, and demonstrate significant decreases in lavage fluid surfactant activity (Fig. 3) (57). However, when CD8+ T cells are additionally depleted, CD4+-depleted mice exhibit little evidence of pulmonary inflammation, have normal pulmonary function measurements, and demonstrate improved surfactant function. These studies suggest that CD8+ T-cell-mediated pulmonary inflammation is particularly important in leading to disruption of pulmonary surfactant function and respiratory impairment during PCP. Furthermore, inflammation-associated surfactant deficiencies likely contribute to impaired pulmonary function during PCP in both reconstituted SCID mice and CD4+-depleted mice.
The immunologically reconstituted SCID mouse model of PCP was used to demonstrate that T-lymphocyte-mediated inflammation alters pulmonary surfactant function. However, nonreconstituted P. carinii-infected SCID mice also exhibited abnormal surfactant function during the latter stages of infection, even in the absence of T cells. In the presence of heavy P. carinii burdens these mice exhibit T-cell-independent pulmonary inflammation that is characterized by the accumulation of neutrophils in the lung. Therefore, it is difficult to determine whether P. carinii-induced, T-cell-independent pulmonary inflammation causes surfactant disruption or if P. carinii directly interferes with normal surfactant function. During advanced PCP heavy P. carinii burdens may initiate T-cell-independent pathways of lung inflammation that inactivate surfactant. Alternatively, there is such a heavy organism burden that P. carinii itself may sequester, inactivate, or inhibit synthesis of surfactant components, leading to decreased surfactant activity.
Recent studies have demonstrated that P. carinii infection can alter the level and composition of surfactant phospholipids and associated proteins in the lungs and the BAL fluid of rodents. P. carinii binds SP-A and -D and may contribute to functionally impaired surfactant by sequestering these components in an inactive form (31, 33, 36). P. carinii-infected, steroid-treated rats have phosphatidylglycerol deficiencies in the lavage fluid and consequently functionally impaired surfactant (52). In addition, surfactant phospholipid secretion is inhibited in isolated alveolar type II cells following infection with P. carinii in vivo and after treatment with P. carinii gpA in vitro (29, 35, 52). Finally, decreased SP-B and -C expression during P. carinii infection in immunodeficient mice is associated with increased minimum surface tension in the LA surfactant fraction (3, 4). Together, these studies demonstrate that P. carinii infection negatively affects surfactant. However, they have not examined the contribution of the host inflammatory response to observed surfactant abnormalities. Our data suggest that despite the direct interaction between P. carinii and surfactant (31, 33, 59), P. carinii itself has little demonstrable direct effect on surfactant function in vivo. Rather, inflammation-mediated mechanisms of surfactant dysfunction appear to be more significant during in vivo P. carinii infection.
Beers et al. have demonstrated that SP-B mRNA and protein levels are decreased in the lungs of P. carinii-infected SCID and CD4+-depleted mice (4). Although we found slightly decreased SP-B mRNA levels in certain groups of P. carinii-infected mice, we could not demonstrate a correlation between SP-B mRNA levels and surfactant abnormalities. At 9 weeks after initial exposure to P. carinii, both reconstituted and nonreconstituted SCID mice demonstrated a 25% decrease in SP-B mRNA levels. However, only the reconstituted mice exhibited surfactant abnormalities, indicating that factors other than the SP-B deficiency were interfering with surfactant function in this model of PCP. Furthermore, nonreconstituted, P. carinii-infected SCID mice actually showed an increase in SP-B mRNA levels at a state of disease when significantly impaired surfactant function was observed (at 15 weeks after exposure to P. carinii). Finally, we were unable to document a change in SP-B mRNA levels in either CD4+- or CD4+- and CD8+-depleted P. carinii-infected mice at 34 days after inoculation. Thus, we were unable to demonstrate any correlation between SP-B mRNA levels and surfactant dysfunction under the experimental conditions examined here.
Inflammation-related changes in surfactant homeostasis may lead to increased surface tension in the lung and consequently respiratory impairment. In both models of PCP-related inflammation examined herein, T-lymphocyte, macrophage, and neutrophil numbers are all elevated in the BAL fluid. Activated T lymphocytes may serve to amplify the inflammatory response by secreting monocyte chemotactic factors, such as RANTES and lymphotactin (23, 40). They may also activate macrophages through the release of gamma interferon and interleukin-3 (10, 47) or through cognate receptor-ligand interactions (48). Activated macrophages release numerous proteases that may lead to surfactant inactivation (37). In addition, macrophages release tumor necrosis factor alpha (TNF-α), which has negative effects on surfactant synthesis (2, 34, 56) and may also play a role in the recruitment and activation of neutrophils (54). Large numbers of neutrophils were found in BAL fluid from both reconstituted, P. carinii-infected mice and P. carinii-infected CD4+-depleted mice coincident with surfactant abnormalities (57). Upon degranulation, neutrophils secrete many proteases, including elastase, that can destroy surfactant components and compromise its surface-active properties (28, 38). Together, the actions of these cell populations may serve to inhibit surfactant function and contribute to respiratory impairment during PCP. This may help to explain the finding of decreased SP-B protein levels reported in mice with PCP (4).
Our experiments did not address the specific mechanisms responsible for inflammation-mediated surfactant dysfunction during PCP in animals. A host of mediators and substances capable of affecting pulmonary surfactant and its activity are present in the lung during PCP. TNF-α, for example, inhibits gene expression of SP-A and -B (34, 56), as well as the synthesis of surfactant phospholipids in alveolar epithelial cells (2). TNF-α also increases protease release from macrophages and neutrophils and can contribute to pulmonary edema by increasing the permeability of the alveous-capillary barrier (35, 37, 45). The significantly increased protein-to-phospholipid ratios present in BAL fluid from injured, reconstituted animals are consistent with lung surfactant inactivation due to plasma proteins or related protein inhibitors in the alveolar spaces. Multiple studies have shown that plasma and blood proteins can impair the surface tension-lowering ability of pulmonary surfactant (18–20, 24, 42). In addition, both pulmonary edema fluid and fibrinogen have been shown to inactivate pulmonary surfactant (25, 49). Edema fluid is leaked into the lung during PCP-related inflammation, and recent studies have demonstrated that fibrinogen is synthesized in the lung during PCP (44). Alternatively, cell membrane lipids and a variety of other endogenous compounds present in the lungs during inflammatory injury can also interact physically with lung surfactant to reduce its activity (27, 32, 41). Proteases and phospholipases induced by inflammation can also contribute to surfactant dysfunction by degrading active components and by generating byproducts such as lysophospholipids and fluid-free fatty acids that themselves inhibit surface tension lowering (14, 21, 55). It is also possible that active large surfactant aggregates could be depleted or altered in PCP, since this is known to occur in a number of other forms of acute lung injury (11, 13, 26). A shift from LA to small-aggregate subtypes of lung surfactant can lead to a reduction in surface activity despite an apparently normal overall amount of lavageable phospholipid. Further studies investigating specific pathways of surfactant dysfunction in PCP will be necessary to define the specific importance of different inhibition mechanisms.
In summary, we have demonstrated that T-cell-mediated inflammation inactivates pulmonary surfactant, contributing to PCP-associated respiratory impairment. Consistent with our findings of surfactant abnormalities during PCP, other studies have found that surfactant replacement therapy can improve pulmonary function during PCP (6, 7, 22). In addition to restoring critical components, exogenous surfactant may also benefit PCP patients by inhibiting T-cell-dependent inflammatory responses (5) and diminishing neutrophil function (53). Similarly, steroid therapy has been of some benefit to PCP patients. In addition to their general anti-inflammatory properties steroids can also upregulate surfactant synthesis (58), thereby compensating for the abnormalities observed during PCP. These findings suggest that surfactant therapy in combination with other treatment regimens may prove beneficial in PCP patients.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant HL-59833-02 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Ames B N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 2.Arias-Diaz J, Vara E, Garcia C, Balibrea J L. Tumor necrosis factor-alpha-induced inhibition of phosphatidylcholine synthesis by human type II pneumocytes is partially mediated by prostaglandins. J Clin Investig. 1994;94:244–250. doi: 10.1172/JCI117313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atochina E N, Beers M F, Scanlon S T, Preston A M, Beck J M. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am J Physiol. 2000;278:L599–L609. doi: 10.1152/ajplung.2000.278.3.L599. [DOI] [PubMed] [Google Scholar]
- 4.Beers M F, Atochina E N, Preston A M, Beck J M. Inhibition of lung surfactant protein B expression during Pneumocystis carinii pneumonia in mice. J Lab Clin Med. 1999;133:423–433. doi: 10.1016/s0022-2143(99)90019-7. [DOI] [PubMed] [Google Scholar]
- 5.Borron P, Veldhuizen R A, Lewis J F, Possmayer F, Caveney A, Inchley K, McFadden R G, Fraher L J. Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am J Respir Cell Mol Biol. 1996;15:115–121. doi: 10.1165/ajrcmb.15.1.8679215. [DOI] [PubMed] [Google Scholar]
- 6.Creery W D, Hashmi A, Hutchison J S, Singh R N. Surfactant therapy improves pulmonary function in infants with Pneumocystis carinii pneumonia and acquired immunodeficiency syndrome. Pediatr Pulmonol. 1997;24:370–373. doi: 10.1002/(sici)1099-0496(199711)24:5<370::aid-ppul10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Eijking E P, van Daal G J, Tenbrinck R, Luijendijk A, Sluiters J F, Hannappel E, Lachmann B. Effect of surfactant replacement on Pneumocystis carinii pneumonia in rats. Intensive Care Med. 1991;17:475–478. doi: 10.1007/BF01690770. [DOI] [PubMed] [Google Scholar]
- 8.Enhorning G. Pulsating bubble technique for evaluation of pulmonary surfactant. J Appl Physiol. 1977;43:198–203. doi: 10.1152/jappl.1977.43.2.198. [DOI] [PubMed] [Google Scholar]
- 9.Escamilla R, Prevost M C, Hermant C, Caratero A, Cariven C, Krempf M. Surfactant analysis during Pneumocystis carinii pneumonia in HIV-infected patients. Chest. 1992;101:1558–1562. doi: 10.1378/chest.101.6.1558. [DOI] [PubMed] [Google Scholar]
- 10.Frendl G. Interleukin 3: from colony-stimulating factor to pluripotent immunoregulatory cytokine. Int J Immunopharmacol. 1992;14:421–430. doi: 10.1016/0192-0561(92)90172-h. [DOI] [PubMed] [Google Scholar]
- 11.Gross N J. Surfactant subtypes in experimental lung damage: radiation pneumonitis. Am J Physiol. 1991;260:L302–L310. doi: 10.1152/ajplung.1991.260.4.L302. [DOI] [PubMed] [Google Scholar]
- 12.Hall S B, Bermel M S, Ko K T, Palmer H J, Enhorning G, Notter R H. Approximations in the measurement of surface tension on the oscillating bubble surfactometer. J Appl Physiol. 1993;75:467–478. doi: 10.1152/jappl.1993.75.1.468. [DOI] [PubMed] [Google Scholar]
- 13.Hall S B, Hyde R W, Notter R H. Changes in subphase surfactant aggregates in rabbits injured by free fatty acid. Am J Respir Crit Care Med. 1994;149:1099–1106. doi: 10.1164/ajrccm.149.5.8173747. [DOI] [PubMed] [Google Scholar]
- 14.Hall S B, Lu R Z, Venkitaraman A R, Hyde R W, Notter R H. Inhibition of pulmonary surfactant by oleic acid: mechanisms and characteristics. J Appl Physiol. 1992;72:1708–1716. doi: 10.1152/jappl.1992.72.5.1708. [DOI] [PubMed] [Google Scholar]
- 15.Harmsen A G. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect Immun. 1988;56:1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmsen A G, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman A G, Lawrence M G, Ognibene F P, Suffredini A F, Lipschik G Y, Kovacs J A, Masur H, Shelhamer J H. Reduction of pulmonary surfactant in patients with human immunodeficiency virus infection and Pneumocystis carinii pneumonia. Chest. 1992;102:1730–1736. doi: 10.1378/chest.102.6.1730. [DOI] [PubMed] [Google Scholar]
- 18.Holm B A, Enhorning G, Notter R H. A biophysical mechanism by which plasma proteins inhibit lung surfactant activity. Chem Phys Lipids. 1988;49:49–55. doi: 10.1016/0009-3084(88)90063-1. [DOI] [PubMed] [Google Scholar]
- 19.Holm B A, Notter R H. Effects of hemoglobin and cell membrane lipids on pulmonary surfactant activity. J Appl Physiol. 1987;63:1434–1442. doi: 10.1152/jappl.1987.63.4.1434. [DOI] [PubMed] [Google Scholar]
- 20.Holm B A, Notter R H, Finkelstein J N. Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem Phys Lipids. 1985;38:287–298. doi: 10.1016/0009-3084(85)90022-2. [DOI] [PubMed] [Google Scholar]
- 21.Holm B A, Wang Z, Notter R H. Multiple mechanisms of lung surfactant inhibition. Pediatr Res. 1999;46:85–93. doi: 10.1203/00006450-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Hughes W T, Sillos E M, LaFon S, Rogers M, Woolley J L, Davis C, Studenberg S, Pattishall E, Freeze T, Snyder G, Staton S. Effects of aerosolized synthetic surfactant, atovaquone, and the combination of these on murine Pneumocystis carinii pneumonia. J Infect Dis. 1998;177:1046–1056. doi: 10.1086/515252. [DOI] [PubMed] [Google Scholar]
- 23.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, Zlotnik A. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 24.Keough K M W, Parsons C S, Tweeddale M G. Interactions between plasma proteins and pulmonary surfactant: pulsating bubble studies. Can J Physiol Pharmacol. 1989;67:663–668. doi: 10.1139/y89-106. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Nitta K, Ganzuka M, Inui S, Grossmann G, Robertson B. Inactivation of exogenous surfactant by pulmonary edema fluid. Pediatr Res. 1991;29:353–356. doi: 10.1203/00006450-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Lewis J, Ikegami M, Jobe A. Altered surfactant function and metabolism in rabbits with acute lung injury. J Appl Physiol. 1990;69:2303–2310. doi: 10.1152/jappl.1990.69.6.2303. [DOI] [PubMed] [Google Scholar]
- 27.Lewis J F, Jobe A H. Surfactant and ARDS. Am Rev Respir Dis. 1993;147:218–233. doi: 10.1164/ajrccm/147.1.218. [DOI] [PubMed] [Google Scholar]
- 28.Liau D F, Yin N X, Huang J, Ryan S F. Effects of human polymorphonuclear leukocyte elastase upon surfactant proteins in vitro. Biochim Biophys Acta. 1996;1302:117–128. doi: 10.1016/0005-2760(96)00042-2. [DOI] [PubMed] [Google Scholar]
- 29.Lipschik G Y, Treml J F, Moore S D, Beers M F. Pneumocystis carinii glycoprotein A inhibits surfactant phospholipid secretion by rat alveolar type II cells. J Infect Dis. 1998;177:182–187. doi: 10.1086/513826. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.McCormack F X, Festa A L, Andrews R P, Linke M J, Walzer P D. The carbohydrate recognition domain of surfactant protein A mediates binding to the major surface glycoprotein of Pneumocystis carinii. Biochemistry. 1997;36:8092–8099. doi: 10.1021/bi970313f. [DOI] [PubMed] [Google Scholar]
- 32.Notter R H, Wang Z. Pulmonary surfactant: physical chemistry, physiology, and replacement. Rev Chem Eng. 1997;13:1–118. [Google Scholar]
- 33.O'Riordan D M, Standing J E, Kwon K Y, Chang D, Crouch E C, Limper A H. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J Clin Investig. 1995;95:2699–2710. doi: 10.1172/JCI117972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pryhuber G S, Bachurski C, Hirsch R, Bacon A, Whitsett J A. Tumor necrosis factor-α decreases surfactant protein B mRNA in murine lung. Am J Physiol. 1996;270:L714–L721. doi: 10.1152/ajplung.1996.270.5.L714. [DOI] [PubMed] [Google Scholar]
- 35.Pugin J, Widmer M C, Kossodo S, Liang C M, Preas H L, Suffredini A F. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20:458–464. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 36.Rice W R, Singleton F M, Linke M J, Walzer P D. Regulation of surfactant phosphatidylcholine secretion from alveolar type II cells during Pneumocystis carinii pneumonia in the rat. J Clin Investig. 1993;92:2778–2782. doi: 10.1172/JCI116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ries C, Petrides P E. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe-Seyler. 1995;376:345–355. [PubMed] [Google Scholar]
- 38.Ryan S F, Ghassibi Y, Liau D F. Effects of activated polymorphonuclear leukocytes upon pulmonary surfactant in vitro. Am J Respir Cell Mol Biol. 1991;4:33–41. doi: 10.1165/ajrcmb/4.1.33. [DOI] [PubMed] [Google Scholar]
- 39.Sastry K, Herman G A, Day L, Deignan E, Bruns G, Morton C C, Ezekowitz R A. The human mannose-binding protein gene. Exon structure reveals its evolutionary relationship to a human pulmonary surfactant gene and localization to chromosome 10. J Exp Med. 1989;170:1175–1189. doi: 10.1084/jem.170.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schall T J, Jongstra J, Dyer B J, Jorgensen J, Clayberger C, Davis M M, Krensky A M. A human T-cell specific molecule is a member of a new gene family. J Immumol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 41.Seeger W, Gunther A, Walmrath H D, Grimminger F, Lasch H G. Alveolar surfactant and adult respiratory distress syndrome. Pathogenic role and therapeutic prospects. Clin Investig. 1993;71:177–190. doi: 10.1007/BF00180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeger W, Stohr G, Wolf H R D, Neuhof H. Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J Appl Physiol. 1985;58:326–338. doi: 10.1152/jappl.1985.58.2.326. [DOI] [PubMed] [Google Scholar]
- 43.Sheehan P M, Stokes D C, Hughes W T. Surfactant phospholipids and lavage phospholipase A2 in experimental Pneumocystis carinii pneumonia. Am Rev Respir Dis. 1986;134:526–531. doi: 10.1164/arrd.1986.134.3.526. [DOI] [PubMed] [Google Scholar]
- 44.Simpson-Haidaris P J, Courtney M A, Wright T W, Goss R, Harmsen A, Gigliotti F. Induction of fibrinogen expression in the lung epithelium during Pneumocystis carinii pneumonia. Infect Immun. 1998;66:4431–4439. doi: 10.1128/iai.66.9.4431-4439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephens K E, Ishizaka A, Larrick J W, Raffin T A. Tumor necrosis factor causes increased pulmonary permeability and edema. Comparison to septic acute lung injury. Am Rev Respir Dis. 1988;137:1364–1370. doi: 10.1164/ajrccm/137.6.1364. [DOI] [PubMed] [Google Scholar]
- 46.Sternberg R I, Whitsett J A, Hull W M, Baughman R P. Pneumocystis carinii alters surfactant protein A concentrations in bronchoalveolar lavage fluid. J Lab Clin Med. 1995;125:462–469. [PubMed] [Google Scholar]
- 47.Stevens M G, Exon J H, Olson D P. In vivo effects of interferon-gamma and indomethacin on murine alveolar macrophage activity. Cell Immunol. 1989;123:83–95. doi: 10.1016/0008-8749(89)90270-0. [DOI] [PubMed] [Google Scholar]
- 48.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 49.Strayer D S, Herting E, Sun B, Robertson B. Antibody to surfactant protein A increases sensitivity of pulmonary surfactant to inactivation by fibrinogen in vivo. Am J Respir Crit Care Med. 1996;153:1116–1122. doi: 10.1164/ajrccm.153.3.8630554. [DOI] [PubMed] [Google Scholar]
- 50.Stripp B R, Huffman J A, Bohinski R J. Structure and regulation of the murine Clara cell secretory protein gene. Genomics. 1994;20:27–35. doi: 10.1006/geno.1994.1123. [DOI] [PubMed] [Google Scholar]
- 51.Stripp B R, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 1995;269:L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- 52.Su T H, Natarajan V, Kachel D L, Moxley M A, Longmore W J, Martin W J., II Functional impairment of bronchoalveolar lavage phospholipids in early Pneumocystis carinii pneumonia in rats. J Lab Clin Med. 1996;127:263–271. doi: 10.1016/s0022-2143(96)90094-3. [DOI] [PubMed] [Google Scholar]
- 53.Tegtmeyer F K, Gortner L, Ludwig A, Brandt E. In vitro modulation of induced neutrophil activation by different surfactant preparations. Eur Respir J. 1996;9:752–757. doi: 10.1183/09031936.96.09040752. [DOI] [PubMed] [Google Scholar]
- 54.Tessier P A, Naccache P H, Clark-Lewis I, Gladue R P, Neote K S, McColl S R. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-α. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 55.Wang Z, Notter R H. Additivity of protein and non-protein inhibitors of lung surfactant activity. Am J Respir Crit Care Med. 1998;158:28–35. doi: 10.1164/ajrccm.158.1.9709041. [DOI] [PubMed] [Google Scholar]
- 56.Wispe J R, Clark J C, Warner B B, Fajardo D, Hull W E, Holtzman R B, Whitsett J A. Tumor necrosis factor-alpha inhibits expression of pulmonary surfactant protein. J Clin Investig. 1990;86:1954–1960. doi: 10.1172/JCI114929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright T W, Gigliotti F, Finkelstein J N, McBride J T, An C L, Harmsen A G. Immune-mediated inflammation directly impairs pulmonary function contributing to the pathogenesis of Pneumocystis pneumonia. J Clin Investig. 1999;104:1307–1317. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young S L, Silbajoris R. Dexamethasone increases adult rat surfactant lipids. J Appl Physiol. 1986;60:1665–1672. doi: 10.1152/jappl.1986.60.5.1665. [DOI] [PubMed] [Google Scholar]
- 59.Zimmerman P E, Voelker D R, McCormack F X, Paulsrud J R, Martin W J., II 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J Clin Investig. 1992;89:143–149. doi: 10.1172/JCI115554. [DOI] [PMC free article] [PubMed] [Google Scholar]