Abstract
The effect of MW technology (1,200 Watts for 10 min) on the chemical and sensory composition of five monovarietal wines with different phenolic composition was studied relative to untreated Control wines. MW improved polymeric pigment content by 30, 22 and 31% in Cabernet Sauvignon, Malbec, and Syrah wines, respectively, and anthocyanin extraction and non-tannin phenolics by 24% in Malbec and Syrah wines, respectively. In Nebbiolo and Pinot noir, MW had no effect on phenolics or chromatic characteristics. Anthocyanins in Nebbiolo wines were the lowest and their pigment profile was composed of 18% pyranoanthocyanins, but tannins were the highest, resulting in a tannin to anthocyanin ratio of 16. Pinot noir and Nebbiolo wines had comparable polymeric pigment content, despite dissimilar tannin to anthocyanin ratios, suggesting different mouthfeel characteristics in their respective wines. Conversely, wines of comparable tannin to anthocyanin produced wines of vastly different polymeric pigment content. MW-treated Cabernet Sauvignon wines showed an improved sensory profile.
Keywords: Anthocyanins, Tannins, Wine color, Microwave, Ripeness, Sensory analysis
Highlights
-
•
Five monovarietal wines harvested at overripeness and processed with and without microwave (MW).
-
•
MW improved polymeric pigment content in Cabernet Sauvignon, Malbec, and Syrah wines.
-
•
In Nebbiolo and Pinot noir, MW had no effect on phenolics nor in chromatic characteristics.
-
•
Dissimilar tannin to anthocyanin ratios produced wines with comparable polymeric pigment.
-
•
MW-treated Cabernet Sauvignon wines showed improved sensory profile.
Anthocyanins; Tannins; Wine color; Microwave; Ripeness; Sensory analysis.
1. Introduction
Red wines own much of their sensory characteristics to phenolic compounds, which are present on the skins and seeds of the berries of Vitis vinifera L. and extracted during the maceration process of red winemaking. The term “phenolic compounds” is used to describe phenolic flavonoids, composed of a 15-carbon structure with a C6–C3–C6 configuration bearing hydroxyl and non-hydroxyl substitutions, as opposed to nonflavonoids or simple phenols. Phenolic flavonoids, heretofore referred to as phenolic compounds, are very reactive and can engage in both covalent and non-covalent reactions with other components of the wine matrix. Phenolic reactivity is due to both the weak acidic character as well as the high electron density of the aromatic phenolic rings, furthered by the presence of one or more electron-donating hydroxyl groups (Fulcrand et al., 2006), which enables them to undergo electrophilic substitutions. Electron delocalization and the extended conjugated system of unsaturated bonds in the phenolic backbone also explain the light-absorption properties, and thus, the chromatic characteristics of some of these molecules. Phenolic compounds are located in the pulp or mesocarp of the berry, and, primarily in skins and seeds. The pulp is rich in nonflavonoids, namely hydroxycinnamic acids which are the substrates of enzymatic oxidations (Casassa, 2017). However, skin and seeds are typically richer sources of phenolic compounds. From a chemical and sensory standpoint, the flavonoid family's four most relevant phenolic classes in the skins and seeds of Vitis vinifera L. berries are flavan-3-ols, flavonols, tannins, and anthocyanins (Casassa, 2017). Flavan-3-ols are low-molecular weight monomers mostly located on the seeds, and once extracted into wine, can elicit bitterness (Thorngate and Noble, 1995). Upon polymerization during grape ripening, flavan-3-ols form tannins, and hence flavan-3-ols can be regarded as the “building blocks” of tannins. Flavonols, of which the most abundant is quercetin-3-O-glucoside, are confined to the skins, and act as copigmentation factors thereby influencing wine color through hyperchromic and bathochromic shifts (Boulton, 2001). Flavonols may also be able to elicit bitterness because of their low molecular weight and provide astringency subqualities such as “velvety” (Hufnagel and Hofmann, 2008). Tannins are polymers of monomeric flavan-3-ols, located in both seeds and skins of the grape berry. Tannins have molecular weight sizes ranging from 5,000 to 22,000 DA in seeds and skins skin extracts, respectively (Hanlin et al., 2011), sometimes referred as mean degree of polymerization, or mDP. Upon wine consumption, wine tannins engage non-covalently with a specific set of salivary proteins known as proline-rich proteins, thereby precipitating them, and resulting in the trigeminal tactile sensation of astringency. There is a vast array of quantitative differences in tannin composition and concentration in red wines as a function of the wine varietal. For example, in a set of 1,325 commercial wines, tannin concentration ranged from 30 to 1895 mg/L among all wines measured (Harbertson et al., 2008). From a compositional and sensory viewpoint, a recent study showed that, sensorially, tannins in Cabernet Sauvignon wines were perceived as dryer and with a longer duration of dryness than tannins in Pinot noir wines, which was related to larger tannins in Cabernet Sauvignon wine (mDP of 6.3) than in Pinot noir wines (mDP of 2.4) (Watrelot et al., 2019).
Anthocyanins are pigments responsible for the color of red wines and are present as monomers of six glycosylated forms, namely malvidin, cyanidin, petunidin, peonidin, delphinidin, and pelargonidin (He et al., 2012). Glycosylation occurs at the C3 position whereas acylation at the C6 position of the glucose moiety by esterification with an aromatic (p-coumaric, caffeic, ferulic, and sinapic acids), or an aliphatic acid (acetic, malic, malonic, oxalic, and succinic acids), renders the molecule more stable and add purple and blue hues to the pigment features (Brouillard et al., 2003). As with tannins, wines made from different grape cultivars show large quantitative and qualitative variations in their anthocyanin profile. For example, Cabernet Sauvignon wines are rich in acylated anthocyanins, whereas Pinot noir may have total absence of acylated anthocyanins (Dimitrovska et al., 2011). Nebbiolo, on the other hand, is particularly rich in peonidin derivatives (Guidoni et al., 2002). Upon extraction into wines, anthocyanins can react with low molecular weight carbonyl compounds such as pyruvic acid or acetaldehyde, to form the so-called pyranoanthocyanins. Anthocyanins can also react with tannins of various molecular weights to give rise to polymeric pigments, which are key wine components from both a chemical and sensory viewpoint. Polymeric pigments are a heterogeneous family of winemaking artifacts that are not originally present on grapes but formed during winemaking operations and aging. From a sensory viewpoint, polymeric pigments provide stable color during aging, and desirable mouthfeel characteristics (Weber et al., 2013).
The extraction and retention of phenolic compounds into wine is modulated by multiple factors, including the grape cultivar, clone, and rootstock, geographic origin and climatic conditions, degree of ripeness and the maceration technique applied during winemaking. In general, phenolic extraction and retention from grapes into wine increases with grape ripeness up to a certain point, in such fashion that properly ripe grapes usually display higher phenolic extractability than unripe ones (Garrido-Bañuelos et al., 2019). Because phenolic extractability varies greatly both as a function of grape maturity and vintage, there is a need to achieve a consistent extraction of key phenolic compounds thereby counteracting changes due to maturity levels at harvest time, vintage effects or disease pressure. Numerous novel winemaking treatments are currently being assayed to extract and retain specific phenolic classes. Among them, microwave technology (MW) applied to freshly crushed grapes is a novel and ecologically friendly alternative in winemaking, as it does not require water input or any chemical aids, nor does generate any byproducts (Kwiatkowski et al., 2020). MW technology may promote phenolic extraction by enhancing mass transfer processes such as diffusion (Casassa et al., 2019a, b), or indirectly by decreasing the activity of polyphenol-oxidases through partial enzyme denaturation at the early stages of winemaking, thereby preserving phenolics (Yuan et al., 2021). In Pinot noir, MW technology produced a four-fold increase in tannin concentration, along with a decrease in the native grape yeast-derived populations (Carew et al., 2013). Another study reported that Merlot grapes harvested at three different maturity levels and submitted to MW prior to alcoholic fermentation led to initial early increases in wine color (from 175% to 300%), in unripe fruit, with wines from riper fruit showing no positive effects upon treatment with MW after 150 days post-crushing (Casassa et al., 2019a, b). In Dornfelder wines, MW technology (1,200 Watts, 80 °C), increased the extraction of total phenolics but no effect on color was observed in the finished wines after maturation (Wojdyło et al., 2021). Finally, a recently published multiyear study reported the effects of MW technology (1,200 Watts, 57 °C), applied to musts and stems from monovarietal Cabernet Sauvignon, Merlot, and Syrah wines from California (Casassa et al., 2022). In this study, MW applied to musts resulted in a 278% enhancement in flavonol extraction in Syrah wines, and overall improvements in the extraction of monoglycosilated, acylated and anthocyanin-derived pigments of all the wines. However, the previous study also reported little effect on tannin extraction upon application of MW. Overall, MW technology seems to favor the extraction of pigments into wine, but effects of lower magnitude are usually observed in other phenolic classes. In all the previous studies, there is, however, the underlying confounding factor of grape maturity, which seems to play a critical role in the outcomes of MW technology.
The goal of the present study was to document the chemical effects of MW technology applied to five very dissimilar wine varietals, namely Cabernet Sauvignon, Malbec, Nebbiolo, Pinot noir and Syrah, with focus on phenolic extraction and chromatic characteristics of their resulting wines. Additionally, sensory characteristics imparted by MW technology were determined in Cabernet Sauvignon wines. The selection of these five cultivars aimed at capturing the widest possible range of quantitative and qualitative phenolic variation within Vitis vinifera L., including cultivars whose genetic origins were very dissimilar climatic regions, including maritime climate (Cabernet Sauvignon), cool continental climate (Pinot noir), and semi-alpine, cool climate (Nebbiolo). Unique to this study, these five cultivars were grown in the semi-arid conditions of Mendoza (Argentina). Grapes were purposedly harvested later during the season to ensure relatively high phenolic extractability into wine.
2. Materials and methods
2.1. Grapes
Cabernet Sauvignon, Malbec, Nebbiolo, Pinot noir and Syrah grapes (Vitis vinifera L.) were obtained from two commercial vineyards located in Luján de Cuyo (33° 00′ S, 68° 51′ W), and La Consulta (33° 73′ S, 69° 12′ W), both located in west Mendoza, Argentina (Table 1). The fruit in all cases was harvested targeting >25 Brix, as it is common local practice for production of Premium and Ultra-premium wines (Casassa et al., 2019a, b). A total of 150 kg of fruit were harvested for each variertal into 18-kg plastic boxes for a total of 750 kg. For the grape basic analysis, three independent samples of 30 berries were analyzed at harvest for basic fruit chemistry, including Brix (Atago, Tokyo, Japan), pH (Orion model 701-A, Thermo Scientific, Waltham, MA, USA), and titratable acidity, which was determined manually by titration with 0.1 N NaOH and expressed as g/L of tartaric acid (Table 1).
Table 1.
Basic growing characteristics and chemical composition of the five different grape cvs. submitted to the winemaking treatments. Values represent the average of three independent grape samples replicates taken at harvest followed by the standard error of the mean (n = 3).
| Cultivar | Vintage | Appellation | Trellis system | Harvest date | Brix | pH | Titratable acidity (g/L) |
|---|---|---|---|---|---|---|---|
| Cabernet Sauvignon | 2014 | Luján de Cuyo | Vertical shoot positioning | 04/30 | 26.4 ± 0.1 ba | 3.64 ± 0.01 b | 4.31 ± 0.02 d |
| Malbec | 2015 | La Consulta | Guyot | 04/12 | 27.9 ± 0.1 a | 3.56 ± 0.05 c | 5.43 ± 0.12 b |
| Nebbiolo | 2015 | La Consulta | Vertical shoot positioning | 05/01 | 26.0 ± 0.1 b | 3.53 ± 0.01 c | 6.32 ± 0.05 a |
| Pinot noir | 2015 | Luján de Cuyo | Overhead canopy ("parral") | 02/12 | 25.2 ± 0.3 c | 3.69 ± 0.03 ab | 5.09 ± 0.03 c |
| Syrah | 2015 | Luján de Cuyo | Overhead canopy ("parral") | 03/12 | 27.6 ± 0.1 a | 3.73 ± 0.01 a | 5.07 ± 0.09 c |
| p-value | --- | --- | --- | --- | <0.0001b | 0.001 | <0.0001 |
a Different letters within a column and a same vintage indicate significant differences for Tukey HSD test and p < 0.05.
b Significant p -values (<0.05) are shown in bold fonts.
2.2. Winemaking and application of microwave technology
Upon each harvest, grapes were transported to the INTA experimental winery and processed in the same day of harvest as follows. Grapes were processed with a destemmer-crusher (Metal Liniers model MTL 12, Mendoza, Argentina), and the musts placed into 20-L food-grade plastic fermentors. The experimental design consisted of two winemaking treatments applied to the fruit of each cultivar, namely Control wines (not microwaved), and microwaved-treated wines (MW), with each winemaking treatment replicated three times (n = 3). The MW treatment was applied to musts immediately after crushing. Control wines were produced by adding 50 mg/L of SO2, and allowing a maceration length of 14 days at 24.5 ± 2.5 °C. Two daily punch downs (morning and afternoon, 1 min each) were applied as a part of the cap management protocol. The MW treatments were established by microwaving the musts (∼15 kg of musts/fermentor), in a household microwave oven at 1,200 Watts for 10 min (∼400 Watts/kg), reaching an average temperature of approximately 40 °C (exact average temperature attained ±standard deviation: 41.55 ± 4.11 °C, n = 450), followed by an addition of 50 mg/L of SO2. MW wines were allowed a maceration length of 14 days, following the same cap management regime applied to Control wines. The same MW protocol was applied to all five grape musts to keep this protocol constant while only varying the grape varietal. Inoculation with a commercial yeast strain (EC-1118; Lallemand Inc., Copenhagen, Denmark) at a rate of 0.3 g/L occurred 8 h after crushing in all cases. After completion of alcoholic fermentation and maceration length, wines were racked into 20-L glass carboys an inoculated with a commercial Oenococcus oeni culture (VP-41, Lallemand Inc., Copenhagen, Denmark) to induce the onset of malolactic fermentation (MLF). Malolactic fermentation was considered completed once the wines reached <0.5 g/L of malic acid or when no significant assimilation of malic into lactic acid was observed within 30 days from previous measurement. After completion of MLF, the wines were racked, added with 30 mg/L of free SO2, and allowed to undergo tartaric or cold stabilization for 45 days 1 °C. After completion of the cold stabilization period, wines were racked, adjusted to 0.5 mg/L of molecular SO2, bottled and stored horizontally at controlled and constant temperature (12 ± 1 °C).
2.3. Wine basic analysis
Basic wine parameters, including ethanol content, residual sugars, glucose, fructose, lactic and acetic acid, titratable acidity, and glycerol were determined by a FOSS Wine-Scan (FT120) rapid-scanning infrared Fourier-transform spectrometer (FOSS, Hillerod, Denmark). Density at 20 °C was measured directly with a densimeter (Fite, Buenos Aires, Argentina), and pH with a pH-meter Orion model 701-A (Thermo Scientific, Waltham, MA, USA). Malic acid was measured by enzymatic determination (Vintessential Laboratories, Victoria, Australia), whereas titratable acidity (as g/L tartaric acid) was measured by titration with 0.1 N NaOH as previously described (Iland et al., 2012).
2.4. Spectrophotometric analysis of the wines
Wine phenolic compounds and chromatic parameters were determined spectrophotometrically at pressing and post-bottling, as it was considered necessary to document the effect of MW technology beyond its previously reported effects immediately after pressing (Casassa et al., 2019a, b). Cabernet Sauvignon wines were further analyzed after 3 months of bottle aging. Prior to spectrophotometric analysis, wine samples were centrifuged at 1,600 g (Gelectronic G-49, Buenos Aires, Argentina), and subsequently filtered through a 0.45-μm membrane (Sartorius, Goettingen, Germany). Spectrophotometric measurements were carried out with a PerkinElmer Lambda 25 UV-visible spectrophotometer (PerkinElmer, Hartford, CT, USA). Wine color was determined using the CIELab system, including L∗ (lightness), C∗ (saturation or chroma), H∗ (hue angle), a∗ (green/red component), and b∗ (blue/- yellow component) as described previously (Pérez-Caballero et al., 2003), using MSCV™ software (Grupo de Color de La Rioja, Logroño, Spain).
Total phenolics (as mg/L (+)-catechin equivalents, CE), and anthocyanins (expressed as mg/L malvidin-3-glucoside), were measured as previously detailed (Harbertson et al., 2003). Protein precipitable tannins (mg/L CE), were determined by protein precipitation with bovine serum albumin (BSA, Fraction V, 1 g/L solution), and large polymeric pigments (LPP), and small polymeric pigments (SPP) as previously described (Harbertson et al., 2003). Total polymeric pigments (TPP) were calculated as LPP + SPP and are heretofore referred as “polymeric pigments”.
2.5. HPLC-DAD-mass spectrometry (MS) analysis of the wines
The anthocyanin and anthocyanin-derived pigment composition of Malbec, Nebbiolo, Pinot noir and Syrah wines was determined post-bottling by HPLC-DAD-(ESI)-MS as previously described (Blanco-Vega et al., 2011). The HPLC system consisted of a PerkinElmer Series 200 HPLC-DAD with a quaternary pump and an autosampler (PerkinElmer, Hartford, CT, USA) and a reversed-phase column (Chromolith Performance C18, 100 mm × 4.6 mm i.d., 2 μm; Merck (Darmstadt, Germany)), protected with a Chromolith guard cartridge (10 mm × 4.6 mm). The gradient occurred at 25 °C and consisted of solvent A (water/formic acid, 90:10, v/v) and solvent B (acetonitrile), applied at a flow rate of 1.1 mL/min from 0 to 22 min and 1.5 mL/min from 22 to 35 min. The gradient was as follows: 96 to 85%A from 0 to 12 min; 85 to 85%A from 12 to 22 min; 85 to 70%A from 22 to 35 min; followed by a final 100% methanol wash and column re-equilibration. Diode-array-detection (DAD) was performed from 210 to 600 nm, and the quantification was carried out using peak area measurements at 520 nm. Anthocyanins and anthocyanin derivatives were quantified using a calibration curve (R2 = 0.99) based on malvidin-3-O-glucoside chloride as standard (Extrasynthèse, Genay, France).
2.6. Sensory descriptive analysis of Cabernet Sauvignon wines
Cabernet Sauvignon wines and their replicates of the two treatments (Control and MW), were submitted to sensory descriptive analysis after 3 months of bottle aging. The trained panel consisted of 21 individuals with previous experience in wine sensory analysis, aged 24 to 65 years-old. Training consisted of two sessions to accomplish terminology development, attribute definition and familiarization with the standards. Panelists defined by consensus the following attributes: color saturation, purple hue, vegetal aroma, fruity aroma, bitterness, and astringency), with a provided definition and/or a standard (Supplemental Table 1). One formal evaluation session was held throughout the experiment. The intensity of each attribute was evaluated in a non-structured 10-cm line scale with reference points located at 1 cm of each end of the line. All wines replicates were poured in 25-mL aliquots into ISO wine glasses and presented to each panelist following a balanced, complete block design. Panelists were instructed to rinse their mouth with mineral water and eat a cracker between wines samples following to minimize sensory fatigue and carry-over. Informed consent was obtained from the members of the panel and their identity during data handling was kept confidential.
2.7. Data analysis
The basic fruit chemical composition was analyzed by a one-way analysis of variance (ANOVA). The basic, phenolic, and chromatic composition of the wines of each wine varietal were analyzed at the last sampling point (post-bottling in all wines and 3 months after bottle aging in Cabernet Sauvignon wines), by a series of fixed-effect one-way ANOVAs. In addition, the full data set was reevaluated by a series of fixed effect two-way ANOVAs with interactions, including as main effects the wine varietal and the two winemaking techniques (Control and MW-treated wines), as well as the wine varietal × winemaking treatment interaction (Supplemental Tables 2, 3, 4, and 5). The data generated by the sensory descriptive analysis panel was analyzed by a fixed-effect one-way ANOVA. In all cases, Fisher’s LSD test was used as a post-hoc comparison of means with a 5% level for rejection of the null hypothesis. Partial Least Square Regression analysis (PLSR) was performed using the detailed anthocyanin composition and general phenolic composition of the all the wines as predictors of the chromatic composition of the resulting wines. Data analysis of chemical and sensory data sets, including PLSR, was performed using XLSTAT v. 2019 (Addinsoft, Paris, France).
3. Results and discussion
3.1. Basic chemical composition of the fruit at harvest
Monovarietal wines from five chemically very distinct cultivars grown to full ripeness were made into wine applying MW technology. The selection of these five cultivars aimed at capturing the widest possible range of quantitative and qualitative phenolic variation within Vitis vinifera L., including cultivars whose genetic origins were very dissimilar climatic regions, including maritime climate (Cabernet Sauvignon), cool continental climate (Pinot noir), and semi-alpine, cool climate (Nebbiolo). Unique to this study, these five cultivars were grown in the semi-arid conditions of Mendoza (Argentina). Intendedly, the grapes of all the five cultivars showed very high sugar levels and advanced ripeness at harvest time (Table 1), which can be considered physiological overripeness. The ripest examples were Malbec and Syrah, which reached sugars levels above 27 Brix, whereas Pinot noir achieved comparatively lower sugar levels (∼25 Brix) due to higher yields than the other cultivars (data not shown), and a trellis system (overhead canopy positioning), conducent to that effect.
3.2. Basic chemical composition of the wines
Supplemental Table 2 shows a two-way ANOVA separating the effect of the winemaking treatment, namely MW technology versus Control wines, as well as their interaction. Ethanol levels were aligned with the high sugar levels observed at harvest time. As expected, the factor “wine varietal” significantly affected the basic chemical parameters of the wines. The MW process applied to these five cultivars also affected some of the basic chemical parameters, namely ethanol, titratable acidity, and glycerol, which were higher in MW-treated wines. However, the effect of MW technology on these parameters was of lesser magnitude relative to the effect of the wine varietal, and thus unlikely to be of sensory relevance.
3.3. Phenolic and chromatic composition of the five monovarietal wines
3.3.1. Cabernet Sauvignon wines
Cabernet Sauvignon wines were followed during winemaking and up to 3 months of bottle aging, at which point a formal sensory analysis was also performed on these wines (section 3.5). At pressing, extraction of anthocyanins, tannins, total phenolics and non-tannin phenolics were enhanced by MW, with improvements of 36, 53, 35 and 30% relative to Control wines, respectively (Figure 1A). Because anthocyanins are primarily responsible for wine color, initial improvements of the CIELab parameters L∗ (lightness), C∗ (saturation or chroma), and a∗ (red hue) in favor of MW-treated wines were also observed (Figure 1 B).
Figure 1.
Detailed A) phenolic and, B) chromatic composition (CIELab values), of Cabernet Sauvignon wines processed with and without microwave technology. BA: bottle aging. Different letters in the last sampling point indicate significant differences for Fisher’s LSD test and p < 0.05.
Polymeric pigments encompass an heterogenous chemical array of pigments of variable molecular weight formed either via direct covalent reactions between anthocyanins and tannins, and/or mediated by acetaldehyde, and/or other carbonyl intermediaries (Es-Safi et al., 2000). Polymeric pigments form gradually during wine aging and because they are resistant to bisulfite bleaching (Kumar et al., 2022), they provide stable color and desirable mouthfeel characteristics (Casassa and Harbertson, 2014). The application of MW to Cabernet Sauvignon wines improved polymeric pigment formation at pressing by 35% (Figure 1A). This initial increase can be accounted for by the positive enhancing effect of MW in the initial anthocyanin and tannin extraction in these wines, which act as primary building blocks of polymeric pigments.
After 3 months of bottle aging, total and non-tannin phenolics, polymeric pigments, a∗, b∗ (yellow hue when positive values), and H∗ (hue angle) still showed significant differences favoring MW-treated wines. Of note was a 30% improvement in polymeric pigment content after 3 months of bottle aging in parallel with a general drop in the anthocyanin and tannin content of the MW-treated wines, which in turn equalized the tannin to anthocyanin ratio of Control and MW-treated wines at this time (Figure 2). A previous study in Cabernet Sauvignon wines in which MW technology was applied to both musts and stems prior to alcoholic fermentation reported similar trends whereby MW-treated wines showed initial increases in anthocyanins and tannins relative to their respective Control wines (Casassa et al., 2022). However, in the previous study, some of these improvements, notably that of anthocyanins and total phenolics, remained after 12 months of bottle aging. In the present study, initial enhanced extraction of anthocyanins and tannins derived from the application of MW technology was followed by a decline. Consequently, in the last sampling point the tannin to anthocyanin ratio of Control and MW-treated wines was close to 1 (Figure 2). A study of MW technology applied to Merlot wines made from fruit harvested at contrasting maturity levels showed that MW was more effective in inducing long-lasting (positive) chemical changes in underripe fruit (21.1 Brix) relative to very ripe fruit (25.1 Brix) (Casassa et al., 2019). This observation aligns with the results presented here whereby MW applied to very ripe Cabernet Sauvignon fruit (26.4 Brix) had relatively minor chemical effects. Similarly, in Shiraz harvested with 21, 23 and 25 Brix, ripe fruit (25 Brix), showed enhanced extraction of polymeric phenols into wine, which was correlated with the deconstruction or “opening-up” of the grape pomace during maceration mediated by enhanced de-pectination of cell walls in riper fruit (Garrido-Bañuelos et al., 2019). Moreover, the addition of pectolytic enzymes (Nel et al., 2014), or presence of increasing levels of ethanol have also been shown to favor phenolic extraction in unripe fruit (Canals et al., 2005). When these results are compounded, it appears that unripe fruit may show improved extraction in conditions of higher levels of ethanol, enzymatic activity, or thermal processes that may favor de-pectination and therefore compromise the integrity of cell walls, thereby increasing extractability of cellular components.
Figure 2.
Comparative content of polymeric pigments and tannin to anthocyanin ratios in Cabernet Sauvignon, Malbec, Nebbiolo, Pinot noir and Syrah wines processed with and without microwave technology.
3.3.2. Malbec wines
Malbec wines were assessed at pressing to capture the full range of phenolic compounds extracted after the maceration and reassessed after bottling, once the wines were tartrate-stable and added with SO2 (Figure 3A). As well, the aggregate phenolic data including Malbec wines and that of the other four wine varietals, plus the two winemaking treatments was reassessed by a two-way ANOVA. Malbec wines showed high tannin levels relative to the other wine varietals, with a tannin to anthocyanin ratio of 0.99 (Figure 2), which was in turn related (though not necessarily causatively), with a high content of polymeric pigments (Supplemental Table 3). Malbec wines displayed the highest anthocyanin content (almost 9-fold higher than Nebbiolo), and accordingly, the higher color saturation (C∗), as well as red hue values (a∗), among all the wines (Supplemental Table 4).
Figure 3.
Detailed phenolic and chromatic composition (CIELab values), of A) Malbec, B) Nebbiolo, C) Pinot noir and, D) Syrah wines processed with and without microwave technology. Different letters in the last sampling point indicate significant differences for Fisher’s LSD test and p < 0.05.
In Malbec wines, the application of MW increased anthocyanins by 24% (Figure 3A). However, the remanding phenolic and chromatic parameters, except for polymeric pigments and b∗ values, were unaffected by MW technology. In MW-treated wines, the enhancement of polymeric pigments (22% increase), occurred concomitantly with higher (positive) b∗ values in these wines, which denote yellow hues. Contrastingly, at bottling, Control wines were distinctively blue in hue, as denoted by negative b∗ values. Polymeric pigments are chemically heterogeneous and, as a result, chromatically diverse as well. For example, it has been shown that polymeric pigments display a higher 280 to 520 nm absorbance ratio (Casassa et al., 2013), and lower molar extinction coefficient (Laitila and Salminen, 2020), compared to intact anthocyanins, thus displaying more yellow or orange-red hues. This may explain why MW-treated Malbec wines, having higher polymeric pigment content, showed higher b∗ values (Figure 3A), and possibly contributed with a more red-orange/yellow hue to the overall color displayed by their respective wines.
3.3.3. Nebbiolo wines
Figure 3B shows the evolution from pressing to post-bottling of key phenolic compounds as well as chromatic characteristic of Nebbiolo wines. Supplemental Tables 3 and 4 show these key parameters in the context of the other four wine varietals and winemaking treatments via respective two-way ANOVA. Nebbiolo wines had the lowest anthocyanin content, followed by Pinot noir wines, which showed the second lowest anthocyanin content (Supplemental Table 3). Accordingly, Nebbiolo wines showed the lowest color saturation (C∗) and red hue (a∗), and the highest yellow hue (b∗). However, the tannin content of these wines was the highest, being about 162% higher than that of Pinot noir wines. As a result, Nebbiolo wines showed a tannin to anthocyanin ratio of 16, in sharp contrast with the remaining four wine varietal wines, in which the tannin to anthocyanin ration never exceeded 2 (Figure 2). Total phenolics and non-tannin phenolics were also significantly higher in Nebbiolo wines. Although there are no comparative studies reporting tannin levels in Nebbiolo wines relative to other international wine varietals, a recent study found Nebbiolo wines to be high in total phenolics and a parameter dubbed “tannin specific activity”, which reportedly implies these wines have tannins with high affinity towards salivary proteins (Azevedo et al., 2020). The reaction between wine tannins and salivary proteins, mediated by both hydrogen-bonding and hydrophobic interactions, underpins the molecular basis of astringency perception in wines. Thus, because the Nebbiolo wines of the present study showed exceedingly high levels of reactive tannins, it is plausible these may also display very high levels of astringency. However, this sensory parameter was not determined in the present study.
The application of MW to these very ripe Nebbiolo grapes had barely any effect on the basic phenolic and chromatic composition of their resulting wines (Figure 3B). This could be due to very ripe Nebbiolo grapes having naturally higher extractability at harvest time, implying MW would be less efficient in extracting phenolics in fully ripe fruit. This is indeed plausible, as a study reported that enhanced phenolic extractability in Nebbiolo occurred in denser and thus riper grapes (Rolle et al., 2011). Only a∗ values (red hue) were significantly affected by the winemaking treatments, being higher in Control wines (Figure 3B).
3.3.4. Pinot noir wines
Figure 3C shows the phenolic and chromatic composition of Pinot noir wines from pressing to post-bottling whereas Supplemental Tables 3 and 4 show respective two-way ANOVA of phenolic and chromatic characteristics. Pinot noir wines showed levels of phenolics and chromatic characteristics consistent with those previously observed in Pinot noir wines from the same geographic origin (Casassa et al., 2019). However, tannin levels of the Pinot noir wines of the present study were higher than the reported average tannin content of 348 mg/L measured in a population of 261 Pinot noir wines (Harbertson et al., 2008). As such, the tannin to anthocyanin ratio of these wines was 1.74 (Figure 2).
The application of MW had no effect in any of the phenolic and chromatic characteristics of Pinot noir wines. As alluded before, this lack of effect of the MW treatment could be the result of the naturally higher phenolic extractability of very ripe grapes, whereby an enhancing technology such as MW seemed to exert a lesser or even nil effect on phenolic extractability relative to the untreated Control treatment. Another explanation for this lack of effect of MW could be the working temperature attained in the musts of the present study, which was 40 °C, with a total residence time of 10 min. The working temperature applied in the present study contrast with a previous report of MW technology applied to Pinot noir and resulting in working temperatures of 70 to 71 °C, which, once achieved, were hold for 10 min (Carew et al., 2013). Of note was the fact that Pinot noir wines of the present study had the lowest but comparable polymeric pigment content than Nebbiolo wines, despite drastically different tannin to anthocyanin ratios of 1.74 and 16, respectively (Figure 2).
3.3.5. Syrah wines
Figure 3D shows the evolution of phenolic compounds and chromatic characteristic in Syrah wines processed with and without MW technology. Syrah wines showed the highest content of polymeric pigments (Figure 2, Supplemental Table 3), and were the second most saturated wines after Malbec wines (Supplemental Table 4). In Syrah wines, MW increased total phenolics (18%), non-tannin phenolics (24%) and polymeric pigments (31%) relative to Control wines post-bottling (Figure 3D). A previous report also in Syrah musts treated with MW technology found increases in flavonol content up to 278% at day 1,235 post-crushing (Casassa et al., 2022). Flavonols act as copigmentation factors, engaging non-covalently with anthocyanins and resulting in hyperchromic (i.e., more color) and bathochromic shifts (i.e., blue hues), in the visible spectrum of the wines. Flavonols may also influence mouthfeel properties, such as the perception of a velvety astringency subquality (Hufnagel and Hofmann, 2008), and taste sensations such as bitterness. Whereas in the present study flavonols were not directly determined, it is possible that these were also higher in MW-treated wines, as reflected by the concomitant enhancement of total phenolics in these wines resulting from the application of MW.
As previously stated, Syrah wines showed the highest content of polymeric pigments, which was favored in MW-treated wines. These Syrah wines had tannin to anthocyanin ratios which were comparable with those observed in Pinot noir wines, yet the latter wines showed the lowest polymeric pigment content (Figure 2). Therefore, the results herein presented suggest that wines with very comparable tannin to anthocyanin ratios can produce wines of vastly different polymeric pigment content.
3.4. Detailed anthocyanin composition and Partial Least Square Regression (PLSR) of phenolic and chromatic data
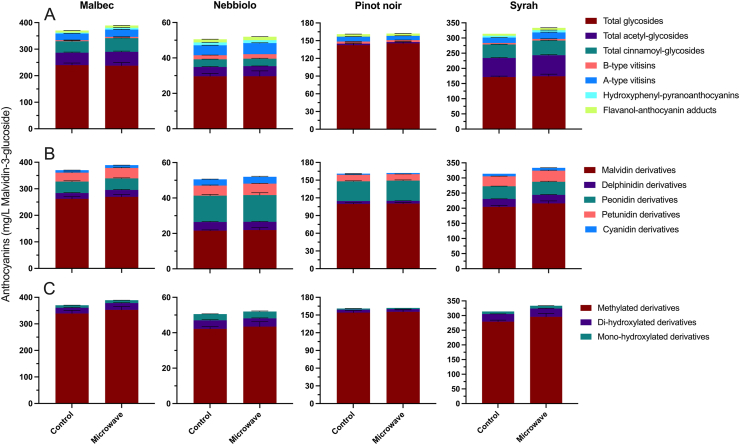
Figure 4 shows the detailed anthocyanin composition of Malbec, Nebbiolo, Pinot noir and Syrah wines. All these wines came from a single vintage, namely 2015 (Table 1). Anthocyanins and anthocyanin derivatives were grouped as a function of the acylation pattern (Figure 4A), the aglycone moiety (Figure 4B), and the methylation/hydroxylation pattern (Figure 4C). Supplemental Figure 1 shows the results of a PLSR analysis in which the detailed anthocyanin composition and general phenolic composition of the wines were used as predictors of the chromatic composition of the resulting wines.
Figure 4.
Comparative anthocyanin composition showing selected anthocyanin classes grouped as a function of A) the acylation pattern and the presence of pyranoanthocyanins; B) the aglycone moiety; and C) the methylation and hydroxylation pattern of the aglycone of Malbec, Nebbiolo, Pinot noir and Syrah wines processed with and without microwave technology. Note the different scale, range, and magnitude in the Y-axis for each monovarietal wine and across the different anthocyanin classes.
The total content of acetyl-glycosides, cinnamoyl-glycosides, A, pyranoanthocyanins (including A- and B-type visitins), and flavonol-anthocyanin adducts were all slightly increased in MW-treated wines (Supplemental Table 5). However, these differences were so small in magnitude that are unlikely to be of any practical or sensory relevance. In contrast, vastly large quantitative differences as a function of each wine varietal were observed. For example, Malbec and Syrah wines showed comparable levels of total anthocyanins in the 300 to 350 mg/L range, whereas Nebbiolo wines showed trace amounts of anthocyanins (∼50 mg/L), which were in turn much lower than the anthocyanin content reported elsewhere for Nebbiolo wines (320 to 370 mg/L post-pressing) (Río Segade, Pace, Torchio, Giacosa, Gerbi and Rolle, 2015). Syrah wines showed the highest concentration of acetyl-glycosides, followed by Malbec wines. Acylation in the anthocyanin moiety generally causes a bathochromic shift towards more purple color in the pigment, possibly due to intramolecular copigmentation reactions (Brouillard et al., 2003). This higher content of acetyl-glycosides may explain the comparatively lower b∗ values observed in Malbec and Syrah wines (Supplemental Table 4). As b∗ values approach zero, or reach negative values, the hue of the wine shifts from yellow (positive b∗ values) to purple/blue (negative b∗ values). The PLSR analysis confirmed this trend whereby Malbec and Syrah wines were inversely related with (positive, i.e. yellow), b∗ values (Supplemental Figure 1).
Whereas Pinot noir wines showed a characteristically high proportion of anthocyanin glycosides along with only trace amounts of acylated anthocyanins (Dimitrovska et al., 2011), Nebbiolo wines showed relatively high levels of type B and especially type A vitisins (Figure 4A). Vitisins A and B are pyranoanthocyanins, formed by covalent reactions between monomeric anthocyanins and either pyruvic acid (vitisin A), or acetaldehyde (vitisin B) (Bakker and Timberlake, 1997). The proportion of pyranoanthocyanins in Nebbiolo wines was high, accounting for 18% of the anthocyanin profile, as compared with Syrah, in which pyranoanthocyanins accounted for only 9% of the anthocyanin profile of these wines. The higher proportion of pyranoanthocyanins, with their characteristic hypsochromic shift bestowing them with orange to orange-brown hues (Bakker and Timberlake, 1997), and comparatively lower molar extinction coefficients relative to that of intact anthocyanins (Håkansson et al., 2003), may explain the typical low color saturation and hue observed in Nebbiolo wines. Thus, the color of both Nebbiolo and Pinot noir wines, characterized by higher values of lightness (L∗), b∗ (yellow/orange hues), and H∗ (hue angle) (Supplemental Figure 1), can be explained by comparatively higher levels of pyranoanthocyanins in these wines.
Important insights were also derived from the analysis of anthocyanins in the finished wines grouped as a function of the aglycone moiety (Figure 4B). The main anthocyanin aglycones included malvidin, delphinidin, cyanidin, peonidin, petunidin and pelargonidin (He et al., 2012), although the later was not found in the current set of wines. Malvidin derivatives dominated the anthocyanin profile of Malbec, Syrah and especially Pinot noir wines, and were relatively unaffected by the application of MW technology. In contrast, Nebbiolo wines showed the highest proportion of peonidin derivatives and a more evenly distribution of anthocyanin aglycones than the other four varietal wines. Peonidin-derivatives show red rather than purple hues due to their O-methylation pattern (Casassa, 2017), and are prevalent, along with malvidin-derivatives, in the anthocyanin profile of Nebbiolo grapes (Guidoni et al., 2002) and wines (Río Segade et al., 2015). The latter is consistent with the results herein presented and suggests that the qualitative anthocyanin composition of Nebbiolo wines, and indeed more generally that of Vitis vinifera L. wines, may be little affected by drastically different climatic, soil, and geographical growing conditions, which will predominately affect their concentration.
Finally, anthocyanins were grouped as a function of the methylation and hydroxylation pattern of the aglycone (Figure 4C). The methylation and hydroxylation pattern of anthocyanins bear implications for both chemical stability and color, because the presence of hydroxyl and methyl groups in the flavylium form of anthocyanins causes electron delocalization within the structure. For example, anthocyanins with more hydroxyl groups in the B ring (such as delphinidin and cyanidin), show bluer hues, whereas the degree of methylation of the B rings (as in malvidin, peonidin and petunidin), causes red hues (He et al., 2012). The most noteworthy insight of this analysis was the almost exclusive presence of methylated derivatives in Pinot noir wines. Methylated derivatives, of which malvidin-3-glucoside is the predominant form, also show enhanced chemical stability (He et al., 2012). This suggest that Pinot noir wine color, while lower in saturation relative to three of the other varietal wines (Supplemental Figure 1), may indeed be more stable over time, as previously suggested by phenolic chemist Raymond Brouillard (Brouillard et al., 2003). Further studies linking chromatic changes observed in Pinot noir wines with that of changes in monomeric anthocyanins, polymeric pigments, and pyranoanthocyanins should be instituted to confirm these results.
Of note as well was the lack of effect of the application of MW on the anthocyanin profile of Nebbiolo and Pinot noir wines. On the other hand, modest increases in most anthocyanin classes were observed in MW-treated Malbec and Syrah wines, relative to their Control counterparts. Overall, the absolute content of most anthocyanins and anthocyanin-derived pigments was significantly higher in Malbec and Syrah wines, which in turn explained comparatively higher values of C∗ (saturation) and a∗ (red hues) in their respective wines (Figure 3A and C). Even though the different anthocyanin classes varied greatly as a function of each of the four wine cultivars, it should be noted that these were harvested at varying times during the vintage, and they were produced from grapes with different cultivation methods (Table 1) which could further explain observed differences in the detailed anthocyanin composition.
3.5. Sensory analysis of Cabernet Sauvignon wines
A trained panel of 21 experienced assessors was convened to describe the sensory characteristics of MW-treated and untreated Cabernet Sauvignon wines. Figure 5 shows a spider plot with the comparative sensory profile of Control and MW-treated Cabernet Sauvignon wines, as well as significance levels of p < 0.05, p < 0.01 and p < 0.0001. Relative to Control wines, MW-treated wines were higher in most of the descriptive sensory terms except for vegetal, which showed less intensity in MW-treated wines. On the other hand, color saturation and purple hue, among color descriptors, and fruity aroma and astringency, among aroma and tactile descriptors, were enhanced in MW-treated wines. These sensory results are consistent with MW-treated wines showing higher values of a∗ (red hue), and lower b∗ values (blue hues when negative), than their Control counterparts (Figure 1B). As previously discussed, polymeric pigments were 30% higher in Cabernet Sauvignon MW-treated wines, but because these may have comparatively lower molar extinction coefficients than intact anthocyanins, it is expected that enhance polymeric pigment formation may have a larger impact in astringency perception than in color saturation, which was indeed observed in these wines (Figure 5). It is also possible that not only overall astringency, but astringency subqualities may be different in these MW-treated wines. This is because polymeric pigments, which were enhanced by MW-treated Cabernet Sauvignon wines, have potentially desirable mouthfeel properties (Casassa and Harberston, 2014) that may trascend astringency. Likewise, MW-treated wines showed enhanced bitterness, which could be related to the higher non-tannin phenolic content of these wines (Figure 1A). Non-tannin phenolics include monomeric flavan-3-ols, flavanols as well as small dimers that cannot precipitate proteins (Harbertson et al., 2014), but because of their smaller molecular weight, they can enter taste pores within the taste bud structure and elicit bitterness (Cheynier, 2006). Thus, comparatively higher content of non-tannin phenolic in MW-treated Cabernet Sauvignon wines explain enhanced perceived bitterness in these wines.
Figure 5.
Spider plot of sensory attributes of Cabernet Sauvignon wines processed with and without microwave technology evaluated by a sensory panel after 3 months of bottle aging. (∗), (∗∗), (∗∗∗) denote significant differences for Fisher’s LDS test and p < 0.05, 0.01 and 0.001, respectively.
Finally, MW-treated Cabernet Sauvignon wines showed enhanced fruitiness and a decrease in vegetal aromas. A previous study in which Grenache, Carignan and Fer wines were treated by heat during 2 h at 70 °C, reported enhanced fruitiness in the resulting wines, which was ascribed to enhanced ester formation and terpene retention (Geffroy et al., 2015), in agreement with the results of the present study. Vegetal aromas in Cabernet Sauvignon are attributed to isobutyl-methoxypyrazine and isopropyl-methoxy-pyrazine, collectively referred to as pyrazines. Pyrazines seem to be susceptible to thermal degradation, and thus thermal processing of musts may help curb their content (Hashizume et al., 1998). A study in which Merlot musts were treated with MW technology found a decreased in the perception of herbaceous aromas in MW-treated wines (Casassa et al., 2022). This agrees with the present results and suggest that, on the aggregate, MW technology can be used as a processing tool to decrease vegetal aromas in varietals known to have pyrazines such as Cabernet Sauvignon, Merlot and Carmenère, among others. However, these results must be confirmed by analysis of these and other key volatile compounds by GC-MS.
4. Conclusions
The present study included five different wine varietals, chosen on the basis of their dissimilar phenolic composition, which were systematically treated with the same MW-technology protocol. The wines were followed throughout winemaking to assess the impact of MW technology, as well as the comparative impact of the inherent phenolic and chromatic profile of each wine varietal. The overarching conclusion regarding the application of MW technology was one of a moderate impact of this technology on most phenolic classes. Variations in phenolic composition due to each wine varietal were therefore more prominent than those elicited by the application of MW technology. Whereas previous literature has reported effects of larger magnitude upon application of MW technology to grapes and musts, the modest improvement observed in the present study may be related to fruit maturity and phenolic extractability. Indeed, the present study used overripe grapes, which are expected to have naturally high phenolic extractability. In such conditions, the application of MW may only and temporarily boost mass transfer processes, which are transient and ultimately may be ineffectual for phenolic retention over time.
Another potential explanation for the modest effects achieved by MW could be that of a subpar working temperature during the MW process. Considering these assumptions, it is recommended to direct research efforts towards the understanding of the effects of high-temperature MW technology (>50 °C) applied to unripe fruit. The application of high-temperature MW processing would also be recommended for processing fruit affected by fungal diseases such as powdery mildew or Botrytis sp., which may force the winemaker to harvest earlier than planned, in which conditions there is a risk of low phenolic extractability into wine. Likewise, higher working temperatures, in the range of 60 to 70 °C, may achieve positive results even in ripe fruit. Optimization studies (residence time and effective working temperature) should be also carried out in the future, as different wine varietals may require different optimal ranges for these two parameters.
The results of present study, by surveying wines with vastly different phenolic composition, suggest that wines with vastly different tannin to anthocyanin ratios (T:A), produced comparable levels of polymeric pigments. For example, Nebbiolo (T:A of 16) and Pinot noir (T:A of 1.6) resulted in comparable levels of polymeric pigments. However, even though comparable in absolute amounts, these polymeric pigments are very likely compositionally different between the two wine varietals. The implication of this is that Nebbiolo wines may own much of their astringency to native tannins rather than polymeric pigments. We suggest that the intensively high, drying astringency sensation often perceived in iconic Nebbiolo-based wines such as Barolo and Barbaresco from Piemonte (Italy), may be explained (although not exclusively), by very high levels of native tannins. Conversely, this study has also shown that wines with vastly different levels of polymeric pigments had very similar and comparable T:A. For example, with a T:A of 2, Pinot noir wines resulted in about 0.6 AU of polymeric pigments, whereas the same T:A ratio in Syrah wines resulted in about 4 AU of polymeric pigments. Although this ratio relativizes differences in tannin concentrations between wine varietals, our results suggest that the formation of polymeric pigments is not solely modulated by anthocyanins and tannins but possibly by other factors including wine pH, free SO2 levels, ethanol and polysaccharide contents. In the case of Syrah, it is also possible that much of the perceived astringency and astringency subqualities observed in these wines may be derived from polymeric pigments rather than from native tannins. As such, potentially positive astringency subqualities such as “velvety”, “suede” or “round”, sometimes used to typify the mouthfeel of Syrah wines, may be related with enhanced polymeric pigment content in these wines. Such a hypothesis will have to be tested by follow-up studies determining the detailed polymeric pigment composition of varietal wines and relating that to astringency and astringency subqualities.
Declaration
Author contribution statement
L. Federico Casassa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Martin L. Fanzone, Santiago E. Sari: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Instituto Nacional de Tecnología Agropecuaria (INTA) [MZASJ-1251101].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
INTA is thanked for financial and logistical support of the project as well as for donation of the grapes. INTA personnel are thanked for help during harvest and winemaking. Members of the sensory panel are acknowledged and thanked for their commitment with the sensory part of this study. Vanesa Garcia is acknowledged for skillful analytical support and Esteban Bolcato for support during the winemaking of the experiment wines.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Azevedo J., Brandão E., Soares S., Oliveira J., Lopes P., Mateus N., de Freitas V. Polyphenolic characterization of Nebbiolo red wines and their interaction with salivary proteins. Foods. 2020;9(12):1867. doi: 10.3390/foods9121867. https://mdpi.com/2304-8158/9/12/1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J., Timberlake C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997;45(1):35–43. [Google Scholar]
- Blanco-Vega D., López-Bellido F.J., Alía-Robledo J.M., Hermosín-Gutiérrez I. HPLC–DAD–ESI-MS/MS characterization of pyranoanthocyanins pigments formed in model wine. J. Agric. Food Chem. 2011;59(17):9523–9531. doi: 10.1021/jf201546j. [DOI] [PubMed] [Google Scholar]
- Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: a critical review. Am. J. Enol. Vitic. 2001;52(2):67–87. https://ajevonline.org/content/52/2/67 [Google Scholar]
- Brouillard R., Chassaing S., Fougerousse A. Why are grape/fresh wine anthocyanins so simple and why is it that red wine color lasts so long? Phytochemistry. 2003;64(7):1179–1186. doi: 10.1016/s0031-9422(03)00518-1. [DOI] [PubMed] [Google Scholar]
- Canals R., Llaudy M.C., Valls J., Canals J.M., Zamora F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005;53(10):4019–4025. doi: 10.1021/jf047872v. [DOI] [PubMed] [Google Scholar]
- Carew A.L., Sparrow A., Curtin C.D., Close D.C., Dambergs R.G. Microwave maceration of Pinot Noir grape must: sanitation and extraction effects and wine phenolics outcomes. Food Bioprocess Technol. 2013;7:954–963. [Google Scholar]
- Casassa L.F. In: Phenolic Compounds - Natural Sources, Importance and Applications. Soto-Hernandez M., Palma-Tenango M., Garcia-Mateos M.d.R., editors. InTech; Rijeka: 2017. Flavonoid phenolics in red winemaking. Ch. 06) [Google Scholar]
- Casassa L.F., Gannett P.A., Steele N.B., Huff R. Multi-year study of the chemical and sensory effects of microwave-assisted extraction of musts and stems in Cabernet Sauvignon, Merlot and Syrah wines from the Central Coast of California. Molecules. 2022;27(4):1270. doi: 10.3390/molecules27041270. https://mdpi.com/1420-3049/27/4/1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casassa L.F., Harbertson J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014;5(1):83–109. doi: 10.1146/annurev-food-030713-092438. [DOI] [PubMed] [Google Scholar]
- Casassa L.F., Larsen R.C., Beaver C.W., Mireles M.S., Keller M., Riley W.R., Smithyman R., Harbertson J.F. Impact of extended maceration and Regulated Deficit Irrigation (RDI) in Cabernet Sauvignon wines: characterization of proanthocyanidin distribution, anthocyanin extraction, and chromatic properties. J. Agric. Food Chem. 2013;61(26):6446–6457. doi: 10.1021/jf400733u. [DOI] [PubMed] [Google Scholar]
- Casassa L.F., Sari S.E., Bolcato E.A., Diaz-Sambueza M.A., Catania A.A., Fanzone M.L., Raco F., Barda N. Chemical and sensory effects of cold soak, whole cluster fermentation, and stem additions in Pinot noir wines. Am. J. Enol. Vitic. 2019;70(1):19–33. [Google Scholar]
- Casassa L.F., Sari S.E., Bolcato E.A., Fanzone M.L. Microwave-assisted extraction applied to Merlot grapes with contrasting maturity levels: effects on phenolic chemistry and wine color. Fermentation. 2019;5(1):15. https://mdpi.com/2311-5637/5/1/15 [Google Scholar]
- Cheynier V. In: Flavonoids, Chemistry, Biochemistry and Applications. Øyvind K.R.M., Andersen M., editors. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2006. Flavonoids in wine; pp. 263–318. [Google Scholar]
- Dimitrovska M., Bocevska M., Dimitrovski D., Murkovic M. Anthocyanin composition of vranec, Cabernet Sauvignon, Merlot and Pinot noir grapes as indicator of their varietal differentiation. Eur. Food Res. Technol. 2011;232(4):591–600. [Google Scholar]
- Es-Safi N.-E., Cheynier V., Moutounet M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their Implication in food color change. J. Agric. Food Chem. 2000;48(12):5946–5954. doi: 10.1021/jf000394d. [DOI] [PubMed] [Google Scholar]
- Fulcrand H., Dueñas M., Salas E., Cheynier V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006;57(3):289–297. https://ajevonline.org/content/57/3/289 [Google Scholar]
- Garrido-Bañuelos G., Buica A., Schückel J., Zietsman A.J.J., Willats W.G.T., Moore J.P., Du Toit W.J. Investigating the relationship between cell wall polysaccharide composition and the extractability of grape phenolic compounds into Shiraz wines. Part II: extractability during fermentation into wines made from grapes of different ripeness levels. Food Chem. 2019;278:26–35. doi: 10.1016/j.foodchem.2018.10.136. [DOI] [PubMed] [Google Scholar]
- Geffroy O., Lopez R., Serrano E., Dufourcq T., Gracia-Moreno E., Cacho J., Ferreira V. Changes in analytical and volatile compositions of red wines induced by pre-fermentation heat treatment of grapes. Food Chem. 2015;187:243–253. doi: 10.1016/j.foodchem.2015.04.105. [DOI] [PubMed] [Google Scholar]
- Guidoni S., Allara P., Schubert A. Effect of cluster thinning on berry skin anthocyanin composition of Vitis vinifera cv. Nebbiolo. American Journal of Enology and Viticulture. 2002;53(3):224–226. https://ajevonline.org/content/53/3/224 [Google Scholar]
- Håkansson A.E., Pardon K., Hayasaka Y., de Sa M., Herderich M. Structures and colour properties of new red wine pigments. Tetrahedron Lett. 2003;44(26):4887–4891. [Google Scholar]
- Hanlin R.L., Kelm M.A., Wilkinson K.L., Downey M.O. Detailed characterization of proanthocyanidins in skin, seeds, and wine of Shiraz and Cabernet Sauvignon wine grapes (Vitis vinifera) J. Agric. Food Chem. 2011;59(24):13265–13276. doi: 10.1021/jf203466u. [DOI] [PubMed] [Google Scholar]
- Harbertson J.F., Hodgins R.E., Thurston L.N., Schaffer L.J., Reid M.S., Landon J.L., Ross C.F., Adams D.O. Variability of tannin concentration in red wines. Am. J. Enol. Vitic. 2008;59(2):210–214. https://ajevonline.org/content/59/2/210 [Google Scholar]
- Harbertson J.F., Kilmister R.L., Kelm M.A., Downey M.O. Impact of condensed tannin size as individual and mixed polymers on bovine serum albumin precipitation. Food Chem. 2014;160:16–21. doi: 10.1016/j.foodchem.2014.03.026. 0. [DOI] [PubMed] [Google Scholar]
- Harbertson J.F., Picciotto E.A., Adams D.O. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003;54(4):301–306. https://ajevonline.org/content/54/4/301 [Google Scholar]
- Hashizume K., Kida S., Samuta T. Effect of steam treatment of grape cluster stems on the methoxypyrazine, phenolic, acid, and mineral content of red wines fermented with stems. J. Agric. Food Chem. 1998;46(10):4382–4386. [Google Scholar]
- He F., Liang N.-N., Mu L., Pan Q.-H., Wang J., Reeves M.J., Duan C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules. 2012;17(2):1571–1601. doi: 10.3390/molecules17021571. https://mdpi.com/1420-3049/17/2/1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel J.C., Hofmann T. Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine. J. Agric. Food Chem. 2008;56(4):1376–1386. doi: 10.1021/jf073031n. [DOI] [PubMed] [Google Scholar]
- Iland P., Bruer N., Edwards G., Caloghiris S., Wilkes E. Patrick Iland Wine Promotions Pty Ltd; Adelaide, Australia: 2012. Chemical Analysis of Grapes and Wine Techniques and Concepts. [Google Scholar]
- Kumar L., Tian B., Harrison R. Interactions of Vitis vinifera L. cv. Pinot Noir grape anthocyanins with seed proanthocyanidins and their effect on wine color and phenolic composition. Lebensm. Wiss. Technol. 2022;162 [Google Scholar]
- Kwiatkowski M., Kravchuk O., Skouroumounis G.K., Taylor D.K. Microwave-assisted and conventional phenolic and colour extraction from grape skins of commercial white and red cultivars at veraison and harvest. J. Clean. Prod. 2020;275 [Google Scholar]
- Laitila J.E., Salminen J.-P. Relevance of the Concentrations and sizes of oligomeric red wine pigments to the color intensity of commercial red wines. J. Agric. Food Chem. 2020;68(11):3576–3584. doi: 10.1021/acs.jafc.9b07941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A.P., van Rensburg P., Lambrechts M.G. The Influence of different winemaking Techniques on the extraction of grape tannins and anthocyanins. S. Afr. J. Enol. Vitic. 2014;35(2):304–320. [Google Scholar]
- Pérez-Caballero V., Ayala F., Echávarri J.F., Negueruela A.I. Proposal for a new standard OIV method for determination of chromatic characteristics of wine. Am. J. Enol. Vitic. 2003;54(1):59–62. https://ajevonline.org/content/54/1/59 [Google Scholar]
- Río Segade S., Pace C., Torchio F., Giacosa S., Gerbi V., Rolle L. Impact of maceration enzymes on skin softening and relationship with anthocyanin extraction in wine grapes with different anthocyanin profiles. Food Res. Int. 2015;71:50–57. [Google Scholar]
- Rolle L., Río Segade S., Torchio F., Giacosa S., Cagnasso E., Marengo F., Gerbi V. Influence of grape density and harvest date on changes in phenolic composition, phenol extractability indices, and instrumental texture properties during ripening. J. Agric. Food Chem. 2011;59(16):8796–8805. doi: 10.1021/jf201318x. [DOI] [PubMed] [Google Scholar]
- Thorngate J.H., Noble A.C. Sensory evaluation of bitterness and astringency of 3R(−)-epicatechin and 3S(+)-catechin. J. Sci. Food Agric. 1995;67(4):531–535. [Google Scholar]
- Watrelot A.A., Heymann H., Waterhouse A.L. Red wine dryness perception related to physicochemistry. J. Agric. Food Chem. 2019;68(10):2964–2972. doi: 10.1021/acs.jafc.9b01480. [DOI] [PubMed] [Google Scholar]
- Weber F., Greve K., Durner D., Fischer U., Winterhalter P. Sensory and chemical characterization of phenolic polymers from red wine obtained by gel permeation chromatography. Am. J. Enol. Vitic. 2013;64(1):15–25. [Google Scholar]
- Wojdyło A., Samoticha J., Chmielewska J. Effect of different pre-treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.127888. [DOI] [PubMed] [Google Scholar]
- Yuan J.-F., Hou Z.-C., Wang D.-H., Qiu Z.-J., Gong M.-G., Sun J.-R. Microwave irradiation: effect on activities and properties of polyphenol oxidase in grape maceration stage. Food Biosci. 2021;44 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.