Abstract
Dental implantology has always emphasized silver nanoparticles (AgNPs) for various applications due to their biocompatibility, antibacterial activity, and increased surface volume ratio offered by these particles. It is utilized to a large extent in the dental implant industry as a surface modification, biocompatible constituent and composite material. AgNPs may be produced inexpensively, sustainably, and environmentally responsibly by utilizing technologies that extract the plant material. The phytochemical components that are contained in plants make them a better, non-toxic, and more cost-effective alternative to both physical and chemical approaches. Because the size and shape of AgNP depend on their synthesis method and technique, and because the efficacy and toxicity of AgNP depend on both size and shape, synthesis methods and techniques have recently become the focus of a significant amount of research attention. In this review, we discussed Plant Extracted Ag-NP's whose sizes range up to 100nm. This review also focuses on recent research advancements in the Plant Extracted synthesis of AgNPs, as well as their characterization methodologies, current obstacles, future possibilities, and applications in dental implantology.
Keywords: Synthesis, Plant extract, Ag-NP'S (silver nanoparticle), Characterization, Dental implant
Synthesis, Plant extract,Ag-NP's (Silver nanoparticle), Characterization, Dental Implant.
1. Introduction
The utilization of nanoparticles lies at the heart of nanotechnology, which is sometimes defined as a science that spans several disciplines [1].The Physio-chemical characteristics of metals vary as they approach the nanoscale, and the properties of nanoparticles are distinct from those of bulk metal [2].Due to their unique physical and biochemical characteristics in contrast to their macro- and micro-complements, Ag-NP's have attracted interest among the numerous nanomaterials now in use [3].The availability of increased surface area to volume ratios is the primary factor contributing to the high efficiency of silver nanoparticles [4].
Physical, chemical, and biological methods can all be used to create Ag-NP's. The chemical approach is poisonous and expensive, and the physical methods take a lot of energy to sustain the high pressure and temperature needed for the reaction [5].Nanomaterials' drawbacks, such as their toxicity toward bone cells, their variable biocompatibility depending on size, surface, and composition, and their high price, have prompted the development of biochemical methods or biosynthesis, such as the use of biomolecular extracts from plants [6, 7].The availability of a wide variety of metabolites with great reduction potentials, worldwide distribution, safe handling, low waste and energy costs, and large and accessible reserves make plant extracts very popular [8, 9].
Size, crystalline structure, composition, and other physical properties of nanoparticles have all been determined through various methods of study.In many situations, assessing physical characteristics requires utilizing more than one method.Since each approach has its advantages and disadvantages, picking the right one can be challenging; a combinatorial characterization is often necessary.Characterizing NPs requires addressing important parameters such as size and form [10, 11, 12].The success of Nps in different contexts hinges on how well we can characterize them.Many different methods exist for characterizing substances.
In dentistry, silver nanoparticles are used to make antibacterial chemicals that improve dental implants. They can be used in conjunction with acrylic resins for fabricating removable dentures during prosthetic treatments, composite resins for direct restoration during restorative treatments, endodontic irrigants and obturation materials during endodontic procedures, orthodontic adhesives, and titanium coating during dental implant procedures [13].A quantitative study found that small Ag-NPs inhibit antibacterial activity by releasing more Ag-NP ions. Nanoparticles' antimicrobial properties can be improved and more work can be done in improving those certain characteristics [14]. Aggregation of Ag-NPs decreases their surface area to volume ratio and activity in numerous areas (antimicrobial, catalyst, etc.). The phytochemical method can fix this issue since it contains potent reducing and capping agents. The future of phytochemical nanoparticle synthesis rests on the genetic engineering of plant genomes to improve the production of reduction and capping agents [1]. Nanoparticle release from customized implants can elicit a cytotoxic response, although few efforts have been made to produce nano-engineered coverings with adequate mechanical stability [15, 16, 17]. Our main objective in this review is to discuss the use and effectiveness of Ag–Np in dental implants, the synthesis processes related to shape, size, and toxicity, its effects, an improvement on dental implants, and why plant extraction is preferable in this regard. We will also discuss potential future research into using plant-extracted Ag-Nps more effectively and engagingly in dental implantology.
2. Importance of Ag-NP's in dental implants
Peri-implantitis, brought on by the buildup of bacterial biofilm on dental implant surfaces, is a leading cause of implant failure. Altering the nanotopography of a surface is proposed to reduce bacterial attachment to implants [18, 19]. Because of AgNP's exceptional antibacterial qualities, they are one of the most popular alternatives for use in dental implant doping and repair [13]. Ag electrostatically binds to the cytoplasmic membrane and the bacterial cell wall, disrupting the cellular structure of the bacterium [15, 20]. Surface modification with Ag NPs is highly recommended for dental implants because of their ability to promote osteogenesis and soft-tissue integration [21]. To provide synergistic antibacterial (S. epidermidis, S. mutans, and E. coli) and osteogenic (human osteoblast-like cells, SAOS-2) properties, Ag-NP's have been deposited on Ti implants via anodic spark deposition [22]. Ag-NP's did not show any signs of cytotoxicity when deposited on sand-blasted, big grit, and acid-etched titanium, but they did limit the growth ofS.aureus and F. nucleatum. These findings imply that titanium implants coated with Ag-NP's can be provided with complementary antibacterial and osteogenic capabilities, which bodes well for their secure and long-term therapeutic uses [23, 24]. Against S. aureus and P. aeruginosa, Ag NPs extracted from plants showed bactericidal activity [25].Because Ag-NP's have such distinctive properties, there is a significant amount of interest in utilizing them in dental implants.
3. Synthesis processes
Both bottom-up and top-down methods can be used for the synthesis of Ag-NP's [26]. Using a top-down methodology, Ag-NP's are produced using a variety of physical techniques that scale down the starting material from bulk to nanoscale [27]. Metal NPs' physicochemical characteristics are mostly determined by their surface structure, which varies greatly from case to case [28]. The high temperature and pressure settings necessary for the synthesis process need a great deal of energy, which is another disadvantage of this method [28, 29]. Several processes, including thermolysis, pyrolysis, radiation-induced, and lithography, fall under this category [28, 29, 30].
The self-assembly technique is a name for the bottom-up strategy [31]. These techniques rely largely on the assemblage of produced NPs into a final nanomaterial of the required size via different biological and chemical processes [32]. For a reasonable price, this approach offers a considerably increased possibility of fabricating Ag-NP's with improved chemical compositional homogeneity and reduced surface imperfection [32]. The necessity for nonpolar organic solvents, toxic chemicals, synthesized capping agents, and some other stabilizing agents severely restrict the biological applications of these chemical techniques [32, 33, 34].
Research teams are aimed at developing greener, safer, more effective, and more compatible alternatives to traditional chemical synthesis [35]. As a result, scientists have been focusing on the biosynthesis technique for creating Ag-NP's. Due to their biocompatibility and low environmental impact, plant extracts have become a popular choice for making silver nanoparticles (Ag-NP's) [36].
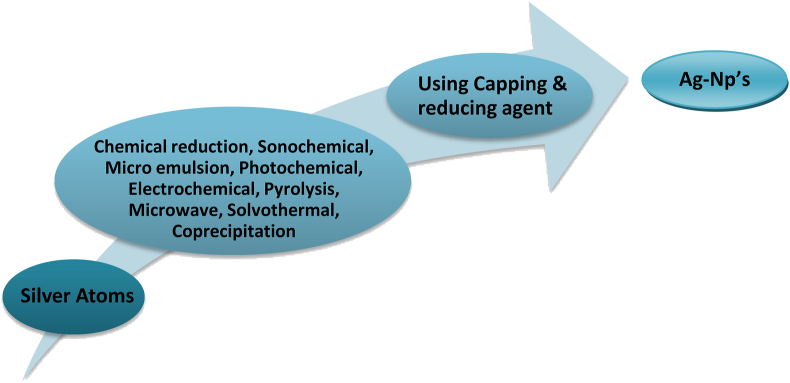
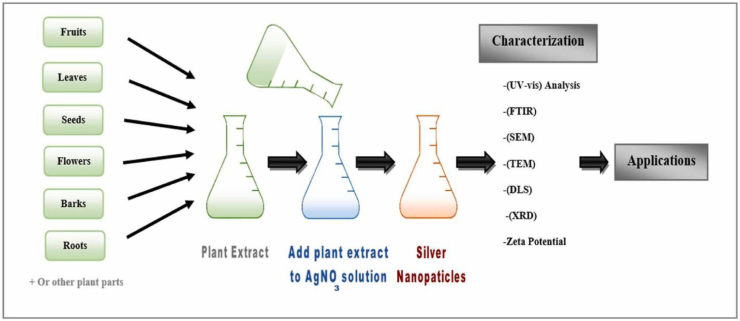
In the process of biological production of silver nanoparticles, the organism functions as a capping agent, reducing agent, or stabilizing agent by converting Ag + to Ag0 [37]. As a result of their low price, high yields, and low toxicity to both humans and the environment, natural compounds generated from plants have become increasingly popular for use in biological processes in recent years [38]. Figure 1 shows the development of nanoparticles through various methods.
Figure 1.
Developing nanoparticles through various methods [39].
4. Chemical method
The tools needed for chemical approaches are typically less complicated and more easily accessible, which is a major plus. It is well known that the reducing agent plays a key role in converting silver ions to the metallic state, where they can then combine to create silver nanoparticles [40]. Size control and capping were accomplished with polyvinylpyrrolidone (PVP), and silver nanoparticles were stabilized with sodium borohydride and trisodium citrate [41]. Borohydride, 2-mercaptoethanol, citrate, and thio-glycerol are only a few of the chemicals and molecules employed in the manufacture of silver nanoparticles, and they are all extremely costly and potentially dangerous.Making silver nanoparticles of a specific size is quite challenging, and stopping them from sticking together needs extra work [42]. There is a great deal of potentially harmful and poisonous byproducts created during synthesis [43]. The main advantage of this technology is the synthesizing of nanoparticles with predefined form and size; nevertheless, environmental concerns have been raised due to the use of harmful chemicals, and severe reaction conditions such as high temp, pressure, and poisonous by-product [44, 45, 46]. Figure 2 shows the chemical method of bottom-down approach for the synthesis of AgNPs.
Figure 2.
Chemical method of Bottom-down Approach for Ag-NP's Synthesis.
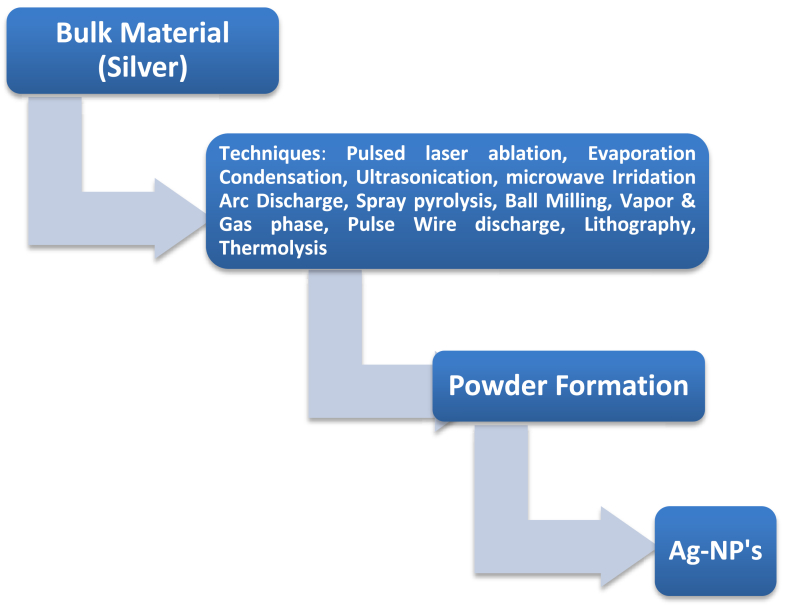
5. Physical method
Ultrasonication, microwave (MW) irradiation, and electrochemical procedures are examples of the "top-down" physical process used most often in the creation of nanoparticles by synthesis. The most common physical techniques include evaporation and condensation, as well as laser removal.The material is evaporated in a pontoon and then pointed at the burner to create a carrier gas [47]. It was discovered that polydisperse nanoparticles were produced when the heater surface temperature was held constant throughout time.These nanoparticles of silver were smooth and did not aggregate [48]. Plasma catalysis [49] and laser ablation [50, 51] are two examples of physical techniques.Conventional approaches are time-consuming and resource-intensive since they need the use of specialized equipment and the expertise of trained personnel.These techniques, as their names imply, involve the application of intense heat and light, both of which can be harmful, and the created nanoparticles are less stable [52]. Physical methods have the advantages of being quick, requiring no harmful chemicals, and being able to employ radiation as a reduction agent.However, physical processes include drawbacks such as wasteful energy use, uneven product distribution, and the presence of solvents in the final product [53]. Figure 3 shows the physical method of top-down approach for the synthesis of AgNPs.
Figure 3.
Physical method of Top-down Approach for Ag-NP's Synthesis.
6. Biosynthesis method
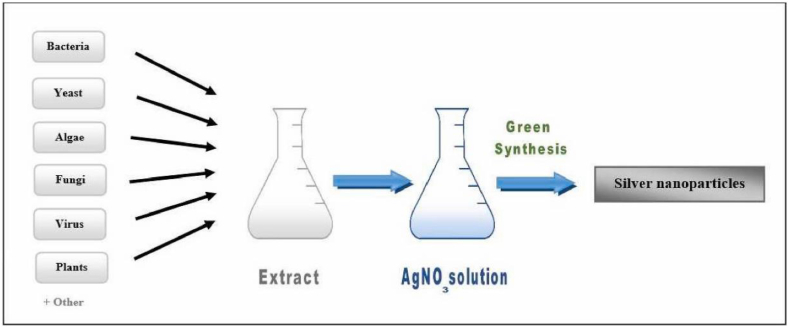
Implementing physical and chemical procedures to create silver nanoparticles is not only inefficient but also harmful to the environment. Therefore, it is crucial to create a system that is both cost-effective and safe for the environment, one that does not make use of dangerous chemicals [51] and voids the hazards of chemical and physical manufacturing methods. These gaps can be filled by biological approaches, which have several uses in health management due to their ability to regulate a wide range of biological processes. The employment of microorganisms like fungi, bacteria, and yeast, as well as natural materials like plants, are all examples of biological manufacturing processes. All of these factors have contributed to the widespread adoption of this strategy for the medicinal use of nanoparticles [36]. Purification of Ag-NP's necessitates cell lysis, adherence of microorganisms on nanoparticles' surface increases the risk of infection, and the microbial mode necessitates the search for potent strains producing Ag-NP's, growth and maintenance of microbial strain on expensive media, and less flexibility of pH and temperature during nano synthesis [1]. Plant material as a reducing agent in silver nanoparticle manufacturing has various benefits. In addition to being readily available, safe for handling, inexpensive, requiring almost little maintenance, and environmentally friendly are just a few of its many benefits [55]. Figure 4 shows the biosynthesis method for the extraction of AgNPs.
Figure 4.
AgNP's extraction by biosynthesis method [54].
6.1. Mechanism of silver nanoparticles biosynthesis
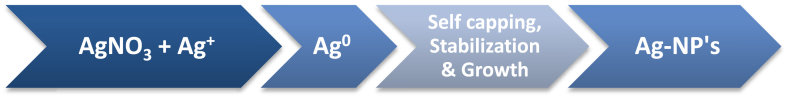
Enzymes are the primary component of Ag-NP's production, whether it takes place within or outside of cells. Researchers have shown that nitrate reductase, which is dependent on the cofactor NADH, is essential for the production of AgNP. This enzyme helps nanoparticles form by moving electrons from the nitrate atom to the metal ion. It does this by acting as an electron shuttle [56, 57, 58, 59, 60]. The "factories" used to create silver nanoparticles are made out of a broad variety of plants, including therapeutic herbs. The potential mechanism of enzymatic AgNP production is typically comparable to that of microorganisms. However, compared to microorganisms, plant cells include a complex of varied antioxidant metabolites preventing the oxidation and degradation of cellular components. Therefore, the compounds used to stabilize and cap the nanoparticles are one-of-a-kind [61]. Due to their anti-inflammatory, antioxidant, anticancer, and other actions, they are especially crucial for the future of Ag-NP's in practical applications [62]. Evidently, "capping" agents can selectively attach to certain Nano crystal facets in order to alter the ratio of their respective surface areas by altering the corresponding surface free energy [63]. Thus, nanoparticle "capping" can perform various key activities, including preventing nanoparticle agglomeration, reducing toxicity, and improving antibacterial capabilities; also, these compounds can increase the bacterial cell affiliation and activity of Ag-NP's [64, 65]. Figure 5 shows the synthesis of AgNPs and related biochemical procedures.
Figure 5.
Ag-NP's (silver Nanoparticle) synthesis and related biochemical procedures [53].
6.2. Plant extract based Ag-NP's synthesis process
Producing silver nanoparticles (Ag-NPs) in a live plant system was originally described in a study with Alfalfa sprouts, which was the first plant used in this way to produce metallic NPs [66]. Intracellular or extracellular plant-based synthesis can usually happen. The plant synthesizes intracellularly, whereas extracellularly in vitro. By using Phyto-compounds for precursor reduction, Ag-NP's may be synthesized directly from plant extracts through an extracellular route.Due to their biological potential, Ag-NP's are synthesized extracellularly from plant extracts. Scientific studies have shown that eliminating the extraction and purification steps makes plant extracts preferable for extracellular synthesis over intracellular synthesis [67, 68].Typically, a solvent solution, stabilizing agents, and reducing agents are needed for the extracellular production of Ag-NP's [69]. Phyto compounds in plant extract reduce and stabilize Silvernanoparticles.Since water is often utilized as the solvent solution, synthesis of Ag-NP's is thought of as environmentally friendly [31, 70]. The complex process of maintaining cell cultures is simplified by using plants and plant extracts in nanoparticle manufacturing, and this is seen as a benefit over using microorganisms [67]. In the presence of plant extracts, Ag-NP's take on two distinct morphologies throughout the process: the highly reactive face-centered cubic (fcc) structure, and the more strongly stabilized spherical form. The activity of Ag-NP's in a wide range of biological settings is well established, and it is known that their preferred development in the (111) plane and spherical form is responsible for this [69, 71, 72, 73, 74, 75, 76, 77, 78]. Figure 6 shows the biosynthesis method for the extraction of AgNPs from plants. Table 1 shows the synthesis of AgNPs from different plants.
Figure 6.
AgNP's extraction from plants by biosynthesis method [54].
Table 1.
A list of Plant-Based Synthesized Ag-NP's ranging in size from 1-100nm and having spherical and FCC (face-centered cubic) shapes [38, 79, 80].
| Scientific Name | Common Name | Plants Extract | Precursor | Shape | Size (nm) | Characterization technique | Ref. |
|---|---|---|---|---|---|---|---|
| Angelicaepubescentis | Female ginseng | Root | AgNO3 | Almost spherical | 12.48 | UV-Vis Spectroscopy, SPR analysis, FE-TEM | [81] |
| Coriandrum sativum | Coriander | Leaf | AgNO3 | Spherical | 37 | UV-Vis Spectroscopy, FESEM/EDX, FTIR, XRD, BET analysis, |
[82] |
| Benzoin gum | Loban | Plant | AgNO3 | Spherical | 12–38 | UV-Vis Spectroscopy, FE-TEM, FTIR, XRD |
[83] |
| Cucurbita maxima | Squash | Petals | AgNO3 | Roughly Spherical | 76.1 | UV-Vis spectroscopy, DLS, Zetasizer, FTIR, Fe-SEM |
[84] |
| Acorus calamus | Calamus | Rhizome | AgNO3 | Roughly Spherical | 59.02 ± 1.3 | UV-Vis spectroscopy, DLS, Zetasizer, FTIR, Fe-SEM |
[84] |
| Plumeria alba | West Indian-jasmine | Flower | AgNO3 | Spherical shape | 36.19 | UV-visible spectroscopy, DLS, SEM/EDX, FTIR, TGA/DSC, and XRD | [85] |
| Abutilon indicum | Indian lantern-flower | Leaf | AgNO3 | Spherical | 5–25 | UV-Spectroscopy, Zeta Sizer, DLS, TEM, EDAX, SEM, XRD, FTIR, DSC |
[86] |
| Solanum trilobatum | Purple fruited pea | Fruit | AgNO3 | Spherical | 12.50–41.90 | UV-Spectroscopy, TEM, EDAX, SEM, XRD, FTIR |
[87] |
| Rosa indica | cyme rose | Petals | AgNO3 | Spherical | 23.52–60.83 | UV-Spectroscopy, TEM, EDX, SEM, XRD, FTIR |
[88] |
| Rosa damascena | damask rose | Petals | AgNO3 | Spherical | 74–94 | UV-Spectroscopy, DLS, XRD, FTIR, HRTEM, FE-SEM, EDAX |
[89] |
| Achillea biebersteinii | Yellow milfoil | Flowers | AgNO3 | Spherical | 12 ± 2 | UV-Spectroscopy, DLS, TEM, EDX | [90] |
| Piper longum | Indian long pepper | Fruit | AgNO3 | Spherical | 46 | UV-Spectroscopy, DLS, FTIR, SEM. | [91] |
| Artemisia tournefortiana | Aerial part | AgNO3 | Spherical | 22.89 ± 14.82 | UV-Spectroscopy, DLS, XRD, FTIR, HRTEM, EDX |
[92] | |
| Artemisia marschalliana | Field wormwood | Aerial part | AgNO3 | Spherical | 5–50 | UV- Vis spectroscopy, SEM, TEM, XRD, FTIR |
[93] |
| Amomum villosum | Malabar cardamom | Fruit | AgNO3 | Spherical | 5–15 | UV-Spectroscopy, DLS, XRD, FTIR, HRTEM, EDX SAED |
[94] |
| Excoecariaagallocha | River poison tree | Leaf | AgNO3 | Spherical | 23 and 42 | UV-Spectroscopy, DLS, XRD, FTIR, FESEM, EDAX |
[95] |
| Cornus officinalis | Asiatic dogwood | Fruit | AgNO3 | Almost spherical | 11.7 | UV-Spectroscopy, DLS, XRD, FTIR, FESEM, EDAX |
[96] |
| Taraxacumofficinale | Dandelion | Leaf | AgNO3 | Spherical | 5–30 | UV-Spectroscopy, DLS, XRD, FTIR, HR-TEM |
[97] |
| Indigofera tinctoria | Indian indigo | Leaf | AgNO3 | Spherical | 16.46 | UV-vis. spectroscopy, FTIR spectroscopy, XRD, TEM, EDX, and AFM |
[73] |
| Syzygiumjambos | Rose-apple | Leaf and bark | AgNO3 | Spherical | 5–8 | UV-vis. Spectroscopy, TEM, XRD, FTIR, NMR |
[98] |
| Hydrastis canadensis | Goldenseal | Whole plant | AgNO3 | Spherical | 90.87 | UV- Vis spectroscopy, DLS, TEM, FTIR, AFM, XRD, CD |
[99] |
| Thuja occidentalis | Arborvitae | Plant | AgNO3 | Spherical | 90.87 | UV- Vis spectroscopy, DLS,TEM, FTIR, AFM, XRD, CD |
[99] |
| Mentha arvensis | wild mint | Leaf | AgNO3 | Spherical | 3–9 | UV- Vis spectroscopy, EDX, DLS, FTIR, AFM, XRD |
[100] |
| Anthemisatropatana | Plant | AgNO3 | Spherical | 38.89 | UV-Vis Spectroscopy, TEM, SEM, XRD, FTIR |
[101] | |
| Ficus religiosa | Peepul tree | Leaf | AgNO3 | Spherical | 21 | UV-Vis Spectroscopy, TEM, FTIR, GCMS, DLS |
[102] |
| Sesbania grandiflora | Vegetable hummingbird | Leaf | AgNO3 | Spherical | 10–25 | UV- Vis spectroscopy, EDX, TEM, FTIR, AFM, XRD |
[103] |
| Syzygiumcumini | Java plum | Leaf | AgNO3 | Spherical | 5–30 | TEM,SAED, XRD, EDX | [104] |
| Phytolacca decandra | Pokeweed | Dried plant | AgNO3 | Spherical | 90.87 | UV- Vis spectroscopy, DLS, TEM, FTIR, AFM, XRD, CD |
[99] |
| Gelsemium sempervirens | Evening trumpet flower | Dried plant | AgNO3 | Spherical | 90.87 | UV- Vis spectroscopy, DLS, TEM, FTIR, AFM, XRD, CD |
[99] |
| Allium sativum | Garlic | Fruit | AgNO3 | Spherical | 3–6 | UV-Vis Spectroscopy, TEM,XRD, EDX, FTIR |
[105] |
| Zingiber officinale | Ginger | Fruit | AgNO3 | Spherical | 3–22 | UV-Vis Spectroscopy, TEM,XRD, EDX, FTIR |
[106] |
| Origanum vulgare | Oregano | Leaf | AgNO3 | Spherical | 63–85 | UV- Vis spectroscopy, FE-SEM, DLS,XRD, FTIR |
[107] |
| Eucalyptus globulus | Tasmanian bluegum | Leaves | AgNO3 | Spherical | 1.9–4.3 and 5–25 | UV-visible spectroscopy, XRD, TEM, SEM-EDX, FTIR | [108] |
| Phyllanthus niruri | Gale of the wind | Leaves | AgNO3 | Crystalline FCC and spherical | 30–60 | UV-visible spectroscopy, XRD, SEM-EDX, FTIR | [109] |
| Bryophyllum and Asiatic Pennywort | Leaves | Crystalline FCC and spherical | 18–21 | UV-visible spectroscopy, XRD, TEM | [110] | ||
| AgNO3 | |||||||
| Eucalyptus leucoxylon | White ironbark | Leaves | AgNO3 | FCC and spherical | 50 | UV-visible spectroscopy, XRD, SEM, TEM, | [111] |
| Desmodiumtriflorum | Threeflowerticktrefoil | Plant Broth | AgNO3 | Spherical | 10 | UV-visible spectroscopy, XRD, TEM | [112] |
| Achyranthes aspera L. | Devil's horsewhip | Leaves | AgNO3 | Spherical | 1.0 ± 18.3 | UV-visible spectroscopy, TEM | [113] |
| Calotropis procera | Giant-milkweed | Latex serum | AgNO3 | Spherical | 12.33 | UV-visible spectroscopy, XRD, TEM, FITR | [114] |
| Allium cepa | Onion | Plant | AgNO3 | Spherical | 33.6 | UV-visible spectroscopy, DLS, TEM | [115] |
| Prunus persica | Peach gum | Peach gum powder | AgNO3 | FCC | 23.56 ± 7.87 | FITR | [116] |
| Solanum lycopersicum | Tomato | Fruit | AgNO3 | Spherical | 10 | UV-visible spectroscopy, FITR, SEM, TEM | [117] |
| Juglans Regia L. | Walnut | Leaves | AgNO3 | Almost spherical | 10–50 | UV-visible spectroscopy, TEM | [118] |
| Chenopodium album | Lambsquarters | Leaves | AgNO3 | Almost spherical | 10–30 | UV-VIS, XRD, EDX, FITR, TEM | [119] |
| Mentha piperita | Pudina | Leaves | AgNO3 | Spherical | 5–30 | SEM, FITR | [120] |
| Catharanthus roseus | Madagascar periwinkle | Leaves | Crystalline FCC and spherical | 20 | UV-vis spectrum, XRD, FTIR | [121] | |
| AgNO3 | |||||||
| Terminalia arjuna | Arjun | Bark | AgNO3 | Spherical | 2–100 | UV-vis spectroscopy, FT-IR, XRD, SEM, and DLS | [122] |
| Vitex negundo | Chinese chaste tree | Leaves | AgNO3 | FCC and spherical | 5 and 10–30 | UV-vis spectroscopy, TEM, XRD | [123] |
6.3. Why it is better than other processes
In comparison to the employment of chemical, physical, and microbiological approaches, plant extracts for the production of Ag-NP's offer various benefits. Extracts from plants can be used to create nanoparticles without the need for hazardous reducing and capping chemicals, radiation, high temperatures, a specific microbial strain, or an expensive growing medium. Antimicrobial, medication delivery, and water purification are all examples of applications where microbial synthesis presents risks of infection and contamination throughout the synthesis process [124, 125, 126]. It takes a lot of time and effort to keep a pure culture of bacteria going so that they can synthesize a compound. Nanoparticle creation in plants is more rapid and they are immune to these limitations [124, 125, 126]. According to Botanic Gardens Conservation International (BGCI), there are around 321,212 plant species on earth. As a result, scientists have had access to a sizable class of plants to synthesize nanoparticles. Phytochemicals and other plant derivatives are among the wide category of plant extracts with the dual capabilities of reducing and stabilizing agents. Since the phytochemicals' –OH groups and carbonyl groups have been oxidized, they may serve as a reducing agent and a capping agent for the stability of nanostructured materials [27]. Because the availability of the reducing agent is greater in the extract than in the whole plant, aqueous plant extracts are typically used in biogenic synthesis for the manufacture of noble nanoparticles [127]. Waste materials and the synthesized goods themselves both originate from plant extracts found in nature, making this method more eco-friendly overall [128]. This bio-based technique for synthesizing nanoparticles allows for improved repeatability of the process and enhanced stability of the generated nanoparticles. Thus, this plant-based nanoparticle synthesis is suited for industrial-scale production with more efficient cost investment; it is also environmentally benign and safe for therapeutic application in humans [129].
7. Characterization techniques
In order to comprehend and regulate the NPs production process, it is crucial to conduct a thorough characterization of the Ag-NP's [130]. For instance, the agglomeration, spatial reasoning, geometric proportions, Brownian motion, intercalation, and scattering of NPs can only be determined by characterizing Ag-NP's. Elements, particle sizes, crystal size, porosity, solubility, surface characteristics, water sorption, and surface morphology are some other parameters that need to be analyzed [131, 132, 133].
8. UV-Vi's spectrophotometry
The creation of nanoparticles may be confirmed and their stability monitored by UV visible spectrometry measurement, which is the most frequent quantitative method. The technique of UV visible spectroscopy is quick, sensitive, and easy to use. Additionally, it may choose and choose amongst several nanoparticles. After completion of synthesis, a brownish yellow color was seen in the AgNO3, and absorption spectra appeared within 410 and 430 nm. In other words, it provides a numerical representation of the sample's solution's exposure to ultraviolet (UV) and visible light [79].
9. X-ray diffraction analysis (XRD)
Based on the insight it provides into the degree of crystallinity, it is frequently employed in the research of nanoparticles, biomolecules, polymers, and other similar substances. It provides a rough estimate of the various chemical groups and particle sizes and provides quantitative information on the resolution of various chemical compounds. This research is based on the fact that various crystals will generate unique diffraction patterns when subjected to a monochromatic X-ray beam. Using Bragg's equation, the interference pattern created by these diffracted X-rays may reveal whether a material is crystalline or polycrystalline. Measurements from this method are expressed in Angstroms (Å) (1 Å = 0.1 nm) [79].
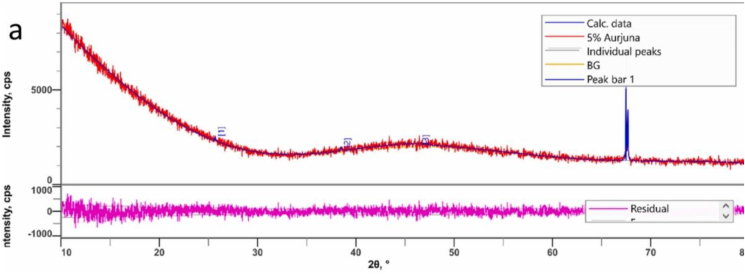
The transmission electron microscope (TEM) is the most widely used and effective method for studying silver nanoparticles. It is often employed to get the size and shape-related quantitative parameters of Ag-NP's.The sample is subjected to an electron beam, and the resulting interaction is captured on a photographic plate. Due to its tremendous resolving power, TEM can identify and measure even the tiniest of nanoparticles.Compared to SEM, TEM has superior spatialre solution power. Multiple high-resolution microscopy techniques that use a stream of high-energy electrons are now being developed by scientists in this field of nano-science and technology [79]. Figure 7 shows an XRD image.
Figure 7.
Example of XRD image of synthesized bioplastic at (a) 5% Arjuna [134].
10. Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) uses the repulsion and attraction of electrons between beams directed through a sample and atoms at various depths to create images. Detectors record the secondary electrons that have been reflected and convert them into visuals. Right now, scanning electron microscopy (SEM) is the best microscopy approach for identifying and characterizing nanoparticles of varying sizes and shapes. Data concerning the nanoscale surface morphology of particles can also be determined. SEM's use is favored because of its ability to resolve particles smaller than 10 nm [79]. Example of SEM image can be seen in Figures 8(a) and 8(b).
Figure 8.
Example of SEM image of mild steel surface (a) uninhibited solution, (b) inhibited solution [135].
11. Dynamic light scattering (DLS)
Nanoparticles in suspension can have their size distribution and poly-disperse nature analyzed by dynamic light scattering (DLS).In most cases, this is the method of choice when attempting to ascertain a size. This approach estimates the hydrodynamic radius of a particle in Brownian motion, which is the key to understanding how it works. When a particle in the solution is hit with a laser beam, it scatters the light at varying intensities. Particle sizes were calculated by analyzing the fluctuations' intensities using the Stokes-Einstein relationship equation. From 20-200 nm, DLS consistently measures particle sizes [79].
12. Fourier transform infrared spectroscopy (FTIR)
Chemical bonds and functioning molecules on a surface can be evaluated by FTIR spectroscopy. Additionally, it may be used to analyze the physical characteristics of artificially created nanoparticles to identify the biomolecules involved in nanoparticle creation. It was utilized by researchers to identify the chemical ingredient employed in the creation of nanoparticles in leaf or other plant extracts. The principle behind this method is that whenever infrared radiation strikes a sample, a portion of its energy is consumed and the rest is released. By analyzing the sample's absorbance and transmittance values, the spectrum may identify the sample. To investigate the effect of plant extract in the reduction of silver, FTIR is a non-intrusive, appropriate, significant, and incredibly easy technology [79].
The FTIR spectra shift due to the presence of carbohydrates and phenolic compounds in the pure plant extracts. Carbohydrates, phenolic compounds and flavonoid compounds present in the pure plant extracts reduce the Ag ion and form AgNPs. Besides, they are also responsible for the stabilization of AgNPs and increases antimicrobial activities [136].
13. Energy-dispersive X-ray spectroscopy (EDX)
Applications of EDX in nanotechnology have been well documented as an essential tool for determining a sample's elemental makeup.The X-ray spectra of any given nanoparticle may be used to determine its elemental makeupsince each element has a distinct atomic structure that results in a distinct collection of peaks [137].
14. Transmission electron microscopy (TEM)
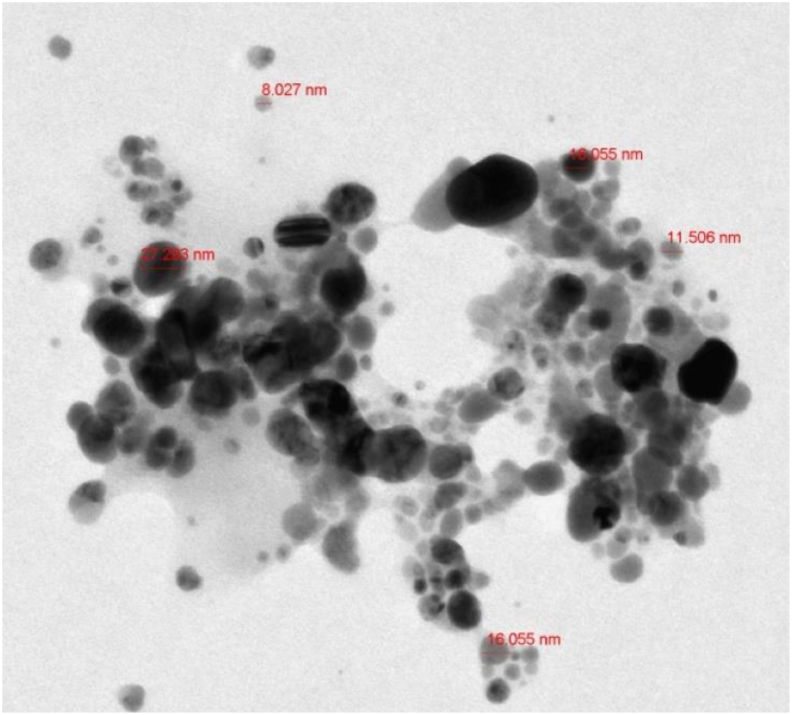
Transmission electron microscopy involves passing a stream of electrons through a very thin specimen, where they interact with the object and create a picture [80]. As part of getting AgNPs ready for TEM investigation, a trace quantity is spread onto carbon-coated copper grids. The AgNPs are embedded in an ultrathin, dried grid that a beam of light travels through and reacts to [138]. This picture is formed as a result of an interaction between electrons passing through the AgNPs. A magnifying lens or other imaging equipment records a blown-up version of the scene [139]. The antibacterial capabilities of AgNPs can be improved by the use of medicinal plants in their synthesis, and the size and shape of the resulting AgNPs can be controlled in this process as well [140, 141]. An example of TEM image can be seen in Figure 9.
Figure 9.
Example of a TEM image of AgNPs synthesized by A. fleurentiniorum extract [142].
15. Auger electron spectroscopy (AES)
As an outcome of the collisions between an electron beam and the atoms permanently stationed at a sample's surface, atomic emission spectroscopy (AES) is a surface-sensitive analytical method [143]as well as a top-notch nanotech analysis tool [144].
16. Low-energy ion scattering (LEIS)
It is generally known that LEIS has the highest sensitivity to the surface of any technology now in use for surface analysis.The approach allows one to determine the structure and elemental content of a material [145, 146, 147]. Strong LEIS is an essential surface analysis technique that plays a significant role in the analysis of SAM-functionalized nanomaterials [148].
17. Applications of Ag-NP'sin dental implant
The antibacterial and antifungal uses of Ag-NPs are extensive. Numerous dental composites feature an antimicrobial coating made of silver nanoparticles (Ag-NPs) [149]. Multiple dental treatments can benefit from using silver nanoparticles (Ag-NP's), which have been extensively studied for their antibacterial properties. Acrylic resins, Nanocomposites, adhesives, resin comonomers, intracanal medicines, and implant coatings have all been shown to benefit from Ag-NP's' potent antibacterial action in in vitro experiments [20].
Adding Ag-NP's to dental composites has been shown to improve their antifungal properties, while another study indicated that doing so decreased microbial colonization of dental implant covering materials [150]. Because of their inability to promote infection, devices based on silver nanoparticles are frequently employed in dental implants. Researchers have demonstrated that silver nanoparticles are efficient against Gram-negative and Gram-positive bacteria [151, 152]. The antibacterial activity of Ag2O nanoparticles prepared from the root extract of Ficus benghalensis was evaluated in vitro against dental bacterial strains [153].
Researchers found that silver nanoparticles made using aqueous Mangifera indica leaf powder have the potential to be used as dental fillings due to their flake-like form and small size (32.4 nm).Glass ionomer cement (GIC) was used to increase the mechanical characteristics of the created silver Nano biomaterials, which in turn increased their resistance to bacteria like S.aureus and E. coli. Combining these Nanoscale bio material-reinforced GIC with traditional dental implant materials as a composite or coating them on the surface may increase their mechanical strength [154]. Silver nanoparticles produced with Oleo europaea extract (white pepper oleoresin) showed increased antibacterial activity against oral infections. Because of their ability to inhibit microbial growth, they can be useful as a surface modification for traditional dental implants [155, 156, 157].
Dental implant insertion is complicated by alveolar bone loss, a frequent medical issue. In order to successfully restore the alveolar ridge, it is imperative that a barrier be placed in between bone graft and thus the gingiva. This will prevent the regeneration of fibrotic tissue, reduce the risk of bacterial infection, and stimulate bone growth. A study showed that using AgNP-coated collagen membranes after implanting bone grafts in alveolar ridge repair can reduce the risk of infection [158].
A study shows that 30 nm-sizedAgNP's doped with Ti Alloy showed better antimicrobial and biocompatibility [159]. As a surface modifier of dental implants, Ag-NP'scoated Ti showed better antimicrobials against S. mutans and P. gingivalis [160].
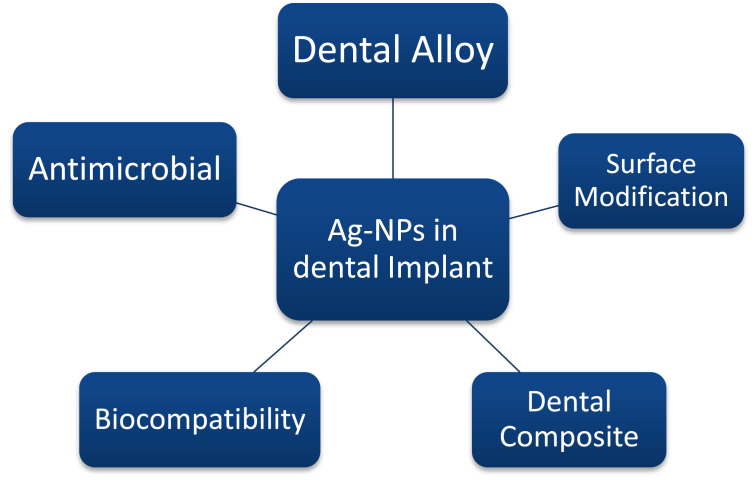
All of thisresearch showed that Nano biomaterials have great potential for use in dental implants.Potentially, innovative Phyto Nano biomaterials for dental implants might be more easily manufactured as a result of developments in contemporary synthesis procedures [155, 156, 157]. Applications of AgNPs in dental implants can be seen in Figure 10.
Figure 10.
Ag–Np's Applications in dental Implants.
18. Current challenges and future prospects
Nano biomaterials made from plants are showing promise as a surface modification for dental implants, and related in vitro research is gaining traction.However, there are also several obstacles and major shortfalls in plant-mediated Nano biomaterial manufacturing.While bionanomaterials produced by plant-mediated synthesis tend to be more stable than those produced via more traditional methods, their speed of production makes them less ideal for some applications [157].
-
•
Small Ag-NP's (d < 10 nm), according to the results of a quantitative investigation, display strong suppression of antibacterial activity due to its increased release of Ag-NP's ions. However, they centrifuged a solution with a very high concentration of bigger Ag-NP's, removed the supernatant containing the Ag-NP's ions, and then utilized just the pellet fraction containing the particles. The results are on par with those of smaller Ag-NP's. Based on these findings, we can only surmise that there are certain characteristics of nanoparticles that are responsible for their antibacterial action, and more work can be done in improving those certain characteristics [14].
-
•
Aggregation of Ag-NP's is a major issue since it reduces their surface area to volume ratio and, therefore, their activity in several sectors (antimicrobial, catalyst, etc.). Because it contains powerful reducing and capping agents, the phytochemical approach can be used to solve this issue as well. The future potential of phytochemical synthesis of nanoparticles lies in the manipulation of plant genomes using genetic engineering technologies in order to increase the production of active principles responsible for the reduction and capping agents [1].
-
•
Delamination or nanoparticle release from modified implants can trigger a cytotoxic reaction, but minimal efforts have been undertaken to assure the effective production of nano-engineered coatings on commercial implants with sufficient mechanical stability [15, 16, 17].
-
•
Bio-fabrication of Ag-NP's via plant extracts is a hot topic in the scientific community right now, although the specific mechanism involved in this process is not extensively researched. The processes involved in the production of Ag-NP's from plant extracts have only been speculated upon by a few researchers so far [31, 70]. Because of the wide variety of phytoconstituents that are present as well as the variation of plant extracts, it can be challenging to identify the specific stabilizing and reducing agent that is responsible for the formation of nanoparticles as well as their ability to maintain their stability [161].
-
•
Up until this point, it was believed that phytocompounds such as proteins, organic acids, and secondary metabolites such as terpenoids, flavonoids, and phenolic acid could be used effectively as stabilizing and reducing agents in the bio fabrication of silver nanoparticles (Ag-NP's). However, it is more likely that several Phyto compounds found in plant extracts work in concert to reduce metal precursors [161, 162].
-
•
It should be highlighted that the Nano biomaterials created recently for dental applications using plant extracts are freestanding Nano biomaterials. This means that in the near future, nano biocomposites may be fabricated using plant extracts, which is great news for a broad variety of commercial uses, such as surface-modified dental implants with special biological properties [157].
Last but not least, there are numerous papers and reviews discussing ways to enhance plant-extracted Ag-NP's, including how to lessen their toxicity, increase their effectiveness, enhance their antibacterial activity, etc. However, there is less research on how these plant-extracted Ag-NP's are specifically enhanced in the application of dental implants, suggesting that this area needs more study.
19. Conclusion
Since the beginning of modern dentistry, dental implants have been recognized as a reliable method of providing efficient medical therapy. Researchers consistently engage in comprehensive research intending to advance the dental implant industry. In order to accomplish this goal, nanoparticles have brought about a highly practical advancement in this area. When compared to other nanoparticles, silver nanoparticles (AgNPs) stand out as an excellent choice since they possess a variety of advantageous qualities. It has been used in a variety of dental implant disciplines. Ag-NPs can be utilized for antibacterial activity, biocompatibility, improved strength, and surface modification. In the realm of nanoparticles, factors such as form, size, and toxicity hold a significant amount of importance. These factors are necessary for the action of Ag-NP. In recent years, researchers have been making efforts to acquire silver nanoparticles (Ag-NPs) from a variety of sources, with the goals of achieving the desired shape and size in order to enhance the material's activity in dental implants, as well as acquiring them in a less toxic manner and a less toxic type. Furthermore, the synthesis of plant extracts is one of the most viable approaches for obtaining these highly effective Ag-Nps. Despite the vast amount of published research on plant-extracted silver nanoparticles and their application in dental implants, there is still a need for research into the subject of optimizing the form, size, and purity of plant-extracted silver nanoparticles for use in dental implant applications. In the end, we can say that plant-extractable Ag-NPs have a bright future thanks to advancements in synthesis, and they also have enormous utility in the dental implantation industry.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Boraseet al. H.P. Plant extract: a promising Biomatrix for Ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. May 2014;173(1):1–29. doi: 10.1007/s12010-014-0831-4. [DOI] [PubMed] [Google Scholar]
- 2.Song J.Y., Kim B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. Jan. 2009;32(1):79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- 3.Jeong S.H., Yeo S.Y., Yi S.C. The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mater. Sci. 2005;40(20):5407–5411. Oct. [Google Scholar]
- 4.Durán N., Marcato P.D., Conti R.D., Alves O.L., Costa F.T.M., Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 2010;21(6):949–959. [Google Scholar]
- 5.Rajasekharreddy P., Usha Rani P., Sreedhar B. Qualitative assessment of silver and gold nanoparticle synthesis in various plants: a photobiological approach. J. Nanoparticle Res. 2010;12(5):1711–1721. [Google Scholar]
- 6.Ha S.-W., Viggeswarapu M., Habib M.M., Beck G.R. Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent. Acta Biomater. 2018;82:184–196. doi: 10.1016/j.actbio.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasouli R., Barhoum A., Uludag H. A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018;6(6):1312–1338. doi: 10.1039/c8bm00021b. [DOI] [PubMed] [Google Scholar]
- 8.Yazdanianet al. M. The potential application of green-synthesized metal nanoparticles in dentistry: a comprehensive review. Bioinorg. Chem. Appl. 2022;2022:1–27. doi: 10.1155/2022/2311910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emam H.E. Accessibility of green synthesized nanopalladium in water treatment. Results Eng. 2022;15 [Google Scholar]
- 10.Minelli C. Measuring nanoparticle properties: are we high and dry or all at sea? Inst. Phys. 2016 Book of Abstracts. [Google Scholar]
- 11.Ahmed H.B., Mikhail M.M., El-Sherbiny S., Nagy K.S., Emam H.E. pH responsive intelligent nano-engineer of nanostructures applicable for discoloration of reactive dyes. J. Colloid Interface Sci. 2020;561:147–161. doi: 10.1016/j.jcis.2019.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Emam H.E., Saad N.M., Abdallah A.E.M., Ahmed H.B. Acacia gum versus pectin in fabrication of catalytically active palladium nanoparticles for dye discoloration. Int. J. Biol. Macromol. 2020;156:829–840. doi: 10.1016/j.ijbiomac.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Bapat R.A., et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C. 2018;91:881–898. doi: 10.1016/j.msec.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 14.Mathur P., Jha S., Ramteke S., Jain N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomedicine Biotechnol. 2018;46:115–126. doi: 10.1080/21691401.2017.1414825. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Gulati K., Li Z., Di P., Liu Y. Dental implant nano-engineering: Advances, limitations and future Directions. Nanomaterials. 2021;11(10):2489. doi: 10.3390/nano11102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T., Gulati K., Wang N., Zhang Z., Ivanovski S. Bridging the gap: Optimized fabrication of robust titania nanostructures on complex implant geometries towards clinical translation. J. Colloid Interface Sci. Nov. 2018;529:452–463. doi: 10.1016/j.jcis.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 17.T. Li, K. Gulati, N. Wang, Z. Zhang, and S. Ivanovski, “Understanding and augmenting the stability of therapeutic nanotubes on anodized titanium implants,” Mater. Sci. Eng. C, vol. 88, pp. 182–195. [DOI] [PubMed]
- 18.Fernandez C.C., et al. Applications of silver nanoparticles in dentistry: Advances and Technological innovation. Int. J. Mol. Sci. Mar. 2021;22(5):2485. doi: 10.3390/ijms22052485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besinis A., Hadi S.D., Le H.R., Tredwin C., Handy R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology. Mar. 2017;11(3):327–338. doi: 10.1080/17435390.2017.1299890. [DOI] [PubMed] [Google Scholar]
- 20.Noronha V.T., et al. Silver nanoparticles in dentistry. Dent. Mater. Oct. 2017;33(10):1110–1126. doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Cao H., Liu X., Meng F., Chu P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials. Jan. 2011;32(3):693–705. doi: 10.1016/j.biomaterials.2010.09.066. [DOI] [PubMed] [Google Scholar]
- 22.Della Valle C., et al. A novel antibacterial modification treatment of titanium capable to improve Osseointegration. Int. J. Artif. Organs. Oct. 2012;35(10):864–875. doi: 10.5301/ijao.5000161. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed O., et al. Plant extract-synthesized silver nanoparticles for application in dental Therapy. Pharmaceutics. Feb. 2022;14(2):380. doi: 10.3390/pharmaceutics14020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y., et al. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomedicine. Oct. 2015:6659. doi: 10.2147/IJN.S92110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores C.Y., Miñán A.G., Grillo C.A., Salvarezza R.C., Vericat C., Schilardi P.L. Citrate-capped silver nanoparticles showing good bactericidal effect against both Planktonic and Sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl. Mater. Interfaces. Apr. 2013;5(8):3149–3159. doi: 10.1021/am400044e. [DOI] [PubMed] [Google Scholar]
- 26.Narayanan K.B., Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. Apr. 2010;156(1–2):1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Mittal A.K., Chisti Y., Banerjee U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. Mar. 2013;31(2):346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Tregueret al. M. Dose rate effects on Radiolytic synthesis of Gold−Silver bimetallic Clusters in solution. J. Phys. Chem. B. May 1998;102(22):4310–4321. [Google Scholar]
- 29.Li Y., Duan X., Qian Y., Yang L., Liao H. Nanocrystalline silver particles: synthesis, agglomeration, and Sputtering induced by electron beam. J. Colloid Interface Sci. Jan. 1999;209(2):347–349. doi: 10.1006/jcis.1998.5879. [DOI] [PubMed] [Google Scholar]
- 30.Tsai S.C., Song Y.L., Tsai C.S., Yang C.C., Chiu W.Y., Lin H.M. Ultrasonic spray pyrolysis for nanoparticles synthesis. J. Mater. Sci. Jun. 2004;39(11):3647–3657. [Google Scholar]
- 31.Thakkar K.N., Mhatre S.S., Parikh R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine Nanotechnol. Biol. Med. Apr. 2010;6(2):257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Velidandi A., Dahariya S., Pabbathi N.P.P., Kalivarathan D., Baadhe R.R. A review on synthesis, applications, toxicity, risk assessment and limitations of plant extracts synthesized silver nanoparticles. NanoWorld J. 2020;6(3) [Google Scholar]
- 33.Gangula A., Podila R., M R., Karanam L., Janardhana C., Rao A.M. Catalytic reduction of 4-Nitrophenol using biogenic gold and silver nanoparticles derived from Breyniarhamnoides. Langmuir. Dec. 2011;27(24):15268–15274. doi: 10.1021/la2034559. [DOI] [PubMed] [Google Scholar]
- 34.Devi Th.B., Ahmaruzzaman Md., Begum S. A rapid, facile and green synthesis of Ag@AgCl nanoparticles for the effective reduction of 2,4-dinitrophenyl hydrazine. New J. Chem. 2016;40(2):1497–1506. [Google Scholar]
- 35.Prabhu S., Poulose E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. Dec. 2012;2(1):32. [Google Scholar]
- 36.Almatroudi A. Silver nanoparticles: synthesis, characterisation and biomedical applications. Open Life Sci. Nov. 2020;15(1):819–839. doi: 10.1515/biol-2020-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanmuganathanet al. R. Synthesis of silver nanoparticles and their biomedical applications - a comprehensive review. Curr. Pharm. Des. 2019;25(24):2650–2660. doi: 10.2174/1381612825666190708185506. [DOI] [PubMed] [Google Scholar]
- 38.Rafique M., Sadaf I., Rafique M.S., Tahir M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomedicine Biotechnol. Oct. 2017;45(7):1272–1291. doi: 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- 39.Dung Dang T.M., Tuyet Le T.T., Fribourg-Blanc E., Dang M.C. Influence of surfactant on the preparation of silver nanoparticles by polyol method. Adv. Nat. Sci. Nanosci. Nanotechnol. Jun. 2012;3(3) [Google Scholar]
- 40.Zewde B., Ambaye A., Iii J.S., Raghavan D. Vol. 14. 2016. A Review of Stabilized Silver Nanoparticles – Synthesis, Biological Properties, Characterization, and Potential Areas of Applications. [Google Scholar]
- 41.Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganaie S.U., Abbasi T., Abbasi S.A. Green synthesis of silver nanoparticles using an otherwise Worthless weed Mimosa (Mimosapudica): Feasibility and process development toward shape/size control. Part. Sci. Technol. Nov. 2015;33(6):638–644. [Google Scholar]
- 43.Zhu J., Liu S., Palchik O., Koltypin Y., Gedanken A. Shape-controlled synthesis of silver nanoparticles by Pulse Sonoelectrochemical methods. Langmuir. Aug. 2000;16(16):6396–6399. [Google Scholar]
- 44.Wang T.C., Rubner M.F., Cohen R.E. Polyelectrolyte Multilayer Nanoreactors for preparing silver nanoparticle composites: Controlling metal concentration and nanoparticle size. Langmuir. Apr. 2002;18(8):3370–3375. [Google Scholar]
- 45.Brichkinet al. S.B. The use of reversed micelles for the synthesis of nanoparticles. High Energy Chem. Dec. 2008;42(7):516–521. [Google Scholar]
- 46.Calderón-Jiménez B., Johnson M.E., Montoro Bustos A.R., Murphy K.E., Winchester M.R., Vega Baudrit J.R. Silver nanoparticles: Technological advances, Societal impacts, and Metrological Challenges. Front. Chem. Feb. 2017;5 doi: 10.3389/fchem.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung J.H., Cheol Oh H., Soo Noh H., Ji J.H., Soo Kim S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. Dec. 2006;37(12):1662–1670. [Google Scholar]
- 48.Ali S.W., Rajendran S., Joshi M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym. Jan. 2011;83(2):438–446. [Google Scholar]
- 49.Shrivastava S., Bera T., Roy A., Singh G., Ramachandrarao P., Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. Jun. 2007;18(22) doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- 50.Tejamaya M., Römer I., Merrifield R.C., Lead J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in Ecotoxicology media. Environ. Sci. Technol. Jul. 2012;46(13):7011–7017. doi: 10.1021/es2038596. [DOI] [PubMed] [Google Scholar]
- 51.Iravani S. Bacteria in nanoparticle synthesis: current status and future prospects. Int. Sch. Res. Not. Oct. 2014;2014:1–18. doi: 10.1155/2014/359316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsupikhe R.F., Shameli K., Ahmad M.B., Ibrahim N.A., Zainudin N. Green sonochemical synthesis of silver nanoparticles at varying concentrations of κ-carrageenan. Nanoscale Res. Lett. 2015;10(1):302. doi: 10.1186/s11671-015-0916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikhailova E.O. Silver nanoparticles: mechanism of action and Probable bio-application. J. Funct. Biomater. Nov. 2020;11(4):84. doi: 10.3390/jfb11040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alharbi N.S., Alsubhi N.S., Felimban A.I. Green synthesis of silver nanoparticles using medicinal plants: characterization and application. J. Radiat. Res. Appl. Sci. Sep. 2022;15(3):109–124. [Google Scholar]
- 55.Fahmy T.Y.A., Mobarak F. Green nanotechnology: a short cut to beneficiation of natural fibers. Int. J. Biol. Macromol. Jan. 2011;48(1):134–136. doi: 10.1016/j.ijbiomac.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Jeevan P., Ramya K., Rena A.E. Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas aeruginosa. Indian J. Biotechnol. 2012;5 [Google Scholar]
- 57.Cho K.-H., Park J.-E., Osaka T., Park S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta. Nov. 2005;51(5):956–960. [Google Scholar]
- 58.Morsi E., Garbuz D., Gross A.E. Total hip arthroplasty with shelf grafts using uncemented cups. A long-term follow-up study. J. Arthroplasty. Jan. 1996;11(1):81–85. doi: 10.1016/s0883-5403(96)80164-1. [DOI] [PubMed] [Google Scholar]
- 59.Anil Kumar S., et al. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007;29(3):439–445. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- 60.Zomorodianet al. K. Biosynthesis and characterization of silver nanoparticles by Aspergillus species. BioMed Res. Int. 2016;2016:1–6. doi: 10.1155/2016/5435397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad R. Synthesis of silver nanoparticles in Photosynthetic plants. J. Nanoparticles. Sep. 2014;2014:1–8. [Google Scholar]
- 62.Mashwani Z.-R., Khan T., Khan M.A., Nadhman A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: current status and future prospects. Appl. Microbiol. Biotechnol. Dec. 2015;99(23):9923–9934. doi: 10.1007/s00253-015-6987-1. [DOI] [PubMed] [Google Scholar]
- 63.Xia Y., Xia X., Peng H.-C. Shape-controlled synthesis of Colloidal metal Nanocrystals: Thermodynamic versus Kinetic products. J. Am. Chem. Soc. Jul. 2015;137(25):7947–7966. doi: 10.1021/jacs.5b04641. [DOI] [PubMed] [Google Scholar]
- 64.Roy A., Bulut O., Some S., Mandal A.K., Yilmaz M.D. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–2702. doi: 10.1039/c8ra08982e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Rafie M.H., El-Naggar M.E., Ramadan M.A., Fouda M.M.G., Al-Deyab S.S., Hebeish A. Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization. Carbohydr. Polym. Aug. 2011;86(2):630–635. [Google Scholar]
- 66.Gardea-Torresdey J.L., Gomez E., Peralta-Videa J.R., Parsons J.G., Troiani H., Jose-Yacaman M. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. Feb. 2003;19(4):1357–1361. [Google Scholar]
- 67.Makarov V.V., et al. ‘Green’ nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae. Jan. 2014;6(1):35–44. [PMC free article] [PubMed] [Google Scholar]
- 68.Khatoon N., Mazumder J.A., Sardar M. Biotechnological applications of green synthesized silver nanoparticles. J. Nanosci. Curr. Res. 2017;2(1) [Google Scholar]
- 69.Du J., Hu Z., Dong W., Wang Y., Wu S., Bai Y. Biosynthesis of large-sized silver nanoparticles using Angelica keiskei extract and its antibacterial activity and mechanisms investigation. Microchem. J. Jun. 2019;147:333–338. [Google Scholar]
- 70.Rai M., Yadav A., Gade A. Crc 675—current trends in Phytosynthesis of metal nanoparticles. Crit. Rev. Biotechnol. Jan. 2008;28(4):277–284. doi: 10.1080/07388550802368903. [DOI] [PubMed] [Google Scholar]
- 71.Arya G., Kumari R.M., Gupta N., Kumar A., Chandra R., Nimesh S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: reaction optimization, antimicrobial and catalytic activities. Artif. Cells Nanomedicine Biotechnol. Jul. 2018;46(5):985–993. doi: 10.1080/21691401.2017.1354302. [DOI] [PubMed] [Google Scholar]
- 72.Saratale R.G., Shin H.S., Kumar G., Benelli G., Kim D.-S., Saratale G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2) Artif. Cells Nanomedicine Biotechnol. Jan. 2018;46(1):211–222. doi: 10.1080/21691401.2017.1337031. [DOI] [PubMed] [Google Scholar]
- 73.Vijayan R., Joseph S., Mathew B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomedicine Biotechnol. May 2018;46(4):861–871. doi: 10.1080/21691401.2017.1345930. [DOI] [PubMed] [Google Scholar]
- 74.Behravan M., Hossein Panahi A., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. Mar. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 75.Botcha S., Prattipati S.D. Green synthesis of silver nanoparticles using Hyptissuaveolens (L.) Poit leaf extracts, their characterization and cytotoxicity evaluation against PC-3 and MDA-MB 231 cells. Biologia (Bratisl.) Jul. 2019;74(7):783–793. [Google Scholar]
- 76.Huang F., Long Y., Liang Q., Purushotham B., Swamy M.K., Duan Y. Safed Musli(Chlorophytumborivilianum L.) Callus-mediated biosynthesis of silver nanoparticles and evaluation of their antimicrobial activity and cytotoxicity against human Colon cancer cells. J. Nanomater. Feb. 2019;2019:1–8. [Google Scholar]
- 77.TaghavizadehYazdiet al. M.E. Eco-friendly and plant-based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity, and photocatalytic effects. Mater. Technol. Jul. 2019;34(8):490–497. [Google Scholar]
- 78.Dauthal P., Mukhopadhyay M. Noble metal nanoparticles: plant-mediated synthesis, Mechanistic aspects of synthesis, and applications. Ind. Eng. Chem. Res. Sep. 2016;55(36):9557–9577. [Google Scholar]
- 79.Hembram K.C., Kumar R., Kandha L., Parhi P.K., Kundu C.N., Bindhani B.K. Therapeutic prospective of plant-induced silver nanoparticles: application as antimicrobial and anticancer agent. Artif. Cells Nanomedicine Biotechnol. 2018;46(sup3):S38–S51. doi: 10.1080/21691401.2018.1489262. [DOI] [PubMed] [Google Scholar]
- 80.Patel P., Agarwal P., Kanawaria S., Kachhwaha S., Kothari S.L. In: Nanotechnology and Plant Sciences. Siddiqui M.H., Al-Whaibi M.H., Mohammad F., editors. Springer International Publishing; Cham: 2015. Plant-based synthesis of silver nanoparticles and their characterization; pp. 271–288. [Google Scholar]
- 81.Markus J., et al. Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens Maxim. Nanoscale Res. Lett. Dec. 2017;12(1):46. doi: 10.1186/s11671-017-1833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sathishkumaret al. P. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol., B. Oct. 2016;163:69–76. doi: 10.1016/j.jphotobiol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Du J., Singh H., Yi T.-H. Antibacterial, anti-biofilm and anticancer potentials of green synthesized silver nanoparticles using benzoin gum (Styrax benzoin) extract. Bioprocess Biosyst. Eng. Dec. 2016;39(12):1923–1931. doi: 10.1007/s00449-016-1666-x. [DOI] [PubMed] [Google Scholar]
- 84.Nayak D., Pradhan S., Ashe S., Rauta P.R., Nayak B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. Nov. 2015;457:329–338. doi: 10.1016/j.jcis.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 85.Mata R., Reddy Nakkala J., Rani Sadras S. Catalytic and biological activities of green silver nanoparticles synthesized from Plumeria alba (frangipani) flower extract. Mater. Sci. Eng. C. Jun. 2015;51:216–225. doi: 10.1016/j.msec.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 86.Park J., et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011;47(15):4382. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- 87.Ramaret al. M. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. Apr. 2015;140:223–228. doi: 10.1016/j.saa.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 88.Manikandan R., et al. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. Mar. 2015;138:120–129. doi: 10.1016/j.saa.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 89.Venkatesan B., Subramanian V., Tumala A., Vellaichamy E. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac. J. Trop. Med. Sep. 2014;7:S294–S300. doi: 10.1016/S1995-7645(14)60249-2. [DOI] [PubMed] [Google Scholar]
- 90.Baharara J., Namvar F., Ramezani T., Hosseini N., Mohamad R. Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Mol. Basel Switz. Apr. 2014;19(4):4624–4634. doi: 10.3390/molecules19044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reddy N.J., Nagoor Vali D., Rani M., Rani S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C. Jan. 2014;34:115–122. doi: 10.1016/j.msec.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 92.Baghbani-Arani F., Movagharnia R., Sharifian A., Salehi S., Shandiz S.A.S. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortianaRchb extract. J. Photochem. Photobiol., B. Aug. 2017;173:640–649. doi: 10.1016/j.jphotobiol.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Ardestaniet al. M.S. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomedicine. Apr. 2016:1835. doi: 10.2147/IJN.S99882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soshnikovaet al. V. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif. Cells Nanomedicine Biotechnol. Jan. 2018;46(1):108–117. doi: 10.1080/21691401.2017.1296849. [DOI] [PubMed] [Google Scholar]
- 95.Bhuvaneswari R., Xavier R.J., Arumugam M. Facile synthesis of multifunctional silver nanoparticles using mangrove plant Excoecariaagallocha L. for its antibacterial, antioxidant and cytotoxic effects. J. Parasit. Dis. Mar. 2017;41(1):180–187. doi: 10.1007/s12639-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He Y., et al. Synthesis, characterization and evaluation cytotoxic activity of silver nanoparticles synthesized by Chinese herbal Cornus officinalis via environment friendly approach. Environ. Toxicol. Pharmacol. Dec. 2017;56:56–60. doi: 10.1016/j.etap.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 97.Saratale R.G., Benelli G., Kumar G., Kim D.S., Saratale G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018;25(11):10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- 98.Dutta P.P., et al. Antimalarial silver and gold nanoparticles: green synthesis, characterization and in vitro study. Biomed. Pharmacother. Jul. 2017;91:567–580. doi: 10.1016/j.biopha.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 99.Das S., Das J., Samadder A., Bhattacharyya S.S., Das D., Khuda-Bukhsh A.R. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids Surf. B Biointerfaces. Jan. 2013;101:325–336. doi: 10.1016/j.colsurfb.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Banerjee P.P., et al. Mentha arvensis (Linn.)-mediated green silver nanoparticles trigger caspase 9-dependent cell death in MCF7 and MDA-MB-231 cells. Breast Cancer Targets Ther. Apr. 2017;ume 9:265–278. doi: 10.2147/BCTT.S130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dehghanizade S., Arasteh J., Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif. Cells Nanomedicine Biotechnol. Jan. 2018;46(1):160–168. doi: 10.1080/21691401.2017.1304402. [DOI] [PubMed] [Google Scholar]
- 102.Nakkala J.R., Mata R., Sadras S.R. Green synthesized nano silver: synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J. Colloid Interface Sci. Aug. 2017;499:33–45. doi: 10.1016/j.jcis.2017.03.090. [DOI] [PubMed] [Google Scholar]
- 103.Rai M., Kon K., Ingle A., Duran N., Galdiero S., Galdiero M. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl. Microbiol. Biotechnol. 1961, Mar. 2014;98(5):1951. doi: 10.1007/s00253-013-5473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balakumaran M.D., Ramachandran R., Kalaichelvan P.T. Exploitation of endophytic fungus, Guignardiamangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. Sep. 2015;178:9–17. doi: 10.1016/j.micres.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Bhattacharyya S.S., et al. Rapid green synthesis of silver nanoparticles from silver nitrate by a homeopathic mother tincture Phytolacca Decandra. Zhong Xi Yi Jie He Xue Bao. May 2012;10(5):546–554. doi: 10.3736/jcim20120510. [DOI] [PubMed] [Google Scholar]
- 106.Otunola G., Afolayan A., Ajayi E., Odeyemi S. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiber officinale, and Capsicum frutescens. Pharmacogn. Mag. 2017;13(50):201. doi: 10.4103/pm.pm_430_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sankar R., Karthik A., Prabu A., Karthik S., Shivashangari K.S., Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces. Aug. 2013;108:80–84. doi: 10.1016/j.colsurfb.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 108.Ali K., Ahmed B., Dwivedi S., Saquib Q., Al-Khedhairy A.A., Musarrat J. Microwave Accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and Antibiofilm activity on clinical Isolates. PLoS One. Jul. 2015;10(7) doi: 10.1371/journal.pone.0131178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suresh U., et al. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae) Parasitol. Res. Apr. 2015;114(4):1551–1562. doi: 10.1007/s00436-015-4339-9. [DOI] [PubMed] [Google Scholar]
- 110.Saikia D., Gogoi P.K., Phukan P., Bhuyan N., Borchetia S., Saikia J. Green synthesis of silver nanoparticles using Asiatic Pennywort and Bryophyllum leaves extract and their antimicrobial activity. Adv. Mater. Lett. Mar. 2015;6(3):260–264. [Google Scholar]
- 111.Rahimi-Nasrabadi M., Pourmortazavi S.M., Shandiz S.A.S., Ahmadi F., Batooli H. Green synthesis of silver nanoparticles using Eucalyptus leucoxylon leaves extract and evaluating the antioxidant activities of extract. Nat. Prod. Res. Nov. 2014;28(22):1964–1969. doi: 10.1080/14786419.2014.918124. [DOI] [PubMed] [Google Scholar]
- 112.Ahmad N., Sharma S., Singh V.N., Shamsi S.F., Fatma A., Mehta B.R. Biosynthesis of silver nanoparticles from Desmodiumtriflorum: a novel approach towards weed utilization. Biotechnol. Res. Int. Nov. 2011;2011:1–8. doi: 10.4061/2011/454090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gude V., Upadhyaya K., Prasad M.N.V., Rao N.V.S. Green synthesis of gold and silver nanoparticles using Achyranthes aspera L. Leaf extract. Adv. Sci. Eng. Med. Mar. 2013;5(3):223–228. [Google Scholar]
- 114.Mohamed N.H., Ismail M.A., Abdel-Mageed W.M., Mohamed Shoreit A.A. Antimicrobial activity of green silver nanoparticles from endophytic fungi isolated from Calotropis procera (Ait) latex. Microbiology. Sep. 2019;165(9):967–975. doi: 10.1099/mic.0.000832. [DOI] [PubMed] [Google Scholar]
- 115.A. Saxena, R. M. Tripathi, and R. P. Singh, “BIOLOGICAL SYNTHESIS OF SILVER NANOPARTICLES BY USING ONION (ALLIUM CEPA) EXTRACT AND THEIR ANTIBACTERIAL ACTIVITY,” p. 6.
- 116.Yang N., Wei X.-F., Li W.-H. Sunlight irradiation induced green synthesis of silver nanoparticles using peach gum polysaccharide and colorimetric sensing of H2O2. Mater. Lett. Sep. 2015;154:21–24. [Google Scholar]
- 117.Umadevi M., Bindhu M.R., Sathe V. A novel synthesis of Malic acid capped silver nanoparticles using Solanum lycopersicums fruit extract. J. Mater. Sci. Technol. Apr. 2013;29(4):317–322. [Google Scholar]
- 118.Korbekandi H., Asghari G., Jalayer S.S., Jalayer M.S., Bandegani M. Nanosilver particle production using juglans regia L. (Walnut) leaf extract. Jundishapur J. Nat. Pharm. Prod. 2013;8(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- 119.Dwivedi A.D., Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. Oct. 2010;369(1–3):27–33. [Google Scholar]
- 120.Parashar U.K., Saxena P.S., Srivastava A. Bioinspired synthesis of silver nanoparticles. Dig. J NanomaterBiostruct. 2009;4 [Google Scholar]
- 121.Al-Shmgani H.S.A., Mohammed W.H., Sulaiman G.M., Saadoon A.H. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif. Cells Nanomedicine Biotechnol. 2017;45(6):1234–1240, Aug. doi: 10.1080/21691401.2016.1220950. [DOI] [PubMed] [Google Scholar]
- 122.Ahmed Q., Gupta N., Kumar A., Nimesh S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif. Cells Nanomedicine Biotechnol. Aug. 2017;45(6):1192–1200. doi: 10.1080/21691401.2016.1215328. [DOI] [PubMed] [Google Scholar]
- 123.Zargaret al. M. Green synthesis and antibacterial effect of silver nanoparticles using Vitex Negundo L. Molecules. Aug. 2011;16(8):6667–6676. doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sintubin L., Verstraete W., Boon N. Biologically produced nanosilver: current state and future perspectives. Biotechnol. Bioeng. Oct. 2012;109(10):2422–2436. doi: 10.1002/bit.24570. [DOI] [PubMed] [Google Scholar]
- 125.Salunkhe R.B., Patil S.V., Patil C.D., Salunke B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae) Parasitol. Res. Sep. 2011;109(3):823–831. doi: 10.1007/s00436-011-2328-1. [DOI] [PubMed] [Google Scholar]
- 126.Gan P.P., Li S.F.Y. Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev. Environ. Sci. Biotechnol. 2012;11(2):169–206. [Google Scholar]
- 127.Huang J., et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. Mar. 2007;18(10) [Google Scholar]
- 128.Lalitha A., Subbaiya R., Ponmurugan P. Vol. 8. 2013. Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. [Google Scholar]
- 129.Pasupuletiet al. V.R. Biogenic silver nanoparticles using Rhinacanthusnasutus leaf extract: synthesis, spectral analysis, and antimicrobial studies. Int. J. Nanomedicine. Sep. 2013:3355. doi: 10.2147/IJN.S49000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Chung I.-M., Park I., Seung-Hyun K., Thiruvengadam M., Rajakumar G. Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res. Lett. Dec. 2016;11(1):40. doi: 10.1186/s11671-016-1257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abou El-Nour K.M.M., Eftaiha A., Al-Warthan A., Ammar R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. Jul. 2010;3(3):135–140. [Google Scholar]
- 132.Khezerlou A., Alizadeh-Sani M., Azizi-Lalabadi M., Ehsani A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. Oct. 2018;123:505–526. doi: 10.1016/j.micpath.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 133.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. Nov. 2019;12(7):908–931. [Google Scholar]
- 134.Chowdhury M., Hossain N., Noman T.I., Hasan A., Shafiul A., Mohammod Abul K. Biodegradable, physical and microbial analysis of tamarind seed starch infused eco-friendly bioplastics by different percentage of Arjuna powder. Results Eng. Mar. 2022;13 [Google Scholar]
- 135.Hossain N., Chowdhury M.A., Rana M., Hassan M., Islam S. Terminalia arjuna leaves extract as green corrosion inhibitor for mild steel in HCl solution. Results Eng. Jun. 2022;14 [Google Scholar]
- 136.Siakavellaet al. I.K. Effect of plant extracts on the characteristics of silver nanoparticles for topical application. Pharmaceutics. Dec. 2020;12(12):1244. doi: 10.3390/pharmaceutics12121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jafarizad A., Safaee K., Gharibian S., Omidi Y., Ekinci D. Biosynthesis and in-vitro study of gold nanoparticles using Mentha and Pelargonium extracts. Procedia Mater. Sci. 2015;11:224–230. [Google Scholar]
- 138.Castro L., Blázquez M.L., Muñoz J.A., González F., García-Balboa C., Ballester A. Biosynthesis of gold nanowires using sugar beet pulp. Process Biochem. May 2011;46(5):1076–1082. [Google Scholar]
- 139.Oveset al. M. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater. Sci. Eng. C. Aug. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 140.Akintelu S.A., Bo Y., Folorunso A.S. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. J. Chem. Dec. 2020;2020:1–12. [Google Scholar]
- 141.Kumara Swamy M., Sudipta K.M., Jayanta K., Balasubramanya S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. Jan. 2015;5(1):73–81. [Google Scholar]
- 142.Salmen S.H., Alharbi S.A. Silver nanoparticles synthesized biogenically from Aloe fleurentiniorum extract: characterization and antibacterial activity. Green Chem. Lett. Rev. Jan. 2020;13(1):1–5. [Google Scholar]
- 143.Niehus H., Heiland W., Taglauer E. Low-energy ion scattering at surfaces. Surf. Sci. Rep. May 1993;17(4–5):213–303. [Google Scholar]
- 144.Brongersma H., Draxler M., Deridder M., Bauer P. Surface composition analysis by low-energy ion scattering. Surf. Sci. Rep. Mar. 2007;62(3):63–109. [Google Scholar]
- 145.Goebl D., Bruckner B., Roth D., Ahamer C., Bauer P. Low-energy ion scattering: a quantitative method? Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. Jul. 2015;354:3–8. [Google Scholar]
- 146.Rafati A., ter Veen R., Castner D.G. Low-energy ion scattering: determining overlayer thickness for functionalized gold nanoparticles: HS-LEIS overlayer thickness quantification of functionalized AuNPs. Surf. Interface Anal. Nov. 2013;45(11–12):1737–1741. doi: 10.1002/sia.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eckeet al. G. Vol. 58. 2007. ANALYSIS OF NANOSTRUCTURES BY MEANS OF AUGER ELECTRON SPECTROSCOPY; p. 6. [Google Scholar]
- 148.Mogk D.W. Application of Auger electron spectroscopy to studies of chemical weathering. Rev. Geophys. 1990;28(4):337. [Google Scholar]
- 149.He W., Liu X., Kienzle A., Müller W.E.G., Feng Q. Vitro Uptake of silver nanoparticles and their toxicity in human Mesenchymal Stem cells derived from bone Marrow. J. Nanosci. Nanotechnol. Jan. 2016;16(1):219–228. doi: 10.1166/jnn.2016.10728. [DOI] [PubMed] [Google Scholar]
- 150.Magalhãeset al. A.P.R. Nanosilver application in dental cements. ISRN Nanotechnol. 2012:1–6. [Google Scholar]
- 151.FaghriZonooz N., Salouti M. Extracellular biosynthesis of silver nanoparticles using cell filtrate of Streptomyces sp. ERI-3. Sci. Iran. 2011;18(6):1631–1635. [Google Scholar]
- 152.Mukunthan K., Elumalai E., Patel T.N., Murty V.R. Catharanthus roseus: a natural source for the synthesis of silver nanoparticles. Asian Pac. J. Trop. Biomed. Aug. 2011;1(4):270–274. doi: 10.1016/S2221-1691(11)60041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manikandan V., et al. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech. May 2017;7(1):72. doi: 10.1007/s13205-017-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sundeep D., Vijaya Kumar T., Rao P.S.S., Ravikumar R.V.S.S.N., Gopala Krishna A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. May 2017;6(1–2):57–66. doi: 10.1007/s40204-017-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paul R.P., Roy A., M A.M., Shanmugam R. Antibacterial activity of white pepper oleoresin mediated silver nanoparticles against oral pathogens. J. Evol. Med. Dent. Sci. Aug. 2020;9(33):2352–2355. [Google Scholar]
- 156.Umai D., Vikranth A., Meenambiga S.S. A study on the green synthesis of silver nanoparticles from Olea europaea and its activity against oral pathogens. Mater. Today Proc. 2021;44:3647–3651. [Google Scholar]
- 157.Jeevanandam J., Danquah M.K., Pan S. Plant-derived nanobiomaterials as a potential Next generation dental implant surface modifier. Front. Mater. Apr. 2021;8 [Google Scholar]
- 158.Chen P., et al. Fabrication of a silver nanoparticle-coated collagen membrane with anti-bacterial and anti-inflammatory activities for guided bone regeneration. Biomed. Mater. Oct. 2018;13(6) doi: 10.1088/1748-605X/aae15b. [DOI] [PubMed] [Google Scholar]
- 159.Kirmanidouet al. Y. Assessment of cytotoxicity and antibacterial effects of silver nanoparticle-doped titanium alloy surfaces. Dent. Mater. Sep. 2019;35(9):e220–e233. doi: 10.1016/j.dental.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 160.Choi S.-H., Jang Y.-S., Jang J.-H., Bae T.-S., Lee S.-J., Lee M.-H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. Jul. 2019;17(3) doi: 10.1177/2280800019847067. [DOI] [PubMed] [Google Scholar]
- 161.Shankar S.S., Rai A., Ahmad A., Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. Jul. 2004;275(2):496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 162.Hossain Nayem, Islam Mohammad Aminul, Chowdhury Mohammad Asaduzzaman, Alam Ashraful. Advances of nanoparticles employment in dental implant applications. Applied Surface Science Advances. 2022;12 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.