Abstract
One of the contributing causes of hypertension or exacerbation of ischemic heart disease (IHD) has been suggested to be environmental pollution and occupational exposure to particular metals, such as lead. Here, we describe a 43‐year‐old man with a history of oral opium with symptoms of IHD due to chest pain.
Keywords: hypertension, ischemic heart disease, lead poisoning, opium addiction
Lead poisoning can cause abdominal and epigastric pain that may be mistaken for an acute abdomen or acute coronary syndrome.
1. INTRODUCTION
In recent years, lead poisoning caused by consuming opium contaminated with lead has become a significant public health issue in Iran. 1 Lead (Pb) is widely used and present in the human environment as a result of its beneficial properties. Lead is a bluish‐gray metal with a high poisonous level. It is a well‐known metal with several industrial applications due to its low melting point, density, and resistance to corrosion. It can lead to both acute and chronic poisoning in individuals. 2 , 3
Like other heavy metals, lead has an effect on a number of organ systems. Abdominal colic pain, loss of appetite, constipation, myalgia, a diminished libido, agitation, irritability, convulsions, and anemia are non‐specific clinical symptoms of lead poisoning. 4 Abdominal pain from lead poisoning may be mistaken for acute abdominal pain. 5 Examples of lead poisoning in the industry or environment include opium and its derivatives, contaminated soil, contaminated food, industrial emissions, and car exhaust. 6 Factors such as physical inactivity, high serum lipids or cholesterol, HTN, family history of ischemic heart disease (IHD), obesity, smoking, and gender are the main risk factors for IHD.
Ischemic heart disease has also been associated with environmental pollution and occupational exposure to heavy metals such as lead, arsenic, mercury, and cobalt. Lead is utilized mostly in several industrial processes. 7 The production and recycling of batteries for the automobile industry is one of the main applications of lead. Lead exposure is not only common for workers in battery manufacturing facilities but can also occur via ingestion, inhalation, or, rarely, direct skin contact. The negative effects of lead can be seen in all of the organ systems of the body, such as the neurological system, cardiovascular, skeletal, renal, hematopoietic, and reproductive systems. 7
Lead exposure has been associated with hearing loss and dental damage. 8 , 9 Also, the risk of atherosclerotic cardiovascular disease and HTN may increase significantly. 10 , 11 Herein, we present a 43‐year‐old man with a history of oral opium who was admitted to the cardiac care unit (CCU) with symptoms of epigastric pain, sweating, nausea, and vomiting with a primary diagnosis of IHD and was treated with a chelator for lead poisoning.
2. CASE PRESENTATION
In May 2022, a 43‐year‐old man with a history of ingesting opium orally for 20 years was referred to an emergency department (ED) in northern Iran with symptoms of epigastric pain, sweating, weakness, and fatigue. Around 3 months ago, the patient experienced intermittent abdominal pain. The colic‐like abdominal pain would exacerbate in the evening and would radiate to the back. The patient also reported weight loss and experiencing nausea, vomiting, constipation, and decreased appetite. He mentioned that he defecates once a week and has difficult bowel movements.
On examination, the sclera was not icteric, the conjunctiva was pale and anemic, and auscultation of the heart and lungs was normal. The abdomen was firm with generalized tenderness. The blood pressure (BP) is 150/100 mmHg, the heart rate (HR) is 98 beats/min, the respiratory rate (RR) is 18 breaths/min, and the body temperature is 36.8°C.
Abdominal and pelvic ultrasounds were normal. The abdomen radiography and chest X‐ray performed with Vesipak solution did not show any abnormalities except abundant feces with an opaque substance in the colon (Figure 1A,B). Also, in the performed electrocardiography (ECG), there is a slight ST depression in leads 2, 3, and aVF (Figure 2). The results of his laboratory findings are shown in the Table 1. Due to high troponin (cTnI), cardiac counseling was requested immediately. In echocardiography, the ejection fraction (EF) was 40%. The patient was admitted to the CCU ward. He was consulted for toxicology, gastroenterology, and surgery. A gastrointestinal endoscopy was shown to be normal. The patient's oral opium was discontinued. Treatment with drugs such as pentaprazole 40 mg daily, ondansetron 4 mg twice a day (BID), aspirin 80 mg daily, enoxaparin 40 mg daily, and morphine sulfate was started. Also, methadone was started at a dose of 25 mg BID to control withdrawal symptoms, and lactulose syrup 10 ml three times a day (TDS) was administered. The fecal impaction improved by performing a 200 cc glycerin enema with normal saline, and the patient's abdominal pain was completely improved.
FIGURE 1.
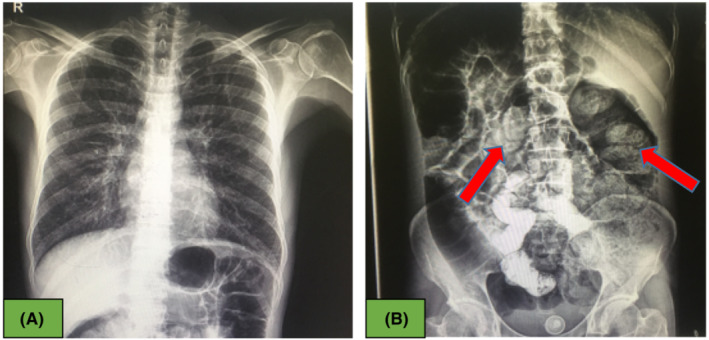
(A) Normal chest X‐ray, (B) Abdominal x‐ray showed fecal impaction in the colon with red arrow.
FIGURE 2.
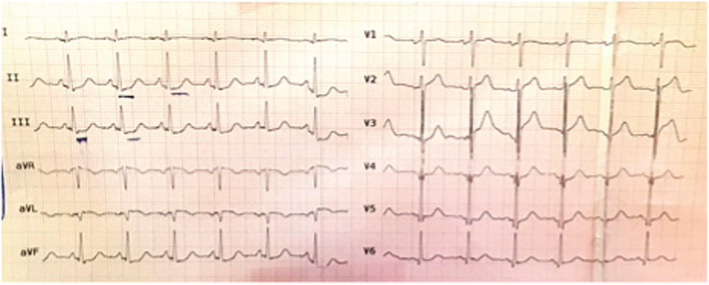
Slight ST depression in leads 2, 3, and aVF.
TABLE 1.
Baseline laboratory results
| Parameter | Value | Reference value | Unit |
|---|---|---|---|
| Na | 131 | 135–145 | mEq/L |
| K | 3.5 | 3.5–5 | mEq/L |
| BUN | 21 | 13–43 | mg/dl |
| Cr | 1.4 | 0.6–1.2 | mg/dl |
| LDH | 613 | 100–190 | U/L |
| CPK | 3145 | 24–195 | U/L |
| CPKMB | 85 | – | U/L |
| TG | 71 | <150 | mg/dl |
| Chol total | 146 | <200 | mg/dl |
| FBS | 92 | 70–110 | mg/dl |
| Amylase | 33 | 80–86 | U/L |
| Lipase | 25 | Up to 60 | U/L |
| AST | 111 | 10–40 | U/L |
| ALT | 50 | ˂45 | U/L |
| ALP | 173 | 80–306 | U/L |
| Bil total | 1.5 | 0.3–1.2 | mg/dl |
| Bil direct | 0.6 | <0.3 | mg/dl |
| Hb | 9.4 | 12–16 | gr/dl |
| Hct | 29 | 36–48 | % |
| MCV | 82 | 80–96 | Fl |
| HDL | 52 | >40 | mg/dl |
| LDL | 83 | <100 | mg/dl |
| Lead | 87.6 | <10 | μg/dl |
| Troponin(cTnI) | 5606 | <0.04 | ng/ml |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Bil, bilirubine; BUN, blood urea nitrogen; Chol, cholesterol; CPK, creatine phosphokinase; Cr, creatinine; Hct, hematocrit; HDL, high‐density lipoprotein; LDH, lactate dehydrogenase; LDL = low‐density lipoprotein; MCV = mean corpuscular volume; Retic, reticulocyte; TG, triglycerides.
A request for blood lead levels (BLL) was made due to colic abdominal pain, anemia (microcytic‐hypochromic), and the consumption of oral opium with suspicion of lead poisoning. The BLL was 87.6 μg/dl. The diagnosis of lead poisoning was confirmed, and treatment was performed with chelation therapy such as deep intramuscular injection (IM) of British Anti‐Lewisite (BAL) at a dosage of 4 mg/kg (200 mg TDS for 5 days) has also been begun for lead poisoning. After 4 h, a continuous slow intravenous infusion (IV) of calcium disodium edentate (CaNa2EDTA) with a dosage of 30 mg/kg (200 mg four times a day for 7 days) was started. Following that, oral succimer was begun at a rate of 10 mg/kg every 8 h for 5 days, then every 12 h for 14 days.
Following treatment, BLL was reduced to less than 35 μg/dl and hemoglobin (HB) was increased to 11.5 g/dl. There was no fecal or urinary incontinence, and the patient's gastrointestinal symptoms had improved significantly, with all para‐clinical results being normal. Finally, the patient was discharged from the hospital in good general condition.
Written informed consent was obtained from the patient for the publication of this report. This report was approved by the Mazandaran University of Medical Science Ethics Committee (NO: IR.MAZUMS.REC.1399.7850) and was carried out in accordance with the Helsinki Declaration Principles. Also, CARE guidelines and methodology were followed in this study.
3. DISCUSSION
Acute lead poisoning occurs with gastrointestinal symptoms, cognitive disorders, and emotional disorders, but chronic poisoning causes fatigue, weakness, decreased brain and cognitive function, motor impairment, psychiatric disorders, and, in some cases, a decrease in nerve conduction velocity. 12 Experimental animals exposed to both acute and chronic lead poisoning developed HTN, dyslipidemia, atherosclerosis, and vascular and cardiac problems. 13 In human autopsy studies, lead exposure was associated with atherosclerotic plaques in the aorta. 14 Elevated blood or bone lead levels in humans have been associated with increases in cardiovascular mortality. 15
Individuals with preexisting chronic kidney disease (CKD), HTN, or diabetes mellitus are more likely to develop lead nephropathy. 16 In addition, it has been shown that a significant number of patients with essential HTN, CKD with unknown etiology, and renal impairment without a history of observable lead exposure have increased lead burdens. 17 , 18
In our patient, immediately after receiving the test result of high serum lead level, oral opium consumption was discontinued, and induction of defecation was done with the onset of glycerin enema and lactulose syrup, as well as with chelation therapy. Fortunately, the patient's symptoms improved.
In research conducted on 497 male workers at a battery recycling center, Ghiasvand et al. showed an association between blood lead levels (BLLs) and IHD risk factors such as HTN, hyperlipidemia, and hyperglycemia. For 281 participants, the mean BLL was >40 μg/dl, ≤40 μg for 216 subjects, and the mean BLL was 43.3 μg/dl. This study revealed no correlation between TG, fasting blood sugar (FBS), or FBS and high BLL or diastolic blood pressure (DBP). The public health burden of IHD is likely to be reduced by focusing on health‐protective measures, individual behavioral changes, and frequent periodic medical assessment with an emphasis on DBP in lead‐exposed workers. 19 A recent systematic study by Navas‐Asin et al. found a correlation between prolonged lead exposure and high blood pressure. In addition, a limited number of studies have reported a relationship between lead exposure and cardiovascular events in the general population, such as coronary artery disease (CAD), stroke, and peripheral arterial disease. 20
The significant correlation between long‐term lead exposure and HTN has been conclusively shown by several studies in experimental animals. In animals with normal genetic structure, prolonged exposure to low levels of lead leads to chronic HTN. The mechanisms of lead‐induced HTN have been the subject of several studies. These causes include oxidative stress, a dysfunctional nitric oxide (NO) system, inflammation, vasoactive hormone dysregulation, and changes in cellular Ca2 transport and intracellular Ca2 distribution. 21
Blood lead levels of more than 100 μg/dl are usually associated with encephalopathy. Azadeh et al. showed the presence of gastrointestinal, hematologic, and neurological symptoms (sensory and motor neuropathy) and a wrist drop in a 24‐year‐old woman with a history of oral opium consumption, and the BLL was 95.7 μg/dl. The BLL decreased after treatment with chelation, and the patient's symptoms improved. 22 According to Mohararie et al., a family had two cases of severe lead poisoning caused by oral opium consumption. They were admitted to the hospital for anemia, icterus, and neurological symptoms such as delirium, hyperirritability, and unconsciousness. The elderly patient had low consciousness and neuropathy symptoms, such as paralysis and absent deep tendon reflexes. The BLL of the patients showed that both of them had high levels of lead (≥150 μg/dl). Unfortunately, one of the patients died due to a cardiovascular collapse. 23
4. CONCLUSION
Lead poisoning should be considered in the differential diagnosis for any opium‐dependent patient with gastrointestinal, hematologic, neurological, or chest pain associated with sweating and hypertension. Early detection of lead poisoning in opium users would benefit from BLL assessment because delay in treatment can lead to irreversible disorders in vital organs. Blood lead level is a method of early detection that may be suitable for the patient.
AUTHOR CONTRIBUTIONS
ASh and ZZ were involved in the interpretation and collecting of data and editing of the manuscript. MAS involved in writing and preparing the final version of the manuscript. MS was responsible for collecting data and submitting the manuscript. All authors reviewed the paper and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ETHICAL APPROVAL
This report was approved by the Mazandaran University of Medical Science Ethics Committee (NO: IR.MAZUMS.REC.1399.7850) and was carried out in accordance with the Helsinki Declaration Principles.
CONSENT
Written informed consent was obtained from the patient for the publication of this case report.
ACKNOWLEDGEMENT
None declared.
Abedi Samakoosh M, Sharifpour A, Soleymani M, Zakariaei Z. Lead poisoning with unusual manifestations in an opium‐addicted man. Clin Case Rep. 2022;10:e06774. doi: 10.1002/ccr3.6774
DATA AVAILABILITY STATEMENT
The data are available to the correspondent author and can be obtained upon request.
REFERENCES
- 1. Alinejad S, Aaseth J, Abdollahi M, Hassanian‐Moghaddam H, Mehrpour O. Clinical aspects of opium adulterated with lead in Iran: a review. Basic Clin Pharmacol Toxicol. 2018;122:56‐64. [DOI] [PubMed] [Google Scholar]
- 2. Azizi MH, Azizi F. Lead poisoning in the world and Iran. Int J Occup Environ Med. 2010;1:81‐87. [PubMed] [Google Scholar]
- 3. Pourmand A, Khedir Al‐Tiae T, Mazer‐Amirshahi M. Perspective on lead toxicity, a comparison between the United States and Iran. Daru. 2012;20:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henretig FM. In: Nelson LS, ed. Lead. Gold Frank's Toxicologic Emergencies. 9th ed. McGraw Hill; 2011:1266‐1280. [Google Scholar]
- 5. Froutan H, Kashefi Zadeh A, Kalani M, et al. Lead toxicity: a probable cause of abdominal pain in drug abusers. Med J Islam Repub Iran. 2011;25:16‐20. [Google Scholar]
- 6. Mokri A. Brief overview of the status of drug abuse in Iran. Arch Iran Med. 2002;5:184‐190. [Google Scholar]
- 7. White D, Cory‐Slechta A, Gilbert E, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225(1):1‐27. [DOI] [PubMed] [Google Scholar]
- 8. Lanphear BP, Hornung R, Khoury J, et al. Environmental health perspectives. Low‐level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brudevold F, Steadman LT. The distribution of lead in human enamel. J Dent Res. 1956;35(3):430‐437. [DOI] [PubMed] [Google Scholar]
- 10. Bargman R. Dietary factors in essential hypertension. Prog Food Nutr Sci. 1985;9(1–2):109‐147. [PubMed] [Google Scholar]
- 11. Cheng Y, Schwartz J, Sparrow D, et al. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension, the normative aging study. Am J Epidemiol. 2001;153(2):164‐171. [DOI] [PubMed] [Google Scholar]
- 12. Dobbs MR, ed. Clinical Neurotoxicology: Syndromes, Substances, Environments. 1st ed. Saunders; 2009:259‐272. [Google Scholar]
- 13. Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. 2011;2011:870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443‐2449. [DOI] [PubMed] [Google Scholar]
- 15. Navas‐Acien A, Selvin E, Sharrett AR, Calderon‐Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196‐3201. [DOI] [PubMed] [Google Scholar]
- 16. Ekong EB, Jaar BG, Weaver VM. Lead‐related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70:2074‐2084. [DOI] [PubMed] [Google Scholar]
- 17. Batuman V, Landy E, Maesaka JK, Wedeen RP. Contribution of lead to hypertension with renal impairment. N Engl J Med. 1983;309:17‐21. [DOI] [PubMed] [Google Scholar]
- 18. Sanchez‐Fructuoso AI, Torralbo A, Arroyo M, et al. Occult lead intoxication as a cause of hypertension and renal failure. Nephrol Dial Transplant. 1996;11:1775‐1780. [PubMed] [Google Scholar]
- 19. Ghiasvand M, Aghakhani K, Salimi A, Kumar R. Ischemic heart disease risk factors in lead exposed workers: research study. J Occup Med Toxicol. 2013;8(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navas‐Acien A, Guallar E, Silbergeld EK, et al. Lead exposure and cardiovascular disease—a systematic review. Environ Health Perspect. 2007;115:472‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaziri ND, Sica D. Lead‐induced HTN: role of oxidative stress. Curr Hypertens Rep. 2004;6:314‐320. [DOI] [PubMed] [Google Scholar]
- 22. Azadeh H, Gholami F, Zakariaei Z, et al. Bilateral wrist drop due to Lead poisoning in a young woman with opium addiction. Clin Med Insights Case Rep. 2022;15. doi: 10.1177/11795476221103813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moharari RS, Khajavi MR, Panahkhahi M, Mojtahedzadeh M, Najafi A. Loss of consciousness secondary to lead poisoning – case reports. Middle East J Anaesthesiol. 2009;20:453‐455. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available to the correspondent author and can be obtained upon request.


