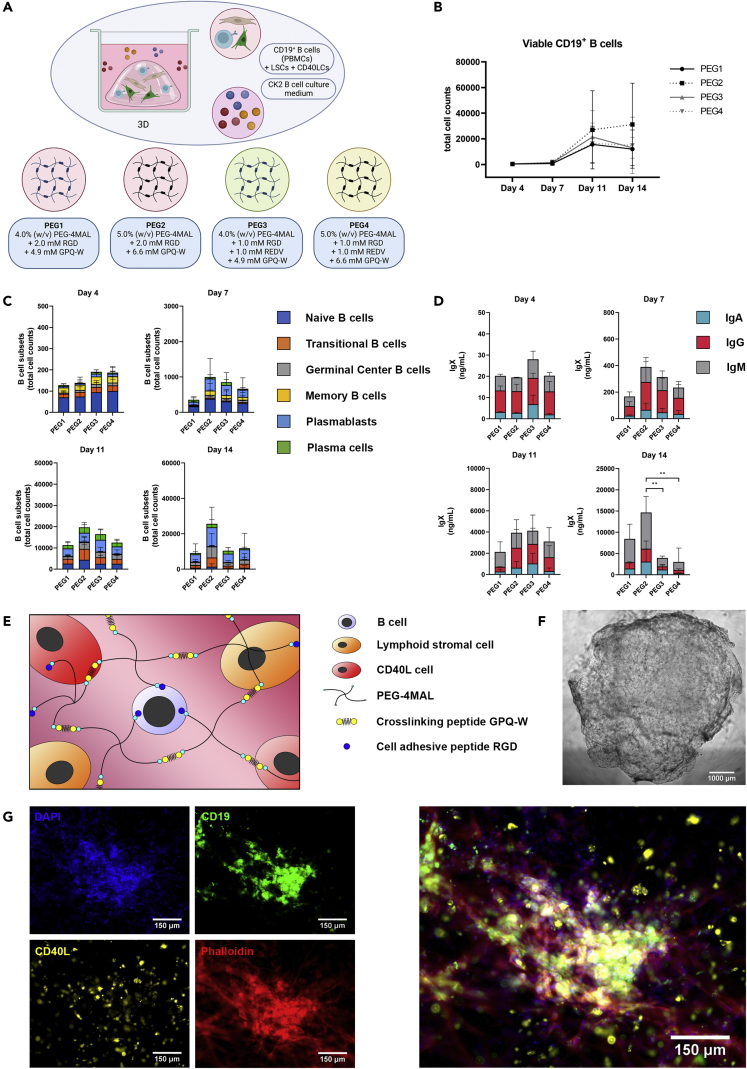
Figure 3.
Optimization of the functionalized hydrogel that is used as a 3D matrix for the 3D in vitro B-cell co-cultures (see also Figure S2)
CD19+ B-cells (n = 3), CD40LCs and LSCs were incorporated in the varying hydrogel compositions (3D, Table 2) and cultured in CK2 (Table 1).
(A) Schematic overview of the studied 3D co-culture conditions.
(B) Absolute number of viable CD19+ B cells over time.
(C) B cell subsets (total cell counts) within each 3D culture of the absolute number of cells per well on days 4, 7, 11 and 14, of the six B-cell subsets.
(D) Production of IgX (IgM, IgG, and IgA) in the culture supernatant on days 7 and 14.
(B-D) Data showing the mean ± SD (n = 3). ∗∗ = p > 0.01.
(E) Schematic representation of the selected PEG2 5.0% (w/v) PEG-4MAL and 2.0 mM RGD functionalized hydrogel, forming a stable 3D hydrogel when crosslinked using a GPQ-W cross-linking peptide. CD19+ B-cells, CD40LCs, as well as LSCs are homogeneously distributed throughout the hydrogel upon cross-linking.
(F) Brightfield image of a 40 μL crosslinked PEG2 (5.0% (w/v) PEG-4MAL and 2.0 mM RGD) 3D co-culture, scalebar represents 1000 μm.
(G) Fluorescent images of the PEG2 3D co-cultures, illustrating the possibility of cellular tracing and staining. Blue + green + red cells are CD19+ B-cells, blue + yellow + red cells are CD40LCs, blue + red cells are LSCs (blue = DAPI, green = CD19 immunostaining, yellow = DiI labeling, red = phalloidin, scale bars represent 150 μm).