Abstract
Although Candida dubliniensis is a close genetic relative of Candida albicans, it colonizes and infects fewer sites. Nearly all instances of candidiasis caused by C. dubliniensis are restricted to the oral cavity. As cell surface hydrophobicity (CSH) influences virulence of C. albicans, CSH properties of C. dubliniensis were investigated and compared to C. albicans. Growth temperature is one factor which affects the CSH status of stationary-phase C. albicans. However, C. dubliniensis, similar to other pathogenic non-albicans species of Candida, was hydrophobic regardless of growth temperature. For all Candida species tested in this study (C. albicans, C. dubliniensis, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis), CSH status correlated with coaggregation with the anaerobic oral bacterium Fusobacterium nucleatum. Previous studies have shown that CSH status of C. albicans involves multiple surface proteins and surface protein N-glycans. The hydrophobic surface glycoprotein CAgp38 appears to be expressed by C. albicans constitutively regardless of growth temperature and medium. C. dubliniensis expresses a 38-kDa protein that cross-reacts with the anti-CAgp38 monoclonal antibody; however, expression of the protein was growth medium and growth temperature dependent. The anti-CAgp38 monoclonal antibody has been shown to inhibit adhesion of C. albicans to extracellular matrix proteins and to vascular endothelial cells. Since protein glycosylation influences the CSH status of C. albicans, we compared the cell wall mannoprotein content and composition between C. albicans and C. dubliniensis. Similar bulk compositional levels of hexose, phosphate, and protein in their N-glycans were determined. However, a component of the C. albicans N-glycan, acid-labile phosphooligomannoside, is expressed much less or negligibly by C. dubliniensis, and when present, the oligomannosides are predominantly less than five mannose residues in length. In addition, the acid-labile phosphooligomannoside profiles varied among the three strains of C. dubliniensis we tested, indicating the N-glycan of C. dubliniensis differs from C. albicans. For C. albicans, the acid-labile phosphooligomannoside influences virulence and surface fibrillar conformation, which affects exposure of hydrophobic surface proteins. Given the combined role in C. albicans of expression of specific surface hydrophobic proteins in pathogenesis and of surface protein glycosylation on exposure of the proteins, the lack of these virulence-associated CSH entities in C. dubliniensis could contribute to its limited ability to cause disseminated infections.
In 1993, Coleman et al. (5) reported that certain atypical isolates of Candida albicans were a distinct species. These atypical isolates were obtained from oropharyngeal specimens of adult human immunodeficiency virus (HIV)-infected individuals, especially individuals receiving fluconazole. Subsequent phenotypic and genotypic analyses supported the validity of placing the atypical isolates into a new taxon, Candida dubliniensis (58). Recently, C. dubliniensis has been recovered from oral samples of HIV-seropositive pediatric patients (3). Other studies have shown that C. dubliniensis is a member of the normal flora of a low percentage of healthy (non-HIV-infected) individuals (33, 35, 47). Despite being closely related to C. albicans, C. dubliniensis appears to have a restricted range of host sites which it colonizes or infects, as retrospective studies of archived clinical isolates revealed few isolates associated with sites other than the oropharyngeal and vaginal mucocutaneous areas (6, 35, 47, 55). However, C. dubliniensis has been reported to be a rare agent of fungemia in immunocompromised patients (2, 45). C. albicans, on the other hand, is capable of invading and parasitizing nearly any body site.
In vitro phenotypic studies have shown that C. dubliniensis has few characteristics that distinguish it from C. albicans (6, 55, 57). None of these characteristics are unique to C. dubliniensis, as rare isolates of C. albicans can express one or more of the characteristics. Examples of such characteristics include the inability to grow at 45°C, the production of multiple terminal chlamydoconidia, and assimilation of xylose. C. dubliniensis appears to have greater expression than C. albicans of some characteristics generally considered to be associated with virulence, such as aspartyl protease production and possibly adhesion to buccal epithelial cells (7, 8, 12, 44), although its ability to bind to mucin appears similar to that of C. albicans (9). C. dubliniensis also appears to more easily develop resistance to fluconazole, which is commonly used to treat oropharyngeal candidiasis (6, 56, 57). Jabra-Rizk et al. (34) have shown that C. dubliniensis, but not C. albicans, coaggregates to the oral bacterium Fusobacterium nucleatum when the yeast is grown at 37°C. While these characteristics may help explain how C. dubliniensis could outcompete C. albicans on the oral mucosa, especially upon exposure to fluconazole, it is unclear what limits its overall invasive potential compared to C. albicans.
The expression of cell surface hydrophobicity (CSH) by C. albicans has been correlated with increased virulence compared to cell surface hydrophilicity (1, 13). How CSH specifically influences virulence is unknown, but hydrophobic cells compared to hydrophilic cells are more adherent to host and inanimate substrata (including mucin, epithelial cells, endothelial cells, and extracellular matrix proteins), more resistant to phagocytosis, and more germination competent (1, 9, 22–24, 43; P. M. Glee, J. E. Cutler, E. E. Benson, R. F. Bargatze, and K. C. Hazen, submitted for publication). CSH expression by C. albicans incubated at 37°C occurs depending on growth conditions, cell morphology, and growth phase and has been demonstrated to occur in chronic candidiasis (13). Nearly homogeneous hydrophobic cell populations can be obtained by the laboratory convenience of growth to stationary phase at 23°C, while nearly homogeneous hydrophilic cell populations are obtained by growth to stationary phase at 37°C depending on growth medium (22). Coaggregation with F. nucleatum by C. albicans occurred when cells were grown at 23°C but not with cells grown at 37°C, suggesting that coaggregation may be linked to surface hydrophobicity (34). On the other hand, C. dubliniensis coaggregated with F. nucleatum regardless of its growth temperature. These observations suggest that expression of CSH may be different between C. albicans and C. dubliniensis.
In this study, we evaluated various CSH properties of C. dubliniensis and compared them to C. albicans. The basis of these studies is our observation that CSH of C. albicans is due to the direct contribution of multiple surface proteins and the indirect contribution of surface protein N-mannosylation groups (26, 27, 31, 41, 42). One such protein, CAgp38, has been recently demonstrated to contribute strongly to attachment of hydrophobic cells to vascular endothelial cells when the yeast cells are exposed to physiologic shear by bulk flow (Glee et al., submitted for publication). However, in order for the hydrophobic proteins to be exposed to the extracellular milieu, protein mannosylation must be modified to cause the surface fibrils, which are composed of high-molecular-mass mannoproteins, to alter conformation and unmask the hydrophobic surface regions. Jabra-Rizk et al. recently reported that the surface fibrils of C. dubliniensis do not vary in architecture between cells grown at 25 and 37°C and that the fibrils resemble those seen on hydrophobic C. albicans cells (27, 36). The mannosylation modification that appears primarily responsible for the change in the C. albicans N-glycan chain involves the acid labile β-1,2-oligomannoside group (41, 42). Here we report that C. dubliniensis differs from C. albicans in its ability to express CSH and in its CSH-related glycoprotein components.
MATERIALS AND METHODS
Isolates and growth conditions.
The yeast isolates used in this study are listed in Table 1. Isolates were grown and subsequently subcultured (inoculum concentration of 1 × 106 to 3 × 106 cells/ml) in Sabouraud dextrose broth (SDB) or phosphate-buffered yeast nitrogen base supplemented with 2% glucose (YNB2G) at 23 or 37°C to stationary phase (26 to 28 h) (29, 43). All assays were performed on cells which were harvested from the second subculture (third pass in the indicated medium). Cells were washed at least three times with deionized water prior to use in experiments, and their morphology was assessed microscopically to assure that the cells were yeast forms.
TABLE 1.
Yeast isolates and references describing their origins
| Isolate | Sourcea | Reference |
|---|---|---|
| C. albicans LGH1095 | Blood culture | 32 |
| C. albicans LGH870 | Blood culture | 32 |
| C. albicans A9wt | Oral | 62 |
| C. albicans B311 | Gastric wash | 21 |
| C. dubliniensis CD36 | Oral | 58 |
| C. dubliniensis NCPF 3108b | Bronchopneumonia (lung isolate, 1957) | 54 |
| C. dubliniensis R2g | Oral | 60 |
| C. dubliniensis R3b | Oral | 60 |
| C. dubliniensis R16b white | Oral | 60 |
| C. dubliniensis CBS 8500 | Blood culture | 45 |
| C. dubliniensis CBS 8501 | Blood culture | 45 |
| C. dubliniensis M41654 | Right atrium biopsy | K. C. Hazen, T. Winner, and D. Kaufman, unpublished data |
| C. glabrata ZB5 | Clinical isolate | 43 |
| C. kefyr ZB2 | Clinical isolate | 43 |
| C. krusei ZB4 | Clinical isolate | 43 |
| C. parapsilosis ZB3 | Clinical isolate | 43 |
| C. tropicalis ZB1 | Clinical isolate | 43 |
All isolates were obtained from humans.
Previously designated Candida stellatoidea.
CSH assay.
CSH was measured by the hydrophobic microsphere assay (13, 28). Microsphere attachment was assessed by bright-field microscopy at a magnification of ×400. The percentage of cells with three or more attached microspheres was recorded as the percent hydrophobicity. A cell is defined as the unit composed of buds and parent structures. The ratio of cell spheres to cells (S/C ratio) was determined for each population (30). This was done to assure that the cell populations are relatively similar in their S/C ratios. The S/C ratios between C. albicans and C. dubliniensis were statistically evaluated using a two-tailed t test.
Coaggregation assay.
Coaggregation analysis was performed as described by Jabra-Rizk et al. (34) on cells grown at 37°C. Briefly, 100 μl each of a 1% (vol/vol) suspension of F. nucleatum and 10% (vol/vol) yeast suspension were mixed for 20 s. The mixture was then allowed to sit for 3 min at room temperature before the relative degree of coaggregation was assessed. CSH and coaggregation properties were assayed on the same population of yeast cells. F. nucleatum suspensions were kindly provided to us by Mary Ann Jabra-Rizk (University of Maryland). Prior to testing for each experiment, the F. nucleatum suspension was qualitatively evaluated for reproducibility by testing coaggregation with standard C. albicans and C. dubliniensis isolates.
Cell wall protein extraction.
Limited cell wall digestion to release primarily surface proteins was achieved with lyticase, a β-1,3 glucanase (Sigma Chemical Co., St. Louis, Mo.), as described elsewhere (41).
Cell wall carbohydrate composition analysis.
Cell wall glycans of C. albicans LGH1095 and C. dubliniensis CD36 were isolated and separated into mannan and glucan fractions as described previously (41, 42). Briefly, cells are grown to stationary phase (26 h; third transfer) and washed and the cell pellet is dried. Initial extraction of glycan from the dried cells is based on the method of Peat et al. (49). The water soluble glycan (glucan and mannan) is precipitated with sodium acetate and absolute ethanol, pelleted by centrifugation, and dialyzed against distilled water overnight at 4°C. Following dialysis, mannan is isolated using a strategy based on that used by Lloyd (40) and Okubo et al. (48), which involves the use of 8% (wt/vol) cetyltrimethylammonium bromide (Calbiochem, La Jolla, Calif.).
To obtain acid-labile mannan, purified bulk mannan samples (approximately 10 mg) were dissolved in 10 mM HCl to 10 mg/ml in a 1.5-ml microcentrifuge tube (48). Each tube was sealed with a cap lock (PGC Scientific) and heated in a boiling water bath for 60 min. The tubes were cooled to room temperature, and the solutions were neutralized with 1 N NaOH. The samples were transferred to 15-ml Corex tubes, and the acid-stable mannoprotein component was precipitated by the addition of 3 volumes of cold ethanol (42). Precipitation was extended by incubation at −20°C overnight. The tubes were centrifuged at 10,000 × g, the supernatant fluid was transferred to borosilicate tubes (2 by 75 mm), and the solutions were dried in a centrifugal vacuum evaporator. Dried pellets were dissolved in 250 μl of distilled water, and carbohydrate content was determined by the assay described below.
Protein, hexose, and phosphate compositions of mannan and glucan were determined as described previously (42) by the bicinchoninic acid assay (Pierce Chemical) (53), the phenol-sulfuric acid method of Dubois et al. (10) using mannose as the standard, and the method of Chen et al. (4) using sodium phosphate as the standard, respectively. Data for each component (protein, hexose, and phosphorus) were compared among the several isolates by analysis of variance followed by Bonferroni post-hoc tests for pairwise comparisons. Significance was tested at the α = 0.05 level.
Western blots.
Cell wall proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% polyacrylamide for enzymatic extracts; 7.5% polyacrylamide for mannan) and transferred to nitrocellulose membranes at a constant voltage of 50 V overnight in Towbin buffer (25 mM Tris, 192 mM glycine [pH 8.3], 20% methanol) (61). Transfer buffer for mannan blots contained 0.1% sodium dodecyl sulfate (Transblot; Bio-Rad). Blots were stained with Ponceau S red, and when necessary, the various membrane panels were subsequently separated. After destaining, the panels were probed with antibodies as described previously (41). Briefly, cell wall protein blots were probed with the monoclonal antibody (MAb) 6C5-H4CA (an immunoglobulin G2a [IgG2a]) at a 1:5,000 dilution (final concentration, 240 ng/ml) MAb 6C5-H4CA recognizes a C. albicans hydrophobic wall protein with a mass of 38 kDa (designated CAgp38) (43). An irrelevant mouse IgG2a served as a control (final concentration, 200 ng/ml). For blots of isolated mannan, MAb B6 and MAb B6.1 (provided by Jim Cutler at Montana State University) were used at a 1:1,000 dilution (final concentration, 3.5 μg/ml). MAb B6.1 recognizes β-1,2-mannotriose, and MAb B6 detects an epitope within the acid-stable region of cell wall mannan (16). Mouse IgM (Kappa, TEPC 183; Sigma) diluted 1:2,000 (final concentration, 3.5 μg/ml) was used as a control. Alkaline phosphatase-conjugated goat anti-mouse IgM (μ-chain specific; Sigma) diluted 1:1,000 served as the secondary antibody.
Labeling of oligosaccharides by ANTS and electrophoretic separation.
Acid-labile oligosaccharides were labeled with 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS) (Molecular Probes, Eugene, Oreg.) by the method of Jackson and Williams (37, 38). β-1,4-Oligomannosides (Man2-Man6; V-Labs, Covington, La.) along with an α-1,4-oligoglucoside ladder (Bio-Rad) were also labeled with ANTS and used as standards (42).
Labeled oligosaccharides were separated by electrophoresis using precast oligosaccharide profiling gels (Glyko or Bio-Rad) and the conditions previously described by Masuoka and Hazen (42). One microgram of total hexose was loaded per lane. Tracking dye (sample buffer containing bromophenol blue, xylene cyanole FF, and Thorin) was added into the β-1,4-oligomannoside standard well. Following electrophoresis, the gels were imaged using a GlycoDoc imager (Bio-Rad). The G4 band in the glucose ladder represents 25 pmol of maltotetraose.
N-glycan antisera.
C. albicans and C. dubliniensis isolates, grown in SDB, were tested for agglutination with antisera against various N-glycan components (Candida Check Kit; Iatron Laboratories, Inc., Tokyo, Japan). Washed cells were suspended to 5 × 108 cells/ml in phosphate-buffered saline. Equal volumes of cell suspension and antisera (15 μl) were mixed at room temperature for 2 min. The cell suspension was mixed with buffer as a negative control. Agglutination was graded as negative, weak positive, moderate positive, or strong positive in accordance with the manufacturer's directions.
RESULTS
Morphology.
All isolates grew as round-to-oval yeasts in SDB and YNB2G at both 23 and 37°C except C. dubliniensis strain NCPF 3108, which produced hyphae and pseudohyphae when grown at 37°C. In addition, C. dubliniensis isolates produced cell units with higher S/C ratios than C. albicans at stationary phase (P < 0.001 for all of the S/C values except C. dubliniensis NCPF 3108 at 37°C [Table 2]). When C. dubliniensis cultures were incubated an additional 24 h, no change in the S/C ratios was seen (data not shown).
TABLE 2.
Effect of growth temperature and medium on CSH status of C. dubliniensis and C. albicans isolates grown to stationary phase
| Growth medium | Isolate | Growth temp (°C)
|
|||
|---|---|---|---|---|---|
| 23
|
37
|
||||
| S/C | CSH (%) | S/C | CSH (%) | ||
| SDB | C. albicans LGH1095 | 1.2 | 94.5 ± 1.3 | 1.7 | 1.6 ± 0.1 |
| C. albicans LGH870 | 2.3 | 97.8 ± 1.9 | 1.5 | 1.3 ± 1.0 | |
| C. dubliniensis CD36 | 2.7 | 98.4 ± 0.5 | 2.3 | 99.0 ± 0.8 | |
| C. dubliniensis NCPF 3108 | 2.7 | 49.7 ± 2.5 | Hyphal | NDa | |
| C. dubliniensis R3b | 2.5 | 80.2 ± 8.6 | 2.7 | 97.0 ± 1.0 | |
| C. dubliniensis R16b white | 2.7 | 87.7 ± 2.5 | 2.6 | 96.8 ± 1.2 | |
| C. dubliniensis CBS 8500 | 2.9 | 94.0 ± 2.0 | 1.7 | 90.7 ± 3.1 | |
| C. dubliniensis CBS 8501 | 3.5 | 98.7 ± 0.6 | 2.2 | 97.5 ± 2.2 | |
| C. dubliniensis M41654 | 2.1 | 95.2 ± 2.1 | 2.1 | 96.5 ± 1.2 | |
| YNB2G | C. albicans LGH1095 | 1.2 | 94.5 ± 1.3 | 1.2 | 1.3 ± 0.5 |
| C. albicans LGH870 | 1.3 | 97.0 ± 1.2 | 1.2 | 0.6 ± 0.5 | |
| C. dubliniensis CD36 | 2.5 | 99.5 ± 0.8 | 2.7 | 99.8 ± 0.4 | |
| C. dubliniensis NCPF 3108 | 2.3 | 78.7 ± 2.1 | Hyphal | ND | |
| C. dubliniensis R3b | 1.7 | 98.5 ± 2.6 | 2.4 | 90.4 ± 11.1 | |
| C. dubliniensis R16b white | 2.1 | 99.7 ± 0.5 | 1.9 | 95.8 ± 1.8 | |
| C. dubliniensis CBS 8500 | 2.7 | 80.3 ± 1.5 | 2.0 | 90.3 ± 3.0 | |
| C. dubliniensis CBS 8501 | 2.3 | 99.7 ± 0.6 | 2.4 | 97.5 ± 1.4 | |
| C. dubliniensis M41654 | 1.4 | 99.0 ± 0.9 | 2.9 | 94.3 ± 1.7 | |
ND, not done.
CSH.
All isolates of C. dubliniensis, except strain NCPF 3108, exhibited high levels (>80%) of CSH when grown to stationary phase at either 23 or 37°C, while C. albicans was, as previously shown (32), hydrophobic when grown at 23°C but not at 37°C (Table 2). Microsphere attachment to C. dubliniensis cells was abundant and distributed in a pattern similar to the distribution typically seen with hydrophobic C. albicans cells (not shown). Thus, the high CSH levels of C. dubliniensis is not an artifact of the criterion used to designate a cell as hydrophobic with the microsphere assay (three or more attached microspheres per cell).
Coaggregation.
Jabra-Rizk et al. (34) have reported that C. dubliniensis strains grown at either 23 or 37°C coaggregate with F. nucleatum, while coaggregation between C. albicans and F. nucleatum is growth temperature dependent. That observation was confirmed here (Table 3). In addition, other Candida species coaggregated with F. nucleatum. All of these species were also hydrophobic. The only species grown at 37°C that did not exhibit coaggregation was C. albicans.
TABLE 3.
Correlation of CSH, coaggregation with F. nucleatum, and MAb CA6C5 antigen detection for various Candida species grown in SDB
| Isolate | Growth temp (°C) | CSH (%) | CoAga | 6C5 Agb |
|---|---|---|---|---|
| C. albicans LGH1095 | 23 | 94.5 ± 1.3 | 4+ | 4+ |
| 37 | 1.6 ± 0.1 | +/− | 4+ | |
| C. dubliniensis CD36 | 23 | 98.4 ± 0.5 | 3+ | 0 |
| 37 | 99.0 ± 0.8 | 3+ | +/− | |
| C. tropicalis ZB1 | 37 | 98.2 ± 1.7 | 4+ | +/− (54 kDa) |
| C. glabrata ZB5 | 37 | 95.5 ± 2.1 | 2+ | 0 |
| C. kefyr ZB2 | 37 | 97.7 ± 1.3 | 3+ | 1+ (36 kDa) |
| C. parapsilosis ZB3 | 37 | 81.0 ± 10.6 | 1+ | 0 |
| C. krusei ZB4 | 37 | 60.5 ± 1.9 | 2+ | + (50 kDa) |
Coaggregation with F. nucleatum. Scoring is in accordance with the method of Jabra-Rizk et al. (34): 0, no visible aggregates in the cell suspension; 1+, small uniform coaggregates in the suspension; 2+, coaggregates that are easily seen but no immediate settling of coaggregates; 3+, large coaggregates which settle rapidly and leave some turbidity in the supernatant fluid; 4+, large coaggregates which settle immediately and leave a clear supernatant fluid; +/−, weakly visible.
Band intensity with MAb CA6C5. Scoring was as follows: 0, no detectable band; 1+, faint band; 2+ obvious band but thin; 3+, dark band but thin; 4+, dark thick band; +/−, weakly visible. Values in parentheses are molecular masses of detected bands.
Hydrophobic protein detection.
MAb 6C5CA recognizes a hydrophobic surface protein antigen of C. albicans designated CAgp38. The designation as a hydrophobic surface protein is based on retention of the protein on a hydrophobic interaction chromatography column (27, 29). The antigen is evident in Western blots of wall digests of C. albicans cells grown at either 23 or 37°C (Table 3). C. dubliniensis had no detectable antigen when grown at 23°C and weakly positive reactivity in digests from cells grown at 37°C. The mass of the detected antigen was 38 kDa. MAb 6C5-H4CA detected a reactive antigen in digests of several other Candida species, but the signal was relatively weak and the masses of the detected antigens differed among the species.
Expression of the antigen recognized by MAb 6C5-H4CA by C. dubliniensis was, unlike C. albicans, also medium dependent as cells grown in YNB2G gave more signal than cells grown in SDB (Fig. 1). This result was consistent for all isolates tested, including the recently recovered bloodstream isolates CBS 8500 and CBS 8501 reported by Meis et al. (45).
FIG. 1.
Representative Western blot showing CAgp38 as detected by MAb 6C5-H4CA in cell wall preparations of C. albicans and C. dubliniensis grown in two media and at two temperatures. Lanes were loaded with 15 μg of total protein. No signal was observed in control lanes probed with irrelevant IgG2a plus secondary antibody or secondary antibody alone (not shown).
Cell wall mannan composition.
Compositional analysis of hexose, phosphate, and protein content in bulk mannan or glucan demonstrated no significant differences between C. albicans and C. dubliniensis (Table 4). Similarly, the carbohydrate/protein, phosphate/protein, and carbohydrate/phosphate ratios did not differ (not shown).
TABLE 4.
Bulk cell wall mannan composition of C. albicans and C. dubliniensis grown to stationary phase in SBD at 23 and 37°C
| Organism | 23°C
|
37°C
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSH (%) | Protein (%)a | Hexose (%)a | Phosphate (%)a | Acid-labile mannan (%)a | CSH (%) | Protein (%)a | Hexose (%)a | Phosphate (%)a | Acid-labile mannan (%)a | |
| C. albicans A9wt | 96.0 (3.7) | 6.7 (0.5) | 74.3 (0.6) | 1.66 (0.13) | 5.90 (0.44) | 3.0 (2.0) | 4.6 (0.4) | 74.6 (6.0) | 1.99 (0.25) | 3.13 (0.50) |
| C. dubliniensis CD36 | 96.3 (1.5) | 4.9 (0.3) | 82.2 (10.0) | 1.53 (1.23) | 0.13 (0.10) | 70.8 (6.8) | 5.3 (0.9) | 88.0 (2.6) | 1.97 (1.16) | 0.11 (0.03) |
| C. dubliniensis R3b | 88.5 (3.5) | 4.5 (0.8) | 85.0 (5.2) | 4.53 (2.6) | 0.09 (0.04) | 98.0 (1.0) | 6.2 (1.1) | 88.6 (6.4) | 1.27 (0.63) | 0.11 (0.07) |
Values are the percent (dry weight) of mannan and are expressed as the sample mean (sample standard deviation) for three different sample preparations.
C. dubliniensis produced much less acid-labile mannan than C. albicans. The acid-labile form comprises 3 to 6% of total mannan from C. albicans A9 (Table 4). Acid-labile mannan from hydrophobic cells is roughly twice the percentage of that from hydrophilic cells. This is likely reflected in the presence of the longer oligosaccharides in the hydrophobic cell acid-labile mannan (Fig. 2). The acid-labile component of C. dubliniensis mannan is an order of magnitude lower than that of C. albicans (Table 4).
FIG. 2.
Representative FACE analysis of the acid-labile material from C. albicans and C. dubliniensis mannan and β-1,4-oligomannoside and α-1,4-oligoglucoside standards. Lanes for each experimental sample were loaded with 1 μg of hexose. M, mannose; G, glucose.
Comparison of acid labile β-1,2-oligomannoside.
Acid-labile oligomannosides from each isolate were also compared by fluorophore-assisted carbohydrate electrophoresis (FACE). This allowed not only visualization of quantitative differences but also profile differences in the individual oligosaccharides comprising each sample (Fig. 2). As has been previously reported (14, 42), the specific monosaccharide composition and location and anomeric configuration of the glycosidic linkages affect electrophoretic mobility. Thus, the β-1,4-oligomannosides do not comigrate with their corresponding α-1,4-oligoglucosides. However, inclusion of ladders with known degrees of polymerization provides a useful framework.
When equal masses of carbohydrate are compared, the acid-labile oligomannoside profile from C. albicans yeast cells grown at 23 and 37°C were similar to those obtained previously (42); the hydrophobic cells appear to have a broad range of oligomannoside lengths, while the hydrophilic cells appear to have oligomannoside lengths that are predominantly less than six residues (Fig. 2). Comparison of acid-labile mannan among the three isolates shows that FACE results mirror those of the colorimetric assay. Mannan from C. dubliniensis CD36 contains less acid-labile mannan than C. albicans. Further, acid-labile mannan at both growth temperatures contains proportionately less of the longer oligomannosides relative to their C. albicans counterparts. Nevertheless, the profile of CD36 acid-labile mannan from 23°C-grown cells, compared to that from 37°C-grown cells, is similar to those from C. albicans, with the acid-labile mannan from 23°C-grown cells containing oligomannosides with higher degrees of polymerization.
The R3b isolate contained the least amount of acid-labile mannan, and this, too, is reflected in the FACE results. The bands observed in acid-labile mannan from R3b cells grown at either temperature are very weak in intensity. In addition, the pattern of bands in the R3b acid-labile mannan appears to be different from CD36. Both of those profiles differed from that of strain R2g (not shown).
Detection of mannan epitopes by Western blotting.
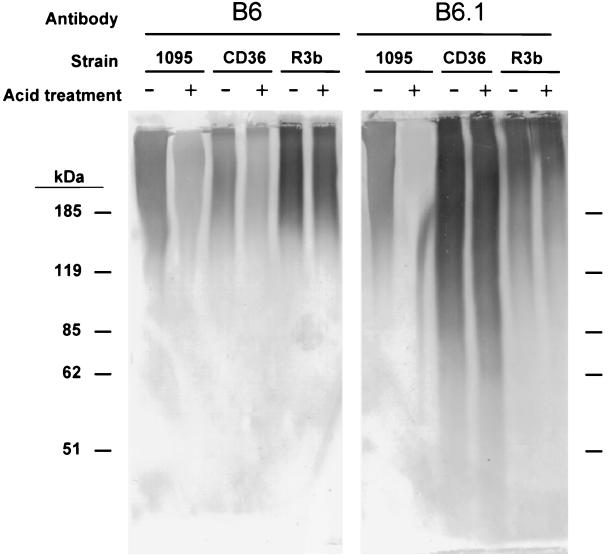
A difference in the staining patterns was observed when cell wall digests from C. albicans and C. dubliniensis were probed on Western blots with MAb B6 and B6.1, which recognize the acid stable region and β-1,2-mannotriose respectively, of C. albicans N-glycans (Fig. 3). Both antibodies produced signal over comparable molecular mass ranges in the C. albicans mannan. However, in mannan isolated from the C. dubliniensis type strain, CD36, MAb B6.1 recognized mannoproteins over a much wider molecular mass range than B6. The reverse appeared to be true for the strongest signal in mannan from a second C. dubliniensis isolate, R3b.
FIG. 3.
Representative Western blot of C. albicans (A9wt) and C. dubliniensis (CD36 and R3b) mannan. A set mass (5 mg) of mannan was dissolved and subjected to mild acid hydrolysis (+) or mock treated (−). Lanes were loaded on the basis of constant (20 μg) total protein. No signal was observed in control lanes probed with irrelevant mouse IgM plus secondary antibody or secondary antibody alone (not shown).
The reactivity of MAb B6.1 against the C. albicans preparation was diminished when the wall digests were acid treated to remove the acid-labile group (Fig. 3). Acid treatment of the C. dubliniensis wall digests did not affect the reactivity of MAb B6.1. This result was unexpected given the previous results. It suggests that a β-1,2-mannotriose group may be present on the acid-stable region of C. dubliniensis N-glycans.
Comparison of N-mannan epitopes using Iatron antisera.
Each isolate was tested against the entire panel of Iatron antisera to ensure detection of any differences in recognized mannan structure. Serotype B C. albicans strains LGH1095, A9, and LGH870 displayed moderate to strong agglutination with factors 1, 4, and 5. Strain LGH870 also showed a weak positive agglutination with factor 13b. Serotype A strain C. albicans B311 showed moderate to strong agglutination with factors 1, 4, 5, and 6. These results are in accordance with standard results provided in the kit.
All C. dubliniensis strains (CD36, NCPF 3108, CBS 8500, CBS 8501, R2g, R3b, and R16b) demonstrated moderate to weak agglutination with factors 1, 4, 5, and 6. One strain, R3b, also gave a very weak positive reaction with factor 13. These results confirm and extend those seen by previous groups, indicating that C. dubliniensis is serotype A based on these sera (46, 52, 58), and confirm the interpretation of the B6.1 Western blot results that the β-1,2-oligotrimannose group may be present on the acid-stable region of C. dubliniensis N-glycans. The results also indicate that, overall, C. albicans and C. dubliniensis express similar N-glycan epitopes.
DISCUSSION
Stationary-phase C. albicans exhibits growth temperature-dependent expression of surface hydrophobicity. C. dubliniensis, a close genetic relative of C. albicans, does not. Similar to the other pathogenic Candida species tested here, C. dubliniensis was hydrophobic when grown at 37°C. While it is evident from the present data (and a similar observation with 45 other C. dubliniensis isolates [M. A. Jabra-Rizk et al., personal communication]) that CSH expression correlates with coaggregation to F. nucleatum and may influence oral candidiasis, the role of surface hydrophobicity in pathogenesis of the various Candida species at other host sites is less clear. One explanation is that CSH of C. albicans influences virulence but is not the sole determinant. That is, CSH is simply one of several contributing virulence determinants (7). An alternative explanation is that surface hydrophobicity is the manifestation of several surface molecules which are involved in pathogenesis. Lack of one of these molecules may not decrease apparent CSH levels but could affect virulence by influencing which body sites can be infected.
Evidence supporting the latter explanation comes, in part, from other investigations and the study presented here. For example, C. dubliniensis appears to express higher levels of aspartyl proteases than C. albicans and to adhere as well as and possibly better than C. albicans to buccal epithelial cells (7, 8, 12, 44). However, except for a few rare instances, the only host body site that is infected by C. dubliniensis is the oropharynx, while C. albicans can attack many body sites. In the present study, expression by C. albicans of a surface hydrophobic protein, CAgp38, occurs at similar levels regardless of growth temperature or medium (Fig. 1). CAgp38 has been shown to be involved in adherence to vascular endothelial cells under physiologic shear (Glee et al., submitted for publication) and, along with other hydrophobic surface proteins, to contribute to adherence to extracellular matrix proteins (43). Other pathogenic species tested here do not express the protein, although some species appear to weakly express a single protein with a cross-reactive epitope but one differing in mass from CAgp38. These results suggest CAgp38 is not required for coaggregation.
The lack of expression of the epitope (and possibly the epitope-bearing protein) could explain in part how these species are less virulent than C. albicans. In the case of C. dubliniensis, expression of the cross-reactive, 38-kDa antigen was both temperature and medium dependent. The reduced expression of the protein was not due to insufficient cell wall glucanase digestion (which would suggest altered retention in the wall), as the protein was not detected in wall preparations from cells exposed to more-extensive digestion (data not shown). C. dubliniensis appears to have the genetic capability of producing the protein, but whether conditions in vivo induce such expression is unknown. If not, then the lack of expression of this protein may diminish the ability of C. dubliniensis to bind to endothelial cells under physiologic shear during hematogenous dissemination.
Meis et al. (45) recently reported the recovery of several C. dubliniensis isolates from cases of fungemia. While these isolates could potentially be more virulent than oral isolates, we did not observe any difference between these isolates and other C. dubliniensis isolates for expression of the MAb 6C5CA antigen. If CAgp38 expression does play a role in dissemination of C. dubliniensis, then it is possible that these isolates were more easily induced to produce the antigen due to an unknown factor particular to the patients from whom the isolates were recovered. Further studies are needed to address this speculation.
The ability of C. albicans to modify its CSH status may provide an additional pathogenic advantage over Candida species, including C. dubliniensis, which do not. We have previously suggested that the ability to modify CSH status could be achieved by several mechanisms (25, 41, 42): increased production and surface localization of hydrophobic cell wall proteins, alteration in the length or density of surface fibrils, and modification of the surface fibril mannan to cause fibrillar conformational change resulting in exposure of protein hydrophobic groups. In the case of C. albicans, the third of these mechanisms appears to be responsible (42). Our previous data suggest that the conformational change of the fibrils is accomplished by modification of the acid-labile β-1,2-oligomannoside chain length (42).
Jabra-Rizk et al. (36) reported that the surface fibrils of C. dubliniensis resemble those of hydrophobic C. albicans. However, the method used by those investigators for preserving and examining those fibrils is more prone to distort the fibrillar ultrastructure and does not provide similar resolution and wall integrity as the freeze-fracture method employed by Hazen et al. (27), who originally described the ultrastructure of hydrophobic C. albicans. It is possible that the fibrils are the same length as those of C. albicans but are more irregularly arranged, allowing the hydrophobic surface to be exposed. This result is supported in part by the observations that no differences were seen in the bulk mannan compositional analysis between C. albicans and C. dubliniensis and that the two species share similar epitopes (as detected by the Iatron factor antisera).
The acid-labile mannans within the N-glycans did differ between C. albicans and C. dubliniensis. Significantly less acid-labile oligomannoside is present on the N-glycans of C. dubliniensis, and there is variation in the acid-labile oligomannoside profile among strains and within a strain grown at 23 and 37°C. These results suggest that the acid-labile group does not have as important a role in exposing the hydrophobic surface as it does for C. albicans. The sample loaded in the experimental lanes in Fig. 2 were normalized to a given mass such that each lane contains 1 μg of carbohydrate, as determined by the colorimetric assay. Because the C. dubliniensis samples showed bands of lesser or negligible intensity although containing the same amount of carbohydrate, we conclude that the majority of mannose in the acid-labile fractions is the monosaccharide form. Due to the acrylamide composition of the oligosaccharide profiling gels, the monosaccharide component cannot be resolved from the unconjugated ANTS front.
Mannose, as a monosaccharide, in the acid-labile fraction may explain some of the elemental composition results (Table 4). Acid-labile mannan is thought to be produced by two enzymes, one that attaches the initial mannophosphate group and one that extends the chain (59). If initiation of the chain occurs but elongation is limited or nonexistent, then the percentage of phosphorus could remain at levels like those seen in C. albicans while the amount of carbohydrate in the acid-labile fraction is greatly reduced.
The present analysis does not exclude the possibility that the acid-stable region of the N-mannosyl groups of C. dubliniensis are unlike those of C. albicans and could thus account, perhaps along with the reduced amount in the acid-labile oligomannosides, for the differences in the fibrillar ultrastructure while having little effect on the bulk compositional analysis.
The difference between C. dubliniensis and C. albicans in their acid-labile mannan may contribute to their differential virulence capabilities. Besides influencing CSH expression and thus CSH-mediated pathogenic events of C. albicans, the acid-labile group, which is a homoanomeric β-1,2-oligomannoside, has been implicated directly and indirectly in the virulence of C. albicans. Poulain and colleagues have shown that β-1,2-oligomannoside can serve to signal tumor necrosis factor alpha production and may be involved in adhesion of C. albicans to macrophages (11, 39, 50). Stimulation of an immune response to the β-1,2-oligomannoside group as well as MAbs directed against the β-1,2-oligomannoside have been shown to protect mice against a lethal challenge of C. albicans (15, 17–20, 51).
Taken together, these results indicate that C. albicans and C. dubliniensis differ in CSH-related protein and mannosylation features. While C. dubliniensis, unlike C. albicans, expresses CSH regardless of growth conditions, the variable expression of the hydrophobic wall protein CAgp38, which is involved in adhesion of C. albicans to vascular endothelium, along with the negligible production of acid-labile β-1,2-oligomannoside, which appears to have immunomodulatory activity, may affect the ability of C. dubliniensis to disseminate from the oral cavity and to invade other host sites. This differential ability to express these two important CSH-related entities could therefore partially explain how C. dubliniensis is less virulent than C. albicans. The results also demonstrate that merely being hydrophobic does not necessarily make a yeast more virulent than a less hydrophobic yeast (all other virulence traits being equal); rather, the moieties which are responsible for making the cell hydrophobic are more important determinants of pathogenic potential.
ACKNOWLEDGMENTS
We gratefully appreciate the willingness of Mary Ann Jabra-Rizk to provide us with the suspensions of F. nucleatum and to show us how to perform the coaggregation assay. We also thank Susan A. Howell (St. Johns Hospital, London, England) and Jacques F. G. M. Meis (University Hospital Nijmegen, Nijmegen, The Netherlands) for providing many of the C. dubliniensis isolates used in this study. The kind gift of MAbs B6 and B6.1 from Jim E. Cutler (Montana State University, Bozeman) is also gratefully appreciated.
This study was supported in part by Public Health Service grants R01 AI31048 and R01 AI43997 (to K.C.H.) from the National Institutes of Health.
REFERENCES
- 1.Antley P P, Hazen K C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 1988;56:2884–2890. doi: 10.1128/iai.56.11.2884-2890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt M E, Harrison L H, Pass M, Sofair A N, Huie S, Morrison C J, Warnock D W, Hajjeh R A. Candida dubliniensis fungemia: the first four cases in North America. Emerg Infect Dis. 2000;6:46–49. doi: 10.3201/eid0601.000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D M, Jabra-Rizk M A, Falkler W A, Baqui A A M A, Meiller T F. Identification of Candida dubliniensis in a study of HIV-seropositive pediatric dental patients. Pediatr Dent. 2000;22:234–238. [PubMed] [Google Scholar]
- 4.Chen P S, Jr, Toribara T Y, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 5.Coleman D C, Bennett D E, Sullivan D J, Gallagher P J, Henman M C, Shanley D B, Russell R J. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit Rev Microbiol. 1993;19:61–82. doi: 10.3109/10408419309113523. [DOI] [PubMed] [Google Scholar]
- 6.Coleman D C, Sullivan D J, Bennett D E, Moran G P, Barry H J, Shanley D B. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 8.Cutler J E, Han Y, Miller D. Fungal factors implicated in pathogenesis. In: Howard D H, editor. The mycota. Berlin, Germany: Springer-Verlag; 1996. pp. 3–29. [Google Scholar]
- 9.de Repentigny L, Aumount F, Bernard K, Belhumeur P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun. 2000;68:3172–3179. doi: 10.1128/iai.68.6.3172-3179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 11.Fradin C, Jouault T, Mallet A, Mallet J-M, Camus D, Sinaÿ P, Poulain D. β-1,2-Linked oligomannosides inhibit Candida albicans binding to murine macrophage. J Leukoc Biol. 1996;60:81–87. doi: 10.1002/jlb.60.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Gilfillan G D, Sullivan D J, Haynes K, Parkinson T, Coleman D C, Gow N A R. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology. 1998;144:829–838. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 13.Glee P M, Sundstrom P, Hazen K C. Expression of surface hydrophobic proteins by Candida albicans in vivo. Infect Immun. 1995;63:1373–1379. doi: 10.1128/iai.63.4.1373-1379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goins T L, Cutler J E. Relative abundance of oligosaccharides in Candida species as determined by fluorophore-assisted carbohydrate electrophoresis. J Clin Microbiol. 2000;38:2862–2869. doi: 10.1128/jcm.38.8.2862-2869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Kanbe T, Cherniak R, Cutler J E. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Y, Kanbe T, That T C C-T, Cutler J E. Candida mannan can induce protective antibodies in mice. In: Suzuki S, Suzuki M, editors. Fungal cells in biodefense mechanisms. Tokyo, Japan: Sarkon Publishing Co.; 1997. pp. 155–159. [Google Scholar]
- 18.Han Y, Morrison R P, Cutler J E. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998;66:5771–5776. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Y, Riesselman M H, Cutler J E. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect Immun. 2000;68:1649–1654. doi: 10.1128/iai.68.3.1649-1654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Y, Ulrich M A, Cutler J E. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis. 1999;179:1477–1484. doi: 10.1086/314779. [DOI] [PubMed] [Google Scholar]
- 21.Hasenclever H F. Comparative pathogenicity of Candida albicans for mice and rabbits. J Bacteriol. 1959;78:105–109. doi: 10.1128/jb.78.1.105-109.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazen B W, Hazen K C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988;56:2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazen K C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989;57:1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazen K C, Brawner D L, Riesselman M H, Cutler J E, Jutila M A. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazen K C, Glee P M. Cell surface hydrophobicity and medically important fungi. Curr Top Med Mycol. 1995;6:1–37. [PubMed] [Google Scholar]
- 26.Hazen K C, Glee P M. Hydrophobic cell wall protein glycosylation by the pathogenic fungus Candida albicans. Can J Microbiol. 1994;40:266–272. doi: 10.1139/m94-043. [DOI] [PubMed] [Google Scholar]
- 27.Hazen K C, Hazen B W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 1992;60:1499–1508. doi: 10.1128/iai.60.4.1499-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazen K C, Hazen B W. A polystyrene microsphere assay for detecting surface hydrophobicity variations within Candida albicans populations. J Microbiol Methods. 1987;6:289–299. [Google Scholar]
- 29.Hazen K C, Hazen B W. Surface hydrophobic and hydrophilic protein alterations in Candida albicans. FEMS Microbiol Lett. 1993;107:83–88. doi: 10.1111/j.1574-6968.1993.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 30.Hazen K C, Hazen B W. Temperature-modulated physiological characteristics of Candida albicans. Microbiol Immunol. 1987;31:497–508. doi: 10.1111/j.1348-0421.1987.tb03112.x. [DOI] [PubMed] [Google Scholar]
- 31.Hazen K C, Lay J-G, Hazen B W, Fu R C, Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990;58:3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazen K C, Plotkin B J, Klimas D M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect Immun. 1986;54:269–271. doi: 10.1128/iai.54.1.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabra-Rizk M A, Baqui A A M A, Kelley J I, Falkler W A, Jr, Merz W G, Meiller T F. Identification of Candida dubliniensis in a prospective study of patients in the United States. J Clin Microbiol. 1999;37:321–326. doi: 10.1128/jcm.37.2.321-326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jabra-Rizk M A, Falkler W A, Jr, Merz W G, Kelley J I, Baqui A A M A, Meiller T F. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J Clin Microbiol. 1999;37:1464–1468. doi: 10.1128/jcm.37.5.1464-1468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jabra-Rizk M A, Falkler W A, Merz W G, Baqui A A M A, Kelley J I, Meiller T F. Retrospective identification and characterization of Candida dubliniensis isolates among Candida albicans clinical laboratory isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected individuals. J Clin Microbiol. 2000;38:2423–2426. doi: 10.1128/jcm.38.6.2423-2426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabra-Rizk M A, Falkler W A, Merz W G, Kelley J I, Baqui A A A M, Meiller T F. Candida dubliniensis and Candida albicans display surface variations consistent with observed intergeneric coaggregation. Rev Iberoam Micol. 1999;16:187–193. [PubMed] [Google Scholar]
- 37.Jackson P. The use of polyacrylamide-gel electrophoresis for the high-resolution separation of reducing saccharides labelled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid. Biochem J. 1990;270:705–713. doi: 10.1042/bj2700705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson P, Williams G R. Polyacrylamide gel electrophoresis of reducing saccharides labeled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid: application to the enzymological structural analysis of oligosaccharides. Electrophoresis. 1991;12:94–96. doi: 10.1002/elps.1150120118. [DOI] [PubMed] [Google Scholar]
- 39.Jouault T, Lepage G, Bernigaud A, Trinel P-A, Fradin C, Wieruszeski J-M, Strecker G, Poulain D. β-1,2-Linked oligomannosides from Candida albicans act as signals for tumor necrosis factor alpha production. Infect Immun. 1995;63:2378–2381. doi: 10.1128/iai.63.6.2378-2381.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lloyd K O. Isolation, characterization, and partial structure of peptido galactomannans from the yeast form of Cladosporium werneckii. Biochemistry. 1970;9:3446–3453. doi: 10.1021/bi00819a025. [DOI] [PubMed] [Google Scholar]
- 41.Masuoka J, Hazen K C. Cell wall mannosylation determines Candida albicans cell surface hydrophobicity. Microbiology. 1997;143:3015–3021. doi: 10.1099/00221287-143-9-3015. [DOI] [PubMed] [Google Scholar]
- 42.Masuoka J, Hazen K C. Differences in the acid-labile component of Candida albicans mannan from hydrophobic and hydrophilic yeast cells. Glycobiology. 1999;9:1281–1286. doi: 10.1093/glycob/9.11.1281. [DOI] [PubMed] [Google Scholar]
- 43.Masuoka J, Wu G, Glee P M, Hazen K C. Inhibition of Candida albicans attachment to extracellular matrix by antibodies which recognize hydrophobic cell wall proteins. FEMS Microbiol Immunol. 1999;24:421–429. doi: 10.1111/j.1574-695X.1999.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 44.McCullough M, Ross B, Reade P. Characterization of genetically distinct subgroup of Candida albicans strains isolated from oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1995;33:696–700. doi: 10.1128/jcm.33.3.696-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meis J F G M, Ruhnke M, De Pauw B E, Odds F C, Siegert W, Verweij P E. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerg Infect Dis. 1999;5:150–153. doi: 10.3201/eid0501.990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercure S, Sénéchal S, Auger P, Lemay G, Montplaiser S. Candida albicans serotype analysis by flow cytometry. J Clin Microbiol. 1996;34:2106–2112. doi: 10.1128/jcm.34.9.2106-2112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Odds F C, Van Nuffel L, Dams G. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J Clin Microbiol. 1998;36:2809–2873. doi: 10.1128/jcm.36.10.2869-2873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okubo Y, Shibata N, Ichikawa T, Chaki S, Suzuki S. Immunochemical study on bakers' yeast mannan prepared by fractional precipitation with cetylmethylammonium bromide. Arch Biochem Biophys. 1981;212:204–215. doi: 10.1016/0003-9861(81)90360-x. [DOI] [PubMed] [Google Scholar]
- 49.Peat S, Whelan W J, Edwards T E. Polysaccharides of baker's yeast. Part IV. Mannan. J Chem Soc. 1961;1:29–34. [Google Scholar]
- 50.Poulain D, Jouault T, Trinel P A. Immunoreactivity of Candida albicans β-1, 2 linked oligomannosides and phospholipomannan. In: Suzuki S, Suzuki M, editors. Fungal cells in biodefense mechanisms. Tokyo, Japan: Sarkon Publishing Co.; 1997. pp. 175–173. [Google Scholar]
- 51.Poulain D, Robert R, Mesnard F, Sendid B, Lepage G, Camus D. Clearances of Candida albicans-derived α- and β-linked mannose residues in sera from patients with candidiasis. Eur J Clin Microbiol Infect Dis. 1997;16:16–20. doi: 10.1007/BF01575114. [DOI] [PubMed] [Google Scholar]
- 52.Schoofs A, Odds F C, Colebunders R, Ieven M, Goossens H. Use of specialised isolation media for recognition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 53.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan D, Bennett D, Henman M, Harwood P, Flint S, Mulcahy F, Shanley D, Coleman D. Oligonucleotide fingerprinting of isolates of Candida species other than C. albicans and of atypical Candida species from human immunodeficiency virus-positive and AIDS patients. J Clin Microbiol. 1993;31:2124–2133. doi: 10.1128/jcm.31.8.2124-2133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan D, Coleman D. Candida dubliniensis: an emerging opportunistic pathogen. Curr Top Med Mycol. 1997;8:15–25. [PubMed] [Google Scholar]
- 56.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan D J, Moran G, Donnelly S, Gee S, Pinjon E, McCartan B, Shanley D B, Coleman D C. Candida dubliniensis: an update. Rev Iberoam Micol. 1999;16:72–76. [PubMed] [Google Scholar]
- 58.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki A, Takata Y, Oshie A, Tezuka A, Shibata N, Kobayashi H, Okawa Y, Suzuki S. Detection of β-1,2-mannosyltransferase in Candida albicans cells. FEBS Lett. 1995;373:275–279. doi: 10.1016/0014-5793(95)01061-i. [DOI] [PubMed] [Google Scholar]
- 60.Timmins E M, Howell S A, Alsberg B K, Noble W C, Goodacre R. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy. J Clin Microbiol. 1998;36:367–374. doi: 10.1128/jcm.36.2.367-374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whelan W L, Delga J M, Wadsworth E, Walsh T J, Kwon-Chung K J, Calderone R, Lipke P N. Isolation and characterization of cell surface mutants of Candida albicans. Infect Immun. 1990;58:1552–1557. doi: 10.1128/iai.58.6.1552-1557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]